Abstract
Astrocytes secrete cytokines and neurotrophic factors to neurons, consistent with a neurosupportive role for astrocytes. However, in ischemic or metabolic insults, the function of astrocytic gap junctions composed mainly from connexin43 (Cx43) remains controversial. We have previously shown that heterozygous Cx43 null mice subjected to middle cerebral artery occlusion exhibited significantly enhanced stroke volume and apoptosis compared to wild-type mice. In this study, we used mice in which the human GFAP promoter-driven cre transgene deletes the floxed Cx43 gene in astrocytes, excluding the effects from reduced Cx43 expression in many other cell types as well as astrocytes. We induced focal brain ischemia in mice lacking Cx43 in astrocytes [Cre(+)] and control littermates [Cre(−)]. Cre(+) mice showed a significantly increased stroke volume and enhanced apoptosis, detected by terminal dUTP nick-end labeling and caspase-3 immunostaining, compared to Cre(−) mice. Inflammatory response assessed by the microglial marker CD11b was amplified in the penumbra of Cre(+) mice compared to that of Cre(−) mice. Our results suggest that astrocytic gap junctions could be important for the regulation of neuronal apoptosis and the inflammatory response after stroke. These findings support the view that astrocytes play a critical role in neuroprotection during ischemic insults.
Ischemic stroke causes not only neuronal loss but also gliosis and inflammation in the surrounding ischemic core.1 Astrocytes are activated in the lesion and accumulate glycogen for energy supply under ischemic conditions.2 Activated astrocytes maintain the extracellular glutamate concentration3 and reduce neuronal vulnerability to free radicals.4,5 Therefore, astrocytes are considered to protect neurons from ischemic insults. However, under ischemic conditions, the role of astrocytic gap junctions is still controversial. Astrocytic gap junctions remain open during ischemia6 and it is assumed that the wave of spreading depression passes through astrocytic gap junction channels causing expansion of stroke volume.7,8 On the other hand, glial cells protect neurons from hypoxic depolarization.9 In primary neuron-glial co-culture and in hippocampal slice culture, gap junctional intercellular communication (GJIC) between astrocytes decreases neuronal vulnerability to oxidative stress.10 Furthermore, blocking astrocytic gap junctions increases neuronal death induced by glutamate cytotoxicity in neuron-astrocyte co-cultures.11
Astrocytic gap junctions are mainly composed of the channel protein, connexin43 (Cx43).12,13 Although Cx43-deficient mice [Cx43(−/−)] die immediately after birth,14 heterozygous Cx43 null mice [Cx43(+/−)] are viable. Cultured Cx43(+/−) astrocytes exhibit reduced Cx43 protein expression and intercellular Ca2+ signaling.15,16 We have reported that Cx43(+/−) mice showed significantly larger infarct volume17 and enhanced apoptosis compared to wild-type mice after middle cerebral artery (MCA) occlusion,18 demonstrating that astrocytic gap junctions may decrease apoptotic neuronal damage in ischemic brain injury.
Inflammation also plays a critical role in cerebral ischemia. Macrophages, microglia, astrocytes, and neurons can secrete various cytokines.19,20 Moreover, it has been reported that co-cultured macrophages down-regulate the expression of Cx43 in astrocytes21 and that microglia, stimulated by cytokines, express Cx43 and might communicate with astrocytes through gap junctions.22 However, the relationship between astrocytic function and inflammatory response in the pathology of the brain ischemic lesion is not yet understood.
In this study, we used mice with astrocyte-directed ablation of Cx4323 to analyze the neuroprotective role of astrocytic gap junctions in focal brain ischemia. In Cx43(+/−) mice, Cx43 is compromised not only in astrocytes but also in many other cell types and organs, including the heart and vasculature. In the present study, the phenotype of mice lacking the Cx43 gene [Cx43(fl/fl), hGFAPcre] in astrocytes is independent of the decreased expression of Cx43 in other type cells, thus yielding more specific and definitive results. We demonstrate that astrocytic gap junctions play a critical role in decreasing neuronal apoptosis and inflammation after ischemic brain infarction.
Materials and Methods
Conditional Knockout Mice
Mice lacking Cx43 in astrocytes were generated by interbreeding mice harboring two floxed alleles of connexin43 (Cx43fl/fl)24 and mice carrying an hGFAP-cre transgene, driving cre expression in neuroglial progenitor cells.25 Parental generations used to obtain offspring for the current study were as follows: Cx43fl/fl × Cx43fl/fl, hGFAP-cre. The mice had a mixed background of 6.25% 129P2/OlaHsd; 6.25% FVB/N; 87.5% C57BL/6. In our study, we compared mice lacking Cx43 in astrocytes, denoted as Cre(+), with littermate controls, expressing both floxed Cx43 alleles in astrocytes, denoted as Cre(−). Cell-type-directed excision of the Cx43 coding region led to activation of a lacZ reporter gene, embedded in the Cx43 floxed allele, in cells with Cx43 promoter activity. Therefore, cre-mediated recombination leading to loss of Cx43 expression could be monitored by X-Gal staining.23 All mice were generated and genotyped23 in the Institute of Genetics, University of Bonn, Germany.
