Abstract
The involvement of proinflammatory cytokines interleukin (IL)-1 and tumor necrosis factor (TNF) in crescentic glomerulonephritis (GN) is well established. Recently the requirement of intrinsic renal cell participation via their production of TNF in crescentic GN was demonstrated. The current studies address the relative contributions of leukocyte and intrinsic renal cell-derived IL-1β in the induction of TNF production and glomerular injury by studying bone marrow chimeric mice. Leukocyte-derived IL-1β was critical in the development of crescentic renal injury because IL-1β−/−→WT (absent leukocyte IL-1β) chimeric mice had significantly attenuated TNF expression and were protected from the development of crescentic GN. In contrast, WT→IL-1β−/− chimeric mice (intact leukocyte but absent renal IL-1β) developed similar TNF expression and crescentic GN to wild-type mice. To determine the cellular target for IL-1 in this model, IL-RI chimeric mice were studied. IL-1RI−/−→WT chimeric (absent leukocyte IL-1RI expression) mice showed no attenuation of crescentic GN, whereas in the absence of renal IL-1RI (WT→IL-1RI−/− chimeras), glomerular TNF expression and the development of crescentic GN were significantly decreased. These studies demonstrate that leukocytes are the major cellular source of IL-1β, and that IL-1β acts principally via the IL-1RI on intrinsic renal cells to induce TNF expression and crescentic glomerular injury.
Cytokine involvement in the pathogenesis of crescentic glomerulonephritis (GN) has been demonstrated in numerous human and experimental studies. Crescentic GN is characterized by the presence of delayed type hypersensitivity effectors (CD4+ T cells, macrophages, and fibrin) and the formation of crescents, and is associated with a Th1 cell-mediated response toward planted glomerular antigens.1–7 Based on in vitro and in vivo data, it is generally accepted that both infiltrating inflammatory cells and intrinsic renal cells synthesize and release cytokines, however the extent of intrinsic renal cell contributions to the effector phase of immune renal injury has only recently been assessed.
The active role of resident cells in mediating inflammatory injury is becoming increasingly apparent, with studies in arthritis,8 experimental autoimmune encephalomyelitis,9–11 and GN12–15 demonstrating that local parenchymal cells initiate and perpetuate the effector inflammatory response. Several studies have established an important function for central nervous system resident cells in the pathogenesis of experimental autoimmune encephalitis. In particular, bone marrow (BM) chimeras have demonstrated the capacity for resident central nervous system cells to present Ag, express MHC class II and CD40, and to control T-cell accumulation via chemokine synthesis.11,16–18 However, another study of experimental autoimmune encephalitis in tumor necrosis factor (TNF) chimeric animals demonstrated that leukocytes contribute >99% of total TNF present within the inflamed central nervous system and are the essential source of TNF that drives chemokine production (MCP-1 and TCA-3) by resident central nervous system stromal cells.19
In experimental GN, studies using BM chimeric mice have demonstrated that intrinsic renal cells are actively involved in directing and influencing renal injury. Intrinsic renal cells contribute toward the formation of glomerular crescents, renal leukocyte accumulation, and impairment of renal function via their production of interferon-γ, interleukin (IL)-12, TNF, and expression of MHC class II and CD40 molecules.12–15,20 In addition, it has been demonstrated that the relative contribution of particular cytokines and their cellular sources may vary. Some cytokines (interferon-γ, IL-12) are produced by both intrinsic renal cells and leukocytes, whereas others (TNF) are expressed principally by intrinsic renal cells.
