Abstract
Transcription of the fecABCDE ferric citrate transport genes of Escherichia coli K-12 is initiated by a signaling cascade from the cell surface into the cytoplasm. FecR receives the signal in the periplasm from the outer membrane protein FecA loaded with ferric citrate, transmits the signal across the cytoplasmic membrane, and converts FecI in the cytoplasm to an active sigma factor. In this study, it was shown through the use of a bacterial two-hybrid system that, in the periplasm, the C-terminal FecR237-317 fragment interacts with the N-terminal FecA1-79 fragment. In the same C-terminal region, amino acid residues important for the interaction of FecR with FecA were identified by random and site-directed mutagenesis. They were preferentially located in and around a leucine motif (residues 247 to 268) which was found to be highly conserved in FecR-like proteins. The degree of residual binding of FecR mutant proteins to FecA was correlated with the degree of transcription initiation in response to ferric citrate in the culture medium. Three randomly generated inactive FecR mutants, FecR(L254E), FecR(L269G), and FecR(F284L), were suppressed to different degrees by the mutants FecA(G39R) and FecR(D43E). One FecR mutant, FecR (D138E, V197A), induced fecA promoter-directed transcription constitutively in the absence of ferric citrate and bound more strongly than wild-type FecR to FecA. The data showed that FecR interacts in the periplasm with FecA to confer ferric citrate-induced transcription of the fec transport genes and identified sites in FecR and FecA that are important for signal transduction.
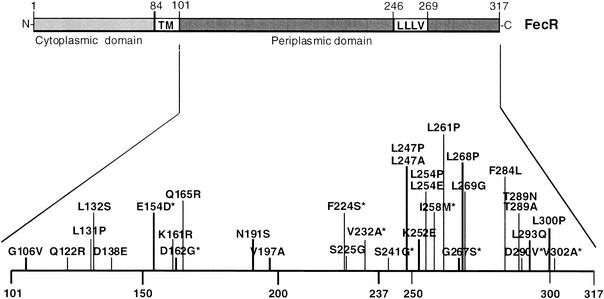
In Escherichia coli K-12, transcription of the ferric citrate transport genes fecABCDE is controlled by a signal transduction mechanism that starts from the cell surface (3, 4, 12). Binding of ferric citrate to the outer membrane FecA protein initiates a signal that is transmitted across the cytoplasmic membrane by FecR (15), resulting in an active FecI sigma factor that directs the RNA polymerase core enzyme to the promoter of the fecABCDE transport genes (1, 6, 22, 23). The C-terminal domain of FecR (Fig. 1) is located in the periplasm (32), interacts with FecA (7, 15), and receives the signal from ferric citrate-loaded FecA. The N-terminal region of FecR is located in the cytoplasm and interacts with FecI (7, 17, 27). FecR contains a stretch of hydrophobic amino acids between residues 85 and 100 that spans the cytoplasmic membrane (32).
FIG. 1.
Model of functional domains of FecR. TM, transmembrane-spanning segment between residues 85 and 100.
FecI belongs to the class of sigma factors that respond to extracytoplasmic stimuli (ECF) (10, 13, 16, 20, 33). ECF sigma factors are usually controlled by anti-sigma factors. No role as an anti-sigma factor has been uncovered for FecR. Instead, FecR is necessary for FecI to function as a sigma factor. To support this finding further, we generated point mutations in fecR by random and site-directed mutagenesis; the mutants obtained showed reduced or no transcription of the fecABCDE operon and were affected in binding of FecR to FecA. The mutations also revealed sites of interaction between FecR and FecA. One FecR mutant displayed a constitutive phenotype and bound more strongly than wild-type FecR to FecA. Many of the mutations were located within and close to a region that is conserved in FecR-like proteins. The motif is composed of repeating heptapeptides flanked by three leucine residues and one valine residue (Fig. 1). It resembles leucine zipper motifs contained in certain prokaryotic and eukaryotic gene-regulatory proteins (2, 14, 35), and they are also highly conserved in FecR-like proteins (Fig. 1). However, since the leucine zipper is not perfectly conserved (valine replaces leucine at one site, and proline is contained in several repeats) and the motif is located in the periplasm and does not bind to DNA, we use the term leucine motif. The data further support the involvement of FecR in signal transduction in a way that cannot be reconciled with a simple anti-sigma factor activity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The E. coli strains and plasmids used in this study are listed in Table 1. Cells were grown in tryptone-yeast extract medium (TY) or nutrient broth medium (NB) as described previously (1). Antibiotics were used at the following concentrations: ampicillin, 50 μg/ml; chloramphenicol, 40 μg/ml; and tetracycline, 12 μg/ml.
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Genotypea | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | Δ(argF lac) U196endA1recA1hsdR17(rK− mK+) supE44 thi-1 gyrA1 relA1 (F′ φ80 ΔlacZΔM15) | 11 |
| AA93 | Δfec aroB Δ(argF lac)U169 araD139 rspL150 relA1 deoC1 flbB5301 ptsF25 rbsR | 22 |
| SU202 | lexA71::Tn5 sulA211 sulA(op408/op+)::lacZ Δ(lacIPOZYA)169/F′ lacIqlacZΔM15::Tn9 | 5 |
| Plasmids | ||
| pSV66 | pHSG576 fecI fecR fecA | 12 |
| pMS604 | ori ColE1 TetrlexA1-87-fos zipper | 5 |
| pDP804 | ori p15A AmprlexA1-87408-jun zipper | 5 |
| plexFecA | pDP804 lexA1-87408-fecA1-79 | This study |
| plexAN | pMS604 lexA1-87 | This study |
| plexRC | pMS604 lexA1-87-fecR101-317 | This study |
| plex237 | pMS604 lexA1-87-fecR237-317 | This study |
