Abstract
Estrogens and estrogen-receptor signaling function in establishing and regulating the female immune system and it is becoming increasingly evident that they may play a similar role in males. We report that B10.PL/SnJ male mice with a disrupted estrogen receptor-1 (α) gene (Esr1−/−) develop less severe clinical experimental allergic encephalomyelitis (EAE) compared to either Esr1+/− or wild-type (Esr1+/+) controls when immunized with myelin basic protein peptide Ac1-11 (MBPAc1-11). In contrast, the disease course in B10.PL/SnJ male mice with a disrupted estrogen receptor-2 (β) gene (Esr2−/−) does not differ from that of wild-type (Esr2+/+) mice. However, Esr2+/− mice do develop more severe clinical disease with an earlier onset indicating that heterosis at Esr2 plays a significant role in regulating EAE in males. No significant differences in central nervous system histopathology or MBPAc1-11-specific T-cell responses as assessed by proliferation and interleukin-2 production were observed as a function of either Esr1 or Esr2 genotype. An analysis of cytokine/chemokine secretion by MBPAc1-11-specific T cells revealed unique Esr1 and Esr2 genotype-dependent regulation. Interferon-γ secretion was found to be negatively regulated by Esr1 whereas interleukin-6 and tumor necrosis factor-α secretion exhibited classical Esr2 gene dose responses. Interestingly, MCP-1 displayed distinctively unique patterns of genotype-dependent regulation by Esr1 and Esr2. The contribution of the hematopoietic and nonhematopoietic cellular compartments associated with the heterotic effect at Esr2 in regulating the severity of clinical EAE was identified using reciprocal hematopoietic radiation bone marrow chimeras generated between male wild-type and Esr2+/− mice. Wild-type → Esr2+/− mice exhibited EAE equivalent in severity to that seen in Esr2+/− → Esr2+/− control constructs; both of which were more severe than the clinical signs observed in Esr2+/− → wild-type and wild-type → wild-type mice. These results indicate that the heterotic effect at Esr2 is a function of the nonhematopoietic compartment.
In females both the humoral and cell-mediated immune responses are more active than in males1 and extensive data indicate that estrogens play a significant role in regulating this immunological sexual dimorphism.2,3 To be a direct target for estrogens either the cells of the immune system, or the nonhematopoietically derived cellular constituents that support their development and function, must express the appropriate cognate receptors. The first classical intracytoplasmic estrogen receptor (iER) cloned was estrogen receptor-1 (α) (Esr1).4 Among cells relevant to the functioning of the immune system, Esr1 is expressed by thymocytes,5,6 thymic epithelial cells,6 T cells,7,8 B lymphocytes and their precursors,9,10 and nonhematopoietic bone marrow cells.9,11 The existence of a second iER, estrogen receptor-2 (β) (Esr2), was identified12 and has been shown to be expressed by nonhematopoietic bone marrow cells13 and in the human thymus and spleen.14,15 The precise roles of these two receptors in regulating cell-specific responses are still ill-defined. However, it is becoming evident that Esr1 and Esr2 may be responsible for regulating different biological functions based on their expression patterns, localization profiles, and protein structures.16,17 In addition to these two cytoplasmic receptors, there may also be a membrane-associated ER that is believed to play a role in mediating nongenomic responses18 as well as a number of estrogen-related receptor genes (α, β, γ, and receptor β-like 1; Esrra, Esrrb, Esrrg, and Esrrbl1, respectively) that function as ligand-regulated transcription factors that play critical roles in multiple aspects of development, cellular differentiation, and homeostasis.19
The effects of estrogens and ER signaling may be prenatal, postnatal, or both16,17 and their involvement in reproduction, growth, and certain organ systems, including the central nervous system (CNS), is well documented in males.16,20 However, their role in the male immune system is beginning to be more fully appreciated. Others and we have shown that Esr1 is involved in normal thymic development and T lymphopoiesis21,22 and in regulating B-cell development9,10 in male mice. However, the role of iERs in autoimmune disease in males is uncharted. In the current study, we use male B10.PL/SnJ congenic mice possessing either a disrupted estrogen receptor-1 (Esr1−/−) or estrogen receptor-2 (Esr2−/−) gene to assess the role of these two iERs in experimental allergic encephalomyelitis (EAE), the principal animal model of multiple sclerosis. In this study we report that the severity of EAE and the secretion of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and MCP-1 by encephalitogenic T cells in male mice exhibit distinctively unique Esr1 and Esr2 genotype-dependent regulation.
Materials and Methods
Animals
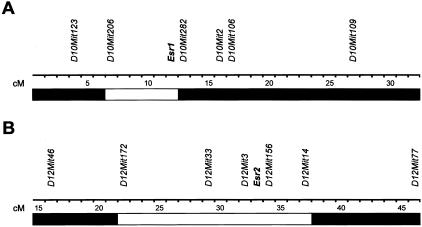
B10.PL/SnJ mice were purchased from the Jackson Laboratory, Bar Harbor, ME. B10PL.129P2(B6)-Esr1tm1Unc (abbreviated B10.PL-Esr1tm1Unc or Esr1−/−) and B10PL.129P2(B6)-Esr2tm1Unc (abbreviated B10.PL-Esr2tm1Unc or Esr2−/−) mice were generated by backcrossing B6.129P2-Esr1tm1Unc and B6.129P2-Esr2tm1Unc mice to B10.PL/SnJ mice for 10 generations.23,24 This represents a total of a minimum of 20 backcross generations on the C57BL background with respect to 129P2 genomic contamination. Mice were selected at each generation by polymerase chain reaction-based genotyping using primers specific for the disrupted Esr1 and Esr2 alleles. The congenic intervals encompassing the two disrupted loci are presented in Figure 1. Animals were maintained in accordance with the Animal Welfare Act and the Public Health Service Policy on the Humane Care and Use of Laboratory Animals.
Figure 1-4129.
Congenic intervals for B10.PL-Esr1 (A) and B10.PL-Esr2 (B) mice. Boundaries of the congenic intervals for both loci were determined using informative microsatellite markers distinguishing 129-strain alleles from C57BL/6J and C57BL/10J strain alleles. Genotyping was performed as previously described.26–28 □, 129-strain-derived alleles; ▪, C57BL/6J and/or C57BL/10J alleles.
Induction of Active EAE
Induction of active disease was as previously described.25 Briefly, on day 0 mice were immunized with 400 μg of myelin basic protein peptide Ac1-11 (MBPAc1-11) (Beckman Institute, Palo Alto, CA) emulsified in complete Freund’s adjuvant (CFA) containing 200 μg of Mycobacterium tuberculosis H37Ra (Difco Laboratories, Detroit, MI) by subcutaneous injection over four sites on the flank. On the day of immunization mice received by intraperitoneal injection 75 ng of pertussis toxin (PTX) (List Biological Laboratories Inc., Campbell, CA). Forty-eight hours later each mouse received an additional 200 ng of PTX by intraperitoneal injection.
