Abstract
Our objective was to determine the role of the cryptococcal virulence factor urease in pulmonary-to-central nervous system, dissemination, invasion, and growth. C. neoformans H99, the urease knockout strain (ure1) derived from H99, and the urease restored strain ure1+URE1−1 were used for thestudies. The absence of cryptococcal urease (ure1infection) resulted in significant protection from the high mortality observed in H99-infected mice. All H99-infected mice had extremely high cryptococcal loads in their brains at the time of death, whereas only two of six animals that died of ure1 infection had detectable C. neoformans in the brain. Histological analysis of the blood-to-brain invasion by C. neoformans H99 demonstrated wedging of the yeasts in small capillaries, altered structure of microvessel walls, formation of mucoid cysts initiated in the proximity of damaged microcapillaries, and the absence of an inflammatory response. Direct inoculation of H99, ure1, and ure1+URE1−1 into the brain demonstrated that urease was not required to grow in the brain. However, the dissemination patterns in the brain, spleen, and other organs after intravenous inoculation indicated that cryptococcal urease contributes to the central nervous system invasion by enhancing yeast sequestration within microcapillary beds (such as within the brain) during hematogenous spread, thereby facilitating blood-to-brain invasion by C. neoformans.
Dissemination into the central nervous system (CNS) and the subsequent development of meningitis/encephalitis is a major cause of mortality during infections with opportunistic yeast Cryptococcus neoformans.1–3 Although high CNS tropism characterizes C. neoformans; the cryptococcal virulence factors that contribute to this high CNS invasiveness are largely unknown.4–6 So far only laccase, an enzyme catalyzing cryptococcal melanin production, has been demonstrated to be critical for the survival of C. neoformans in the brain.7,8 As more data about cryptococcal virulence factors becomes available, it is also becoming apparent that a combination of virulence factors rather than a single virulence factor is most likely involved in multistep process of cryptococcal dissemination and CNS invasion.7,9–13 However, understanding how C. neoformans transfers from the primary sites of infection into the CNS is incomplete. The steps in pulmonary-to-CNS dissemination include escape out of the primary infection site, fungemia, and transport through the blood, transfer from the blood into the CNS, and colonization/growth in the CNS.7,14–16 The most frequently proposed mechanism of cryptococcal CNS invasion involves the initial transfer of circulating cryptococci into the cerebrospinal fluid/subarachnoid space and the development of leptomeningitis. Subsequent progression of the infection from the brain surface into the cortex, and cryptococcal growth along the Virchow-Robin spaces into the deeper parts of the brain is thought to lead to fatal brain infection. Alternatively, it has been proposed that C. neoformans can directly cross the blood brain barrier at the level of small cerebral capillaries. This suggestion was based on the observation that in some cases cryptococcal cysts develop within the brain in the absence of meningitis (parenchymal form of cryptococcal meningoencephalitis)3 and the finding of isolated cryptococcal lesions in the proximity of small microvessels in necropsy studies.1 On the cellular level, a transfer across the endothelial barrier could occur via direct extravasation, endothelial transcytosis,17 or with help of mononuclear phagocytes serving as Trojan horses.15 Even though a direct evidence of C. neoformans transfer into the CNS has not been presented to date, it is possible that more than one mechanism (route) can lead C. neoformans into the CNS and in each case, different virulence factors may be important.
Urease was recently identified as an important virulence factor of C. neoformans.18 Although urease-negative isolates have been sporadically reported,19–21 the overwhelming majority of C. neoformans strains produce this extracellular enzyme and invest a significant amount of energy in urease production.19 The mechanism by which urease could mediate the virulence in opportunistic yeasts is unknown. To study the role of cryptococcal urease, a urease knockout strain (ure1) was produced by targeted deletion of urease gene from H99.18 Strains H99 and the newly developed ure1 and the ure1+URE1−1 (strain ure1− rescued with a wild-type copy of URE1 gene) provide a valuable tool to study the role of urease as a cryptococcal virulence factor. Using these strains, it has been demonstrated that urease expression by C. neoformans has a detrimental effect on the survival of mice infected via the intravenous or intranasal route.18 The mechanism by which cryptococcal urease contributes to the high mortality is unknown, but mortality in both clinical and experimental cryptococcosis primarily correlates with the invasion of the CNS.22–26 When cryptococci were directly injected into the cerebrospinal fluid of corticosteroid-pretreated rabbits, urease expression did not affect growth in the CNS.18 This result suggested that urease was not required for the optimal growth of C. neoformans in the CNS; however, it did not negate the possible role for urease in the dissemination process. Our objective was to determine the role of cryptococcal urease in pulmonary-to-CNS dissemination, invasion, and growth.
Materials and Methods
Cultures of C. neoformans
For this study, the wild-type strain H99 and two genetically modified derivatives were used: ure1 (urease-negative transformant of H99 with a selective disruption of native urease gene, URE1), and ure1+URE1−1 (strain ure1− rescued with a wild-type copy of URE1 gene). H99 is a widely studied clinical isolate of serotype A. This strain is virulent in immunocompetent mice and is characterized by capsule, laccase/melanin, phospholipase B, and urease activity.9,18,27–29 The development of ure1 and ure1+URE1−1 strains was previously described.18 For infection, yeasts recovered from frozen 10% glycerol stocks were grown to stationary phase (at least 72 hours) in Sabouraud dextrose broth (1% neopeptone, 2% dextrose; Difco, Detroit, MI) at 36°C on a shaker. The cultures were then washed in nonpyrogenic saline (Travenol, Deerfield, IL), counted on a hemocytometer, and diluted to the required concentrations in sterile nonpyrogenic saline.
Mice
Mice (C57BL/6×129F2; Jackson Laboratories, Bar Harbor, ME) were selected to study the effects of cryptococcal urease because they are able to clear the low-mid virulent strains of C. neoformans but succumb to the highly virulent ones.25,30,31 These mice were housed under specific pathogen-free conditions in enclosed filter top cages at the University of Michigan Unit for Laboratory Animal Medicine. Clean food and water were given ad libitum. The mice were handled and maintained using microisolator techniques with daily veterinarian monitoring. This study was approved and monitored by the University Committee on the Use and Care of Animals at the University of Michigan.
