Abstract
This study was performed to quantitate and characterize the mononuclear phagocytes (MPs) in human immunodeficiency virus encephalopathy (HIVE) by immunohistochemistry in an effort to gain insights into potential mechanisms of central nervous system (CNS) accumulation. Single- and double-labeled studies using antibodies against CD14, CD16, CD68, proliferating cell nuclear antigen (PCNA), Ki-67, von Willebrand factor, and HIV-1 p24 were performed using brain tissue from patients with HIVE, HIV-1 infection without encephalitis, and seronegative controls. A substantial increase in MPs was observed in CNS tissue from patients with HIVE, relative to seronegative controls and patients with acquired immune deficiency syndrome but without encephalitis, as determined by CD68 and CD16 immunohistochemistry. A large proportion of CD16+ MPs in HIVE CNS tissue were PCNA+, but do not appear to be proliferating, based on limited Ki-67 positivity. Although virtually all cells positive for HIV-1 p24 were PCNA+, there were many PCNA+ cells where HIV-1 p24 expression was not detected. PCNA positivity was also observed in some endothelial cells and ependymal cells in HIVE CNS. Our results would support a role for HIV-1-induced alterations in MP trafficking and homeostasis in the pathogenesis of HIVE.
Human immunodeficiency virus type 1 (HIV-1)-associated neurological disorders, including dementia and peripheral neuropathies, affect ∼30% of adults and almost all children with HIV-1 infection and acquired immune deficiency syndrome (AIDS).1–3 HIV-1 dementia complex is characterized histologically as HIV encephalitis (HIVE) with accumulations of perivascular macrophages, multinucleated giant cells, nodular lesions with areas of focal necrosis, and white matter thinning.4–6 The transmission of virus to the central nervous system (CNS) by macrophages during acute HIV-1 infection has been proposed to account for HIV-1 dementia complex and HIVE, even though both occur much later in the course of the disease.7
Unchecked, an active reservoir of HIV-1 infection in the CNS would presumably promote the development of clinical dementia. Accordingly, viral load in CNS and cerebral spinal fluid correlates with the severity of dementia.8,9 Cells productively infected with HIV-1 in the CNS appear to be cleared by the immune system early in the course of infection, before immune deficiency. Studies in primate and murine models emphasize the role of T cells in the control and clearance of the active HIV-1 reservoir in the CNS and support the notion that changes in immune status and loss of control of viremia contribute to the development of HIVD.10,11 The active reservoir of HIV-1 infection in the CNS, therefore, likely reflects a balance between the accumulation and clearance of infected cells.
There is considerable debate as to whether peripheral blood-derived macrophages and/or resident microglia constitute the major CNS reservoir of productive HIV-1 infection. By standard immunohistochemical methods, CD14 and CD45(LCA) expression is detectable in perivascular macrophages, but not in resident microglia.12–15 Studies by our group and others have used these immunophenotypic markers to characterize mononuclear phagocytes (MPs) in the CNS in HIVE.16,17 In the HIVE brain, the perivascular MPs are CD14- and CD45(LCA)-positive. These perivascular MPs also express the FcγIII receptor (CD16) and HLA-DR, and are, for the most part, productively infected, as determined by HIV-1 p24 immunostaining.16 In addition, monocytic cells within the nodular lesions and multinucleated giant cells are CD14+/CD45(LCA)+ and CD16+/HIV-1 p24+.16 The prominent CD16 expression on these perivascular macrophages is evidence for immunophenotypic similarity with circulating MPs in HIVD, supporting the hypothesis put forth by Pulliam and colleagues,18 that an increased proportion of circulating monocytes in individuals with HIVD leads to CNS invasion and contributes to the pathogenesis of HIV-1 in the CNS. The immunophenotype of the perivascular MPs in HIVE, therefore, shows phenotypic similarity with that of MPs expanded in the peripheral blood in HIV/AIDS and even more so in the setting of HIVD. Importantly, these perivascular macrophages are HIV-1 p24-positive,16 suggesting the role of these cells as a productive reservoir of HIV-1 infection and a likely source of cerebral spinal fluid viral load. This conclusion is supported by studies in macaques with SIVE.17
A second cellular reservoir of HIV-1 infection in the CNS consists of large numbers of HIV-1-infected (HIV-1 p24+) cells with ramified microglial morphology. In these microglial cells, the level of expression of both CD14 and CD45(LCA) is generally below the level of detection using conventional immunohistochemical methods, in contrast to monocytes/macrophages derived from circulation that stain positively for these markers. The expression of CD16 within this parenchymal MP population in HIVE suggests microglial activation and provides a marker in common with the perivascular macrophages. We considered the possibilities that the parenchymal CD14−/CD45(LCA)−/CD16+ cells may represent activated resident microglia that are activated and/or infected. Alternatively, they may represent CD14+/CD45(LCA)+ and CD16+/HIV-1 p24+ cells that have previously invaded the CNS from the periphery and taken on microglial characteristics, ie, loss of CD45(LCA) and CD14 expression, on differentiation. These alternatives are not necessarily mutually exclusive. To begin to address the issue of MP proliferation and/or invasion into the CNS, we performed quantitative immunohistochemical analysis of MP subsets in HIVE using markers for proliferation [proliferating cell nuclear antigen (PCNA) and Ki-67], activation (CD16), HIV-1 infection (HIV-1 p24), and monocyte/macrophage markers (CD14 and CD68).