Evaluation of Clinical and Histopathological EAE
Starting on day 7 after immunization the mice were examined daily for clinical signs of EAE according to the following scale: 0, no signs; 1, limp tail; 1.5, moderate hind limb weakness with difficulty in righting; 2, moderate hind limb weakness without ability to right itself; 2.5, moderate hind limb weakness (waddling gait) without ability to right itself; 3, moderately severe hind limb weakness with the ability to walk upright for only a few steps; 3.5, moderately severe hind limb weakness with paralysis of one limb; 4, severe hind limb weakness; 4.5, severe hind limb weakness with mild forelimb weakness; 5, paraplegia with no more than moderate forelimb weakness; 5.5, paraplegia with severe forelimb weakness (quadriplegia); and 6, moribund condition. Clinical disease parameters assessed were incidence and mortality, and the quantitative traits (QTs): day of onset of clinical signs, cumulative disease score (CDS), disease index (DI), peak score, and severity index (SI).26,27
Brains and spinal cords (SCs) were dissected from calvaria and vertebral columns, respectively, and fixed by immersion in 10% phosphate-buffered formalin (pH 7.2). After adequate fixation, they were trimmed and representative transverse sections-embedded in paraffin, sectioned at 5 μm, and mounted on glass slides. Sections were stained with hematoxylin and eosin (H&E) for routine evaluation and Luxol fast blue-periodic acid-Schiff reagent for demyelination. Representative areas of the brain and SC, including brain stem, cerebrum, cerebellum, and the cervical, thoracic, and lumbar segments of the SC, were selected for histopathological evaluation based on previous studies.28 The following components of the lesions were assessed: 1) severity of the lesion as represented by each component of the histopathological assessment; 2) extent and degree of myelin loss and tissue injury (swollen axon sheaths, swollen axons, and reactive gliosis); 3) severity of the acute inflammatory response (predominantly neutrophils); and 4) severity of the chronic inflammatory response (lymphocytes/macrophages). A score was assigned separately to the entire brain and SC for each lesion characteristic based on a subjective scale ranging from 0 to 5. A score of 0 indicates no lesions; 1 indicates minimal; 2, mild; 3, moderate; 4, marked; and 5, severe lesions. Occasional mice had eosinophils admixed with the dominant neutrophilic inflammatory response.
Proliferation Assays
Single cell suspensions of draining lymph node cells were prepared.29–31 Draining lymph node cells (4 × 105 cells/well) were plated on standard 96-well flat-bottom tissue culture plates for 72 hours at 37°C and 7% CO2 with and without antigen and in the presence of 0.5 μCi of 3H-thymidine during the last 18 hours. Cells were harvested onto glass fiber filters, and thymidine uptake was determined by liquid scintillation. Data are expressed as corrected mean counts per minute.
Cytokine Assays
Single cell splenocyte suspensions from immunized mice were prepared and suspended at 4 × 106 cells/ml in stimulation media with 20 μg/ml of MBPAc1-11. Cell culture supernatants were recovered at 72 hours and frozen at −70°C. IL-4 secretion was quantitated by enzyme-linked immunosorbent assay (PharMingen, San Diego, CA) and IFN-γ, TNF-α, IL-2, IL-6, IL-5, IL-10, IL-12, and MCP-1 were simultaneously detected using the mouse inflammation and Th1/Th2 cytokine CBA kits from BD Biosciences (San Jose, CA).29–31 Fifty μl of sample were mixed with 50 μl of the mixed capture beads and 50 μl of the mouse inflammation phycoerythrin-conjugated detection antibodies. The tubes were incubated at room temperature for 2 hours in the dark, followed by a wash step. The samples were then resuspended in 400 μl of wash buffer before acquisition on the FACScan (BD Bioscience, San Jose, CA). The data were analyzed using the CBA software. Standard curves were generated for each cytokine using the mixed bead standard provided in the kit and the concentration of cytokine in the cell supernatant was determined by interpolation from the appropriate standard curve. Means and standard deviations were determined using data from individual animals.
Radiation Bone Marrow Chimeras
Radiation bone marrow chimeras were constructed as previously described.32,33 Briefly, host mice were lethally irradiated and reconstituted by the intravenous injections of 107 T-cell-depleted donor bone marrow cells. In this way the following radiation chimeras were generated: wild-type → wild-type, Esr2+/− → Esr2+/−, wild-type → Esr2+/− and Esr2+/− → wild-type. Chimeras were studied for EAE at 4 to 6 weeks after reconstitution.
Statistical Analysis
Analysis of variance was used to test for significant differences in QT values by genotype for the two lines. Where indicated posthoc one-way multiple comparisons with Tukey’s and Hsu’s family error rates of 0.1 were used to determine the significance of differences in clinical QT values as a function of genotype. Logistic regression with a Wald chi-square test was used to test for significant qualitative differences by genotype. In all tests, P values ≤0.05 were used as the significance threshold.
Results
EAE in B10.PL/SnJ, B10.PL-Esr1+/−, and B10.PL-Esr1tm1Unc Male Mice
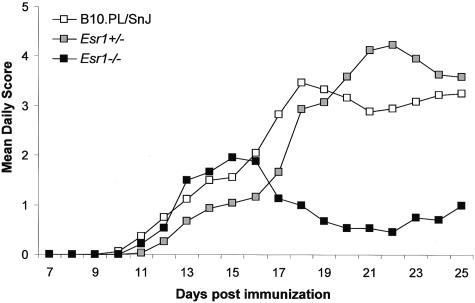
To study the role of Esr1 in regulating susceptibility to EAE in male mice, homozygous wild-type (B10.PL/SnJ), heterozygous B10.PL-Esr1+/− (Esr1+/−), and homozygous Esr1-deficient B10.PL-Esr1tm1Unc (Esr1−/−) littermates were immunized with MBPAc1-11 + CFA + PTX, and monitored for clinical EAE (Figure 2). No significant difference in disease onset, peak score, or mortality was detected between wild-type, Esr1+/−, and Esr1−/− mice (Table 1). However, compared to wild-type and Esr1+/− mice, Esr1−/− males presented with a significant reduction in disease incidence and average CDS. The lower CDS in the Esr1−/− mice was also reflected as significant reductions in the severity and disease indices, SI and DI, respectively. Because the peak of disease in the wild-type and Esr1+/− mice occurred between days 18 and 25 after immunization, we stratified the clinical disease course into acute-early (day 7 to day 16) and chronic-late (day 17 to day 25) and reevaluated the clinical QTs (Table 2). No significant differences were seen for any of the parameters as a function of genotype during the acute-early phase of the disease. In contrast, Esr1−/− mice had significantly reduced CDS, SI, and DI compared to wild-type and Esr1+/− mice during the chronic-late phase of disease. These results indicate that Esr1 signaling plays a significant role in regulating the severity of the clinical signs seen in male mice during the chronic-late phase of the disease but not during the acute-early phase.
Figure 2-4129.
Clinical disease profiles comparing ERKO wild-type B10.PL/SnJ (n = 18), B10.PL-Esr1+/− (n = 15), and B10.PL-Esr1tm1Unc (n = 12) mice. Male littermates were injected with 400 μg of MBPAc1-11 + CFA + PTX. Starting on day 7 the animals were monitored daily for clinical signs of EAE and the data are plotted as the mean daily score for all animals studied.
Table 1.
