Abstract
The tissue inhibitor of metalloproteinase-1 (TIMP1) is expressed in a subset of malignant lymphomas and can inhibit tumor spread and promote cell survival. Recent data suggest that TIMP1 expression may be regulated by signal transducer and activator of transcription (STAT)-3. Thus, we tested the hypothesis that TIMP1 expression is related to STAT3 activation in lymphomas, with a focus on anaplastic large cell lymphomas (ALCLs), which are known to express high levels of phosphorylated/active STAT3 (pSTAT3). Specific inhibition of STAT3 with a dominant-negative construct led to concentration-dependent down-regulation of TIMP1 expression in two anaplastic lymphoma kinase (ALK)+ ALCL cell lines, Karpas 299 and SU-DHL-1. Using cDNA microarrays, ALK+ ALCL cell lines consistently expressed the highest TIMP1 level among 29 lymphoma cell lines of various subtypes. The association between TIMP1 expression and high level of STAT3 activation was validated by Western blots and immunostaining using antibodies specific for pSTAT3 and TIMP1. We further evaluated the relationship between TIMP1 expression and STAT3 activation in 43 ALCL tumors (19 ALK+ and 24 ALK−) using immunohistochemistry and a tissue microarray. The TIMP1+ group had a mean of 64% pSTAT3+ cells as compared to 23% pSTAT3+ cells in the TIMP1− group (P = 0.002). As expected, TIMP1 positivity was higher in the ALK+ group (15 of 19, 79%) compared with the ALK− group (5 of 24, 21%; P = 0.0002) because NPM-ALK restricted to ALK+ tumors was previously shown to activate STAT3. In conclusion, STAT3 directly contributes to the high level of TIMP1 expression in ALK+ ALCL, and TIMP1 expression correlates with high level of STAT3 activation in ALCL. TIMP1, as a downstream target of STAT3, may mediate the anti-apoptotic effects of STAT3.
The tissue inhibitors of metalloproteinases (TIMPs) constitute a family of secreted proteins, of which their known primary function is inhibition of the degradative action of matrix metalloproteinases. In pathological states, TIMPs have been shown to inhibit tumor invasion and metastasis through regulation of extracellular homeostasis.1–3 Overexpression of TIMP1, one of the TIMP family members, was shown to inhibit tumor growth and metastasis of T-cell lymphoma in transgenic mice.4 In addition to its roles in regulating the extracellular matrix, TIMP1 also possesses anti-apoptotic and differentiation properties in B cells as well as in breast cancer cells.5,6 In B-cell lymphomas, TIMP1 expression correlates with the high histological grade and is associated with worse clinical outcome.7–9 TIMP1 is also constitutively expressed in some Hodgkin’s lymphoma cell lines and tumors.10 Regulation of TIMP1 expression is incompletely understood, but previous studies have shown that interleukin (IL)-6 and IL-10 up-regulate TIMP1 expression.11–15 Recent data also suggest that TIMP1 expression may be regulated by STAT3 because the binding site for STAT3 has been found in the promoter region of the TIMP1 gene in rat cells.16 Nevertheless, the exact role of STAT3 in regulating TIMP1 expression in human neoplasms has not been examined.
The signal transducers and activators of transcription (STATs) are members of a ubiquitously expressed family of transcription factors activated in response to growth factors and cytokines.17,18 STAT3 has been shown to be an oncogene.19 Many types of human cancer express constitutively active STAT3.20–24 Several downstream targets of STAT3 have been identified in various cell types, many of which are associated with the regulation of apoptosis and cell proliferation, such as Bcl-XL, Mcl-1, cyclin D3, c-Myc, and p21waf-1 17. Recently, it has been shown that the chimeric NPM-ALK oncoprotein, which results from the t(2;5)(p23;q35) seen in a substantial subset of anaplastic large cell lymphomas (ALCLs),25 is capable of binding to and phosphorylating STAT3 at the Tyr705 residue.26 We recently also have confirmed the finding that activation of STAT3 by phosphorylation is strongly associated with anaplastic lymphoma kinase (ALK) expression in ALCL tumors.27
With this background, we hypothesized that TIMP1 expression is related to STAT3 activation in malignant lymphoma. In particular, we evaluated TIMP1 expression in ALK+ ALCL cell lines and tumors because these cells have a relatively high level of STAT3 activation, which is related to the presence of NPM-ALK. We also assessed for a direct role of STAT3 in contributing to TIMP1 expression in ALK+ ALCL cell lines by using a dominant-negative STAT3 construct. Our results provide evidence that STAT3 plays a direct role in up-regulating TIMP1 in ALK+ ALCL, and demonstrate that TIMP1 expression correlates with high level of STAT3 activation in lymphomas.
Materials and Methods
cDNA Microarray Analysis of 29 Lymphoma Cell Lines
The lymphoma cell lines analyzed by cDNA microarrays in this study are listed in Table 1. Three ALK+ ALCL cell lines, Karpas 299, SR-786, and SU-DHL-1 were used in this study. In addition, four Hodgkin’s lymphoma cell lines, (L428, L1236, MDA-E, and MDA-V), eight diffuse large B-cell lymphoma cell lines (CJ, EJ-1, FL318, JM, JMEA, JP, MS, and SKI-DLCL1), six Burkitt lymphoma cell lines (CA 46, Daudi, Ramos, BCHN-1, Raji, and ST 486), four mantle cell lymphoma cell lines (JeKo-1, M-1, Mino, and SP53), and four body cavity effusion lymphoma cell lines (BC-1, BC-2, BC-3 and BC-4) were included. All cell lines were grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Life Technologies, Inc., Grand Island, NY), supplemented with 10% heat-inactivated fetal bovine serum, 10,000 U/ml penicillin (Sigma, St. Louis, MO), 10 mg/ml streptomycin (Sigma), and 200 mmol/L l-glutamine (Life Technologies), and incubated at 37°C in 5% CO2. We performed cDNA microarray analysis with RNA extracted from these lymphoma cell lines using the Trizol reagent. Briefly, cDNA microarray expression profile analysis was performed with Clontech Human Cancer 1.2 cDNA nylon membrane (Clontech, Palo Alto, CA). Radiolabeled cDNA probes were synthesized by reverse transcription from 5 μg of total RNA using the manufacturer’s protocol with P32-dATP (Amersham Biosciences, Piscataway, NJ). The images were quantitated using the Image Quantification Software Array Vision from Imaging Research Inc. (St. Catharines, Ontario, Canada). The signal intensities and the local background intensities were determined, and the background-subtracted signal intensities were used for analysis using a program designed at the University of Texas M.D. Anderson Cancer Center for microarray analysis. As a structure detection method, principal component analysis was applied to this set of data, with six groups corresponding to six subtypes of lymphoma.28 Two group statistics, in which the log-transformed normalized data along with the different groups of samples as a vector, were used to obtain gene-by-gene means and variance for two groups. The significant t-statistics were those with values higher than the cutoff.
