Abstract
Increased expression of hyaluronan (HA) has been associated with both acute renal injury and progressive renal disease, although the functional significance of this remains unclear. There is overwhelming evidence that transforming growth factor (TGF)-β1 is critical to the development of progressive renal disease. Recent studies suggest an interaction between HA and TGF-β signaling in cancer cell biology. The aim of this study was to examine the potential role of HA as a modulator of TGF-β1 function in renal proximal tubular epithelial cells (PTC). Under resting conditions, co-localization of the principal receptor for HA, CD44, and both the TGF-β type I and type II receptors was demonstrated by immunoprecipitation and western analysis and further confirmed by immunocytochemistry and confocal microscopy. Stimulation of PTC with TGF-β1 led to increased synthesis of both type III and type IV collagen assessed by Western analysis. Addition of HA did not alter collagen synthesis, but abrogated TGF-β1-mediated increase in type III and type IV collagen. This effect was blocked by the addition of a blocking antibody to CD44 and also by inhibition of MAP kinase kinase (MEK) activity. Furthermore HA decreased TGF-β1 activation of a luciferase-SMAD responsive construct, and decreased translocation of SMAD4 into the cell nucleus. We have previously demonstrated an anti-migratory effect of TGF-β1 in a scratch wounding model. As with HA antagonism of TGF-β1 extracellular matrix generation, HA reduced the anti-migratory effect of TGF-β1 in a CD44-dependent manner. In contrast to the effect of TGF-β1 on collagen synthesis, which is SMAD-dependent, the anti-migratory effect of TGF-β1 in this model is known to be dependent of activation of RhoA. In the presence of HA, TGF-β1-mediated activation of RhoA was also abrogated in a CD44-dependent manner. The results suggest that co-localization of CD44 and TGF-β receptors facilitate modulation of both SMAD and non-SMAD-dependent TGF-β1-mediated events by HA. Our results therefore suggest that alteration of HA synthesis may represent an endogenous mechanism to limit renal injury.
Progression of renal disease is known to correlate with the degree of renal interstitial fibrosis, and much interest has focused on the role of the renal proximal tubular epithelial cell (PTC) in its pathogenesis. PTC may contribute to the pathogenesis of renal fibrosis directly by alterations in the production of components of extracellular matrix (ECM), and indirectly by the production of pro-fibrotic cytokines.1–5
Transforming growth factor-β1 (TGF-β1), which is the prototypic member of the TGF-β superfamily, exerts a broad range of biological activities. It plays pivotal roles during embryonic development where it is involved in induction of cell differentiation and organogenesis. TGF-β1 has been implicated in the pathogenesis of renal fibrosis in both experimental and human disease.6–10 A major function of TGF-β1 is to regulate the expression of genes, the products of which contribute to the formation and degradation of ECM.11–15 Generally, TGF-β1 leads to the accumulation of ECM by decreasing the synthesis of proteases and by increasing the levels of protease inhibitors.16 It also increases the expression of integrins through which ECM proteins such as fibronectin and collagen interact with cells.17,18 In vitro studies also suggest that TGF-β1 induces phenotypic alterations in PTC using intermediate filament markers and reorganization of the cytoskeleton with cells as indicators of a “fibroblastic” phenotype.19 Studies using normal rat PTC also suggest that TGF-β1 is a key mediator regulating differentiation of PTC into α-SMA positive cells.20 Not only is there strong evidence that TGF-β1 is a key mediator of progressive renal fibrosis, but attenuation of its action has been postulated to be a target for therapeutic intervention in numerous disease models.7,8,21,22 Understanding the mechanisms, which regulate TGF-β1-dependent responses, is therefore an important goal.
Hyaluronan (HA) is an ubiquitous connective tissue polysaccharide which in vivo is present as a high molecular mass component of ECM. In the normal kidney HA is expressed in the interstitium of the renal papilla only, and alteration in papillary interstitial HA has been implicated in regulating renal water handling by affecting physiochemical characteristics of the papillary interstitial matrix and influencing the interstitial hydrostatic pressure.23 Although HA is not a major constituent of the normal renal corticointerstitium, it is known to be expressed around PTC following renal injury caused by diverse diseases.24–27 Increased deposition of interstitial HA has also been correlated with renal function in progressive renal disease associated with IgA nephropathy.28 A recent study suggest that HA promotes the signaling interaction between the principal cell surface receptor for HA, CD44, and the TGF-β type I receptor in metastatic breast tumor cells. The functional significance of alterations in the expression of HA associated with renal injury, however, is not clear. The aim of the current study was to define the relationship between CD44 and TGF-β type I receptor and the potential modulating influence of HA on TGF-β1-dependent signaling and function in PTC.
Materials and Methods
Materials
Antibodies for Western blot analysis, immunoprecipitation, and immunocytochemistry and the final working dilution were as follows.
For Western blot analysis and immunoprecipitation, rat polyclonal anti-CD44 antibody (dilution 1:1000) was from Calbiochem (Nottingham, UK); rabbit polyclonal anti-TGFβ type I receptor antibody (dilution 1:500) was from Santa Cruz Biotechnology (Wiltshire, UK); rabbit polyclonal anti-TGFβ type II receptor antibody (dilution 1:500) was from Santa Cruz Biotechnology; goat polyclonal anti-type III collagen antibody (dilution 1:250) was from Chemicon International (Temecula, CA); rabbit polyclonal anti-type IV collagen antibody (dilution 1:500) was from ICN Pharmaceuticals (Basingstoke, UK); Rhotekin Rho-binding domain (RBD; recombinant protein expressed in Escherichia coli) was from Upstate Biotechnology (Buckinghamshire, UK); mouse monoclonal anti-RhoA antibody (dilution 1:400) was from Santa Cruz Biotechnology. All HRP conjugated secondary antibodies (1:10,000) were from Sigma (Poole, UK).
For immunocytochemistry, rat polyclonal anti-CD44 antibody (dilution 1:100) was from Calbiochem; rabbit polyclonal anti-TGFβ type I receptor antibody (dilution 1:100) was from Santa Cruz Biotechnology; rabbit polyclonal anti-Smad4 antibody (dilution 1:100) was from Santa Cruz Biotechnology. All FITC and TRITC conjugated secondary antibodies (dilution 1:100) were from Sigma.
Other reagents used were: recombinant TGF-β from R&D Systems (Oxford, UK); mouse monoclonal anti-CD44 blocking antibody from Ancell (Bayport, MN); MAP kinase kinase (MEK) inhibitor PD98059 from Calbiochem. Hyaluronan (Lot No. F1750762) in the form of freeze-dried white powder was kindly provided by Denki Kagaku Kogyo K. K., Japan.