Surgery
All procedures were approved by the Animal Care and Veterinary Services of the University of Western Ontario. Adult male Cre(+) mice (n = 10) and Cre(−) mice (n = 10), each weighing 30 to 40 g, were anesthetized with sodium pentobarbital (65 mg/kg i.p.). Each animal was secured to a heating pad by using surgical tape to hold the tail to the pad and the head was held securely in place using a stereotaxic frame. The rectal temperature was maintained at 37.5°C throughout the surgery and after the procedure until the animal regained consciousness. Under anesthesia, the right MCA was exposed according to a procedure described previously.18 Briefly, an incision was made on the right side of the head from the anterior of the ear to the corner of the eye horizontally and from the corner of the eye vertically 5 mm. The squamosal bone was exposed by folding the temporal muscle. By using a fine electronic-powered dental drill, a small burr hole was made ∼2 mm in diameter on the bone. The overlying dura mater was removed to expose the MCA. Then, the MCA was occluded above and below the rhinal fissure by using an electronic coagulator (Codman; Codman & Shurtleff Inc., Randolph, MA). After confirming no recanalization, the skin incision was closed with sutures. All animals were subsequently given free access to water and food.
Histological Analysis
Four days after surgery, animals were placed under deep anesthesia with an overdose of sodium pentobarbital (130 mg/kg i.p.) and perfused transcardially first with 10 mmol/L phosphate-buffered saline (PBS), pH 7.4, and then with 4% paraformaldehyde, pH 7.4, in PBS. The brains were removed and fixed in 4% paraformaldehyde and cryoprotected in 20% sucrose in PBS. For the immunohistochemical staining with CD11b antibody (Pharmingen, San Diego, CA), brains were removed and cryoprotected without fixation. Coronal sections were cut with a cryostat microtome at a thickness of 20 μm for measuring the infarct volume and 7 μm for terminal dUTP nick-end labeling (TUNEL) and immunohistochemical staining.
Measurement of Stroke Volume
The brain slices were mounted sequentially onto glass microscope slides and stained with thionin to allow measurement of infarct size as previously described.17,18 Damage was assessed by using a Leitz Diaplan microscope with high-power magnification (×16). Pictures of the brain sections were taken with a digital camera at ×1.6 power magnification and analyzed with a computer-assisted image analysis system (SigmaScan Pro, version 4; SSPS Inc., Chicago, IL). The infarct areas in each section were measured by subtracting the remaining area of the lesioned ipsilateral hemisphere from the contralateral hemisphere. The areas of infarction on each slice were summed and multiplied by slice thickness to give the volume of infarction.
TUNEL Staining
TUNEL staining was performed according to protocol of the Apoptosis Detection System, Fluorescein (Promega, Madison, WI). Briefly, brain sections (7 μm thickness) mounted on glass slides were rehydrated and washed in 0.85% NaCl for 5 minutes and in PBS for 5 minutes. Then, sections were fixed in 4% paraformaldehyde for 15 minutes, washed in PBS, and incubated with 20 μg/ml proteinase K solution for 10 minutes. After washing in PBS, sections were postfixed in 4% paraformaldehyde for 5 minutes. Equilibration buffer was added onto the slides, then 10 minutes later, TdT enzyme and nucleotide mix were added and incubated for 1 hour at 37°C. The reaction was stopped with 2× standard saline citrate. The slides were washed and counterstained with propidium iodide. The sections were examined under a photomicroscope (Zeiss Axiophot; Carl Zeiss, New York, NY). TUNEL-stained cells were counted and averaged from six randomly picked 0.09-mm2 areas in the ischemic penumbra at the level of the brain sections shown in the third row of Figure 1A.
Figure 1.
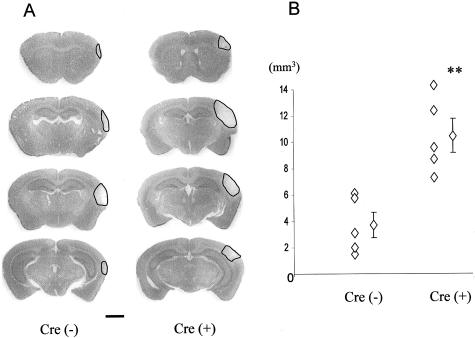
A: Representative micrographs of thionin-stained brain sections of Cre(−) and Cre(+) mice assessed 4 days after MCA occlusion. Black lines indicate infarct area. The Cre(+) brain shows a larger stroke area than the Cre(−) brain. B: The infarct volume was determined for Cre(−) and Cre(+) mice. Left line of diamonds are individual data and diamond with bar indicates average volume ± SEM. Cre(+) mice exhibited a significantly larger infarct volume compared to Cre(−) mice; **, P < 0.01. Scale bar, 1 mm.
Immunohistochemistry
Brain sections (7 μm thickness) were mounted on glass slides and were blocked with 10% goat serum, 1% bovine serum albumin, and 0.3% Tween 20 in PBS for 1 hour at room temperature. The sections were then incubated with β-gal (Promega), Cx43 (Sigma, St. Louis, MO), Cx30 (Zymed, South San Francisco, CA), Cx26 (Zymed), cleaved caspase-3 (Cell Signaling, Beverly, MA), CD11b, and glial fibrillary acidic protein (GFAP; Sigma) antibodies (dilution was 1:250, 1:1000, 1:500, 1:500, 1:100, 1:50, and 1:1000, respectively) in 1% bovine serum albumin and 0.3% Tween 20 in PBS. After washing in PBS, sections were reacted with appropriate secondary antibodies (Alexa Fluor; Molecular Probes Inc., Eugene, OR) in 1% bovine serum albumin and 0.3% Tween 20 in PBS for 1 hour. Counter staining of nuclei was visualized by Hoechst 33342 (Sigma). The sections were observed under a photomicroscope (Zeiss Axiophot).
Measurement of Immunoreactivity
In this study, we focused on the lesions of interest as the cortex within the range of 400 μm from the edge of the necrotic region. All of the following assessments were performed at the level of the brain sections shown in the third row of Figure 1A.