Table 1.
Lymphoma Cell Lines Used for cDNA Microarray
| Anaplastic large cell lymphoma | Karpas 299, SR 786, SU-DHL-1 |
| Burkitt lymphoma | BCHN1, CA 46, Daudi, Raji, Ramos, ST 486 |
| Body cavity effusion lymphoma | BC-1, BC-2, BC-3, BC-4 |
| Hodgkin’s lymphoma | L428, L1236, MDA-E, MDA-V |
| Large B-cell lymphoma | CJ, EJ-1, FL318, JMEA, JP, JM, MS, SKI-DLCL1 |
| Mantle cell lymphoma | JEKO-1, M-1, MINO, SP53 |
Cell lines in bold type were also further assessed for TIMP1 expression by Western blots and/or immunocytochemistry.
Microarrays of ALCL Cell Lines and Tumors
For the preparation of the lymphoma cell line microarray, 23 lymphoma cell lines were included, as shown in Table 2. Most (16 of 23) of these cell lines were included in the cDNA microarray studies. Additional cell lines were Jurkat (T-cell acute lymphoblastic leukemia), SKW-3 (T-cell prolymphocytic leukemia), HH, and HUT 78 (Sezary syndrome), and FN, LP, and LR (diffuse large B-cell lymphoma). ALL cell lines were kept in conditions described above, grown to mid-logarithmic phase, fixed overnight in buffered formalin, and embedded in paraffin. Two cores of each cell block were used to create the lymphoma cell line microarray.
Table 2.
Summary of the Cell Lines Used in This Study, and Their Expression of TIMP1 and pSTAT3 by Immunohistochemistry and Western Blots
| Cell lines | Type of cell lines | TIMP-1 by ICP | TIMP1 by WB | pSTAT3 by IH | PSTAT3 by WB |
|---|---|---|---|---|---|
| Karpas 299 | Anaplastic large cell lymphoma, ALK-positive | + | Strongly + | + | Strongly + |
| SU-DHL-1 | Anaplastic large cell lymphoma, ALK-positive | + | Strongly + | + | Strongly + |
| SR-786 | Anaplastic large cell lymphoma, ALK-positive | + | Strongly + | + | Strongly + |
| Jurkat | T-cell acute lymphoblastic leukemia | − | Weakly + | − | − |
| SKW-3 | T-cell prolymphocytic leukemia | − | − | − | − |
| HUT-78 | Sezary syndrome | − | − | − | − |
| HH | Sezary syndrome | − | − | − | − |
| MS | Diffuse large cell lymphoma, t(14;18)-positive | − | − | − | − |
| CJ | Diffuse large cell lymphoma, t(14;18)-positive | − | − | − | − |
| EJ-1 | Diffuse large cell lymphoma, t(14;18)-positive | − | − | − | − |
| LP | Diffuse large cell lymphoma | − | − | − | − |
| LR | Diffuse large cell lymphoma | − | − | − | − |
| JMEA | Diffuse large cell lymphoma | − | − | − | − |
| FN | Diffuse large cell lymphoma | − | Weakly + | − | − |
| SKI-DLCL1 | Diffuse large cell lymphoma | − | Weakly + | − | Weakly + |
| SP53 | Mantle cell lymphoma | − | − | − | Weakly + |
| Mino | Mantle cell lymphoma | − | Weakly + | − | − |
| BC-1 | BC-lymphoma | − | − | − | − |
| BC-3 | BC-lymphoma | − | Weakly + | − | Weakly + |
| MDA-V | Hodgkin lymphoma | − | − | − | − |
| L428 | Hodgkin lymphoma | − | − | − | − |
| CA-46 | Burkitt lymphoma | − | − | − | − |
| Daudi | Burkitt lymphoma | − | − | − | − |
ICP, immunocytochemistry using paraffin-embedded, cell line microarray (the presence of any definitive immunostained cells was regarded as positive); WB, Western blots; ND, Not determined; IH, immunohistochemistry; BC-lymphoma, body cavity effusion lymphoma.
Cell lines in bold type were also previously included in the cDNA microarray studies.
For the preparation of the ALCL tumor tissue microarray, only tumors that fulfilled the diagnostic criteria of ALCL as defined in the World Health Organization classification25 were included. All ALCL tumors were uniformly positive for CD30, negative for CD20 and PAX5, and were of either T cell (37 cases) or null-cell (6 cases) immunophenotype. Nineteen (44%) tumors were ALK+ and 24 (56%) were ALK−. All ALCL tumor specimens were pretreatment biopsy samples that were fixed in formalin, routinely processed, and embedded in paraffin. To construct the ALCL tissue microarrays, three representative cores from each tumor were selected from the paraffin tissue blocks. Triplicate cores from two reactive lymph nodes were included in the tissue microarrays as internal controls.
