Abstract
Ischemia-reperfusion of the intestine produces a set of inflammatory mediators, the origin of which has recently been shown to involve pancreatic digestive enzymes. Matrix metalloproteinase-9 (MMP-9) participates in a variety of inflammatory processes including myocardial, hepatic, and pancreatic ischemia-reperfusion. In the present study, we explore the role of neutrophil-derived MMP-9 in acute intestinal ischemia-reperfusion and its interaction with pancreatic trypsin. Male Sprague-Dawley rats were subjected to 45 minutes of superior mesenteric arterial occlusion followed by 90 minutes of reperfusion. In situ zymography of the proximal jejunum reveals increased gelatinase activity in the intestinal wall after ischemia-reperfusion. Gel electrophoresis zymography and immunofluorescence co-localization suggests that this gelatinase activity is derived from MMP-9 released from infiltrating neutrophils. The role of intraluminal trypsin in this process was investigated using an in vivo isolated jejunal loop model of intestinal ischemia-reperfusion. Trypsin increased the inflammatory response after reperfusion, with an augmented neutrophil infiltration of the intestinal wall. Furthermore, trypsin stimulated a rapid conversion of neutrophil-released proMMP-9 into the lower molecular weight enzymatically active MMP-9. This process represents a powerful in vivo pathophysiological mechanism for trypsin-induced MMP-9 activation and is likely to play a central role in the development of acute intestinal inflammation and shock.
Intestinal ischemia-reperfusion (I/R) is a localized pathology that often leads to multi-organ dysfunction and shock.1,2 Intestinal I/R serves to generate several inflammatory mediators including reactive oxygen metabolites, platelet-activating factor, leukotriene B4, and proinflammatory cytokines.3–8 Reperfusion of the ischemic intestine leads to the production of myocardial depressant factor (MDF) resulting in compromised cardiac function and the exacerbation of shock.9 We have recently shown that MDF-like inflammatory mediators are produced during intestinal I/R by proteolytic cleavage of the extracellular matrix, in a pancreatic protease-dependent mechanism.10 Other studies suggest that protease digestion of extracellular matrix proteins may reveal hidden bioactive epitopes or release proinflammatory mediators, collectively termed matricryptic effectors.11
Among the pancreatic proteases involved in the production of inflammatory mediators, trypsin stands out as an important player. In addition to its role as a mediator of morphological injury in intestinal I/R,12 trypsin yields potent small molecular weight leukocyte activators during digestion of intestine as well as other organs.13 In addition, trypsin may activate complement14 or stimulate protease activated receptor-2 in the microvasculature15 or in neutrophils.16
Even though the pancreatic digestive enzymes in the lumen of the intestine are powerful by themselves, other proinflammatory proteases may be involved in intestinal injury as well. Matrix metalloproteinase-9 (MMP-9) is a structurally complex metalloproteinase (endoproteases with zinc in its active center) family that is involved in the inflammatory and immune responses.17–19 MMP-9 has recently been implicated in a variety of cardiovascular pathologies. After myocardial I/R neutrophils release MMP-9, resulting in a robust extracellular matrix turnover.20 MMP-9 also participates in the pathophysiology of hemorrhagic stroke.21 Inhibition of matrix metalloproteinases, including MMP-9, during hepatic I/R decreases cell injury in the liver.22 MMP-9 is involved in both the pathogenesis of pancreatitis and in its associated lung injury.23,24 Despite these findings, the involvement of MMP-9 in intestinal I/R has not been investigated.
We hypothesize that MMP-9 levels and activity are elevated in the intestinal wall during acute I/R, a situation that promotes inflammation and tissue injury. MMP-9 is released as an inactive proenzyme that can be activated in vitro by trypsin through a “cysteine-switch” mechanism.25,26 Although pancreatic trypsin could represent an in vivo mechanism for MMP-9 activation in pancreatitis23 and inflammatory bowel disease,27 a physiological role for this process has not been identified. Pancreatic trypsin entering a disrupted intestinal wall could serve as both a proinflammatory stimulus as well as a mechanism of acute MMP-9 activation.
Thus, the aim of this study is to investigate the presence of MMP-9 in intestinal I/R and the role of pancreatic trypsin in its activation. Following superior mesenteric arterial occlusion and reperfusion we demonstrate that MMP-9 is highly expressed in the wall of the small intestine and derived mostly from infiltrating neutrophils. We also demonstrate rapid proteolytic activation of MMP-9 by pancreatic trypsin in the wall of the intestine after I/R as a potential mechanism for intestinal injury.
Materials and Methods
Animals and Reagents
Male Sprague-Dawley rats (350 to 450 g, Charles River Laboratories, Wilmington, MA) were housed in a controlled environment and maintained on a standard pellet diet for at least 3 days before the experimental procedures. The experiments were reviewed and approved by the University of California at San Diego Animal Use and Care Committee. All surgical procedures were performed in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals. At the end of the experiment, the animals were euthanized with an intravenous overdose of sodium pentobarbital (120 mg/kg, Abbott, North Chicago, IL). Unless noted otherwise, reagents were purchased from Sigma Chemical Corp. (St. Louis, MO).
Anesthesia and Catheter Placement
After induction of general anesthesia (sodium pentobarbital, 50 mg/kg, i.m., Abbott, Chicago, IL) the right jugular vein was cannulated (PE50 tubing, Clay Adams, Parsippany, NJ) for supplemental anesthesia and fluid replacement. A catheter was inserted into the left femoral artery (PE50, Clay Adams) to assess mean arterial blood pressure. Cardiovascular parameters were continuously recorded with a pressure transducer (Statham, Gould Medical Products, Oxnard, CA, amplified by CTH-2000 amplifier, CB Sciences, St. Dover, NH) and recorded onto a lab computer (G3 Macintosh with AD Instruments, Mountain View, CA). To reduce the risk of respiratory failure, a tracheotomy was performed (PE260 tubing, Clay Adams).
Superior Mesenteric Artery Occlusion
The superior mesenteric artery was isolated through an abdominal midline incision and clamped for 45 minutes (surgical bullclamps, World Precision Instruments, Sarasota, FL) after measurement of the mean arterial blood pressure. The mesenteric collateral arterial arcade at the proximal jejunum and terminal ileum were isolated from the surrounding adipose tissue and ligated before ischemia; pilot studies demonstrated that the occlusion of these collaterals was necessary to produce uniform ischemia in the small intestine. The animals were subsequently observed for 90 minutes of reperfusion. The model produced a significant and consistently maintained systemic hypotension after mesenteric reperfusion (data not shown).