Cell Culture
HK-2 cells (human renal proximal tubular epithelial cells immortalized by transduction with human papilloma virus (HPV) 16 E6/E7 genes29) were cultured in DMEM/Ham’s F12 (Gibco BRL, Paisley, UK) supplemented with 10% FCS (Biological Industries Ltd, Cumbernauld, UK), 2 μmol/L l-glutamine (Gibco BRL), 20 mmol/L HEPES buffer (Gibco BRL), 5 μg/ml insulin, 5 μg/ml transferring (Sigma), 40 ng/ml hydrocortisone (Sigma), and 5 ng/ml sodium selenite (Sigma). Cells were grown at 37°C in 5% CO2 and 95% air. Fresh growth medium was added to cells every 3 to 4 days until confluent. Cells were grown to confluence and serum deprived for 48 hours before experimental manipulation. In all experiments cells were stimulated with either recombinant TGF-β1 and/or HA, under serum-free conditions.
In all aspects of cell biology that we have studied previously, HK-2 cells respond in an identical fashion to primary cultures of human proximal tubular cells.30–33 They are therefore a good model from which general conclusions can be drawn in terms of proximal tubular cell biology.
Immunoblotting/Western Analysis
Briefly confluent monolayers were washed once with cold PBS (Gibco BRL), scraped, and rinsed into 5 ml of cold PBS. After centrifugation at 2500 rpm for 10 minutes, cell pellets were extracted in buffer (150 mmol/L NaCl, 50 mmol/L Tris-Cl, 0.01% NaN3, 2 mmol/L EDTA, 1 mmol/L sodium orthovanadate, 10 μg/ml leupeptin, 25 μg/ml aprotinin) containing 1% Triton X-100 for 30 minutes on ice. Samples were centrifuged at 12,500 rpm for 30 minutes and then the supernatant (Triton-soluble components including membrane and cytosolic fraction) was transferred to a separate tube and kept at −70°C until use.
Analysis of type III and IV collagen in the culture supernatant was performed by standard methodologies. Briefly, equal amounts of culture supernatant were prepared in SDS sample buffer (2% SDS, 10% v/v glycerol, 60 mmol/L Tris, and 0.05% v/v mercaptoethanol) and boiled for 5 minutes before loading onto 10% SDS-PAGE gels. Electrophoresis was carried out under reducing conditions according to the procedure of Laemmli.34 After electrophoresis the separated proteins were transferred to a nitrocellulose membrane (Amersham Pharmacia, Biotech UK Ltd, Buckinghamshire, UK). The membrane was blocked with Tris-buffered saline containing 5% nonfat powdered milk for 1 hour and then incubated with the primary antibody in Tris-buffered saline containing 1% bovine serum albumin and 0.1% Tween 20 (Tris-buffered saline-Tween) overnight at 4°C. The blots were subsequently washed in Tris-buffered saline-Tween and then incubated with an appropriate HRP-conjugated secondary antibody (Sigma) in Tris-buffered saline-Tween. Proteins were visualized using enhanced chemiluminescence (Amersham) according to the manufacturer’s instructions.
Immunoprecipitation
Immunoprecipitation was performed by standard methodologies. Briefly, cell protein samples (200 μg) were pre-cleared with 25 μl of packed protein A cross-linked 4% beaded agarose (Sigma) at 4°C overnight. The beads were removed by centrifugation (13,000 rpm, 10 minutes) and the supernatant collected. Primary antibody (2 μg/ml) was added to the cleared supernatant and incubated at 4°C with constant mixing for 4 hours. The immune complex was captured by the addition of packed agarose protein A beads (50 μl) overnight at 4°C. Beads were washed with RIPA buffer (50 mmol/L Tris, 150 mmol/L NaCl, 0.5% sodium deoxycholate, 10 mmol/L MgCl2, 0.1% SDS, 1% Triton X-100), 30 μl of sample buffer was then added before boiling for 5 minutes. Separation of the beads was achieved by centrifugation (13,000 × g for 10 minutes) and the supernatant removed. Subsequently samples were subject to immunoblot/Western analysis as described above. Specificity of immunoprecipitation was confirmed by negative control reactions performed with either no primary antibody, or IgG control.
Assessment of RhoA Activation
Briefly, following addition of TGF-β1 (10 ng/ml), HA (25 μg/ml), or the combination for up to 2 hours, cell monolayer was rinsed twice with ice-cold Tris-buffered saline (TBS). Cell lysate was obtained by adding Mg2+ buffer (25 mmol/L HEPES, pH 7.5, 150 mmol/L NaCl, 1% Igepal CA-630, 10 mmol/L MgCl2, 1 mmol/L EDTA and 10% glycerol) onto cell monolayer and scraping with cell scraper, followed by centrifugation at 14,000 × g for 5 minutes at 4°C. Supernatant was transferred to a microfuge tube and further incubated with 30 μg of rhotekin RBD-agarose slurry for 45 minutes at 4°C. Agarose beads were then pelleted by brief centrifugation, washed with Mg2+ buffer three times, and subjected to western blot analysis as described above. Activated GTP-RhoA was detected by immunoblotting with specific RhoA antibody.
Immunocytochemistry
Cells were grown in eight-well multichamber slides (Gibco BRL) under serum-free media for 48 hours and then stimulated with either recombinant TGF-β1 (10 ng/ml), HA (25 μg/ml) or the combination of both stimuli. At each time point, cells were rinsed three times in PBS for 5 minutes each, before fixation in 3% paraformaldehyde for 15 minutes at room temperature and subsequent permeabilized with 0.1% Triton in PBS for 5 minutes at room temperature. Following a blocking step (1% BSA/PBS for 1 hour), cells were incubated with the primary antibodies overnight at 4°C, followed by the incubation of FITC-conjugated and/or TRITC-conjugated secondary antibodies. After washing with PBS, cells were mounted with fluorSave reagent (Calbiochem) and analyzed by confocal microscope (Leica TCS 40, Leica Microsystems, Cambridgeshire, UK).
Transient Transfection
The SMAD-responsive promoter (SBE)4-Lux was a gift from Aristidis Moustakas (Ludwig Institute for Cancer Research, Uppsala, Sweden).35 For transfection of the reporter construct, 80 × 103 cells/well were seeded onto a 12-well plate (this density of cells produced a 80% confluence monolayer the following day). The next day cells were transfected with 0.5 μg of the SMAD responsive promoter-luciferase construct, using the mixed lipofection reagent FuGene 6 (Roche, Lewes, UK) at a ratio of 1.5 μl of FuGene to 0.5 μg of DNA in serum-free and insulin-free medium. Transfection efficiency was monitored by co-transfection with a β-galactosidase reporter plasmid. 24 hours after transfection, cells were stimulated with either TGF-β1, or the combination of TGF-β1 and HA. Following lysis of the cells in Reporter Lysis Buffer (Promega, WI) luciferase content was quantified by glow-type luminescence assay (Bright-Glo, Promega), and β-galactosidase activity determined by commercial assay (Promega). Luciferase activity was normalized to β-galactosidase activity.