The cleaved caspase-3-immunoreactive cells were counted and averaged in four different randomly selected areas (300 μm × 300 μm square) from the penumbra in each mouse. Cx30-immunoreactive plaques were counted in four different randomly selected areas (300 μm × 300 μm square) from both the contralateral hemisphere cortex and the penumbral cortex in each mouse. The number of CD11b-immunoreactive cells was counted in three different ×200 microscopic fields, randomly selected from the penumbra in each mouse brain. Then, the numbers were statistically evaluated in each group.
Measurement of Astrogliosis
The area of astrogliosis on the cortex was determined between the edge of necrosis and the edge of astrogliosis where the reactive astrocytes were sparsely observed.26 The measurement was performed in three different randomly selected cortical lesions in each mouse. We have, however, estimated the total gliosis volume using the obtained data. The shape of lesion was assumed to be hemispherical. Then we used the formula, Gv = 2/3π[(3√3Sv/2π) + r]3; Gv is gliosis volume, Sv is infarct volume and r is the width of gliosis. The results were statistically averaged in each group.
Statistics
All data are presented as mean ± SEM values for each group of mice. To compare mean values in two separate groups, we used unpaired t-test. Values of P < 0.05 were considered to be significant.
Results
Reduction of Cx43 Protein Expression in Cre(+) Astrocytes
Cre(−) mice harbor two floxed Cx43 alleles without the hGFAP-cre transgene, so that their astrocytes continuously express Cx43. On the other hand, Cre(+) mice carry the floxed Cx43 alleles and the hGFAP-cre recombinase, resulting in loss of Cx43 in the cells that express hGFAP-cre and Cx43, ie, astrocytes.23 As expected, astrocytes in Cre(−) mice expressed Cx43 in their membrane and cytosol but Cre(+) brains showed strongly reduced Cx43 immunoreactivity, supporting the previous data that Cx43 expression was significantly decreased in Cre(+) astrocytes.23
Increased Stroke Volume in Cre(+) Mice
We measured the infarct volume at 4 days after MCA occlusion in Cre(−) and Cre(+) mice brains to determine whether there was any difference in ischemic tolerance. A larger stroke area was observed in the brains of Cre(+) mice compared to Cre(−) mice (Figure 1A). According to the calculation of the infarct volume, Cre(+) mice showed a threefold larger volume compared to Cre(−) mice (Figure 1B; 10.47 ± 1.28 mm3 and 3.69 ± 0.96 mm3, respectively).
Enhanced Apoptosis in the Penumbra of Cre(+) Mice
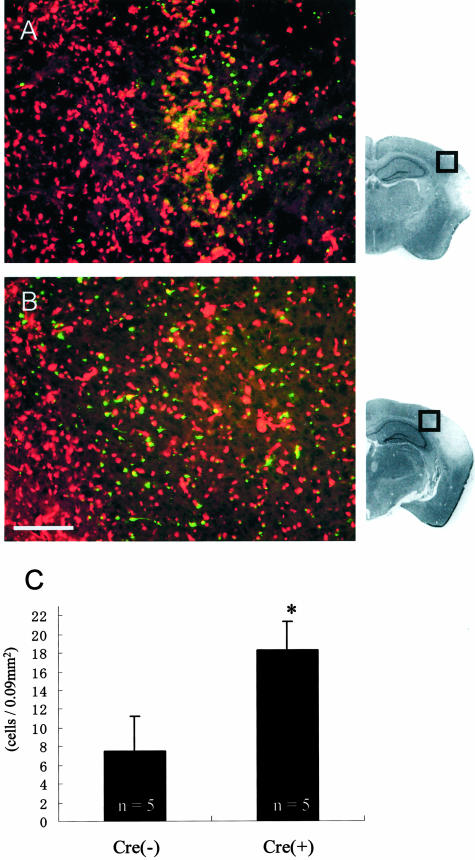
Apoptosis might contribute to delayed neuronal death, causing expansion of the stroke lesion.27 Our previous data showed an increased apoptotic reaction in Cx43(+/−) mice in which astrocytic gap junctional communication was decreased compared to wild-type mice.18 Representative cases demonstrate that the penumbral lesion of Cre(+) mice show enhanced apoptosis compared to Cre(−) mice (Figure 2, A and B). When measured quantitatively, the number of TUNEL-positive cells per unit area in Cre(+) mice was significantly increased compared to Cre(−) mice (Figure 2C; 18.25 ± 3.03 cells/0.09 mm2 and 7.50 ± 3.68 cells/0.09 mm2, respectively).
Figure 2.
TUNEL staining of the penumbral lesion [A: Cre(−) and B: Cre(+) mice]. TUNEL-positive cells appear in green fluorescence and all nuclei are visible with propidium iodide red fluorescence. Representative micrographs show enhanced apoptosis in Cre(+) compared to Cre(−) mice. C: In the cell-counting analysis, the number of apoptotic cells was significantly increased in Cre(+) mice compared to Cre(−) mice; *, P < 0.05. Scale bar, 100 μm. Outlined squares indicated on the micrographs of representative sections attached to A and B indicate the location of immunofluorescent panels, respectively.