Susceptibility to MBPAc1-11-Induced EAE in B10.PL/SnJ, B10.PL-Esr1+/−, and B10.PL-Esr1tm1Unc Male Mice
| Genotype | Incidence | Onset | Peak score | SI | CDS | DI | Mortality |
|---|---|---|---|---|---|---|---|
| B10.PL/SnJ | 18/18 | 15.6 ± 4.8 | 4.2 ± 1.9 | 3.2 ± 1.6 | 35.6 ± 21.6 | 1.9 ± 1.1 | 2/18 |
| Esr1+/− | 15/15 | 16.5 ± 3.6 | 5.0 ± 1.3 | 3.4 ± 1.2 | 34.9 ± 15.6 | 1.8 ± 0.8 | 3/15 |
| Esr1−/− | 8/12 | 12.3 ± 1.0 | 3.3 ± 1.5 | 1.7 ± 0.8* | 14.5 ± 15.8* | 0.8 ± 0.8* | 0/12 |
| χ2 = 12.07 | F = 3.18 | F = 2.73 | F = 4.77 | F = 5.58 | F = 5.94 | χ2 = 2.70 | |
| P value | 0.002 | 0.05 | 0.08 | 0.01 | 0.007 | 0.005 | 0.26 |
Significance of differences based on one-way multiple comparisons with Tukey’s and Hsu’s family error rates at 0.1: Esr1−/− < Esr1+/− = wild-type.
Table 2.
Comparison of Acute-Early and Chronic-Late Stages of EAE in B10.PL/SnJ, B10.PL-Esr1+/−, and B10.PL-Esr1tm1Unc Male Mice
| Genotype | Acute-early |
Chronic-late |
||||||
|---|---|---|---|---|---|---|---|---|
| Peak score | SI | CDS | DI | Peak score | SI | CDS | DI | |
| B10.PL/SnJ | 4.5 ± 1.6 | 3.2 ± 0.7 | 13.7 ± 11.3 | 1.1 ± 0.9 | 3.8 ± 2.0 | 3.3 ± 2.0 | 21.9 ± 14.8 | 3.1 ± 2.1 |
| Esr1+/− | 4.3 ± 1.0 | 2.5 ± 1.2 | 8.7 ± 8.8 | 0.7 ± 0.7 | 4.7 ± 1.5 | 3.8 ± 1.7 | 26.2 ± 12.5 | 3.7 ± 1.8 |
| Esr1−/− | 3.3 ± 1.5 | 2.2 ± 1.1 | 9.9 ± 10.2 | 0.8 ± 0.8 | 2.8 ± 1.3 | 1.2 ± 0.9* | 4.7 ± 6.0* | 0.7 ± 0.9* |
| F = 2.07 | F = 3.30 | F = 1.07 | F = 1.01 | F = 2.97 | F = 5.80 | F = 11.20 | F = 10.93 | |
| P value | 0.14 | 0.05 | 0.35 | 0.37 | 0.06 | 0.006 | <0.001 | <0.001 |
Significance of differences based on one-way multiple comparisons with Tukey’s and Hsu’s family error rates at 0.1: Esr1−/− < Esr1+/− = wild-type.
EAE in B10.PL/SnJ, B10.PL-Esr2+/−, and B10.PL-Esr2tm1Unc Male Mice
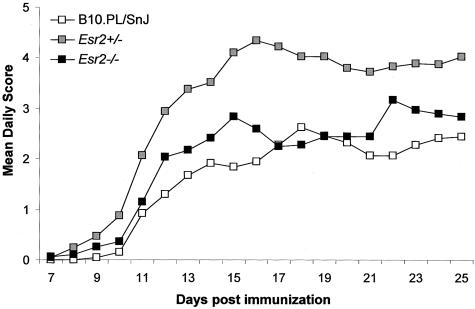
B10.PL/SnJ, B10.PL-Esr2+/− (Esr2+/−), and B10.PL-Esr2tm1Unc (Esr2−/−) littermates were immunized with MBPAc1-11 + CFA + PTX, and monitored for clinical EAE (Figure 3). No significant difference was seen for disease incidence among the three genotypes (Table 3); however, significant differences were detected for average day of onset, peak score, CDS, SI, DI, and mortality. With the exception of the day of onset, the clinical QT values for Esr2+/− mice were significantly increased over wild-type and Esr2−/− mice. These results are consistent with heterosis at Esr2 in the regulation of EAE in male mice. Stratification of the disease course into acute-early and chronic-late resulted in similar differences with respect to each of the clinical QTs studied (data not shown). Similar results were seen in male Esr1+/−Esr2+/− double heterozygotes (Table 4). Esr1+/−Esr2+/− mice developed significantly more severe disease with an earlier onset and greater mortality compared to wild-type controls. Taken together these data suggest that heterosis at Esr2+/− influences the severity of the clinical signs throughout the course of the disease.
Figure 3-4129.
Clinical disease profiles comparing BERKO wild-type B10.PL/SnJ (n = 24), B10.PL-Esr2+/− (n = 32), and B10.PL-Esr2tm1Unc (n = 18) mice. Male littermates were injected with 400 μg of MBPAc1-11 + CFA + PTX. Starting on day 7 the animals were monitored daily for clinical signs of EAE and the data are plotted as the mean daily score for all animals studied.
Table 3.
Susceptibility to MBPAc1-11-Induced EAE in B10.PL/SnJ, B10.PL-Esr2+/−, and B10.PL-Esr2tm1Unc Male Mice
| Genotype | Incidence | Onset | Peak score | SI | CDS | DI | Mortality |
|---|---|---|---|---|---|---|---|
| B10.PL/SnJ | 22/24 | 14.5 ± 4.9* | 3.9 ± 1.7 | 2.8 ± 1.4 | 30.7 ± 20.9 | 1.5 ± 1.0 | 1/24 |
| Esr2+/− | 29/32 | 10.8 ± 2.9* | 5.4 ± 0.6† | 4.3 ± 1.0† | 57.6 ± 27.4† | 3.1 ± 1.5† | 8/32 |
| Esr2−/− | 17/18 | 12.4 ± 4.2* | 4.5 ± 1.3 | 3.1 ± 1.4 | 37.7 ± 22.6 | 2.0 ± 1.2 | 1/18 |
| χ2 = 0.23 | F = 5.38 | F = 9.41 | F = 10.00 | F = 9.26 | F = 11.03 | χ2 = 6.38 | |
| P value | 0.89 | 0.007 | <0.001 | <0.001 | <0.001 | <0.001 | 0.04 |
Significance of differences based on one-way multiple comparisons with Tukey’s and Hsu’s family error rates at 0.1: wild-type > Esr2+/−; wild-type = Esr2−/−; and Esr2+/− = Esr2−/−.
Significance of differences based on one-way multiple comparisons with Tukey’s and Hsu’s family error rates at 0.1: Esr2+/− > Esr2−/− = wild-type.
Table 4.
Susceptibility to MBPAc1-11-Induced EAE in B10.PL/SnJ and B10.PL-Esr1+/−Esr2+/− F1 Hybrid Male Mice
| Genotype | Incidence | Onset | Peak score | SI | CDS | DI | Mortality |
|---|---|---|---|---|---|---|---|
| B10.PL/SnJ | 22/22 | 15.8 ± 5.6 | 3.9 ± 1.9 | 2.7 ± 1.1 | 44.5 ± 21.3 | 2.9 ± 1.1 | 3/22 |
| Esr1+/−Esr2+/− | 25/25 | 12.0 ± 3.4 | 5.4 ± 0.8 | 4.1 ± 1.3 | 74.4 ± 32.5 | 3.9 ± 1.7 | 11/25 |
| P value | 0.006* | 0.001 | 0.008 | 0.008 | 0.001 | 0.001† |
Significance of the differences between the means was determined using the Student’s t-test with unequal sample sizes.
