Abstract
Estrogen receptor (ER)-β is thought to exert anti-proliferative effects in the normal prostate but supports prostate cancer (PCa) cell survival. We previously reported that the receptor’s expression declined as PCa developed in the gland but reappeared in lymph node and bone metastases. To investigate whether hypermethylation was the underlying mechanism for these phenomena, we first identified two CpG islands (CGIs) encompassing 41 CpG dinucleotides, located separately in the untranslated exon 0N and the promoter region of ER-β. Using immunostained, laser capture-microdissected samples from 56 clinical specimens, we demonstrated an inverse relationship exists between the extent of ER-β CGI methylation and receptor expression in normal, hyperplastic, premalignant, and malignant foci of the prostate and in lymph node and bone metastases. Treatment of PCa cell lines (LNCaP and DU145), that express little ER-β mRNA, with a demethylating agent increased levels of receptor expression thus corroborating our in vivo findings that methylation is involved in ER-β silencing. Methylation centers in the promoter region and exon 0N were identified by hierarchical cluster analysis of bisulfite sequencing data obtained from 710 alleles. Methylation at these centers was insignificant in normal epithelium, reached 80 to 90% in grade 4/5 PCa, but declined to less than 20% in bone metastases. In addition, progressive methylation spreading from the exonic CGI to the promoter CGI, which correlated with loss of ER-β expression, was detected in microdissected samples and in cell cultures. Using a new class of methylated oligonucleotides that mediate sequence-specific methylation in cellulo, we demonstrated that methylation of the promoter CGI, but not the exonic CGIs, led to transcriptional inactivation of ER-β. Our results present the first evidence that epigenetic regulation of ER-β is a reversible and tumor stage-specific process and that gene silencing via methylated oligonucleotides may have therapeutic potential in the treatment of advanced PCa.
Estrogens are believed to play a significant role in the pathogenesis of prostate cancer (PCa).1 Until recently, the steroid hormone receptor, estrogen receptor (ER)-α, was thought to mediate all estrogen actions.2 In 1996, a second ER, encoded by ER-β, was identified3,4 and found to differ significantly from ER-α. Unlike ER-α, which is the predominant ER in female reproductive organs, the β-isotype is highly expressed in the male reproductive tract, including the prostate. Although the precise biological function of ER-β is incompletely defined, it has been suggested that the receptor, acting through estrogens, may protect normal prostate epithelial from undergoing unscheduled cell proliferation, neoplastic transformation, and from oxidative injuries.5–11 In contrast, the function of ER-β in PCa cells appears to be associated with cell survival.12–14 We previously reported abundant expression of ER-β in the epithelial compartment of the human prostate.6 In our study we demonstrated that ER-β expression declined as PCa developed and reached higher grades in the primary site but reappeared in lymph node and bone metastases. Based on our findings and the functions proposed for ER-β, we postulate that the receptor plays distinct and different roles at various stages in the evolution and progression of PCa. Thus, a better understanding of how ER-β expression is regulated throughout the natural history of the disease may yield new strategies for the diagnosis, prevention, and treatment of PCa.
Carcinogenesis is now thought to represent both genetic and epigenetic alterations that culminate in the evolution of a malignant phenotype.15 To our knowledge, no mutations in ER-β or loss of heterozygosity at the gene locus have been reported for PCa, whereas recent studies16,17 have demonstrated methylation of CpG-rich 5′-flanking sequences of ER-β in primary PCas and breast cancers as well as in cancer cell lines, thus implicating that cytosine methylation-mediated ER-β regulation may be involved in carcinogenesis. However, these studies have investigated only relatively short sequences of the ER-β 5′-flanking region that span ∼20 CpG dinucleotides and were conducted in whole tissues that included stromal components that express the receptor. Furthermore, the existence of a bonde fide CpG island(s) (CGIs) in the promoter/5′-flanking region of ER-β has not been established. At present, no evidence has been provided to indicate whether this epigenetic mechanism is causally linked to aberrant ER-β expression during PCa evolution and metastatic progression. Direct evidence in support of the cytosine methylation of specific 5′ CpGs that leads to transcriptional inactivation has not been reported.
To address these questions, in the present work, we have performed a detailed analysis of the promoter/5′-untranslated region of ER-β and used RNA ligase-mediated 5′ rapid amplification of cDNA end (RLM-RACE) to verify that transcription of the gene in PCa cells starts at exon 0N. Using an innovative protocol that combined the use of immunocytochemistry with laser capture-microdissection (LCM) of lesions that encompass the entire natural history of PCa, relevant cell culture models, bisulfite sequencing, and clustering analysis we have identified two CGIs, separately located in the promoter and exon 0N of ER-β. Importantly, we present compelling evidence that methylation of specific CpG-rich sequences in the promoter/5′-flanking region of ER-β dynamically regulates expression of ER-β during PCa development and metastatic progression. We further demonstrate higher order phenomena such as preferred methylation at methylation centers, methylation spreading from exon region to promoter, and potential relationships between specific CpGs and transcriptional factor (TF)-binding sites in the 5′-flanking sequence of ER-β. Finally, to delineate the contribution of exonic versus promoter CpG dinucleotides in the gene-silencing process we have designed a novel class of oligonucleotides and used them to achieve sequence-specific methylation in cellulo. Results from these studies indicate that exonic CpGs and promoter CpGs play different roles in methylation-mediated inhibition of ER-β expression. Our results have important implications for furthering our understanding of stage-specific biology of PCa development and progression. Moreover, our findings may lead to the potential use of demethylating agents/methylation oligonucleotides for targeting ER-β gene expression as therapeutic approaches in the chemoprevention and treatment of PCa.
Materials and Methods
Samples
Formalin-fixed, paraffin-embedded sections were obtained from an archival collection of 42 radical prostatectomy specimens, 6 regional lymph nodes with metastases, and 5 biopsies of metastases to the bone. None of the patients who underwent radical prostatectomy had received previous treatment. Only one patient with lymph node metastases was treated with Lupron (TAP Pharmaceuticals Inc., Deerfield, IL) whereas all six patients with bone metastases received treatments with Lupron or Flutamide (Schering Corp., Kenilworth, NJ). The grading of all PIN and cancerous lesions was done on representative hematoxylin and eosin (H&E)-stained sections.
