Abstract
Intestinal-derived endotoxins are importantly involved in alcohol-induced liver injury. Disruption of intestinal barrier function and endotoxemia are common features associated with liver inflammation and injury due to acute ethanol exposure. Zinc has been shown to inhibit acute alcohol-induced liver injury. This study was designed to determine the inhibitory effect of zinc on alcohol-induced endotoxemia and whether the inhibition is mediated by metallothionein (MT) or is independent of MT. MT knockout (MT-KO) mice were administered three oral doses of zinc sulfate (2.5 mg zinc ion/kg body weight) every 12 hours before being administered a single dose of ethanol (6 g/kg body weight) by gavage. Ethanol administration caused liver injury as determined by increased serum transaminases, parenchymal fat accumulation, necrotic foci, and an elevation of tumor necrosis factor (TNF-α). Increased plasma endotoxin levels were detected in ethanol-treated animals whose small intestinal structural integrity was compromised as determined by microscopic examination. Zinc supplementation significantly inhibited acute ethanol-induced liver injury and suppressed hepatic TNF-α production in association with decreased circulating endotoxin levels and a significant protection of small intestine structure. As expected, MT levels remained undetectable in the MT-KO mice under the zinc treatment. These results thus demonstrate that zinc preservation of intestinal structural integrity is associated with suppression of endotoxemia and liver injury induced by acute exposure to ethanol and the zinc protection is independent of MT.
Endotoxemia is an important cofactor in alcohol-induced liver injury.1 Transient increases in circulating endotoxins are associated with increased liver injury in experimental animals exposed to acute bolus doses of alcohol.2 Endotoxins are glycolipid components of the outer wall of gram-negative bacteria that colonize the small and large intestines. Circulating endotoxins are removed from the blood by Kupffer cells, the resident macrophage population of the liver.3,4 Kupffer cells become activated on exposure to endotoxins and subsequently produce proinflammatory mediators such as cytokines, prostaglandins, and reactive oxygen species.5,6 In rats, alcohol-induced liver injury was significantly inhibited when Kupffer cells were eliminated using gadolinium chloride before alcohol insult.7 Furthermore, sterilization of the intestines of rats with polymixin B and neomycin prevented ethanol-induced liver injury.8,9 These data suggest that inhibition of endotoxemia is an important approach to preventing alcohol-induced liver injury.
Under normal physiological conditions, the small intestine serves as an efficient barrier to large antigens such as endotoxins, however, acute ethanol exposure elicits an increase in intestinal permeability to bacterial products.10 Structural damage to intestinal villi (eg, loss of enterocytes and mucosal blebbing) may play a critical role in the increased permeability to endotoxins and indeed has been reported experimentally in rodent models of acute ethanol intoxication.11 Specifically, rats treated acutely with high doses of ethanol were found to have significantly elevated levels of plasma endotoxins compared to those animals treated with low-dose ethanol and the endotoxemia was positively correlated with increased intestinal injury.10 Thus, it appears that structural alterations of the small intestine, induced by high doses of ethanol, are associated with abnormalities in barrier function. Therefore, agents that protect the structural integrity of the small intestine may prevent alterations in barrier function to endotoxins under alcohol challenge.
Zinc supplementation has been illustrated to improve barrier dysfunction in many pathophysiological conditions of the small intestine such as chronic inflammatory bowel or Crohn’s disease (IBD) and ulcerative colitis.12,13 Typically, zinc salts (eg, zinc sulfate) are administered to patients with intestinal permeability disturbances as an adjunct therapy at doses ranging from 100 to 400 mg/day.12,14,15 However, the efficacy of zinc as therapy for alcohol exposure remains unclear due to the fact that alcoholics generally develop intestinal malabsorption syndromes.16 Alcoholics and patients with inflammatory bowel disease are commonly known to be zinc deficient, which may be attributed to chronic intestinal injury and altered trace element homeostasis.17,18 Sturniolo et al12 showed that individuals with chronic intestinal permeability disturbances have plasma zinc concentrations below 100 μg/dL before zinc therapy. After eight weeks of oral zinc sulfate (110 mg three times a day) the average plasma zinc level increased to 130 μg/dL. Importantly, the intestinal permeability was restored in greater than 80% of the patients treated with zinc.
The mechanisms of zinc protection are difficult to study due to the myriad organic ligands that bind this vital trace element (eg, metallothionein (MT)). In response to inflammatory stimuli such as endotoxins, “acute phase” proteins such as MT are induced, however, the effects of many of these endogenous agents following alcohol exposure remain unclear.19,20 A study conducted by Takano et al21 found that MT acted as a cytoprotectant against gastroduodenal mucosal injury caused by ethanol in mice. In that study, MT knockout (MT-KO) mice, in which the MT-I and MT-II genes were interrupted, were treated orally with ethanol and gastric and duodenal lesions were compared with wild-type mice. The number and severity of gastroduodenal lesions were significantly higher in MT-KO mice versus wild-type mice. The results suggested the involvement of intrinsic MT in cytoprotection against ethanol-induced gastroduodenal mucosal injury.
Our recent studies have shown that MT prevents acute alcohol-induced liver injury by inhibition of oxidative stress.22 Further studies have revealed that supplementation of zinc significantly inhibited hepatocyte cell death following ethanol exposure.23,24 Interestingly, we have observed that supplementation of MT-KO mice with zinc also inhibited alcohol-induced liver injury.23 Several studies have shown that MT releases zinc under stress conditions that involve changes in the intracellular redox potential.25,26 Thus, it is possible that zinc may mediate the protective action of MT and MT would function as an endogenous zinc reservoir. The lack of MT in the MT-KO mice would cause a shortage of endogenous storage of zinc and exogenous supplementation with zinc would overcome the shortage.