STAT3 Dominant-Negative Adenovirus Vector and Infection Protocol
The production and characteristics of STAT3 dominant-negative adenovirus vector have been detailed elsewhere.29–31 Briefly, the adenoviral vector was deleted at the E1A region. Using the site-directed mutagenesis technique, the STAT3 cDNA was modified such that the tyrosine705 residue was replaced by phenylalanine. Expression of STAT3 was driven by the rabbit β-actin promoter and cytomegalovirus enhancer. The construct was epitope-tagged with FLAG (DYKDDDDK) (Kodak, Rochester, NY) at the N terminal. Before each experiment, cells were grown in logarithmic phase. Cells were first concentrated to 107/ml, and these highly concentrated cell suspensions were incubated with adenoviral vectors at varying titers to create multiplicity of infection (MOI) ranging from 0 to 100 for 1 hour at 37°C. Subsequently, fresh complete medium was added to dilute the cell suspensions to 106/ml. Cell lysates were prepared after 24 hours of incubation.
Antibodies and Immunostaining
Antibodies reactive with STAT3, pSTAT3 (Tyr705), and TIMP1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FLAG antibody was purchased from Abcam (Cambridge, UK). Immunostaining was performed using standard techniques. Briefly, heat-induced epitope retrieval was performed. 3,3′-Diaminobenzidine/H2O2 (Biogenex, San Ramon, CA) was used as a chromogen, and hematoxylin as the counterstain. For each case, TIMP1 was considered positive when at least 20% of the tumor cells showed definitive cytoplasmic staining. STAT3 was considered to be activated when more than 20% of the neoplastic cells showed unequivocal nuclear immunostaining of pSTAT3, regardless of the staining intensity. Evaluation of TIMP1 and pSTAT3 immunostaining was performed separately without knowing the results of the staining of the other marker or clinical outcome.
Western Blot Analysis
Western blot analysis was performed using standard techniques. Briefly, the cells were washed in phosphate-buffered saline (pH 7.5), and lysed in a buffer containing 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40, 1 mmol/L phenylmethyl sulfonyl fluoride, 5 μg/ml aprotinin (5 μg/ml), sodium vanadate (1 mmol/L), and leupeptin (5 μg/ml). After incubation on ice for 15 minutes, the lysates were subjected to centrifugation at 12,000 rpm, and the supernatants were collected. Protein concentration was determined with a protein assay kit (Bio-Rad, Hercules, CA). Each lane of a 5 to 12% polyacrylamide slab gel received 80 μg of protein. After electrophoresis and transfer to nitrocellulose membranes (Bio-Rad) by electroblotting, blots were probed with specific primary and secondary antibodies and the enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL) according to the manufacturer’s protocol. The antibodies were used at dilutions of 1:500 to 1:1000.
Statistical Analysis
The association between TIMP1 and various parameters was evaluated by Fisher’s exact test. The nonparametric correlation between pSTAT3 expression as a continuous variable (percentage of pSTAT3-positive tumor cells) and TIMP1 expression was assessed by the Mann-Whitney test. All statistical calculations were performed using the StatView software (Abacus Concepts, Inc., Berkeley, CA).
Results
Down-Regulation of TIMP1 Expression in ALK+ ALCL Cells by Inhibiting STAT3
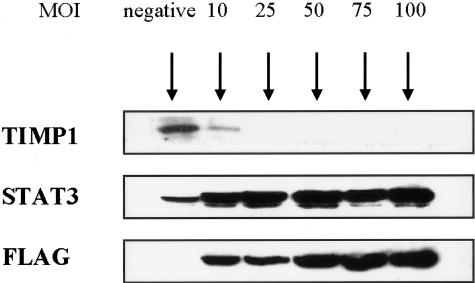
Because the TIMP1 promoter contains the STAT3-binding consensus sequence in rat cells, we determined if STAT3 activation directly contributes to the relatively high level of TIMP1 expression in ALK+ ALCL cells. We blocked STAT3 signaling using an adenoviral vector that expresses a dominant-negative STAT3 construct. Our previous experiment using an adenoviral vector carrying green fluorescent protein construct showed that a MOI of 10 led to >70% of SU-DHL-1 and Karpas 299 cells expressing relatively high levels of green fluorescent signals detectable by flow cytometry. Both SU-DHL-1 and Karpas 299 also had similar efficiency of infection with the adenoviral vector expressing dominant-negative STAT3, as assessed by the intensity of STAT3 and FLAG staining in Western blots (not shown). As exemplified in Figure 1, using Karpas 299, gene transfer of the dominant-negative STAT3 construct led to an increase in the total STAT3 protein level and FLAG level in a dose-dependent manner. With the same experimental conditions, TIMP1 immunoreactivity decreased in a dose-dependent manner and became undetectable at a MOI of 25. Similar experiments using SU-DHL-1 cells also yielded comparable findings. Thus, STAT3 signaling directly contributes to the high level of TIMP1 expression in ALK+ ALCL cell lines.
Figure 1.
Adenoviral vector gene transfer of a dominant-negative STAT3 construct into Karpas 299 led to down-regulation of TIMP1 expression in a dose-dependent manner. MOI represents multiplicity of infection. STAT3 represents the total amount of STAT3, including both exogenous and endogenous STAT3. FLAG staining showed an increased amount of the expressed constructs with higher MOIs.