| plex288 | pMS604 lexA1-87-fecR101-288 | This study |
| plex278 | pMS604 lexA1-87-fecR101-278 | This study |
| plexLZG | pMS604 lexA1-87-fecR227-288 | This study |
| pT7-7 | ori ColE1 phage T7 gene promoter | 28 |
| pAA70 | pT7-7 fecR | 34 |
| pLZ1A | pT7-7 fecR(L247A) | This study |
| pLZ1C | pT7-7 fecR(L247C) | This study |
| pLZ1 | pT7-7 fecR(L247P) | This study |
| pLZ2 | pT7-7 fecR(L254P) | This study |
| pLZ3 | pT7-7 fecR(L261P) | This study |
| pLZ4 | pT7-7 fecR(V268P) | This study |
| plexLZ1A | pMS604 lexA1-87-fecR101-317(L247A) | This study |
| plexLZ1C | pMS604 lexA1-87-fecR101-317(L247C) | This study |
| plexLZ1 | pMS604 lexA1-87-fecR101-317(L247P) | This study |
| plexLZ2 | pMS604 lexA1-87-fecR101-317(L254P) | This study |
| plexLZ3 | pMS604 lexA1-87-fecR101-317(L261P) | This study |
| plexLZ4 | pMS604 lexA1-87-fecR101-317(V268P) | This study |
| pFPV25 | ori ColE1 Ampr contains promoterless gfpmut3 | 30 |
| pGFPA′ | pFPV25 PfecA-gfp | This study |
| pGFPAA′ | pFPV25 fecA PfecA-gfp | This study |
| pHSG576 | pSC101 derivative, Cmr | 29 |
| pMMO203 | pHSG576 fecI fecR | 27 |
| pLCLZ1A | pHSG576 fecI fecR(L247A) | This study |
| pLCLZ1C | pHSG576 fecI fecR(L247C) | This study |
| pLCLZ1 | pHSG576 fecI fecR(L247P) | This study |
| pLCLZ2 | pHSG576 fecI fecR(L254P) | This study |
| pLCLZ3 | pHSG576 fecI fecR(L261P) | This study |
| pLCLZ4 | pHSG576 fecI fecR(V268P) | This study |
| pIS712 | pHSG576 fecI fecR fecA | 15 |
| pOR603 | pHSG576 fecI fecR(T2511C)(L254E) fecA | This study |
| pOR601 | pHSG576 fecI fecR(C2255G/T2256C)(L269G) fecA | This study |
| pOR600 | pHSG576 fecI fecR(T2600C/C2602G)(F284L) fecA | This study |
| pOR603D1 | pHSG576 fecI fecR(T2511C)(L254E) fecA(C3054A)(G39R) | This study |
| pOR601D1 | pHSG576 fecI fecR(C2255G/T2256C) (L269G) fecA(C3054A) (G39R) | This study |
| pOR600D1 | pHSG576 fecI fecR(T2600C/C2602G) (F284L) fecA(C3054A) (G39R) | This study |
| pOR603C3 | pHSG576 fecI fecR(T2511C)(L254E) fecA(G3004C)(D43E) | This study |
| pOR601C3 | pHSG576 fecI fecR(C2255G/T2256C) (L269G) fecA(G3004C) (D43E) | This study |
| pOR600C3 | pHSG576 fecI fecR(T2600C/C2602G) (F284L) fecA(G3004C) (D43E) | This study |
| pORD1 | pHSG576 fecI fecR fecA(C3054A) (G39R) | This study |
| pORC3 | pHSG576 fecI fecR fecA(G3004C) (D43E) | This study |
| pLCIRA | pHSG576 fecI fecR fecA | 27 |
| pIS135 | pHSG576 fecI fecR | 22 |
| pHCIR | pBCKS+fecI fecR1-110 | This study |
| pAS103 | pBCKS+fecI fecR | 27 |
| pMS604fecA1-79 | pMS604 lexA1-87-fecA1-79 | This study |
| pUS11 | pDP804 lexA1-87408-fecR101-317 | 7 |
| pHBlexR2 | pDP804 lexA1-87408-fecR101-317 (T2164A/T2340C) (D138E/V197A) | This study |
| pHBlexR7 | pDP804 lexA1-87408-fecR101-317 (A2235G/A2471G/A2619T) (D162G/S241G/D290V) | This study |
| pHBlexR12 | pDP804 lexA1-87408-fecR101-317 (A2115G/T2142C/A2145G/A2232G/A2322G/A2504G/T2628A) (Q122R/L131P/N132S/K161R/N191S/K252E/L293Q) | This study |
| pHBlexR13 | pDP804 lexA1-87408-fecR101-317 (T2445C/G2548A) (V232A/G267S) | This study |
| pHBlexR15 | pDP804 lexA1-87408-fecR101-317 (A2423G/A2615G) (S225G/T289A) | This study |
| pHBlexR16 | pDP804 lexA1-87408-fecR101-317 (C2616A/T2649C) (T289N/L300P) | This study |
| pHBlexR21 | pDP804 lexA1-87408-fecR101-317 (A2212T/T2421C/A2524G/T2655C) (E154D/F224S/I258M/V302A) | This study |
| pHBlexR22 | pDP804 lexA1-87408-fecR101-317 (G2067T/A2244G) (G106V/Q165R) | This study |
| pHBlcR2 | pHSG576 fecI fecR (T2164A/T2340C) (D138E/V197A) fecA | This study |
| pHBlcR7 | pHSG576 fecI fecR (A2235G/A2471G/A2619T) (D162G/S241G/D290V) fecA | This study |
| pHBlcR12 | pHSG576 fecI fecR (A2115G/T2142C/A2145G/A2232G/A2322G/A2504G/T2628A) (Q122R/L131P/N132S/K161R/N191S/K252E/L293Q) fecA | This study |
| pHBlcR13 | pHSG576 fecI fecR (T2445C/G2548A) (V232A/G267S) fecA | This study |
| pHBlcR15 | pHSG576 fecI fecR (A2423G/A2615G) (S225G/T289A) fecA | This study |
| pHBlcR16 | pHSG576 fecI fecR (C2616A/T2649C) (T289N/L300P) fecA | This study |
| pHBlcR21 | pHSG576 fecI fecR (A2212T/T2421C/A2524G/T2655C) (E154D/F224S/I258M/V302A) fecA | This study |
| pHBlcR22 | pHSG576 fecI fecR (G2067T/A2244G) (G106V/Q165R) fecA | This study |
| pHBhcR2 | pBCKS+fecI fecR (T2164A/T2340C) (D138E/V197A) | This study |
| pHBhcR7 | pBCKS+fecI fecR (A2235G/A2471G/A2619T) (D162G/S241G/D290V) | This study |
| pHBhcR12 | pBCKS+fecI fecR (A2115G/T2142C/A2145G/A2232G/A2322G/A2504G/T2628A) (Q122R/L131P/N132S/K161R/N191S/K252E/L293Q) | This study |
| pHBhcR13 | pBCKS+fecI fecR (T2445C/G2548A) (V232A/G267S) | This study |
| pHBhcR15 | pBCKS+fecI fecR (A2423G/A2615G) (S225G/T289A) | This study |
| pHBhcR16 | pBCKS+fecI fecR (C2616A/T2649C) (T289N/L300P) | This study |
| pHBhcR21 | pBCKS+fecI fecR (A2212T/T2421C/A2524G/T2655C) (E154D/F224S/I258M/V302A) | This study |
| pHBhcR22 | pBCKS+fecI fecR (G2067T/A2244G) (G106V/Q165R) | This study |
Subscripts denote amino acid residues of the encoded proteins. Numbers in parentheses indicate amino acids replaced.
Construction of plasmids.
The truncated fecA1-79 fragment was synthesized by PCR. Plasmid plexFecA was obtained with primers A1XhoI (5′-CCACGGTAGATCTTTATTCTTTTGGTGCG-3′) and A79BglII (5′-CCGCTTTTGCTCTCGAGGTTAATATCGCAC-3′). The resulting fecA fragment was digested with XhoI and BglII and cloned into XhoI- and BglII-restricted pDP804.