Infection with C. neoformans
For the intratracheal and intracranial infections mice were anesthetized by intraperitoneal injection of ketamine/xylazine mix (ketamine/xylazine, 100/6.8 mg/kg/body weight) and restrained on a foam plate. For the intratracheal infection, trachea was exposed by small incision through the skin and separation of underlying tissue. A bent 30-gauge needle (Becton Dickinson, Rutherford, NJ) attached to a tuberculin syringe (BD&Co., Franklin Lakes, NJ) with the adjusted C. neoformans culture was inserted into the trachea. Inoculum (104 yeast cells in 30 μl) was dispensed into the lungs. The skin was closed with cyanoacrylate adhesive. The mice recovered with minimal visible trauma. The intracranial infection was performed as described elsewhere.32,33 Briefly, the inoculum (104 yeast cells in 20 μl) was injected into the central occipital area of a cranium via a 27-gauge needle inserted as shallowly as possible (less than 5 mm). The intravenous infection (105 or 106 yeast cells in 0.25 ml of nonpyrogenic saline) was performed by the tail vein injection of unanesthetized animals. Aliquots of the inoculums were collected and plated on Sabouraud dextrose agar (Difco) to monitor the number of colony forming units (CFU) being delivered. The actual depositions for the different procedures are included in the figure legends. No significant differences between inoculi sizes between three strains at each experiment were observed.
Organ and Blood CFU Assays
At selected time points, mice were humanely euthanized by CO2 asphyxiation, lungs, spleens, kidneys, hearts, and brains were dissected and homogenized in 2 ml of sterile water and serially diluted in sterile water. Blood was collected aseptically via cardiac puncture immediately after euthanasia. Dilutions of organ and blood samples (10 μl each) were plated on Christensen’s urea agar (containing 300 nm of urea and phenol red as a pH indicator) and incubated at room temperature for 48 hours. Colony counts were performed and adjusted to reflect the total CFU/organ. Media color change in proximity of colonies confirmed the urease activity in H99 and ure1+URE1−1, and the absence of urease activity in ure1, isolated from organs of infected animals.
Serum Incubation CFU Assay
Samples of H99, ure1, and ure1 +URE1−1 cultures prepared for intravenous injection were prepared in nonpyrogenic saline at a concentration of 106 cells/ml. These microbial suspensions (10 μl) were combined with freshly isolated mouse serum (90 μl) and incubated for 3 hours on 96-well plate in triplicates for each strain. At the same time 104 cells of each strain were incubated in 100 μl of Saburaud dextrose broth (SDB) as the control. After the incubation samples were serially diluted in sterile water. Dilution samples (10 μl each) were plated on Sabouraud dextrose agar and incubated at room temperature for 48 hours. Colony counts were performed and expressed in log CFU/well.
Peritoneal Macrophage Phagocytosis Assay
Peritoneal macrophages were washed from the peritoneal cavities of uninfected mice, washed, adjusted to 106 cells/ml, and allowed to adhere to the bottom of the Lab-Tek II chamber slides (Nalge Nunc Int., Rochester, NY) for 1 hour. H99, ure-1, or ure1+URE-1−1 organisms preopsonized in mouse serum were added to different chambers (10:1 cell/target ratio) in duplicates. After 3 hours of incubation (37°C, 5% CO2) slides were washed with phosphate-buffered saline to remove free-floating yeast cells, fixed, and stained with Diff-Quick (Dade Behring Inc., Newark, DE). Numbers of ingested yeast were evaluated in 200 macrophages for each culture.
Histology
Lungs were fixed by inflation with 1 ml of 10% neutral buffered formalin, excised en bloc, and immersed in neutral buffered formalin. Brains were carefully excised and immersed in neutral buffered formalin. After tissue processing and paraffin embedding, 2- to 3-μm sections of both organs were cut and stained with mucicarmine and hematoxylin and eosin. Up to 20 consecutive sections of the brain were prepared for examination and analyzed. Sections were analyzed with light microscopy at low and high power to evaluate cryptococcal invasion and size/location of lesions in the brain. Size of the lesions and their associations with anatomical structures was determined by examination of the same lesion on adjacent consecutive sections.
Calculations and Statistics
Data (mean ± SE) for each experimental group were derived from at least two independent infections and analyzed via t-test, one-way or two-way analysis of variance, depending on the number of groups. For individual comparisons of multiple groups, post hoc Tukey’s test was used to calculate P values. Survival between the groups was compared using Kaplan-Meier survival analysis coupled with log rank test. Means with P < 0.05 were considered statistically significant.
Results
Lung-to-Brain Dissemination, CNS Growth, and Neurological Symptoms Accompany the High Mortality in Mice Infected Intratracheally with Urease-Producing C. neoformans H99
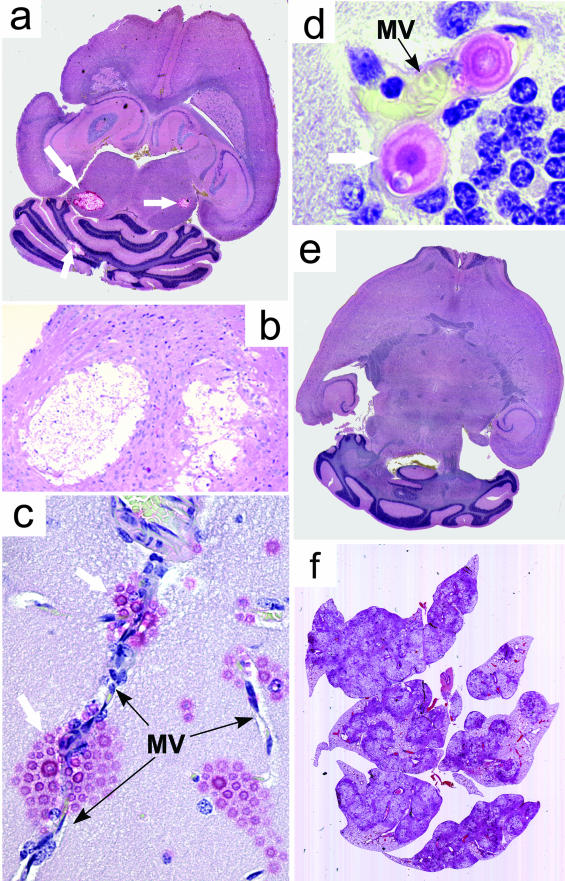
Intratracheal administration of urease-producing C. neoformans H99 resulted in uniform mortality of C57BL/6 × 129F2 mice: 75% of the H99-infected mice died by 4 weeks of infection and 100% of animals died by week 6 of infection. To determine whether the high mortality rate was accompanied by CNS invasion, we monitored moribund animals for the presence of neurological symptoms and performed pathological examination of the brains. Before death, we observed neurological symptoms such as extension of the cerebral portion of cranium, abnormal gait, paralysis, and lethargy. Histological analysis of brains postmortem demonstrated multiple cysts up to 1.5 mm in diameter containing C. neoformans (Figure 1, a and b). This severe brain pathology observed in H99 infection was consistent with neurological symptoms and high mortality because of massive disseminated growth of cryptococci in the brains of infected mice.
Figure 1.