Materials and Methods
Human Tissue Samples
Paraffin-embedded brain tissue sections from patients with HIVE were obtained from the Manhattan Brain Bank National Neuro-AIDS Tissue Consortium.19 Specimens from seronegative and HIV-1-positive adults without CNS disease were obtained from the Drexel University College of Medicine autopsy service. A total of nine HIVE cases, three HIV-1-positive cases without CNS disease, and five seronegative controls were analyzed (Table 1).
Table 1.
Patient Data
| Accession no. | Age/gender | HIV-1 status | CNS pathology | Non-CNS pathology |
|---|---|---|---|---|
| HIVE 01 | 50 M | + | HIVE | Bacterial pneumonia, history of microsporidia, CMV |
| HIVE 02 | 38 F | + | HIVE | Bacterial pneumonia, CMV, ITP, MAI, PCP |
| HIVE 03 | 45 M | + | HIVE | Endocarditis, pneumonia, amyloid |
| HIVE 04 | 47 M | + | HIVE | Wasting, candidal sepsis w/foci in kidneys and lungs, urosepsis |
| HIVE 05 | 46 M | + | HIVE | Acute bronchopneumonia, cachexia, micronodular cirrhosis w/jaundice |
| HIVE 06 | 50 M | + | HIVE | Bronchopneumonia w/diffuse alveolar damage |
| HIVE 07 | 44 M | + | HIVE | Cachexia, esophagitis |
| HIVE 08 | 37 M | + | HIVE | Klebsiella pneumonia, sepsis |
| HIVE 09 | 37 M | + | HIVE | Bronchopneumonia, history of CMV retinitis |
| HIV+/HIVE-01 | 35 M | + | None | CMV pneumonia and cystitis |
| HIV+/HIVE-02 | 41 M | + | Multiple infarctions w/diffuse intraventricular hemorrhage | Autopsy restricted to CNS |
| HIV+/HIVE-03 | 32 M | + | CNS lymphoma | Autopsy restricted to CNS |
| HIV-01 | 67 F | − | Hypertensive/diabetic vasculopathy | Coronary artery disease, hypertension, diabetes |
| HIV-02 | 56 M | − | Hypertension, healed infarctions | S/P heart transplant, pneumonia, chronic renal failure |
| HIV-03 | 22 F | − | Hypoxic/ischemic changes, acute infarction occipital lobe | Metastatic carcinoma, sepsis |
| HIV-04 | 43 M | − | Hypertensive vascular disease, diffuse micro-infarctions | Endocarditis, pulmonary hypertension |
| HIV-05 | 48 M | − | Hypoxic/ischemic changes | Hepatic failure |
Immunohistochemistry
Immunohistochemistry was performed as previously described16 on 4-μm brain tissue sections. Mouse monoclonal anti-human antibodies were used as follows: CD68 (clone KP1; NovaCastra, Newcastle, UK) was used at a 1:50 dilution; CD14 (clone 7, NovoCastra) was used ata 1:50 dilution; CD16 (clone 2H7, NovoCastra) was usedat 1:40; PCNA (clone PC10; DAKO, Carpinteria, CA) was used at a 1:50 dilution; Ki-67 (clone MM1, NovoCastra) used at a 1:25 dilution; and HIV-1 p24 (clone Kal-1, DAKO) used at 1:5. A rabbit polyclonal antibody against human von Willebrand Factor (Chemicon International, Temecula, CA) was used at 1:300. Tonsil and autopsy intestine from HIV-1-seronegative individuals were used as positive controls. Negative controls included tonsil, intestine, and brain tissues incubated in blocking solution with IgG1 and IgG2 isotype control antibodies at concentrations equal to the highest antibody IgG concentration. Primary antibodies were detected with biotinylated anti-mouse (monoclonal primaries), or anti-rabbit (polyclonal primary) IgG, avidin-biotin complex, and alkaline phosphatase-Vector Red (Vector Laboratories, Burlingame, CA) or peroxidase-3,3′-diaminobenzidine (Sigma Chemical Co., St. Louis, MO) according to manufacturers’ instructions. A tyramide signal amplification method was used (Catalyzed Signal Amplification System, DAKO) for detection of Ki-67 as recommended by the manufacturer with the following exceptions: primary antibody was diluted in antibody diluent with background reducing components (DAKO) and allowed to incubate on tissues overnight at room temperature. Endogenous peroxidase was quenched by incubating sections in 3% H2O2/methanol.
Double-label immunohistochemistry for PCNA/CD16 and PCNA/von Willebrand factor was performed by sequential application of primary antibodies to the same tissue section, as previously described.16 Subsequent to overnight exposure with the first primary antibody, sections were incubated with biotinylated secondary antibody followed by avidin-biotin complex and 3,3′-diaminobenzidine. Tissues were then taken back to buffer and blocking reagent, incubated in the second primary antibody overnight, followed by secondary antibody, avidin-biotin complex, and alkaline phosphatase-Vector Red, according to the manufacturer’s instructions.
Double-label immunohistochemistry for PCNA/p24 was performed by sequential application of primary antibodies to the same tissue section, which were revealed by systems with fluorescein and Texas Red tags, as previously described.16 The double-stained sections were examined using an inverted fluorescence microscope (Nikon, Melville, NY) through both fluorescein isothiocyanate and rhodamine filters. A digital photographic system (Princeton Instruments; IP Lab) was used to superimpose images and evaluate the degree of co-localization of the fluorescent stain products.