Both IL-1 and TNF are important cytokines in the pathogenesis of crescentic GN. Human studies have demonstrated elevated expression of both cytokines in biopsies from patients with crescentic GN.21 In experimental GN, administration of soluble TNF receptors or the natural IL-1 receptor antagonist (IL-1ra) to rats developing crescentic GN reduced glomerular injury and prevented crescent formation.22,23 Furthermore, treatment with anti-IL-1β antibodies or anti-TNF antibodies also resulted in a reduction of proteinuria in anti-glomerular basement membrane (GBM) GN.24 Intrinsic renal cells have been shown to be the predominant source of TNF that mediates glomerular inflammation in a murine model of crescentic GN.14 However, the predominant cellular source of IL-1 is controversial because some studies suggest that infiltrating macrophages are the main source of IL-1 in crescentic glomeruli,25–27 whereas others implicate intrinsic renal cells as the major source.28,29
The current study investigates the major cellular source of IL-1β contributing to inflammatory injury in crescentic GN, and the main target for IL-1-mediated proinflammatory effects in crescentic GN. Our results demonstrate that IL-1β is produced principally by BM-derived cells, and predominantly targets intrinsic renal cells via the IL-1RI to mediate renal injury in murine crescentic GN. It also indicates that TNF production by intrinsic renal cells is induced by IL-1β from infiltrating leukocytes acting in a paracrine manner.
Materials and Methods
Mice
Mice homozygous for targeted deletions of IL-1β (a gift from Y. Iwakura, University of Tokyo, Tokyo, Japan)30 and IL-1RI (a gift from A. Satsokar, Ohio State University, OH, USA)31 genes were backcrossed onto a C57BL/6 background. These mice were bred and housed under specific pathogen-free conditions at Monash University (Clayton, Victoria, Australia).
BM Transplantation
IL-1β−/−, IL-1RI−/−, or C57BL/6 wild-type (WT) mice were used for BM transplantation. Donor mice (12 to 14 weeks of age) were sacrificed by CO2 inhalation followed by cervical dislocation. The marrow was harvested from the tibia and femur under sterile conditions and depleted of red blood cells, as previously reported.12 Recipient mice (5 to 6 weeks of age) were lethally irradiated with two exposures of 5.5 Gy at 3 hours apart. Within 6 hours of irradiation, 5 × 106 BM cells were injected into the tail vein. The resulting chimeric mice were housed under specific pathogen-free conditions for 8 weeks to allow BM reconstitution. At this time, circulating leukocyte numbers, lymphocyte subsets (assessed by flow cytometry), and splenic distribution of T cells and macrophages (assessed by immunohistology) were normal, as previously reported.12,13
The effect of irradiation on kidney architecture, function, and development of GN was assessed in WT mice after irradiation and transplantation of WT BM. Sham chimeric (WT→WT) and age-matched nonirradiated mice had similar baseline proteinuria (WT→WT chimeras, 0.94 ± 0.27 mg/24 hours; WT, 0.77 ± 0.2 mg/24 hours) and serum creatinine levels (WT→WT chimeras, 18.84 ± 1.38 μmol/L; WT, 17.51 ± 0.58 μmol/L). Furthermore, kidneys of irradiated mice showed no histological abnormalities on routine light microscopy.
Sham chimeras (WT→WT) developed similar systemic immune responses to the nephritogenic Ag and similar progression of GN to WT mice,12 indicating that irradiation and BM transplantation per se did not affect the development of this disease. The incidence of crescentic glomeruli (WT, 31 ± 3%; sham chimeras, 33 ± 1%) and glomerular infiltration of CD4+ T cells (WT, 1.2 ± 0.1 c/gcs; sham chimeras, 1.1 ± 0.1 c/cgs) and macrophages (WT, 3.3 ± 0.4 c/gcs; sham chimeras, 3.2 ± 0.1 c/gcs) were equivalent. Similarly, there was no difference in functional renal injury between the two groups, indicated by proteinuria (WT, 3.9 ± 0.8 mg/24 hours; sham chimeras, 4.6 ± 0.1 mg/24 hours) and serum creatinine (WT, 35 ± 3 μmol/L; sham chimeras, 36 ± 6 μmol/L).