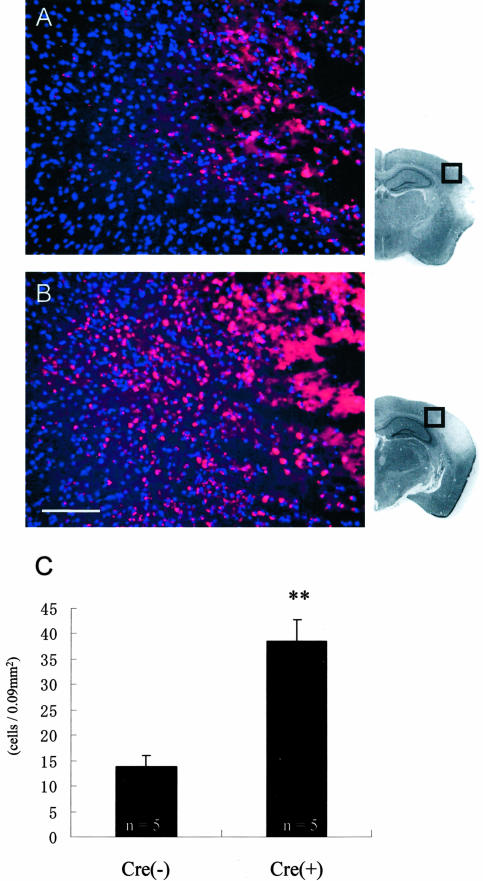
Caspase-3 is one of the key molecules of apoptosis, activated by proapoptotic stimuli such as initiator caspases and cytochrome C.28 Activated caspase-3 is detected as cleaved caspase-3. We observed cleaved caspase-3 immunoreactivity to further characterize the apoptotic response in the penumbra (Figure 3, A and B). Our results indicate that the lesion of Cre(+) mice exhibited increased caspase-3 immunoreactivity compared to that of Cre(−) mice. Quantitative analysis revealed that the caspase-3 immunoreactivity was significantly higher in Cre(+) compared to Cre(−) mice (Figure 3C; 38.5 ± 4.19 and 13.9 ± 2.19 cells/0.09 mm2, respectively), supporting the results of TUNEL staining.
Figure 3.
Representative micrographs of immunohistochemical staining with cleaved caspase-3 antibody in the penumbra of Cre(−) (A) and Cre(+) (B) mice. Caspase-3 immunostaining was seen as red fluorescence and all nuclei were visualized after blue staining with Hoechst 33342. The immunoreactivity of caspase-3 was enhanced in the Cre(+) penumbra compared to Cre(−). C: Quantitative analysis of the number of immunoreactive cells revealed a significant increase in Cre(+) than Cre(−)mice; **, P < 0.01. Scale bar, 100 μm. Outlined squares of representative brain sections attached to A and B indicate the location of immunofluorescent panels, respectively.
Astrogliosis in Cre(+) Mice
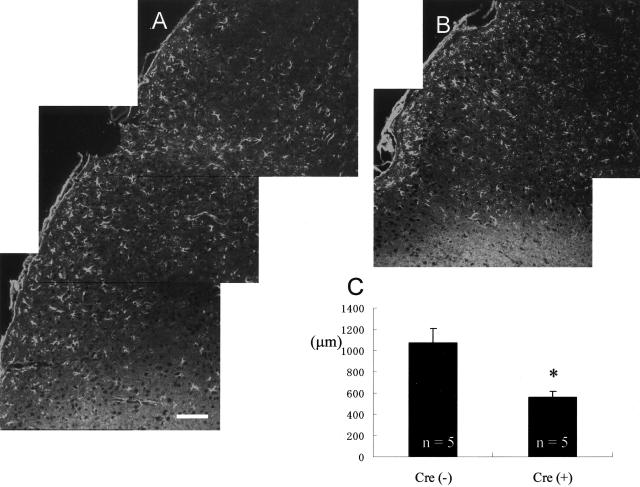
The lack of gap junctions influences the intercellular communication between astrocytes. Excitatory signals, such as Ca2+ and glutamate, can diffuse through gap junctions and regulate the neighboring astrocytic activation after ischemic injury.29,30 Therefore, we analyzed activated astrocytes to evaluate the reactive gliosis after ischemia. Results of our initial analysis of the area of astrogliosis in Cre(+) and Cre(−) brains are shown in Figure 4, A and B. As seen in these representative pictures, astrogliosis appears reduced in Cre(+) cerebral cortex compared to Cre(−) mice. The average width of the astrogliosis was significantly smaller in Cre(+) cortex compared to Cre(−) cortex (Figure 4C; 563.0 ± 55.9 μm and 1072.5 ± 134.0 μm, respectively). However, to determine whether astrogliosis is in fact reduced or if appears to change because of the ischemic core becoming larger in Cre(+) mice, we estimated the total gliosis volume. In this case we found the volume of astrogliosis for Cre(−) mice to be 25.29 mm3 and for Cre(+) to be 23.39 mm3. There was no statistical difference.
Figure 4.
The astrogliosis, detected by an increased level of GFAP immunoreactivity in astrocytes, was enhanced in the brain of Cre(−) compared to Cre(+) mice (A and B, respectively). The average width of the astrogliosis in each group is shown in C. The measured area is described in Materials and Methods section. The area of astrogliosis appears significantly smaller in Cre(+) compared to Cre(−) mice; *, P < 0.05. Scale bar, 100 μm.
Increased Expression Level of Cx30 in Cre(+) Mice
Cx30 and Cx26 are additional connexin proteins that have been shown to be expressed in astrocytic gap junctions.31–33 We determined the expression level of Cx30 and Cx26 in the astrocytes of Cre(−) and Cre(+) mice. We counted and averaged the number of Cx30-immunoreactive plaques in the cortex of contralateral hemisphere and the penumbral cortex of Cre(+) and Cre(−) mice. The number of Cx30-immunoreactive plaques was significantly increased both in the cortex of the contralateral hemisphere and in the penumbral cortex of Cre(+) mice as compared to the Cre(−) mice (281.4 ± 19.47 and 179.3 ± 14.04 counts/0.09 mm2 in contralateral hemisphere cortex and 282.3 ± 32.97 and 137.5 ± 14.89 counts/0.09 mm2 in penumbral cortex, respectively). The Cx26 protein level was not significantly changed both in intact cortex and penumbra (data not shown).