Effect of C. neoformans urease on the CNS pathology. Mice were infected intratracheally with 3.87 log CFU of C. neoformans H99 (a–c) or 4.00 ± 0.09 log CFU of C. neoformans ure-1 (d and e). Brains and lungs of mice that succumbed to these infections were analyzed by light microscopy of sections stained with combined Mucicarmine/H&E stains. a: Cryptococcal cysts located in central nuclei colliculi (bilateral) and in cerebellum 4 weeks after infection with H99. b: Expanding cryptococcal mucoid cysts infiltrating and displacing the brain tissue. Note the absence of inflammatory infiltrate. c: Localization of C. neoformans (white arrows) growth sites in the brain parenchyma adjacent to brain microvessels (MV, black arrows). d: Intravascular cryptococci growing/budding in a thin-walled capillary of cerebellum. Note the close contact of yeast cells with the endothelium, distended vessel wall, and flattened endothelial cells in proximity of yeast cell. e: Brain without the evidence of CNS infection 8 weeks after intratracheal infection with ure-1. f: Pulmonary infection. Note the extensive lung damage and demarcation at the edge of granulomas. Original magnifications: ×6 (a, f); ×33 (b); ×132 (c); ×330 (d).
Mechanism of C. neoformans Entry into the Brain—CNS Dissemination after Pulmonary Infection with H99
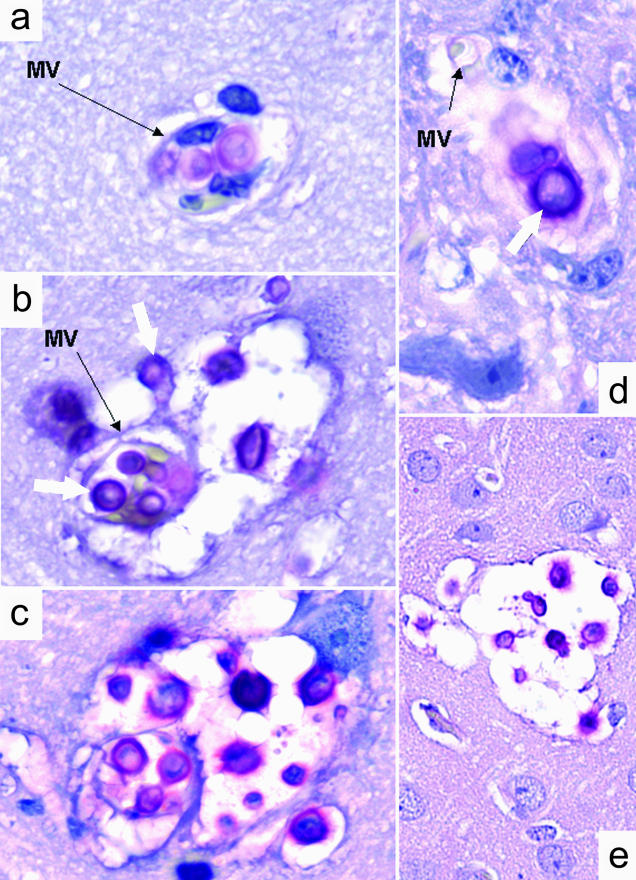
To determine how C. neoformans H99 enters the brain parenchyma during pulmonary-to-CNS dissemination in mice, we performed extensive histological analysis of consecutive brain sections in mice 40 days after intratracheal infection (pulmonary/natural route) as well as 20, 40, and 72 hours after intravenous inoculation. After intratracheal and intravenous inoculations, the cysts in the brains were observed in diverse areas of the brain, including brain stem, colliculus, dentate gyrus, amygdala, cerebellum, and lateral cortex/Ammon horns but not within the leptomeninx. Mucoid cysts were entirely embedded within the brain parenchyma and had no contact with the leptomeningeal surface (determined by analysis of serial brain sections). The newly formed microcysts were located in direct proximity of the microcapillaries (Figure 1c), whereas intravascular cryptococci were found individually or in small clusters (Figure 1d and Figure 2, a and b) frequently in different stages of cell division/budding (Figure 1d and Figure 2; a to c). Growing cryptococci formed a contact with the endothelial surface, and appeared to distend the capillaries (Figure 1d). In some areas the small vessels were completely occluded by intravascular cryptococci as indicated by lack of blood cells in these microvessels (Figure 2c). In both partially and completely occluded microvessels, endothelial cells appeared compressed (Figure 1d; Figure 2, b and c) and their structure was blurred (Figure 2, c and d) and in some areas the capillary wall was no longer visible under the light microscopy (Figure 1d; Figure 2, a and d). Cryptococcal growth into the brain parenchyma progressed from the perivascular space of these occluded microvessels into the deeper layers of brain tissue, suggesting that C. neoformans H99 invaded brain parenchyma via direct transfer across the blood/brain barrier at the sites were cryptococci embolized microcapillaries (Figure 2; a to c). Both intravascular and extravascular growth of C. neoformans occurred without local leukocyte accumulation. Sporadically, individual neutrophils could be found in the proximity of intravascular yeast cells (Figure 1d), however no evidence of intravascular or interstitial granuloma formation could be found at any level of the lesion. The halos of mucopolysaccharide were invisible or minimal around the intravascular yeasts (Figure 1d; Figure 2, a and d) butit became apparent within occluded portions ofmicrovessels (Figure 2c), and within neighboring brain parenchyma (Figure 2; b, c, and e). In all individuals studied, no visible invasion of subarachnoid space was visible. Leptomeningeal surfaces, cerebrospinal fluid in brain ventricles, and Virchow-Robin spaces along the blood vessel entering the brain from its surface and plexus choroids were free of cryptococci, regardless of the widespread infection in the brain parenchyma (data not shown). On the intravenous injection, formation of small mucoid microcysts was observed as early as 20 hours after infection, however multiple microcysts were evident at 36 hours after infection (Figure 2, d and e) indicating that the transfer from blood into the brain parenchyma may occur as early as 20 hours, and clearly before 36 hours from the onset of H99-fungemia. These primary microcysts resembled the small, presumably newly developed cysts that were found in the brains of intratracheal-infected animals (Figure 2; a to c). In summary, invasion of the CNS by C. neoformans H99 occurs in central parts of the brain in the proximity of microvessels occluded by cryptococci rather than from the surface of the brain via the invasion of leptomeninx.
Figure 2.
Photomitograph of C. neoformans transfer from blood into the brain 4 weeks after intratracheal infection with 3.87 log CFU of H99 (a–c) and 36 hours after intravenous infection with 5.02 log CFU of H99 (d and e). a: Sequestration and proliferation of C. neoformans within cerebral MV resulting in discontinuity in capillary wall. b and c: Progressive invasion of C. neoformans (white arrows) into the brain parenchyma from MV and the development of the mucoid-cyst (soap bubble); note the contour of necrotic MV and formation of lesion displacing vascular tissues and brain parenchyma. d: Growth of C. neoformans on the edge of ruptured MV 20 hours after intravenous infection. e: Developed microcysts in the brain 36 hours after the intravenous infection. Mucicarmine stain was used to detect cryptococci within the tissues. Original magnifications: ×330 (a–d); ×132 (e).