Isolated Jejunal Loop Ischemia-Reperfusion
After abdominal midline incision, a jejunal loop (4 to 5 cm long) with a single vascular pedicle was selected. The arterioles of the anastomotic arcade were ligated and the jejunal loop was isolated from the rest of the intestine, whose continuity was re-established, while keeping an intact vascular pedicle from the superior mesenteric artery. The luminal content was carefully rinsed with Krebs-Henseleit buffer and the jejunal loop was subjected to continuous intraluminal perfusion with Krebs-Henseleit buffer under controlled intraluminal pressure, temperature, pH, and osmolarity. In some of the animals, the Krebs-Henseleit buffer was supplemented with bovine pancreatic trypsin (1 mg/ml); this concentration of trypsin in the jejunal lumen falls within its described physiological range.28,29 In the animals subjected to I/R, the vascular pedicle to the jejunal segment was clamped for 45 minutes and the animals were observed during a reperfusion period of 90 minutes.
Intestinal Sample Preparation
After rinsing the intestine with cold phosphate-buffered saline, tissue samples were embedded (TBS Freezing Medium, Triangle Biomedical Sciences, Durham, NC) in disposable base molds (Electron Microscopy Sciences, Ft. Washington, PA). The entire sample was snap-frozen in liquid nitrogen and stored at −80°C until further analysis.
Jejunal samples were homogenized on ice in 10 mmol/L phosphate-buffered saline (Polytron homogenizer; 1:10 w/v, Brinkman Instruments, Westbury, NY). Homogenates were then centrifuged at 14000 × g for 30 minutes at 4°C (Sorvall RC 5C Super Centrifuge, Sorvall, Newtown, CT). The supernatant was aliquoted and stored at −80°C until further analysis. In the preparation of samples from the isolated jejunal loop experiments the homogenization buffer contained the serine and cysteine protease inhibitors Pefabloc SC (500 μg/ml, Roche, Mannheim, Germany), leupeptin (0.5 μg/ml, Calbiochem, La Jolla, CA), aprotinin (1 μg/ml, Roche), and E64 (5 μg/ml, Roche).
Intravital Microscopy
In selected animals the intestinal surface (serosal side up) of the isolated jejunal loop during I/R was viewed by intravital microscopy with epifluorescence.30 Each animal received a single bolus of rhodamine 6G (1 mg/kg, i.v.) to label leukocytes, which were observed in the post-capillary and collecting venules of the submucosa of the intestinal wall. Leukocytes were considered to be rolling if seen moving at a slower speed than adjacent red blood cells, and counted over a period of 1 minute; adherent leukocytes remained stationary for at least 30 seconds and were counted over a vessel length of 100 μm. All measurements were normalized by the vessel diameter. All intravital observations were performed at 90 minutes of reperfusion, or at an equivalent time point in the control experiments.
Neutrophil Isolation and Incubation
Human neutrophils were isolated from peripheral venous blood following dextran-induced erythrocyte sedimentation and centrifugation on Percoll density gradients. Each neutrophil suspension was more than 95% pure in terms of cell type and had more than 98% viable cells. They were suspended in Hank’s buffered saline solution with glucose at a concentration of 1.0 × 106/ml and incubated at 37°C for 60 minutes, with or without pancreatic trypsin or the neutrophil activator phorbol-myristate acetate (PMA). At the end of incubation an aliquot of the suspension was collected to assess neutrophil death by trypan blue exclusion. The cells were then spun down and the supernatant collected and stored at −80°C until further analysis.
Histology
Frozen tissue sections (5 μm) were fixed for 30 minutes in cold methanol. After fixation, the samples were stained with Gill’s hematoxylin and eosin using standard techniques. The sections were dehydrated with an ethanol gradient and mounted using Permount Media (Fisher Chemicals, Springfield, NJ).
Myeloperoxidase Histochemistry
In situ staining for myeloperoxidase was adapted from previously described methods.7 Frozen tissue sections (10 μm) were fixed for 30 minutes in cold methanol. After fixation, the samples were thoroughly rinsed in Krebs-Ringer buffer. Myeloperoxidase-positive cells were detected using a mixture of 10 mg Hanker-Yates reagent (Fluka/Riedel-deHaën, St. Louis, MO), 10 ml Krebs-Ringer buffer, and 100 μl of 3% H2O2 for 5 minutes at room temperature. Stained sections were dehydrated in an ethanol gradient and mounted on a glass slide (Permount Media). The numerical density of myeloperoxidase-stained cells was determined at high magnification. Ten random independent images from multiple sections were generated for both the muscularis and submucosa of each sample. The average numerical density was then recorded for each experimental group using NIH Image software. Repeated studies with identical sections showed a counting error of less than 5%.
In Situ Zymography
Gelatinase activity was detected in situ by visualization of fluorescence from the proteolytic cleavage of intramolecularly quenched fluorescein isothiocyanate (FITC)-labeled DQ-gelatin (Molecular Probes, Eugene, OR).31,32 Briefly, 10-μm frozen sections were equilibrated at room temperature and overlaid with 20 μl of a solution containing 20 μmol/L FITC DQ-gelatin in 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 5 mmol/L CaCl2, 0.2 mmol/L NaN3, 0.02% Brj35, and 0.5% low melting point agarose. The sections were then cover-slipped, allowed to gel for 5 minutes at 4°C and incubated for 4 hours at 37°C. The sections were incubated in the presence of the serine and cysteine protease inhibitors Pefabloc SC (500 μg/ml), leupeptin (0.5 μg/ml), aprotinin (1 μg/ml), and E64 (5 μg/ml). Some of the sections were incubated in the absence of the fluorogenic substrate or in the presence of the metalloproteinase inhibitor ethylenediaminotetracetate (EDTA, 10 mmol/L). As control to assess trypsin infiltration into the intestinal wall, selected sections derived from the isolated jejunal loop were incubated in the absence of serine protease inhibitors.
Immunofluorescence
Frozen tissue sections (5 μm) were applied to Vectabond-treated (Vector Laboratories, Burlingame, CA) slides. The sections were then dried overnight at 37°C to ensure that the sample would adequately adhere to the slide. Fixation was performed with cold acetone for 10 minutes. The tissue sections were stained for neutrophils with a purified mouse anti-rat granulocyte antibody (HIS48; BD PharMingen, San Diego, CA). A FITC-conjugated anti-mouse IgM was used for visualization (Vector Laboratories). Sections were also stained for MMP-9 using a goat polyclonal IgG antibody (C-20; Santa Cruz Biotechnology, Santa Cruz, CA). Visualization was subsequently performed with a Texas Red-conjugated anti-goat IgG (Vector Laboratories). To examine possible bleed-over fluorescence, the staining for the two antigens was also performed in different tissue sections. The anti-MMP-9 antibody used did not cross-react with other metalloproteinases, namely with MMP-2.