Immunohistochemistry
Tissue sections were immunostained for ER-β using a highly specific antibody GC-17 (Biogenix Inc., Mountain View, CA) as previously described.6 At least three replicate sections were immunostained followed by H&E counterstaining. One section was coverslipped while the remaining others were left uncoverslipped for subsequent microdissection. The overall quality, specificity of immunostaining, and the intensity of ER-β expression in benign glands and lesions of interest was evaluated and graded in coverslipped sections. A positive designation was made when at least 90% of cells in the surveyed morphologically normal epithelia were stained. Preneoplastic and neoplastic lesions were considered positive when at least 80% of the cells were immunostained. Cases were designated as mixed when nuclear staining was seen in more than 10% or in less than 80% of cells in a lesion. A negative grade was given for a case when 10% or fewer of the cells in a lesion were positively stained.
LCM and DNA Extraction
Representative normal glands; areas of benign prostatic hyperplasia (BPH); high-grade intraprostatic neoplasia (HG-PIN); grades 3, 4, and 4/5 carcinomas; and PCa metastases were first identified in coverslipped sections and then located in replicate immunostained uncoverslipped sections for microdissection. LCM was performed as previously described.6 A total of 71 foci were dissected from the 42 specimens from radical prostatectomies and 13 metastatic foci. In 15 of the radical prostatectomies, it was possible to microdissect various combinations of morphological entities encompassing the entire natural history of prostate carcinogenesis. DNA was extracted from microdissected samples after digestion with proteinase K at 50°C followed by extraction with phenol-chloroform and precipitation with ethanol.
Cell Culture and Treatment
DU145, LNCaP, and PC3 PCa cancer cell lines were obtained from the American Type Culture Collection (Rockville, MD) and cultured as described.13 Some cultures were treated with the demethylating agent 5′-aza-2′-deoxycytidine (5-Aza-dC) (Sigma, St. Louis, MO) at 0.5 and 1 μmol/L for 72 hours.18
RLM-RACE
Total RNA isolated from PC3 was subjected to dephosphorylation, decapping, and ligation reactions. A series of 5′RACE reactions were performed according to the instructions of GeneRacer kit (Invitrogen, Carlsbad, CA). Several gene-specific primers spanning the exon 1 of ER-β were designed (GR1-490, 5′-AAGGTGTGTTCTAGCGATCTTGCTTC-3′; GR1-421, 5′-TGGCGATGGACCACTAAAGGA-3′; GR1-350, 5′-ACCAGGCCCACCTTCCAAGT-3′). Polymerase chain reaction (PCR) products of interest were gel-purified, cloned, and sequenced.
Bisulfite Sequencing
DNA samples were bisulfite-modified as described.18 Nested PCR was conducted with two sets of primers (ER-β-bis1F, 5′-AGA TTT TTT AAA TTT GAG ATT GGG GTT G-3′ and ER-β-bis1R, 5′-CTT ACC TTA CAA ATA AAC ACA CC-3′ as the outer set, and ER-β-bis2F, 5′-GTT ATT ATT TTT GTG GGT GGA TTA GG-3′ and ER-β-bis2R, 5′-ACC TTA CCT TCT CTA AAA TAC-3′ as the inner set). All primer sequences were devoid of CpG dinucleotides to avoid biased amplification of the methylated alleles. Hot-start PCR was performed at 95°C for 10 minutes, followed by a first round amplification using 40 cycles of 95°C for 30 seconds, 54°C for 45 seconds, and 72°C for 45 seconds and a second amplification involving 45 cycles of 95°C for 30 seconds, 56°C for 45 seconds, and 72°C for 45 seconds. The PCR products were sized in 1.5% agarose gels with ethidium bromide. The correct size band was isolated and its DNA extracted using the QIAquick gel extraction kit (Qiagen, Valencia, CA). The purified DNA was cloned into pGEM-T Easy vector (Promega, Madison, WI). Ten clones were picked for each sample, DNA purified, and sequenced (Macogene Inc., Seoul, Korea).
RNA Isolation and Reverse Transcriptase-PCR
Isolation of RNA from PCa cells and assessment of relative abundance of ER-β mRNA were conducted as reported.13
Methylated Oligonucleotide Treatment
Phosphorothioated oligonucleotides containing 5′-methylcytosines (mC) in CpG dinucleotide were designed and synthesized (Proligo, Boulder, CO). Methylated oligonucleotide 1 (MO1, 5′-GTC AmCG mCGmC GGmC GGT mCGG GmCG T-3′) is a 22-mer sense oligonucleotide that is complementary to a region of human ER-β promoter encompassing CpG sites 3 to 8 (as shown in Figure 1) and MO2 is a sense 25-mer (5′-GCT mCGG GGC AGG GCT AAmC GCC mCGG-3′) spanning from CpG sites 28 to 30. Two unmethylated phosphorothioated oligonucleotides, UMO1 and UMO2, which are identical to MO1 and MO2 in sequence, were purchased (Qiagen) and used as controls. PC3 cells (3 × 105) were seeded in six-well plates and transfected in duplicate with 2 μmol/L of methylated or unmethylated oligonucleotides using Lipofectamine Plus reagent (Invitrogen). Untreated cells and cells treated with various regimens were harvested after 48 hours of transfection and genomic DNA and total cellular RNA isolated using standard protocols. The experiment was repeated once to generate four samples per treatment regimen. Bisulfite sequencing and reverse transcriptase-PCR were performed as described earlier to access the methylation status and transcript abundance of ER-β after transfection with MOs and UMOs.