Migration
Cell migration was examined using a monolayer wounding system as previously described.36 Briefly quiescent cell monolayers were injured by scraping with a sterile 1-ml pipette tip to generate an intersecting area of denuded cells. The monolayer was washed twice with PBS and then incubated with serum-free medium or serum-free medium to which either TGF-β1 or the combination of both TGF-β1 and HA were added. Closure of the denuded area was monitored using an Axiovert 100 mol/L inverted microscope fitted with a digital camera (ORCA-1394, Hamamatsu Photonics, Hamamatsu, Japan), and images of the wounded area captured as a digitized sequence. The rate of motility of cells was calculated as the number of cells entering the central denuded area. Cell number was expressed as cells per mm2 of original denuded area.
Results
Characterization of the Association of CD44 and TGF-β Receptor Expression
The relationship of CD44 to TGF-β type I receptors was examined by immunoprecipitation of CD44 followed by analysis of TGF-β type I receptor by immunoblot and also by immunoprecipitation of the TGF-β type I receptor before immunoblot analysis of CD44. We have previously demonstrated the expression of numerous splice variants of CD44,37 for these experiments we therefore used a polyclonal antibody directed against the common region of CD44.
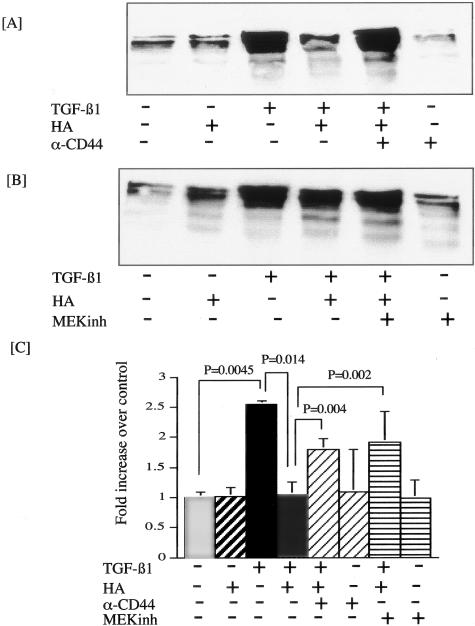
Immunoprecipitation of the TGF-β type I receptor resulted in isolation of both CD44 and the TGF-β type I receptor (Figure 1A). Similarly, immunoprecipitation of CD44 resulted in isolation of both CD44 and the TGF-β type I receptor in unstimulated cells (Figure 1A). Addition of either TGF-β1 or HA either alone or in combination to confluent monolayers of HK-2 cells under serum-free conditions did not influence the association of the two receptors. Furthermore, immunoprecipitation of CD44 followed by immunoblot analysis for the TGF-β type II receptor suggested that these receptor proteins were also associated.
Figure 1.
Co-localization of CD44 and TGF-β receptors. A: Western blot analysis of the association of CD44 and TGF-β receptors. Cell protein was extracted from confluent monolayers of serum deprived HK-2 cells which had been exposed to HA (molecular weight 2 × 106, final concentration 25 μg/ml, recombinant TGF-β1 (10 ng/ml) or the combination of both stimuli, all under serum-free conditions for 48 hours. Subsequently, either the TGF-β1 type I receptor (IP TGF-β RI) or CD44 (IP CD44) were immunoprecipitated and the samples subjected to immunoblot analysis with antibodies to CD44 (IB CD44), TGF-β type I receptor (IB TGF-β RI), or TGF-β type II receptor (IB TGF-β RII). In control experiments cells were exposed to serum-free medium alone for 48 hours. B: Immunocytochemical localization of CD44 and TGF-β type I receptor. Confluent monolayers of HK-2 cells were serum deprived for 48 hours before fixation with 3% paraformaldehyde for 15 minutes at room temperature and permeabilized with 0.1% Triton in PBS for 5 minutes at room temperature. The expression of the CD44 (green) and the TGF-β type I receptor (red) were examined by immunocytochemistry and analyzed by confocal microscopy as detailed in Materials and Methods and their association examined by merging of individual images.
The association between CD44 and the TGF-β type I receptor was further examined by immunocytochemistry and confocal microscopy. Double immunocytochemistry using a FITC conjugated antibody to detect CD44 and a TRITC conjugated antibody to detect the TGF-β type I receptor confirmed co-localized of the two proteins as generation of yellow staining in merged images (Figure 1B).
Modulation of TGF-β1-Dependent Matrix Generation by HA
Alteration in matrix generation was examined by determination of both type III collagen and type IV collagen in cell culture supernatants by Western analysis. Stimulation of confluence monolayers of HK-2 cells led to increased levels of both type III (Figure 2) and type IV collagen (Figure 3). In contrast incubation with HA did not influence the levels of either type III or type IV collagen. Incubation of HK-2 cells with TGF-β1 in the presence of HA led to a decrease in the amount of type III and type IV collagen detected in the cell culture supernatant compared to supernatant collected from cells stimulated with TGF-β1 alone. This decrease in TGF-β1 responsiveness in the presence of HA was prevented by adding TGF-β1 and HA in the presence of a blocking antibody to CD44 (Figures 2A and 3A).
Figure 2.
HA prevents TGF-β1-mediated increase in type III collagen. Confluent monolayers of serum deprived HK-2 cells were stimulated with TGF-β1 and HA as indicated in the presence or absence of either blocking antibody to CD44 (final concentration 5 μg/ml) (A), or the MEK inhibitor PD98059 (10 μmol/L) (B). Supernatant samples were collected 48 hours following addition of stimuli, and equal volumes of supernatant subjected to Western blot analysis for type III collagen. C: Following scanning densitometry, alteration in type III collagen was expressed as fold increase in densitometric ratios of collagen expression over control. The data represent the mean ± SD of six separate experiments.
Figure 3.
HA prevents TGF-β1-mediated increase in type IV collagen. Confluent monolayers of serum-deprived HK-2 cells were stimulated with TGF-β1 and HA as indicated in the presence or absence of either blocking antibody to CD44 (final concentration 5 μg/ml) (A) or MEK inhibitor PD98059 (10 μmol/L) (B). Supernatant samples were collected 48 hours following addition of stimuli and equal volumes of supernatant subjected to Western blot analysis for type IV collagen. C: Following scanning densitometry, alteration in type IV collagen was expressed as fold increase in densitometric ratios of collagen expression over control. The data represent the mean ± SD of five separate experiments.
