Abstract
Neuropilin-1 (NRP-1), a recently identified co-receptor for vascular endothelial growth factor, is expressed by several nongastrointestinal tumor types and enhances prostate cancer angiogenesis and growth in preclinical models. We investigated the expression and regulation of NRP-1 and the effect of NRP-1 overexpression on angiogenesis and growth of human colon adenocarcinoma by immunohistochemistry and in situ hybridization. NRP-1 was expressed in 20 of 20 human colon adenocarcinoma specimens but not in the adjacent nonmalignant colonic mucosa. By reverse transcriptase-polymerase chain reaction analysis, NRP-1 mRNA was expressed in seven of seven colon adenocarcinoma cell lines. Subcutaneous xenografts of stably transfected KM12SM/LM2 human colon cancer cells overexpressing NRP-1 led to increased tumor growth and angiogenesis in nude mice. In in vitro assays, conditioned medium from NRP-1-transfected cell lines led to an increase in endothelial cell migration, but did not affect endothelial cell growth. Epidermal growth factor (EGF) led to induction of NRP-1 in human colon adenocarcinoma cells and selective blockade of the epidermal growth factor receptor (EGFR) decreased constitutive and EGF-induced NRP-1 expression. Blockade of the Erk 1/2 and P38 mitogen-activated protein kinase signaling pathways also led to a decrease in constitutive and EGF-induced NRP-1 expression. These findings demonstrate the ubiquitous expression of NRP-1 in human colon cancer and suggest that NRP-1 may contribute to colon cancer angiogenesis and growth. This study also suggests that EGF and mitogen-activated protein kinase signaling pathways play an important role in NRP-1 regulation in colon cancer cells.
The growth of cancers and the development of metastasis is angiogenesis-dependent. Of the many proangiogenic factors identified, vascular endothelial growth factor (VEGF; also known as vascular permeability factor) is the best characterized. VEGF has been associated with increased angiogenesis and advanced-stage disease in a variety of solid tumor types including colon cancer.1,2 The VEGF family of proteins are highly structurally related proteins including VEGF-A (commonly designated as VEGF), VEGF-B, VEGF-C, VEGF-D, and VEGF-E, and placenta growth factor.3–5 The most prominent and characterized member, VEGF-A, exists as different isoforms based on the number of amino acids: VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206. Most studies suggest that VEGF165 is the most abundant and biologically active isoform.6,7 Members of the VEGF family act primarily via three membrane-bound tyrosine kinase receptors: VEGF receptor-1 (VEGFR-1; Flt-1), VEGFR-2 (Flk-1/KDR), and VEGFR-3 (Flt-4).8 Although these receptors were initially thought to be present only on endothelial cells, recent evidence suggests that VEGF receptors may also be infrequently expressed on tumor cells.9,10
Neuropilin (NRP)-1 was originally described as a 130- to 140-kd cell-surface glycoprotein expressed in the developing Xenopus laevis nervous system.11 Subsequently, it was discovered that this transmembrane glycoprotein is a receptor for the semaphorins/collapsins, a large family of secreted and transmembrane proteins that serve as guidance signals in axonal and neuronal development.12–15 Several studies have also suggested a role for NRP-1 in embryological vasculogenesis and angiogenesis. NRP-1 has been shown to be expressed in the developing skeletal and cardiovascular systems in embryos.12,16 NRP-1 knockout mice suffer from insufficient and delayed vascularization leading to embryonic death,17,18 whereas overexpression of NRP-1 in transgenic mice is lethal because of hemorrhage in the head and neck, excess blood vessel formation, and malformed hearts.16 NRP-1 has also been found to be expressed in adult endothelial cells and, to a lesser degree, in a variety of other tissues including lung, heart, liver, kidney, pancreas, and placenta as well as in osteoblasts and bone marrow stromal cells.19,20
The specific functions of NRP-1 in vessel development and angiogenesis remain to be elucidated.14,19,21 Unlike VEGFR-1, VEGFR-2, and VEGFR-3, NRP-1 does not contain a tyrosine-kinase domain and therefore seems to act as a co-receptor for VEGF165.19 The binding of VEGF165 to NRP-1 is mediated by amino acids residing at the carboxyl-terminal part of the exon 7-encoded peptide of VEGF165.19 In contrast, the binding of VEGF165 to VEGFR-1 and VEGFR-2 occurs via exon 3 and exon 4, respectively,19 thus enabling VEGF165 to bind to both NRP-1 and VEGFR-1 or VEGFR-2 simultaneously. Inhibition of VEGF165 binding to NRP-1 in endothelial cells also decreases VEGF165 binding to VEGFR-2 and subsequent mitogenic activity.22 Furthermore, co-transfection of NRP-1 into VEGFR-2-expressing endothelial cells enhances the binding of VEGF165 to VEGFR-2 and subsequent mitogenic and chemotactic activity as compared to cells expressing VEGFR-2 alone.13,19 Endothelial cells expressing NRP-1 but not VEGFR-2 do not respond to any VEGF isoform, suggesting that NRP-1 is not a signaling receptor for chemotaxis, in and of itself, but rather acts as a co-receptor for VEGFR-2, enhancing VEGF’s activity as an angiogenic factor.19
Expression of NRP-1 has recently been found in prostate cancer, breast cancer, and melanoma cell lines as well as several tumor types from patient specimens.12,23–25 Overexpression of NRP-1 in rat prostate carcinoma cells results in increased tumor growth in vivo as well as increased microvessel density and endothelial cell proliferation.12,14 Prostate tumor cell NRP-1 expression also enhances binding of VEGF165 to these tumor cells.12 Recent studies suggest that VEGF165 has a direct effect on tumor cells mediated through NRP-1. VEGF165 has been shown to act as an autocrine survival factor in NRP-1-positive breast carcinoma cells lacking VEGFR-2, likely occurring via activation of the PI-3 kinase pathway.23 In studies on human tumor specimens, NRP-1 expression correlates with the metastatic potential, stage, and grade of prostate cancer. NRP-1 has also been found to be associated with advanced stage and grade of astrocytoma.24,26
However, the expression and regulation of NRP-1 in gastrointestinal malignancies, including colorectal adenocarcinoma, has not been characterized. In this study, we investigated the expression of NRP-1 in human colon adenocarcinomas and uninvolved colonic mucosa, and the effect of NRP-1 overexpression on tumor growth and angiogenesis. We also investigated mechanisms of induction of NRP-1 in human colon adenocarcinoma cell lines.
Materials and Methods
Tissue Specimens
Specimens of colon adenocarcinoma and adjacent nonmalignant colonic mucosa were obtained from 20 patients at The University of Texas M. D. Anderson Cancer Center immediately after resection under a protocol approved by the institutional review board at M. D. Anderson Cancer Center. Specimens were either frozen in optimum cutting temperature (OCT) solution (Miles, Elkhart, IN) and stored at −80°C or fixed in formalin and embedded in paraffin at the time of their collection. The histopathological diagnosis of colon adenocarcinoma and adjacent nonmalignant mucosa was confirmed by the Department of Pathology. No patients received preoperative chemotherapy or radiation therapy.
Reagents and Chemicals
Recombinant human epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), interleukin-1β (IL-1β), and tumor necrosis factor (TNF)-α were purchased from R&D Systems (Minneapolis, MN). U0126, the extracellular signal-regulated kinase 1/2 (Erk 1/2) mitogen-activated protein kinase (MAPK) inhibitor, was obtained from New England Biolabs (Beverly, MA). The phosphatidylinositol-3 (PI-3) kinase inhibitor Wortmannin was purchased from Sigma Chemical Company (St. Louis, MO). The P38 MAPK inhibitor SB203580 was purchased from Calbiochem (San Diego, CA). The anti-human EGF receptor (EGFR) monoclonal antibody C225 was kindly provided by Daniel J. Hicklin, Ph.D. (ImClone Systems, New York, NY).