Amplified Inflammatory Response in Cre(+) Brain
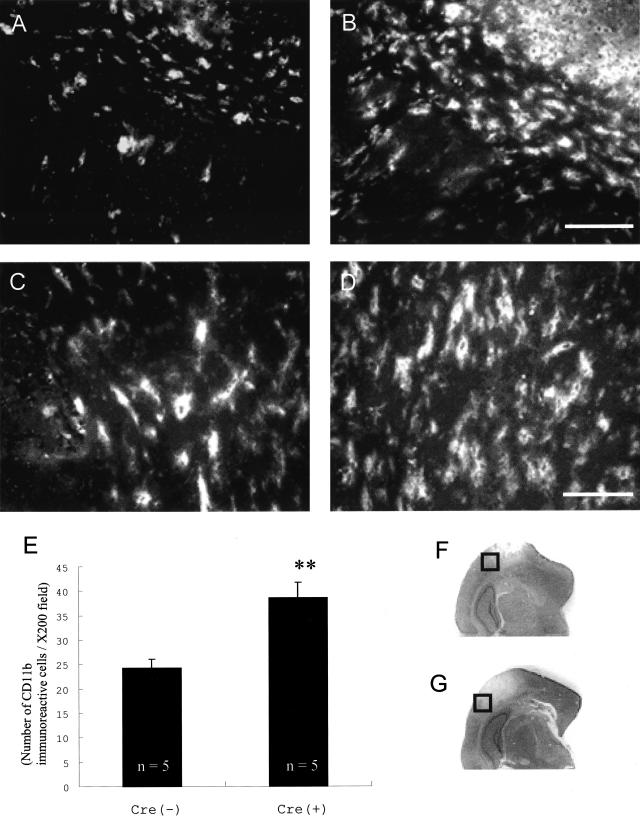
The activity of astrocytes can influence the inflammatory response. Therefore, we observed the inflammatory reaction in the penumbral lesion using anti-CD11b antibody that reacts with the 170-kd αM subunit of Mac-1, expressed on macrophages and microglia.34 Immunohistochemical investigation clearly demonstrated the amplified microglia or macrophage migration into the penumbral lesion in Cre(+) compared to Cre(−) mice (Figure 5; A to D). The number of CD11b-immunoreactive cells was counted and averaged in areas randomly selected from the penumbral lesion (Figure 5E). Cre(+) mice showed a significantly increased number of microglia and macrophages compared to Cre(−) mice (38.60 ± 9.80 and 24.29 ± 1.77 cells/×200 field, respectively).
Figure 5.
CD11b immunohistochemical staining of the penumbral lesion in Cre(−) and Cre(+) mice (A and B, respectively) and high magnifications of the external capsule in Cre(−) and Cre(+) mice (C and D, respectively). Cre(+) mice showed amplified microglial migration into the penumbral lesion. F and G indicate the location of immunofluorescent panels A and B, respectively. E: The number of CD11b-immunoreactive cells in the penumbra was significantly increased in Cre(+) mice; **, P < 0.01. Scale bars, 100 μm (A and B); 50 μm (C and D).
Discussion
Our results clearly demonstrate that astrocytic Cx43 plays an important role in reducing brain damage after ischemic stroke. Cre(+) mice, in which astrocytes were devoid of Cx43, exhibit significantly larger stroke volumes and amplified apoptosis compared to Cre(−) mice that maintain Cx43 expression. This finding supports our previous findings that decreased Cx43 expression amplifies and extends neuronal apoptosis after focal stroke.18 In addition, an amplified inflammatory response occurs in Cre(+) mice as compared to the Cre(−) mice. These results suggest that apoptosis and inflammation may be enhanced presumably because of diminished astrocytic gap junction coupling in Cre(+) mice, resulting in larger ischemic stroke volume.
The Cre-recombinase, whose expression is directed by an hGFAP promoter region, recognizes loxP sites, and catalyzes the removal of the floxed Cx43 coding region in Cre(+) mice. Therefore, the expression of Cx43 is lost only in the cells that express GFAP, ie, astrocytes and ependymal cells as opposed to leptomeningeal cells and cells of the vasculature.23 Although Zhuo and colleagues25 reported that hGFAP-cre mediates deletion of floxed DNA in neurons and in oligodendrocytes as well, no β-galactosidase, indicative of Cx43 inactivation, was observed in the neuronal marker-positive cells of the mice used in the current studies.23 Because neurons and oligodendrocytes have not been shown to express Cx43,35,36 hGFAP-cre-mediated deletion of Cx43 coding DNA is not relevant in those cell types. Therefore, the GJIC is compromised exclusively between astrocytes in Cre(+) mice.23 Previously, we performed focal brain ischemia on Cx43(+/−) mice17,18 rather than Cx43(−/−) mice, because Cx43(−/−) mice die perinatally.14 In Cx43(+/−) mice, although astrocytes show diminished Cx43 expression with reduced GJIC,17 the reduced expression of Cx43 is not restricted to astrocytes. Therefore, this deficiency of Cx43 in other cell types may have contributed to the observed findings. In the current study, the Cre(+) mice specifically have deficient GJIC in the astrocytes, leaving communication between other cell types unaltered.