Gelatin Zymography
Gelatin zymography was performed as previously described.20,23,24 Briefly, SDS-denatured samples under non-reducing conditions were loaded onto 10% polyacrylamide gels containing 0.1% gelatin (Invitrogen, Carlsbad, CA). After electrophoresis, the proteins were re-natured with Triton X-100 and the gel incubated for 18 hours at 37°C in a developing buffer containing 50 mmol/L Tris-HCl, 150 mmol/L NaCl, 5 mmol/L CaCl2, and 0.02% Brj35. In addition some of the gels were incubated in the presence of the metalloproteinase inhibitor EDTA (10 mmol/L) or the serine protease inhibitor Pefabloc SC (500 μg/ml). The gels were subsequently developed with 0.5% Coomassie blue in 40% methanol and 10% acetic acid. The gels were de-stained three times with the same solution lacking Coomassie blue. Pre-stained molecular weight standards (Pierce, Rockford, IL), purified MMP-2/MMP-9 standards (Chemicon, Temecula, CA), and supernatant obtained from activated human neutrophils33 were used for reference. To determine relative activity levels, the zymograms were inverted and densitometry levels were measured with digital image analysis (NIH Image software).
Western Blot
Samples were denatured by SDS in the presence of mercaptoethanol, loaded onto 7.5% polyacrylamide gels and transferred to a PVDF membrane after electrophoresis. Membranes were blocked overnight at 4°C in Tris-buffered saline containing 5% bovine serum albumin and then incubated with anti-rat MMP-9 goat polyclonal IgG antibody (C-20, Santa Cruz Biotechnology) for 90 minutes. After thorough washing, the membranes were incubated with horseradish peroxidase-conjugated anti-goat secondary antibodies for 90 minutes and the bands were detected by chemiluminescence (ECL Western Blotting Detection Kit, Amersham, Piscataway, NJ).
Statistical Analysis
All values are reported as mean ± SD. The unpaired, two-tailed Student’s t-test was performed to determine differences between two experimental groups. To compare more than two experimental groups the one-way analysis of variance test was used with Bonferroni post-hoc test. To correlate different variables the non-parametric Spearman test was performed. P < 0.05 was considered statistically significant.
Results
Intestinal MMP-9 Is Increased following Acute Ischemia-Reperfusion
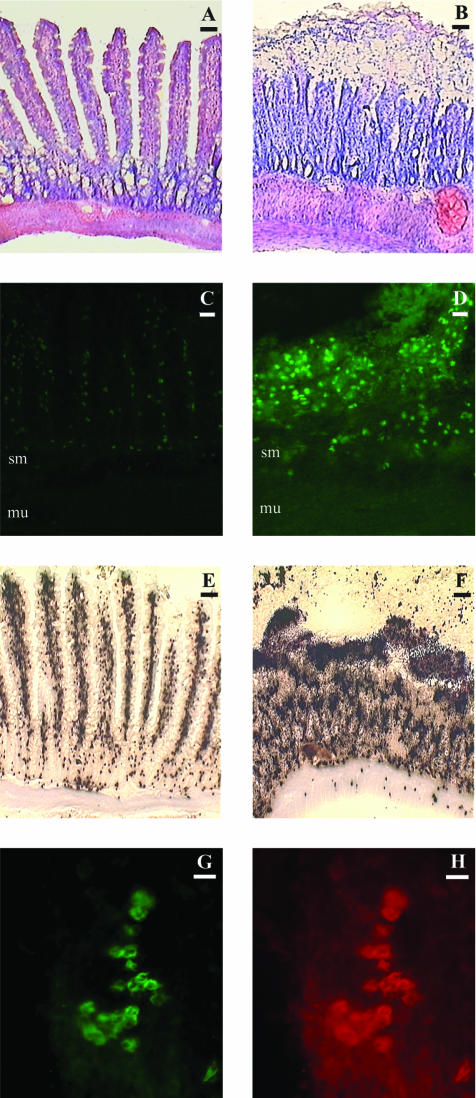
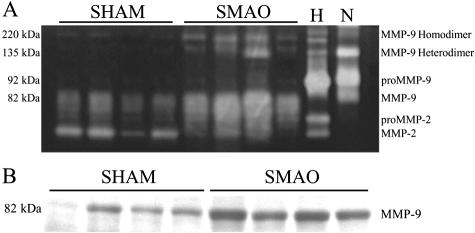
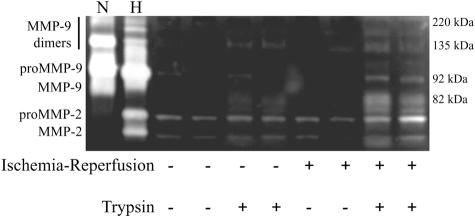
Acute intestinal I/R was accompanied by considerable histological damage, including edema, destruction of villi, and vascular congestion (Figure 1, A and B). In situ zymography revealed increased gelatinolytic activity in I/R, in close correlation with increased morphological tissue damage (Figure 1, C and D). Gelatin zymography demonstrated that this increased activity was in line with significantly increased levels of MMP-9 (P < 0.05 for monomers and P < 0.01 for dimers; data not shown) in the intestinal wall following I/R (Figure 2A). Immunoblotting (Figure 2B) confirmed the results of the zymography.
Figure 1.
A and B: Light microscopic images of intestinal wall sections after staining with Gill’s hematoxylin and eosin. Animals subjected to superior mesenteric arterial occlusion (B) exhibit severe morphological damage to the intestinal wall. In contrast, controls in section (A) maintain overall morphological integrity. Bars, 50 μm. C and D: In situ zymography of animals subjected to superior mesenteric arterial occlusion. Sections from sham-operated controls show little gelatinase activity in the submucosa of the intestine (C). In contrast, SMAO animals exhibit high levels of gelatinase activity throughout the intestinal tissue (D). Application of EDTA eliminates the gelatinase activity in both groups, indicating that the proteolytic activity originates from matrix metalloproteinases. sm, submucosa; mu, muscularis. Bars, 30 μm. E and F: Myeloperoxidase staining of animals subjected to superior mesenteric arterial occlusion. Sham-operated controls show low amounts of myeloperoxidase staining (E), as compared to SMAO animals, which exhibit high levels of myeloperoxidase staining throughout the submucosa and muscularis of the jejunum (F). Bars, 50 μm. G and H: Immunofluorescent staining of neutrophils and MMP-9 in samples subjected to superior mesenteric arterial occlusion. Staining for neutrophils reveals aggregates of infiltrating cells throughout the submucosa (G). Staining of the same section for MMP-9 reveals co-localization of the enzyme to the infiltrating neutrophils (H). Bars, 10 μm.
Figure 2.