MT-KO mice suffer more gastrointestinal damage from acute bolus doses of alcohol than wild-type mice.21 It is interesting to know whether this enhanced toxic effect of alcohol in the small intestine is due to the lack of endogenous zinc that would be stored in MT in the wild-type mice and released under oxidative stress condition, as observed in the liver.23 The present study was thus undertaken using the MT-KO mouse model to address specifically whether zinc can provide protection from alcohol-induced intestinal injury in the absence of MT.
Materials and Methods
Animals
Homozygous MT-KO mice (20 to 25 g, 8 to 10 weeks of age) produced on the 129/Sv genetic background were obtained from Jackson Laboratories (Bar Harbor, Maine). MT-KO mice are phenotypically identical to their wild-type counterparts under normal control conditions. In addition, growth and development as well as reproductive characteristics are unaltered by the MT null mutation. The mice were housed in the animal quarters at the University of Louisville Research Resources Center. They were maintained at 22°C with a 12-hour light/dark cycle and had free access to rodent chow and water. The experimental procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association for Accreditation of Laboratory Animal Care.
Acute Ethanol Challenge
A binge drinking model developed by Carson and Pruett27 was followed for acute ethanol challenge. This model was designed to achieve blood alcohol levels that would produce physiological effects comparable to human binge drinking. Animals were divided into four treatment groups in a 2 × 2 factorial design (+/− zinc, +/− ethanol): 1) isocaloric maltose water control, 2) ethanol, 3) zinc plus isocaloric maltose water, and 4) zinc plus ethanol. The concentration of zinc used in this study was determined from preliminary examination of the induction of increased liver enzyme activity (eg, alanine aminotransferase, ALT) and/or alterations in parenchymal tissue architecture as a function of increasing levels of ZnSO4 (0 to 10 mg/kg). Mice in groups 3 and 4 were given ZnSO4 (Sigma, St. Louis, MO) intragastrically at a dose of 2.5 mg zinc ion/kg body weight, in 12-hour intervals for a total of three doses. In groups 1 and 2, mice were given sterile saline intragastrically following the same schedule as a vehicle control. The animals were fasted for 16 hours and subsequently received the third and final dose of zinc in groups 3 and 4. One hour after the last pretreatment, mice in groups 2 and 4 were administered ethanol (30% w/v) (Sigma-Aldrich, Milwaukee, WI) in a single dose of 6 g/kg body weight by gavage and mice in groups 1 and 3 received isocaloric maltose water by the same route on the same schedule. The time of necropsy for determination of plasma endotoxin levels and analysis of duodenum histopathology was 1.5 hours following ethanol or maltose water administration. For examination of liver TNF-α, liver histopathology, and serum enzymes, the time of necropsy was 6 hours following ethanol or maltose water. Mice were anesthetized with sodium pentobarbital (0.05 mg/g body weight; Abbott Laboratories, North Chicago, IL). Blood was drawn from the dorsal vena cava, livers were perfused and harvested, and 1.0- to 3.0-cm sections of the duodenum were obtained for analysis. Stored tissues were first flash-frozen in liquid nitrogen and then placed in −80°C until analysis.
Histopathological Examination
Liver and intestinal histological slides were prepared as described previously23 and hematoxylin and eosin staining of liver and intestinal sections were observed by light microscopy.
Plasma Endotoxins
Blood samples were drawn from the dorsal vena cava via sterile heparinized syringes. Platelet-rich plasma was obtained by centrifuging the whole blood at 300 × g for 15 minutes at 4°C. Plasma samples were diluted 1:10 with sterile nanopure water, mixed by vortex and placed in a 75°C water bath for 10 minutes. Samples were allowed to cool to room temperature for 10 minutes before colorimetric assay using the limulus ameobocyte lystate (LAL) kit (Biowhittaker, Walkerville, MD). Standards and samples were incubated with LAL for 10 minutes at 37°C followed by incubation with colorimetric substrate for 6 minutes. The reaction was stopped with 25% acetic acid and the absorbance was read in a microplate reader (BIO-TEK Instrument Inc., Winooski, VT) at 405 nm.
Liver TNF-α
Liver samples were minced thoroughly in ice-cold RIPA buffer (150 mmol/L NaCl, 5 mmol/L EDTA, 50 mmol/L Tris base, 0.3% Triton X-100, 0.03% sodium dodecyl sulfate, 0.3% Na-deoxycholate, and 1% protease inhibitor cocktail (pH 7.4)) followed by incubation on ice for 30 minutes. The homogenates were then centrifuged at 15,000 × g for 20 minutes at 4°C. The supernatants were removed to clean tubes and centrifuged again at 15,000 × g for 20 minutes at 4°C. The supernatants of this spin were then used for ELISA assay (Kit No. KMC3012; Biosource, Camarillo, CA).
Alanine Aminotransferase Assay
Serum alanine aminotransferase (ALT, EC 2.6.1.2.) activity was colorimetrically measured using a Diagnostic Kit (Procedure No. 505; Sigma, St. Louis, MO) according to the manufacturer’s instructions.