Consistently High TIMP1 Expression in ALK+ ALCL Cell Lines
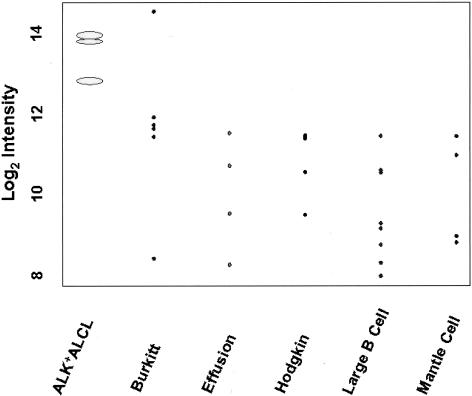
As shown in Figure 2, cDNA microarray analysis showed that TIMP1 was highly expressed in all three cell lines derived from ALK+ ALCL, including Karpas 299, SU-DHL-1, and SR-786. The median expression level of TIMP1 was 1 to 2 log-fold higher than the other lymphoma cell types. The t-statistics in the two group statistical analysis gave a highly significant value (t = −5.2; P < 0.0001) for TIMP1 in the ALK+ ALCL group compared to the other groups. Of the remaining 26 lymphoma cell lines examined, only 1 derived from a Burkitt lymphoma had a comparable expression level of TIMP1. All other Burkitt lymphoma cell lines showed lower levels of TIMP1 expression compared with ALK+ ALCL cells. Thus, high TIMP1 expression in lymphoma cell lines is almost restricted to and is most consistently in the ALK+ ALCL cell type.
Figure 2.
Statistical analysis of the cDNA microarray data with plot of log (base 2) intensity (expression) of each histological group of lymphoma cell lines. All three ALK+ ALCL cell lines consistently had a higher level of TIMP1 expression than cell lines representing the other lymphoma subtypes. Although one Burkitt lymphoma cell line had a high level of TIMP1, this was not a consistent finding in this type of lymphoma.
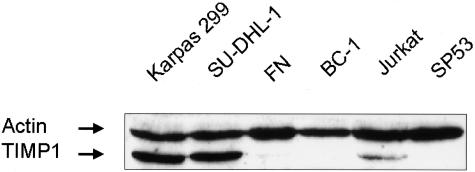
To validate the cDNA data, we performed Western blot analysis and immunocytochemistry using a tissue microarray that included 23 lymphoma cell lines, 16 of which were previously included in the cDNA microarray study. The results are summarized in Table 2 and some of the Western blots are illustrated in Figure 3. Western blots showed that all three ALK+ ALCL cell lines expressed TIMP1 strongly and 5 of 23 cell lines (BC-3, FN, Jurkat, Mino, and SKI-DLCL1) expressed TIMP1 weakly. All other cell lines showed no detectable TIMP1 expression.
Figure 3.
Western blot analysis showed that Karpas 299 and SU-DHL-1 had a relatively high TIMP1 expression (bottom bands). Jurkat and FN cells had detectable TIMP1 but at a lower level. All of the other cell lines were negative. The top bands represent actin, which was expressed at a similar level in all cell lines examined.
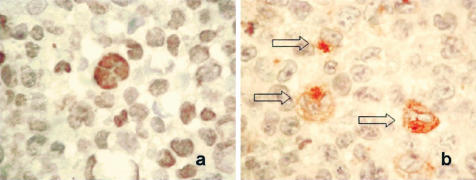
Immunocytochemistry using the lymphoma cell line tissue microarray and an anti-TIMP1 antibody showed that TIMP1 immunoreactivity was detectable only in ALK+ ALCL cell lines; those cell lines that showed a weak TIMP1 band or no detectable TIMP1 band on Western blots had no detectable TIMP1 immunoreactivity in any cells by immunocytochemistry. Figure 4 shows that Karpas 299 cells uniformly expressed pSTAT3 that was localized to the nucleus (Figure 4a), and TIMP1 that was present in the cytoplasm with a distinct Golgi pattern (Figure 4b). The cells that highly expressed TIMP1 were generally the larger lymphoma cells. Because of the fact that staining was confined to the Golgi, the proportion of TIMP1-positive was probably underestimated because our method only allowed us to evaluate a section of the cell. Overall, considering that Western blotting is more sensitive than immunostaining, our results showed concurrence between Western blots and immunocytochemistry, and the findings validate the cDNA microarray data.
Figure 4.
Immunocytochemistry showing expression of pSTAT3 (a) and TIMP1 (b) in Karpas 299 cells in the lymphoma cell line microarray. Many lymphoma cells showed pSTAT3 nuclear staining. A small subset of lymphomas, mostly the large lymphoma cells (open arrows), showed TIMP1 reactivity; both cytoplasmic and Golgi patterns were recognized.
Correlation of TIMP1 Expression with STAT3 Activation in ALCL Tumors
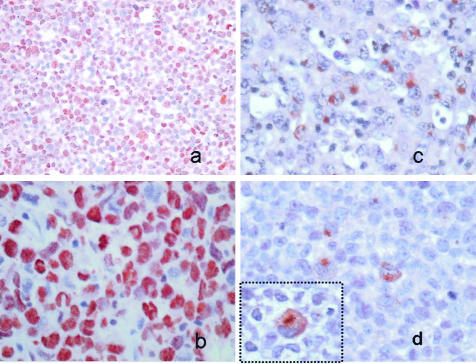
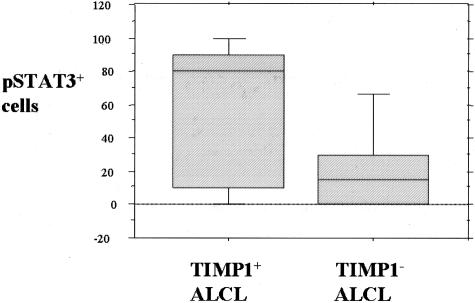
Because simultaneous high expression levels of pSTAT3 and TIMP1 were found only in ALK+ ALCL cell lines, we further investigated if the relationship between TIMP1 expression and STAT3 activation is also present in ALCL tumors. We assessed 43 cases of systemic ALCL tumors (19 ALK+ and 24 ALK−) included in a tissue microarray using immunohistochemistry. The results are summarized in Table 3. Overall, 20 of 43 (47%) tumors were positive for TIMP1. Similar to the cell lines, TIMP1 demonstrated a distinct Golgi staining pattern in most of the positive cases, as shown in Figure 5. TIMP1 expression significantly correlated with ALK expression; 15 of 19 (79%) of ALK+ ALCL tumors compared with 5 of 24 (21%) ALK− ALCL tumors were positive for TIMP1 (P = 0.0002, Fisher’s exact test). We also found that TIMP1 expression significantly correlated with pSTAT3 immunoreactivity, with 13 of 22 (59%) pSTAT3+ ALCL tumors being positive for TIMP1, compared with 3 of 16 (19%) pSTAT3− cases (P = 0.02, Fisher’s exact test). In addition, when TIMP1 expression was correlated with STAT3 activation based on the percentage of pSTAT3 cells (as a continuous variable), TIMP1+ cases demonstrated a significantly higher mean percentage of pSTAT3+ cells compared with TIMP1− cases (64% versus 23%, P < 0.002, Mann-Whitney test; Figure 6).