Effect of Cryptococcal Urease Gene Deletion on Extrapulmonary Dissemination and Host Survival after Intratracheal Inoculation
Our next objective was to determine the contribution of cryptococcal urease to the virulence of C. neoformans H99 in terms of survival and CNS invasion after pulmonary infection in C57BL/6×129F2 mice. Mice were infected intratracheally with H99 (n = 20) or ure1 (n = 21). Cumulative survival and brain pathologies were compared. By week 4 of infection, no mortality had occurred in mice infected with ure1. In contrast, there was 75% mortality in the H99-infected group by week 4. Only 30% of ure1-infected mice died during the 8-week course of infection whereas all of the H99-infected animals died by week 6. Neurological symptoms were not apparent in mice that died from ure1; however, these mice demonstrated a rapid shallow breathing pattern before death. Postmortem analysis of this group of 30% of ure1-infected mice (weeks 6 to 8 after infection, n = 3 for histology) indicated that these mice had no visible evidence of damage in the brain (Figure 1e). However, multiple solidified nodules, defined histologically as granulomas, encompassed almost the whole lungs (Figure 1f). The central portion of these granulomas contained dividing cryptococci with large capsules, surrounded by mixed leukocyte infiltrate. No macroscopic abnormalities were observed in other internal organs. Thus, deletion of the urease gene from C. neoformans prevented long-term cryptococcal infection/pathology in the brain and prolonged host survival after pulmonary inoculation with the fungus.
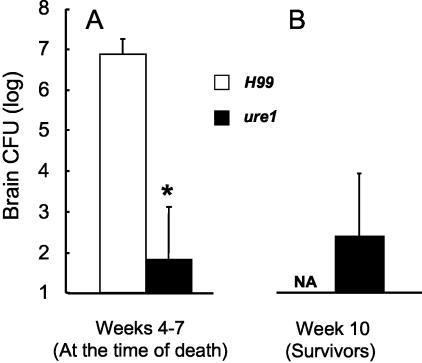
Next, we compared the cryptococcal loads in the lungs and brain at time of the death in H99 versus ure1 infections to determine whether the differences in survival and CNS pathology were associated with differences in pulmonary clearance. Total lung CFU analysis at the time of death revealed high cryptococcal burdens in ure1 infection (7.95 ± 0.70 log CFU, weeks 6 to 7) that was similar to those observed in H99-infected lungs at the time of death (8.70 ± 0.30 log CFU, weeks 3 to 6). These data indicated that longer survival and minimal brain pathology in ure1-infected mice was not because of clearance of the infection from the primary site of infection (the lungs). All mice that died from H99 infection had extremely high cryptococcal loads in their brains (Figure 3A), whereas only two of the six animals that died of ure1 infection cultured positive for C. neoformans in the brain (Figure 3A, limit of detection = 100 CFU). At week 10 after infection, all of the surviving ure1-infected mice were euthanized and the brain CFUs were analyzed (Figure 3B). At week 10, brain CFU was detected only in 33% of the surviving ure1-infected mice. Collectively, these data indicate that nonsurviving ure1-infected mice did not die from CNS infection, but most likely they died from ongoing pulmonary infection.
Figure 3.
Effect of C. neoformans urease on brain CFU loads at the time of death after the intratracheal infection with 4.00 ± 0.09 log CFU (A) and brain CFU in mice surviving the infection with ure1 10 weeks after infection (B). Data, pooled from separate matched experiments, are expressed as the mean CFU per brain ± SE; *, P < 0.05 in comparison with H99 infection, n = 5 to 18 per group.
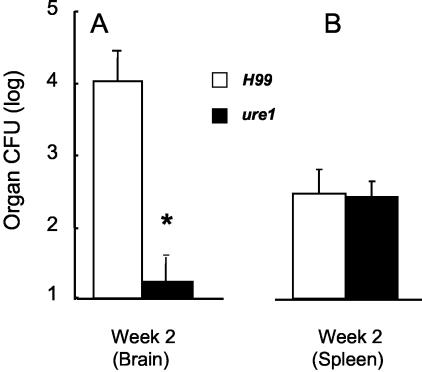
We next assessed whether urease expression facilitated earlier dissemination of C. neoformans from the lung into the blood/spleen and brain. Cryptococcal burdens in brains and spleens (index of hematogenous dissemination from the lung) were assessed on day 16 after infection (a week before any deaths of infected animals). Mice infected with the urease producing strain H99 already had a 1000-fold higher cryptococcal burden in the brain compared to ure1-infected mice (Figure 4A). The frequency of CNS colonization was 94% in H99-infection compared to only 40% of mice infected with ure1. In contrast with the large differences in brain colonization and CFU, the frequency of spleen colonization and distribution of individual spleen CFU was not different between H99- and ure1-infected mice (Figure 4B). Thus, urease did not appear to play a role in extrapulmonary dissemination per se (because the spleens did not differ); rather, this data suggested that urease plays a role in CNS invasion and/or growth in the CNS.
Figure 4.
Role of C. neoformans urease in systemic dissemination of C. neoformans from the lung. Bars represent CFU organ load at 2 weeks after infection with 4.14 ± 0.07 log CFU intratracheally found in the brain (A) and spleen (B). Data, pooled from three separate matched experiments, are expressed as the mean CFU per organ ± SE; *, P < 0.05 in comparison with H99, n = 10 to 18 per group.