Cell Lines
The human colon adenocarcinoma cell lines HT29, SW480, SW620, and RKO, and human umbilical vein endothelial cells (HUVECs) were obtained from the American Type Culture Collection (Manassas, VA). The human colon adenocarcinoma cell lines KM12L4, KM12SM, KM12SMLM2, and KM20 were provided by IJ Fidler, D.V.M., Ph.D. (M. D. Anderson Cancer Center). Colon cancer cell lines were cultured and maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), 2 U/ml penicillin-streptomycin, vitamins, 1 mmol/L sodium pyruvate, 2 mmol/L l-glutamine, and nonessential amino acids at 37°C in 5% CO2 and 95% air. HUVECs were plated onto 0.5% gelatin-coated flasks and maintained in MEM supplemented with 15% FBS, 2 U/ml penicillin-streptomycin, vitamins, 1 mmol/L sodium pyruvate, 2 mmol/L l-glutamine, and nonessential amino acids at 37°C in 5% CO2 and 95% air.
Immunohistochemical Staining for NRP-1 in Frozen Tissue Specimens
Tissue specimens frozen in OCT solution were sectioned (8 to 10 μm thick), mounted on positively charged Superfrost slides (Fisher Scientific, Houston, TX), and air-dried for 30 minutes. Sections were fixed in cold acetone (5 minutes) followed by 1:1 acetone:chloroform (5 minutes) and then acetone (5 minutes) and washed with phosphate-buffered saline (PBS) three times for 3 minutes each time. All samples were incubated with 3% hydrogen peroxide in methanol for 12 minutes at room temperature to block endogenous peroxidase. Sections were then washed three times for 3 minutes each time with PBS (pH 7.5) and incubated for 20 minutes at room temperature in a protein-blocking solution consisting of PBS supplemented with 1% normal goat serum and 5% normal horse serum. The primary antibody directed against NRP-1, polyclonal rabbit anti-NRP-1 (1:150 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA), was applied to the sections, which were incubated overnight at 4°C. Sections were then rinsed three times for 3 minutes in PBS and incubated for 10 minutes in protein-blocking solution. The secondary antibody [peroxidase-conjugated goat anti-rabbit IgG (H+L), Jackson Research Laboratories, Westgrove, PA] was then used at a 1:400 dilution. Sections were washed three times with PBS followed by rinsing with PBS/0.1% Brij detergent. This was followed by incubation with stable diaminobenzidine substrate (Research Genetics, Huntsville, AL), during which the staining was monitored by bright-field microscopy. The reaction was halted by rinsing with ddH2O. The sections were counterstained with Gill’s no. 3 hematoxylin solution (Sigma Chemical), mounted with Universal Mount (Research Genetics), and analyzed with light microscopy. The protocol for control specimens was similar except that the primary antibody was omitted.
Immunofluorescent Staining for NRP-1 and CK-22 in Frozen Tissue Specimens
Tissue specimens frozen in OCT were sectioned and processed as described above. Immunofluorescent staining for NRP-1, cytokeratin-22 (CK-22, an epithelial cell marker), and NRP-1/CK-22 double staining were performed in the manner previously described.27
In Situ Hybridization
In situ hybridization was performed as previously described.28 An anti-sense NRP-1-specific riboprobe was made from a 750-bp 3′ UTR cDNA fragment in Bluescript II KS(+) (Stratagene, La Jolla, CA) by using a digoxigenin RNA-labeling kit (Boehringer Mannheim, Indianapolis, IN) and used in in situ hybridization as previously described.29
RNA Extraction and Northern Blot Analysis
Total RNA was harvested from subconfluent tumor cells in culture by using Trizol Reagent (Life Technologies, Inc., Grand Island, NY) following the manufacturer’s instructions. Northern blot analysis was performed as previously described.30 Briefly, 25 μg of RNA was fractionated on 1% denaturing formaldehyde/agarose gels and transferred to a Hybond-N+ positively charged nylon membrane (Amersham Biosciences, Piscataway, NJ) overnight by capillary elution. After ultraviolet crosslinking at 120,000 mJ/cm2 with an ultraviolet Stratalinker 1800 (Stratagene), the membranes were prehybridized at 65°C for 3 to 4 hours in rapid hybridization buffer (Amersham). The membranes were then hybridized at 65°C overnight with the cDNA probe for NRP-1 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The probed membranes were then washed and autoradiography performed. The cDNA probes used comprised a human NRP-1 450-bp cDNA probe derived from the reverse transcriptase-polymerase chain reaction (RT-PCR) product of PC-3 human prostate cancer cells (purchased from American Type Culture Collection) using the primers 3′-ACGATGAATGTGGCGATACT-5′ and 5′-AGTGCATTCAAGGCTGTTGG-3′ and a GAPDH probe (purchased from American Type Culture Collection). Probes were purified by agarose gel electrophoresis using a Qiaex gel extraction kit (Qiagen, Chatworth, CA). Each cDNA probe was radiolabeled with [α-32P]deoxyribonucleotide triphosphate by the random-priming technique using the Rediprime labeling system (Amersham).
Subcloning of NRP-1 into pcDNA3.1 and Transfection
The full-length cDNA for NRP-1 was subcloned into the BamHI site of pcDNA3.1 (Invitrogen, San Diego, CA) as previously described.27 Vectors containing NRP-1 or vector alone (pcDNA3.1) were transfected into KM12SMLM2 cells by lipofection according to the manufacturer’s protocol (Hoffman-La Roche, Ltd., Basel, Switzerland). Selective medium containing 200 μg/ml of hygromycin was added 48 hours later, and viable colonies were selected and expanded. Cells from subconfluent cultures were then harvested for Western blot analysis and in vivo animal experiments as described below.
Animals and Tumor Inoculation
Eight-week-old male nude mice were obtained from the National Cancer Institute’s Animal Protection Area (Frederick, MD) and acclimated for 1 week while caged in groups of five. Mice were fed a diet of animal chow and water ad libitum throughout the experiment. Mice were randomly assigned to one of three groups (10 mice per group); body weight at assignment was similar among the groups. After cell viability was verified as being ≥80% by trypan blue exclusion, control and experimental KM12SMLM2 cells (1 × 106 cells in 200 μl) were injected by means of a 30-gauge needle and 1-ml syringe subcutaneous in the right flank of the animals. Tumor growth was measured every second to third day. Tumor volume was calculated as (diameter2 × length)/2. All of the animal studies were approved by the Institutional Animal Care and Use Committee of the M.D. Anderson Cancer Center. Animals in all of the three groups were killed 18 days after tumor cell inoculation because of lethargy and the first signs of the moribund state. Tumors were harvested and placed in either 10% formalin for paraffin fixation or OCT.
Immunohistochemical Staining and Quantification of CD31
Frozen tumors from animal experiments were sectioned and processed as described above. Staining and quantification of CD-31 (vessels) was performed as previously described.27 Briefly, CD-31-positive endothelial cells were detected by localized red fluorescence with a rhodamine filter mounted on a Zeiss universal microscope (Carl Zeiss, Thornwood, NY). Tumor vessels were counted 2 mm from the tumor edge in four distinct quadrants at ×100 magnification. Results were confirmed by two observers in a blinded manner. Tumor vessel area was selectively measured in pixels2 using NIH Image 1.62 imaging software. Necrotic areas were excluded.
Determination of Tumor Cell Growth
To determine the effect of NRP-1 overexpression on monolayer cell growth in vitro, NRP-1 and pcDNA3.1 empty vector transfectants were plated into 96-well plates (Becton Dickinson, Franklin Lakes, NJ) at a density of 2000 cells per well in 10% MEM. Cell number was assessed by measuring the mitochondrial reduction of 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) to formazan. MTT was added at 24, 48, and 72 hours after plating at a final concentration of 2.5 mg/ml. After incubation for 90 minutes, the medium and MTT were removed and dimethyl sulfoxide was added for 1 minute. Optical density was measured at 570 nm.