Plasmids pLZ1A and pLZ1 were constructed by site-directed mutagenesis of plasmid pAA70 with primers 2490A (5′-GAAGGATATCGCGACGTTCAGCG-3′) and 2490 (5′-GAAGGATATCCCGAGCTTCAGCG-3′), respectively, and the reverse primer 2490REV (5′-TCAGGATATCCTTCGTCCAGCTTG-3′) for introduction of the leucine substitutions at amino acid 247 and an EcoRV cleavage site at position 2490. The resulting PCR fragments were cleaved with EcoRV and religated. For construction of plasmid pLZ2 by site-directed mutagenesis, primers 2511 (5′-ACCGCCCGGGGAGGTGATAGCCACGCTAA-3′) and 2511REV (5′-CCTCCCCGGGCGGTTTATCGCTCAA-3′) were used to introduce the leucine-to-proline substitution at residue 254 and a SmaI cleavage site at position 2511.
Plasmids pLZ3 and pLZ4 were obtained by PCR with primers 2532 (5′-ACCCGGTACCGCAACGGCGTCGT-3′) and 2552 (5′-ACCCGGTACCGCAACGGCCCGCTGCGCT-3′), respectively, and reverse primers 2532REV (5′-TTGCGGTACCGGGTTGGCGTGGCTAT-3′) and 2552REV (5′-TTGCGGTACCGGGTTAGCGTGGCTAT-3′), respectively, replacing residues 261 and 268, respectively, with proline residues and introducing a KpnI cleavage site.
Plasmid plexAN was constructed by BstEII and XhoI restriction of plasmid pMS604 (5) and religation of the plasmid treated with Klenow polymerase. For the LexA repressor fusion proteins, the fos zipper motif of plasmid pMS604 was replaced with the wild-type or a mutated fecR101-317 fragment. fecR101-317 from plasmids pAA70, pLZ1A, pLZ1, pLZ2, pLZ3, and pLZ4 was amplified by PCR with primers lexfecR8 and lexfecR9 (7), cleaved with BstEII and XhoI, and cloned into BstEII- and XhoI-restricted pMS604, yielding plasmids plexRC, plexLZ1A, plexLZ1, plexLZ2, plexLZ3, and plexLZ4, respectively.
For construction of plasmid pGFPA′, the fecA promoter region was amplified by PCR with primers AA11 (1) and PA2769 (5′-GCCCTAGGTTGTGTTCAGCTATG-3′). The resulting PCR fragment was cleaved with EcoRI and BamHI and cloned into EcoRI- and BamHI-digested pFPV25 (30). The complete fecA-containing fragment was obtained by PCR with primers AA11 and FA5160 (5′-CGGAATTCTAATCACATTCCAGC-3′), restricted with EcoRI, and ligated into EcoRI-cleaved pGFPA′ in the opposite orientation to PfecA::gfp, resulting in plasmid pGFPAA′. To replace wild-type fecR with the leucine zipper mutants, the NdeI-HindIII fragment of plasmids pLZ1A, pLZ1, pLZ2, pLZ3, and pLZ4 was cloned into NdeI- and HindIII-restricted pMMO203 (27), yielding plasmids pLCLZ1A, pLCLZ1, pLCLZ2, pLCLZ3, and pLCLZ4, respectively.
The region of fecR encoding fecR237-317 was obtained by PCR amplification with primers RBstE237 (5′-GCTGGTGACCGAGAGTACAAGCTGGACGAA-3′) and lexfecR9 (5′-CCCCTCGAGTTACAGTGGTGAAATGTTTAT-3′) and plasmid pAA70 as the template. The resulting PCR fragment was digested with BstEII and XhoI and ligated into BstEII- and XhoI-restricted pMS604, yielding plasmid plex237. Plasmid plex288 was obtained by PCR amplification of fecR101-288 with primers lexfecR8 (5′-GAACCGGTGACCTCGGAAACCGGCGAAGGT-3′) and RXhoI288 (5′-GGATCTCGAGTCAATTTTTCAGCGGGAACGTCC-3′) and plasmid pAA70 as the template. Plasmid plex278 encoding fecR101-278 was obtained by PCR amplification with primers lexfecR8 and RXhoI278 (5′-CCCCTCGAGTCACAGCCCGGCAACGGCGGGATC-3′) and plasmid pAA70 as the template. Plasmid plexLZG encoding fecR227-288 was obtained by PCR amplification with primers RBstEII227 (5′-GTTGGTGACCTCTGAGTTTGGCGCAGTG-3′) and RXhoI288 and plasmid pAA70 as the template. The resulting PCR fragments were digested with BstEII and XhoI and cloned into BstEII- and XhoI-restricted pMS604.
fecR224-317 was randomly mutagenized by PCR with primers FecR1 (5′-CTGGAGTATGGCATATGAATC-3′) and FecIR3 (5′-GGGAATTATTAAGCTTACAGTGG-3′) and plasmid pSV66 as the template. The resulting fecR fragments were cleaved with PstI and HindIII and cloned into the PstI- and HindIII-restricted plasmid pIS712 fecIRA, yielding plasmids pOR603, pOR601, and pOR600.
Mutated fecA genes were amplified by PCR with primers AA4 (5′-CCGTTAGAATTCAGTCTATTACGC-3′) and AA13 (5′-GGCGTGGCGGATCCCCAGCAGCAGGCC-3′) and pIS712 as the template. The fecA fragments were digested with EcoRI and DsaI and ligated into EcoRI- and DsaI-cleaved plasmid pOR600, yielding plasmids pOR600D1 and pOR600C3. The mutated fecA fragments of plasmids pOR600D1 and pOR600C3 were cleaved with EcoRI and DsaI and cloned into EcoRI- and DsaI-restricted plasmids pIS712, pOR603, and pOR601, yielding plasmids pORD1, pORC3, pOR603D1, pOR601D1, pOR603C3, and pOR601C3, respectively.
The periplasmic domain of FecR, representing the region from amino acids 101 to 317, was randomly mutagenized by PCR with primers LexFecR1 (5′-CGCCTCGAGGGATCTAGATCGGAAACCGGCGAAGGT-3′) and LexFecR2 (5′-GGAAGATCTTCCACCTAGTTTACAGTGGTGAAATGTT-3′) and plasmid pSV66 as the template. The mutated fecR fragments were cloned into pDP804 by replacing the XhoI-BglII fragment containing the Jun zipper motif, resulting in plasmids pHBlexR2, pHBlexR7, pHBlexR12, pHBlexR13, pHBlexR15, pHBlexR16, pHBlexR21, and pHBlexR22. The fecR point mutations were identified by DNA sequencing.