Role of Cryptococcal Urease in Facilitating C. neoformans Growth in the Lungs, Spleen, and Brain
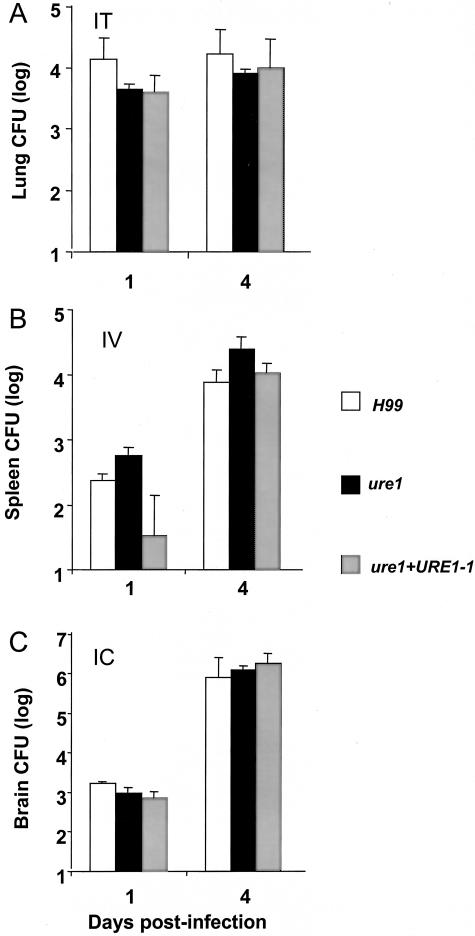
Our next objective was to determine whether urease promoted a direct growth advantage for Cryptococcus in the brain. A single dose of C. neoformans was inoculated via intratracheal, intravenous, or intracranial injections and the organ loads were measured on days 1 and 4 after inoculation. These latter experiments now also used ure1+URE1−1, the rescued ure1 C. neoformans strain in which the wild-type copy of URE1 was stably, ectopically reinserted into the genome. After delivery into the lungs, H99, ure1, and ure1+ URE1−1 all demonstrated similar pulmonary growth rates during the early phase of infection (Figure 5A). After intravenous injection, the splenic CFU loads of C. neoformans strains were identical (Figure 5B). Most importantly, direct intracranial inoculation of H99, ure1, and ure1+URE1−1 demonstrated that urease was not required to grow in the brain (Figure 5C). This latter result is consistent with the previous report that urease is not required for the growth of C. neoformans in the brains of corticosteroid-treated rabbits18 and rules out species-specific differences in our results.
Figure 5.
Effect of C. neoformans urease on organism survival (day 1) and growth (day 4) within organs after direct delivery. Mice were inoculated with 4.05 ± 0.06 log CFU intratracheally (A), 3.90 ± 0.05 log CFU intracranially (B), or 4.72 ± 0.17 log CFU intravenously of H99 (C); ure1; or urease revertant ure1+URE1−1 (ure1 with reinserted urease gene). CFU in target organs were determined in lungs (A), spleens (B), and brains (C). Bars represent mean ± SE, n = 5 to 6 animals per group from three separate matched infections; *, P < 0.05.
Role of Cryptococcal Urease in Facilitating Hematogenous Spread and Microcapillary Sequestration
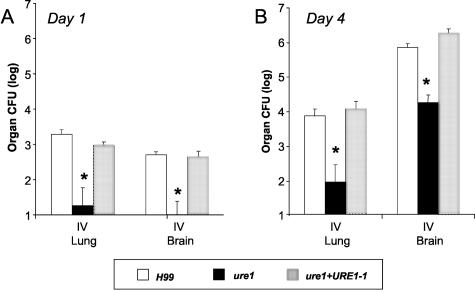
We wished to address the alternate interpretation of Figure 4: urease did facilitate extrapulmonary dissemination as reflected by the brain colonization differences and the early differences in spleen CFU are lost by day 16 (Figure 4). For these experiments, all groups of mice were given an intravenous bolus of C. neoformans to uniformly simulate fungemia and analyze the hematogenous spread into the brain. CFUs were analyzed at days 1 and 4. Under these conditions of identical blood fungal loads, nearly 100-fold higher brain CFU was observed with the urease-expressing strains (H99 and ure1+URE1−1) compared to ure1 on both days 1 and 4 after intravenous injection (Figure 6). Interestingly, lung CFU was also 100-fold higher for the urease-producing strains compared to the urease-deficient strain (Figure 6). Comparison of the lung CFU data between Figures 5 and 6 may seem contradictory, but the major difference is the route of entry into the organs. Figure 5A (intratracheal) is measuring direct growth in the alveolar space of the parenchymal tissue of the organ. In contrast, Figure 6 is measuring growth after a process of microcapillary implantation (growth within the vessels of analyzed organ and/or within the parenchyma). Most importantly, the dissemination patterns in the brain and spleen after intravenous inoculation (Figures 5 and 6) are consistent with that observed on day 16 intratracheal-infected mice; supporting the model that urease plays a role in a pathogenesis step that occurs between hematogenous spread and the growth in the brain parenchyma such as microcapillary sequestration and/or trans-endothelial invasion.
Figure 6.
Effect of C. neoformans urease on the transfer and subsequent growth of organism in brain and lung after intravenous infection. Mice were injected intravenously with H99, ure1, or ure1 +URE1−1 (4.72 ± 0.17 log CFU). Brain and lung CFU were determined at day 1 and 4 days after infection. Bars represent mean ± SE, n = 4 to 6 animals per group; *, P < 0.05.
Role of Urease Expression in Microcapillary Sequestration by C. neoformans
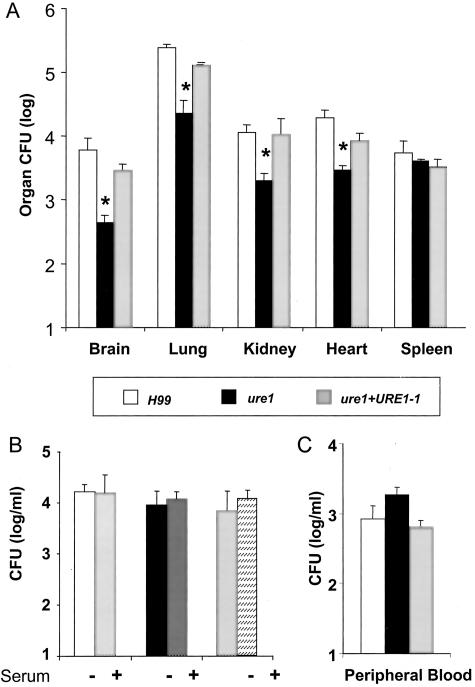
To determine whether increased sequestration within small capillaries was a mechanism by which cryptococcal urease promoted CNS infection during hematogenous spread, CFU organ loads were measured in numerous organs at 3 hours after bolus intravenous inoculation. This is a long enough period of time for microembolization of cryptococci to occur, but short enough that replication will not significantly affect organ CFU levels (C. neoformans in vitro doubling time at 37°C is ∼2.6 hours). Consistent with the day 1 and 4 time points, C. neoformans accumulation in the spleen at 3 hours was not different in the presence or absence of urease expression (Figure 7A). In contrast, CFU in organs with closed capillary beds (brain, lung, heart, kidney) was more than 10-fold lower for urease-negative ure1 compared to the urease-positive H99 or ure1+URE1−1. We next set up experiments to exclude the possibility that the decreased accumulation of ure1 in major organs was because of increased susceptibility to serum anti-microbial factors, phagocytosis, or all mechanisms of intravascular clearance combined. All three C. neoformans strains were cultured in fresh 90% mouse serum at 37°C for 3 hours, incubated with freshly isolated peritoneal macrophages, or analyzed in the blood 3 hours after intravenous injection. Deletion of the urease gene from C. neoformans did not affect the ability of the yeast to survive in serum (Figure 7B), change the number of cells ingested by macrophages (data not shown), or affect the numbers of circulating cryptococci throughout this time period (Figure 7C). Thus, cryptococcal urease activity enhances yeast sequestration within small capillaries, such as within the brain, during hematogenous spread rather than providing protection against anti-cryptococcal factors present in the blood.