Previous studies have demonstrated that CD44-mediated alteration in cell function may be associated with the MAP kinase signaling cascade,38 and we have previously demonstrated activation of this cascade following addition of exogenous HA to HK-2 cells.39 We therefore examined the role of MAP kinase signaling in HA-mediated abrogation of TGF-β1 stimulation of collagen synthesis by the addition of the MEK inhibitor PD98059 (Calbiochem). Stimulation with both TGF-β1 and HA in the presence of the MEK inhibitor prevented antagonism of the effect of TGF-β1 on both type III and type IV collagen generation (Figures 2B and 3B). Densitometric analysis of six individual experiments confirmed statistically significant stimulation of type III collagen following TGF-β1 stimulation, which was abrogated by addition of HA (Figure 2C). Furthermore, densitometry confirmed statistically significant prevention of HA-mediated abrogation of TGF-β1 stimulated collagen synthesis by either blocking antibody to CD44 or inhibition of MEK by PD98059. Similarly densitometric analysis of five individual experiments confirmed an identical pattern on the synthesis of type IV collagen following TGF-β1 stimulation and stimulation by TGF-β1 in the presence of HA (Figure 3C).
Decreased SMAD-Dependent Signaling
TGF-β1-dependent alterations in collagen generation have previously been demonstrated to be dependent on nuclear translocation of Smad3/Smad4 complexes.40–42 Confirmation of activation of the SMAD signaling pathway was sought using the (SBE)4-Lux reporter which contains 4 repeats of the CAGACA sequence identified as a SMAD binding element. Addition of TGF-β1 led to a 14-fold increase in luciferase activity of the reporter construct (Figure 4A). Addition of HA did not increase the signal above control values, but addition of HA in the presence of TGF-β1 led to a significant and dose-dependent decrease in luciferase activity (Figure 4A). At a dose of 25 μg/ml of HA, this represented a 32% reduction in luciferase activity compared to that generated by TGF-β1 alone (TGF-β, 14.05 ± 2.0 versus TGF-β+HA-9.6 ± 0.814, mean fold increase in luciferase activity over control, mean ± SD, n = 9, P = 0.0001). Interestingly this effect of HA was only seen with HA of a high molecular weight (2 × 106) while HA of much lower molecular weight (65,000) did not antagonize the effect of TGF-β1.
Figure 4.
Antagonism of TGF-β1-mediated increase SMAD-dependent signaling. A: HK-2 cells were transfected with a SMAD-responsive promoter (SBE)4-Lux, before stimulation with TGF-β1 (1 ng/ml) or either low molecular weight HA (LMW-HA molecular weight 65,000) or high molecular weight HA (molecular weight 2 × 106) at a concentration of 25 μg/ml, all under serum-free conditions. Cells were also stimulated with the combination of TGF-β1 (1 ng/ml) and increasing doses of high molecular HA as indicated. At the highest dose of high molecular weight HA cells were stimulated with TGF-β1 and a blocking antibody to CD44 (final concentration 5 μg/ml). B: In a separate series of experiments serum deprived monolayers of HK-2 cells were stimulated with TGF-β1 (1 ng/ml) and high molecular weight HA (25 μg/ml) either alone, in combination, or in combination together with increasing doses of the MEK inhibitor PD98059 as indicated. All stimuli were applied for 24 hours. Data represent mean ± SD, n = 9, *P < 0.05 compared to TGF-β1 alone, #P < 0.005 compared to TGF-β1 and HA in the absence of PD98059.
As with the effect of HA on TGF-β1-mediated alteration in collagen synthesis, addition of a blocking antibody to CD44 prevented antagonism of TGF-β1-mediated increase in luciferase activity of the SMAD responsive reporter construct (TGF-β+HA 9.6 ± 0.814, versus TGF-β+HA+CD44 antibody 13.26 ± 1.78, mean fold increase in luciferase activity over control, mean ± SD, n = 4, P = 0.0001). The luciferase activity following addition of TGF-β1, HA and blocking antibody was statistically no different to addition of TGF-β1 alone (Figure 4A).
In a separate series of experiments abrogation of TGF-β1-dependent activation of the SMAD reporter construct was also prevented by addition of the MEK inhibitor PD98059 in a dose-dependent fashion (Figure 4B). This effect was significant at all doses of PD98059 used, such that luciferase activity in the presence of TGF-β1 and HA together with PD98059 was statistically no different to activity following addition of TGF-β1 alone (TGF-β, 14.05 ± 12.0; MEKi 1 μmol/L 9.6 ± 1.9; MEKi 25 μmol/L 12.5 ± 1.52; MEKi 50 μmol/L 14.02 ± 2.23, mean fold increase in luciferase activity over control, mean ± SD, n = 4, P < 0.029).
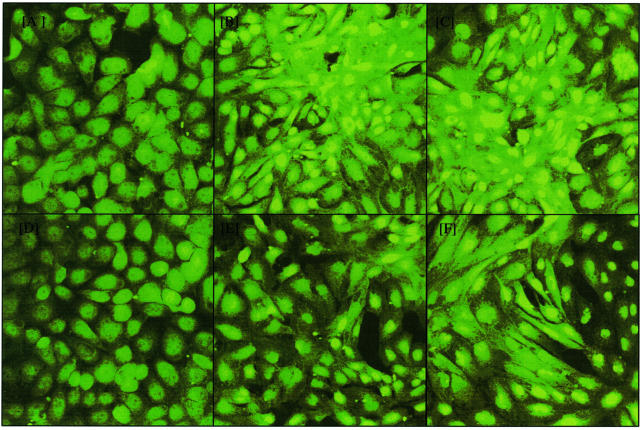
Modulation of SMAD-dependent signaling was also examined by immunocytochemistry and confocal imaging of SMAD4. Addition of TGF-β1 led to the expected nuclear translocation of Smad4. Addition of HA together with TGF-β1 led to a decrease in the intensity of nuclear Smad4 translocation (Figure 5). As with attenuation of luciferase activity of the SMAD responsive reporter construct, stimulation of cells with TGF-β1 in the presence of HA did not completely abolish SMAD4 translocation.
Figure 5.
Nuclear translocation of SMAD 4 is attenuated by high molecular weight HA. Confluent monolayers of serum deprived HK-2 cells were stimulated with increasing doses of recombinant TGF-β1 (0, A and D; 1 ng/ml, B and E; 10 ng/ml, C and F) either alone (A–C) or in the presence of 25 μg/ml HMW-HA (E and F). In separate experiments cells were stimulated with 25 μg/ml of HMW-HA alone (D). Cells were fixed by addition of 3% paraformaldehyde for 15 minutes at room temperature and permeabilized with 0.1% Triton in PBS for 5 minutes at room temperature 48 hours following application of the stimuli. SMAD 4 localization was examined by immunocytochemistry and analyzed by confocal microscopy.
Modulation of TGF-β1 Anti-Migratory Effect in the Scratch Wound System
We have previously demonstrated in a scratch wound model of cell migration that TGF-β1 has profound anti-migratory effects related to alteration in focal adhesion assembly and RhoA activation.36 To determine whether the effects of HA were specifically related to TGF-β1-dependent SMAD signaling, or to a more general inhibition of TGF-β1-mediated signaling, we sought to examine the effect of HA on the anti-migratory effect of TGF-β1 in this experimental system.