Serum and kidney TNF and IL-1β cytokine production in sham chimeric (WT→WT) animals (n = 5) were equivalent to levels observed in normal C57BL/6 mice (n = 3) (TNF levels in serum: WT→WT chimeras, 0.03 ± 0.01 ng/ml and WT, 0.05 ± 0.01 ng/ml; kidney: WT→WT, 5.2 ± 1.1 ng/g and WT, 5.1 ± 0.6 ng/g; IL-1β levels in kidney: WT→WT, 6.6 ± 0.8 ng/g and WT, 7.0 ± 0.5 ng/g).
The efficiency of BM reconstitution was assessed by BM transplantation between CD45 congenic mice (Ly5.1 and Ly5.2). Detection of donor and recipient leukocytes by flow cytometry using allele-specific monoclonal antibodies demonstrated that 98 ± 1% of BM cells, 92 ± 1% of PBMCs, and 90 ± 2% of splenic leukocytes were of donor origin in the Ly5.1→WT (Ly 5.2) chimeric mice.13
Repopulation of intrinsic renal cells by BM-derived precursors was assessed by transplantation of green fluorescent protein-positive BM into irradiated WT mice. Eight weeks after transplantation, green fluorescent protein expression was detected in 1.9 ± 0.3 cells per glomerular cross-section, indicating that ∼5% of cells in normal mouse glomeruli were BM derived. Because kidneys were not perfused some of these cells may be circulating leukocytes.14
Induction of Crescentic GN
Crescentic anti-GBM was induced in mice by an intravenous injection of a total of 14.4 mg of sheep anti-mouse GBM globulin [in 900 μl of phosphate-buffered saline (PBS)] divided equally into two doses given 3 hours apart. The development of crescentic GN was assessed 21 days later in WT mice and four groups of irradiated BM transplanted chimeric mice. These were, IL-1β−/−→WT chimeras in which IL-1β−/− BM was transplanted into WT recipients, WT→IL-1β−/− chimeras in which WT BM was transplanted into IL-1β−/− recipients, IL-1RI−/−→WT chimeras in which IL-1RI−/− BM was transplanted into WT recipients, and WT→IL-1RI−/− chimeras in which WT BM was transplanted into IL-1RI−/− recipients. All mice were 13 to 14 weeks of age at the time of administration of anti-GBM globulin.
Histological Assessment of Glomerular Injury
Glomerular Crescent Formation
Kidney tissue was fixed in Bouin’s fixative, embedded in paraffin, and 3-μm sections were stained with periodic acid-Schiff’s reagent. Glomeruli were considered to exhibit crescent formation when two or more layers of cells were observed in Bowman’s space. A minimum of 50 glomeruli was assessed to determine the crescent score for each animal.
Glomerular CD4+ T Cell and Macrophage Accumulation
Kidney tissue and spleen was fixed in periodate/lysine/paraformaldehyde. Six-μm cryostat-cut sections were stained to demonstrate CD4+ T cells and macrophages with monoclonal antibodies, GK1.5 and M1/70, respectively, using a three-layer immunoperoxidase technique.2 A minimum of 20 equatorially sectioned glomeruli were assessed per animal and results expressed as cells per glomerular cross-section (c/gcs).
Functional Assessment of Glomerular Injury
Proteinuria
Mice were housed individually in cages to collect urine before administration of anti-GBM globulin and throughout the final 24 hours of the experiment. Urinary protein concentrations were determined by a modified Bradford method.32 Before induction of GN, all groups of mice had 24-hour urine protein excretion in the normal range (0.5 to 2.0 mg/24 hours).
Serum Creatinine
Serum creatinine concentrations were measured by an enzymatic creatininase assay.