Statistical Analysis
The number of CD68+, CD14+, CD16+, and Ki-67+ cells was determined for each case by averaging the number of positive cells observed over 10 random microscopic fields at ×400 magnification. This magnification allowed for the discrimination of cellular features, including overlying nuclei in perivascular inflammatory clusters, and yielded an adequate number of countable profiles/field for data collection. Data were obtained using two independent observers for a total of 20 microscopic fields. The mean of the two averages was accepted as the average number of positive cells per field for each case. In the case of CD68, the total number of positive cells and total parenchymal-positive cells were counted separately. The means for the three groups, HIV-, HIV+/HIVE−, and HIVE+, were compared by one-way analysis of variance followed by Tukey-Kramer multiple comparisons posttest using Graph Pad Prism version 3.00 software, San Diego CA.
Results
Increased brain macrophages in HIVE have been previously reported.20 In the studies presented here, we extend our previous studies identifying two immunophenotypically distinct subpopulations of activated MPs in HIVE CNS tissue. Here we performed quantitative immunophenotypic analysis with additional markers to evaluate mechanisms involved in the accumulation of MPs in HIVE, ie, increased trafficking into the CNS and/or MP proliferation. We previously reported an abundance of CD14+/CD16+ MPs localized perivascularly in HIVE. These cells appeared morphologically and immunophenotypically distinct from the parenchymal microglial cells, which expressed CD16 as a marker of MP activation, however, CD14 expression was not generally detectable by our immunohistochemical methods in these ramified MPs. To quantitate the total MPs in brain specimens from patients with HIVE, HIV-1 infection (AIDS) but without encephalitis and seronegative controls, immunohistochemistry was performed using a monoclonal antibody against CD68, a molecule of unknown function found intracellularly in monocytes and macrophages. Analysis of variance and Tukey-Kramer multiple comparisons analyses revealed a statistically significant increase in total CD68+ cells in HIVE brain tissue sections when compared to brain tissue from patients with HIV-1 infection without encephalitis (P = 0.016) and seronegative controls (P = 0.002) (Figure 1A). Clusters of CD68+ cells, generally observed in the perivascular spaces of HIVE samples, where the individual cell borders could not be distinguished, were counted as a single cell. As such, the actual number of total brain macrophages in HIVE we report here are most likely an underestimate of the total number of MPs in HIVE brain tissue. Because of the reduced density of parenchymal MPs, a more accurate quantitation of MPs in parenchyma could be obtained. Here, the total number of parenchymal CD68+ cells was also significantly increased in HIVE (Figure 1B). Similar increases in parenchymal MPs in HIVE were observed with tissue sections stained for CD16, relative to HIV+/HIVE− (P = 0.03) or normal (P = 0.005) (Figure 1C). Although we previously characterized the majority of parenchymal CD16+ MPs in HIVE as CD14−,16 we had noted scattered CD14+ cells with short processes. We observed restricted areas of parenchymal MP expression of CD14 in three of nine HIVE cases; overall there was only limited parenchymal CD14 positivity in HIVE brain. As illustrated graphically in Figure 1D, the difference in parenchymal CD14+ cells between seronegative, HIV-1/AIDS without encephalitis, and HIVE brain sections did not reach statistical significance. We cannot rule out the possibility that an increase in CD14+ MPs in parenchyma in HIVE would reach significance with a larger number of samples. The P value was lower, P = 0.114, yet did not reach statistical significance after combining the HIV/AIDS and seronegative groups and comparing the total non-encephalopathy group with HIVE by Student’s t-test (assuming unequal variances).
Figure 1.
CD68, CD14, and CD16 analysis of variance box plots. A significant number of total brain MPs (A), as determined by CD68 positivity, were observed in HIVE when compared to HIV-1-infected individuals without dementia (P = 0.016) and seronegative controls (P = 0.002). HIV+/HIVE− versus HIV− was not statistically significant (P = 0.861). Similarly, parenchymal CD68+ MPs (B) demonstrated statistical significance between HIVE and HIV-1 without dementia (P = 0.003) and HIVE and seronegative individuals (P = 0.001). CD16+ cells in HIVE parenchyma were also present in statistically greater numbers, as compared to HIV+/HIVE− (P = 0.03), as well as seronegative controls (P = 0.005) (C), however, CD14+ parenchymal cells did not increase significantly between controls and HIVE brains (D). Results for all comparisons were verified by the Tukey-Kramer comparison of means posttest.
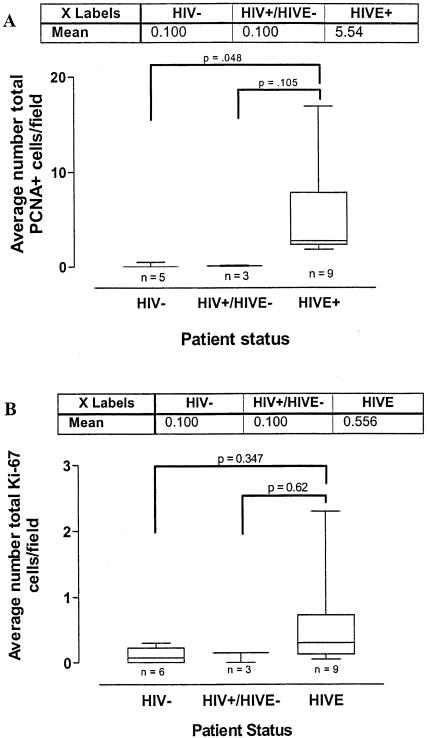
To begin to address potential mechanisms that could account for the increase in brain monocytes/macrophages in HIVE, we performed immunohistochemical analysis using antibodies recognizing markers associated with proliferation, ie, PCNA and Ki-67. PCNA+ nuclei were observed in the parenchyma of both cortex and white matter, as well as around blood vessels in patients with HIVE (Figure 2, C and G). Nodular lesions, including multinucleated giant cells, were also PCNA+ (Figure 2I). A smaller number of positive cells (an average of 0.1 cells per microscopic field) were seen perivascularly in the brains of patients with HIV-1 infection but without encephalitis (Figure 2F). Brain tissue from seronegative controls were negative for PCNA staining (Figure 2, A and E). The number of PCNA-positive cells was significantly increased in HIVE relative to seronegative controls using the Tukey-Kramer test for multiple comparisons (P = 0.048) (see Figure 4A). The difference between HIV-1/AIDS and HIVE did not reach significance in multiple comparison tests, however the number of patients is small with reasonably large standard deviations because of local variations in staining within specimens. Furthermore, after combining the two HIV-1/AIDS and control groups into HIVE− and HIVE+, the encephalopathy group had increased PCNA positivity relative to the patients without encephalopathy (P = 0.01) by Student’s t-test, assuming unequal variances as a conservative approach.