Determination of Endothelial Cell Growth in Response to Conditioned Media
To determine the effect of conditioned media from NRP-1-transfected KM12SMLM2 colon adenocarcinoma cells on the growth of endothelial cells, NRP-1 and pcDNA3.1 empty vector transfectants were grown to 80% confluence in 10% MEM. The media was then changed to 1% MEM for 48 hours. This media was then collected, centrifuged to remove cellular debris, and given to HUVECs plated in 96-well plates in vitro for 24 to 48 hours. Cell number was then assessed by the MTT assay as described above.
Determination of Tumor Cell and Endothelial Cell Migration
NRP-1- and pcDNA 3.1-transfected KM12SMLM2 cells were seeded onto hydrated 24-well migration plates (Becton Dickinson) at a density of 70,000 cells/well (membrane insert) in FBS-free MEM. MEM containing 10% FBS or 10% FBS plus VEGF165 (10 ng/ml) were then added to the bottom well as chemoattractants and cells were incubated for 48 hours. Nonmigrated cells were then removed and migrated cells were fixed and stained using the Diff-Quick fixative (Dade Behring, Deerfield, IL) using the manufacturer’s instructions. Migrated cells were counted in five distinct areas at ×100 magnification.
HUVECs were seeded onto hydrated 24-well migration plates (Becton Dickinson) at a density of 40,000 cells/well (membrane insert) in FBS-free MEM. MEM containing 1% FBS or conditioned medium from NRP-1- and pcDNA 3.1 (empty vector)-transfected KM12SM/LM2 cells, as described above, were then added to the bottom well as chemoattractants; cells were incubated for 6 hours. Nonmigrated cells were then removed and migrated cells were fixed and stained using the Diff-Quick fixative (Dade Behring) using the manufacturer’s instructions. Migrated cells were counted in five distinct areas at ×100 magnification.
Immunoprecipitation and Western Blot Hybridizations
Human colon cancer cells were rinsed twice with ice-cold PBS and lysed with protein lysis buffer [20 mmol/L sodium phosphate (pH 7.4), 150 mmol/L NaCl, 1% Triton X-100, 5 mmol/L ethylenediaminetetraacetic acid, 5 mmol/L phenylmethyl sulfonyl fluoride, 1% aprotinin, 1 μg/ml leupeptin, and 500 μmol/L Na3VO4]. In addition, resultant tumors from in vivo studies were homogenized on ice and protein was extracted as described above. For Western blot hybridization, aliquots (50 μg) of protein were subjected to electrophoresis on 8% polyacrylamide gels followed by electrotransfer to nitrocellulose membranes (Schleicher & Schleicher, Keene, NH). Membranes were blocked with 5% fat-free milk in 0.1% Tween 20 in PBS. The primary antibodies used in this study were 1:150 dilution of rabbit polyclonal anti-NRP-1 (Santa Cruz) and 1:1000 dilutions of rabbit polyclonal anti-phospho-specific p44/42 MAPK (Erk 1/2) antibody, anti-phospho-specific P38 MAPK antibody, anti-phospho-specific Akt antibody, and anti-β-actin antibody (all from New England Biolabs). The membranes were then washed and treated with the secondary antibody labeled with horseradish peroxidase (anti-rabbit immunoglobulin from donkey at a 1:3000 dilution; Amersham). Protein bands were visualized using a commercially available chemiluminescence kit (Amersham). Before reprobing, the membranes were washed with stripping solution [100 mmol/L 2-mercaptoethanol, 2% sodium dodecyl sulfate, and 62.5 mmol/L Tris-HCl (pH 6.7)] for 30 minutes at 50°C. For immunoprecipitation, 500-μg aliquots of protein were incubated with 10 μl of NRP-1 antibody (H-286, Santa Cruz Biotechnology) at 4°C for 1 hour. The protein was then precipitated overnight at 4°C with 25 μl of Protein A/G plus Agarose (Santa Cruz Biotechnology) per the manufacturer’s instructions. The precipitated protein was then washed, denatured, and used for Western blot hybridization as described above using a different NRP-1 antibody (A-12, Santa Cruz Biotechnology) at a 1:200 dilution.
Effects of Cytokines on NRP-1 mRNA and Protein Levels in Human Colon Cancer Cell Lines
To determine the effects of the cytokines EGF, IGF-1, IL-1β, and TNF-α on NRP-1 mRNA expression, human colon cancer cells (HT29, KM12L4, and SW-480) were grown to subconfluence in standard medium as described above, and the medium was changed to 5% FBS-containing medium overnight. Cells were then incubated with EGF (50 ng/ml), IGF-1 (100 ng/ml), IL-1β (10–50 ng/ml), or TNF-α (10 to 20 ng/ml) in 1% FBS-containing medium for various times ranging from 4 to 24 hours. Total RNA was extracted and NRP-1 mRNA expression was determined by Northern blot analysis as described below. For immunoprecipitation, cells were treated with various cytokines for 48 hours and protein was then harvested for NRP-1 levels as described above.
Effect of Increasing Doses of EGF on NRP-1 mRNA Induction in HT29 Cells
To determine the effect of increasing doses of EGF on NRP-1 mRNA expression, HT29 human adenocarcinoma cells were grown to subconfluence in standard medium as described above and the medium was changed to medium containing 5% FBS overnight. Cells were the incubated with EGF (0, 0.01, 0.1, 1, 5, 50, and100 ng/ml) for 24 hours in medium containing 1% FBS. Total RNA was extracted and NRP-1 mRNA expression was determined by Northern blot analysis as described above.
Effect of C225 of EGF-Induced NRP-1 mRNA in HT29 Cells
To determine the effect of blocking the EGF receptor (EGFR) on NRP-1 expression, HT29 cells grown under conditions described above were pretreated with the EGFR monoclonal antibody C225 (10 μg/ml) for 1 hour in 1% FBS-containing medium, followed by the addition of EGF (50 ng/ml) for 24 hours. Total RNA was extracted and Northern blot analysis was performed as described above.
Determination of EGF’s Effects on Erk 1/2, Akt, and P38 Phosphorylation in HT29 Cells
To determine the effect of EGF on the protein levels and phosphorylation of the signaling intermediates Erk 1/2, Akt, and P38, cells grown under the conditions described above were incubated with EGF (50 ng/ml) for 0, 10, 15, 30, or 60 minutes in 1% FBS-containing medium, and cell lysates were obtained. Phosphorylated and total protein levels were determined by Western blot analysis as described above.
Determination of Effects of Erk1/2, Akt, and P38 MAPK Inhibition on NRP-1 Induction by EGF
To determine the effects of inhibiting activation of Erk 1/2, Akt, and P38 on NRP-1 induction, HT29 cells grown under the conditions described above were pretreated with 200 nmol/L Wortmannin, 10 μmol/L U0126, or 25 μmol/L SB203580 for 1 hour in 1% FBS-containing medium, followed by the addition of EGF (50 ng/ml) for 24 hours. In preliminary studies, doses of the signaling inhibitors demonstrated inhibition of the intended pathways without increasing cell apoptosis. Total RNA was extracted, and Northern blot analysis was done as described above.
Statistical and Densitometric Analysis
Tumor volume, tumor mass, the number of CD31 cells, and the total vessel area were compared using unpaired Student’s t-tests (InStat for Macintosh; GraphPad Software, San Diego, CA). P ≤ 0.05 was deemed significant. Densitometric analysis of autoradiographs was performed using NIH Image Analysis software (V1.62) from the National Institutes of Health (Bethesda, MD) to quantify the results of Northern blot analysis.