Blood Alcohol
Blood alcohol levels (BAL) were determined by an alcohol dehydrogenase (ADH) assay (Procedure No. 332-UV, Sigma).
Intestinal MT
Tissue MT levels were determined by a cadmium-hemoglobin affinity assay. Briefly, intestinal tissues were homogenized in four volumes of 10 mmol/L Tris-HCl buffer (pH 7.4) at 4°C. After centrifugation of the homogenate at 10,000 × g for 15 minutes, 200 μl of supernatant was transferred to microtubes for MT analysis as described previously.23
Intestinal, Hepatic, and Plasma Zinc Levels
Zinc concentrations were determined by inductively coupled argon plasma emission spectroscopy (Jarrel-Ash, Model 1140, Waltham, MA) at the Grand Forks Human Nutrition Research Center (GFHNRC), Grand Forks, North Dakota. Briefly, intestinal and hepatic tissues were lyophilized and subsequently digested with nitric acid and hydrogen peroxide. Zinc concentrations were expressed as μg/g dry tissue for intestine and liver and μg/dL for plasma.
Statistical Analysis
Data were expressed as mean ± SD (n = 5 to 8) and analyzed according to a 2 × 2 (zinc versus ethanol) factorial experimental design. After a significant interaction was detected by the two-way analysis of variance, the significance of the main effects was further determined. The level of significance was considered at P < 0.05.
Results
The relationship between MT and zinc in protection from acute ethanol-induced small intestinal injury was initially characterized by measuring MT concentrations in the duodenum following zinc and/or ethanol treatment. MT-KO mice were administered three doses of zinc sulfate at 2.5 mg/kg by gavage in 12-hour intervals before a single oral dose of ethanol (6 mg/kg). One and one-half hours after ethanol administration, the animals were sacrificed. Snap-frozen sections of duodenal and ileal tissue were subsequently processed for measurement of MT concentration. As shown in Table 1, neither zinc nor ethanol caused significant changes in MT in the duodenum or ileum of MT-KO mice, in which MT concentrations were near the detection limit of the assay. In contrast, both zinc and ethanol caused significant increases in MT levels in the intestine of wild-type mice (Table 1). To determine whether the absence of MT affected zinc status in the small intestine, total zinc levels of the duodenum and ileum were examined. In general, compared to wild-type mice, MT-KO mice had lower levels of zinc in all tissues examined including plasma, however, these differences were significant only in the duodenum and liver (Table 2). MT-KO mice exhibited an interesting biodistribution pattern of zinc where levels in the plasma of animals treated with zinc or ethanol alone were comparable to controls. Paradoxically, in mice exposed to zinc plus ethanol, a significant increase in plasma zinc was observed (Table 2). In addition, acute ethanol exposure appeared to decrease zinc concentrations in the ileum and zinc treatment alone caused an increase. However, the varying effects of the different treatments on zinc levels in the intestine did not appear to alter the amount of zinc deposited into the liver, where it was found that zinc, ethanol, and zinc plus ethanol produced approximately equal increases in zinc content compared to control (Table 2).
Table 1.
Metallothionein Levels in Proximal and Distal Small Intestine of Mice
| MT (μg/g tissue) | Con | EtOH | Zn | Zn+EtOH |
|---|---|---|---|---|
| KO | ||||
| Duodenum | 1.92 ± 0.14 | 1.70 ± 0.29 | 2.21 ± 0.79 | 1.86 ± 0.50 |
| Ileum | 1.07 ± 0.37 | 1.66 ± 0.66 | 0.84 ± 0.15 | 0.71 ± 0.42 |
| WT | ||||
| Duodenum | 7.13 ± 2.71* | 26.42 ± 15.02*† | 22.44 ± 8.54*† | 5.45 ± 2.51* |
| Ileum | 1.85 ± 0.21* | 18.55 ± 4.87*† | 4.24 ± 4.10 | 2.92 ± 0.85* |
Data are means ± S.D. (n = 4).
, Significantly different from corresponding KO (p < 0.05).
, Significantly different from control (p < 0.05).
Table 2.
Zinc Levels in Metallothionein-Knockout (KO) and Wild-Type (WT) Mice
| Zn (μg/g tissue) | Con | EtOH | Zn | Zn+EtOH |
|---|---|---|---|---|
| KO | ||||
| Duodenum | 110.34 ± 6.43 | 110.67 ± 9.91 | 109.31 ± 4.64 | 116.41 ± 2.01 |
| Ileum | 95.53 ± 7.35 | 87.93 ± 14.87 | 115.36 ± 25.75 | 89.06 ± 13.85 |
| Liver | 82.94 ± 4.64 | 92.15 ± 4.68 | 91.43 ± 3.12 | 90.18 ± 4.10 |
| Plasma (μg/dL) | 97.40 ± 13.01 | 129.0 ± 26.23 | 113.37 ± 16.01 | 136.03 ± 14.68* |
| WT | ||||
| Duodenum | 128.12 ± 9.49† | 131.06 ± 6.86† | 141.91 ± 14.22† | 137.48 ± 14.56† |
| Ileum | 105.66 ± 12.52 | 82.85 ± 13.36 | 116.47 ± 45.36 | 110.01 ± 53.45 |
| Liver | 112.74 ± 1.24† | 111.75 ± 10.31† | 103.51 ± 8.48† | 113.18 ± 8.57† |
| Plasma (μg/dL) | 125.87 ± 62.59 | 145.10 ± 35.46 | 130.08 ± 37.99 | 141.70 ± 51.52 |
Data are means ± S.D. (n = 4).