Figure 2.
PCNA and CD16 immunohistochemistry. A and E: Normal brain; B and F: HIV-1 without encephalopathy; C, G, and I: HIVE; D, H, and J: CD16 immunohistochemistry in HIVE brain tissue. The top row (A–D) illustrates white matter. E to H: Blood vessels. I and J: Microglial nodules in the vicinity of blood vessels (arrows). In HIVE, PCNA+ cells were observed in white matter, perivascularly, and within microglial nodules (C, G, and I, arrows). A small number of PCNA+ cells were observed perivascularly in patients with HIV-1 infection but without dementia (F, arrow). Some cells observed within blood vessels, not associated with brain tissue, were also PCNA+ in HIV+/HIVE− and HIVE+ brain tissue (F and I, arrowheads). CD16+ cells observed in white matter of patients with HIVE had a ramified morphology (D, arrows) and were also seen perivascularly and within microglial nodules in HIVE (H and J). Original magnifications, ×40.
Figure 4.
PCNA and Ki-67 analysis of variance box plots. A: The total number of PCNA+ cells was significantly increased in HIVE relative to seronegative controls using the Tukey-Kramer test for multiple comparisons (P = 0.048). Although the difference between HIV-1/AIDS and HIVE did not reach significance (P = 0.105), the number of patients is small with a large SD because of local variations in staining within specimens. After dividing the patients into HIVE− and HIVE+, the encephalopathy group demonstrated increased PCNA positivity relative to the patients without encephalopathy (P = 0.01) by Student’s t-test, assuming unequal variances. In contrast, the small increase in the number of Ki-67-positive cells in HIVE relative to HIV-1/AIDS and seronegative controls, was not significant (B) and did not reach statistical significance after combining the HIV-1/AIDS and control groups into HIVE− and HIVE+ (P = 0.089). Results for all comparisons were verified by the Tukey-Kramer comparison of means posttest.
Because PCNA positivity is indicative of either DNA replication or DNA repair, additional studies were performed using an antibody against human Ki-67, a more definitive marker of cell proliferation. In contrast to the results with PCNA, Ki-67 positivity was detected in a relatively small number of parenchymal nuclei in HIVE specimens (Figure 3C). Rare positive cells were also observed in the perivascular cuffs in HIVE (Figure 3F), but not in multinucleated giant cells (Figure 3H) as well as in brain tissue from patients with HIV-1/AIDS but without encephalitis and seronegative controls (Figure 3; A, B, D, and E). Although there was a small increase in the number of Ki-67-positive cells in HIVE relative to HIV-1/AIDS and seronegative controls, the differences were not statistically significant (Figure 4B). Additionally, these differences did not reach statistical significance after combining the HIV-1/AIDS and control groups (P = 0.089). Human autopsy intestine, as a positive control, showed a large proportion of positive nuclei confirming the sensitivity of the tyramide signal amplification technique used in this study in postmortem tissue (Figure 3G).
Figure 3.
Ki-67 immunohistochemistry. A and D: Normal brain; B and E: HIV-1 without encephalopathy; C, F, and H: HIVE; G: small intestine from a seronegative individual. The top row (A–C) illustrates white matter. D–F: Blood vessels. H: A multinucleated giant cell within a microglial nodule. PCNA+ brain tissue from patients with HIVE showed only occasional Ki-67-labeled nuclei, as demonstrated in C (white matter) and F (blood vessel). H: Multinucleated giant cells did not show Ki-67 positivity. Rare positivity was observed in white matter of patients with HIV-1 infection without dementia (B) and seronegative controls (A). E: Limited positive cells were also located perivascularly in HIV+/HIVE− brain tissue. G: Autopsy intestine from a seronegative individual shows abundant nuclear Ki-67 positivity with the CSA technique. Original magnifications: ×40 (A–G); ×100 (H).
Previously, we reported CD16 expression in two populations of cells representing the major reservoirs of HIV-1 infection in the CNS, namely the CD14+ perivascular macrophage and CD14-negative parenchymal ramified microglial cells (also shown in Figure 2D).16 With single-labeling immunohistochemistry, CD16+ cells were once again observed perivascularly and within microglial nodules (Figure 2, H and J). To determine the relationship between these activated MPs and PCNA expression, PCNA/CD16 double-label immunohistochemistry was performed on the same CNS tissues. In HIVE, a considerable number of PCNA+ cells were also CD16+ in the perivascular space (Figure 5C), in parenchyma (areas not in obvious association with blood vessels) (Figure 5, A and B) and within nodular lesions (Figure 5, D and E). Many multinucleated giant cells, not associated with nodules, were also positive for both antigens (Figure 5F).