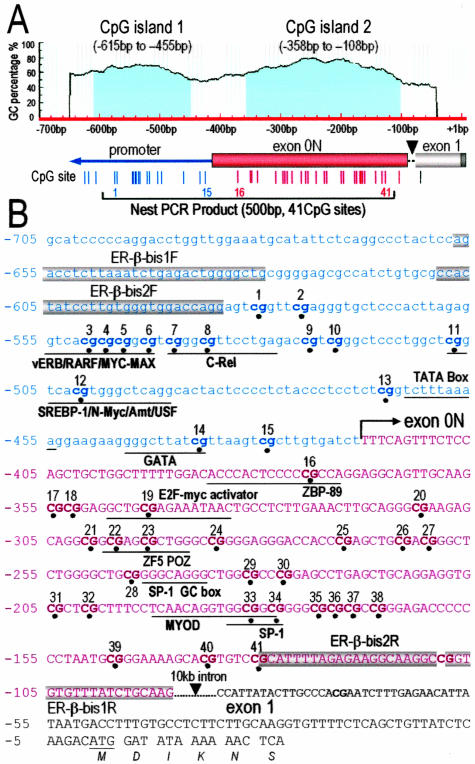
Figure 1.
ER-β 5′-flanking region (AF191544) structure and primer design for bisulfite sequencing. A: Two CGIs (island 1, −615 to −455; island 2, −362 to −108) were identified. Individual CpG sites are indicated as vertical lines and numbered from 1 to 41. Island 1 contains CpG sites 1 to 15 located in the promoter and island 2 contains CpG sites 16 to 41 located in exon 0N. A 10,958-bp intron region between exon 0N and exon 1 (AL161756) is indicated by a black arrowhead. The 500-bp nested PCR-amplified region is indicated by a line. B: Sequence of ER-β promoter, exon 0N, and exon 1 region. The 5′ transcription start site is designated as + 1. The start site of exon 0N is indicated by a bent arrow. Potential transcription factor binding sites are indicated. The two round primers for PCR are dark-blocked.
Statistical Analyses
A nonparametric test developed by Cuzick19 was used to examine association of increased/decreased methylation of the ER-β 5′-flanking sequence with initiation and progression of PCa. Analysis of variance tests were used to test if the overall methylation of the entire CpG-rich region (the promoter CGI plus the exonic CGI) was different among groups (normal glands, BPH, HG-PIN, grade 3 carcinoma, grade 4/5 carcinoma, lymph node metastases, and bone metastases). Posthoc tests were performed with Dunnett’s test, which controls for type I experiment-wise error at 0.05 for comparisons of all classes of lesions against normal. Hierarchical cluster analyses20 were performed on the entire sequence data set, which consisted of methylation status of CpG sites in a total of 710 alleles to establish clusters of CpG sites with high and low probability of co-methylation within the said region.
GenBank Accession Numbers
The National Center for Biotechnology Information human genome sequence for ER-β promoter is AF191544 and for the 10-kb intron between exon 0N and exon 1, AL161756.
Results
Identification of Two CGIs Separately Located in the Promoter and the Untranslated First Exon of ER-β
Li and co-workers21 cloned and partially characterized a 2.1-kb 5′-flanking sequence of ER-β from PCa cells that they referred to as the proximal promoter of the gene. Hirata and colleagues22 reported transcription from an untranslated exon termed 0N in endometrial cells. Zhao and colleagues17 claimed that they observed methylation in exon 0N in breast cancer but provided no data in support of actual transcription at this exon in their studies. Prompted by these reports, we conducted a detailed reanalysis of this region in PCa cells. We noted a sequence matching exon 0N in the DNA sequence under investigation (Figure 1). RLM-RACE was therefore used to identify full-length ER-β transcripts. Our results (data not shown) consistently revealed the existence of a transcript that is 13 bp longer than previously reported by Li and associates21 but agreed with the results reported by Moore and colleagues.23 This finding indicated that the correct transcription start site of ER-β in PCa cells should be located as indicated in Figure 1B, ie, matching where exon 0N starts. Based on this new finding, the region 5′ to this start site is now referred to as the promoter region in this article whereas the region 3′ to this site is recognized as in the untranslated exon 0N. An extensive genomic BLAST search was then conducted and found a 10-kb intron between exon 0N and exon 1 that was omitted in the originally published sequence.21 Existence of this intron has now been indicated in Figure 1. Based on our view of this recharacterized region, we conducted computational analyses18 and identified two putative CGIs containing 41 CpG sites in this region (Figure 1A). We deduced that CpG dinucleotides (sites) 1 to 15 (from −615 to −455) are located in the promoter region and CpG sites 16 to 41 (from −358 to −108) reside in exon 0N of ER-β (Figure 1, A and B), which we now refer to as promoter CGI and exonic CGI, respectively. Primers were designed for nested PCR to amplify a 500-bp sequence that encompasses all 41 CpG sites in both CGIs. Bisulfite sequencing was used to study the methylation status of each CpG site within this region.
Correlation of Hypermethylation of ER-β CGIs and Loss of Receptor Expression Occurs in Primary PCas but Receptor Re-Expression with Decreased Methylation of These CGIs Is Found in Lymph Node and Bone Metastases
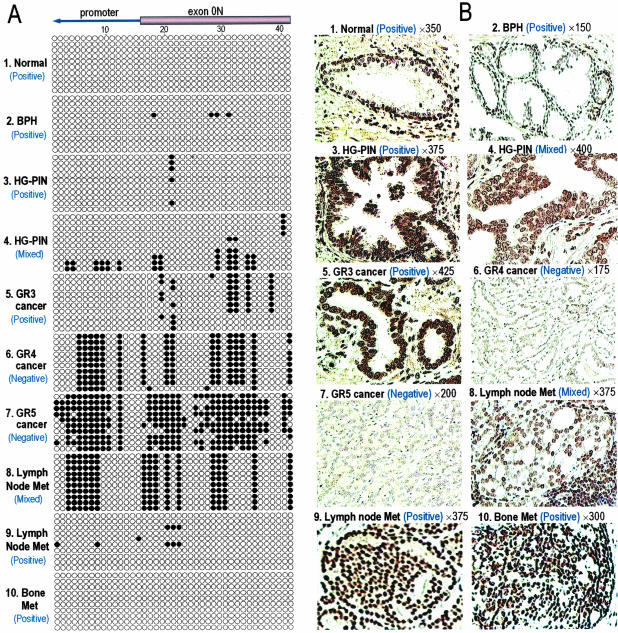
We then correlated the methylation status of the two ER-β CGIs in immunostained-microdissected samples of normal epithelium: BPH; HG-PIN; and grades 3, 4, and 4/5 carcinomas of the prostate and lymph node; and bone metastases individually with ER-β expression in the microdissected foci (Figure 2). Methylation of CpG sites in this region was absent or extremely rare in normal glands and BPH samples (Figure 2A, 1 and 2), whereas ER-β immunostaining was strongly positive in these glands (Figure 2B, 1 and 2). The majority of HG-PIN had either unmethylated (Figure 2A, 3) or modest methylated (Figure 2A, 4) ER-β CGIs and were correspondingly positive (Figure 2B, 3) or mixed (Figure 2B, 4) for receptor staining. The extent of ER-β methylation and loss of receptor expression was most pronounced in the high-grade cancers (Figure 2A, 6 and 7, and Figure 2B, 6 and 7). Cancerous foci exhibiting heavy (Figure 2A, 9) or minimal (Figure 2A, 8) ER-β CGI methylation were found in all lymph node metastases. Figure 2B illustrates a case with mixed receptor staining (Figure 2B, 8) alongside a case with uniformly strong ER-β staining of PCa cells (Figure 2B, 9). PCa cells of bone metastases were uniformly positive for ER-β immunostaining (Figure 2B, 10) and possessed unmethylated ER-β CGIs (Figure 2A, 10).