, Significantly different from control (p < 0.05).
, Significantly different from corresponding KO (p < 0.05).
MT-KO mice were then used to evaluate the MT-independent zinc effects on acute ethanol-induced intestinal structural damage. The major route of ethanol metabolism is through the zinc-dependent enzyme ADH. Several isoforms of ADH exists in the liver and stomach. Therefore, to eliminate potential effects of zinc on ethanol absorption from the gastrointestinal system as a mechanism of protection, the BAL of mice 1.5 hours after acute exposure to ethanol were measured. The results showed that there were no significant differences in BAL between ethanol-treated mice (0.52 ± 0.22%, w/v) and zinc plus ethanol-treated mice (0.47 ± 0.03%, w/v).
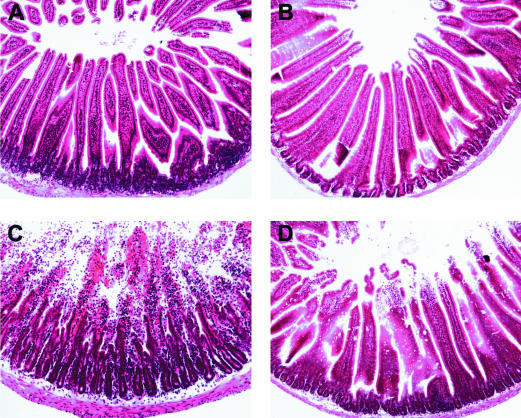
Next, the effect of zinc on alcohol-induced lesions in small intestine was examined. Hemorrhagic erosions of the duodenum were observed in acute ethanol-treated mice (data not shown). These macroscopic lesions were observed over the entire length of the duodenum, in varying degrees of severity, however, the most significant damage was consistently observed in those areas adjacent to the pylorus. Zinc pretreatment prevented this injury as evidenced by an absence of gross hemorrhage in the duodenum. Closer examination of duodenal tissue from acute ethanol-treated mice revealed significant damage that included hemorrhage, desquamation of epithelial cells, and denudation and massive degeneration of villi (Figure 1). The duodenum of mice pretreated with zinc suffered similar cellular lesions, however, the severity was much less in comparison with those mice treated with alcohol only.
Figure 1.
Histopathological changes in the duodenum of MT-KO mice 1.5 hours after gastric administration of ethanol. A, isocaloric maltose water; B, zinc; C, ethanol; D, zinc and ethanol. Ethanol treatment caused massive degeneration of duodenal villi and hemorrhage. Zinc pretreatment inhibited these morphological anomalies. Hematoxylin and eosin staining; magnification, × 260.
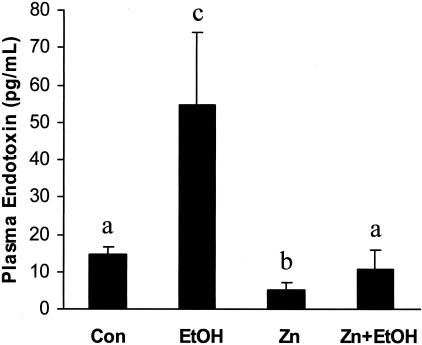
The consequences of the ethanol-induced lesions in small intestine and the effects of zinc were determined by examining circulating endotoxin levels. As illustrated in Figure 2, acute ethanol exposure elicited a fourfold increase in plasma endotoxin (54.7 ± 19.6 pg/ml) as compared to control animals (14.4 ± 2.2 pg/ml). Zinc treatment alone appeared to be beneficial as shown by a significant decrease in basal concentrations of circulating endotoxins (5.1 ± 2.2 pg/ml) compared to controls. More importantly, zinc pretreatment significantly inhibited acute ethanol-induced elevation in plasma endotoxin (10.8 ± 5.0 pg/ml).
Figure 2.
Plasma endotoxin levels in MT-KO mice 1.5 hours after gastric administration of ethanol. Con, control; EtOH, ethanol; Zn, zinc; Zn+EtOH, zinc and ethanol. Data are mean ± SD values (n = 5 to 8). Groups that do not share the same letter are significantly different from each other (P < 0.05).
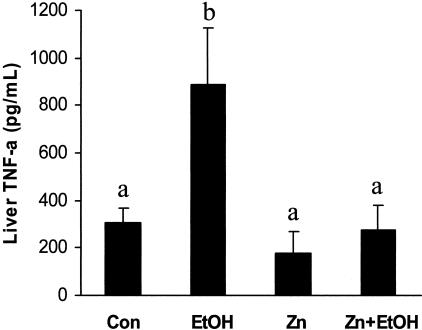
Since increased portal delivery of circulating endotoxin leads to activation of Kupffer cells and subsequent production of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) in the liver, we examined the effect of acute ethanol exposure on hepatic TNF-α levels. As shown in Figure 3, acute ethanol exposure caused an approximate threefold increase in the liver TNF-α (886.8 ± 240.3 pg/ml) as compared to control animals (305.2 ± 62.1 pg/ml). Zinc pretreatment completely abolished this ethanol-induced elevation of liver TNF-α levels (276.2 ± 103.6 pg/ml).
Figure 3.
Intrahepatic tumor necrosis factor (TNF-a) levels in mice 6 hours after gastric administration of ethanol. Con, control; EtOH, ethanol; Zn, zinc; Zn+EtOH, zinc and ethanol. Data are mean ± SD values (n = 5 to 8). Groups that do not share the same letter are significantly different from each other (P < 0.05).