Table 3.
Association between ALK-1 and TIMP1 in 43 Cases of ALCL
| TIMP1-positive | TIMP1-negative | Total cases | |
|---|---|---|---|
| ALK-1-positive | 15 (78.9%) | 4 (21.1%) | 19 |
| ALK-1-negative | 5 (20.8%) | 19 (79.2%) | 24 |
P = 0.0002 (Fisher’s exact test).
Figure 5.
Immunohistochemistry using anti-pSTAT3 (a and b) and anti-TIMP1 (c and d) applied to the ALCL tissue microarray, with strong expression of pSTAT3 in a case of ALK+ ALCL as shown in a (low magnification) and b (high magnification). In the same case, TIMP1 is highly expressed, as shown in c and d. The inset in d highlights the Golgi TIMP1 staining of the lymphoma cells.
Figure 6.
Box plot illustrating that TIMP1+ ALCL tumors have a higher mean number of pSTAT3+ cells than TIMP1− ALCL tumors.
Correlation of TIMP1 Expression with Clinical Parameters in ALCL Tumors
TIMP1 expression correlated with progression-free survival (PFS) in 36 patients with ALCL tumors (14 ALK+, 22 ALK−) and available clinical follow-up data. In the ALK+ group, the 5-year PFS was 89% for patients with TIMP1-positive tumors, compared with 50% for patients with TIMP1-negative tumors. Because of the small number of patients in this analysis, the difference in PFS between the two groups is not statistically significant (P = 0.5 by log rank). For the ALK− group, 5-year PFS was 27% for patients with TIMP1-positive tumors, compared with 74% for patients with TIMP1-negative tumors (P = 0.05 by log rank).
Expression of TIMP1 in Peripheral T-Cell Lymphomas
To further support that ALK+ ALCL cells have relatively high TIMP1 expression compared to other T-cell neoplasms, we examined nine cases of peripheral T-cell lymphomas for TIMP1 using immunohistochemistry and a protocol similar to that used for immunocytochemistry described in Methods and Materials. All nine cases of peripheral T-cell lymphomas had no detectable TIMP1 or pSTAT3 expression.
Discussion
The primary function of TIMPs in physiological states is to inhibit the degradation effects of metalloproteinases. In pathological states, it has been shown that TIMPs may inhibit tumor invasion and metastasis and control tumor growth through regulating extracellular matrix homeostasis.9 TIMP1 overexpression in a transgenic mouse model has been shown to inhibit tumor growth and metastasis of a T-cell lymphoma.4 There is evidence that TIMPs also may have functions not directly related to cell migration, and these functions include growth-promoting as well as anti-apoptotic effects.5,6,32 For instance, TIMP1 can protect germinal center B cells from apoptosis.5 In fact, high levels of TIMP1 in serum correlates with poor clinical outcome in patients with some types of carcinoma.33,34 Most likely, the correlation between TIMP1 levels and worse survival is attributed to the cell proliferative and anti-apoptotic effects of TIMP1.
Regulation of gene expression of TIMPs is not completely understood. In rat cells, the TIMP1 promoter has been found to have bindings sites for AP-1 and STAT3, and the presence of these two transcriptional factors is required for full activation of TIMP1 expression.16 A number of cytokines are implicated in up-regulating TIMP1 production in a variety of cell types. For instance, IL-6 has been shown to stimulate TIMP1 production in fibroblasts,35 but not in a number of lymphoid cell lines.9 In plasma cells, it has been shown that TIMP1 production can be induced by a combination of tumor necrosis factor-α and IL-1β.36 In mononuclear phagocytes, IL-10 stimulates secretion of TIMP1.11 In fact, TIMP1 and IL-10 may form an autocrine loop in non-Hodgkin’s lymphomas, and TIMP1 expression in B-cell nonlymphoma patients correlates closely with IL-10 expression and with a high histological grade in these patients. In summary, it is likely TIMP1 production in different cell types is regulated by different cytokines, and it is possible that STAT3 may be the signal transducer for these cytokines because all of the above-mentioned cytokines can activate STAT3.
In normal lymphoid cells, TIMP1 expression is restricted to B cells.8 In T cells, TIMP1 expression has been shown to increase in response to viral transactivators of HTLV-1 and HTLV-2.5,37 As shown in this study, ALK+ ALCL is a rather unusual type of T-cell neoplasms in that a relatively high level of TIMP1 is expressed. Other than ALCL, we did not observe any TIMP1 expression in all peripheral T-cell lymphomas examined and only one of four T-cell lymphoma cell lines showed weak reactivity on Western blots in this study. One recent study using cDNA microarray analysis also showed that stimulated T cells have relatively low levels of TIMP1 compared to ALK+ ALCL.38 In B-cell lymphomas, expression of TIMP1 is more highly expressed in high-grade than low-grade lymphomas.39 Previously, it has been reported that the TIMP1 gene promoter carries STAT3-response element, suggesting that TIMP1 expression may be regulated by STAT3.16 Functional blockage of STAT3 has been shown to inhibit thrombin-driven TIMP1 expression in human glomerular mesangial cells,40 although a possible role of the STAT3 signaling pathway in regulating TIMP1 expression in neoplastic cells has not been examined previously.