Significance of the difference in mortality was determined by chi-square test with one degree of freedom: χ = 10.62.
Histological EAE in B10.PL/SnJ, Heterozygous, B10.PL-Esr1tm1Unc, and B10.PL-Esr2tm1Unc Male Mice
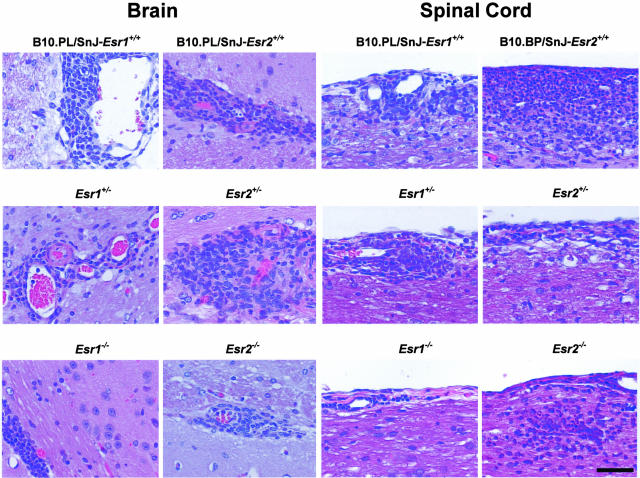
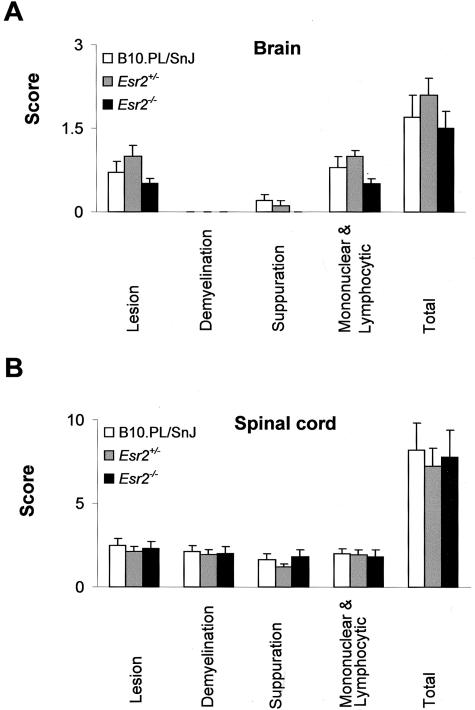
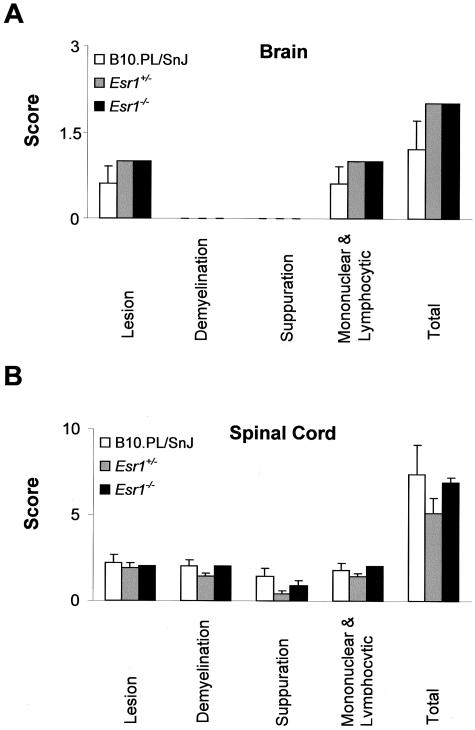
Histopathological assessment of each of the previously defined QTs28 was performed at day 25 after immunization (Figure 4). Briefly, these represent the overall severity of the lesions observed, extent and degree of myelin loss and tissue injury (swollen axon sheaths, swollen axons, and reactive gliosis), severity of the acute inflammatory response (predominantly neutrophils), and severity of the chronic inflammatory response (lymphocytes/macrophages). No significant differences were seen for any of the histopathological QTs studied in the brain or SC as a function of either Esr1(Figure 5) or Esr2 genotype (Figure 6). Similar results were observed for the histopathological QTs in the brains and SCs between wild-type and Esr1+/−Esr2+/− double heterozygotes (data not shown).
Figure 4-4129.
Histopathological lesions in the brains and SCs of male B10.PL/SnJ wild-type Esr1 and Esr2 line mice, Esr1 and Ers2 heterozygotes, and Esr1 and Esr2 knockout mice 25 to 26 days after injection. Brain lesions consisted of mononuclear cell (lymphocyte and macrophages) perivascular inflammation at the level of the external capsule (white matter myelinated tracts) of the cerebrum. Lesions in the SC consisted of a mixed inflammatory cell response consisting of neutrophils and mononuclear cells (lymphocyte and macrophages) in the pia mater and subjacent neuroparenchymal white matter (myelinated tracts). H&E stain. Scale bar, 50 μm (applies to all images).
Figure 5-4129.
Histopathological QTs in the brains (A) and SCs (B) of B10.PL/SnJ, B10.PL-Esr1+/−, and B10.PL-Esr1−/− male mice. Sections were stained with H&E for routine evaluation and Luxol fast blue-periodic acid-Schiff reagent for demyelination. Sections from representative areas were scored in a semiquantitative manner on a scale from 0 to 5 for each of the previously defined histopathological QTs.28 Data are expressed as the mean ± SD. The total score is the average of the sum of the individual trait values.
Figure 6-4129.
Histopathological QTs in the brains (A) and SCs (B) of B10.PL/SnJ, B10.PL-Esr2+/−, and B10.PL-Esr2−/− male mice. Sections were stained with H&E for routine evaluation and Luxol fast blue-periodic acid-Schiff reagent for demyelination. Sections from representative areas were scored in a semiquantitative manner on a scale from 0 to 5 for each of the previously defined histopathological QTs.28 Data are expressed as the mean ± SD. The total score is the average of the sum of the individual trait values.