A: Gel electrophoresis zymography of intestinal tissue subjected to superior mesenteric arterial occlusion. The four left lanes are from a representative control (SHAM) each, the four right lanes from a representative I/R (SMAO) animal each (n = 8 in each group). Animals subjected to intestinal ischemia-reperfusion present significantly higher level of MMP-9 (82 kd). Higher molecular weight gelatinase bands (220 kd and 135 kd, corresponding to MMP-9 homodimers and heterodimers, respectively) are also increased, suggesting that neutrophils are a major source of MMP-9 in this setting. Lane H represents human MMP-9/MMP-2 standards and lane N represents neutrophil-derived MMP-9 standards. Addition of EDTA to the developing buffer abolished the bands, while Pefabloc SC did not cause changes. Samples from experimental animals contained 2.5 μg of protein/well. B: Western blot analysis of MMP-9 in SMAO animals after intestinal ischemia-reperfusion and SHAM controls (representative of n = 8 in each group). Samples from experimental animals contained 45 μg of protein/well.
Neutrophils Are the Source of Intestinal MMP-9 in Acute Ischemia-Reperfusion
The zymogram in Figure 2A showed the presence of increased levels of MMP-9 homo and heterodimers (at 220 kd and 135 kd, respectively) after intestinal I/R. Since the dimeric forms of MMP-9 (most notably the heterodimers) are highly suggestive to originate from the neutrophil,34–37 we sought to evaluate the role of infiltrating neutrophils in MMP-9 accumulation. Histochemical staining for the neutrophilic enzyme myeloperoxidase yielded an important infiltration of neutrophils all across the intestinal wall after I/R (Figure 1, E and F). The density of neutrophils was significantly increased in both the submucosa and muscularis (P < 0.001 and P < 0.01, respectively; data not shown). Interestingly, in each animal the accumulation of MPO-positive cells in the submucosa correlated positively with the densitometric measurement of MMP-9 monomers (r = 0.56; P = 0.02) and MMP-9 dimers (r = 0.68; P = 0.004) in the zymogram. A denser staining at the tips of the destroyed villi coincides with the topographic localization of increased gelatinolytic activity by in situ zymography. In addition, immunofluorescence for both MMP-9 and infiltrating neutrophils showed a high degree of co-localization within the intestinal wall (Figure 1, G and H), further supporting a role for neutrophil-mediated accumulation of MMP-9 in this setting.
Intraluminal Trypsin Increases Neutrophil Infiltration in Intestinal Ischemia-Reperfusion
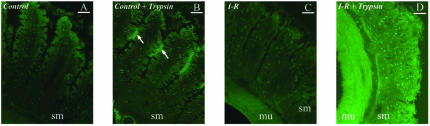
We next investigated whether intraluminal pancreatic trypsin could play a role in neutrophil-induced MMP-9 release and activation. We first developed an in vivo model for controlling the luminal content of a single jejunal loop to which the vascular supply could be occluded and later reestablished, thus creating a model for small intestine single loop I/R. In situ detection of serine protease activity shows the influx of trypsin into the intestinal wall in this model following ischemia and reperfusion (Figure 3).
Figure 3.
In situ detection of serine protease activity in the intestinal wall. The panels show non-ischemic control intestine (A), control intestine with intraluminal trypsin lavage (B), intestine after ischemia-reperfusion (C), intestine after ischemia-reperfusion with trypsin in the lumen of the intestine (D). When trypsin is added under control conditions, its presence is observed in the intraluminal area as bright fluorescent patches (arrows) between the villi, but not inside the intestinal wall (B). Under I/R, the addition of trypsin results in bright background fluorescence permeating the intestinal wall (D). sm, submucosa; mu, muscularis. Bars, 20 μm.
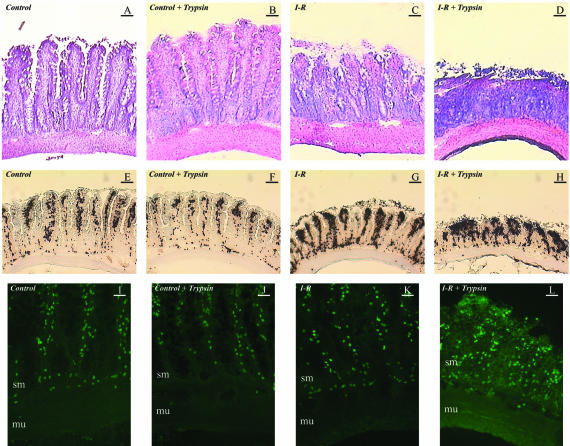
Histology showed that the induction of I/R in this model was associated with morphological injury accentuated by the presence of trypsin in the luminal content (Figure 4, A to D). Histology also suggested that I/R in the presence of trypsin was associated with an increased inflammatory infiltrate.
Figure 4.
A–D: Bright-field images of intestinal sections after Gill’s hematoxylin and eosin staining. The four lanes (from left to right) show non-ischemic control intestine (first row), control intestine with intraluminal trypsin lavage (second row), control intestine after ischemia and reperfusion (third row), intestine after ischemia-reperfusion with trypsin in the lumen of the intestine (fourth row). Ischemia-reperfusion generates morphological damage that is potentiated by trypsin. Bars, 50 μm. E–H: Histochemistry for myeloperoxidase in jejunal sections for the same groups outlined in the description of A–D. The images demonstrate an increase in neutrophil infiltration during intestinal ischemia-reperfusion. Moreover, the presence of luminal trypsin in intestinal ischemia-reperfusion is associated to increased morphological damage with a more diffuse and infiltrative distribution of the heavier neutrophilic component. Bars, 50 μm. I–L: In situ zymography for gelatinase activity in the same groups as outlined in A–D. Ischemia-reperfusion in the presence of trypsin is associated to a major increase in the damaged wall of the intestine. This activity is abolished by EDTA, confirming matrix metalloproteinases as its source. sm, submucosa; mu, muscularis. Bars, 30 μm.