Figure 2.
A: Maps of methylated sites in individual cloned DNA fragments from representative microdissected prostate tissue samples. Each row represents one sequenced allele. Ten alleles from each microdissected sample were cloned and sequenced. Each circle represents a CpG site, a filled circle indicates methylation, and an open circle indicates no methylation. “Positive,” “mixed,” and “negative” refer to ER-β protein expression status. B: Light micrographs showing representative ER-β expression status in tissue sections before microdissection. Magnifications are as indicated. 1: Normal human prostate acinus with immunostaining predominantly found in basal cell nuclei. 2: A nodule of BPH with immunopositive basal cells. 3: A HG-PIN lesion showing positive immunostaining. 4: A HG-PIN lesion with mixed positively and negatively stained (arrow) epithelial cell nuclei. 5: Grade 3 PCa with uniformly positively stained neoplastic cells. 6: Grade 4 PCa. Neoplastic cells are negatively stained. 7: Grade 5 PCa with uniformly negatively stained neoplastic cells. 8: Metastases in a lymph node with mixed expression. Note the intense staining of adjacent lymphocytes (bottom right corner). 9: Positive immunostaining of metastatic PCa cells in a lymph node. 10: Positive immunostaining in PCa cells metastasized to bone.
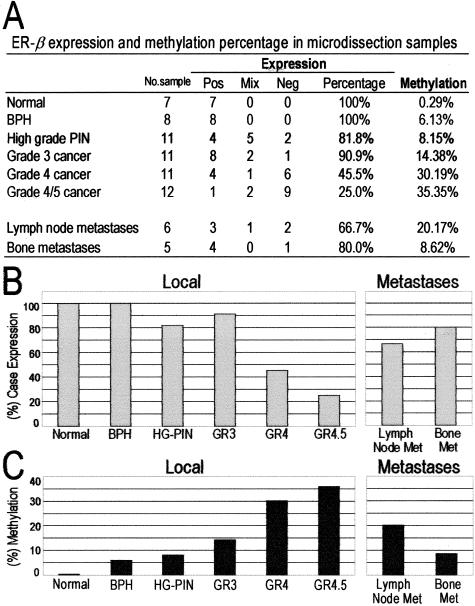
We then compared the extent of ER-β CGI methylation with ER-β expression among different morphological entities of normal and malignant prostate tissues in a semiquantitative manner (Figure 3). Foci with morphologically normal glands (7 of 7), BPH foci (8 of 8), HG-PIN (9 of 11), and grade 3 cancers (10 of 11) expressed ER-β. However, the percentage of methylated CpG sites in their ER-β CGIs increased progressively from 0.29 to 14%. Progression to higher grade tumors (grades 4 and 4/5) was associated with a dramatic reduction in receptor-positive samples (5 of 11 and 3 of 12, respectively), whereas the total percentage of methylated CpG sites increased to 30% and 35%, respectively. When compared with high-grade primary site PCas, four of six (67%) lymph node and four of five (80%) bone metastases expressed ER-β, whereas the extent of ER-β CGI methylation decreased to 20% and 8.6%, respectively. Cuzick’s test showed a significantly increasing trend in the percentage of methylated sites in ER-β CGIs from normal epithelium to grade 4/5 tumors (z = 9.09, P < 0.0001) and a significantly decreasing trend in methylated sites from grade 4/5 tumors, lymph node metastases, to bone metastases (z = −5.31, P < 0.0001). Analysis of variance and Dunnett’s tests revealed significantly higher overall methylation of ER-β CGIs among different classes of premalignant and malignant lesions than of normal and hyperplastic glands (F = 59.57 and P < 0.0001). Collectively, these data indicate a direct correlation between hypermethylation of ER-β CGIs and loss of receptor expression in primary PCas whereas the reverse was found in lymph nodes and bone metastases.
Figure 3.
Expression and methylation status of ER-β in microdissected normal epithelium, BPH, high-grade PIN, and local and metastatic PCas. A: Shown are the numbers of samples, defined by morphological types, that showed positive, mixed, or negative immunostaining for ER-β. Percentage of positive receptor expression represents the combined number of positive and mixed-stained microdissected samples from the total number microdissected for each morphological entity. The total number of methylated CpG sites in the entire population of alleles belonging to a single morphological entity was calculated as a percentage of methylated versus unmethylated CpGs. B: Percentage of samples positive or mixed for ER-β expression in local sites and metastases. C: Percentage of methylated CpG sites of the total number of alleles analyzed for each morphological entity in the prostate or metastases.