Demonstration of Renal IL-1β and IL-1RI Expression
IL-1β and IL-1RI expression in renal tissues were detected using monoclonal antibodies: anti-mouse IL-1β (clone 30311; R&D Systems/Bio Scientific, Gymea, NSW, Australia) and anti-mouse IL-1RI (clone 35F5; BD Biosciences/PharMingen, San Diego, CA) directly conjugated to Alexa 488 and 594 (Molecular Probes, Eugene OR), respectively. Cryostat-cut snap-frozen kidney tissue sections (6 μm) were blocked with 10% normal rat serum in 5% bovine serum albumin/PBS and then incubated with individual antibodies at a final dilution of 1 in 40 for 60 minutes at room temperature. Confocal images of IL-1β and IL-1RI renal expression were collected using a Bio-Rad (Hampstead, UK) confocal inverted Nikon microscope equipped with an air-cooled 25-mW argon/krypton laser, as previously described.13 Digital images were collected using Bio-Rad laser sharp 2000 version 4.1 software.
Assessment of Glomerular TNF Expression
Glomerular TNF expression was detected using a phycoerythrin-conjugated rat anti-mouse TNF antibody (clone MP6-XT22, BD Biosciences/PharMingen). Cryostat-cut snap-frozen kidney tissue sections (6 μm) were blocked with 10% normal rat serum in 5% bovine serum albumin/PBS and then incubated with the phycoerythrin-rat-anti-mouse TNF antibody at a final dilution of 1 in 40 for 60 minutes at room temperature. In a blinded protocol on a minimum of 50 glomeruli per mouse, glomeruli were scored semiquantitatively (0 to 3+), with 0 being equivalent to background and 3+ equal to the most intense fluorescence in any glomerulus.
Measurement of TNF and IL-1β
Serum and kidney tissue samples were collected from normal WT (n = 5), IL-1β−/−→WT (n = 5), WT→IL-1β−/− (n = 5), and IL-1RI−/−→WT (n = 5) and WT→IL-1RI−/− (n = 5) chimeric mice with GN. Kidney samples were homogenized in 0.1 mol/L NaCl, 0.05 mol/L Tris-Cl, 2% v/v Triton X-100 together with protease inhibitors (Roche Diagnostics, Melbourne, Australia). The homogenate was spun at 14,000 rpm for 15 minutes and the supernatant was collected. Tissue extracts and serum were stored at −70°C until assayed. TNF and IL-1β were measured by enzyme-linked immunosorbent assay (ELISA), with a detection limit of 0.031 ng/ml and 0.061 ng/ml, respectively. Microtiter plates were coated with anti-mouse TNF (1 μg/ml) or anti-mouse IL-1β (4 μg/ml) as the capture antibody (R&D Systems, NSW, Australia). Plates were washed in 0.05% Tween/PBS and blocked with 1% bovine serum albumin in PBS for 2 hours at 37°C. Plates were then washed and incubated with recombinant murine TNF or IL-1β (R&D Systems) at serial dilutions between 4.0 and 0.031 ng/ml dilutions or with serum, spleen, and kidney extracts at 4°C overnight. Plates were washed and bound mouse TNF or IL-1β was detected using biotinylated anti-mouse TNF or biotinylated IL-1β antibodies (R&D Systems) at a concentration of 200 or 500 ng/ml, respectively, for 1 hour at room temperature. Plates were washed and streptavidin-horseradish peroxidase (1:2000 in 1% bovine serum albumin/PBS; Chemicon, Melbourne, Australia) was added for 20 minutes at room temperature followed by TMB (Sigma Aldrich Pty. Ltd., NSW, Australia), the chromogenic substrate. All samples were assayed in duplicate, the assay was performed twice and results were calculated as concentration/wet weight of tissue (ng/g) or ng/ml for serum.