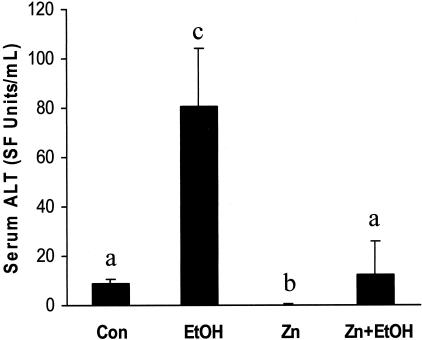
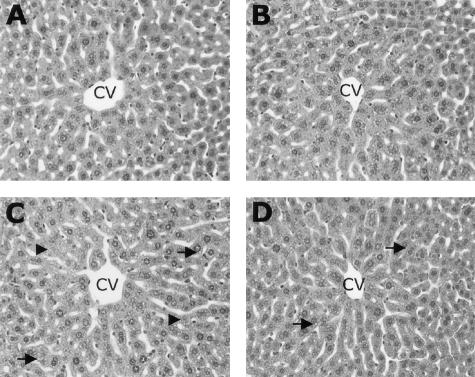
Next, to determine whether zinc inhibition of endotoxemia and the subsequent suppression of TNF-α cytokine production correlate with reduced liver injury, serum levels of alanine transaminase (ALT) were examined. Acute ethanol exposure elicited a 10-fold increase in serum (ALT) levels compared to control, and zinc pretreatment prevented this alcohol-induced effect (Figure 4). To further illustrate the protective effect of zinc on alcohol-induced liver injury, hepatic histopathological changes were examined, as shown in Figure 5. The primary histopathological change observed in mouse liver following acute ethanol exposure was an accumulation of small fat droplets (microvesicular steatosis) in hepatocytes. In addition, mild necrosis characterized by swollen hepatocytes with absence of or faded nuclei, particularly in the perivenous regions of the liver, was observed. Zinc pretreatment decreased the number and volume of fat vesicles in the hepatocytes and diminished liver necrosis induced by acute alcohol exposure.
Figure 4.
Serum ALT levels in MT-KO mice 6 hours after gastric administration of ethanol. Con, control; EtOH, ethanol; Zn, zinc; Zn+EtOH, zinc and ethanol. Data are mean ± SD values (n = 5 to 8). Groups that do not share the same letter are significantly different from each other (P < 0.05).
Figure 5.
Histopathological changes in the livers of mice 6 hours after gastric administration of ethanol. A, isocaloric maltose water; B, zinc; C, ethanol; D, zinc and ethanol. The major histopathological change induced by ethanol in mouse liver was microvesicular steatosis (arrows). In addition, ethanol administration caused hepatocyte enlargement with nuclear dissolution indicative of necrosis (arrowheads). Zinc pretreatment suppressed these ethanol-induced alterations. CV, central vein. Hematoxylin and eosin staining; magnification, × 260.
Discussion
Alterations in small intestinal barrier function are suggested to play a critical role in alcohol-induced liver injury.1,9,28,29 In the virtual absence of endotoxin translocation across the intestinal epithelium (ie, gut sterilization), alcohol at concentrations of up to 17 g/kg/day has been shown to cause insignificant liver damage in rat models.30 The data obtained from this study using a MT-KO mouse model revealed that treatment of these mice with zinc significantly inhibited alcohol-induced endotoxemia and liver injury. Pretreatment with zinc preserved the small intestinal structural integrity, corresponding to a complete abolishment of endotoxemia and inhibition of liver damage. Collectively, these results demonstrated that zinc inhibition of acute alcohol-induced liver inflammation and injury is associated with preservation of small intestinal barrier function and this inhibitory effect of zinc is independent of MT.
It is well known that zinc is a potent inducer of MT production in multiple organ systems.31,32 Thus, it has long been proposed that zinc inhibition of tissue damage is mediated by increased MT synthesis. Zinc supplementation indeed caused an elevation of MT concentrations in the duodenum of wild-type mice (Table 1). However, our results obtained from the MT-KO mouse model under the identical treatment conditions brings into question the involvement of MT in zinc cytoprotection. In our previous studies, we have also shown that zinc supplementation significantly inhibited ethanol-induced oxidative stress and early alcohol liver injury in MT-KO mice.23 Therefore, this present study together with our previous observations demonstrate the MT-independent protective effect of zinc. However, the role of MT in cytoprotection cannot be minimized. Several studies have shown that MT releases zinc when the intercellular environment becomes more oxidized.26,33 In our previous studies, we have also observed that MT-KO mice suffer more liver damage from acute ethanol exposure than wild-type mice, presumably due to the difference in the availability of endogenously stored zinc in MT.23 This present study indeed illustrates that MT-KO mice have lower levels of endogenous zinc in the small intestine compared to wild-type mice (Table 2), but supplementation with zinc before alcohol exposure led to an equal protection in both wild-type and MT-KO mice. It is possible that MT serves as an important source of labile zinc and without this endogenous reserve, the organ systems would experience more damage unless exogenously supplemented zinc is available, as demonstrated in this study.