Results
Immunohistochemical Staining of Colon Adenocarcinomas for NRP-1
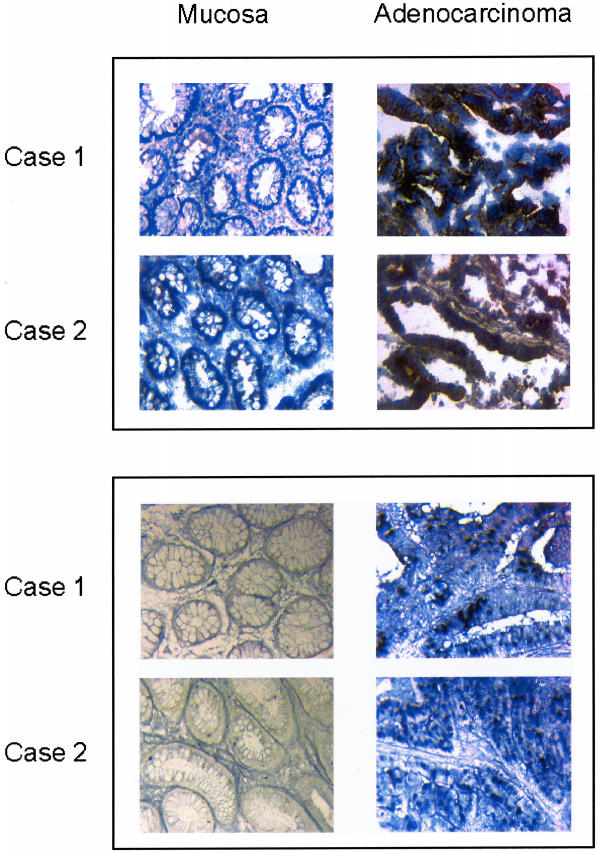
To investigate whether NRP-1 is expressed by human colon adenocarcinoma and/or adjacent nonmalignant mucosa, immunoperoxidase staining was performed on 20 frozen human colon adenocarcinoma specimens. NRP-1 protein was expressed in all 20 adenocarcinoma specimens studied but was not detectable in the adjacent nonmalignant colonic mucosa in any specimen (Figure 1, top). To further ascertain the origin of NRP-1 production, immunofluorescent double staining for NRP-1 and CK-22 (an epithelial cell marker), as previously described,27 was performed on 10 colon adenocarcinoma specimens. NRP-1 expression was localized to the adenocarcinoma epithelial cells in all specimens (data not shown).
Figure 1.
Representative tissue sections investigating NRP-1 protein and NRP-1 mRNA expression in nonmalignant human colonic mucosa and in colon adenocarcinoma. Top: Frozen sections were immunohistochemically stained, and representative images were obtained by light microscopy. The presence of NRP-1 is indicated by brown staining. NRP-1 protein was detected in the epithelium of all cancers but was not detected in nonmalignant colonic epithelium. Bottom: Paraffin-embedded sections were examined by in situ hybridization for NRP-1 mRNA expression. NRP-1 expression is indicated by the presence of purple-blue staining. NRP-1 mRNA was detected in the epithelium of all cancers but was not detected in nonmalignant colonic epithelium. Original magnifications, ×100.
Detection of NRP-1 mRNA by in Situ Hybridization
To determine the expression of NRP-1 mRNA in human colon adenocarcinoma and nonmalignant colonic mucosa, in situ hybridization was performed on 20 human colon adenocarcinoma specimens. NRP-1 mRNA expression was present in all adenocarcinoma specimens but was not detectable in the adjacent nonmalignant colonic mucosa (Figure 1, bottom) in agreement with the results of immunohistochemical staining.
RT-PCR Analysis for Expression of NRP-1 in Colon Adenocarcinoma Cell Lines
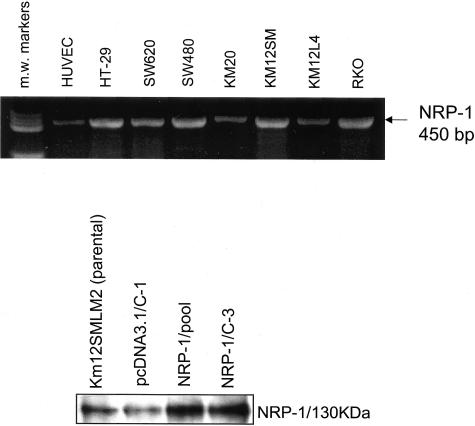
The expression of NRP-1 mRNA by human colon adenocarcinoma cell lines was examined by RT-PCR. In all seven cell lines tested, NRP-1 mRNA was constitutively expressed (Figure 2, top). HUVECs, used as a positive control, also expressed the NRP-1 transcript.
Figure 2.
NRP-1 expression in parental and transfected colon carcinoma cell lines. Top: NRP-1 mRNA expression in seven human colon adenocarcinoma cell lines. Cells were grown to 80% confluence, total RNA was harvested, and RT-PCR was performed to examine NRP-1 mRNA expression. HUVECs served as a positive control. PCR molecular weight (m.w.) markers shown in lane 1. Bottom: NRP-1 protein levels in transfected KM12SMLM2 human colon cancer cells. Parental KM12SMLM2 human colon cancer cells, pcDNA 3.1 (empty vector) transfectants, and NRP-1 transfectants were grown to 80% confluence and protein was harvested for analysis of NRP-1 levels by immunoprecipitation.
Effects of NRP-1 Transfection on Tumor Growth and Vessel Counts
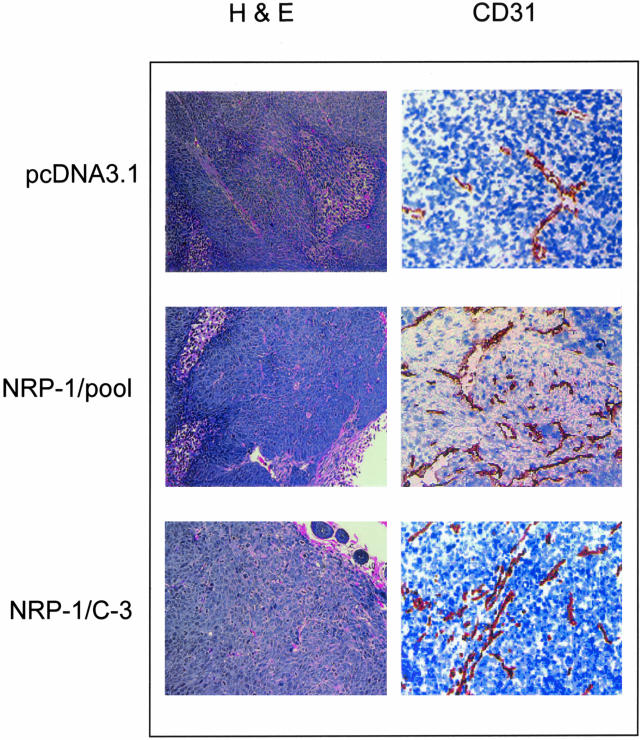
KM12SM/LM2 were chosen for transfection studies because these cells are transfected with high efficiency in our laboratory. NRP-1-transfected clones were screened for exogenous NRP-1 protein expression by immunoprecipitation and Western blot analysis (Figure 2, bottom). Vector-only transfected cells were used as controls. We selected a clone (C-3) with high NRP-1 expression and the pooled clones of NRP-1 transfectants for in vivo studies. Tumors in mice with NRP-1-transfected KM12SM/LM2 cells were fourfold to sixfold larger in mass (P < 0.05) and sevenfold to ninefold larger in volume (P < 0.01) than those from the pcDNA3.1 (empty vector)-transfected cells (Table 1). NRP-1 levels in harvested tumors were examined by immunoprecipitation and were higher in NRP-1 transfectants when compared to pcDNA3.1 empty vector transfectants (data not shown). Immunohistochemical staining (hematoxylin and eosin) of these tumors revealed no differences in tumor cell necrosis or morphology (see Figure 4). Staining for CD31, however, revealed that NRP-1-transfected tumors contained a larger number of vessels in addition to a higher total vessel area when compared to pcDNA3.1-transfected tumors (Table 1, Figure 3).
Table 1.