Figure 7.
Effect of C. neoformans urease on vascular sequestration at different organs and numbers of circulating cryptocci after intravenous infection. A: Mice were injected intravenously with 5.67 ± 0.09 log CFU of H99, ure1, or ure1 +URE1−1. There was no significant difference between the inoculi of the various groups. CFUs were determined at 3 hours after infection. n = 5 animals per group. B: H99, ure1, or ure1 +URE1−1 were incubated in fresh 90% murine serum for 3 hours in triplicates on 96-well plates on a shaker at 37°C. C: Cryptococcal blood CFU at 3 hours after infection (log/ml). n = 4 animals/group. Bars represent mean ± SE; *, P < 0.05.
Discussion
This is the first study to demonstrate that pathogen-expressed urease can play a role in CNS invasion. In this model of C. neoformans infection, urease is critically important for CNS invasion leading to enhanced mortality of infected immunocompetent C57BL/6×129F2 mice. Even though the ability to invade CNS is likely to be mediated by a whole group of cryptococcal virulence factors, our finding was quite unexpected. Urease is a major site-specific virulence factor in bacteria that infects the stomach mucosa or urinary tract.34–38 Urease enables these microbes to survive in the presence of low pH. Our study demonstrates that urease plays a completely different role in the pathogenesis of infection with C. neoformans. Urease promotes cryptococcal sequestration within the microcapillaries, which seems to be a critical step in dissemination to the brain.
Our data support the model that increased intravascular sequestration is an important mechanism by which urease expression facilitates invasion of the brain by C. neoformans. As demonstrated in these studies, a 3-hour time frame is not sufficient to produce any significant changes between the strains with respect to growth/survival in mouse serum, in vitro phagocytosis by peritoneal macrophages, or numbers of circulating yeast, but a difference in intravascular organ CFU does occur by 3 hours. This model of microcapillary sequestration preceding brain invasion is consistent with other studies using a rat C. neoformans fungemia model.39 In these studies, intravascular granulomas began to form in different organs within hours after intravenous infection.39 This is also consistent with our observation that urease promotes C. neoformans accumulation in organs with closed capillary networks (Figure 7) but not in an organ with an open vascular system, the spleen. In the CNS, cryptococcal invasion appears to occur within brain capillaries in which cryptococci form microemboli, proliferate, and gain access to the brain parenchyma, seemingly via disruption of the vessel wall (Figure 2). This observation in animal models is consistent with the autopsy reports of cryptococcal encephalitis patients that describe C. neoformans organisms to be localized in the proximity of cerebral microvessels.1 Altogether, these data suggest that urease-mediated sequestration of C. neoformans to the endothelial surface of microcapillaries of different organs is an important step in pathogenesis of cryptococcal dissemination.
Urease appears to be more critical for the sequestration of C. neoformans in microcapillaries the fewer organisms are present in the blood. The requirement for urease in embolization decreases as the level of C. neoformans fungemia increases. For example, the most significant role for cryptococcal urease in increasing brain CFU (>1000-fold increase) was observed after intratracheal infection with 4 log CFU (Figures 3a and 4a). During the bolus intravenous injection of 5 log CFU, the effect of urease was less pronounced but still dramatic (100-fold increase in CNS load of H99 compared to ure1, Figure 6). There is an even smaller (but still significant) difference in brain CFU after intravenous injection of 6 log CFU (Figure 7). Thus, urease is critical for virulence in infections in which slow pulmonary growth of C. neoformans is followed by gradually occurring dissemination and is less important when high bolus doses are injected intravenously during which microembolization may be promoted by increased aggregation of injected yeast cells.
The mechanism by which urease facilitates microvascular sequestration remains unknown. One of the possibilities could be production of ammonia from the urea and/or other nitrogenous products present in host plasma. Yeast-derived urease is extremely efficient in hydrolyzing urea even in significantly diluted normal serum samples.40 We could further speculate that the production of ammonia in direct proximity of endothelial cells could result in the increased cryptococcal adherence to endothelium17 either by strictly mechanical effects (change in shape of microvessel), or by an induction of adhesion molecules on the surface of endothelial cells. Additionally, ammonia could potentially promote endothelial cell toxicity and/or affect astrocytes leading to the opening of endothelial cell junctions and weakening the integrity of the blood brain barrier.7,41 These latter effects however would occur downstream from sequestration and would further increase cryptococcal transfer from the intravascular compartment. Another possibility involves urease-mediated utilization of other nitrogenous moieties found in serum/on the surface of endothelial cells, which in turn would affect the adherence of C. neoformans to endothelial cells.
Even though urease has been demonstrated to be critical for the transfer of C. neoformans from blood into the brain, urease expression has no role in the survival/growth of C. neoformans in the brain (Figure 4). Only laccase (an other extracellular enzymatic factor produced by C. neoformans) has been clearly demonstrated to facilitate intracerebral growth of this organism.8,42 Consistent with the requirement for laccase in CNS infection, our laboratory has performed experiments demonstrating that a laccase-deficient strain produces a minimal CNS infection after intratracheal infection.43 All C. neoformans strains used in this study (H99, ure1, ure1+URE1−1) were laccase-positive. The ability of these strains to produce laccase was consistent with their ability to grow in the brain on direct injection into the brain. These data indicate that urease and laccase play a synergistic role in CNS invasion, with urease promoting the early steps of C. neoformans invasion through brain microcapillaries and laccase promoting yeast survival and replication within the brain parenchyma. Blood-brain invasion and growth of C. neoformans in the CNS is a multistep process mediated by distinct virulence factors at each step, including at least two extracellular enzymes urease and laccase.
Even though the effect of urease deletion was striking in C. neoformans H99 infection, urease may play different roles in different types of cryptococcal infections. C. neoformans H99 infection is characterized by very rapid dissemination into the CNS and a paucity of inflammation in the infected areas of the brain. The histological appearance of brain cryptococcomas in our model closely resembles those found in the subsets of human pathologies of human immunodeficiency virus-positive patients that succumbed to C. neoformans infections.1,15 It also resembles the lesions that develop in mice infected with another clinical C. neoformans isolate.15 A common element in all of these studies is minimal or completely absent inflammatory response within the CNS despite the presence of infiltrative/invasive cryptococcal growth within the brain parenchyma. In some cases of human CNS invasion, referred to as the parenchymal form,3 mucoid cysts in the brain are found in the absence of meningitis, as it occurs in our model. Our study demonstrates that C. neoformans H99 appears on both sides of the blood brain barrier in the absence of an inflammatory reaction or intracellular forms of C. neoformans, reported in other studies.15,17 One of these reports proposed that translocation of C. neoformans from the blood into the brain occurs via movement of Cryptococcus-containing monocytes across the endothelial barrier serving as Trojan horses.15 In our study, massive translocation of C. neoformans from the blood into brain occurs via microcapillary sequestration, extracellular growth, and most likely endothelial disruption without involvement of macrophages. Thus disseminated C. neoformans infection and transfer across the blood brain barrier can occur without meningitis, infection of cerebrospinal fluid, and the involvement of intravascular macrophages.