Because ALK+ ALCL has a relatively high level of STAT3 activation, we hypothesized that ALK+ ALCL also has a relatively high level of TIMP1 expression compared with other lymphoma types. Results from the cDNA microarray studies using 29 lymphoma cell lines representing a wide range of lymphoma types supported our hypothesis. With the exception of one Burkitt lymphoma cell line, ALK+ ALCL cell lines expressed TIMP1 at a level higher than that of all other lymphoma cell lines. We confirmed this finding by examining TIMP1 expression in a panel of lymphoma cell lines using a tissue microarray and immunocytochemistry. In addition, using tissue microarrays, we identified that TIMP1 expression significantly correlates with STAT3 activation in ALCL. Lastly, gene transfer of STAT3DN into two ALK+ ALCL cell lines, Karpas 299 and SU-DHL-1, led to down-regulation and complete abrogation of TIMP1 expression in these cells. Together, our data strongly support the concept that high levels of TIMP1 expression in lymphomas correlate with STAT3 activation, and that STAT3 directly contributes to the high level of TIMP1 expression in ALK+ ALCL.
Because it has been previously shown by us and others26,27 that ALK expression significantly correlates with STAT3 activation in ALCL, it is not surprising to observe that ALK positivity also significantly correlated with TIMP1 expression in these tumors in our study. Although we did not include T-cell lymphoma cell lines in our initial cDNA analysis, results from Western blot/immunocytochemical studies of four T-cell lymphoma cell lines, as well as results from immunohistochemical studies of nine cases of peripheral T-cell lymphoma support that high TIMP1 expression is unique to ALK+ ALCL, tumors that also frequently have STAT3 activation. In addition, Gaiser and colleagues38 have recently reported that TIMP1 is strongly expressed in ALK+ ALCL cell lines compared to stimulated T cells.
Although we concluded that there is a correlation between STAT3 activation and TIMP1 expression in lymphoma, exceptions do exist. Subsets of Burkitt lymphoma cell lines are known to produce high levels of TIMP1, and the mechanism of TIMP1 production may be related to Epstein-Barr viral infection of these cells.9 In addition, the identification of cell lines, as summarized in Table 1, showing relatively low levels of TIMP1 expression in the absence of detectable pSTAT3 in Western blots suggests that TIMP1 expression is not strictly dependent on the STAT3 signaling pathway. Likewise, TIMP1 expression was detectable by immunohistochemistry in two pSTAT3− ALCL tumors (Table 4). The mechanism responsible for TIMP1 expression in these pSTAT3− cells remains to be investigated. On the other hand, cells with STAT3 activation may not necessarily be coupled with high TIMP1 expression. For instance, as shown in Table 4, 9 of 22 cases of pSTAT3+ ALCL showed no detectable TIMP1 by immunohistochemistry. The lack of TIMP1 immunoreactivity in these cases may be because of suboptimal fixation, but we have also considered the possibility that TIMP1 was expressed at a low level that is below the detection sensitivity of immunohistochemistry. In fact, it has been shown that STAT3 may require the presence of another transcriptional factor, AP-1, for full activation of TIMP1 expression in rat cells.16
Table 4.
Association between pSTAT3 and TIMP1 in 38 Cases of ALCL
| TIMP1-positive | TIMP1-negative | Total cases | |
|---|---|---|---|
| pSTAT3-positive | 13 (59.1%) | 9 (40.9%) | 22 |
| pSTAT3-negative | 3 (18.7%) | 13 (81.3%) | 16 |
P = 0.02 (Fisher’s exact test).
Previous studies indicated that stromal cells, macrophages, and fibroblasts produce TIMP1. In our study, we detected TIMP1 immunostaining in the cytoplasm of neoplastic cells. TIMP1+ ALCL tumors often have a Golgi staining pattern, supporting the concept that TIMP1 is produced by the neoplastic cells. Considering the cytokine nature of TIMP1, it is likely that the lymphoma cells produce TIMP1, which is subsequently secreted in the microenvironment where TIMP1 eventually binds to its specific cell-surface ligands to exert its biological effects. Thus, TIMP1 may function in an autocrine/paracrine pathway, to promote cell proliferation and survival. Disruption of this pathway may have therapeutic implication for ALK+ ALCL tumors.
The exact biological significance of the high level of TIMP1 expression in ALK+ ALCL remains to be investigated. In view of its function in some Burkitt lymphoma cell lines, TIMP1 may contribute to the pathogenesis of ALK+ ALCL in two ways. First, it antagonizes the actions of metalloproteinases and inhibits spreading of cells into the stroma. In this regard, it is tempting to hypothesize that TIMP1 production by ALK+ ALCL cells may explain the distinct sinusoidal infiltration pattern of these tumors, which is one of the distinguishing features of ALCL.25 Second, it has been shown that TIMP1 has anti-apoptotic effects. We recently have shown that selective blockade of STAT3 signaling pathway using a dominant-negative STAT3 construct induced apoptosis in ALK+ ALCL cell lines (HM Amin et al, Oncogene, in press). It will be of great interest to determine whether deregulation of TIMP1 is a key factor contributing to apoptosis in this experimental model.
The adenoviral vector used in this study has been previously used in a number of studies.29–31 The biological effects of the dominant-negative STAT3 construct are secondary to a single mutation at residue 705tyrosine→phenylalanine. This mutation prevents tyrosine phosphorylation at this site, which is believed to be crucial in mediating dimerization and nuclear localization of STAT3.19 Our findings from our immunoprecipitation studies support this concept, because STAT3DN did not show evidence of tyrosine phosphorylation in Karpas 299 cells. The exact mechanism underlying the dominant-negative effects of this construct is not clear. Nevertheless, our immunoprecipitation studies showed that the expression of dominant-negative STAT3 induces down-regulation of the endogenously expressed tyrosine-phosphorylated STAT3. It is likely that dominant-negative STAT3 competes with the endogenous STAT3 for the binding sites on NPM-ALK, and thereby inhibiting STAT3 activation.