Effect of NRP-1 Transfection on Tumor Growth
| Tumor mass (gm) | Tumor volume (mm3) | Tumor vessel count/HPF | Total tumor vessel area (pixels2) | |
|---|---|---|---|---|
| pcDNA 3.1 | 0.09 ± 0.05 | 87 ± 42 | 19 ± 2 | 4848 ± 644 |
| NRP-1 pool | 0.55* ± 0.13 | 736† ± 164 | 37‡ ± 2 | 9161§ ± 1364 |
| NRP-1 C-3 | 0.38* ± 0.12 | 607† ± 187 | 30‡ ± 2 | 9898¶ ± 743 |
P < 0.05 versus pcDNA3.1;
P < 0.01 versus pcDNA3.1;
P < 0.01 versus pcDNA3.1;
P = 0.02 versus pcDNA3.1;
P = 0.0005 versus pcDNA3.1
Figure 4.
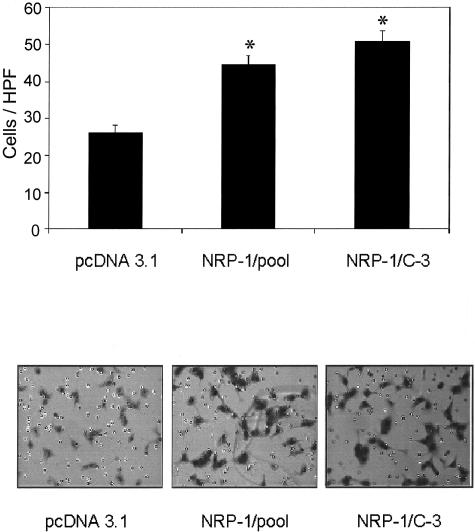
Effect of conditioned medium from NRP-1 transfectants on endothelial cell migration. HUVECs were plated into migration assay chambers using conditioned medium from NRP-1 or pcDNA 3.1 (empty vector)-transfected KM12SMLM2 human colon cancer cells as chemoattractants, as described. After 6 hours, migrated HUVECs were fixed, stained, and then counted in five distinct areas. Top: Mean number of migrated HUVECs per HPF in response to conditioned medium from pcDNA3.1 and NRP-1 transfectants (*, P < 0.001 versus pcDNA 3.1). Bottom: Representative micrographs showing the presence of migrated HUVECs (purple cells) in response to conditioned media. Original magnifications, ×100.
Figure 3.
Immunohistochemical analyses of tumors with increased NRP-1 expression. Representative photomicrographs demonstrating H&E-staining and tumor vessel staining (CD31) in tumors from mice injected with KM12SMLM2 human colon cancer cells transfected with pcDNA 3.1 (empty vector) or NRP-1. The presence of CD-31-stained vessels is indicated by the reddish-brown staining. Original magnifications: ×100 (H&E); ×200 (CD31).
Effect of NRP-1 Transfection on Tumor Cell Migration and Growth in Vitro
Transfection with NRP-1 led to a greater than twofold increase (P < 0.0001) in the migration of KM12SM/LM2 cells in standard medium and a fivefold increase (P < 0.0001) in response to VEGF165 compared to pcDNA3.1 controls (data not shown). Tumor cell growth in vitro, as measured by MTT assay at 24, 48, and 72 hours after seeding, was not significantly different among the NRP-1 transfectants compared to pcDNA3.1 vector-only transfectants.
Effect of NRP-1 Transfection on Endothelial Cell Migration and Cell Number
Conditioned medium from NRP-1-transfected KM12SM/LM2 cells led to a greater than twofold increase (P < 0.001) in the in vitro migration of HUVEC cells as compared to conditioned medium from pcDNA3.1 (empty vector) controls (Figure 4). In vitro exposure of HUVECs to conditioned media from NRP-1-transfected KM12SM/LM2 cells did not lead to a change in cell number, as determined by MTT assay, when compared to conditioned media obtained from empty vector-transfected KM12SM/LM2 cells (data not shown).
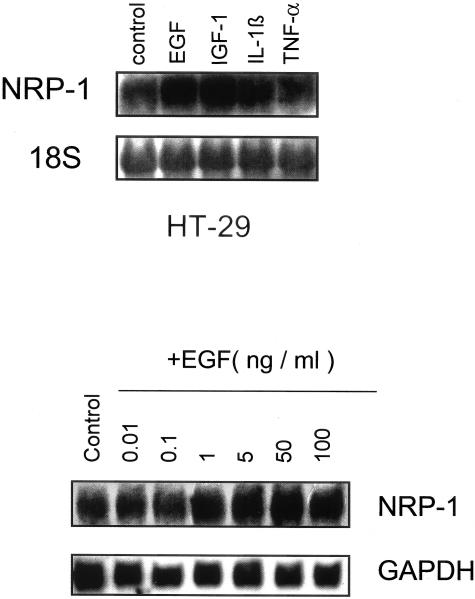
Effects of EGF, IGF-1, IL-1β, and TNF-α on NRP-1 mRNA in Colon Adenocarcinoma Cell Lines
Because overexpression of NRP-1 led to increased tumor growth and angiogenesis, we sought to determine factors that lead to NRP-1 induction. In preliminary studies, we found that the cell line with the greatest cytokine-mediated induction of NRP-1 was the HT29 cell line. Therefore, for cytokine and signaling studies we focused our investigations on this cell line. To examine the role of different cytokines in the regulation of NRP-1 in human colon adenocarcinoma, HT29 cells were treated with EGF, IGF-1, IL-1β, or TNF-α for 4 to 24 hours after which cells were harvested for measurement of NRP-1 mRNA levels by Northern blot analysis. EGF increased NRP-1 mRNA expression (Figure 5, top), with maximum expression observed at 24 hours (2.5-fold by densitometric analysis). Treatment with IGF-1 also increased NRP-1 mRNA levels (twofold by densitometric analysis), but in contrast, incubation with IL-1β or TNF-α did not (Figure 5, top). Additional time points up to 48 hours after stimulation and higher doses of IL-1β (50 ng/ml) and TNF-α (20 ng/ml) also failed to increase NRP-1 mRNA levels (data not shown). Similar experiments performed with KM-12L4 and SW-480 human colon adenocarcinoma cells also showed increased NRP-1 mRNA levels (twofold to fourfold as measured by densitometry) in response to EGF.
Figure 5.
Effect of cytokines on NRP-1 mRNA induction in HT29 human colon adenocarcinoma cells. Top: HT29 cells were grown to 80% confluence and treated with EGF (50 ng/ml), IGF-1 (100 ng/ml), IL-1β (10 ng/ml), or TNF-α (10 ng/ml) for 24 hours. Total RNA was then harvested and Northern blot analysis for NRP-1 was performed. Ethidium bromide staining was done to detect 18S RNA, which served as a loading control. Bottom: Effect of increasing doses of EGF on NRP-1 mRNA expression in HT29 human adenocarcinoma cells. Cells were grown to 80% confluence and treated with varying doses of EGF as indicated for 24 hours. Total RNA was then harvested for Northern blot analysis of NRP-1 mRNA expression.
Effects of EGF, IGF-1, IL-1β, and TNF-α on NRP-1 Protein Levels in HT29 Colon Adenocarcinoma Cells
To determine whether these various cytokines also led to increases in NRP-1 protein production, HT29 cells were treated with EGF, IGF-1, IL-1β, or TNF-α for 48 hours after which cells were harvested for measurement of NRP-1 protein levels by immunoprecipitation. In agreement with the findings of NRP-1 mRNA levels, EGF and IGF-1 both led to an increase in NRP-1 protein levels as compared to controls, whereas IL-1β and TNF-α did not (data not shown).
NRP-1 Induction by EGF in Human Colon Adenocarcinoma Cells Is Dose-Dependent
HT29 cells were treated with escalating doses of EGF (0 to 100 ng/ml) for 24 hours after which NRP-1 mRNA levels were analyzed. There was an increase in NRP-1 expression with increasing concentration of EGF beginning at 1 ng/ml with maximum mRNA induction (2.1-fold increase versus control, as determined by densitometric analysis) at 50 ng/ml (Figure 5, bottom). This dose of EGF was therefore used in subsequent experiments.