Our final comment would be a speculation on a possibility of the global importance of urease in disseminating infections. High urease expression is an uncommon property of C. neoformans compared with other pathogenic yeasts19 and so is its high CNS tropism. Among other pathogenic yeasts, Coccidioides immitis also expresses urease and it also causes disseminated disease with CNS infection after spore inhalation.44–48 Even though C. neoformans is the first microbial species reported to use urease to facilitate its dissemination into the CNS via sequestration within the microvessels, the possibility exists that urease could be used by other microbial species to facilitate their spread into the CNS.
Acknowledgments
We thank Rebecca S. Wiesner, Rod McDonald, Nicole Falkowski, Mia Lui, and Rishi Surana for their assistance in different parts of this project; and the Undergraduate Research Opportunity Program at University of Michigan for the support.
Footnotes
Address reprint requests to Michal Olszewski, University of Michigan Medical Center, Division of Pulmonary and Critical Care Medicine, 6301 MSRB III, 1150 W. Medical Center Dr., Ann Arbor, Michigan 48109-0642. E-mail: olszewsm@umich.edu.
Supported by the Veteran’s Administration (merit grants to M.A.O. and G.B.T., Research Enhancement Award Program grant to G.B.T. and M.A.O.), the National Institutes of Health (NHLBI R01-HL63670 to G.B.H., NHLBI R01-HL65912 to G.B.H., NHLBI R01-HL51082 to G.B.T., NHLBI T32-HL07749 to G.B.H. and M.A.O., and RO1-AI28388 to J.R.P.), and the Burroughs-Wellcome Fund (to G.B.H.).
References
- Lee SC, Dickson DW, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol. 1996;27:839–847. doi: 10.1016/s0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- Kovacs JA, Kovacs AA, Polis M, Wright WC, Gill VJ, Tuazon CU, Gelmann EP, Lane HC, Longfield R, Overturf G, Macher AM, Fauci AS, Perrillo JI, Bennett JI, Masur H. Cryptococcosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1985;103:533–538. doi: 10.7326/0003-4819-103-4-533. [DOI] [PubMed] [Google Scholar]
- Berkefeld J, Enzensberger W, Lanfermann H. Cryptococcus meningoencephalitis in AIDS: parenchymal and meningeal forms. Neuroradiology. 1999;41:129–133. doi: 10.1007/s002340050717. [DOI] [PubMed] [Google Scholar]
- Manelis J, Reichenthal E, Merzbach D, Haschman N, Peyser E. Cryptococcus neoformans meningitis. Report of a case and review of cryptococcosis in Israel. Confin Neurol. 1973;35:304–311. [PubMed] [Google Scholar]
- Poo QM, Paul FM. Cryptococcus neoformans meningitis in a school girl. J Singapore Paediatr Soc. 1977;19:17–23. [PubMed] [Google Scholar]
- Yalaburgi SB, Mohapatra KC. Cryptococcus neoformans meningitis: a case report. S Afr Med J. 1980;57:1011–1012. [PubMed] [Google Scholar]
- Barluzzi R, Brozzetti A, Mariucci G, Tantucci M, Neglia RG, Bistoni F, Blasi E. Establishment of protective immunity against cerebral cryptococcosis by means of an avirulent, non melanogenic Cryptococcus neoformans strain. J Neuroimmunol. 2000;109:75–86. doi: 10.1016/s0165-5728(00)00319-2. [DOI] [PubMed] [Google Scholar]
- Salas S, Bennett J, Kwon-Chung K, Perfect J, Williamson P. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Cox GM, Perfect JR, Huffnagle GB. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun. 2003;71:1538–1547. doi: 10.1128/IAI.71.3.1538-1547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KL, Murphy JW. What makes Cryptococcus neoformans a pathogen? Emerg Infect Dis. 1998;4:71–83. doi: 10.3201/eid0401.980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GM, Harrison TS, McDade HC, Taborda CP, Heinrich G, Casadevall A, Perfect JR. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun. 2003;71:173–180. doi: 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasipura SD, Ault JG, Behr MJ, Chaturvedi V, Chaturvedi S. Characterization of Cu, Zn superoxide dismutase (SOD1) gene knock-out mutant of Cryptococcus neoformans var. gattii: role in biology and virulence. Mol Microbiol. 2003;47:1681–1694. doi: 10.1046/j.1365-2958.2003.03393.x. [DOI] [PubMed] [Google Scholar]
- Wilder JA, Olson GK, Chang YC, Kwon-Chung KJ, Lipscomb MF. Complementation of a capsule deficient Cryptococcus neoformans with CAP64 restores virulence in a murine lung infection. Am J Respir Cell Mol Biol. 2002;26:306–314. doi: 10.1165/ajrcmb.26.3.4479. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Perfect JR. Washington DC: ASM Press; Cryptococcus neoformans. 1998:pp 289–294. [Google Scholar]
- Chretien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis. 2002;186:522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- Mozaffarian N, Casadevall A, Berman JW. Inhibition of human endothelial cell chemokine production by the opportunistic fungal pathogen Cryptococcus neoformans. J Immunol. 2000;165:1541–1547. doi: 10.4049/jimmunol.165.3.1541. [DOI] [PubMed] [Google Scholar]
- Chen SH, Stins MF, Huang SH, Chen YH, Kwon-Chung KJ, Chang Y, Kim KS, Suzuki K, Jong AY. Cryptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J Med Microbiol. 2003;52:961–970. doi: 10.1099/jmm.0.05230-0. [DOI] [PubMed] [Google Scholar]
- Cox GM, Mukherjee J, Cole GT, Casadevall A, Perfect JR. Urease as a virulence factor in experimental cryptococcosis. Infect Immun. 2000;68:443–448. doi: 10.1128/iai.68.2.443-448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BL, Roberts GD. Rapid selective urease test for presumptive identification of Cryptococcus neoformans. J Clin Microbiol. 1979;10:380–381. doi: 10.1128/jcm.10.3.380-381.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruane PJ, Walker LJ, George WL. Disseminated infection caused by urease-negative Cryptococcus neoformans. J Clin Microbiol. 1988;26:2224–2225. doi: 10.1128/jcm.26.10.2224-2225.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava AJ, Negroni R, Bianchi M. Cryptococcosis produced by a urease negative strain of Cryptococcus neoformans. J Med Vet Mycol. 1993;31:87–89. [PubMed] [Google Scholar]