Plasmid pMS604fecA1-79 encodes the N-terminal region of FecA from amino acids 1 to 79. This plasmid was obtained by replacing the BstEII-XhoI fragment containing the Fos zipper on plasmid pMS604 with sequence encoding the N-terminal region of the mature FecA protein. The gene was amplified from plasmid pSV66 with oligonucleotides LexFecA3 (5′-GAACCGGTGACCGGATCTAGAGCACAGGTTAATATCGGA-3′) and LexFecA2 (5′-TTCCCCCTCGAGTCCACTAGTTTCTTTTGGTGCGGGCGC-3′). For construction of plasmids pHBlcR2, pHBlcR7, pHBlcR12, pHBlcR13, pHBlcR15, pHBlcR16, pHBlcR21, and pHBlcR22, the DNA encoding the N-terminal region of the mature FecR protein was amplified with primers fecR1 (5′-CTGGAGTATGGCATATGAATC-3′) and AgeIWTfecR (5′-ATCCCTACCGGTTTCCCGCTGCCAGAGCTGCCA-3′) with plasmid pSV66 as the template. The mutated periplasmic part of FecR was amplified by PCR with oligonucleotides AgemutFecR (5′-ATCCCTACCGGTGAAGGTCTGCGGGCAGATTAC-3′) and the reverse primer pDP804HindIII (5′-CGTTGCCAAGCTTCTTTTACCCCTGCATCTTTG-3′) with plasmid pHBlexR2, pHBlexR7, pHBlexR12, pHBlexR13, pHBlexR15, pHBlexR16, pHBlexR21, or pHBlexR22 as the template. The two AgeI-digested PCR fragments were ligated and cloned into plasmid pLCIRA, replacing the NdeI-HindIII region encoding wild-type FecR.
To obtain plasmids pHBhcR2, pHBhcR7, pHBhcR12, pHBhcR13, pHBhcR15, pHBhcR16, pHBhcR21, and pHBhcR22, wild-type fecR was cut out of plasmid pHCIR with NdeI and HindIII and replaced with the NdeI- and HindIII-digested mutated fecR of plasmids pHBlcR2, pHBlcR7, pHBlcR12, pHBlcR13, pHBlcR15, pHBlcR16, pHBlcR21, and pHBlcR22, respectively.
Recombinant DNA techniques.
Standard techniques (25) or the protocols of the suppliers were used for the isolation of plasmid DNA, PCR, digestion with restriction endonucleases, ligation, transformation, and agarose gel electrophoresis. DNA was sequenced by the dideoxy chain termination method (26) with the AutoRead sequencing kit (Pharmacia Biotech, Freiburg, Germany). The reaction products were sequenced on an A.L.F. DNA sequencer (Pharmacia Biotech).
Determination of β-galactosidase activity.
β-Galactosidase activities were determined according to Miller (19) and Giacomini et al. (9). To determine the induction level, cells were grown in NB medium with no additions or supplemented with 50 μM 2,2′-dipyridyl or 1 mM citrate. For the LexA-based repression system, cells were grown in TY medium supplemented with 1 mM isopropylthiogalactopyranoside (IPTG).
GFP measurements.
Cells were grown in NB medium containing supplementations as indicated. Green fluorescent protein (GFP) was quantified by fluorometry in a Bio-Tek FL500 microplate fluorescence reader (Bio-Tek Instruments Inc., Winooski, Vt.). Specific activity of GFP in bacterial cultures was expressed as relative fluorescence intensity at 530 nm of cells adjusted to an optical density of 0.5 at 578 nm in phosphate-buffered saline (30).
Similarity search and sequence alignments.
A global similarity search of the current National Center for Biotechnology Information nucleic acid databases with the advanced Blast search and the specialized Blast search of finished and unfinished microbial genomes was used to look for amino acid sequences homologous to the FecR sequence. Preliminary sequence data for Bordetella pertussis and Pseudomonas syringae were obtained from the Institute for Genomic Research website at http://www.tigr.org. The sequence data for Pseudomonas were from the Sanger Center and can be obtained from ftp://ftp.sanger.ac.uk/pub/yyy. Sequences of Pseudomonas aeruginosa were obtained from the Pseudomonas Genome Project at http://www.pseudomonas.com/data.html. Protein sequences were aligned with ClustalW.
RESULTS
Binding sites of FecR on FecA.
The N-terminal domain of FecA (residues 1 to 79) interacts in vivo and in vitro with the C-terminal domain of FecR (residues 101 to 317) (7). To localize the region of FecR that interacts with FecA specifically, a bacterial LexA-based two-hybrid system was used. LexA is a transcriptional repressor that binds as a homodimer to the sulA promoter. It consists of an N-terminal DNA-binding domain and a C-terminal dimerization domain. To determine heterodimerization, the C-terminal domain can be replaced with the dimerization domains of other proteins. To prevent homodimerization of LexA hybrid proteins, the promoter of sulA is mutated so that wild-type LexA binds to one site and mutated LexA408 binds to the other site. Dimerization of the hybrid proteins was assessed by repression of chromosomal PsulA::lacZ transcription of the reporter strain E. coli SU202. The LexA1-87408 DNA-binding domain was fused to the N terminus of FecA1-79 and the LexA1-87 DNA-binding domain was fused to the N terminus of FecR101-317 and to N- and C-terminally truncated derivatives of FecR101-317. Control measurements involved LexA-Fos combined with LexA-FecA1-79 as a negative control and LexA-FecR101-317 combined with LexA-FecA1-79 as a positive control (Table 2).
TABLE 2.
Interaction of truncated FecR101-317 derivatives with FecA1-79
| Plasmid | Proteins | β-Galactosidase activitya (Miller units) |
|---|---|---|
| pMS604 | LexA1-87WT-Fos | 478 |
| plexRC | LexA1-87WT-FecR101-317 | 45 |
| plex237 | LexA1-87WT-FecR237-317 | 65 |
| plex288 | LexA1-87WT-FecR101-288 | 221 |
| plex278 | LexA1-87WT-FecR101-278 | 453 |
| plexLZG | LexA1-87WT-FecR227-288 | 453 |
Determined by using the bacterial two-hybrid LexA-based system in E. coli SU202 sulA-lacZ, which, in addition to FecR derivatives, synthesized LexA1-87408-FecA1-79 encoded by plexFecA. WT, wild type.
The truncated FecR237-317 derivative combined with FecA1-79 repressed PsulA::lacZ transcription (Table 2). A more central fragment, FecR101-288, and FecA1-79 showed less repression (4.9-fold higher β-galactosidase activity than fully repressed cells). Deletion of a further 10 residues at the C terminus, resulting in FecR101-278, and also FecR227-288 led to no repression. The data indicate that a region encompassing residues 237 to 317 of FecR is required for the interaction with FecA1-79.
Point mutations in FecR leucine motif reduce binding to FecA.