T-Cell Proliferative Responses and Cytokine/Chemokine Production by B10.PL/SnJ, Heterozygous, B10.PL-Esr1tm1Unc, and B10.PL-Esr2tm1Unc Mice
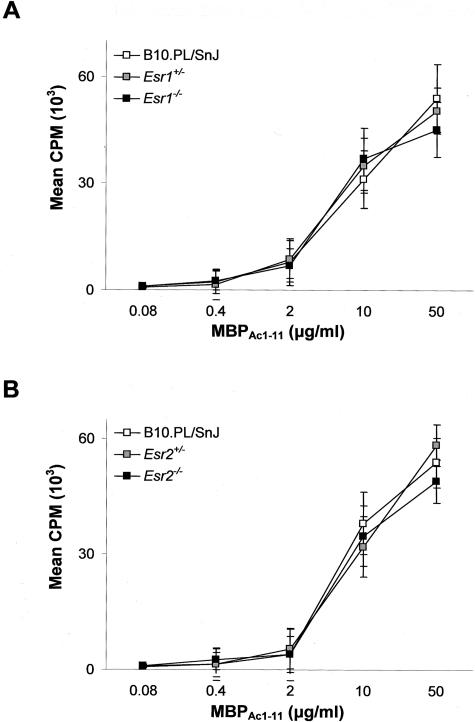
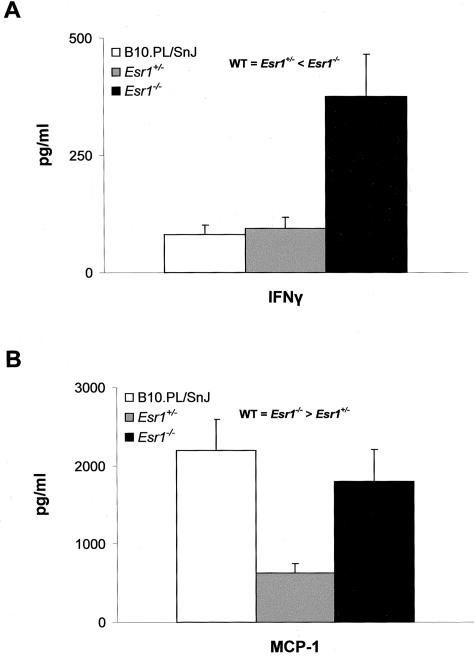
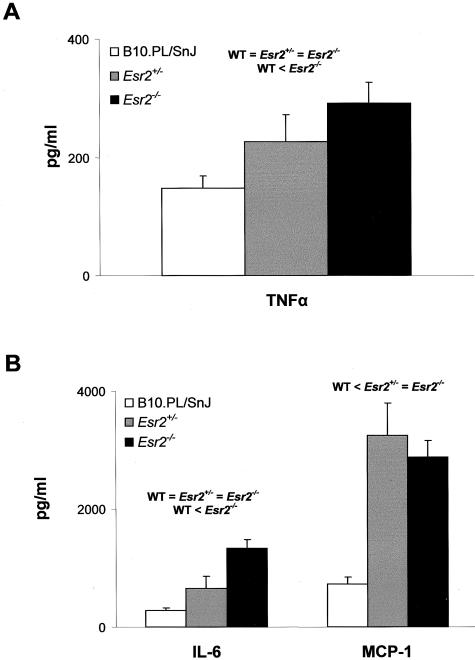
A variety of T-cell parameters were examined in an effort to delineate a mechanistic basis for the inhibition of chronic-late phase EAE in Esr1−/− mice and the heterotic effect at Esr2. Significant difference in the in vitro T-cell proliferative responses to MBPAc1-11 as a function of either the Esr1(Figure 7A) or Esr2(Figure 7B) genotype were not seen. In contrast, cytokine/chemokine production differed significantly. Of the cytokines studied, no significant differences in secretion were seen for IL-2, IL-4, IL-5, IL-10, and IL-12 (data not shown). IFN-γ secretion was found to be significantly negatively regulated by Esr1(Figure 8A) but uninfluenced by Esr2 (data not shown); whereas TNF-α (Figure 9A) and IL-6 (Figure 9B) secretion both exhibited classical Esr2 gene dose responses. Interestingly, MCP-1 secretion displayed distinctively unique patterns of genotype-dependent regulation by Esr1 and Esr2. MCP-1 production in Esr1+/− mice was significantly less than either wild-type or Esr1−/− mice (Figure 8B); whereas wild-type mice produced significantly less MCP-1 than Esr2+/− and Esr2−/− mice. As with clinical disease, these results are consistent with the existence of ER-mediated regulation of TNF-α, IL-6, and MCP-1 secretion by encephalitogen-specific CD4+ T cells in male mice.
Figure 7-4129.
MBPAc1-11-specific T-cell proliferative responses for Esr1 line mice (A) and Esr2 line mice (B). Data are expressed as mean cpm ± SD based on triplicate wells for three mice.
Figure 8-4129.
IFN-γ (A) and MCP-1 (B) secretion by MBPAc1-11-specific T cells from B10.PL/SnJ (□), B10.PL-Esr1+/− (░⃞), and B10.PL-Esr1−/− (▪). Cytokine production was determined by either enzyme-linked immunosorbent assay or cytometric bead assay.
Figure 9-4129.
TNF-α (A) and IL-6 and MCP-1 (B) secretion by MBPAc1-11-specific T cells from B10.PL/SnJ (□), B10.PL-Esr2+/− (░⃞), and B10.PL-Esr2−/− (▪). Cytokine production was determined by either enzyme-linked immunosorbent assay or cytometric bead assay.
EAE in Reciprocal Radiation Bone Marrow Chimeras between B10.PL/SnJ and B10.PL-Esr2+/− Male Mice
Reciprocal and control hematopoietic radiation bone marrow chimeras between wild-type and Esr2+/− male mice were constructed to map the functional component mediating the heterotic effect at Esr2 to either the hematopoietic or nonhematopoietic cellular compartments (Table 5). Wild-type → wild-type and Esr2+/− → wild-type mice exhibited delayed onset and less severe disease as determined by SI, CDS, and DI compared to wild-type → Esr2+/− and Esr2+/− → Esr2+/− mice. These results indicate that the heterotic effect at Esr2 maps to the nonhematopoietic compartment.
Table 5.
Susceptibility to MBPAc1-11-Induced EAE in Radiation Bone Marrow Chimeras between B10.PL/SnJ and B10.PL-Esr2+/− Male Mice
| Genotype | Incidence | Onset | Peak score | SI | CDS | DI | Mortality |
|---|---|---|---|---|---|---|---|
| B10.PL/SnJ → B10.PL/SnJ | 6/6 | 14.2 ± 3.3 | 4.5 ± 1.3 | 2.8 ± 1.1 | 36.4 ± 15.0 | 1.9 ± 0.8 | 0/6 |
| B10.PL/SnJ → Esr2+/− | 6/6 | 9.7 ± 1.5* | 5.6 ± 0.4 | 4.6 ± 0.9* | 74.5 ± 15.0* | 3.9 ± 0.8* | 2/6 |
| Esr2+/− → B10.PL/SnJ | 6/6 | 13.7 ± 3.2 | 4.1 ± 1.7 | 3.6 ± 1.4 | 38.4 ± 22.8 | 2.0 ± 1.2 | 1/6 |
| Esr2+/− → Esr2+/− | 6/6 | 9.8 ± 1.6* | 5.3 ± 0.5 | 4.5 ± 1.0* | 68.6 ± 19.7* | 3.6 ± 1.0* | 1/6 |
| F = 5.54 | F = 2.34 | F = 5.26 | F = 6.98 | F = 7.26 | χ2 = 2.40 | ||
| P value | 0.006 | 0.10 | 0.008 | 0.002 | 0.002 | 0.49 |
Significance of differences based on one way multiple comparisons with Tukey’s and Hsu’s family error rates of 0.1: wild-type → wild-type = Esr2+/− → wild-type ≠ wild-type → Esr2+/− and Esr2+/− → Esr2+/−.
Discussion
The function of estrogens and iERs has been well documented in female reproduction16,17 and these ligand receptor pairs are believed to play a role in the sexual dimorphism observed in autoimmune diseases such as multiple sclerosis34 and its animal model EAE.26–28 The fact that Esr1 is required for normal thymic development and maintenance of optimal T lymphopoiesis21,22 and in regulating B-cell development9,10 in male mice, combined with the observation that males are equally susceptible to estrogen treatment in preventing and modulating disease susceptibility,35 suggest that iERs may play a significant role in regulating autoimmune disease of the CNS in males. In this study we demonstrate that both Esr1 and Esr2 play unique roles in modulating clinical EAE in male mice.