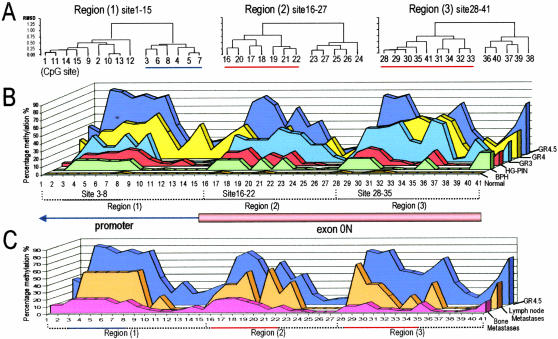
Identification of Three Methylation Centers by Hierarchical Cluster Analysis
Bisulfite sequencing data were further analyzed to obtain methylation frequencies of individual CpG sites among normal glands and different classes of prostatic lesions (Figure 4). Hierarchical cluster analyses identified three CpG clusters with high levels of methylation activity in the ER-β CGIs (Figure 4A), which we referred as methylation centers. During PCa development and progression in the primary site, cytosine methylation increased in these three centers (Figure 4B): CpG, 3 to 8 in the promoter CGI; and 16 to 22 and 28 to 35 in the exonic CGI. Methylation frequencies were less than 10% in all three centers in normal glands, reached 80 to 90% in high-grade cancers (Figure 4B), and then declined to ≈40% in lymph node metastases and <10% in bone metastases (Figure 4C). We noted that these methylation centers coincided with putative TF-binding sites or cis-regulatory elements, which were identified by the same method described previously.24 For example, CpG 3 to 8 coincided with a MYC/MAX and a c-Rel site, CpG 16 to 22 resided in a region with an E2F-myc activator and a ZF5 POZ site, and CpG 28 to 35 is located in a sequence that contains two Sp-1 sites (Figure 1B).
Figure 4.
Methylation patterns in the proximal promoter region and exon 0N of ER-β. A: Hierarchical cluster analysis of bisulfite sequencing data revealed three clusters of CpGs that exhibited high levels of methylation (underscored) compared with neighboring CpGs. B: Methylation patterns in ER-β CGIs observed in normal glands; BPH; HG-PIN; and grades 3, 4, and 4/5 cancers. C: Methylation patterns in ER-β CGIs observed in grade 4/5 cancers and of metastatic deposits to lymph nodes and bone.
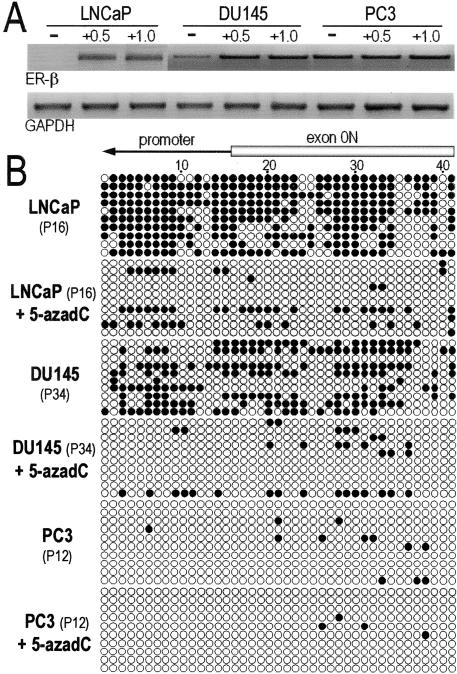
Methylation Status of the ER-β CGIs in LNCaP, DU145, and PC3 PCa Cell Lines before and after Treatment with 5-Aza-dC
To corroborate our in vivo data, three PCa cell lines expressing different levels of ER-β mRNA (PC3 > DU145 > LNCaP) were subjected to methylation analyses (Figure 5A). The extent of methylation in ER-β CGIs was the heaviest in LNCaP, followed by DU145 (Figure 5B). In contrast, this region was unmethylated in PC3 cells. Treatment of LNCaP and DU145 cells with 5-Aza-dC caused demethylation of ER-β CGIs and a concomitant restoration or increase in the expression of receptor mRNA (Figure 5A). Treatment of PC3 cells with 5-Aza-dC had no effects on methylation status and levels of mRNA expression. These in vitro results support the notion that DNA hypermethylation is a key determinant of ER-β expression.
Figure 5.
Expression of mRNA and patterns of DNA methylation in ER-β CGIs in PCa cell lines. A: Relative abundance of ER-β mRNA in RNA isolated from DU145, LNCaP, and PC3 PCa cell lines before and after treatment with 0.5 and 1.0 μmol/L 5-aza-dC. B: Representative methylation patterns in ER-β CGIs observed in DU145, LNCaP, and PC3 before and after treatment with 5-aza-dC.
Evidence of DNA Methylation Spreading from Exonic CGI to Promoter CGI
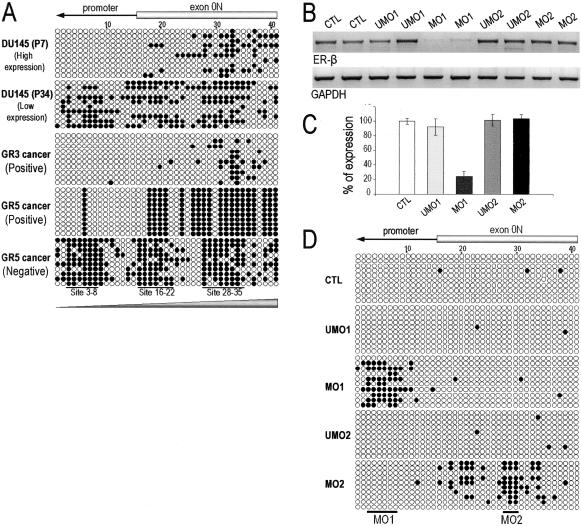
To further reveal other high-order methylation phenomena, we analyzed methylation changes and expression of ER-β mRNA in DU145 cells passaged in culture and found a progressive decrease in gene expression from early passages (less than 7 passages) to high passages (more than 25 passages) (data not shown). This decrease was attended by an increase in methylation of ER-β CGIs in a manner consistent with methylation spreading from the exonic CGI to the promoter CGI (Figure 6A, top two panels). Methylation spreading toward the 5′ promoter region was also observed in vivo in microdissected cancer foci with methylation occurred mostly in the exonic CGI in lower grade (grade 3) cancers and extended to the promoter CGI in higher grade cancers (grade 4/5) (Figure 6A, bottom three panels). Furthermore, high-grade cancers positive for receptor expression were more likely to have methylation restricted to the exonic CGI, whereas those that had both CGIs methylated lacked ER-β immunostaining.