Effect of the EGFR Monoclonal Antibody C225 on EGF-Induced NRP-1 Expression
HT29 cells were pretreated with the EGFR monoclonal antibody C225 before the addition of EGF (50 ng/ml). The presence of C225 completely abolished EGF-induced NRP-1 mRNA expression as determined by Northern blot analysis (data not shown).
Effect of EGF on the PI-3 and MAPK Signal Transduction Pathways
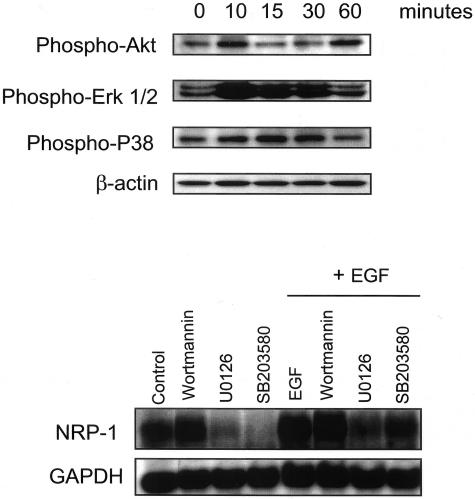
To determine the signaling pathways induced by EGF, HT29 cells were treated with EGF and harvested at various time points for analysis of signaling intermediates by Western blotting. As shown in Figure 6, top, EGF induced Erk 1/2 phosphorylation by 10 minutes, and phosphorylation of Erk 1/2 returned to baseline levels by 60 minutes. Phosphorylation of P38 was also increased by 15 to 30 minutes and returned to baseline levels by 60 minutes. Phosphorylation of Akt was transiently increased at 10 minutes and 60 minutes.
Figure 6.
EGF induction of signaling pathways and regulation of NRP-1. Top: Effect of EGF on intracellular signaling pathways in HT29 human adenocarcinoma cells. Cells were grown to 80% confluence and treated with EGF (50 ng/ml) for the indicated times. Protein was harvested for Western blot analysis of the indicated signaling intermediates. Bottom: Northern blot demonstrating effect of signaling inhibitors on EGF-induced NRP-1 mRNA expression. HT29 cells were grown to 80% confluence and pretreated with Wortmannin (200 nmol/L, PI-3 kinase inhibitor), U0126 (10 μmol/L, Erk 1/2 MAPK inhibitor), or SB20358 (50 μmol/L, P38 MAPK inhibitor) for 1 hour before treatment with EGF (50 ng/ml) or vehicle (MEM containing 1% FBS) for 24 hours. Total RNA was then harvested for analysis of NRP-1 mRNA expression as described in text.
Regulation of EGF Induction of NRP-1 by the Erk and P38 MAPK Pathways
To investigate the roles of different signal transduction pathways in EGF-induced NRP-1 expression in human colon adenocarcinoma cells, HT29 cells were pretreated with the PI-3 kinase inhibitor Wortmannin, the Erk 1/2 MAPK inhibitor U0126, or the P38 MAPK inhibitor SB203580 before the addition of EGF. Inhibition of the Erk 1/2 MAPK pathway or the P38 MAPK pathway abrogated both constitutive and EGF-induced NRP-1 mRNA expression (Figure 6, bottom). Inhibition of the PI-3 kinase pathway, however, had no effect on either constitutive or EGF-induced NRP-1 expression.
Discussion
The expression of NRP-1 has been recently been described in numerous tumor systems, including prostate, pituitary, squamous cell, and breast cancers.14 To our knowledge, this is the first study to describe the expression of the novel VEGF receptor NRP-1 in human colon adenocarcinoma. NRP-1 expression and production were present in all human colon adenocarcinoma specimens but not in the nonmalignant colonic mucosa. Constitutive expression of NRP-1 mRNA was also present in all human colon adenocarcinoma cell lines tested consistent with studies on human tissues. Overexpression of NRP-1 by human colon adenocarcinoma cells led to significantly increased tumor growth as well as increased tumor vessel counts and overall vessel area. These findings suggest that NRP-1 is associated with the growth and development of colon adenocarcinoma as well as increased angiogenesis in vivo. Furthermore, the demonstration that increased NRP-1 expression led to increased tumor cell migration in response to VEGF suggests that this increase in tumor growth is associated with a direct biological effect on tumor cell behavior. Miao and colleagues12 recently reported similar findings in prostate cancer. Using AT2.1 rat prostate carcinoma cells transfected with NRP-1 using a tetracycline-induced promoter, treatment with the tetracycline homologue, doxycycline (Dox), led to increased NRP-1 expression, increased VEGF165 binding, and increased cell motility.14 When rats injected with these tumors were fed Dox, NRP-1 synthesis was induced and the resulting tumors were larger and exhibited increased microvessel density, proliferating endothelial cells, dilated blood vessels, and decreased apoptosis12 providing the first evidence that NRP-1 overexpression leads to increased tumor growth and angiogenesis in vivo.
The precise mechanisms by which NRP-1 expression and subsequent angiogenesis lead to increased tumor growth are still unclear. Although conditioned media from NRP-1-transfected colon cancer cells failed to have any direct effect on the growth rate of endothelial cells in this study, endothelial cell migration was significantly increased suggesting that a soluble mediator may be partially responsible for the observed increase in angiogenesis and tumor growth. In all of the colon tumor lines tested, VEGFR2 was not expressed by RT-PCR or enzyme-linked immunosorbent assay, although VEGFR1 was (data not shown). Because it has been shown that VEGF-A binding to both NRP-1 and VEGFR2 on endothelial cells leads to increased mitogenic and chemotactic activity,19,22 we, as have others, theorize that VEGF may bind NRP-1 on tumor cells and VEGFR2 on endothelial cells simultaneously increasing endothelial cell activity and providing a juxtacrine mechanism for NRP-1 induction of angiogenesis and tumor growth.12
Several lines of evidence support the fact that angiogenesis is important in the growth and metastasis of colon cancer. A large number of angiogenic factors have been shown to regulate angiogenesis in colon cancer, with VEGF appearing to be one of the most important.27,31–35 For example, studies have demonstrated that VEGF is increasingly produced during progression from nonmalignant colonic mucosa to adenoma and finally to adenocarcinoma,36,37 that the expression of VEGF and its receptor correlates with the development of colon metastasis,1 and that increased VEGF expression by tumors is associated with decreased survival in patients with node-negative colon cancer.38 Similarly, anti-VEGF therapies have been associated with decreased tumor vascularity, tumor growth, carcinomatosis, and metastasis and increased survival in animal models.39–42 This study provides further support for the role of VEGF in colon cancer growth and angiogenesis via its newly discovered receptor NRP-1.
Following the demonstration that NRP-1 is expressed in colon cancer contributes to increased tumor growth, we sought to determine NRP-1’s potential regulatory factors. The epidermal growth factor receptor (EGFR) has been shown to regulate a variety of different angiogenic factors, including VEGF, in several tumor types.43,44 Recently, EGF has been shown to regulate NRP-1 expression in astrocytoma cells, but the signaling intermediates that regulated this induction were not investigated.26 In our study, EGF increased NRP-1 mRNA expression to varying degrees in all human colon adenocarcinoma cell lines studied. Three cell lines were studied to verify that the effect of EGF on NRP-1 induction was not a phenomenon of a single cell line. This increase in NRP-1 mRNA expression was also associated with an increase in NRP-1 protein levels. The finding that EGFR activation led to NRP-1 induction further supports a role for EGF in angiogenesis and growth of colon cancer.45 Furthermore, the addition of a monoclonal antibody to the EGFR (C225) abrogated EGF-induced NRP-1 mRNA expression lending further support to the role of EGFR in the regulation of NRP-1. IGF-1 also increased NRP-1 expression and protein production, a finding that has not been previously reported. TNF-α, an inflammatory cytokine, has recently been reported to increase VEGFR-2 and NRP-1 expression in human vascular endothelial cells.46 In our study, however, TNF-α and IL-1β failed to significantly increase NRP-1 expression in any of the colon adenocarcinoma cell lines tested.