Because of the relatively small number of ALCL patients included in our study, we did not observe any statistically significant difference in PFS in TIMP1-positive and -negative ALCL patients within the ALK+ ALCL group. The problem is further compounded by the fact that most ALK+ ALCL patients have TIMP1 expression. Nevertheless, in the ALK− group, patients with TIMP1 expression showed a shorter PFS (P = 0.05). This may correlate with the results of some of the previous studies that TIMP1 is associated with worse clinical outcome and clinical aggressiveness of high-grade lymphoma.39 Results from one of our recent studies also suggest that TIMP1 is associated with shortened overall survival in diffuse large B-cell lymphoma patients (manuscript in preparation). Further studies will be warranted to confirm the finding of TIMP1 being a prognostic indicator for ALCL patients.
In conclusion, our data show that high TIMP1 expression correlates with STAT3 activation in malignant lymphoma, and that selective inhibition of STAT3 results in TIMP1 down-regulation in ALK+ ALCL. Considering the known functions of TIMP1, it seems to mediate at least some of the anti-apoptotic effects of STAT3. Our results also demonstrate that selective targeting of STAT3 signaling pathway could represent a potential therapeutic modality in ALCL by the down-regulation of known anti-apoptotic proteins such as TIMP1.
Footnotes
Address reprint requests to Raymond Lai, Department of Laboratory Medicine and Pathology, University of Alberta, 4B1, 8440 112 St., Edmonton, Alberta, Canada T6G 2B7. E-mail: raymondmail_65@yahoo.com.
Supported by the Tobacco Settlement Fund as appropriated to The University of Texas M. D. Anderson Cancer Center by the Texas Legislature, the generous donations from the Kadoorie Foundation, and the Cancer Center Support Grant to M.D. Anderson Cancer Center.
References
- Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer. 1999;79:1347–1355. doi: 10.1038/sj.bjc.6690217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G. Matrix metalloproteinases and their inhibitors. Acta Orthop Scand Suppl. 1995;266:55–60. [PubMed] [Google Scholar]
- Henriet P, Blavier L, Declerck YA. Tissue inhibitors of metalloproteinases (TIMP) in invasion and proliferation. APMIS. 1999;107:111–119. doi: 10.1111/j.1699-0463.1999.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Kruger A, Fata JE, Khokha R. Altered tumor growth and metastasis of a T-cell lymphoma in Timp-1 transgenic mice. Blood. 1997;90:1993–2000. [PubMed] [Google Scholar]
- Guedez L, Courtemanch L, Stetler-Stevenson M. Tissue inhibitor of metalloproteinase (TIMP)-1 induces differentiation and an antiapoptotic phenotype in germinal center B cells. Blood. 1998;92:1342–1349. [PubMed] [Google Scholar]
- Li G, Fridman R, Kim HR. Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res. 1999;59:6267–6275. [PubMed] [Google Scholar]
- Mansoor A, Birkedal-Hansen B, Lim M, Guedez L, Stetler-Stevenson WG, Stetler-Stevenson M. TIMP-1 expression correlates with histologic grade in B-cell lymphomas. Mod Pathol. 1997;10:130A. (Abstract) [Google Scholar]
- Stetler-Stevenson M, Mansoor A, Lim M, Fukushima P, Kehrl J, Marti G, Ptaszynski K, Wang J, Stetler-Stevenson WG. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood. 1997;89:1708–1715. [PubMed] [Google Scholar]
- Kossakowska AE, Urbanski SJ, Janowska-Wieczorek A. Matrix metalloproteinases and their tissue inhibitors—expression, role and regulation in human malignant non-Hodgkin’s lymphomas. Leuk Lymphoma. 2000;39:485–493. doi: 10.3109/10428190009113379. [DOI] [PubMed] [Google Scholar]
- Oelmann E, Herbst H, Zuhlsdorf M, Albrecht O, Nolte A, Schmitmann C, Manzke O, Diehl V, Stein H, Berdel WE. Tissue inhibitor of metalloproteinases 1 is an autocrine and paracrine survival factor, with additional immune-regulatory functions, expressed by Hodgkin/Reed-Sternberg cells. Blood. 2002;99:258–267. doi: 10.1182/blood.v99.1.258. [DOI] [PubMed] [Google Scholar]
- Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304–2310. doi: 10.1172/JCI118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossakowska AE, Edwards DR, Prusinkiewicz C, Zhang MC, Guo D, Urbanski SJ, Grogan T, Marquez LA, Janowska-Wieczorek A. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood. 1999;94:2080–2089. [PubMed] [Google Scholar]
- Stearns ME, Wang M, Hu Y, Garcia FU, Rhim J. Interleukin 10 blocks matrix metalloproteinase-2 and membrane type 1-matrix metalloproteinase synthesis in primary human prostate tumor lines. Clin Cancer Res. 2003;9:1191–1199. [PubMed] [Google Scholar]
- Stearns ME, Garcia FU, Fudge K, Rhim J, Wang M. Role of interleukin 10 and transforming growth factor beta1 in the angiogenesis and metastasis of human prostate primary tumor lines from orthotopic implants in severe combined immunodeficiency mice. Clin Cancer Res. 1999;5:711–720. [PubMed] [Google Scholar]
- Guedez L, Mansoor A, Birkedal-Hansen B, Lim MS, Fukushima P, Venzon D, Stetler-Stevenson WG, Stetler-Stevenson M. Tissue inhibitor of metalloproteinases 1 regulation of interleukin-10 in B-cell differentiation and lymphomagenesis. Blood. 2001;97:1796–1802. doi: 10.1182/blood.v97.6.1796. [DOI] [PubMed] [Google Scholar]