An intact intestinal mucosa is critical for barrier function. In this study, we found that zinc treatment inhibited damage in the proximal small intestine in the absence of MT production (Figure 1). In mice acutely exposed to ethanol, we observed significant hemorrhagic lesions and massive damage to the villi in the proximal duodenum, indicative of severe mucosal injury (Figure 1). It has been proposed that intestinal hemorrhage is associated with a significant localized inflammatory response in the mucosa and serosa, which may be attributed to intestinal endotoxemia.34,35 In general, there are two pathways of endotoxin permeability, paracellular and transcellular.36 Previous data suggests that exposure to acute doses of ethanol decreased barrier function against small and large antigens, with the latter implicating an increase in transcellular permeability via epithelial layer disruption.37,38 Some studies have even reported the translocation of intact bacteria through the small bowel following acute ethanol exposure.39 In this study, we illustrated a significant protection of the structural integrity of the duodenum in MT-KO mice treated with zinc before acute ethanol exposure (Figure 1) and this protection was positively correlated with abrogation of endotoxemia (Figure 2).
Bacterial lipopolysaccharides (LPS) flowing into the liver from portal blood are effectively removed from the circulation by Kupffer cells.3,4 LPS-binding protein (LBP) forms complexes with endotoxins, and these complexes then serve as ligands for CD14 receptors on Kupffer cells.40,41 Stimulated Kupffer cells subsequently produce significant levels of prooxidants, via inducible nitric oxide synthase (iNOS), and proinflammatory cytokines such as TNF-α through activation of nuclear factor kappa-B (NF-κB).3,5,6 Eliminating Kupffer cells with gadolinium chloride, knocking out p47PHOX or iNOS, or adenoviral delivery of IκB superrepressor gene have all been shown to significantly inhibit alcohol-induced liver injury in experimental animals.7,42–44 Therefore, inhibiting liver inflammation upstream of Kupffer cell activation (ie, suppressing endotoxemia) is an important aspect of therapeutic intervention. Our results showed that zinc pretreatment decreased acute ethanol-induced liver TNF-α production to levels comparable to control animals (Figure 3). This observation suggests that zinc inhibition of intestinal injury and subsequent endotoxemia is significantly associated with the virtual quiescence of Kupffer cells following acute ethanol exposure in mice. Furthermore, these zinc effects correlate with suppressed pathological changes in the liver parenchyma. Zinc treatment not only inhibits hepatocyte necrotic cell death (Figures 4 and 5), but also diminishes ethanol-induced fat accumulation. The mechanisms underlying these zinc effects in the liver remain unclear and will be the focus of future investigation.
Although our results indicate zinc is protective independent of MT, the possibility that other zinc-binding proteins mediate the protective effect(s) of zinc is not excluded. Several reports have provided evidence of zinc involvement in structure/function of biological molecules that are intimately involved in inhibition of liver injury (eg, A20, ZAS3, Cu/Zn SOD).45–47 In addition, there are a significant number of regulatory proteins whose dynamic changes and their relation to Zn and MT status, particularly during alcohol intoxication, are unclear.48 For example, interleukin-6 (IL-6) induces uptake of zinc by hepatocytes and is critically involved in inhibition of liver inflammation and injury via STAT3 induction, which is positively regulated by zinc binding proteins.49–51
A potential drawback of using zinc as therapy for alcohol-induced damage is the superphysiological concentrations of the salt required for protection. While zinc concentrations administered to humans and experimental animals for treatment of intestinal permeability disturbances (eg, IBD) far exceeds the FDA recommended daily allowance (9 to 11 mg elemental zinc/day), it has previously been shown that ZnSO4 is not toxic in humans at levels up to 650 mg/day (≈ 10 mg/kg/day).52 Furthermore, in rats, the lethal dose (LD50) of ZnSO4 has been established to be 1370 mg/kg.53 Therefore, the oral dose of 2.5 mg/kg ZnSO4 used in this mouse model is well below what might be considered toxic in humans and rodents.
In summary, the data obtained from this study clearly demonstrate that zinc is a potent protective agent in the small intestine and that this protection translates into significant inhibition of acute ethanol-induced liver injury. Furthermore, preservation of small intestinal structural integrity is independent of MT. Future studies will examine the mechanisms of action of zinc in the cytoprotection against alcohol toxicity.
Acknowledgments
We thank Lisa Zhang and Raymond Ortines for technical assistance.
Footnotes
Address reprint requests to Dr. Y. James Kang, Department of Medicine, University of Louisville School of Medicine, 511 S. Floyd St., MDR 530, Louisville, KY 40202. E-mail: yjkang01@louisville.edu.
Supported in part by NIH grants AA13601 (to Z.Z.), HL59225 (to Y.J.K.) and HL63760 (to Y.J.K.), the University of Louisville School of Medicine, and The Veterans Administration, Louisville, Kentucky.
Y.J.K. is a Distinguished University Scholar of the University of Louisville.