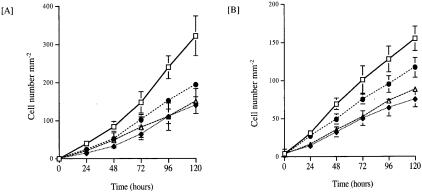
As we have previously reported,36 addition of TGF-β1 led to a marked inhibition of cell migration as compared to control experiments. Quantification of the number of cells entering the denuded area confirmed the anti-migratory effect of TGF-β1. Significant inhibition of cell migration being evident at all time points beyond 24 hours following mechanical injury of the monolayer and addition of 10 ng/ml of TGF-β1 (Figure 6A) (24 hours, control 39.4 ± 2.9 versus TGF-β1 13.5 ± 7.0 P = 0.002; 72 hours, control 146.0 ± 28.3 versus TGF-β1 63.5 ± 14.7 P = 0.005; 120 hours, control 322.5 ± 52.7 versus TGF-β1 140.3 ± 22.6 P = 0.026; mean cell number mm−2 ± SD, n = 3).
Figure 6.
Quantification of inhibition of wound closure following addition of TGF-β1. A: Confluent monolayers of HK-2 cells were “scratched” as described in Materials and Methods. Subsequently the rate of cell migration following addition of 10 ng/ml of TGF-β1 (♦), the combination of TGF-β1 (10 ng/ml) and HMW-HA (25 μg/ml) either in the presence (▵) or absence (•) of blocking antibody to CD44 was assessed. In control experiments cells were exposed to serum-free medium alone (□). Cell migration was assessed by directly counting the number of cells migrating into the intersecting denuded area at each of the time points indicated. B: In parallel experiments following creating of a wounded area the rate of cell migration following addition of 10 ng/ml of TGF-β1 (♦), the combination of TGF-β1 (10 ng/ml) and HMW-HA (25 μg/ml) either in the presence (▵) or absence (•) of 10 μmol/L of the MEK inhibitor PD98059 was assessed. In control experiments cells were exposed to serum-free medium alone (□). Cell migration was assessed by directly counting the number of cells migrating into the intersecting denuded area at each of the time points indicated. The data represent the mean ± SD of three separate experiments and is expressed as the number of cells mm−2 of denuded area. P < 0.05 TGF-β1 versus control, and TGF-β1 versus TGF-β1+HA at all time points beyond 24 hours. P-NS TGF-β1 versus TGF-β1 + HA + blocking antibody to CD44, and TGF-β1 versus TGF-β1 + HA + PD98059.
At all time points addition of TGF-β1 in the presence of HA significantly attenuated the anti-migratory effect of TGF-β1 (24 hours, TGF-β1 13.5 ± 7.0 versus TGF-β+HA 23.7 ± 1.6 P = 0.03; 72 hours, TGF-β1 63.5 ± 14.7 versus TGF-β1 + HA 101.2 ± 15.8 P = 0.019; 120 hours, TGF-β1 140.3 ± 22.6 versus TGF-β1 + HA 193.2 ± 10.8 P = 0.010; mean cell number mm−2 ± SD, n = 3). This attenuation of the anti-migratory effect of TGF-β1 by HA was overcome by addition of a blocking antibody to CD44, such that at all time points studied there was no statistical difference between cell migration following addition of TGF-β1 alone as compared with TGF-β1 together with HA and blocking antibody to CD44 (Figure 6A).
In a separate series of experiments abrogation of TGF-β1-dependent cell migration by HA was also prevented by addition of the MEK inhibitor PD98059 (Figure 6B). At all time points studied there was no statistical difference between cell migration following addition of TGF-β1 alone as compared with TGF-β1 together with HA and PD98059.
We have previously demonstrated that the anti-migratory effect of TGF-β1 in the scratch wound system is related to activation of RhoA.36 Increased expression of activated RhoA was demonstrated following GTP-Rho immunoprecipitation followed by RhoA immunoblot analysis (Figure 7). Addition of HA alone did not affect RhoA activation. Co-incubation of cells with TGF-β1 and HA inhibited TGF-β1-mediated activation of RhoA (Figure 7A). Inhibition of RhoA activation in the presence of both TGF-β1 and HA was prevented by the presence of the blocking antibody to CD44 (Figure 7B) and also by inhibition of MEK with the inhibitor PD98059 (Figure 7C). Densitometric analysis of three separate experiments confirmed statistically significant inhibition of TGF-β1-mediated activation of RhoA by HA, and that this effect could be prevented by either blocking antibody to CD44, or inhibition of MEK by PD98059 (Figure 7D).
Figure 7.
Modulation of TGF-β1-mediated activation of RhoA. A: Confluent monolayers of HK-2 cells were stimulated with either recombinant TGF-β1 (10 ng/ml), HMW-HA (25 μg/ml), or both stimuli applied together for time periods up to 120 minutes as indicated. In parallel experiments cells were stimulated with TGF-β1 (10 ng/ml), HMW-HA (25 μg/ml) or the combination of the stimuli either in the presence or absence of 5 μg/ml of a blocking antibody to CD44 (B), or the MEK inhibitor PD98059 (C) at a concentration of 10 μmol/L. Activation of RhoA was assessed by immunoprecipitation of GTP-RhoA as described in Materials and Methods. D: Following scanning densitometry, alteration in RhoA was expressed as fold increase in densitometric ratios of RhoA expression over control. The data represent the mean ± SD of three separate experiments *P < 0.05 versus both control and TGF-β+HA.
Discussion
The importance of pathological changes in the renal cortical interstitium is now well recognized, suggesting that interstitial fibrosis represents a crucial development leading to progressive renal dysfunction.43–48 With increasing awareness of the importance of these pathological interstitial changes, interest has focused on the role of cells, such as PTC, in the initiation of a fibrotic response. We have demonstrated that PTC may contribute to the pathogenesis of renal fibrosis directly by alterations in the production of components of ECM, and indirectly by the production of pro-fibrotic cytokines such as TGF-β1.1–5 The evidence implicating TGF-β1 in the pathogenesis of renal fibrosis is now overwhelming. In contrast although increased expression of HA has been documented in both acute injury24 and progressive fibrosis28 its role in the pathogenesis of these disease processes is not clear.