Figure 5.
PCNA/CD16 immunohistochemistry. All panels show PCNA/CD16 double immunohistochemistry on brain tissue from a patient with HIVE (HIVE 08). In HIVE, PCNA (brown) and CD16 (pink) double-positive cells were observed in white matter (A and B), around blood vessels (C), and within microglial nodules (D and E). B: Inset of A. E: Inset of D. Arrows point to cells with co-localized PCNA and CD16 expression. Some PCNA+ cells in white matter and around blood vessels did not express detectable levels of CD16 (A–C, arrowheads). Multinucleated giant cells, not associated with microglial nodules, were also positive for both antigens (F). Original magnifications: ×40 (A–E); ×100 (F).
Although PCNA staining often co-localized with CD16, not all CD16+ cells were PCNA+. Conversely, not all PCNA+ cells were CD16+. Some PCNA+/CD16− cells observed along blood vessel walls were oblong, suggesting endothelial cell morphology. Double-label immunohistochemistry, using antibodies against PCNA and von Willebrand factor, confirmed the PCNA positivity of some endothelial cells (Figure 6, A and B) in HIVE brain tissue.
Figure 6.
PCNA/von Willebrand factor immunohistochemistry. All panels show PCNA/von Willebrand factor double immunohistochemistry on brain tissue from a patient with HIVE (HIVE 08). In brain tissue from patients with HIVE, several endothelial cells, which are positive for von Willebrand Factor (pink), were also PCNA-positive (brown). Arrows indicate double-positive cells. Original magnifications: ×40 (A); ×100 (B).
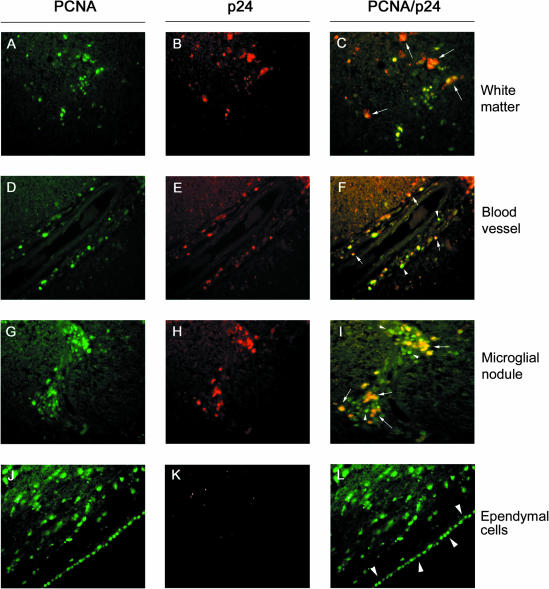
To determine whether PCNA expression occurred in association with productive HIV-1 infection, double-label immunofluorescence was performed on CNS tissues using antibodies against PCNA and the HIV-1 p24 gag protein. PCNA/HIV-1 p24 double-labeling revealed that HIV-1 p24 co-localized with PCNA in approximately half of the PCNA+ cells observed in the parenchyma, around blood vessels, and within microglial nodules (Figure 7; C, F, and I, arrows) of patients with HIVE. In contrast, virtually all of the cells positive for HIV-1 p24 antigen were also positive for PCNA. Multinucleated giant cells were also generally positive for both antigens (Figure 7C, short-tailed arrows). Ependymal cells were positive for PCNA, but not HIV-1 p24 (Figure 7; J to L). Additional cells were PCNA-positive, but HIV-1 p24-negative in Figure 7, J and L, apparently within the subependymal region.
Figure 7.
PCNA/p24 immunofluorescence. All panels show PCNA/p24 immunofluorescence on brain tissue from a patient with HIVE (HIVE 08). fluorescein isothiocyanate-labeled PCNA+ cells (green) were observed in white matter (A), around blood vessels (D), within microglial nodules (G), and in ependymal cells (J). Texas Red-labeled HIV-1 p24+ cells (red) were observed in white matter (B), around blood vessels (E), and within microglial nodules (H), but not in ependymal cells (K). Approximately half of the PCNA+ cells were also p24+ (yellow) (C, F, and I). Arrows point to cells that are positive for PCNA and p24. Some multinucleated giant cells, not associated with nodules, in white matter were also positive for both antigens (C, short-tailed arrows). Although virtually all p24+ cells were PCNA+, many PCNA+ cells were negative for HIV-1 p24, including cells located in white matter, perivascularly, and within microglial nodules (C, F, and I, arrowheads), as well as all ependymal cells (L, arrowheads). Original magnifications, ×40.