- Bugno M, Graeve L, Gatsios P, Koj A, Heinrich PC, Travis J, Kordula T. Identification of the interleukin-6/oncostatin M response element in the rat tissue inhibitor of metalloproteinases-1 (TIMP-1) promoter. Nucleic Acids Res. 1995;23:5041–5047. doi: 10.1093/nar/23.24.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- Gouilleux-Gruart V, Gouilleux F, Desaint C, Claisse JF, Capiod JC, Delobel J, Weber-Nordt R, Dusanter-Fourt I, Dreyfus F, Groner B, Prin L. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood. 1996;87:1692–1697. [PubMed] [Google Scholar]
- Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai SK, Nichols GL, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol. 1997;159:4720–4728. [PubMed] [Google Scholar]
- Ni Z, Lou W, Lee SO, Dhir R, DeMiguel F, Grandis JR, Gao AC. Selective activation of members of the signal transducers and activators of transcription family in prostate carcinoma. J Urol. 2002;167:1859–1862. [PubMed] [Google Scholar]
- Delsol G, Ralfkiaer E, Stein H, Wright D, Jaffe ES. Anaplastic large cell lymphoma. Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Lyon: IARC Press; Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. 2001:pp 230–235. [Google Scholar]
- Zamo A, Chiarle R, Piva R, Howes J, Fan Y, Chilosi M, Levy DE, Inghirami G. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene. 2002;21:1038–1047. doi: 10.1038/sj.onc.1205152. [DOI] [PubMed] [Google Scholar]
- Khoury JD, Medeiros LJ, Rassidakis GZ, Yared MA, Tsioli P, Leventaki V, Schmitt-Graeff A, Herling M, Amin HM, Lai R. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK(+) and ALK(−) anaplastic large cell lymphoma. Clin Cancer Res. 2003;9:3692–3699. [PubMed] [Google Scholar]
- Raychaudhuri S, Stuart JM, Altman RB. Principal components analysis to summarize microarray experiments: application to sporulation time series. Pac Symp Biocomput. 2000:455–466. doi: 10.1142/9789814447331_0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada K, Tone E, Fujio Y, Matsui H, Yamauchi-Takihara K, Kishimoto T. Activation of gp130 transduces hypertrophic signals via STAT3 in cardiac myocytes. Circulation. 1998;98:346–352. doi: 10.1161/01.cir.98.4.346. [DOI] [PubMed] [Google Scholar]
- Osugi T, Oshima Y, Fujio Y, Funamoto M, Yamashita A, Negoro S, Kunisada K, Izumi M, Nakaoka Y, Hirota H, Okabe M, Yamauchi-Takihara K, Kawase I, Kishimoto T. Cardiac-specific activation of signal transducer and activator of transcription 3 promotes vascular formation in the heart. J Biol Chem. 2002;277:6676–6681. doi: 10.1074/jbc.M108246200. [DOI] [PubMed] [Google Scholar]
- Negoro S, Kunisada K, Fujio Y, Funamoto M, Darville MI, Eizirik DL, Osugi T, Izumi M, Oshima Y, Nakaoka Y, Hirota H, Kishimoto T, Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001;104:979–981. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- Hayakawa T. Multiple functions of tissue inhibitors of metalloproteinases (TIMPs): a new aspect involving osteoclastic bone resorption. J Bone Miner Metab. 2002;20:1–13. doi: 10.1007/s774-002-8440-0. [DOI] [PubMed] [Google Scholar]
- Holten-Andersen MN, Murphy G, Nielsen HJ, Pedersen AN, Christensen IJ, Hoyer-Hansen G, Brunner N, Stephens RW. Quantitation of TIMP-1 in plasma of healthy blood donors and patients with advanced cancer. Br J Cancer. 1999;80:495–503. doi: 10.1038/sj.bjc.6690384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K, Maguire T, McGreal G, McDermott E, O’Higgins N, Duffy MJ. High levels of tissue inhibitor of metalloproteinase-1 predict poor outcome in patients with breast cancer. Int J Cancer. 1999;84:44–48. doi: 10.1002/(sici)1097-0215(19990219)84:1<44::aid-ijc9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Sato T, Ito A, Mori Y. Interleukin 6 enhances the production of tissue inhibitor of metalloproteinases (TIMP) but not that of matrix metalloproteinases by human fibroblasts. Biochem Biophys Res Commun. 1990;170:824–829. doi: 10.1016/0006-291x(90)92165-v. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Tedla N, Lloyd A, Wakefield D. Expression of matrix metalloproteinases by human plasma cells and B lymphocytes. Eur J Immunol. 1998;28:1773–1784. doi: 10.1002/(SICI)1521-4141(199806)28:06<1773::AID-IMMU1773>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Uchijima M, Sato H, Fujii M, Seiki M. Tax proteins of human T-cell leukemia virus type 1 and 2 induce expression of the gene encoding erythroid-potentiating activity (tissue inhibitor of metalloproteinases-1, TIMP-1). J Biol Chem. 1994;269:14946–14950. [PubMed] [Google Scholar]
- Gaiser T, Thorns C, Merz H, Noack F, Feller AC, Lange K. Gene profiling in anaplastic large-cell lymphoma-derived cell lines with cDNA expression arrays. J Hematother Stem Cell Res. 2002;11:423–428. doi: 10.1089/152581602753658619. [DOI] [PubMed] [Google Scholar]
- Kossakowska AE, Urbanski SJ, Huchcroft SA, Edwards DR. Relationship between the clinical aggressiveness of large cell immunoblastic lymphomas and expression of 92 kDa gelatinase (type IV collagenase) and tissue inhibitor of metalloproteinases-1 (TIMP-1) RNAs. Oncol Res. 1992;4:233–240. [PubMed] [Google Scholar]
- Chen X, Liu W, Wang J, Wang X, Yu Z. STAT1 and STAT3 mediate thrombin-induced expression of TIMP-1 in human glomerular mesangial cells. Kidney Int. 2002;61:1377–1382. doi: 10.1046/j.1523-1755.2002.00283.x. [DOI] [PubMed] [Google Scholar]