FecR237-317, which was sufficient for binding to FecA1-79, contains a conserved leucine motif within residues 247 to 268 (Fig. 1 and 2). To determine whether the leucine motif is important for binding of FecR to FecA1-79, the leucine and valine residues were replaced with proline residues. In addition, the first leucine residue was replaced with alanine and cysteine residues.
FIG. 2.
Alignment of leucine motifs of FecR homologues. Similarity search and sequence alignment were done as described in Materials and Methods. Note the highly conserved leucine residues of the leucine motif corresponding to positions 247, 254, 261, and 268 of E. coli FecR. See additional information in Martinez-Bueno et al. (18), Nelson et al. (21), and Visca et al. (31).
E. coli SU202 was transformed with plasmids carrying lexA-fecA1-79 and the mutated lexA-fecR101-317 fusion genes. All leucine-to-proline mutations resulted in higher levels of PsulA::lacZ transcription than wild-type FecR101-317 (Table 3). Proline residues introduced at positions 247 and 268 reduced binding to FecA1-79 less than proline substitutions at positions 254 and 261 in the middle of the leucine zipper-like motif (Table 3). Proline distorts an α-helix, particularly when it is located in the middle of an α-helix. Alanine at position 247 slightly reduced binding to FecA1-79, and cysteine exerted a somewhat stronger effect. To eliminate the possibility that differences in activity were caused by different amounts of protein, Western analysis was performed with an anti-LexA antibody. The expression level of the hybrid proteins was low, but similar amounts of the proteins were observed (data not shown). The amounts of the plasmid-encoded FecR derivatives were higher than the amounts of chromosomally encoded wild-type FecR. These results demonstrate the important role played by the conserved leucine residues in binding of FecR to FecA.
TABLE 3.
Binding of mutated FecR101-317 to FecA1-79
| Plasmid | FecR proteins | β-Galactosidase activitya (Miller units) |
|---|---|---|
| pSM604 | LexA1-87WT-Fos | 446 |
| plexRC | LexA1-87WT-FecR101-317 | 36 |
| plexLZ1A | LexA1-87WT-FecR (L247A) | 54 |
| plexLZ1C | LexA1-87WT-FecR (L247C) | 94 |
| plexLZ1 | LexA1-87WT-FecR (L247P) | 170 |
| plexLZ2 | LexA1-87WT-FecR (L254P) | 367 |
| plexLZ3 | LexA1-87WT-FecR (L261P) | 376 |
| plexLZ4 | LexA1-87WT-FecR (L268P) | 112 |
Determined by using the bacterial two-hybrid LexA-based system in E. coli SU202 sulA-lacZ, which, in addition to FecR derivatives, synthesized LexA1-87408-FecA1-79 encoded by plexFecA. WT, wild type.
Randomly generated point mutations in FecR affect binding to FecA.
To determine additional FecR residues that are important for the interaction with FecA1-79, binding of PCR-mutated FecR101-317 to FecA was studied in the LexA-based two-hybrid system. The LexA1-87 DNA-binding domain was fused to the N terminus of FecA1-79, and the LexA1-87408 DNA-binding domain was fused to the N terminus of FecR101-317. Blue colonies on TY agar plates supplemented with IPTG and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) were selected from E. coli SU202 transformed with mutagenized fecR101-317 and wild-type fecA1-79 because they indicated lack of repression of PsulA::lacZ and consequently lack of heterodimerization. All FecR mutants except FecR(D138E, V197A) displayed high transcription levels (Table 4). The mutations were clustered in the regions from residues 131 to 165 and from 224 to 302 (Fig. 1).
TABLE 4.
Binding of mutated FecR101-317 to FecA1-79
| Plasmid | FecR protein | β-Galactosidase activitya (Miller units) |
|---|---|---|
| pDP804 | LexA1-87408-Jun | 636 |
| pUS11 | LexA1-87408-FecR101-317 | 134 |
| pHBLexR2 | LexA1-87408-FecR101-317 (D138E, V197A) | 93 |
| pHBLexR7 | LexA1-87408-FecR101-317 (D162G, S241G, D290V) | 542 |
| pHBLexR12 | LexA1-87408-FecR101-317 (Q122R, L131P, N132S, K161R, N191S, K252E, L293Q) | 607 |
| pHBLexR13 | LexA1-87408-FecR101-317 (V232A, G267S) | 516 |
| pHBLexR15 | LexA1-87408-FecR101-317 (S225G, T289A) | 537 |
| pHBLexR16 | LexA1-87408-FecR101-317 (T289N, L300P) | 688 |
| pHBLexR21 | LexA1-87408-FecR101-317 (E154D, F224S, I258M, V302A) | 754 |
| pHBLexR22 | LexA1-87408-FecR101-317 (G106V, Q165R) | 731 |
Determined by using the bacterial two-hybrid LexA-based system in E. coli SU202 sulA-lacZ, which, in addition to FecR derivatives, synthesized LexA1-87-FecR1-79 encoded by pMS604fecA1-79.
FecR point mutants display reduced activation of FecI.
The site-directed and randomly generated FecR point mutants were tested to determine whether reduction of binding to FecA affected transcription initiation of the fec transport genes. The DNA fragment encoding the wild-type leucine zipper-like motif of FecR was replaced with the mutated fragment, and the resulting fecR mutant genes were cloned on the low-copy-number plasmid pHSG576 together with wild-type fecI. After confirming that the FecR mutant proteins were contained in the membrane fraction in amounts similar to that of wild-type FecR (data not shown), transcription of the fec transport genes was determined in E. coli AA93 lacking the fec genes but carrying the reporter plasmid pGFPAA′, which encodes complete fecA and PfecA::gfp, the green fluorescence protein gene under the control of the fecA promoter. The promoter upstream of fecA controls transcription of the fecABCDE transport genes (6). E. coli AA93/pGFPAA′ was transformed with plasmids carrying the fecR mutant genes and the fecI wild-type gene.
The FecR mutant proteins in the leucine motif induced ferric citrate-dependent fecA transcription less than wild-type FecR (low fluorescence activity; Table 5). The mutations that most strongly reduced PfecA::gfp transcription also most strongly reduced dimerization (high β-galactosidase activity; Table 3). Induction depended on the inducer, ferric citrate (Table 5). Only background fluorescence was detected in E. coli AA93(pGFPAA′) transformed with the vector plasmid pHSG576. This result supports the involvement of the leucine motif in fecA promoter activity mediated by FecI.
TABLE 5.
Induction activity of FecR leucine motif mutantsa
| Plasmid | FecR protein | Relative fluorescence
|
|
|---|---|---|---|
| NB medium | NB medium + citrate | ||
| pHSG576 | None | 3 | 4 |
| pMMO203 | FecR | 22 | 356 |
| pLCLZ1A | FecR(L247A) | 21 | 285 |
| pLCLZ1C | FecR(L247C) | 21 | 235 |
| pLCLZ1 | FecR(L247P) | 18 | 107 |
| pLCLZ2 | FecR(L254P) | 25 | 28 |
| pLCLZ3 | FecR(L261P) | 10 | 36 |
| pLCLZ4 | FecR(V268P) | 11 | 174 |
Induction was determined in E. coli AA93 Δfec(pGFPAA′ fecA, PfecA::gfp). The plasmids listed encoded FecI in addition to the listed FecR derivatives.