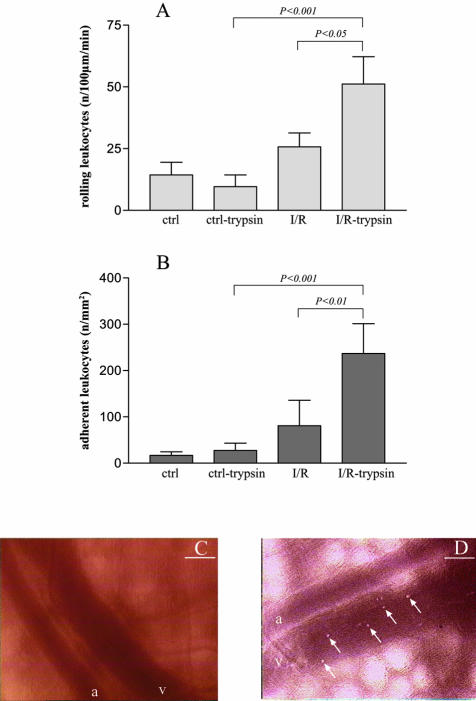
Histochemistry for the neutrophil enzyme myeloperoxidase showed that intraluminal trypsin potentiates the increase in neutrophil infiltration induced by I/R (Figure 4, E to H), namely in the submucosal area (P < 0.01 for I/R with trypsin versus I/R alone or control with trypsin; data not shown). In addition, intravital microscopy allowed to demonstrate that trypsin causes a significant increase in leukocyte rolling and adhesion in the post-capillary and early collecting venules of the jejunal submucosa following I/R (Figure 5).
Figure 5.
A: The number of rolling leukocytes (per length of post-capillary venule and per time) derived from the intravital microscopic record of the isolated intestinal loop at a location on the surface of the jejunum. Four cases are shown: control intestine (ctrl), control intestine with trypsin in the lumen of the intestine (ctrl-trypsin), intestine after ischemia-reperfusion (I/R), and intestine after ischemia-reperfusion with trypsin (I/R-trypsin). The number of rolling leukocytes are increased significantly in ischemia-reperfusion only when pancreatic trypsin is present, compared to animals with ischemia-reperfusion without trypsin or control animals with trypsin. N = 4 to 5 in each group. B: The number of adherent leukocytes per endothelial surface area is also significantly increased in ischemia-reperfusion animals in the presence of intraluminal trypsin versus ischemia-reperfusion without trypsin or control animals with trypsin. Groups (n = 4 to 5) are the same as in A. C and D: Representative intravital microscopy images from submucosal collecting venules in an animal from group ctrl-trypsin (C) and I/R-trypsin (D). In C, no detectable leukocytes adhere to the wall of the venule. The arrows point at individual leukocytes adherent to the vessel wall; a stands for arteriole, v for venule. Bars, 100 μm.
Trypsin Increases MMP-9 in the Intestinal Wall and Activates Its Proform
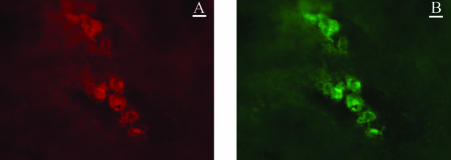
We next showed that this model is also associated with increased presence of neutrophil-derived MMP-9 in the intestinal wall. Using in situ zymography, we identified an increase in gelatinolytic activity in the reperfused intestinal wall only in the presence of trypsin (Figure 4, I to L). The topographical localization of gelatinolytic activity coincided with the increased submucosal localization of myeloperoxidase-positive cells, mostly neutrophils with segmented nucleus. Co-localization of infiltrating neutrophils with MMP-9 activity was consistently detected by dual immunofluorescence staining (Figure 6).
Figure 6.
Example of dual immunofluorescence labeling of intestinal wall submucosa from the isolated jejunal loop with antibody against MMP-9 (A) and neutrophils (B) following ischemia-reperfusion in the presence of pancreatic trypsin. The images were taken at the same position and show direct co-localization of MMP-9 and neutrophils. The co-localization shown here is the same in all other parts of the tissue (results not shown). Bars, 10 μm.
Gelatin zymography served to further characterize the role of trypsin in inducing MMP-9 infiltration in the intestinal wall (Figure 7). This technique showed that in acute small intestinal I/R the presence of trypsin was associated with a significant accumulation of MMP-9. The zymographic evidence supports the hypothesis that MMP-9 is neutrophilic in origin, since it also shows bands for MMP-9 dimers.
Figure 7.
Gelatin gel zymography (representative of n = 4 in each of the four groups) due to MMP-9 activity in intestinal loops of tissue derived from (−) sham controls (four left lanes) and after (+) ischemia and reperfusion (four right lanes) with (+) and without (−) pancreatic trypsin. In this experiment MMP-9 appears mostly in its active form (82 kd). Lane H represents human MMP-9/MMP-2 standards and lane N represents neutrophil-derived MMP-9 standards. Addition of EDTA to the developing buffer abolished the bands, while Pefabloc SC did not cause changes. Samples from experimental animals contained 25 μg of protein/well.
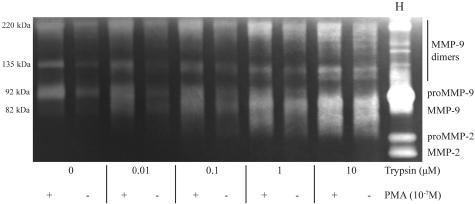
Zymography showed consistently that in acute intestinal I/R MMP-9 appears mostly in its active form (predominantly at 82 kd), suggesting that intraluminal pancreatic trypsin released into the intestinal wall could play a role in rapid activation of MMP-9. To investigate this possible phenomenon, we incubated isolated neutrophils with pancreatic trypsin in the presence of PMA, a neutrophil activator that causes degranulation of neutrophil tertiary granules and release of MMP-9. In Figure 8 we show that while PMA alone caused substantial release of proMMP-9 from the neutrophils into the supernatant, the presence of trypsin was required to induce the fast conversion of this metalloproteinase into its active form. Trypsin alone caused some level of neutrophil activation, with proMMP-9 release and activation in line with previous experiments.23 In our experimental setting the release of MMP-9 into the supernatant was not caused by trypsin-dependent neutrophil death, as assessed by trypan blue exclusion (data not shown).
Figure 8.
Zymography (representative of three independent experiments) from the supernatants of human neutrophils with (+) and without (−) PMA stimulation. There is release of significant levels of proMMP-9, which is converted to active lower molecular weight forms by the addition of pancreatic trypsin (shown at different concentrations). Lane H represents human MMP-9/MMP-2 standards.
Discussion
The current results indicate that during intestinal I/R the levels of MMP-9 increase in the wall of the small intestine. MMP-9 was found to be mostly neutrophil-associated. Pancreatic trypsin, which following opening of the intestinal mucosal barrier during I/R penetrates into the intestinal wall, facilitates accumulation of neutrophils and MMP-9 in the intestine. In addition, trypsin serves as an effective activator of the MMP-9 proform released from neutrophils.
In the present experiments, high levels of MMP-9 were identified in the intestinal wall after only 90 minutes of reperfusion. The evidence is in line with the observations of a rapid and intense expression of endothelial-derived adhesion molecules in the intestinal microcirculation with subsequent neutrophil trapping.38 Neutrophil-derived MMP-9 is stored in tertiary granules and released on stimulation. In contrast to the primary neutrophilic granules, the tertiary granules of neutrophils are usually the first to be degranulated requiring the lowest level of stimulation.19 Thus, neutrophils may rapidly release MMP-9 following entrapment in the intestinal microcirculation.