Our results clearly demonstrated that the association of CD44 and TGF-β1 receptors facilitated attenuation of PTC response to TGF-β1. More specifically we have demonstrated both a decrease in synthesis of collagen in response to TGF-β1 and also decreased nuclear translocation of SMAD 4 when cells were stimulated with TGF-β1 in the presence of HA. HA-dependent inhibition of TGF-β1 function was blocked by both blocking antibody to CD44 and inhibition of the MAP-kinase signaling pathway. TGF-β-mediated alterations in extracellular matrix synthesis are regulated by the SMAD family of signaling intermediates.49 As central mediators of TGF-β family signals, SMADs are subject to many different regulatory mechanisms. One mode of regulation is phosphorylation of MAP-kinase sites in the linker region.50 Nuclear accumulation of SMAD1 are antagonized by activation of the Erk MAP-kinase pathway, a mechanism which may underlie the ability of fibroblast growth factor to oppose the effects of BMP2 during limb bud outgrowth or tooth development.50 Other receptor-regulated SMADs also have potential; MAP-kinase phosphorylation sites in their linker region. As antagonism of TGF-β1-SMAD-mediated events such as increased collagen synthesis are prevented by inhibition of MAP kinase, it is interesting to speculate that this may be related to CD44-mediated activation of the MAP kinase cascade and subsequent phosphorylation of TGF-β1-regulated SMAD proteins.
In addition to the SMAD signaling intermediates, numerous other pathways of signal transduction, such as MAP kinase and Rho GTPases, have been identified following TGF-β1 receptor activation.51–57 Our recent studies in a scratch wound model demonstrated that the addition of TGF-β1 led to a marked inhibition of cell migration, increased expression of paxillin and vinculin and their incorporation into dense focal adhesion plaques. This was associated with increased association of focal adhesion components with the F-actin cytoskeleton. The effect on migration and focal adhesion reorganization, was abrogated by inhibitors of the RhoA downstream target ROCK. The data in this paper demonstrate that HA inhibits TGF-β1-mediated activation of RhoA and that this is associated with blunting of the anti-migratory effect of TGF-β1 in the scratch wound model of cell migration. This data together with attenuation of TGF-β1-dependent SMAD-mediated stimulation of collagen synthesis suggest that the effect of HA on TGF-β1 modulation of PTC function is not restricted to one specific post-receptor signaling pathway, but results in inhibition of all post receptor-ligand binding events.
In addition to a role in chronic fibrosis, altered HA expression has been documented in association with the acute renal injury in renal ischemia.24 Interestingly, recent studies performed in a rodent model of ischemic injury demonstrated elevation of TGF-β1 expression, seen predominantly in the proximal tubule, occurring as early as 12 hours post-ischemia, during the phase of cellular proliferation and persisted for as long as 14 days post-ischemia well into the phase of remodeling.58 Recovery of renal function is dependent in large part on the restoration of proximal tubular cell integrity and function, a process which is initially dependent on replenishing the population of proximal tubular cells by a wave of proliferation occurring 1 to 3 days post-injury.59 This wave of proliferation therefore occurs in the presence of elevated levels of TGF-β1which in itself has potent antiproliferative effects on PTC.60 Following the initial proliferative phase, restoration of PTC structure and function is dependent on numerous other events such as migration along a modified tubular basement membrane.61 This also occurs in the presence of elevated levels of TGF-β1, which in itself has anti-migratory effects on PTC.36 It is interesting to speculate therefore that increased expression of hyaluronan around injured cortical tubules may be involved in the recovery from acute ischemic injury by opposing the effects of TGF-β1 thus allowing both cell proliferation and cell migration in the face of elevated levels of TGF-β1 which if unopposed would be expected to inhibit both of these important components of the healing process.
Our results demonstrating attenuation of TGF-β1 signaling by HA are in contrast to recent reports using metastatic breast tumor cells in which CD44 interaction with the TGF-β receptor I kinase increases SMAD2/SMAD3 phosphorylation.62 Furthermore, in these studies, activation of the TGF-β receptor by HA also phosphorylated CD44, which enhanced its binding interaction with the cytoskeleton modulating breast tumor cell migration. TGF-βs elicit their signaling effects by binding mainly to three cell-surface receptors: type I (RI), type II (RII), and type III (RIII). RI and RII are serine/threonine kinases that form heteromeric complexes, and are necessary for TGF-β signaling, which is initiated when the ligand induces assembly of a heteromeric complex of type II and type I receptors. The RII kinase then phosphorylates RI on a conserved glycine-serine rich domain. This activates the RI kinase, which subsequently recognizes and phosphorylates downstream signaling partners such as members of the intracellular receptor regulated SMADs (R-SMADs) signal transduction pathway,63 and non-SMAD kinase pathways such as RhoA and p38MAP kinase.52,54,64 It is now well established that TGF-β1 plays a complex role in carcinogenesis. In human breast cancer, decreased expression of the TGF-β type II receptor in hyperplasias correlates with increased risk of developing invasive breast cancer, and in invasive breast cancer loss of receptors correlates with higher tumor grade, suggesting that TGF-βs are tumor suppressors early in carcinogenesis.65 However, advanced tumors overexpress TGF-β-ligand, suggesting a switch to a pro-oncogenic role in advanced disease.66 In addition to alteration in TGF-β1 type II receptor expression, expression of a truncated form or a splice variant associated with the development of malignancy may also result in defective ligand binding and aberrant TGF-β1 response. We speculate that differences in the expression pattern of the TGF-β1 receptor subtypes and their association with CD44 may explain why HA attenuated TGF-β1 signaling in our nonmalignant epithelial cell line, while in the breast tumor cell line HA triggered a TGF-β1 like signaling cascade. One important difference between our results and those derived from the breast tumor epithelial cells may relate to the degree of co-localization of CD44 with the TGF-β1 type II receptor. In our experiments CD44 immunoprecipitation resulted in detection of both TGF-β1 type I and type II receptors in equal abundance. In contrast, in the malignant cells, CD44 was associated predominantly with TGF-β1 type I receptors and to a much lesser extent with the type II receptors.
In summary we have demonstrated in renal proximal tubular epithelial cells, through co-localization of TGF-β1 and HA signaling receptors, that HA acts to antagonize TGF-β1-mediated alterations in cell function. In terms of renal disease, increased expression of HA is known to occur in both acute and chronic models of injury, and it is interesting to speculate that its role is to facilitate repair and limit progressive fibrotic effects which underlie progressive renal dysfunction.
Footnotes
Address reprint requests to Dr. A.O. Phillips, Institute of Nephrology, University of Wales College of Medicine, Heath Park, Cardiff CF14 4XN. E-mail: phillipsao@cf.ac.uk.
Supported by a senior Fellowship from GlaxoSmithKline (to A.O.P.).