- Abe K, Kadota J, Ishimatsu Y, Iwashita T, Tomono K, Kawakami K, Kohno S. Th1-Th2 cytokine kinetics in the bronchoalveolar lavage fluid of mice infected with Cryptococcus neoformans of different virulences. Microbiol Immunol. 2000;44:849–855. doi: 10.1111/j.1348-0421.2000.tb02573.x. [DOI] [PubMed] [Google Scholar]
- Blackstock R, Buchanan KL, Adesina AM, Murphy JW. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect Immun. 1999;67:3601–3609. doi: 10.1128/iai.67.7.3601-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle GB, Chen GH, Curtis JL, McDonald RA, Strieter RM, Toews GB. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3516. [PubMed] [Google Scholar]
- Olszewski MA, Huffnagle GB, Traynor TR, McDonald RA, Cook DN, Toews GB. Regulatory effects of macrophage inflammatory protein 1alpha/CCL3 on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infect Immun. 2001;69:6256–6263. doi: 10.1128/IAI.69.10.6256-6263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Qifeng X, Tohyama M, Qureshi MH, Saito A. Contribution of tumour necrosis factor-α (TNF-α) in host defence mechanism against Cryptococcus neoformans. Clin Exp Immunol. 1996;106:468–474. doi: 10.1046/j.1365-2249.1996.d01-870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Gibbons J, Garcia-Rivera J, Casadevall A, Williamson PR. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun. 2001;69:5589–5596. doi: 10.1128/IAI.69.9.5589-5596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade HC, Cox GM. A new dominant selectable marker for use in Cryptococcus neoformans. Med Mycol. 2001;39:151–154. doi: 10.1080/mmy.39.1.151.154. [DOI] [PubMed] [Google Scholar]
- Liu L, Wakamatsu K, Ito S, Williamson PR. Catecholamine oxidative products, but not melanin, are produced by Cryptococcus neoformans during neuropathogenesis in mice. Infect Immun. 1999;67:108–112. doi: 10.1128/iai.67.1.108-112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyetko MR, Sud S, Chen GH, Fuller JA, Chensue SW, Toews GB. Urokinase-type plasminogen activator is required for the generation of a type 1 immune response to pulmonary Cryptococcus neoformans infection. J Immunol. 2002;168:801–809. doi: 10.4049/jimmunol.168.2.801. [DOI] [PubMed] [Google Scholar]
- Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol. 2000;164:2021–2027. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- Craven PC, Graybill JR, Jorgensen JH. Ketoconazole therapy of murine cryptococcal meningitis. Am Rev Respir Dis. 1982;125:696–700. doi: 10.1164/arrd.1982.125.6.696. [DOI] [PubMed] [Google Scholar]
- Hector RF, Yee E. Evaluation of Bay R 3783 in rodent models of superficial and systemic candidiasis, meningeal cryptococcosis, and pulmonary aspergillosis. Antimicrob Agents Chemother. 1990;34:448–454. doi: 10.1128/aac.34.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley HL, Hu LT, Foxal PA. Helicobacter pylori urease: properties and role in pathogenesis. Scand J Gastroenterol. 1991;187:S39–S46. [PubMed] [Google Scholar]
- Chen G, Fournier RL, Varanasi S, Mahama-Relue PA. Helicobacter pylori survival in gastric mucosa by generation of a pH gradient. Biophys J. 1997;73:1081–1088. doi: 10.1016/S0006-3495(97)78140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher DM, Griffith DP, Yawn D, Rossen RD. Role of urease in pyelonephritis resulting from urinary tract infection with Proteus. J Infect Dis. 1975;131:177–181. doi: 10.1093/infdis/131.2.177. [DOI] [PubMed] [Google Scholar]
- Peerbooms PG, Marian A, Verweij JJ, MacLaren DM. Urinary virulence of Proteus mirabilis in two experimental mouse models. Infect Immun. 1982;36:1246–1248. doi: 10.1128/iai.36.3.1246-1248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orazio SE, Collins CM. Characterization of a plasmid-encoded urease gene cluster found in members of the family Enterobacteriaceae. J Bacteriol. 1993;175:1860–1864. doi: 10.1128/jb.175.6.1860-1864.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka H, Sakaguchi N, Sano K, Ito M. Intravascular granuloma induced by intravenous inoculation of Cryptococcus neoformans. Mycopathologia. 1996;133:149–158. doi: 10.1007/BF02373022. [DOI] [PubMed] [Google Scholar]
- Naslund B, Stahle L, Lundin A, Anderstam B, Arner P, Bergstrom J. Luminometric single step urea assay using ATP-hydrolyzing urease. Clin Chem. 1998;44:1964–1973. [PubMed] [Google Scholar]
- Taylor-Robinson SD, Jackson N, Buckley C. Helicobacter pylori, ammonia and the brain. Gut. 1997;40:805–806. doi: 10.1136/gut.40.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Mazzolla R, Pitzurra L, Puliti M, Saleppico S, Bistoni F. Biomolecular events involved in anticryptococcal resistance in the brain. Infect Immun. 1995;63:1218–1222. doi: 10.1128/iai.63.4.1218-1222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Peter R, Williamson, Ryan S, Fajardo, Huffnagle GB. CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Infect Immun. 2004;72:1693–1699. doi: 10.1128/IAI.72.3.1693-1699.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GT. Ammonia production by Coccidioides immitis and its possible significance to the host-fungus interplay. Vanden Bossche H, Stevens DA, Odds FC, editors. Bethesda: National Foundation for Infectious Disease; Host-Fungus Interplay. 1997:pp 247–263. [Google Scholar]
- Mirbod F, Schaller RA, Cole GT. Purification and characterization of urease isolated from the pathogenic fungus Coccidioides immitis. Med Mycol. 2002;40:35–44. doi: 10.1080/mmy.40.1.35.44. [DOI] [PubMed] [Google Scholar]
- Kamberi P, Sobel RA, Clemons KV, Stevens DA, Pappagianis D, Williams PL. A murine model of coccidioidal meningitis. J Infect Dis. 2003;187:453–460. doi: 10.1086/367961. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Mazowiecki M, Bonds LA, Cohn DL, Wilson ML. Coccidioidomycosis meningitis with massive dural and cerebral venous thrombosis and tissue arthroconidia. Arch Pathol Lab Med. 2000;124:310–314. doi: 10.5858/2000-124-0310-CMWMDA. [DOI] [PubMed] [Google Scholar]
- Pryor WH, Jr, Huizenga CG, Splitter GA, Harwell JF., Jr Coccidioides immitis encephalitis in two dogs. J Am Vet Med Assoc. 1972;161:1108–1112. [PubMed] [Google Scholar]