The randomly generated fecR mutant genes were cloned into the low-copy-number plasmid pLCIRA fecIRA to replace the sequence of wild-type fecR encoding residues 101 to 317. The transcription levels were measured in E. coli AA93 carrying the reporter plasmid pGFPA′ PfecA::gfp. All FecR mutants conferred lower transcription levels than wild-type FecR, which depended on ferric citrate (Table 6). Only FecR(D138E, V197A) displayed constitutive PfecA::gfp transcription that was only slightly increased by ferric citrate. This FecR mutant bound to FecA even more strongly than wild-type FecR (Table 4). The level of PfecA::gfp transcription was correlated with binding of FecR to FecA with the exception of FecR(S225G, T289A), which showed strongly reduced binding to FecA1-79 but a rather high transcription level (58%) in the presence of ferric citrate. Inactivity of the mutated FecR could be caused by instability of the point mutants. Therefore, their amounts were estimated in uninduced and induced cells by Western blot analysis with anti-FecR antibodies. FecR(D162G, S241G, D290V), FecR(V232A, G267S), and FecR(E154D, F224S, I258 M, V302A) were not detectable in the blot (data not shown), which implied that their low binding to FecA and low induction of PfecA::gfp were caused by small amounts of protein.
TABLE 6.
Induction activity of FecR mutantsa
| Plasmid | Protein | Relative fluorescence (% of wild-type)
|
Stability | |
|---|---|---|---|---|
| NB medium | NB medium + citrate | |||
| pIS135 | FecR | 7 | 8 | Wild type |
| pLCIRA | FecR | 17 | 100 | Wild type |
| pHBlcR2 | FecR(D138E, V197A) | 43 | 74 | As wild type |
| pHBlcR7 | FecR(D162G, S241G, D290V) | 7 | 8 | Unstable |
| pHBlcR12 | FecR(Q122R, L131P, N132S, K161R, N191S, K252E, L293Q) | 7 | 10 | As wild type |
| pHBlcR13 | FecR(V232A, G267S) | 22 | 18 | Unstable |
| pHBlcR15 | FecR(S225G, T289A) | 15 | 58 | As wild type |
| pHBlcR16 | FecR(T289N, L300P) | 16 | 14 | As wild type |
| pHBlcR21 | FecR(E154D, F224S, I258M, V302A) | 10 | 12 | Unstable |
| pHBlcR22 | FecR(G106V, Q165R) | 7 | 10 | As wild type |
Induction was determined in E. coli AA93 Δfec(pGFPA′ fecA, PfecA::gfp). The plasmids listed encoded FecA and FecI in addition to the listed FecR derivatives except pIS135, which encoded FecI but no FecA.
Mutations in fecA that restore transcription initiation of fecR mutants.
Another approach to identify interacting regions of FecA and FecR used genetic suppression analysis. fecR point mutants were isolated and analyzed for restoration of transcription initiation of the fec transport genes by fecA point mutants. A fecR fragment encoding FecR224-317 was mutagenized by PCR, and the fecR genes were cloned into the low-copy-number plasmid pIS712 fecIRA. E. coli AA93 Δfec(pGFPA′ PfecA::lacZ) was transformed with the mutagenized plasmids, and white and pink transformant colonies were picked on MacConkey agar plates containing 1 mM citrate. Citrate and iron in the nutrient agar form ferric citrate. Transformants containing the wild-type plasmid pIS712 formed red colonies. The mutated fecR genes of three white colonies were sequenced and shown to contain a leucine-to-glutamine change at position 254 (L254E), 269 (L269G), or 284 (F284L) (Table 7). To confirm the results obtained on the MacConkey agar plates, the inducing activity of the mutated fecR genes was determined in E. coli AA93 Δfec(pGFPA′ PfecA::gfp) transformed with the fecR mutant plasmids. Cells were grown in NB medium with and without added citrate, and their relative fluorescence was determined. All three mutants displayed a fluorescence of 7 to 9% of that of wild-type fecR in the presence of citrate (Table 7).
TABLE 7.
Induction activity of FecR and FecA mutantsa
| Plasmid | Protein | Relative fluorescence
|
|
|---|---|---|---|
| NB medium | NB medium + citrate | ||
| pHSG576 | None | 40 | 45 |
| pIS712 | FecR FecA | 65 | 856 |
| pOR603 | FecR(L254E) FecA | 67 | 70 |
| pOR601 | FecR(L269G) FecA | 59 | 66 |
| pOR600 | FecR(F284L) FecA | 58 | 63 |
| pOR603D1 | FecR(L254E) FecA(G39R) | 65 | 78 |
| pOR601D1 | FecR(L269G) FecA(G39R) | 63 | 318 |
| pOR600D1 | FecR(F284L) FecA(G39R) | 48 | 108 |
| pOR603C3 | FecR(L254E) FecA(D43E) | 65 | 139 |
| pOR601C3 | FecR(L269G) FecA(D43E) | 65 | 124 |
| pOR600C3 | FecR(F284L) FecA(D43E) | 47 | 289 |
| pORD1 | FecR FecA(G39R) | 45 | 801 |
| pORC3 | FecR FecA(D43E) | 43 | 764 |
Induction was determined in E. coli AA93 Δfec(pGFPA′ fecA, PfecA::gfp). The plasmids listed encoded FecI in addition to the listed FecR and FecA derivatives.
To identify residues in the FecA N terminus that bind FecR, mutants with mutations in fecA that suppressed the fecR missense mutations were isolated. fecA was mutagenized by PCR, and the fragments comprising residues 1 to 156 were inserted into plasmid pIS712 fecIRA encoding each of the fecR mutations. E. coli AA93 Δfec harboring the reporter plasmid pMMO1034 PfecA::lacZ was transformed with each of the fecIRA derivatives. Transformants that formed red colonies on MacConkey agar plates were picked, plasmids were isolated from them, and the isolated fecA fragments were sequenced. Two mutants were isolated; one contained a G39R replacement, and the other contained a D43E replacement. The relative fluorescence of E. coli AA93(pGFPA′ PfecA::gfp) and of the same strain transformed with each of the fecIRA mutant derivatives was measured to verify the results obtained on the MacConkey agar plates and to obtain quantitative data. The mutated FecA proteins increased PfecA::gfp transcription 1.6- to 4.8-fold (Table 7). Some allele specificity was observed; for example, FecR(L269G) combined with FecA(G39R) yielded a relative fluorescence of 318, whereas FecR(L269G) combined with FecA(D43E) yielded a relative fluorescence of 124. Both FecA derivatives were highly active in combination with wild-type FecA (Table 7).