The observation that heterozygous null mice18 and Cre(+) mice had a similar stroke volume was puzzling. The finding that wild-type mice18 had a larger stroke volume compared to Cre(−) mice was even more surprising, because Cre(−) mice carry a floxed Cx43 gene and show Cx43 expression decreased by 50% in cerebrum (M.T., unpublished data) as well as in cerebellum.23 CD-1 mice were used as subjects in the Cx43(+/−) study whereas the Cre(+) and Cre(−) mice have a C57BL/6 background. We observed that C57BL/6 wild-type mice exhibited the same infarct volume as that of Cre(−) mice (unpublished data), which also had a genetic background composed mainly of C57BL/6, suggesting that the CD-1 mice, on reduction of wild-type Cx43 levels by 50%, may be more susceptible to ischemic insult as compared to C57BL/6 mice. Moreover, previous reports have demonstrated a significantly different stroke volume between different strains of wild-type mice, and variations of vascular anatomy or intrinsic factors yet to be determined were assumed to contribute to these differences.37,38
Brain injury was assessed at 4 days after stroke. After ischemic insults, focal pan-necrosis occurs immediately39 and apoptosis follows in 1 to 2 days.27 Inflammation and gliosis reach a maximum at 3 to 5 days after stroke.40 Therefore, it is reasonable to evaluate the results of apoptosis and the maximal inflammatory response at 4 days after stroke. In fact, we reported that a significant difference in apoptosis between Cx43(+/+) and Cx43(+/−) mice was observed at 4 days after MCA occlusion although there was no difference at 1 day after stroke.18
In general, the penumbral lesion is defined as the region in which cerebral blood flow decreases to 20 to 40% of the normal condition and the tissue is electrically silent, but the possibility of improvement remains.41 Moreover, spreading depression that causes an increase in GFAP expression in astrocytes42 is considered a deterious factor in this lesion.41 Because our observations were focused on the alteration of astrocytes in which gap junctions were already compromised, we could not use traditional determinants of the penumbral lesion, so that we selected areas closely adjacent to the ischemic core for analysis.
Apoptosis plays a critical role in delayed neuronal death after cerebral ischemia. In general, apoptosis becomes maximal from 24 to 48 hours after an ischemic insult.27 In this study, intense apoptosis characterized by TUNEL labeling and cleaved caspase-3 immunohistochemical staining was still observed in the penumbra of Cre(+) mice at 4 days after MCA occlusion. Moreover, we observed an elevated apoptosis and caspase-3 activation only in the area of astrogliosis in both Cre(−) and Cre(+) mice. After an ischemic insult, astrocytic gap junctions in the core become internalized, while they remains intact at the penumbra.43 The authors of this latter study suggested that the gap junction-facilitated removal of cytotoxic substances from the penumbra counteracts an expansion of the ischemic lesion. Taken together, we can further conclude that astrocytic gap junctions composed of Cx43 may play a critical role in spatially buffering the apoptotic initiators and cytotoxic factors away from the penumbral lesion.
It is also critical to maintain the energy resources for the survival of cells in the penumbra because blood supply is dramatically reduced under ischemia. Recently, Zonta and colleagues44 reported that astrocytes can regulate arteriolar dilation by Ca2+ oscillation. Ca2+ waves can pass through gap junctions between astrocytes. Therefore, arterioles may not dilate in the Cre(+) penumbra because astrocytic gap junctions were compromised, resulting in diminished blood supply in the lesion.
We also evaluated the reactivity of astrocytes after MCA occlusion. Activated astrocytes dramatically increase the expression of GFAP.45 Using this criteria, we observed the activated astrocytes by immunostaining the tissue with a minimal amount of antibody against GFAP.26 We found that the area of astrogliosis was significantly smaller in the penumbra of Cre(+) mice as compared to Cre(−) mice. Meanwhile, we observed that inflammation was enhanced in the Cre(+) lesion while activated microglia and macrophages were present within the gliosis lesion in both Cre(−) and Cre(+) mice. In general, an ischemic insult in the central nervous system causes massive inflammation as well as gliosis. Activated astrocytes produce cytokines, such as interleukin-1, interleukin-6, interferon-γ, and tumor necrosis factor-α46 and interact with inflammatory cells. Therefore, altered astrocytic gap junctions may affect astrocytic activation, causing a stronger inflammatory response. In this context, the apparently smaller gliosis observed in Cre(+) mice may suggest that the ischemic core expanded more widely into the gliosis lesion in Cre(+) than in Cre(−) mice. In fact, we found no statistical difference in the volume of gliosis comparing Cre(+) and Cre(−) mice. Thus, the apparently smaller gliosis observed in Cre(+) mice could result from the larger infarct volume. However, it has been reported that ischemic tolerance causes a stronger gliosis and milder inflammation after a second ischemia as compared to a nonischemic tolerance group.47 Further investigation may be needed to determine whether there is a smaller astrogliosis in the stroke lesion of Cx43-compromised animals.
Cx30 is another connexin expressed by astrocytes in the mature central nervous system31 and has been reported to be up-regulated twofold in Cre(+) mice.23 We also observed that the expression level of Cx30 was increased twofold in the cortex of the contralateral hemisphere. Cx30 was up-regulated to a similar extent in the penumbral cortex of Cre(+) mice, suggesting that the expression level of Cx30 was not further increased even under the ischemic stress. In previous experiments with heterozygous Cx43 null mice, we observed an up-regulation of Cx30 specifically in the lesioned area.18 Differences between the two studies might be because of selective ablation of Cx43 in astrocytes as opposed to decreased Cx43 expression in multiple cell types or because of the fact that Cx30 is already maximally up-regulated in mice lacking astrocytic Cx43. The issue of connexin up-regulation is complex: Cx43 expression has been reported to increase responding to the surrounding area of necrosis after ischemic insult.48 Different connexins may have different functions under normal and pathological conditions.35 Thus, the gap junctional function served by Cx30 may differ from that of Cx43 in an ischemic insult.
Cx43 gap junction hemichannel gating is also affected under pathological conditions. Contreras and colleagues49 reported that inhibition of glycolytic and oxidative metabolism induces Cx43 hemichannels to open while reducing GJIC between astrocytes. Astrocytic gap junction hemichannels may take up metabolic substances from the extracellular space and contribute to the maintenance of neuronal survival under ischemic conditions. Therefore, impediment or reduction of astrocytic gap junction hemichannels may decrease astrocytic neuroprotection.