We found unusually pronounced levels of neutrophil-derived MMP-9 during intestinal I/R. Previous acute inflammation models have demonstrated significant increases in MMP-9 when loading zymographic gels with approximately 100 μg of protein per sample.20–24 In the present experiments, zymography gels were loaded with 2.5 μg suggesting that the strong bands we obtain were significantly stronger than those from other organs. This is probably due to the fact that intestinal I/R represents a particularly aggressive pathophysiological process, where the reperfusion injury is potentiated by an inflammatory reaction originating from the lumen contents. There is strong evidence that the invasion of the intestinal wall by resident bacterial flora and endotoxin is one of the key events that induces local inflammation, although their involvement in the pathogenesis of associated systemic inflammation and multiple organ injury is still uncertain.39 We have previously demonstrated that the luminal pancreatic proteases are also key mediators involved in augmented intestinal inflammation,1,10,40 and their intraluminal inhibition is associated with decreased neutrophil infiltration.41 In the present experiments, we clearly show that luminal pancreatic trypsin produces an increased inflammatory response that translates into a rising neutrophil infiltration into the intestinal wall.
MMP-9 plays a prominent role in the regulation of leukocyte biology in the context of the inflammatory response.18,19 A major activity of MMP-9 is the cleavage of extracellular matrix proteins (eg, denatured collagens I and II, collagen IV, and laminin), thereby playing an important role in cell migration and extracellular matrix remodeling.17 Several cytokines and growth factors are reported to be clipped by MMP-9 yielding a lower molecular weight protein with more potent biological activity.19 The cleavage may be particularly important for interleukin-8, a neutrophil chemoattractant chemokine, which in turn activates the release of MMP-9 by neutrophils and may thereby create a positive feedback loop.42 The extracellular matrix itself also contains signals that control cell shape, migration, and survival.43,44 Tissue injury may result in the proteolytic modification of existing extracellular matrix proteins and exposure of biologically active matricryptic sites that can provide important signals within the injured tissue.11,17,45 More specifically, neutrophils have been cited as sources of enzymes that commonly contribute to the enzymatic exposure of matricryptic sites,46,47 while MMP-9 itself generates proinflammatory neo-epitopes.18
Lumen-derived pancreatic trypsin may serve to rapidly activate MMP-9 during I/R of the small intestine. Trypsin is a potent in vitro activator of proMMP-9,25,26 but its in vivo relevance was regarded to be limited, due to compartmentalization of these enzymes. Several groups have reported a potential role for trypsin in the activation of MMP-9 in neoplasia growth,48 acute pancreatitis,23 and inflammatory bowel disease.27 During acute intestinal I/R, we observe that pancreatic trypsin invades the intestinal wall through the ruptured mucosal barrier and acts as an acute activator of MMP-9 released by infiltrating neutrophils.
Other mechanisms may exist to enhance the proteolytic activity of neutrophil-derived MMP-9. Although mast cell-derived tryptase is not a potent activator of matrix metalloproteinases, mast cell-derived chymase can activate MMP-9.49 In our experimental settings, though, this is less likely to play a central role since intestinal mast cells release low levels of chymase.50 Lipopolysaccharide-associated proteinases have the ability to activate MMP-9 zymogens in vitro.51 In addition, lipopolysaccharide produces chemotactic gradients that will attract neutrophils to the damaged intestine, further enhancing the activity of MMP-9.52 Intestinal lumen-derived lipopolysaccharide and pancreatic trypsin could thus serve as co-factors in the recruitment and activation of MMP-9 in intestinal I/R.
A considerable body of evidence suggests that intestinal I/R is frequently followed by shock and multi-organ failure.53 The process is accompanied by production of powerful inflammatory mediators with a variety of pathophysiological activities, including the suppression of different cell and organ functions and the exacerbation of shock symptoms.9,54 In the past, a number of inflammatory mediators have been proposed, including reactive oxygen and nitrogen metabolites, platelet-activating factor, leukotriene B4, endotoxin, and cytokines.3–8 But most interventions against these mediators have shown limited success against the deleterious progression of shock.6
Recently we have obtained evidence that pancreatic digestive enzymes may produce not only activating but also cytotoxic mediators for leukocytes and other cells in the microcirculation.55,56 We hypothesize that pancreatic digestive enzymes enter the wall of the intestine on elevation of the mucosal epithelial permeability after I/R. The production of inflammatory mediators in mesenteric I/R can be inhibited by blockade of digestive proteases in the lumen of the intestine, a procedure that attenuates many symptoms of experimental shock.1,10,40,41,57,58 Inflammatory mediators are readily produced in vitro from intestinal tissue in the presence of digestive enzymes, among which pancreatic trypsin serves as a particularly potent producer of mediators for leukocyte activation.13 The inflammatory mediators produced by proteolytic digestion of the intestinal wall consist of both peptide and lipid components, many of which with molecular weight below 10 kd.59 This process of proteolytic digestion of matrix may reveal hidden bioactive epitopes, previously denoted as toxic or matricryptic peptides.2,11
The current evidence shows that in addition to its ability to generate toxic peptides, pancreatic trypsin may also serve to enhance the activity of other proteases such as that of MMP-9, and possibly amplify tissue damage produced by proteolytic enzymes. Neutrophil-derived MMP-9 may act in collaboration with pancreatic digestive enzymes in generating inflammatory mediators during intestinal I/R. Thus, there may be multiple enzymatic mechanisms that could lead to release of inflammatory mediators after intestinal I/R into the intestinal venous circulation and into the lymphatics. On one hand, inflammatory mediators can be directly produced by proteolytic cleavage with pancreatic digestive enzymes,13 which is in line with the evidence that inhibition of pancreatic digestive enzymes during intestinal I/R reduces inflammation in shock.1,41 Matrix metalloproteinases may also play a role in the creation of proteolytic-derived inflammatory mediators. The results obtained in this study might not only help in defining a unique pathophysiological role for MMP-9 and pancreatic trypsin as proinflammatory agents in intestinal I/R, but also provide an important insight into developing potential therapeutic interventions for intestinal I/R and other intestinal inflammatory pathologies. The inhibition of pancreatic enzyme activity has served as an effective way to prevent formation of inflammatory peptides. Its effectiveness may be further enhanced by concomitant inhibition of MMP-9. Results from animal experiments (pharmacological inhibition and MMP-9 knockout animals) in several models of ischemia-reperfusion and inflammation show feasibility of MMP-9 blockade.60–67 The feasibility to control the progression of intestinal ischemia-reperfusion in this manner remains to be explored under pre-clinical and clinical conditions.