Discussion
Our results demonstrate an increase in the number of macrophages/microglia in the CNS in HIVE, in agreement with earlier reports suggesting an important role for MPs in the development of HIVE.5,6,20 Our previous studies demonstrated that there are two phenotypically distinct MP cell populations in HIVE CNS tissue. The perivascular macrophages are CD14+/CD16+, whereas the CD16+ cells in white matter, in areas not clustered around blood vessels, are CD14− with ramified morphology. These CD16+ MP populations seem to represent the predominant sources of CNS virus production in HIVE.16,21 In HIVE, perivascular macrophages share a similar immunophenotype with activated monocytes/macrophages in circulation,22 supporting a similar origin. The origin of the CD16+ ramified cells in parenchyma remains uncertain. The characterization of MPs accumulating around blood vessels and in microglial nodules as perivascular macrophages, rather than resident microglia, is based on differential expression of CD14 and CD45/LCA. Using standard immunohistochemical techniques (as in this study), these markers are detected on perivascular macrophages, but not resident microglia.14,23 Microglia are indeed capable of expressing CD14 on activation in vivo and in vitro.24–28 Under normal conditions, the low level of CD14 expression in microglia is detectable in tissue by signal amplification techniques or by fluorescence-activated cell sorting analysis of MPs isolated ex vivo (not used in this study). Because our results demonstrated an increase in MPs expressing CD16 and CD14, both possible markers for microglial activation, we were interested in evaluating the total number of MPs in HIVE CNS. Using immunohistochemical analysis for CD68, a constitutive marker expressed on macrophages and microglia, we demonstrate a significant accumulation of CD68+ MPs, both perivascularly and within the brain parenchyma, consistent with the reported overall increase in brain tissue macrophages in HIVE.20 Although CD14+ cells are increased perivascularly in HIVE, we did not observe a significant increase in CD14 positivity in parenchymal tissue relative to HIV-1/AIDS without dementia or seronegative controls. There is an increase, however, in parenchymal CD16+ cells representing ramified parenchymal MPs. Microglia have been reported to proliferate in response to a variety of pathological processes in both animal and human studies.29–31 We therefore evaluated macrophage/microglial proliferation as a potential mechanism that could contribute to the increase in perivascular and parenchymal MPs using antibodies against PCNA and Ki-67.
Immunohistochemical analysis of brain tissue from patients with HIVE revealed that many cells in white matter, around blood vessels, and within microglial nodules were, in fact, positive for PCNA. A small number of PCNA+ cells were in the proximity of blood vessels in brain tissue from patients with HIV-1 infection but without encephalitis, whereas no PCNA positivity was observed in seronegative controls. Additionally, many PCNA+ cells appeared to be MPs, based on the large number of these cells that were also positive for CD16. PCNA positivity was also observed in some endothelial cells along blood vessel lumina, a finding confirmed by co-localization with the endothelial cell marker, von Willebrand Factor. We detected few Ki-67 cells in all of the HIVE cases, suggesting that PCNA expression in HIVE is not a consequence of proliferation of these cells. In support of these findings, PCNA+, but Ki-67−, MPs were found to be abundant in SIV-infected macaques with encephalitis (SIVE).32 The overall increase in the number of perivascular and parenchymal MPs we observed in this study supports a model in which both perivascular and parenchymal reservoirs of HIV-1 infection accumulate as a result of increased trafficking and CNS invasion. These results emphasize the role of invading macrophages and new resident microglia as major contributors to the active reservoir of HIV-1 infection in HIVE CNS tissue. These observations and interpretation are supported by a recent study in SIV-infected macaques, in which activated monocytes with high CD16 expression were observed in circulation during the peak of acute infection and again during AIDS.33
Our results do not rule out the possibility that MP or MP precursor proliferation is altered outside the CNS, ie, in bone marrow. The number of CD14+/CD16+ cells has been demonstrated to be increased in circulation as well as in CNS tissue. If these activated MPs are indeed more invasive as has been suggested,18 the detection of high levels in both circulating and CNS compartments might suggest an increase in the generation kinetics of these cells, or an increase in survival time, possibly even recirculation of tissue MPs back into the circulation. Interestingly, bone marrow from patients with HIV-1 infection and AIDS has been reported to contain a statistically significant increase in PCNA+ macrophages, when compared to normal marrow, but these cells did not express detectable levels of topoisomerase IIα, a marker for DNA replication.34 In the absence of corroborating evidence with topoisomerase IIα, it was proposed that the observed PCNA expression was because of virus-induced DNA damage, in accordance with the involvement of PCNA in unscheduled DNA repair. The PCNA+ cells, however, were not analyzed for viral gene expression in that study. In a recent study in SIVE, co-localization of SIV p27 antigen with PCNA in bone marrow macrophages has been observed.33
In our study, HIV-1 infection was clearly associated with PCNA expression in CNS, in agreement with a similar study in SIVE reported by Williams and colleagues.32 PCNA/p24 double-labeling revealed that all cells positive for p24 were PCNA+. In contrast, not all PCNA-expressing cells appeared to be productively infected, in which no more than half of the PCNA+ cells also had detectable levels of HIV-1 p24. Although many of the PCNA+/p24− cells were in the vicinity of productively infected cells, there were also large numbers of PCNA+/p24− cells that did not appear to be within the proximity of infected cells. We cannot exclude the possibility, however, that neighboring infected cells were outside the plane of the section. Additionally, PCNA positivity was observed in endothelial cells, without additional evidence for neovascularization in CNS. PCNA positivity was also observed in ependymal cells, which reportedly do not proliferate in adults.35 These results suggest that PCNA expression is induced by viral infection (ie, by the expression of viral gene products) and possibly by indirect mechanisms as well. The observation of PCNA-positive cells in the vicinity of MPs where HIV-1 p24 was not detectable might suggest that PCNA expression may be also induced through cytokine dysregulation, secreted viral proteins, or other diffusible factors.36
Our results demonstrate a 10- to 20-fold increase in the total number of CD68+ macrophages/microglia in HIVE CNS tissue. Accordingly, no more than 5 to 10% of the microglia are probably long-term residents in this disease. Our results support the potential role of altered monocyte/macrophage trafficking in the pathogenesis of HIVE. In our study, many PCNA+ cells were also seen within blood vessel lumina. Therefore, cells that are already PCNA+ might enter the CNS compartment from the periphery. In support of this notion, sequence analysis of virus found in brain has been best correlated with virus in bone marrow.37 Although these results suggested trafficking of infected monocytes from bone marrow to the CNS, it is also possible that CNS and bone marrow are invaded by a common source of monocytes/macrophages.