The EGF system is comprised of its receptor, EGFR and its ligands, EGF, transforming growth factor-α, heparin-binding EGF-like growth factor (HB-EGF),47 amphiregulin, betacellulin, epiregulin, and epigen.48 Activation of EGFR has been implicated in the progression and metastasis of colorectal cancer. A large percentage of colon cancer cell lines49–51 and specimens52,53 express EGFR, and both transforming growth factor-α and EGF have been shown to increase colon adenocarcinoma cell growth in vitro.54 The expression of EGFR has been shown to be higher in colon adenocarcinoma than in adjacent normal mucosa,55,56 and higher in advanced stage human specimens compared to less advanced specimens.52 Increased EGFR expression by human colon adenocarcinoma has also been correlated with increased hepatic metastases57 and metastatic potential in vivo.58 Although the mechanism by which EGF may increase colon cancer growth is less clear, results from this study suggest that enhancement of NRP-1 expression by EGF may provide one possible explanation and further studies are warranted.
The intracellular signaling pathways governing NRP-1 induction have not been fully elucidated. A study in astrocytoma cells suggested that the ras-GTP (MAPK) signaling pathway may regulate NRP-1 expression.26 Consistent with those results in our study, constitutive expression of NRP-1 in colon adenocarcinoma also appears to be regulated, in part, by the Erk 1/2 MAPK signaling pathway. Considering that the EGFR monoclonal antibody abrogated baseline expression of NRP-1, this may be, in part, because of autocrine activation of the Erk 1/2 MAPK pathway. The addition of EGF in the presence of an Erk 1/2 MAPK inhibitor was able to induce NRP-1, albeit to a significantly lesser degree suggesting that the Erk MAPK pathway may be partially, but not solely, responsible for EGF-induction of NRP-1. Baseline and EGF-induced NRP-1 expressions in our system were also decreased by inhibition of the P38 MAPK signaling pathway, a finding that has not been previously described. Although constitutive NRP-1 expression was essentially abolished by inhibition of the P38 pathway, the addition of EGF was still able to increase NRP-1 expression despite P38 inhibition, suggesting that multiple intracellular signaling pathways are important in EGF-induction of NRP-1 expression. In contrast, although EGF transiently increased phosphorylation of Akt in a biphasic manner, the PI-3 kinase pathway does not seem to play a role in the constitutive or EGF-induced expression of NRP-1 in this system.
In summary, our study suggests that the novel VEGF receptor NRP-1 is expressed in human colon adenocarcinoma but not in nonmalignant colonic mucosa and is associated with increased tumor growth and angiogenesis. Although the exact mechanisms by which NRP-1 regulates tumorigenesis and angiogenesis are still unclear, EGFR activation may play an important role, lending further support to EGFR’s potential involvement in the growth of colon cancer. The regulation of NRP-1 expression appears to involve, at least in part, EGFR-dependent signaling pathways that have also been shown to be important in modulating the production of other angiogenic factors including VEGF. Further in vitro studies, particularly involving the NRP-1 promoter, as well as further in vivo studies investigating tumor growth, survival, and the molecular interaction between tumor cells and the surrounding endothelial cells are necessary to ultimately determine both the mechanism and the role of this novel VEGF receptor in the pathogenesis and progression of human colon adenocarcinoma.
Acknowledgments
We thank Donna Reynolds for assistance with immunohistochemistry, Melissa Burkett of the Department of Scientific Publications for editorial assistance, and Rita Hernandez of the Department of Surgical Oncology for manuscript preparation.
Footnotes
Address reprint requests to Lee M. Ellis, M.D., Department of Surgical Oncology, Box 444, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030-4009. E-mail: lellis@mdanderson.org.
Supported, in part, by the National Institutes of Health (T-32 training grant CA-09599 to A.A.P. and S.A.A. and cancer center support grant CA-16672 to M.K.), the Gillson Longenbaugh Foundation (to L.M.E.), the Jon and Susie Hall Fund for Colon Cancer Research (to L.M.E.), and the National Cancer Institute (grants 37392 and 45448 to M.K.).
A.A.P. and F.F. contributed equally to this work.
References
- Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- Price DJ, Miralem T, Jiang S, Steinberg R, Avraham H. Role of vascular endothelial growth factor in the stimulation of cellular invasion and signaling of breast cancer cells. Cell Growth Differ. 2001;12:129–135. [PubMed] [Google Scholar]
- Clauss M. Molecular biology of the VEGF and the VEGF receptor family. Semin Thromb Hemost. 2000;26:561–569. doi: 10.1055/s-2000-13213. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:RE21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol. 2001;280:C1375–C1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Alitalo K. Signaling via vascular endothelial growth factor receptors. Exp Cell Res. 1999;253:117–130. doi: 10.1006/excr.1999.4707. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor. Eur J Cancer. 1996;32A:2413–2422. doi: 10.1016/s0959-8049(96)00387-5. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. EMBO J. 1999;13:9–22. [PubMed] [Google Scholar]
- Pidgeon GP, Barr MP, Harmey JH, Foley DA, Bouchier-Hayes DJ. Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer. 2001;85:273–278. doi: 10.1054/bjoc.2001.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal PM, Failla CM, Pagani E, Odorisio T, Schietroma C, Falcinelli S, Zambruno G, D’Atri S. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J Invest Dermatol. 2000;115:1000–1007. doi: 10.1046/j.1523-1747.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- Fujisawa H, Takagi S, Hirata T. Growth-associated expression of a membrane protein, neuropilin, in Xenopus optic nerve fibers. Dev Neurosci. 1995;17:343–349. doi: 10.1159/000111304. [DOI] [PubMed] [Google Scholar]
- Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. EMBO J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- Fuh G, Garcia KC, de Vos AM. The interaction of neuropilin-1 with vascular endothelial growth factor and its receptor flt-1. J Biol Chem. 2000;275:26690–26695. doi: 10.1074/jbc.M003955200. [DOI] [PubMed] [Google Scholar]
- Miao HQ, Klagsbrun M. Neuropilin is a mediator of angiogenesis. Cancer Metastasis Rev. 2000;19:29–37. doi: 10.1023/a:1026579711033. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nakamura F, Jin Z, Kalb RG, Strittmatter SM. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat Neurosci. 1998;1:487–493. doi: 10.1038/2203. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci USA. 2002;99:3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Tordjman R, Ortega N, Coulombel L, Plouet J, Romeo PH, Lemarchandel V. Neuropilin-1 is expressed on bone marrow stromal cells: a novel interaction with hematopoietic cells? Blood. 1999;94:2301–2309. [PubMed] [Google Scholar]
- Soker S, Fidder H, Neufeld G, Klagsbrun M. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7-encoded domain. J Biol Chem. 1996;271:5761–5767. doi: 10.1074/jbc.271.10.5761. [DOI] [PubMed] [Google Scholar]
- Soker S, Gollamudi-Payne S, Fidder H, Charmahelli H, Klagsbrun M. Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial cell proliferation by a peptide corresponding to the exon 7-encoded domain of VEGF165. J Biol Chem. 1997;272:31582–31588. doi: 10.1074/jbc.272.50.31582. [DOI] [PubMed] [Google Scholar]