DISCUSSION
FecR consists of three domains: the periplasmic signal sensor, the transmembrane signal transmitter, and the cytoplasmic signal receiver that conveys the signal to the FecI sigma factor. Previously, we have reported that the C terminus of FecR101-317 binds to the N terminus of FecA1-79 (7). In this study, the site of FecR to which FecA binds was more accurately delineated. The region comprising residues 237 to 317 of FecR was found to be sufficient for binding to FecA1-79. Further-truncated FecR C termini displayed low or no interaction with FecA1-79. The binding site of FecR contains the leucine motif, whose role was assessed by replacing the leucine residues with alanine or proline residues. Interaction, as evidenced by repression by the mutated LexA1-87-FecR101-317 proteins, was 1.5- to 10-fold lower than repression by wild-type LexA1-87-FecR101-317, depending on the nature of the introduced amino acid and the position within the motif. The strongest effects were observed with proline substitutions in the center of the motif, as found previously for prokaryotic leucine zipper motifs (2, 14, 35). The transcription-inducing activity of the FecR mutant proteins was correlated with the degree of binding of mutated FecR101-317 to FecA1-79. The FecR leucine zipper mutant proteins that showed the lowest degree of dimerization showed the lowest level of PfecA::gfp transcription.
Suppression of fecR mutations by fecA mutations supported the functionally relevant binding of the periplasmic regions of FecA and FecR. The independently isolated inactive FecR mutations L254E, L269G, and F284L in FecA wild-type cells were located within or near the leucine motif (residues 247 to 268), even though the entire periplasmic fragment, FecR101-317, was randomly mutagenized. Restoration of transcription induction of the FecR mutants depended on the FecA mutants and the presence of ferric citrate. The FecA mutations were located close to each other at the beginning of the N terminus of FecA. FecA(G39R), with the mutation of a small neutral residue to a large basic residue, combined with FecR(L254E) and FecR(L269G) increased the transcription activity at least to 1.5-fold compared to that of wild-type FecA. The FecA(D43E) mutant is particularly noteworthy because the difference between wild-type and mutant FecA is just a CH2 group. Despite this small change, FecA(D43E) restored the transcription activity of the FecR point mutations, especially of FecR(F284L). The complementing mutants did not display strict allele specificity, which leads us to conclude that the mutations mainly affect the conformations of the interacting regions and do not reveal interacting amino acid side chains.
The two-hybrid system offered the possibility to screen for FecR mutants that no longer interact with FecA. Such mutants should not repress PsulA::lacZ transcription. Of the eight FecR mutants isolated, three were not identifiable on Western blots and were therefore unstable. One mutant, FecR(D138E, V197A), bound to FecA better than wild-type FecR; it repressed PsulA::lacZ transcription more strongly than wild-type FecR. This mutant transcribed PfecA::gfp constitutively, which implied that it did not require ferric citrate for induction. FecR(D138E, V197A) in the absence of ferric citrate already assumes a conformation that activates FecI. FecR(D138E, V197A) might reflect the conformation of FecR activated by FecA in the presence of ferric citrate. This could mean that in the ferric citrate-dependent wild-type FecR, ferric citrate induces binding of FecR to FecA.
The data presented in this paper further support the concept that FecR does not act as a simple anti-sigma factor. FecR is involved in signal transduction from the cell surface and is required for FecI activity. A model consistent with the data proposes interaction of FecR with FecA, as has been demonstrated previously with a bacterial two-hybrid system (7). Upon binding of ferric citrate, FecA undergoes major and minor conformational changes that involve mainly extracellular loops of the β-barrel and the central plug domain, as revealed by the FecA crystal structure (8). Connected to the plug domain is the TonB box, which is involved in the interaction with TonB (24), and the N-terminal extension, which interacts with FecR (7, 15). Unfortunately, the crystal structure does not disclose the conformation of the N-terminal extension located in the periplasm; the extension is flexible in the ferric citrate-loaded and unloaded structure. It does, however, show that upon binding of ferric citrate, the TonB box region becomes disordered. The structural change in FecA is conveyed to FecR, which presumably reacts with a structural change. The structural change in FecR is communicated into the cytoplasm, where interaction with FecI produces active FecI, which subsequently acts as a sigma factor. FecI is released from FecR and binds to the fec promoter. The dependence of FecI activity on FecR could be caused by instability of FecI in the absence of FecR or by FecI's assuming a different, active conformation when it dissociates from FecR. The high constitutive inducing activity of FecR1-85, which binds to FecI (27), might be caused by the stabilization of FecI and spontaneous dissociation of FecI from FecR in the absence of the signal.
Analysis of microbial genome sequences reveals at least 52 fecI and fecR homologs in more than 20 different genera with high abundance in certain bacteria, such as Pseudomonas, Caulobacter, and Nitrosomonas. FecIR of E. coli might become the paradigm for these transcription regulatory systems. As in E. coli, the genes homologous to fecR are preceded by genes homologous to fecI and are followed by fecA homologs. The promoters of the fecA homologs resemble extracytoplasmic sigma factor (ECF)-dependent promoters, and the encoded proteins contain an N-terminal extension that is not present in other TonB-dependent outer membrane transport proteins that have no role in transcription initiation. The pairwise identity between E. coli FecR and the FecR homologues ranges from 24 to 37%. They exhibit the highest sequence identity in the C-terminal region: residues 242 to 317 of FecR, in the leucine motif, with hydrophobic residues at all four conserved positions (Fig. 2). Motif search programs do not reveal the FecR leucine-like zipper motif, probably because of the α-helix-breaking proline at position 253 of FecR. As this study shows, the leucine motif is part of the FecR binding site for binding to FecA. Other FecR homologs that contain proline or arginine instead of proline might bind to FecA through the leucine motif. Another signature of FecR proteins is the tryptophan residues in the N-proximal end, which in E. coli are essential for FecR activity. These tryptophan residues are highly conserved among FecR-like proteins from a variety of gram-negative bacteria and are only occasionally replaced with other aromatic amino acids (27).