Humoral Immune Responses to Sheep Globulin
Mouse anti-sheep globulin antibody titers were measured by ELISA on serum collected at the end of each experiment. Assays were performed using microtiter plates coated with sheep globulin at a concentration of 10 μg/ml and bound mouse Ig was detected using horseradish peroxidase-conjugated sheep anti-mouse Ig antibody (Amersham, Little Chalfont, UK), as previously described.4
Experimental Design and Statistical Analysis
The development of GN was studied in a total of 20 WT mice, 5 IL-1β−/−→WT chimeric mice, 10 WT→IL-1β−/− chimeric mice, 5 IL-1RI−/−→WT chimeric mice, and 19 WT→IL-1RI−/− chimeric mice. Results are expressed as the mean ± SEM. The statistical significance was determined by one-way analysis of variance, followed by Newman-Keuls post hoc test.
Results
Leukocyte-Derived IL-1β Is Required for the Full Development of Crescentic GN
Renal IL-1β Expression
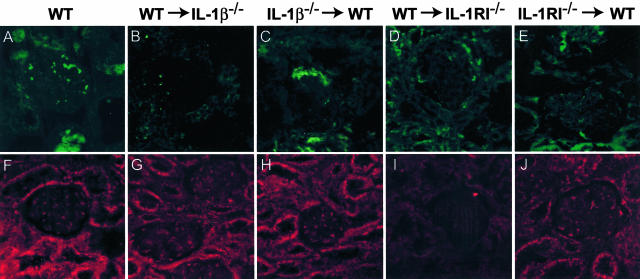
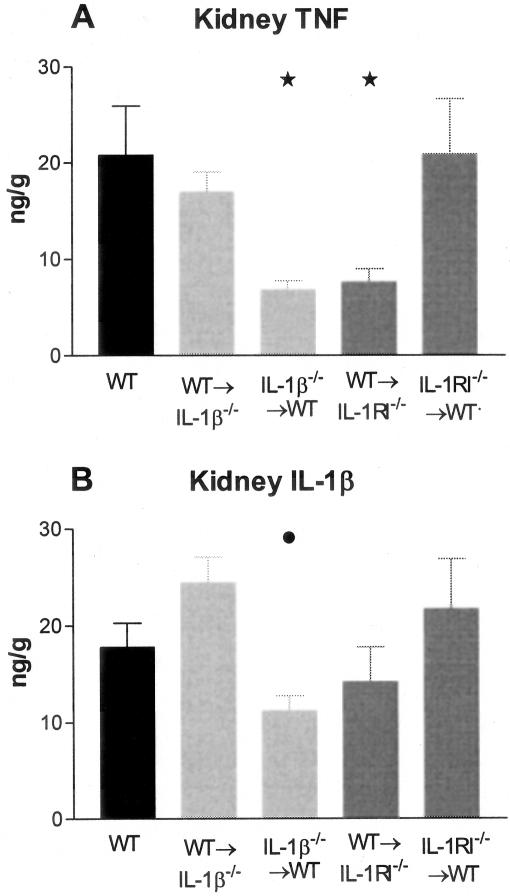
IL-1β was expressed in interstitial and intraglomerular areas as well as on tubular cells. Expression in glomeruli was associated with both leukocytes and intrinsic renal cells. Prominent IL-1β expression was observed in WT mice with GN (Figure 1A). In WT→IL-1β−/− chimeric mice (Figure 1B) intrinsic renal cell expression was absent, however occasional intraglomerular cells were positive for IL-1β. Co-localization with Mac-1 demonstrated that these IL-1β-expressing cells were macrophages (not shown). In IL-1β−/−→WT chimeras, tubular and intraglomerular IL-1β was observed (Figure 1C). In the IL-RI chimeric (both WT→IL-1RI−/− and IL-1RI−/−→WT) groups (Figure 1, D and E, respectively) IL-1β expression was unaffected and similar to the expression observed in WT mice. Measurement of IL-1β protein by ELISA demonstrated that renal IL-1β was significantly reduced in IL-1β−/−→WT chimeric mice compared to WT→IL-1β−/−, this indicating that a major contribution of leukocytes to renal IL-1β occurs in this model (Figure 2B). Renal IL-1β in WT→IL-1β−/− chimeras was not different from WT mice with GN. Serum levels of IL-1β were undetectable in all groups (<0.031 ng/ml).