- Bachelder RE, Crago A, Chung J, Wendt MA, Shaw LM, Robinson G, Mercurio AM. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- Latil A, Bieche I, Pesche S, Valeri A, Fournier G, Cussenot O, Lidereau R. VEGF overexpression in clinically localized prostate tumors and neuropilin-1 overexpression in metastatic forms. Int J Cancer. 2000;89:167–171. doi: 10.1002/(sici)1097-0215(20000320)89:2<167::aid-ijc11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Fakhari M, Pullirsch D, Abraham D, Paya K, Hofbauer R, Holzfeind P, Hofmann M, Aharinejad S. Selective upregulation of vascular endothelial growth factor receptors neuropilin-1 and -2 in human neuroblastoma. Cancer. 2002;94:258–263. doi: 10.1002/cncr.10177. [DOI] [PubMed] [Google Scholar]
- Ding H, Wu X, Roncari L, Lau N, Shannon P, Nagy A, Guha A. Expression and regulation of neuropilin-1 in human astrocytomas. Int J Cancer. 2000;88:584–592. doi: 10.1002/1097-0215(20001115)88:4<584::aid-ijc11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Ahmad SA, Liu W, Jung YD, Fan F, Reinmuth N, Bucana CD, Ellis LM. Differential expression of angiopoietin-1 and angiopoietin-2 in colon carcinoma. A possible mechanism for the initiation of angiogenesis. Cancer. 2001;92:1138–1143. doi: 10.1002/1097-0142(20010901)92:5<1138::aid-cncr1431>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: in vivo expression and antitumor activity. Proc Natl Acad Sci USA. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. New York: Oxford University Press,; In Situ HybridizationA Practical Approach. 1998 [Google Scholar]
- Jung YD, Nakano K, Liu W, Gallick GE, Ellis LM. Extracellular signal-regulated kinase activation is required for up-regulation of vascular endothelial growth factor by serum starvation in human colon carcinoma cells. Cancer Res. 1999;59:4804–4807. [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Hsu S, Huang F, Friedman E. Platelet-derived growth factor-B increases colon cancer cell growth in vivo by a paracrine effect. J Cell Physiol. 1995;165:239–245. doi: 10.1002/jcp.1041650204. [DOI] [PubMed] [Google Scholar]
- Ellis LM, Takahashi Y, Liu W, Shaheen RM. Vascular endothelial growth factor in human colon cancer: biology and therapeutic implications. Oncologist. 2000;5:11–15. doi: 10.1634/theoncologist.5-suppl_1-11. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Cleary KR, Mai M, Kitadai Y, Bucana CD, Ellis LM. Significance of vessel count and vascular endothelial growth factor and its receptor (KDR) in intestinal-type gastric cancer. Clin Cancer Res. 1996;2:1679–1684. [PubMed] [Google Scholar]
- Takahashi M, Kawabe T, Ogura K, Maeda S, Mikami Y, Kaneko N, Terano A, Omata M. Expression of vascular endothelial growth factor at the human gastric ulcer margin and in cultured gastric fibroblasts: a new angiogenic factor for gastric ulcer healing. Biochem Biophys Res Commun. 1997;234:493–498. doi: 10.1006/bbrc.1997.5974. [DOI] [PubMed] [Google Scholar]
- Wong MP, Cheung N, Yuen ST, Leung SY, Chung LP. Vascular endothelial growth factor is up-regulated in the early pre-malignant stage of colorectal tumour progression. Int J Cancer. 1999;81:845–850. doi: 10.1002/(sici)1097-0215(19990611)81:6<845::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748–753. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR, Ellis LM. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997;132:541–546. doi: 10.1001/archsurg.1997.01430290087018. [DOI] [PubMed] [Google Scholar]
- Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen RM, Ahmad SA, Liu W, Reinmuth N, Jung YD, Tseng WW, Drazan KE, Bucana CD, Hicklin DJ, Ellis LM. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br J Cancer. 2001;85:584–589. doi: 10.1054/bjoc.2001.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen RM, Tseng WW, Vellagas R, Liu W, Ahmad SA, Jung YD, Reinmuth N, Drazan KE, Bucana CD, Hicklin DJ, Ellis LM. Effects of an antibody to vascular endothelial growth factor receptor-2 on survival, tumor vascularity, and apoptosis in a murine model of colon carcinomatosis. Int J Oncol. 2001;18:221–226. [PubMed] [Google Scholar]
- Bruns CJ, Liu W, Davis DW, Shaheen RM, McConkey DJ, Wilson MR, Bucana CD, Hicklin DJ, Ellis LM. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89:488–499. [PubMed] [Google Scholar]
- Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M, Tamatani T, Fukui K, Yuki T, Yoshida H, Bando T, Hoque MO, Kamogashira T, Ogino K, Nishino N, Suzuki T, Sato M. Identification of EGF as an angiogenic factor present in conditioned medium from human salivary gland adenocarcinoma cell clones with varying degrees of metastatic potential. Cancer Lett. 1994;84:189–198. doi: 10.1016/0304-3835(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Ciardiello F, Bianco R, Damiano V, Fontanini G, Caputo R, Pomatico G, De Placido S, Bianco AR, Mendelsohn J, Tortora G. Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res. 2000;6:3739–3747. [PubMed] [Google Scholar]
- Giraudo E, Primo L, Audero E, Gerber HP, Koolwijk P, Soker S, Klagsbrun M, Ferrara N, Bussolino F. Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem. 1998;273:22128–22135. doi: 10.1074/jbc.273.34.22128. [DOI] [PubMed] [Google Scholar]
- Mangrulkar RS, Ono M, Ishikawa M, Takashima S, Klagsbrun M, Nowak RA. Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium. Biol Reprod. 1995;53:636–646. doi: 10.1095/biolreprod53.3.636. [DOI] [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Bradley SJ, Garfinkle G, Walker E, Salem R, Chen LB, Steele G., Jr Increased expression of the epidermal growth factor receptor on human colon carcinoma cells. Arch Surg. 1986;121:1242–1247. doi: 10.1001/archsurg.121.11.1242. [DOI] [PubMed] [Google Scholar]
- Wan CW, McKnight MK, Brattain DE, Brattain MG, Yeoman LC. Different epidermal growth factor growth responses and receptor levels in human colon carcinoma cell lines. Cancer Lett. 1988;43:139–143. doi: 10.1016/0304-3835(88)90226-1. [DOI] [PubMed] [Google Scholar]
- Huang S, Trujillo JM, Chakrabarty S. Proliferation of human colon cancer cells: role of epidermal growth factor and transforming growth factor alpha. Int J Cancer. 1992;52:978–986. doi: 10.1002/ijc.2910520625. [DOI] [PubMed] [Google Scholar]
- Yasui W, Sumiyoshi H, Hata J, Kameda T, Ochiai A, Ito H, Tahara E. Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res. 1988;48:137–141. [PubMed] [Google Scholar]
- Moorghen M, Ince P, Finney KJ, Watson AJ, Harris AL. Epidermal growth factor receptors in colorectal carcinoma. Anticancer Res. 1990;10:605–611. [PubMed] [Google Scholar]
- Huang SA, Lin PF, Fan D, Price JE, Trujillo JM, Chakrabarty S. Growth modulation by epidermal growth factor (EGF) in human colonic carcinoma cells: constitutive expression of the human EGF gene. J Cell Physiol. 1991;148:220–227. doi: 10.1002/jcp.1041480206. [DOI] [PubMed] [Google Scholar]
- Ozgul C, Karaoz E, Erdogan D, Dursun A. Expression of epidermal growth factor receptor in normal colonic mucosa and in adenocarcinomas of the colon. Acta Physiol Hung. 1997;85:121–128. [PubMed] [Google Scholar]
- Borlinghaus P, Wieser S, Lamerz R. Epidermal growth factor, transforming growth factor-alpha, and epidermal growth factor receptor content in normal and carcinomatous gastric and colonic tissue. Clin Invest. 1993;71:903–907. doi: 10.1007/BF00185601. [DOI] [PubMed] [Google Scholar]
- Radinsky R, Risin, Fan, Dong, Bielenberg, Bucana CD, Fidler IJ. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- Parker C, Roseman BJ, Bucana CD, Tsan R, Radinsky R. Preferential activation of the epidermal growth factor receptor in human colon carcinoma liver metastases in nude mice. J Histochem Cytochem. 1998;46:595–602. doi: 10.1177/002215549804600505. [DOI] [PubMed] [Google Scholar]