References
- Phillips AO, Topley N, Steadman R, Morrisey K, Williams J. Induction of TGF-β1 synthesis in D-glucose primed human proximal tubular cells: differential stimulation by the macrophage derived pro-inflammatory cytokines IL-1β and TNFα. Kidney Int. 1996;50:1546–1554. doi: 10.1038/ki.1996.470. [DOI] [PubMed] [Google Scholar]
- Phillips AO, Morrisey R, Steadman R, Williams JD. Polarity of stimulation and secretion of TGF-β1 by proximal tubular cells. Am J Pathol. 1997;150:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- Phillips AO, Morrisey K, Steadman R, Williams JD. Decreased degradation of collagen and fibronectin following exposure of proximal tubular cells to glucose. Exp Nephrol. 1999;7:449–462. doi: 10.1159/000020624. [DOI] [PubMed] [Google Scholar]
- Morrisey K, Steadman R, Williams JD, Phillips AO. Renal proximal tubular cell fibronectin accumulation in response to glucose is polyol pathway dependent. Kidney Int. 1999;55:160–167. doi: 10.1046/j.1523-1755.1999.00248.x. [DOI] [PubMed] [Google Scholar]
- Jones SG, Morrisey T, Williams JD, Phillips AO. TGF-β1 stimulates release of pre-formed bFGF from renal proximal tubular cells. Kidney Int. 1999;56:83–91. doi: 10.1046/j.1523-1755.1999.00517.x. [DOI] [PubMed] [Google Scholar]
- Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β1. Nature. 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Tomooka S, Kagami S. Antagonists of transforming growth factor-β: a novel approach to treatment of glomerulonephritis and prevention of glomerulosclerosis. Kidney Int. 1992;41:566–570. doi: 10.1038/ki.1992.83. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA, Yamamoto T, Harper JR, Yamasguchi Y, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor β protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor-β is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Noble NA, Miller DE, Border WA. Sustained expression of TGF-β1 underlies development of progressive kidney fibrosis. Kidney Int. 1994;45:916–927. doi: 10.1038/ki.1994.122. [DOI] [PubMed] [Google Scholar]
- Border WA, Okuda S, Languino LR, Ruoslahti E. Transforming growth factor-β regulates production of proteoglycans by mesangial cells. Kidney Int. 1990;37:689–695. doi: 10.1038/ki.1990.35. [DOI] [PubMed] [Google Scholar]
- Bassols A, Massague J. Transforming growth factor β regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988;263:3039–3045. [PubMed] [Google Scholar]
- Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type 1 collagen mRNA levels by transforming growth factor-β. J Biol Chem. 1987;262:6443–6446. [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- Nakamura T, Miller D, Ruoslahti E, Border WA. Production of extracellular matrix by glomerular epithelial cells is regulated by transforming growth factor-β1. Kidney Int. 1992;41:1213–1221. doi: 10.1038/ki.1992.183. [DOI] [PubMed] [Google Scholar]
- Overall CM, Wrana JI, Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-β. J Biol Chem. 1989;264:1860–1869. [PubMed] [Google Scholar]
- Ignotz RA, Massague J. Cell adhesion protein receptors as targets for transforming growth factor-β action. Cell. 1987;51:189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Kagami S, Border WA, Ruoslahti E, Noble N. Coordinated expression of β1 integrins and transforming growth factor-β induced matrix proteins in glomerulonephritis. Lab Invest. 1993;69:68–76. [PubMed] [Google Scholar]
- Humes HD, Nakamura T, Cieslinski DA, Miller D, Emmons RV, Border WA. Role of proteoglycans and cytoskeleton in the effects of TGF-β1 on renal proximal tubule cells. Kidney Int. 1993;43:575–584. doi: 10.1038/ki.1993.85. [DOI] [PubMed] [Google Scholar]
- Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor β regulates tubular epithelial myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- Isaka Y, Tsujie M, Ando Y, Nakamura H, Kaneda Y, Imai E, Hori M. Transforming growth factor β1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int. 2000;58:1885–1892. doi: 10.1111/j.1523-1755.2000.00360.x. [DOI] [PubMed] [Google Scholar]
- Akagi Y, Isaka Y, Arai M, Kaneko T, Takenaka M, Moriyama T, Kaneda Y, Ando A, Orita Y, Kamada T, Ueda N, Imai E. Inhibition of TGF-β1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int. 1996;50:148–155. doi: 10.1038/ki.1996.297. [DOI] [PubMed] [Google Scholar]
- Hansell P, Göransson V, Odlind C, Gerdin B, Hällgren R. Hyaluronan content in the kidney in different states of body hydration. Kidney Int. 2000;58:2061–2068. doi: 10.1111/j.1523-1755.2000.00378.x. [DOI] [PubMed] [Google Scholar]
- Lewington AJP, Padanilam BJ, Martin DR, Hammeramn MR. Expression of CD44 in kidney after acute ischemic injury in rats. Am J Physiol. 2000;278:R247–R254. doi: 10.1152/ajpregu.2000.278.1.R247. [DOI] [PubMed] [Google Scholar]
- Sibalic V, Fan X, Loffing J, Würthrich RP. Upregulated renal tubular CD44, hyaluronan, and osteopontin in kdkd mice with interstitial nephritis. Nephrol Dial Transplant. 1997;12:1344–1353. doi: 10.1093/ndt/12.7.1344. [DOI] [PubMed] [Google Scholar]
- Wells A, Larsson E, Hanas E, Laurent T, Hallgren R, Tufvesson G. Increased hyaluronan in acutely rejecting human kidney grafts. Transplantation. 1993;55:1346–1349. doi: 10.1097/00007890-199306000-00025. [DOI] [PubMed] [Google Scholar]
- Wells AF, Larsson E, Tengblad A, Fellstrom B, Tufvesson G, Klareskog L, Laurent TC. The localisation of hyaluronan in normal and rejected human kidneys. Transplantation. 1990;50:240–243. doi: 10.1097/00007890-199008000-00014. [DOI] [PubMed] [Google Scholar]
- Sano N, Kitazawa K, Sugisaki T. Localization and roles of CD44, hyaluronic acid and osteopontin in IgA nephropathy. Nephron. 2001;89:416–421. doi: 10.1159/000046113. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- Fraser DJ, Wakefield L, Phillips AO. Independent regulation of transforming growth factor-β1 transcription and translation by glucose and platelet-derived growth factor. Am J Pathol. 2002;161:1039–1049. doi: 10.1016/s0002-9440(10)64265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen U, Thomas G, Glant T, Phillips AO. Regulation of inter-alpha-trypsin inhibitor (IaI) and tumour necrosis factor-stimulated gene 6 (TSG-6) expression in human renal proximal tubular epithelial cells. Kidney Int. 2001;60:126–136. doi: 10.1046/j.1523-1755.2001.00779.x. [DOI] [PubMed] [Google Scholar]
- Jones SG, Jones S, Phillips AO. Regulation of renal proximal tubular epithelial cell hyaluronan generation: implications for diabetic nephropathy. Kidney Int. 2001;59:1739–1749. doi: 10.1046/j.1523-1755.2001.0590051739.x. [DOI] [PubMed] [Google Scholar]