Figure 1.
Renal localization of IL-β (green, A–E) and IL-1RI (red, F–J) in WT and chimeric mice with GN, detected by immunofluorescence, and captured by confocal microscopy. A: In glomeruli of WT mice, cytoplasmic staining of infiltrating leukocytes with occasional patchy staining of resident glomerular cells was observed. Tubular areas were also positive for IL-1β. B: In WT→IL-1β−/− chimeras IL-1β was only detected on macrophages infiltrating the kidney, no renal expression was detected. C: In IL-1β−/−→WT chimeric animals, IL-1β expression was sparsely observed in glomeruli and on tubular cells. In IL-1RI chimeric mice, WT→IL-1RI−/− (D), and IL-1RI−/−→WT (E), IL-1β expression was observed in a similar pattern to WT animals. Renal expression of IL-1RI in WT (F), WT→IL-1β−/− (G), and IL-1β−/−→WT (H) mice was similar, with the receptor observed to be present on cells in the glomerulus and on tubules. I: In contrast the WT→IL-1RI−/− chimeras had absent renal IL-1RI expression with limited expression detected on infiltrating inflammatory cells. J: The expression of IL-1RI in IL-1RI−/−→WT chimeras was not noticeably reduced compared with WT mice, this indicating that the prominent expression observed in WT and IL-1β chimeric mice to be by intrinsic renal cells. Confocal immunofluorescence, under oil immersion. Original magnifications, ×600.
Figure 2.
Renal TNF and IL-1β levels were measured in WT and chimeric mice with GN. Kidney samples were homogenized and protein extracted, results expressed as concentration (ng) per tissue wet weight (g). A: Kidney TNF expression was significantly reduced in IL-1β−/−→WT and WT→IL-1RI−/− chimeric mice compared to WT mice (*, P < 0.05). B: Renal IL-1β expression was reduced in IL-1β−/−→WT chimeras compared to WT→IL-1β−/− chimeras (•, P < 0.05).
Similar Development of Crescentic GN Is Observed in WT and IL-1β−/−→WT Chimeric Mice
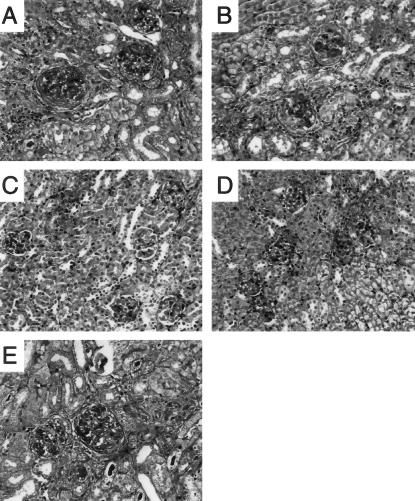
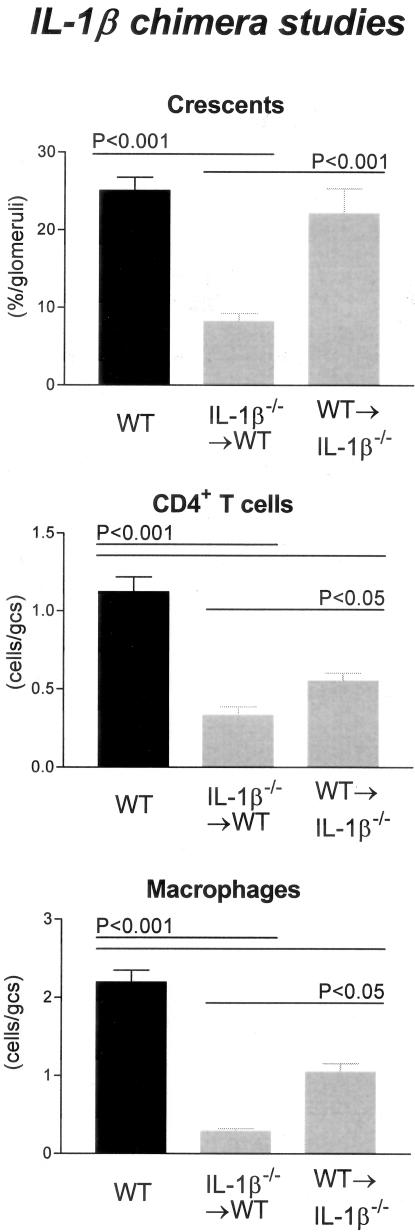