References
- Mathurin P, Deng Q-G, Keshavarzian A, Choudray S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Ikejima K, Bradford B, Rivera C, Kono H, Brenner DA, Thurman RG. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115:443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- Saba TM. Physiology and pathophysiology of the reticuloendothelial system. Arch Intern Med. 1970;126:1031–1052. [PubMed] [Google Scholar]
- Nolan JP. Endotoxin, reticuloendothelial function, and liver injury. Hepatology. 1981;1:458–465. doi: 10.1002/hep.1840010516. [DOI] [PubMed] [Google Scholar]
- Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells). Eur J Biochem. 1990;192:245–261. doi: 10.1111/j.1432-1033.1990.tb19222.x. [DOI] [PubMed] [Google Scholar]
- Arthur MJP, Kowalski-Saunders P, Wright R. Effect of endotoxin on release of reactive oxygen intermediates by rat hepatic macrophages. Gastroenterology. 1988;95:1588–1594. doi: 10.1016/s0016-5085(88)80082-9. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Rivera CA, Bradford BU, Seabra V, Thurman RG. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am J Physiol. 1998;275:G1252–G1258. doi: 10.1152/ajpgi.1998.275.6.G1252. [DOI] [PubMed] [Google Scholar]
- Tamai H, Kato S, Horie Y, Ohki E, Yokoyama H, Ishii H. Effect of acute ethanol administration on the intestinal absorption of endotoxin in rats. Alcohol Clin Exp Res. 2000;24:390–394. [PubMed] [Google Scholar]
- Baraona E, Pirola RC, Lieber CS. Small intestinal damage and changes in cell population produced by ethanol ingestion in the rat. Gastroenterology. 1974;66:226–234. [PubMed] [Google Scholar]
- Sturniolo GC, Di Leo V, Ferronato A, D’Odorico A, D’Inca R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm Bowel Dis. 2001;7:94–98. doi: 10.1097/00054725-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Sturniolo GC, Fries W, Mazzon E, Di Leo V, Barollo M, D’Inca R. Effect of zinc supplementation on intestinal permeability in experimental colitis. J Lab Clin Med. 2002;139:311–315. doi: 10.1067/mlc.2002.123624. [DOI] [PubMed] [Google Scholar]
- Kelly R, Davidson GP, Townley RR, Campbell PE. Reversible intestinal mucosal abnormality in acrodermatitis enteropathica. Arch Dis Child. 1976;51:219–222. doi: 10.1136/adc.51.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder TP, van der Sluys Veer A, Verspaget HW, Griffioen G, Pena AS, Janssens AR, Lamers CB. Effect of oral zinc supplementation on metallothionein and superoxide dismutase concentrations in patients with inflammatory bowel disease. J Gastroenterol Hepatol. 1994;9:472–477. doi: 10.1111/j.1440-1746.1994.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Dinsmore W, Callender ME, McMaster D, Todd SJ, Love AH. Zinc absorption in alcoholics using zinc-65. Digestion. 1985;32:238–242. doi: 10.1159/000199243. [DOI] [PubMed] [Google Scholar]
- Hendricks KM, Walker WA. Zinc deficiency in inflammatory bowel disease. Nutr Rev. 1988;46:401–408. doi: 10.1111/j.1753-4887.1988.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Akar N, Arcasoy A. Alcohol and zinc deficiency: contribution to villous atrophy. J Intern Med. 1990;228:412–413. [PubMed] [Google Scholar]
- Rofe AM, Philcox JC, Coyle P. Trace metal, acute phase and metabolic response to endotoxin in metallothionein-null mice. Biochem J. 1996;314:793–7. doi: 10.1042/bj3140793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingalewski C, Theodorakis NG, Yang J, Beck SC, De Maio A. Distinct expression of heat shock and acute phase genes during regional hepatic-ischemia reperfusion. Am J Physiol. 1996;271:R634–R640. doi: 10.1152/ajpregu.1996.271.3.R634. [DOI] [PubMed] [Google Scholar]
- Takano H, Satoh M, Shimada A, Sagai M, Yoshikawa T, Tohyama C. Cytoprotection by metallothionein against gastroduodenal mucosal injury caused by ethanol in mice. Lab Invest. 2000;80:371–377. doi: 10.1038/labinvest.3780041. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, James Kang Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp Biol Med. 2002;227:214–222. doi: 10.1177/153537020222700310. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Sun X, Lambert JC, Saari JT, Kang YJ. Metallothionein-independent zinc protection from alcoholic liver injury. Am J Pathol. 2002;160:2267–2274. doi: 10.1016/S0002-9440(10)61174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Zhou Z, Kang YJ. Suppression of Fas-mediated signaling pathway is involved in zinc inhibition of ethanol-induced liver apoptosis. Exp Biol Med. 2003;228:406–412. doi: 10.1177/153537020322800411. [DOI] [PubMed] [Google Scholar]
- Jiang L-J, Maret W, Vallee BL. The glutathione redox couple modulates zinc transfer from metallothionein to zinc-depleted sorbitol dehydrogenase. Proc Natl Acad Sci USA. 1998;95:3483–3488. doi: 10.1073/pnas.95.7.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. Oxidative metal release from metallothionein via zinc-thiol/disulfide interchange. Proc Natl Acad Sci USA. 1994;91:237–241. doi: 10.1073/pnas.91.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson EJ, Pruett SB. Development and characterizaton of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcohol Clin Exp Res. 1996;20:132–138. doi: 10.1111/j.1530-0277.1996.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Bhagwandeen BS, Apte M, Manwarring L, Dickeson J. Endotoxin induced hepatic necrosis in rats on an alcohol diet. J Pathol. 1987;151:47–53. doi: 10.1002/path.1711520107. [DOI] [PubMed] [Google Scholar]