- Morrisey K, Evans RA, Wakefield L, Phillips AO. Translational regulation of renal proximal tubular epithelial cell transforming growth factor-β1 generation by insulin. Am J Pathol. 2001;159:5:1905–1915. doi: 10.1016/s0002-9440(10)63037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-(β) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci. 1999;112:4557–4568. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- Tian YC, Phillips AO. TGF-β1 mediated inhibition of HK-2 cell migration. J Am Soc Nephrol. 2003;14:631–640. doi: 10.1097/01.asn.0000053418.56286.5e. [DOI] [PubMed] [Google Scholar]
- Jones SG, Ito T, Phillips AO. Regulation of proximal tubular epithelial cell CD44 mediated binding and internalisation of hyaluronan. Int J Biochem Cell Biol. 2003;35:1361–1377. doi: 10.1016/s1357-2725(03)00040-2. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce protein tyrosine kinase activity in endothelial cells and activate a cytoplasmic signal transduction pathway resulting in proliferation. Lab Invest. 1998;78:987–1003. [PubMed] [Google Scholar]
- Ito T, Williams JD, Al-Assaf S, Phillips GO, Phillips AO. Hyaluronan and proximal tubular epithelial cell migration. Kidney Int. 2004;65:823–833. doi: 10.1111/j.1523-1755.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- Ingaki Y, Mamura M, Kanamaru Y, Greenwel P, MNemoto T, Takehara K, Dijke P, Nakao A. Constitutive phosphorylation and nuclear localisation of Smad3 are correlated with increased collagen gene transcription in activated hepatic stellate cells. J Cell Physiol. 2001;187:117–123. doi: 10.1002/1097-4652(2001)9999:9999<00::AID-JCP1059>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-β: involvement of Smad3. J Invest Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor β1 stimulation of α2(I)-collagen (COL1A2) transcription. J Biol Chem. 2000;275:39237–39245. doi: 10.1074/jbc.M003339200. [DOI] [PubMed] [Google Scholar]
- Mackensen-Haen S, Bohle A, Christensen J, Wehrmann M, Kendziorra H, Kokot F. The consequences for renal function of widening of the interstitium and changes in the tubular epithelium of the renal cortex and outer medulla in various renal diseases. Clin Nephrol. 1992;37:70–77. [PubMed] [Google Scholar]
- Wehrmann M, Bohle A, Held H, Schumm G, Kendziorra H, Pressler H. Long-term prognosis of focal sclerosing glomerulonephritis: an analysis of 250 cases with particular regard to tubointerstitial changes. Clin Nephrol. 1990;33:115–122. [PubMed] [Google Scholar]
- Wehrmann M, Bohle A, Bogenschutz O, Eissele R, Freislederer A, Ohlschlegel C, Schumm G, Batz C, Gartner HV. Long-term prognosis of chronic idiopathic membranous glomerulonephritis. Clin Nephrol. 1989;31:67–76. [PubMed] [Google Scholar]
- Mackensen-Haen S, Bader R, Grund KE, Bohle A. Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clin Nephrol. 1981;15:167–171. [PubMed] [Google Scholar]
- Bogenschutz O, Bohle A, Batz C, Wehrmann M, Pressler H, Kendziorra H, Gartner HV. IgA Nephritis: on the importance of morphological and clinical parameters in the long-term prognosis of 239 patients. Am J Nephrol. 1990;10:137–147. doi: 10.1159/000168068. [DOI] [PubMed] [Google Scholar]
- Bader R, Bader H, Grund KE, Mackensen-Haen S, Christ H, Bohle A. Structure and function of the kidney in diabetic glomerulosclerosis: correlations between morphological and functional parameters. Pathol Res Pract. 1980;167:204–216. doi: 10.1016/S0344-0338(80)80051-3. [DOI] [PubMed] [Google Scholar]
- Tian YC, Fraser DJ, Attisano L, Phillips AO. TGF-β1 mediated alterations of renal proximal tubular epithelial cell phenotype. Am J Physiol. 2003;285:F130–142. doi: 10.1152/ajprenal.00408.2002. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-β1 family mediator Smad1. Nature. 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-β1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int. 1999;56:1710–1720. doi: 10.1046/j.1523-1755.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- Ravanti L, Häkkinen L, Larjava H, Saariaoho-Kere U, Foschi M, Han J, Kähäri VM. Transforming growth factor-β induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:37292–37300. doi: 10.1074/jbc.274.52.37292. [DOI] [PubMed] [Google Scholar]
- Isono M, Iglesias-De La Cruz MC, Chen S, Hong SW, Ziyadeh FN. Extracellular signal-regulated kinase mediates stimulation of TGF-β1 and matrix by high glucose in mesangial cells. J Am Soc Nephrol. 2001;11:2222–2230. doi: 10.1681/ASN.V11122222. [DOI] [PubMed] [Google Scholar]
- Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-β-induced mobilization of actin cytoskeleton requires signalling by Small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. Activations of ERK1/2 and JNK by transforming growth factor β negatively regulate Smad3-induced alkaline phosphatase activity and mineralization in mouse osteoblastic cells. J Biol Chem. 2002;277:36024–36031. doi: 10.1074/jbc.M206030200. [DOI] [PubMed] [Google Scholar]
- Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signalling in transforming growth factor-β-mediated transcription. J Biol Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- Basile DP, Rovak JM, Martin DR, Hammerman MR. Increased transforming growth factor β1 expression in regenerating rat renal tubules following ischemic injury. Am J Physiol. 1996;270:F500–F509. doi: 10.1152/ajprenal.1996.270.3.F500. [DOI] [PubMed] [Google Scholar]
- Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, cFos, and clusterin in the post-ischemic kidney. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Choi HJ, Lee JH, Woo CH, Kim JH, Han HJ. High glucose inhibits renal proximal tubule cell proliferation and involves PKC, oxidative stress and TGF-β1. Kidney Int. 2001;59:1695–1705. doi: 10.1046/j.1523-1755.2001.0590051695.x. [DOI] [PubMed] [Google Scholar]
- Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–1178. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Singleton PA, Zhu H, Zhou B. Hyaluronan promotes signalling interaction between CD44 and the transforming growth factor β receptor I in metastatic breast tumor cells. J Biol Chem. 2002;277:39703–39712. doi: 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-β signal transduction. J Cell Sci. 2001;114:4359–4369. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- Chin BY, Mohsenin A, Li SX, Choi AM, Choi ME. Stimulation of pro-alpha(1)(I) collagen by TGF-β(1) in mesangial cells: role of the p38 MAPK pathway. Am J Physiol. 2001;280:F495–F504. doi: 10.1152/ajprenal.2001.280.3.F495. [DOI] [PubMed] [Google Scholar]
- Gobbi H, Arteaga CL, Jensen RA, Simpson JF, Dupont WD, Olson SJ, Schuyler PA, Plummer WD, Jr, Page DL. Loss of expression of transforming growth factor β type II receptor correlates with high tumour grade in human breast in-situ and invasive carcinomas. Histopathology. 2000;36:168–177. doi: 10.1046/j.1365-2559.2000.00841.x. [DOI] [PubMed] [Google Scholar]
- Gold LI. The role for transforming growth factor-β (TGF-β) in human cancer. Crit Rev Oncog. 1999;10:303–360. [PubMed] [Google Scholar]