Male Esr1−/− mice exhibit less severe disease during the chronic-late phase of the disease course compared to wild-type and Esr1+/− mice, indicating either that Esr1 signaling potentiates and enhances late clinical signs, or that its absence leads to increased expression of immunosuppressive factors. The results of the analysis of the cytokine/chemokine secreted by encephalitogen-specific T cells from Esr1−/− mice indicate that IFN-γ secretion is negatively regulated by Esr1 signaling. This stands in contrast to the report that estrogen exerts a direct stimulatory effect on IFN-γ gene expression in vitro.36 However, Cenci and colleagues,37 recently demonstrated that ovariectomy specifically up-regulates IFN-γ production by Th1 cells in vivo. The negative regulation of IFN-γ secretion by Esr1 provides a mechanistic basis for the inhibition of chronic-late phase disease in Esr1−/− male mice in that a considerable body of evidence exists indicating that IFN-γ has potent disease-inhibitory activity in EAE.38–41 It is worth noting that the negative inhibition of IFN-γ production by encephalitogen-specific T cells may be indirect because of the effects of Esr1 signaling in other hematopoietic cells, particularly dendritic cells,42,43 and may even require direct cell-cell contact.44
In comparison to Esr1−/− mice, Esr2−/− mice are phenotypically identical to wild-type mice with respect to clinical and histological disease. However, a significant heterotic effect at Esr2 is seen; Esr2+/− mice develop more severe disease compared to wild-type and Esr2−/− mice. Heterosis for susceptibility to EAE is not without precedence and is seen in several F1 hybrid combinations.45 Importantly, heterosis for EAE is not seen in (C57BL/6 × 129) F1 hybrid mice41,46 indicating that the heterotic effect at Esr2+/− is a function of the Esr2 genotype rather than 129-strain alleles at linked loci within the congenic interval. The heterotic effect does not seem to be because of increased T-cell proliferative responses or proinflammatory cytokine/chemokine secretion because neither parameter exhibits a significant increase in Esr2+/− mice over wild-type or Esr2−/− mice. This is consistent with the results of the radiation bone marrow chimera studies indicating that the heterotic effects maps to the nonhematopoietic cellular compartment. Additionally, our results suggest that single hit germ line or somatic mutations affecting Esr2 activity have the potential to significantly impact autoimmune disease of the CNS. In this regard, a growing body of evidence indicates that haploinsufficiency at a number of tumor suppressor genes in which a single allele is mutated or disrupted is sufficient to lead to tumorigenesis without inactivation of the second allele.47
The mechanism whereby heterosis at Esr2 influences the severity of the clinical signs associated with EAE as a function of the parenchyma, which includes cells in the CNS, is unknown but may be occurring prenatally, postnatally, or both.16,17 Esr2 has been shown to be required for normal brain development48,49 and recent immunohistochemical staining and expression analysis during postnatal development revealed significant cell-specific changes in the expression of Esr2 in cells of the lumbar tract of the ventral SC,50 the location of the earliest and most significant events that correlate with the severity of the clinical signs of EAE.51–55Esr1 expression was greater during early development, declining with age whereas Esr2 expression was minimal early, increasing with age and peaking at postnatal day 25. Co-localization studies revealed that at postnatal day 25, Esr1 and Esr2 are simultaneously expressed in astrocytes and oligodendrocytes within this region of the SC. Given that the binding of agonist facilitates an activating conformational change that permits iERs to form either α/α, α/β, or β/β dimers mediating receptor functions,56,57 the possibility exists that the heterotic effect at Esr2 is the result of a genotype-based imbalance in the relative proportion of α/α, α/β, or β/β dimer formation.
Heterosis in CNS development is not without precedence. Heterosis for brain myelin content has been reported for a variety of constituents including cerebroside, GM1 ganglioside, and myelin proteins including 2′,3′-cyclic nucleotide, 3′-phosphohydrolase (CNPase), and MBP,58–63 the target encephalitogen used in these studies. Additionally, the expression of myelin proteins, including MBP, CNPase, myelin-associated glycoprotein, and proteolipid protein, all of which are either encephalitogenic or autoantigenic,64 are regulated by steroid hormones.65–70 Similarly, steroid hormones determine the number of neurons in the sexually dimorphic motor nucleus of the lumbar SC.71–73 Thus, it is tempting to hypothesize that heterosis at Esr2 for increased severity of clinical signs of EAE in male mice may be related to the regulation of the expression of SC myelin constituents or the number of SC motor neurons during development. In this regard, B10.S SC homogenate is significantly less effective at eliciting EAE in SJL/J mice compared to autologous SJL/J SC homogenate (unpublished data).74 This is consistent with the concept that susceptibility to EAE, and by inference multiple sclerosis, may in part be determined by genes controlling myelin development, organization, and function.75,76
Footnotes
Address reprint requests to Dr. Cory Teuscher, Department of Medicine, C317 Given Medical Building, University of Vermont, Burlington, VT 05405. E-mail: cteusche@zoo.uvm.edu.
Supported by the National Institutes of Health (grants NS36526 to C.T. and E.P.B., AI4515 to C.T., AI41747 to C.T., AI42376 to H.O., NS23444 to H.O.), the National Multiple Sclerosis Society (grants RG-3129 to C.T. and E.P.B. and RG-3108 to H.O.), the Swedish Cancer Fund, and KaroBio AB.