The notion of altered trafficking kinetics of MPs into the CNS does not necessarily conflict with the notion of a compartmentalized infection in CNS. Although it has been demonstrated that HIV-1 envelope sequences in CNS are most closely related to sequences in peripheral blood and bone marrow,38 it is possible that some MPs are infected as they enter the CNS. Furthermore, there is clearly sequence heterogeneity with respect to different areas of the brain.39 Virus and virus-infected cells entering the CNS from the periphery may present new opportunities for recombination, adaptation, and evolution within the brain microenvironment, as suggested by the mosaic nature of CNS isolates when compared to sequences within the periphery.39 The relationship between the blood compartment and the CNS is further demonstrated by biological comparison with respect to the frequency of co-receptor utilization specificities.38 It is possible that some of the differences that have been observed in comparison of brain sequence with other organs40 may reflect differences in cell populations infected by HIV-1 in particular tissues (ie, the abundance of T cells in lymphoid tissue, but not brain) or, alternatively, mechanisms of immune selection as has been inferred from differences in T-cell epitopes in viruses isolated from different organs.41 Further studies will be needed combining viral-genetic characterization with immunohistochemical analysis to characterize virus within individual cell populations in multiple organs.
In summary, our results demonstrate substantial increases in total numbers of perivascular macrophages and parenchymal microglia in HIVE. The magnitude of this increase, along with the demonstrated increase in circulating activated MPs in HIVD, suggests the role of altered monocyte/macrophage trafficking and homeostasis in the pathogenesis of HIVD. This conclusion is further supported by the response of patients with HIVD to highly active anti-retroviral therapy, in which pharmacotherapeutics poorly penetrate the blood brain barrier,1,42,43 with the concomitant reduction in circulating CD16+ MPs.44 Additional studies will be necessary to elucidate the mechanisms involved in PCNA expression observed in cells of the CNS, particularly the monocyte/macrophage, and the potential role of cytokines and/or viral proteins in promoting up-regulation of this antigen. Furthermore, monocyte/macrophage trafficking studies will be needed to help identify alterations in homeostatic parameters contributing to the accumulation of MPs in the CNS in HIVE.
Acknowledgments
HIVE tissues were provided by S.M. from the Manhattan HIV Brain Bank (1R24MH59724).
Footnotes
Address reprint requests to Jay Rappaport, Ph.D., Center for Neurovirology and Cancer Biology, Temple University, Biology Life Science Bldg., Rm. 246, 1900 N. 12th St., Philadelphia, PA 19122. E-mail: jayrapp@astro.temple.edu.
Supported by the National Institutes of Health, National Institute of Neurodegenerative Disorders and Stroke (grant PO1 NS30916-09 to J.R. and K.K.); and the Howard Hughes Medical Institute through the Undergraduate Biological Sciences Education Program to Temple University.
References
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, Becker JT, Cohen B, McArthur JC. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP, Visscher BR, Concha M, Saah S. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- Vazeux R, Lacroix-Ciaudo C, Blanche S, Cumont MC, Henin D, Gray F, Boccon-Gibod L, Tardieu M. Low levels of human immunodeficiency virus replication in the brain tissue of children with severe acquired immunodeficiency syndrome encephalopathy. Am J Pathol. 1992;140:137–144. [PMC free article] [PubMed] [Google Scholar]
- Rostad SW, Sumi SM, Shaw CM, Olson K, McDougall JK. Human immunodeficiency virus (HIV) infection in brains with AIDS-related leukoencephalopathy. AIDS Res Hum Retroviruses. 1987;3:363–373. doi: 10.1089/aid.1987.3.363. [DOI] [PubMed] [Google Scholar]
- Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987;21:490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- Budka H, Costanzi G, Cristina S, Lechi A, Parravicini C, Trabattoni R, Vago L. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol (Berl) 1987;75:185–198. doi: 10.1007/BF00687080. [DOI] [PubMed] [Google Scholar]
- Meltzer MS, Skillman DR, Gomatos PJ, Kalter DC, Gendelman HE. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- McArthur JC, McClernon DR, Cronin MF, Nance-Sproson TE, Saah AJ, St Clair M, Lanier ER. Relationship between human immunodeficiency virus-associated dementia and viral load in cerebrospinal fluid and brain. Ann Neurol. 1997;42:689–698. doi: 10.1002/ana.410420504. [DOI] [PubMed] [Google Scholar]
- Cinque P, Vago L, Ceresa D, Mainini F, Terreni MR, Vagani A, Torri W, Bossolasco S, Lazzarin A. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998;12:389–394. doi: 10.1097/00002030-199804000-00007. [DOI] [PubMed] [Google Scholar]
- Bell JE, Busuttil A, Ironside JW, Rebus S, Donaldson YK, Simmonds P, Peutherer JF. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J Infect Dis. 1993;168:818–824. doi: 10.1093/infdis/168.4.818. [DOI] [PubMed] [Google Scholar]
- Poluektova LY, Munn DH, Persidsky Y, Gendelman HE. Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis. J Immunol. 2002;168:3941–3949. doi: 10.4049/jimmunol.168.8.3941. [DOI] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Gregersen R, Dorries R, ter Meulen V. Resident macrophages (ramified microglia) of the adult brown Norway rat central nervous system are constitutively major histocompatibility complex class II positive. J Exp Med. 1993;177:1145–1152. doi: 10.1084/jem.177.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulvestad E, Williams K, Mork S, Antel J, Nyland H. Phenotypic differences between human monocytes/macrophages and microglial cells studied in situ and in vitro. J Neuropathol Exp Neurol. 1994;53:492–501. doi: 10.1097/00005072-199409000-00008. [DOI] [PubMed] [Google Scholar]