Acknowledgments
We thank Frank A. Delano for his support throughout this project and Dr. Tony Hugli for critical comments.
Footnotes
Address reprint requests to Dr. Geert W. Schmid-Schönbein, Department of Bioengineering, The Whitaker Institute for Biomedical Engineering, University of California, San Diego, La Jolla, CA 92093. E-mail: gwss@bioeng.ucsd.edu.
Supported by the National Institutes of Health (Grant HL 67825 to G.W.S.S.). H.S.R. was partially supported by a Travel Grant from Fundação Luso-Americana para o Desenvolvimento (Grant FLAD 470/2000).
References
- Mitsuoka H, Kistler EB, Schmid-Schönbein GW. Protease inhibition in the intestinal lumen: attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock. 2002;17:205–209. doi: 10.1097/00024382-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein GW, Hugli TE, Kistler EB, Sofianos A, Mitsuoka H. Pancreatic enzymes and microvascular cell activation in multi-organ failure. Microcirculation. 2001;8:5–14. [PubMed] [Google Scholar]
- Fink MP. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med. 1991;19:627–641. doi: 10.1097/00003246-199105000-00009. [DOI] [PubMed] [Google Scholar]
- Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- Karasawa A, Guo JP, Ma XL, Tsao PS, Lefer AM. Protective actions of a leukotriene B4 antagonist in splanchnic ischemia and reperfusion in rats. Am J Physiol. 1991;261:G191–G198. doi: 10.1152/ajpgi.1991.261.2.G191. [DOI] [PubMed] [Google Scholar]
- Maier RV. Pathogenesis of multiple organ dysfunction syndrome- endotoxin, inflammatory cells, and their mediators: cytokines and reactive oxygen species. Surg Infect (Larchmt) 2000;1:197–205. doi: 10.1089/109629600750018123. [DOI] [PubMed] [Google Scholar]
- Turler A, Schwarz NT, Turler E, Kalff JC, Bauer AJ. MCP-1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am J Physiol. 2002;282:G145–G155. doi: 10.1152/ajpgi.00263.2001. [DOI] [PubMed] [Google Scholar]
- Wanecek M, Weitzberg E, Rudehill A, Oldner A. The endothelin system in septic and endotoxin shock. Eur J Pharmacol. 2000;407:1–15. doi: 10.1016/s0014-2999(00)00675-0. [DOI] [PubMed] [Google Scholar]
- Lefer AM. Role of a myocardial depressant factor in the pathogenesis of circulatory shock. Fed Proc. 1970;29:1836–1847. [PubMed] [Google Scholar]
- Mitsuoka H, Schmid-Schönbein GW. Mechanisms for blockade of in vivo activator production in the ischemic intestine and multi-organ failure. Shock. 2000;14:522–527. doi: 10.1097/00024382-200014050-00005. [DOI] [PubMed] [Google Scholar]
- Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounous G. Pancreatic proteases and oxygen-derived free radicals in acute ischemic enteropathy. Surgery. 1986;99:92–94. [PubMed] [Google Scholar]
- Waldo SW, Rosario HS, Penn AH, Schmid-Schönbein GW. Pancreatic digestive enzymes are potent generators of mediators for leukocyte activation and mortality. Shock. 2003;20:138–143. doi: 10.1097/01.shk.0000073866.47824.ae. [DOI] [PubMed] [Google Scholar]
- Roxvall L, Sennerby L, Johansson BR, Heideman M. Trypsin-induced vascular permeability and leukocyte accumulation in hamster cheek pouch: the role of complement activation. J Surg Res. 1990;49:504–513. doi: 10.1016/0022-4804(90)90175-2. [DOI] [PubMed] [Google Scholar]
- Vergnolle N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- Howells GL, Macey MG, Chinni C, Hou L, Fox MT, Harriott P, Stone SR. Proteinase-activated receptor-2: expression by human neutrophils. J Cell Sci. 1997;110:881–887. doi: 10.1242/jcs.110.7.881. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, Proost P, Van Damme J. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851–859. [PubMed] [Google Scholar]
- Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, Burns AR, Rossen RD, Michael L, Entman M. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation. 2001;103:2181–2187. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- Planas AM, Sole S, Justicia C, Farre ER. Estimation of gelatinase content in rat brain: effect of focal ischemia. Biochem Biophys Res Commun. 2000;278:803–807. doi: 10.1006/bbrc.2000.3881. [DOI] [PubMed] [Google Scholar]
- Cursio R, Mari B, Louis K, Rostagno P, Saint-Paul MC, Giudicelli J, Bottero V, Anglard P, Yiotakis A, Dive V, Gugenheim J, Auberger P. Rat liver injury after normothermic ischemia is prevented by a phosphinic matrix metalloproteinase inhibitor. EMBO J. 2002;16:93–95. doi: 10.1096/fj.01-0279fje. [DOI] [PubMed] [Google Scholar]
- Keck T, Balcom JH, Fernandez-del Castillo C, Antoniu BA, Warshaw AL. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology. 2002;122:188–201. doi: 10.1053/gast.2002.30348. [DOI] [PubMed] [Google Scholar]
- Muhs BE, Patel S, Yee H, Marcus S, Shamamian P. Increased matrix metalloproteinase expression and activation following experimental acute pancreatitis. J Surg Res. 2001;101:21–28. doi: 10.1006/jsre.2001.6244. [DOI] [PubMed] [Google Scholar]
- Duncan ME, Richardson JP, Murray GI, Melvin WT, Fothergill JE. Human matrix metalloproteinase-9: activation by limited trypsin treatment and generation of monoclonal antibodies specific for the activated form. Eur J Biochem. 1998;258:37–43. doi: 10.1046/j.1432-1327.1998.2580037.x. [DOI] [PubMed] [Google Scholar]
- Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlton JF, Whiting CV, Tunmore D, Bregenholt S, Reimann J, Claesson MH, Bland PW. The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis. Am J Pathol. 2000;157:1927–1935. doi: 10.1016/S0002-9440(10)64831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green GM, Nasset ES. Importance of bile in regulation of intraluminal proteolytic enzyme activities in the rat. Gastroenterology. 1980;79:695–702. [PubMed] [Google Scholar]
- Miyasaka K, Green GM. Effect of partial exclusion of pancreatic juice on rat basal pancreatic secretion. Gastroenterology. 1984;86:114–119. [PubMed] [Google Scholar]
- Massberg S, Eisenmenger S, Enders G, Krombach F, Messmer K. Quantitative analysis of small intestinal microcirculation in the mouse. Res Exp Med (Berl) 1998;198:23–35. doi: 10.1007/s004330050086. [DOI] [PubMed] [Google Scholar]