We have previously demonstrated the important role IL-1β has in the development of crescentic GN, with a 50% reduction in crescent formation and marked attenuation of glomerular leukocyte recruitment and functional parameters of renal injury in IL-1β-deficient mice developing anti-GBM GN.33 WT mice and chimeric mice with IL-1β expression restricted to cells derived from the BM (WT→IL-1β−/− chimeras) developed proliferative GN (Figure 3, A and B, respectively) with a similar incidence of glomerular crescent formation, 25.1 ± 1.7% and 22.1 ± 3.2%, respectively (Figure 4), indicating that leukocyte-derived IL-1β is sufficient for the development of crescents.
Figure 3.
The histological appearance of crescentic glomerular injury in WT and chimeric mice 21 days after administration of sheep anti-mouse GBM globulin. A: WT mice developed proliferative GN with glomerular crescent formation, deposition of glomerular periodic acid-Schiff-positive material and cellular interstitial infiltrate. Proliferative changes and crescents were also observed in WT→IL-1β−/− chimeric (B) and IL-1RI−/−→WT chimeric (E) mice with GN. IL-1β−/−→WT chimeric (C) and WT→IL-1RI−/− chimeric (D) mice developed mild proliferative GN, with only occasionally observed crescent formation. Periodic acid-Schiff-stained sections. Original magnifications, ×400.
Figure 4.
Histological features of glomerular injury in WT and IL-1β chimeric mice with GN: glomerular crescent formation, glomerular CD4+ T cell accumulation, and glomerular macrophage accumulation. Control WT mice with GN developed severe GN with a high incidence of glomerular crescent formation. In contrast IL-1β−/−→WT chimeric mice had a significant reduction in the proportion of glomeruli affected. WT→IL-1β−/− chimeric mice had a similar incidence of crescent formation to WT controls, which was significantly higher than IL-1β−/−→WT chimeric mice. Glomerular accumulation of CD4+ T cells and macrophages was significantly reduced in both chimeric groups, with a trend to a greater reduction observed in IL-1β−/−→WT chimeras because inflammatory cellular infiltrate is significantly reduced compared to WT→IL-1β−/− chimeras.
Leukocyte recruitment in WT→IL-1β−/− chimeras, specifically glomerular CD4+ T cells (0.6 ± 0.05 c/gcs; P < 0.001; compare WT) and macrophages (1.1 ± 0.11 c/gcs; P < 0.001; compare WT) was significantly less than in WT (CD4+ T cells, 1.1 ± 0.09; macrophages, 2.2 ± 0.15; Figure 4) mice but significantly greater than previously observed in IL-1β-deficient mice.