- Williams K, Bar-Or A, Ulvestad E, Olivier A, Antel JP, Yong VW. Biology of adult human microglia in culture: comparisons with peripheral blood monocytes and astrocytes. J Neuropathol Exp Neurol. 1992;51:538–549. doi: 10.1097/00005072-199209000-00009. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, Cavert W, Marra C, Grant I, Singer EJ. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27:326–335. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Becher B, Antel JP. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18:1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Becher B, Fedorowicz V, Antel JP. Regulation of CD14 expression on human adult central nervous system-derived microglia. J Neurosci Res. 1996;45:375–381. doi: 10.1002/(SICI)1097-4547(19960815)45:4<375::AID-JNR6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Rivest S. Role of microglial-derived tumor necrosis factor in mediating CD14 transcription and nuclear factor kappa B activity in the brain during endotoxemia. J Neurosci. 2000;20:3456–3468. doi: 10.1523/JNEUROSCI.20-09-03456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix S, Feinstein D, Rivest S. The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol. 1998;8:625–640. doi: 10.1111/j.1750-3639.1998.tb00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beschorner R, Nguyen TD, Gozalan F, Pedal I, Mattern R, Schluesener HJ, Meyermann R, Schwab JM. CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury. Acta Neuropathol (Berl) 2002;103:541–549. doi: 10.1007/s00401-001-0503-7. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Schluesener HJ, Gozalan F, Meyermann R, Schwab JM. Infiltrating CD14+ monocytes and expression of CD14 by activated parenchymal microglia/macrophages contribute to the pool of CD14+ cells in ischemic brain lesions. J Neuroimmunol. 2002;126:107–115. doi: 10.1016/s0165-5728(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Amat JA, Ishiguro H, Nakamura K, Norton WT. Phenotypic diversity and kinetics of proliferating microglia and astrocytes following cortical stab wounds. Glia. 1996;16:368–382. doi: 10.1002/(SICI)1098-1136(199604)16:4<368::AID-GLIA9>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Schonrock LM, Kuhlmann T, Adler S, Bitsch A, Bruck W. Identification of glial cell proliferation in early multiple sclerosis lesions. Neuropathol Appl Neurobiol. 1998;24:320–330. doi: 10.1046/j.1365-2990.1998.00131.x. [DOI] [PubMed] [Google Scholar]
- Williams K, Schwartz A, Corey S, Orandle M, Kennedy W, Thompson B, Alvarez X, Brown C, Gartner S, Lackner A. Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;161:575–585. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W-K, Crey S, Avarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003;74:650–656. doi: 10.1189/jlb.0503207. [DOI] [PubMed] [Google Scholar]
- Titius BR, Thiele J, Schaefer H, Kreipe H, Fischer R. Ki-S1 and proliferating cell nuclear antigen expression of bone marrow macrophages. Immunohistochemical and morphometric study including reactive (inflammatory) myelitis, secondary aplastic anemia, AIDS, myelodysplastic syndromes and primary (idiopathic) osteomyelofibrosis. Acta Haematol. 1994;91:144–149. doi: 10.1159/000204320. [DOI] [PubMed] [Google Scholar]
- Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tang XP, McArthur JC, Scott J, Gartner S. Analysis of human immunodeficiency virus type 1 gp160 sequences from a patient with HIV dementia: evidence for monocyte trafficking into brain. J Neurovirol. 2000;6(Suppl 1):S70–S81. [PubMed] [Google Scholar]
- Ohagen A, Devitt A, Kunstman KJ, Gorry PR, Rose PP, Korber B, Taylor J, Levy R, Murphy RL, Wolinsky SM, Gabuzda D. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J Virol. 2003;77:12336–12345. doi: 10.1128/JVI.77.22.12336-12345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A, Marsden M, Halcrow K, Hughes ES, Brettle RP, Bell JE, Simmonds P. Mosaic structure of the human immunodeficiency virus type 1 genome infecting lymphoid cells and the brain: evidence for frequent in vivo recombination events in the evolution of regional populations. J Virol. 1999;73:8720–8731. doi: 10.1128/jvi.73.10.8720-8731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty S, Stevenson M. Predominance of distinct viral genotypes in brain and lymph node compartments of HIV-1-infected individuals. Viral Immunol. 1991;4:123–131. doi: 10.1089/vim.1991.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Ignacio CC, Torriani F, Havlir D, Fitch NJ, Richman DD. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. J Virol. 1997;71:2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 1999;13:1249–1253. doi: 10.1097/00002030-199907090-00015. [DOI] [PubMed] [Google Scholar]
- Enting RH, Hoetelmans RM, Lange JM, Burger DM, Beijnen JH, Portegies P. Antiretroviral drugs and the central nervous system. AIDS. 1998;12:1941–1955. doi: 10.1097/00002030-199815000-00005. [DOI] [PubMed] [Google Scholar]
- Amirayan-Chevillard N, Tissot-Dupont H, Capo C, Brunet C, Dignat-George F, Obadia Y, Gallais H, Mege JL. Impact of highly active anti-retroviral therapy (HAART) on cytokine production and monocyte subsets in HIV-infected patients. Clin Exp Immunol. 2000;120:107–112. doi: 10.1046/j.1365-2249.2000.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]