- Mook ORF, Van Overbeek C, Ackema EG, Van Maldegem F, Frederiks WM. In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem. 2003;51:821–829. doi: 10.1177/002215540305100613. [DOI] [PubMed] [Google Scholar]
- Yi CF, Gosiewska A, Burtis D, Geesin J. Incorporation of fluorescent enzyme substrates in agarose gel for in situ zymography. Anal Biochem. 2001;291:27–33. doi: 10.1006/abio.2001.5017. [DOI] [PubMed] [Google Scholar]
- Pender SL, Breese EJ, Gunther U, Howie D, Wathen NC, Schuppan D, MacDonald TT. Suppression of T cell-mediated injury in human gut by interleukin 10: role of matrix metalloproteinases. Gastroenterology. 1998;115:573–583. doi: 10.1016/s0016-5085(98)70136-2. [DOI] [PubMed] [Google Scholar]
- Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985;260:2493–2500. [PubMed] [Google Scholar]
- Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- Kjeldsen L, Bainton DF, Sengelov H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83:799–807. [PubMed] [Google Scholar]
- Kolkenbrock H, Hecker-Kia A, Orgel D, Kinawi A, Ulbrich N. Progelatinase B forms from human neutrophils: complex formation of monomer/lipocalin with TIMP-1. Biol Chem. 1996;377:529–533. doi: 10.1515/bchm3.1996.377.7-8.529. [DOI] [PubMed] [Google Scholar]
- Hayward R, Lefer AM. Time course of endothelial-neutrophil interaction in splanchnic artery ischemia-reperfusion. Am J Physiol. 1998;275:H2080–H2086. doi: 10.1152/ajpheart.1998.275.6.H2080. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Martindale R. The gastrointestinal tract in critical illness. Curr Opin Clin Nutr Metab Care. 2001;4:547–551. doi: 10.1097/00075197-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Fitzal F, DeLano FA, Young C, Rosario HS, Schmid-Schönbein GW. Pancreatic protease inhibition during shock attenuates cell activation and peripheral inflammation. J Vasc Res. 2002;39:320–329. doi: 10.1159/000065544. [DOI] [PubMed] [Google Scholar]
- Mitsuoka H, Kistler EB, Schmid-Schönbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci USA. 2000;97:1772–1777. doi: 10.1073/pnas.97.4.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- Basbaum CB, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996;148:1345–1350. [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Salo T, Koivunen E, Tyynela J, Konttinen YT, Bergmann U, Tuuttila A, Niemi E, Teronen O, Heikkila P, Tschesche H, Leinonen J, Osman S, Stenman UH. Activation of type IV procollagenases by human tumor-associated trypsin-2. J Biol Chem. 1997;272:21067–21074. doi: 10.1074/jbc.272.34.21067. [DOI] [PubMed] [Google Scholar]
- Fang KC, Raymond WW, Lazarus SC, Caughey GH. Dog mastocytoma cells secrete a 92-kD gelatinase activated extracellularly by mast cell chymase. J Clin Invest. 1996;97:1589–1596. doi: 10.1172/JCI118583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min D, Moore AG, Bain MA, Breit SN, Lyons JG. Activation of macrophage promatrix metalloproteinase-9 by lipopolysaccharide-associated proteinases. J Immunol. 2002;168:2449–2455. doi: 10.4049/jimmunol.168.5.2449. [DOI] [PubMed] [Google Scholar]
- Power C, Wang JH, Sookhai S, Wu QD, Redmond HP. Proinflammatory effects of bacterial lipoprotein on human neutrophil activation status, function, and cytotoxic potential in vitro. Shock. 2001;15:461–466. doi: 10.1097/00024382-200115060-00009. [DOI] [PubMed] [Google Scholar]
- Abello PA, Buchman TG, Bulkley GB. Shock and multiple organ failure. Adv Exp Med Biol. 1994;366:253–268. doi: 10.1007/978-1-4615-1833-4_18. [DOI] [PubMed] [Google Scholar]
- Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg. 1995;32:925–1002. doi: 10.1016/s0011-3840(05)80008-5. [DOI] [PubMed] [Google Scholar]
- Kistler EB, Lefer AM, Hugli TE, Schmid-Schonbein GW. Plasma activation during splanchnic arterial occlusion shock. Shock. 2000;14:30–34. doi: 10.1097/00024382-200014010-00006. [DOI] [PubMed] [Google Scholar]
- Kistler EB, Hugli TE, Schmid-Schönbein GW. The pancreas as a source of cardiovascular cell activating factors. Microcirculation. 2000;7:183–192. [PubMed] [Google Scholar]
- Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- Fitzal F, DeLano FA, Young C, Rosario HS, Junger WG, Schmid-Schönbein GW. Pancreatic enzymes sustain systemic inflammation after an initial endotoxin challenge. Surgery. 2003;134:446–456. doi: 10.1067/s0039-6060(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Kramp WJ, Schmid-Schönbein GW, Coimbra R, Hoyt D, Hugli TE. Characterization of two classes of pancreatic shock factors: functional differences exhibited by hydrophylic and hydrophobic shock factors. Shock. 2003;20:356–362. doi: 10.1097/01.shk.0000082442.66379.90. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois B, Starckx S, Pagenstecher A, Oord J, Arnold B, Opdenakker G. Gelatinase B deficiency protects against endotoxin shock. Eur J Immunol. 2002;32:2163–2171. doi: 10.1002/1521-4141(200208)32:8<2163::AID-IMMU2163>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalu MM, Gao CQ, Schulz R. Matrix metalloproteinase inhibitors attenuate endotoxemia-induced cardiac dysfunction: a potential role for MMP-9. Mol Cell Biochem. 2003;251:61–66. [PubMed] [Google Scholar]
- Maitra SR, Bhaduri S, Valane PD, Tervahartiala T, Sorsa T, Ramamurthy N. Inhibition of matrix metalloproteinases by chemically modified tetracyclines in sepsis. Shock. 2003;20:280–285. doi: 10.1097/00024382-200309000-00014. [DOI] [PubMed] [Google Scholar]
- Romanic AM, Harrison SM, Bao W, Burns-Kurtis CL, Pickering S, Gu J, Grau E, Mao J, Sathe GM, Ohlstein EH, Yue TL. Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc Res. 2002;54:549–558. doi: 10.1016/s0008-6363(02)00254-7. [DOI] [PubMed] [Google Scholar]
- Wielockx B, Lannoy K, Shapiro SD, Itoh T, Itohara S, Vandekerckhove J, Libert C. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nat Med. 2001;7:1202–1208. doi: 10.1038/nm1101-1202. [DOI] [PubMed] [Google Scholar]