Thus far, the neuroprotective role of astrocytic gap junctions is still controversial. Some reports note that astrocytic gap junctions spread the Ca2+ wave after ischemic insult causing the expansion of stroke volume,7,8 suggesting that the astrocytic gap junctions play a deleterious role in ischemic brain damage. In contrast, several reports have shown evidence for a neuroprotective role of astrocytic gap junctions. For example, astrocytic GJIC decreases neuronal vulnerability to oxidative stress10 and inhibition of gap junctions in astrocyte-neuron co-cultures increases neuronal death induced by glutamate cytotoxicity.11 Our findings that the compromised astrocytic gap junctions affect gliosis and inflammation after ischemia could illuminate an additional aspect to the neuroprotective role of astrocytic gap junctions.
In conclusion, Cre(+) mice showed a severe brain stroke lesion with amplified apoptosis and inflammation compared to Cre(−) mice. Because GJIC is specifically compromised in astrocytes of Cre(+) mice, astrocytes may not be able to remove cytotoxic substances and apoptosis-initiating factors from the ischemic core lesion through gap junctions. Moreover, an enhanced inflammatory response was observed in the Cre(+) mice penumbra, suggesting that GJIC between astrocytes may be important in limiting the inflammatory response after ischemic insults. The appropriate regulation of astrocytic gap junctions during ischemia may be a critical factor to enhance the neuroprotective role of astrocytes in stroke.
Footnotes
Address reprint requests to Dr. Christian C.G. Naus, Department of Anatomy and Cell Biology, the University of British Columbia, Vancouver, BC V6T 1Z3. E-mail: cnaus@interchange.ubc.ca.
Supported by the Canadian Institutes of Health Research (to C.C.G.N.); the Heart and Stroke Foundation of Canada (to C.C.G.N.); the Canadian Stroke Network (to C.C.G.N.); the Joint Fellowship of Canadian Institutes of Health Research/Institutes of Neuroscience, Mental Health, and Addiction (to T.N.); the Japan-Canada Program (to T.N.); the Graduiertenkolleg “Pathogenese von Krankheiten des Nervensystems” (to M.T.); and the German Research Association (SFB 400, E3) to the work in the Bonn laboratory.
C.C.G.N. holds a Canada Research Chair in Gap Junctions and Disease.
Present address of M.T.: Howard Hughes Medical Institute, Center for Neurobiology and Behavior, Columbia University, 1051 Riverside Dr., New York, NY 10032.
References
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1997;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Kajihara H, Tsutsumi E, Kinoshita A, Nakano J, Takagi K, Takeo S. Activated astrocytes with glycogen accumulation in ischemic penumbra during the early stage of brain infarction: immunohistochemical and electron microscopic studies. Brain Res. 2001;909:92–101. doi: 10.1016/s0006-8993(01)02640-3. [DOI] [PubMed] [Google Scholar]
- Hansson E, Muyderman H, Leonova J, Allansson L, Sinclair J, Blomstrand F, Thorlin T, Nilsson M, Rönnbäck L. Astroglia and glutamate in physiology and pathology: aspects on glutamate transport, glutamate-induced cell swelling and gap-junction communication. Neurochem Int. 2000;37:317–329. doi: 10.1016/s0197-0186(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Kang J, Lin JH, Bueno E, Hansen TW, He L, Liu Y, Nedergaard M. Astrocytic gap junctions remain open during ischemic conditions. J Neurosci. 1998;18:2520–2537. doi: 10.1523/JNEUROSCI.18-07-02520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawanduzy A, Hansen A, Hansen TW, Nedergaard M. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. J Neurosurg. 1997;87:916–920. doi: 10.3171/jns.1997.87.6.0916. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Kokarovtseva L, Perez Velazquez JL. Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab. 2002;22:453–462. doi: 10.1097/00004647-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Largo C, Cuevas P, Herreras O. Is glia disfunction the initial cause of neuronal death in ischemic penumbra? Neurol Res. 1996;18:445–448. doi: 10.1080/01616412.1996.11740449. [DOI] [PubMed] [Google Scholar]
- Blanc EM, Bruce-Keller AJ, Mattson MP. Astrocytic gap junctional communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca2+ homeostasis and cell death. J Neurochem. 1998;70:958–970. doi: 10.1046/j.1471-4159.1998.70030958.x. [DOI] [PubMed] [Google Scholar]
- Ozog MA, Siushansian R, Naus CCG. Blocking gap junctions increases glutamate cytotoxicity. J Neuropathol Exp Neurol. 2002;61:132–141. doi: 10.1093/jnen/61.2.132. [DOI] [PubMed] [Google Scholar]
- Naus CCG, Bechberger JF, Caveney S, Wilson JX. Expression of gap junction genes in astrocytes and C6 glioma cells. Neurosci Lett. 1991;126:33–36. doi: 10.1016/0304-3940(91)90364-y. [DOI] [PubMed] [Google Scholar]
- Giaume C, Fromaget C, el Aoumari A, Cordier J, Glowinski J, Gros D. Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron. 1991;6:133–143. doi: 10.1016/0896-6273(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Reaume AG, De Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Naus CCG, Bechberger JF, Zhang YC, Venance L, Yamasaki H, Juneja SC, Kidder GM, Giaume C. Altered gap junctional communication, intercellular signaling and growth in cultured astrocytes deficient in connexin43. J Neurosci Res. 1997;49:528–540. doi: 10.1002/(SICI)1097-4547(19970901)49:5<528::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Scemes E, Dermietzel R, Spray DC. Calcium waves between astrocytes from cx43 knockout mice. Glia. 1998;24:65–73. doi: 10.1002/(sici)1098-1136(199809)24:1<65::aid-glia7>3.0.co;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siushansian R, Bechberger JF, Cechetto DF, Hachinski VC, Naus CCG. Connexin43 null mutation increases infarct size after stroke. J Comp Neurol. 2001;440:387–394. doi: 10.1002/cne.1392. [DOI] [PubMed] [Google Scholar]