Figure 6.
Changes in methylation patterns consistent with methylation spreading observed in vivo as illustrated in microdissected clinical samples and in vitro as illustrated in low- and high-passaged DU145 cells (A). B: Expression level of ER-β in PC3 cells after 48 hours of treatment with 2 μmol/L of unmethylated (UMO1 and UMO2) and methylated oligonucleotides (MO1 and MO2). Mock transfection was performed to serve as control (CTL). C: Percentage change of ER-β expression after oligonucleotide treatment. Columns represent an average of two individual experiments and the error bar represents SD of each treatment. D: Representative methylation patterns in ER-β CGIs in PC3 after treatment with various oligonucleotides. The targeting regions of the oligonucleotides (MO1 and MO2) were highlighted by solid bars.
Induction of ER-β Silencing by a Novel Methylated Oligonucleotide that Effectively Induces Promoter CGI Methylation
We next formulated a functional study to determine which CGI, when methylated, is responsible for ER-β silencing and if evidence of methylation spreading to neighboring CpGs could be demonstrated. For these studies, we have used a new class of oligonucleotides, known as methylated sense oligonucleotides (MOs),25 to achieve sequence-specific methylation in cellulo. MOs are synthetic sense 20 to 25 mers in which cytosine residues in at least three CpG dinucleotides are replaced with 5′-methylcytosine (m5C). Binding of the synthetic m5CpG probe to one strand of the gene forms a hemimethylated DNA intermediate, which has a replication fork-like structure. Because it is a preferred substrate of DNA methyltransferase 1 (DNMT-1) the process results in cytosine methylation of the first strand of the intermediate. When the synthetic probe leaves the targeted locus, the methylated strand reanneals to its complementary unmethylated DNA strand to form a second hemimethylated substrate for DNMT1 that immediately methylates the second strand. Methylation spreading is believed to follow and extend methylation to neighboring CpG dinucleotides beyond the targeted region. Two novel MOs and their complementary unmethylated counterparts were designed to target promoter CpG 3 to 8 (MO1) and exonic CpG 28 to 30 (MO2). Our results showed that transfection of PC-3 cells with MO1 or MO2 induced selectively regional methylation of either the promoter CGI (mostly between CpGs 3 to 8) or the exonic CGI (mostly between CpG 19 to 34), respectively (Figure 6D). Most importantly, transcription of ER-β was significantly suppressed in PC-3 cells transfected with MO1 but not in those transfected with MO2 (Figure 6, B and C). These data provided direct evidence that methylation of specific CpGs (CpG sites 3 to 8) in the promoter CGI is responsible for transcriptional silencing of the gene. In contrast, methylation of CpGs in the exonic CGIs alone exerted little inhibition on gene expression. Methylation spreading from the targeted sequence to the 5′ direction was clearly evident in cells transfected with MO1.
Discussion
A detailed study of the 5′-flanking region of the ER-β gene enabled us to identify two CGIs, one located in the proximal promoter region and the other in the untranslated first exon, known as 0N. Microdissection of foci with known receptor immunostaining status was then used to correlate changes in methylation statuses of ER-β CGIs with receptor expression in various lesions that comprise the entire natural history of PCa. A clear inverse relationship was observed between extent of methylation in ER-β CGIs and receptor expression in normal/hyperplastic glands, HG-PIN lesions, various grades of primary-site cancers, and metastases. Progressive hypermethylation of ER-β CGIs was accompanied by loss of receptor expression in primary PCas as they evolved from low- to high-grade lesions. Paradoxically, strong receptor expression was observed in metastatic PCas that possessed only lightly methylated ER-β CGI sequences. These findings clearly indicate that ER-β expression is epigenetically regulated by methylation throughout the entire natural history of prostatic carcinogenesis and metastasis. Because the methylation of specific genes can be detected in DNA from diagnostic biopsies,26 ER-β methylation status could be a helpful potential adjunct to morphological criteria for a more precise stage-specific identification of primary and recurrent PCas.
Findings from cell culture experiments corroborated our observations in vivo. We noted that ER-β CGIs were extensively methylated in LNCaP and high-passage DU145 PCa cell lines that expressed low levels of ER-β mRNA. In contrast, the CGIs were unmethylated in PC3 cells that expressed high levels of the transcript. Treatment of LNCaP and DU145 cells with a demethylating agent reduced methylation of cytosine in both CGIs and increased expression of ER-β mRNA.
Hypermethylation-associated silencing of anti-tumor genes has been proposed as an epigenetic mechanism of tumorigenesis.15,27 In our current work, we found evidence that ER-β CGI methylation may repress expression of ER-β during initiation and progression of PCa in the primary site. Intriguingly, strong expression of ER-β, along with a corresponding paucity of methylation was found in the majority of metastases, particularly those involving bone. The presence of high levels of ER-β in metastatic PCa cells may indicate that the receptor confers a selective advantage for subclones of primary PCa cells to metastasize and/or establish themselves in distant sites. Alternatively, the same phenotype could arise if primary PCa cells, with heavily methylated ER-β CGIs, are demethylated after exposure to local factors in metastatic sites and re-express the receptor. Given the proposed function of ER-β in protecting normal prostate epithelial cells from undergoing aberrant cell proliferation5,6,10 and its possible role in enhancing survival of PCa cells,12–14 we conclude that repression of ER-β expression in local PCa and re-expression of the gene within metastases may facilitate both early tumor development and subsequent metastatic progression.