- Arai M, Nakano S, Okuno F, Hirano Y, Sujita K, Kobayashi T, Ishii H, Tsuchiya M. Endotoxin-induced hypercoagulability: a possible aggravating factor of alcoholic liver disease. Hepatology. 1989;9:846–851. doi: 10.1002/hep.1840090609. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Khettry U, Hossein Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver disease. Proc Soc Exp Biol Med. 1993;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- Coyle P, Philcox JC, Carey LC, Rofe AM. Metallothionein: the multipurpose protein. Cell Mol Life Sci. 2002;59:627–647. doi: 10.1007/s00018-002-8454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Cousins RJ. Metallothionein expression in animals: a physiological perspective on function. J Nutr. 2000;130:1085–1088. doi: 10.1093/jn/130.5.1085. [DOI] [PubMed] [Google Scholar]
- Giles GI, Tasker TM, Jacob C. Oxidation of biological thiols by highly reactive disulfide-S-oxides. Gen Physiol Biophys. 2002;21:65–72. [PubMed] [Google Scholar]
- Shibayama Y. An experimental study on the pathogenesis of acute haemorrhagic enteropathy: significance of congestion and endotoxaemia. J Pathol. 1986;148:169–174. doi: 10.1002/path.1711480207. [DOI] [PubMed] [Google Scholar]
- Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961–965. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason AB, Jarocka-Cyrta E, Faria J, Wild GE. Small bowel review: part II. Can J Gastroenterol. 1997;11:159–165. doi: 10.1155/1997/536830. [DOI] [PubMed] [Google Scholar]
- Lavo B, Colombel JF, Knutsson L, Hallgren R. Acute exposure of small intestine to ethanol induces mucosal leakage and prostaglandin E2 synthesis. Gastroenterology. 1992;102:468–473. doi: 10.1016/0016-5085(92)90092-d. [DOI] [PubMed] [Google Scholar]
- Beck IT, Dinda PK. Acute exposure of small intestine to ethanol: effects on morphology and function. Dig Dis Sci. 1981;26:817–38. doi: 10.1007/BF01309614. [DOI] [PubMed] [Google Scholar]
- Tabata T, Tani T, Endo Y, Hanasawa K. Bacterial translocation and peptidoglycan translocation by acute ethanol administration. J Gastroenterol. 2002;37:726–731. doi: 10.1007/s005350200118. [DOI] [PubMed] [Google Scholar]
- Su GL, Rahemtulla A, Thomas P, Klein RD, Wang SC, Nanji AA. CD14 and lipopolysaccharide binding protein expression in a rat model of alcoholic liver disease. Am J Pathol. 1998;152:841–849. [PMC free article] [PubMed] [Google Scholar]
- Lukkari TA, Jarvelainen HA, Oinonen T, Kettunen E, Lindros KO. Short-term ethanol exposure increases the expression of Kupffer cell CD14 receptor and lipopolysaccharide binding protein in rat liver. Alcohol Alcohol. 1999;34:311–319. doi: 10.1093/alcalc/34.3.311. [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteel GE, Gabele E, Wheeler MD, Uesugi T, Kadiiska M, Connor HD, Mason RP, Thurman RG. Inducible nitric oxide synthase is necessary for alcohol-induced liver injury: studies with knock-out mice. Hepatology. 2001;36:339A. [Google Scholar]
- Uesugi T, Froh M, Arteel GE, Bradford BU, Gabele E, Wheeler MD, Thurman RG. Delivery of IκB superrepressor gene with adenovirus reduces early alcohol-induced liver injury in rats. Hepatology. 2001;34:1149–1157. doi: 10.1053/jhep.2001.29400. [DOI] [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JW, Allen CE, Wu LC. Inhibition of NF-κB by ZAS3, a zinc-finger protein that also binds to the κB motif. Proc Natl Acad Sci USA. 2003;100:12301–12306. doi: 10.1073/pnas.2133048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A, Mahmood A, Pathak R, Dhawan D. Effect of zinc on hepatic lipid peroxidation and antioxidative enzymes in ethanol-fed rats. J Appl Toxicol. 2002;22:207–210. doi: 10.1002/jat.851. [DOI] [PubMed] [Google Scholar]
- Sato M, Sasaki M, Hojo H. Differential induction of metallothionein synthesis by interleukin-6 and tumor necrosis factor-α in rat tissues. Int J Immunopharmacol. 1994;16:187–195. doi: 10.1016/0192-0561(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Schroeder JJ, Cousins RJ. Interleukin 6 regulates metallothionein gene expression and zinc metabolism in hepatocyte monolayer cultures. Proc Natl Acad Sci USA. 1990;87:3137–3141. doi: 10.1073/pnas.87.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streetz KL, Wustefeld T, Klein C, Kallen KJ, Tronche F, Betz UA, Schutz G, Manns MP, Muller W, Trautwein C. Lack of gp130 expression in hepatocytes promotes liver injury. Gastroenterology. 2003;125:532–543. doi: 10.1016/s0016-5085(03)00901-6. [DOI] [PubMed] [Google Scholar]
- Rodel B, Tayassoli K, Karsunky H, Schmidt T, Bachmann M, Schaper F, Heinrich P, Shuai K, Elsasser HP, Moroy T. The zinc finger protein Gfi-1 can enhance STAT3 signaling by interacting with the STAT3 inhibitor PIAS3. EMBO J. 2000;19:5845–5855. doi: 10.1093/emboj/19.21.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Select Committee on GRAS Substances Bethesda, MD: Life Sciences Research Office, Federation of American Societies for Experimental Biology; Evaluation of the Health Aspects of Certain Zinc Salts as Food Ingredients (SCOGS-21) 1973 [Google Scholar]
- RTECS: Database compiled by the National Institute of Occupational Safety and Health, U.S. Department of Health and Human Services, 1997 [Google Scholar]