- Nakase T, Fushiki S, Naus CCG. Astrocytic gap junctions composed by connexin43 reduce apoptotic neuronal damage in cerebral ischemia. Stroke. 2003;34:1987–1993. doi: 10.1161/01.STR.0000079814.72027.34. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Dobbertin A, Schmid P, Gelman M, Glowinski J, Mallat M. Neurons promote macrophage proliferation by producing transforming growth factor-b2. J Neurosci. 1997;17:5305–5315. doi: 10.1523/JNEUROSCI.17-14-05305.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roauch N, Glowinski CFC, Giaume C. Brain macrophages inhibit gap junctional communication and downregulate connexin 43 expression in cultured astrocytes. Eur J Neurosci. 2002;15:403–407. doi: 10.1046/j.0953-816x.2001.01868.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Eckardt D, Theis M, Willecke K, Bennett MVL, Saez JC. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-γ and tumor necrosis factor-a. Proc Natl Acad Sci USA. 2001;98:4190–4195. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Frisch C, Söhl G, Teubner B, Euwens C, Huston J, Steinhauser C, Messing A, Heinemann U, Willecke K. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci. 2003;23:766–776. doi: 10.1523/JNEUROSCI.23-03-00766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, de Wit C, Schlaeger TM, Eckardt D, Krüger O, Döring B, Risau W, Deutsch U, Pohl U, Willecke K. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis. 2001;29:1–13. doi: 10.1002/1526-968x(200101)29:1<1::aid-gene1000>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- Fernaud-Espinosa I, Nieto-Sampedro M, Bovolenta P. Differential activation of microglia and astrocytes in aniso- and isomorphic gliotic tissue. Glia. 1993;8:277–291. doi: 10.1002/glia.440080408. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portere-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Budinhardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- Giaume C, Tabernero A, Medina JM. Metabolic trafficking through astrocytic gap junctions. Glia. 1997;21:114–123. [PubMed] [Google Scholar]
- Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- Nagy JI, Patel D, Ochalski PAY, Stelmack GJ. Connexin 30 in rodent, cat and human brain: selective expression in grey matter astrocytes, co-localization with connexin 43 at gap junctions and late developmental appearance. Neuroscience. 1999;88:447–468. doi: 10.1016/s0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE. Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. J Comp Neurol. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MVL, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- Roauch N, Avignone E, Même W, Koulakoff A, Venance L, Blomstrand F, Giaume C. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Theis M, Söhl G, Speidel D, Kühn R, Willecke K. Connexin43 is not expressed in principal cells of mouse cortex and hippocampus. Eur J Neurosci. 2003;18:267–274. doi: 10.1046/j.1460-9568.2003.02740.x. [DOI] [PubMed] [Google Scholar]
- Maeda K, Hata R, Hossmann KA. Regional metabolic disturbances and cerebrovascular anatomy after permanent middle cerebral artery occlusion in C57Black/6 and SV129 mice. Neurobiol Dis. 1999;6:101–108. doi: 10.1006/nbdi.1998.0235. [DOI] [PubMed] [Google Scholar]
- Majid A, He YY, Gidday JM, Kaplan SS, Gonzales ER, Park TS, Fenstermacher JD, Wei L, Choi DW, Hsu CY. Differences in vulnerability to permanent focal cerebral ischemia among 3 common mouse strains. Stroke. 2000;31:2707–2714. doi: 10.1161/01.str.31.11.2707. [DOI] [PubMed] [Google Scholar]
- Clark RK, Lee EV, Fish CJ, White RF, Price WJ, Jonak ZL, Feuerstein GZ, Barone FC. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study. Brain Res Bull. 1993;31:565–572. doi: 10.1016/0361-9230(93)90124-t. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann NY Acad Sci. 1997;825:179–193. doi: 10.1111/j.1749-6632.1997.tb48428.x. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP. The ischemic penumbra: twenty years on. Cerebrovasc Brain Metab Rev. 1995;7:297–323. [PubMed] [Google Scholar]
- Kraig RP, Dong L, Thisted R, Jaeger CB. Spreading depression increases immunohistochemical staining of glial fibrillary acidic protein. J Neurosci. 1991;11:2187–2198. doi: 10.1523/JNEUROSCI.11-07-02187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WE, Ochalski PA, Hertzberg EL, Nagy JI. Immunorecognition, ultrastructure and phosphorylation status of astrocytic gap junctions and connexin43 in rat brain after cerebral focal ischaemia. Eur J Neurosci. 1998;10:2444–2463. doi: 10.1046/j.1460-9568.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann K-A, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia. 2000;31:95–103. doi: 10.1002/1098-1136(200008)31:2<95::aid-glia10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- Kato H, Kogure K, Araki T, Itoyama Y. Astroglial and microglial reactions in the gerbil hippocampus with induced ischemic tolerance. Brain Res. 1994;664:69–76. doi: 10.1016/0006-8993(94)91955-0. [DOI] [PubMed] [Google Scholar]
- Hossain MZ, Peeling J, Sutherland GR, Hertzberg EL, Nagy JI. Ischemia-induced cellular redistribution of the astrocytic gap junctional protein connexin43 in rat brain. Brain Res. 1994;652:311–322. doi: 10.1016/0006-8993(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Mennett MV, Sáez JC. Metabolic inhibition induces opening of unapposed connexin43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]