Our ability to amplify a very long amplicon (500 bp) that contains both ER-β CGIs, to obtain sequence data from adequate numbers of alleles derived from a single bisulfite-modified DNA sample, and to analyze multiple microdissected foci of the same morphological entity has enabled us to generate a large data set. This has allowed us to follow and compare changes in methylation at individual CpG sites, to identify methylation clusters, and to detect higher order phenomena such as methylation seeding and spreading.15 The use of hierarchical cluster analyses has proven superior to visual inspection in identifying methylation centers. Three methylation centers within the two ER-β CGIs were identified by these analyses that closely approximate the three regions where we observed the most dramatic changes in methylation status during PCa evolution and progression. Our data indicate that methylation may first occur as a stochastic phenomenon at low frequencies, in the three methylation centers in normal, hyperplastic, and dysplastic epithelia, via a process known as methylation seeding.18 This concept proposes that the transient methylation of one or a few CpG dinucleotides facilitates more permanent and extensive methylation of the neighboring sites within the region.
It is of particular interest that these methylation centers are located in consensus DNA sequences of TF-binding sites or cis-regulatory elements (Figure 1). It has been demonstrated that DNA methylation can interfere with protein-DNA interaction, recruitment of histone deacetylases, and the induction of chromatin condensation necessary for gene inactivation.15,28 Several methyl CpG-binding proteins can also compete with TFs for the same DNA sequences.28 On the basis of this body of knowledge, it is reasonable to speculate that the DNA sequences comprising the methylation centers uncovered in our study may also be the elements responsible for regulating ER-β gene expression by TFs.
The second methylation-related phenomenon, methylation spreading,29 appeared to occur within the two CGIs of ER-β. In this regard, results from the analysis of LCM specimens indicate that exonic CpGs became methylated first, before those in the promoter region. Findings from our cell culture experiments corroborated our observations in LCM specimens. ER-β CGIs were virtually unmethylated in the promoter CGIs in low-passaged DU145 PCa cells that strongly express the receptor. However in high-passaged DU145 cells methylation had extended to the promoter region and was attended by a significant loss of ER-β mRNA expression. It has been suggested that CGIs located in promoters are less accessible to DNA methyltransferases because of steric hindrance from transcription-initiation complexes and binding of TFs to upstream enhancer sequences.28 In contrast, CpGs in exonic CGIs are less likely to encounter this limitation and thus have a higher propensity for becoming methylated first, an event that facilitates methylation spreading to promoter CpGs.29 Previous studies have shown that once promoters are methylated they are stably inactivated and the gene is ultimately silenced.30,31 Although the phenomenon of methylation spreading has been demonstrated in several experimental systems,15 our data provide the first correlative evidence that it occurs in PCa and parallels gene silencing during the initiating phases and subsequent progression of these cancers in the primary site.
Importantly, using methylated sense oligonucleotides, the present study provides the first direct evidence in support of differential roles played by the two CGIs in ER-β regulation. In PC-3 cells both ER-β CGIs are unmethylated and the receptor is expressed at high levels. Exposure of PC-3 cells to MO1 that targets CpG 3 to 8 in the promoter CGI inhibited ER-β expression whereas MO2 that causes methylation in the exonic CGI did not suppress gene expression. These results are in agreement with the concept that methylation of exonic CpGs does not directly contribute to gene silencing but might facilitate methylation spreading whereas methylation of specific CpGs in the promoter regions are responsible for gene silencing.
Late-stage PCa and particularly bone metastasis is a major challenge for the management of patients with progressive disease because no treatments are currently available that effectively alter its outcome. Our finding of strong ER-β expression and low-methylation status of the promoter CGI in bone metastases, together with the possibility that the receptor enhances survival of PCa cells12–14 suggests that the use of MOs to inhibit ER-β transcription by direct gene silencing may provide a novel therapeutic option for the treatment of advanced PCa. Future experiments are needed to compare the efficacy of MOs to existing therapeutic oligonucleotides that target inhibition of gene expression by either suppressing translation as in the case of anti-sense oligonucleotides32 or by inducing mRNA degradation such as siRNA and ribozymes.33
In summary, we have conducted a detailed analysis of cytosine methylation of the ER-β with correlative information on receptor expression from individually immunostained-microdissected morphological entities that encompass the entire natural history of PCa. Moreover, we are first to use algorithms commonly used in gene profiling for the identification of methylation centers which has facilitated our documentation of other higher order methylation events. This protocol, supplemented with data from cell cultures, allowed us to conclude that dynamic increases in methylation at the three CpG centers, along with spreading in the two ER-β CGIs, plays a key role in regulating ER-β expression during the development and progression of PCa. Because ER-β undergoes stage-specific methylation and expression changes this information may contribute to our understanding of the development and progression of PCa and could be useful for the prognosis of the cancer. Furthermore, the use of MOs to inhibit ER-β transcription by methylation-directed gene silencing may provide a novel therapeutic option for the treatment of metastatic PCa.
Acknowledgments
We thank Dr. Gerald Murphy (University of Florida Medical School) and Dr. Mary Ellen Taplin (University of Massachusetts Medical School) for providing us with sections from six lymph node metastases and five bone metastases biopsies, respectively.
Footnotes
Address reprint requests to Shuk-Mei Ho, Ph.D., 364 Plantation St., Rm. 504, Lazare Research Bldg., Department of Surgery, University of Massachusetts Medical School, Worcester, MA 01605-2324. E-mail: shuk-mei.ho@umassmed.edu.
Supported in part by the National Institutes of Health (grants DK61084 and CA94221), the Department of Defense (award PC030595), and the Richard M. Lucas Foundation for Cancer Research (to J.E.M.).