Acknowledgments
We thank Martina Ochs and In Sook Kim for the initial isolation of FecR mutants and Karen A. Brune for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (BR330/19-1,-3) and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Angerer, A., S. Enz, M. Ochs, and V. Braun. 1995. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. FecI belongs to a new subfamily of σ70-type factors that respond to extracytoplasmic stimuli. Mol. Microbiol. 18:163-174. [DOI] [PubMed] [Google Scholar]
- 2.Boss, A., A. Nussbaum-Shochat, and O. Amster-Choder. 1999. Characterization of the dimerization domain in BglG, an RNA-binding transcriptional antiterminator from Escherichia coli. J. Bacteriol. 181:1755-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, V. 1995. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol. Rev. 16:295-307. [DOI] [PubMed] [Google Scholar]
- 4.Braun, V. 1997. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch. Microbiol. 167:325-331. [DOI] [PubMed] [Google Scholar]
- 5.Dmitrova, M., G. Younes-Cauet, P. Oertel-Buchheit, D. Porte, M. Schnarr, and M. Granger-Schnarr. 1998. A new LexA-based genetic system for monitoring and analyzing protein heterodimerization in Escherichia coli. Mol. Gen. Genet. 257:205-212. [DOI] [PubMed] [Google Scholar]
- 6.Enz, S., V. Braun, and J. H. Crosa. 1995. Transcription of the region encoding the ferric dicitrate-transport system in Escherichia coli: similarity between promoters for fecA and for extracytoplasmic function sigma factors. Gene 163:13-18. [DOI] [PubMed] [Google Scholar]
- 7.Enz, S., S. Mahren, U. H. Stroeher, and V. Braun. 2000. Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J. Bacteriol. 182:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 9.Giacomini, A., B. Corich, F. J. Ollero, A. Squartini, and M. P. Nuti. 1992. Experimental conditions may affect reproducibility of the β-galactosidase assay. FEMS Microbiol. Lett. 100:87-90. [DOI] [PubMed] [Google Scholar]
- 10.Gross, C. A., C. Chan, A., Dombroski, T. Gruber, M. Sharp, J. Tupy, and B. Young. 1998. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harb. Symp. Quant. Biol. 63:141-155. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan, D. 1983. Studies in transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557.. [DOI] [PubMed] [Google Scholar]
- 12.Härle, C., K. Insook, A. Angerer, and V. Braun. 1995. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 14:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmann, J. D. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47-110. [DOI] [PubMed] [Google Scholar]
- 14.Hu, J. C., E. K. O'Shea, P. S. Kim, and R. T. Sauer. 1990. Sequence requirements for coiled-coils: analysis with λ repressor-GCN4 leucine zipper fusions. Science 250:1400-1403. [DOI] [PubMed] [Google Scholar]
- 15.Kim, I., A. Stiefel, S. Plantör, A. Angerer, and V. Braun. 1997. Transcription induction of the ferric citrate transport genes via the N terminus of the FecA outer membrane protein, the Ton system and the electrochemical potential of the cytoplasmic membrane. Mol. Microbiol. 23:333-344. [DOI] [PubMed] [Google Scholar]
- 16.Lonetto, M. A., K. L. Brown, K. E. Rudd, and M. J. Buttner. 1994. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase σ factors involved in the regulation of extracytoplasmic functions. Proc. Natl. Acad. Sci. USA 91:7573-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahren, S., S. Enz, and V. Braun. 2002. Functional interaction of region 4 of the extracytoplasmic function sigma factor FecI with the cytoplasmic portion of the FecR transmembrane protein of the Escherichia coli ferric citrate transport system. J. Bacteriol. 184:3704-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Bueno, M. A., R. Tobes, M. Rey, and J. L. Ramos. 2002. Detection of multiple extracytoplasmic function (ECF) sigma factors in the genome of Pseudomonas putida KT2440 and their counterparts in Pseudomonas aeruginosa PAO1. Environ. Microbiol. 4:842-855. [DOI] [PubMed] [Google Scholar]
- 19.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Missiakas, D., and S. Raina. 1998. The extracytoplasmic function sigma factors: role and regulation. Mol. Microbiol. 28:1059-1066. [DOI] [PubMed] [Google Scholar]
- 21.Nelson, K. E., C. Weinel, I. T. Paulsen, R. J. Dodson, H. Hilbert, V. A. Martins dos Santos, D. E. Fouts, S. R. Gill, M. Pop, M. Holmes, L. Brinkac, M. Beanan, R. T. DeBoy, S. Daugherty, J. Kolonay, R. Madupu, W. Nelson, O. White, J. Peterson, H. Khouri, I. Hance, P. Chris Lee, E. Holtzapple, D. Scanlan, K. Tran, A. Moazzez, T. Utterback, M. Rizzo, K. Lee, D. Kosack, D. Hoestl, H. Wedler, J. Lauber, D. Stjepandic, J. Hoheisel, M. Straetz, S. Heim, C. Kiewitz, J. Eisen, K. N. Timmis, A. Dusterhoft, B. Tummler, and C. M. Fraser. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4:799-808. [DOI] [PubMed] [Google Scholar]
- 22.Ochs, M., S. Veitinger, I. Kim, D. Welz, A. Angerer, and V. Braun. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: FecR is required for transcription activation by FecI. Mol. Microbiol. 15:119-132. [DOI] [PubMed] [Google Scholar]
- 23.Ochs, M., A. Angerer, S. Enz, and V. Braun. 1996. Surface signaling in transcriptional regulation of the ferric citrate transport system of Escherichia coli: mutational analysis of the alternative sigma factor FecI supports its essential role in fec transport gene transcription. Mol. Gen. Genet. 250:455-465. [DOI] [PubMed] [Google Scholar]
- 24.Ogierman, M., and V. Braun. 2003. In vivo cross-linking of the outer membrane ferric citrate transporter FecA and TonB: studies of the FecA-TonB box. J. Bacteriol. 185:1870-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stiefel, A., S. Mahren, M. Ochs, P. Schindler, S. Enz, and V. Braun. 2001. Control of the ferric citrate transport system of Escherichia coli: mutations in region 2.1 of the FecI extracytoplasmic-function sigma factor suppress mutations in the FecR transmembrane protein. J. Bacteriol. 183:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeshita, S., M. Sato, M. Toba, W. Masahashi, and T. Hashimoto-Gotoh. 1987. High-copy-number and low-copy-number plasmid vectors for lacZ α-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63-74. [DOI] [PubMed] [Google Scholar]
- 30.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 31.Visca, P., L. Leoni, M. J. Wilson, and I. L. Lamont. 2002. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 45:1177-1190. [DOI] [PubMed] [Google Scholar]
- 32.Welz, D., and V. Braun. 1998. Ferric citrate transport of Escherichia coli: functional regions of the FecR transmembrane regulatory protein. J. Bacteriol. 180:2387-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wösten, M. M. 1998. Eubacterial sigma factors. FEMS Microbiol. Rev. 22:127-150. [DOI] [PubMed] [Google Scholar]
- 34.Wriedt, K., A. Angerer, and V. Braun. 1995. Transcriptional regulation from the cell surface: conformational change in the transmembrane protein FecR lead to altered transcription of the ferric citrate transport genes in Escherichia coli. J. Bacteriol. 177:3320-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaku, H., and T. Mizuno. 1997. The membrane-located osmosensory kinase, EnvZ, that contains a leucine zipper-like motif functions as a dimer in Escherichia coli. FEBS Lett. 417:409-413. [DOI] [PubMed] [Google Scholar]