References
- Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J Steroid Biochem. 1989;34:241–251. doi: 10.1016/0022-4731(89)90088-5. [DOI] [PubMed] [Google Scholar]
- Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–384. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Haruki Y, Seiki K, Enomoto T, Fujii H, Sakabe K. Estrogen receptor in the “non-lymphocytes” in the thymus of the ovariectomized rat. Tokai J Exp Clin Med. 1983;8:31–39. [PubMed] [Google Scholar]
- Kawashima I, Seiki K, Sakabe K, Ihara S, Akatsuka A, Katsumata Y. Localization of estrogen receptors and estrogen receptor-mRNA in female mouse thymus. Thymus. 1992;20:115–121. [PubMed] [Google Scholar]
- Stimson WH. Oestrogen and human T lymphocytes: presence of specific receptors in the T-suppressor/cytotoxic subset. Scand J Immunol. 1988;28:345–350. doi: 10.1111/j.1365-3083.1988.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Paavonen T. Hormonal regulation of immune responses. Ann Med. 1994;26:255–258. doi: 10.3109/07853899409147900. [DOI] [PubMed] [Google Scholar]
- Smithson G, Medina K, Ponting I, Kincade PW. Estrogen suppresses stromal cell-dependent lymphopoiesis in culture. J Immunol. 1995;155:3409–3417. [PubMed] [Google Scholar]
- Thurmond TS, Murante FG, Staples JE, Silverstone AE, Korach KS, Gasiewicz TA. Role of estrogen receptor alpha in hematopoietic stem cell development and B lymphocyte maturation in the male mouse. Endocrinology. 2000;141:2309–2318. doi: 10.1210/endo.141.7.7560. [DOI] [PubMed] [Google Scholar]
- Bellido T, Girasole G, Passeri G, Yu XP, Mocharla H, Jilka RL, Notides A, Manolagas SC. Demonstration of estrogen and vitamin D receptors in bone marrow-derived stromal cells: up-regulation of the estrogen receptor by 1,25-dihydroxyvitamin-D3. Endocrinology. 1993;133:553–562. doi: 10.1210/endo.133.2.8393768. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoe Y, Miyaura C, Ohta H, Nozawa S, Suda T. Expression of estrogen receptor beta in rat bone. Endocrinology. 1997;138:4509–4512. doi: 10.1210/endo.138.10.5575. [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Gustafsson JA. Biological role of estrogen and estrogen receptors. Crit Rev Biochem Mol Biol. 2002;37:1–28. doi: 10.1080/10409230290771438. [DOI] [PubMed] [Google Scholar]
- Moggs JG, Orphanides G. Estrogen receptors: orchestrators of pleiotropic cellular responses. EMBO Rep. 2001;2:775–781. doi: 10.1093/embo-reports/kve185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Shiau AK, Coward P, Schwarz M, Lehmann JM. Orphan nuclear receptors: from new ligand discovery technologies to novel signaling pathways. Curr Opin Drug Discov Dev. 2001;4:575–590. [PubMed] [Google Scholar]
- Couse JE, Mahato D, Eddy EM, Korach KS. Molecular mechanism of estrogen action in the male: insights from the estrogen receptor null mice. Reprod Fertil Dev. 2001;13:211–219. doi: 10.1071/rd00128. [DOI] [PubMed] [Google Scholar]
- Staples JE, Gasiewicz TA, Fiore NC, Lubahn DB, Korach KS, Silverstone AE. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J Immunol. 1999;163:4168–4174. [PubMed] [Google Scholar]
- Yellayi S, Teuscher C, Woods JA, Welsh TH, Jr, Tung KS, Nakai M, Rosenfeld CS, Lubahn DB, Cooke PS. Normal development of thymus in male and female mice requires estrogen/estrogen receptor-alpha signaling pathway. Endocrine. 2000;12:207–213. doi: 10.1385/endo:12:3:207. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebo BF, Jr, Adlard K, Schuster JC, Unsicker L, Vandenbark AA, Offner H. Gender differences in protection from EAE induced by oral tolerance with a peptide analogue of MBP-Ac1-11. J Neurosci Res. 1999;55:432–440. doi: 10.1002/(SICI)1097-4547(19990215)55:4<432::AID-JNR4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Butterfield RJ, Sudweeks JD, Blankenhorn EP, Korngold R, Marini JC, Todd JA, Roper RJ, Teuscher C. New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Sudweeks J, Rose J, Teuscher C. Genetic analysis of disease subtypes and sexual dimorphisms in mouse experimental allergic encephalomyelitis (EAE): relapsing/remitting and monophasic remitting/nonrelapsing EAE are immunogenetically distinct. J Immunol. 1999;162:3096–3102. [PubMed] [Google Scholar]
- Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Teuscher C. Identification of genetic loci controlling the characteristics and severity of brain and spinal cord lesions in experimental allergic encephalomyelitis. Am J Pathol. 2000;157:637–645. doi: 10.1016/S0002-9440(10)64574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Adlard K, Zamora A, Vandenbark AA. Estrogen potentiates treatment with T-cell receptor protein of female mice with experimental encephalomyelitis. J Clin Invest. 2000;105:1465–1472. doi: 10.1172/JCI9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma RZ, Gao J, Meeker ND, Fillmore PD, Tung KSK, Watanabe T, Zachary JF, Offner H, Blankenhorn EP, Teuscher C. Identification of Bphs, an autoimmune disease locus, as histamine receptor H1. Science. 2002;297:620–623. doi: 10.1126/science.1072810. [DOI] [PubMed] [Google Scholar]
- Teuscher C, Poynter ME, Offner H, Zamora A, Watanabe T, Fillmore PD, Zachary JF, Blankenhorn EP. Attenuation of Th1 effector cell responses and susceptibility to experimental allergic encephalomyelitis in histamine H2 receptor knockout mice is due to dysregulation of cytokine production by antigen presenting cells. Am J Pathol. 2004;164:883–892. doi: 10.1016/S0002-9440(10)63176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher C, Hickey WF, Korngold R. Experimental allergic orchitis in mice V Resistance to actively induced disease in BALB/cJ substrain mice is mediated by CD4+ T cells. Immunogenetics. 1990;32:34–40. doi: 10.1007/BF01787326. [DOI] [PubMed] [Google Scholar]
- Meeker ND, Hickey WF, Korngold R, Hansen WK, Sudweeks JD, Wardell BB, Griffith JS, Teuscher C. Multiple loci govern the bone marrow-derived immunoregulatory mechanism controlling dominant resistance to autoimmune orchitis. Proc Natl Acad Sci USA. 1995;92:5684–5688. doi: 10.1073/pnas.92.12.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Sierra O, Pacifici R. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA. 2003;100:10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys P, Vermeire K, Heremans H, Billiau A. The protective effect of IFN-gamma in experimental autoimmune diseases: a central role of mycobacterial adjuvant-induced myelopoiesis. J Leukoc Biol. 2000;68:447–454. [PubMed] [Google Scholar]
- Lublin FD, Knobler RL, Kalman B, Goldhaber M, Marini J, Perrault M, D’Imperio C, Joseph J, Alkan SS, Korngold R. Monoclonal anti-gamma interferon antibodies enhance experimental allergic encephalomyelitis. Autoimmunity. 1993;16:267–274. doi: 10.3109/08916939309014645. [DOI] [PubMed] [Google Scholar]
- Furlan R, Brambilla E, Ruffini F, Poliani PL, Bergami A, Marconi PC, Franciotta DM, Penna G, Comi G, Adorini L, Martino G. Intrathecal delivery of IFN-gamma protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J Immunol. 2001;167:1821–1829. doi: 10.4049/jimmunol.167.3.1821. [DOI] [PubMed] [Google Scholar]
- Yang J, Lindsberg PJ, Hukkanen V, Seljelid R, Gahmberg CG, Meri S. Differential expression of cytokines (IL-2, IFN-gamma, IL-10) and adhesion molecules (VCAM-1, LFA-1, CD44) between spleen and lymph nodes associates with remission in chronic relapsing experimental autoimmune encephalomyelitis. Scand J Immunol. 2002;56:286–293. doi: 10.1046/j.1365-3083.2002.01132.x. [DOI] [PubMed] [Google Scholar]
- Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A, Dwyer J, Vandenbark AA, Offner H. Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res. 2002;70:238–248. doi: 10.1002/jnr.10409. [DOI] [PubMed] [Google Scholar]
- Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, Dupont S, Chambon P, Druet P, Bayard F, Guery JC. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur J Immunol. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Yamasaki M, Miyazaki Y, Tachibana H, Yamada K. Estrogenic compounds suppressed interferon-gamma production in mouse splenocytes through direct cell-cell interaction. In Vitro Cell Dev Biol Anim. 2003;39:383–387. doi: 10.1290/1543-706X(2003)039<0383:ECSIPI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Arnon R. Experimental allergic encephalomyelitis-susceptibility and suppression. Immunol Rev. 1981;55:5–30. doi: 10.1111/j.1600-065x.1981.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Frei K, Eugster HP, Bopst M, Constantinescu CS, Lavi E, Fontana A. Tumor necrosis factor alpha and lymphotoxin alpha are not required for induction of acute experimental autoimmune encephalomyelitis. J Exp Med. 1997;185:2177–2182. doi: 10.1084/jem.185.12.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodde R, Smits R. Cancer biology A matter of dosage. Science. 2002;298:761–763. doi: 10.1126/science.1077707. [DOI] [PubMed] [Google Scholar]
- Wang L, Andersson S, Warner M, Gustafsson JA. Morphological abnormalities in the brains of estrogen receptor beta knockout mice. Proc Natl Acad Sci USA. 2001;98:2792–2796. doi: 10.1073/pnas.041617498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER) beta knockout mice reveal a role for ER beta in migration of cortical neurons in the developing brain. Proc Natl Acad Sci USA. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platania P, Laureanti F, Bellomo M, Giuffrida R, Giuffrida-Stella AM, Catania MV, Sortino MA. Differential expression of estrogen receptors alpha and beta in the spinal cord during postnatal development: localization in glial cells. Neuroendocrinology. 2003;77:334–340. doi: 10.1159/000070899. [DOI] [PubMed] [Google Scholar]
- Leibowitz S, Kennedy L. Cerebral vascular permeability and cellular infiltration in experimental allergic encephalomyelitis. Immunology. 1972;22:859–869. [PMC free article] [PubMed] [Google Scholar]
- Oldendorf WH, Towner HF. Blood-brain barrier and DNA changes during the evolution of experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1974;33:616–631. doi: 10.1097/00005072-197410000-00004. [DOI] [PubMed] [Google Scholar]
- Kristensson K, Wisniewski HM. Chronic relapsing experimental allergic encephalomyelitis Studies in vascular permeability changes. Acta Neuropathol (Berl) 1977;39:189–194. doi: 10.1007/BF00691696. [DOI] [PubMed] [Google Scholar]
- Kerlero de Rosbo N, Bernard CC, Simmons RD, Carnegie PR. Concomitant detection of changes in myelin basic protein and permeability of blood-spinal cord barrier in acute experimental autoimmune encephalomyelitis by electroimmunoblotting. J Neuroimmunol. 1985;9:349–361. doi: 10.1016/s0165-5728(85)80035-7. [DOI] [PubMed] [Google Scholar]
- Butter C, Baker D, O’Neill JK, Turk JL. Mononuclear cell trafficking and plasma protein extravasation into the CNS during chronic relapsing experimental allergic encephalomyelitis in Biozzi AB/H mice. J Neurol Sci. 1991;104:9–12. doi: 10.1016/0022-510x(91)90209-p. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ER alpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- McDonnell DP. Mining the complexities of the estrogen signaling pathways for novel therapeutics. Endocrinology. 2003;144:4237–4240. doi: 10.1210/en.2003-0900. [DOI] [PubMed] [Google Scholar]
- Seyfried TN, Yu RK. Heterosis for brain myelin content in mice. Biochem Genet. 1980;18:1229–1237. doi: 10.1007/BF00484350. [DOI] [PubMed] [Google Scholar]
- Ebato H, Seyfried TN, Yu RK. Biochemical study of heterosis for brain myelin content in mice. J Neurochem. 1983;40:440–446. doi: 10.1111/j.1471-4159.1983.tb11302.x. [DOI] [PubMed] [Google Scholar]
- Miskimins R, Ebato H, Seyfried TN, Yu RK. Molecular basis for heterosis for myelin basic protein content in mice. Proc Natl Acad Sci USA. 1986;83:1532–1535. doi: 10.1073/pnas.83.5.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskimins R, Yu RK. Heterosis for myelin content is limited to the central nervous system. J Neurosci Res. 1988;19:364–366. doi: 10.1002/jnr.490190312. [DOI] [PubMed] [Google Scholar]
- Sato C, Yu RK. Heterosis for brain cerebroside synthesis in mice. Dev Neurosci. 1990;12:153–158. doi: 10.1159/000111845. [DOI] [PubMed] [Google Scholar]
- Bigbee JW, Yu DS, Yu RK. Morphometric analysis of the developing optic nerve of the F1 heterotic mouse and its parental strains. Neurosci Lett. 1990;119:179–181. doi: 10.1016/0304-3940(90)90828-w. [DOI] [PubMed] [Google Scholar]
- Morris-Downes MM, McCormack K, Baker D, Sivaprasad D, Natkunarajah J, Amor S. Encephalitogenic and immunogenic potential of myelin-associated glycoprotein (MAG), oligodendrocyte-specific glycoprotein (OSP) and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) in ABH and SJL mice. J Neuroimmunol. 2002;122:20–33. doi: 10.1016/s0165-5728(01)00460-x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Cole R, Chiappelli F, de Vellis J. Differential regulation of oligodendrocyte markers by glucocorticoids: post-transcriptional regulation of both proteolipid protein and myelin basic protein and transcriptional regulation of glycerol phosphate dehydrogenase. Proc Natl Acad Sci USA. 1989;86:6807–6811. doi: 10.1073/pnas.86.17.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdi JM, Kampf K, Campagnoni AT. Translational regulation of myelin protein synthesis by steroids. J Neurochem. 1989;52:321–324. doi: 10.1111/j.1471-4159.1989.tb10935.x. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Newman SL, Campagnoni CW, Verdi JM, Mohandas T, Handley VW, Campagnoni AT. Expression of a novel transcript of the myelin basic protein gene. J Neurochem. 1990;54:2032–2041. doi: 10.1111/j.1471-4159.1990.tb04908.x. [DOI] [PubMed] [Google Scholar]
- Campagnoni AT, Verdi JM, Verity AN, Amur-Umarjee S. Posttranscriptional events in the expression of myelin protein genes. Ann NY Acad Sci. 1990;605:270–279. doi: 10.1111/j.1749-6632.1990.tb42400.x. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Campagnoni AT. Translational regulation by steroids Identification of a steroid modulatory element in the 5′-untranslated region of the myelin basic protein messenger RNA. J Biol Chem. 1990;265:20314–20320. [PubMed] [Google Scholar]
- Campagnoni AT, Verdi JM, Verity AN, Amur-Umarjee S, Byravan S. Posttranscriptional regulation of myelin protein gene expression. Ann NY Acad Sci. 1991;633:178–188. doi: 10.1111/j.1749-6632.1991.tb15608.x. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Breedlove SM, Arnold AP. Sexual dimorphism and the influence of neonatal androgen in the dorsolateral motor nucleus of the rat lumbar spinal cord. Brain Res. 1982;249:309–314. doi: 10.1016/0006-8993(82)90065-8. [DOI] [PubMed] [Google Scholar]
- Wagner CK, Clemens LG. Perinatal modification of a sexually dimorphic motor nucleus in the spinal cord of the B6CD2F1 house mouse. Physiol Behav. 1989;45:831–835. doi: 10.1016/0031-9384(89)90303-x. [DOI] [PubMed] [Google Scholar]
- Korngold R, Feldman A, Rorke LB, Lublin FD, Doherty PC. Acute experimental allergic encephalomyelitis in radiation bone marrow chimeras between high and low susceptible strains of mice. Immunogenetics. 1986;24:309–315. doi: 10.1007/BF00395536. [DOI] [PubMed] [Google Scholar]
- Moscarello MA, Wood DD, Ackerley C, Boulias C. Myelin in multiple sclerosis is developmentally immature. J Clin Invest. 1994;94:146–154. doi: 10.1172/JCI117300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosccaccio GL, Steinman L. Multiple sclerosis: from a myelin point of view. J Neurosci Res. 1996;45:647–654. doi: 10.1002/(SICI)1097-4547(19960915)45:6<647::AID-JNR1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]