References
- Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000;27:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol. 2001;63:165–192. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Horvath LG, Henshall SM, Lee CS, Head DR, Quinn DI, Makela S, Delprado W, Golovsky D, Brenner PC, O’Neill G, Kooner R, Stricker PD, Grygiel JJ, Gustafsson JA, Sutherland RL. Frequent loss of estrogen receptor-beta expression in prostate cancer. Cancer Res. 2001;61:5331–5335. [PubMed] [Google Scholar]
- Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM. Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol. 2001;159:79–92. doi: 10.1016/s0002-9440(10)61676-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano MM, Katzenellenbogen BS. The quinone reductase gene: a unique estrogen receptor-regulated gene that is activated by antiestrogens. Proc Natl Acad Sci USA. 1997;94:2581–2586. doi: 10.1073/pnas.94.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim GJ, Wang L, Andersson S, Nagy N, Kis LL, Zhang Q, Makela S, Warner M, Gustafsson JA. Disruption of the estrogen receptor beta gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci USA. 2003;100:6694–6699. doi: 10.1073/pnas.0731830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S, Loda M. Estrogen receptor beta in prostate cancer: brake pedal or accelerator? Am J Pathol. 2001;159:13–16. doi: 10.1016/s0002-9440(10)61666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson JA. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z, Warner M, Gustafsson JA. Estrogen receptor beta in the prostate. Mol Cell Endocrinol. 2002;193:1–5. doi: 10.1016/s0303-7207(02)00089-8. [DOI] [PubMed] [Google Scholar]
- Kim HT, Kim BC, Kim IY, Mamura M, Seong DH, Jang JJ, Kim SJ. Raloxifene, a mixed estrogen agonist/antagonist, induces apoptosis through cleavage of BAD in TSU-PR1 human cancer cells. J Biol Chem. 2002;277:32510–32515. doi: 10.1074/jbc.M202852200. [DOI] [PubMed] [Google Scholar]
- Lau KM, LaSpina M, Long J, Ho SM. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: regulation by methylation and involvement in growth regulation. Cancer Res. 2000;60:3175–3182. [PubMed] [Google Scholar]
- Neubauer BL, McNulty AM, Chedid M, Chen K, Goode RL, Johnson MA, Jones CD, Krishnan V, Lynch R, Osborne HE, Graff JR. The selective estrogen receptor modulator trioxifene ( LY133314) inhibits metastasis and extends survival in the PAIII rat prostatic carcinoma model. Cancer Res. 2003;63:6056–6062. [PubMed] [Google Scholar]
- Garinis GA, Patrinos GP, Spanakis NE, Menounos PG. DNA hypermethylation: when tumour suppressor genes go silent. Hum Genet. 2002;111:115–127. doi: 10.1007/s00439-002-0783-6. [DOI] [PubMed] [Google Scholar]
- Nojima D, Li LC, Dharia A, Perinchery G, Ribeiro-Filho L, Yen TS, Dahiya R. CpG hypermethylation of the promoter region inactivates the estrogen receptor-beta gene in patients with prostate carcinoma. Cancer. 2001;92:2076–2083. doi: 10.1002/1097-0142(20011015)92:8<2076::aid-cncr1548>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC, Gustafsson JA, Dahlman-Wright K. Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene. 2003;22:7600–7606. doi: 10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]
- Wu MC, Ho SM. PMP24, a gene identified by MSRF, undergoes DNA hypermethylation-associated gene silencing during cancer progression in an LNCaP model. Oncogene. 23:250–259. doi: 10.1038/sj.onc.1207076. [DOI] [PubMed] [Google Scholar]
- Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- Lance GN, Williams WT. A general theory of classificatory sorting strategies. Computer J. 1967;9:373–380. [Google Scholar]
- Li LC, Yeh CC, Nojima D, Dahiya R. Cloning and characterization of human estrogen receptor beta promoter. Biochem Biophys Res Commun. 2000;275:682–689. doi: 10.1006/bbrc.2000.3363. [DOI] [PubMed] [Google Scholar]
- Hirata S, Shoda T, Kato J, Hoshi K. The multiple untranslated first exons system of the human estrogen receptor beta (ER beta) gene. J Steroid Biochem Mol Biol. 2001;78:33–40. doi: 10.1016/s0960-0760(01)00071-1. [DOI] [PubMed] [Google Scholar]
- Moore JT, McKee DD, Slentz-Kesler K, Moore LB, Jones SA, Horne EL, Su JL, Kliewer SA, Lehmann JM, Willson TM. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- Leung YK, Ho JW. Induction of UDP-glucuronosyltransferase 1A8 mRNA by 3-methylcholanthene in rat hepatoma cells. Biochem Pharmacol. 2002;63:767–775. doi: 10.1016/s0006-2952(01)00902-9. [DOI] [PubMed] [Google Scholar]
- Yao X, Hu JF, Daniels M, Shiran H, Zhou X, Yan H, Lu H, Zeng Z, Wang Q, Li T, Hoffman AR. A methylated oligonucleotide inhibits IGF2 expression and enhances survival in a model of hepatocellular carcinoma. J Clin Invest. 2003;111:265–273. doi: 10.1172/JCI15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Groopman JD, Umar A. DNA methylation as a cancer-specific biomarker: from molecules to populations. Ann NY Acad Sci. 2003;983:286–297. doi: 10.1111/j.1749-6632.2003.tb05983.x. [DOI] [PubMed] [Google Scholar]
- Clark SJ, Warnecke PM. DNA methylation analysis in mammalian cells. Methods. 2002;27:99–100. doi: 10.1016/s1046-2023(02)00059-2. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Turner BM. Histone deacetylases: complex transducers of nuclear signals. Semin Cell Dev Biol. 1999;10:179–188. doi: 10.1006/scdb.1999.0299. [DOI] [PubMed] [Google Scholar]
- Turker MS. Gene silencing in mammalian cells and the spread of DNA methylation. Oncogene. 2002;21:5388–5393. doi: 10.1038/sj.onc.1205599. [DOI] [PubMed] [Google Scholar]
- Bird AP, Wolffe AP. Methylation-induced repression—belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- Rountree MR, Bachman KE, Herman JG, Baylin SB. DNA methylation, chromatin inheritance, and cancer. Oncogene. 2001;20:3156–3165. doi: 10.1038/sj.onc.1204339. [DOI] [PubMed] [Google Scholar]
- Stephens AC, Rivers RP. Antisense oligonucleotide therapy in cancer. Curr Opin Mol Ther. 2003;5:118–122. [PubMed] [Google Scholar]
- Lavery KS, King TH. Antisense and RNAi: powerful tools in drug target discovery and validation. Curr Opin Drug Discov Dev. 2003;6:561–569. [PubMed] [Google Scholar]