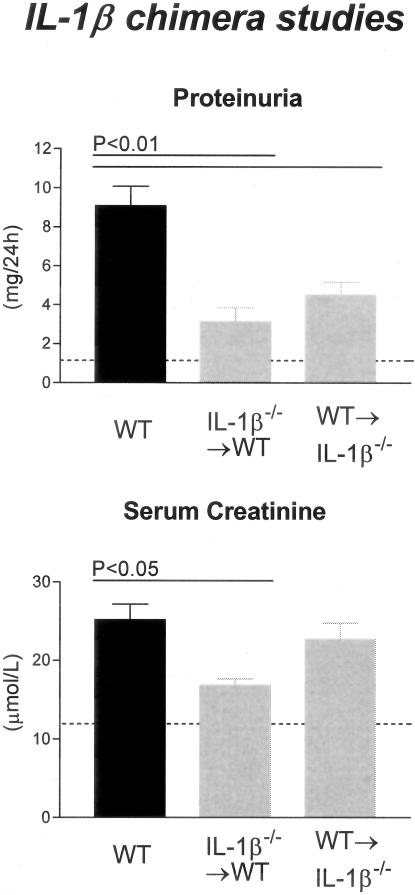
Elevations in serum creatinine were similar in WT and WT→IL-1β−/− chimeras (25.2 ± 2.0 μmol/L, 22.7 ± 2.1 μmol/L, respectively); however, there was a significant reduction in the amount of proteinuria in WT→IL-1β−/− chimeras (4.5 ± 0.7 mg/24 hours, P < 0.01) compared to WT (9.1 ± 1.0 mg/24 hours, Figure 5).
Figure 5.
Functional indices of injury in IL-1β chimeric mice with GN. WT mice with GN developed significant proteinuria, whereas IL-1β chimeric mice had reduced proteinuria compared with WT mice. Elevated serum creatinine demonstrated impaired renal function in WT and WT→IL-β−/− chimeric mice, whereas significantly lower serum creatinine levels were demonstrated in IL-1β−/−→WT chimeric mice. The dotted line indicates the urinary protein excretion and serum creatinine of normal WT mice without GN.
GN Is Less Severe in IL-1β−/−→WT Chimeric Mice
Chimeric mice with absent BM-derived IL-1β but intact renal IL-1β (IL-1β−/−→WT chimeras) showed similar protection from crescentic GN to that observed in mice with complete IL-1β deficiency. Crescent formation was significantly reduced in IL-1β−/−→WT chimeras (8.2 ± 1.0% of glomeruli) compared to WT (P < 0.001) and WT→IL-1β−/− chimeras (P < 0.01, Figures 3C and 4).
Leukocyte recruitment was diminished in chimeric mice with IL-1β expression restricted to renal cells. In IL-1β−/−→WT chimeras the glomerular accumulation of CD4+ T cells (0.3 ± 0.06 c/gcs) and macrophages (0.3 ± 0.04 c/gcs) was significantly reduced compared to WT mice (P < 0.001) and WT→IL-1β−/− chimeras (P < 0.05, Figure 4). Only minor renal impairment was displayed in these chimeric mice consistent with the degree of glomerular crescent formation. Both proteinuria (3.1 ± 0.7 mg/24 hours; P < 0.01; compare WT) and serum creatinine (16.9 ± 0.8μmol/L; P < 0.05; compare WT) were reduced to a similar extent as previously demonstrated in IL-1β-deficient mice (Figure 5).
Expression of the IL-1RI on Intrinsic Renal Cells Is Required for Full Development of Crescentic GN
IL-1RI Expression
IL-1RI expression was detected in glomeruli of WT, WT→IL-1β−/− chimeric, IL-1β−/−→WT chimeric, and IL-1RI−/−→WT chimeric mice (Figure 1; F, G, H, and J, respectively). Intrinsic renal cell expression of IL-1RI was not observed in WT→IL-1RI−/− chimeric mice, however it was detected on intraglomerular leukocytes (Figure 1I).
Crescentic GN Is Diminished in WT→IL-1RI−/− Chimeric Mice
Chimeric mice with the receptor expressed on BM-derived cells but with absent renal expression (WT→IL-1RI−/− chimeras) showed significant protection from crescentic GN and were protected to a similar extent to IL-1RI-deficient mice, IL-1β-deficient mice33 and IL-1β−/−→WT chimeras (Figure 3D).
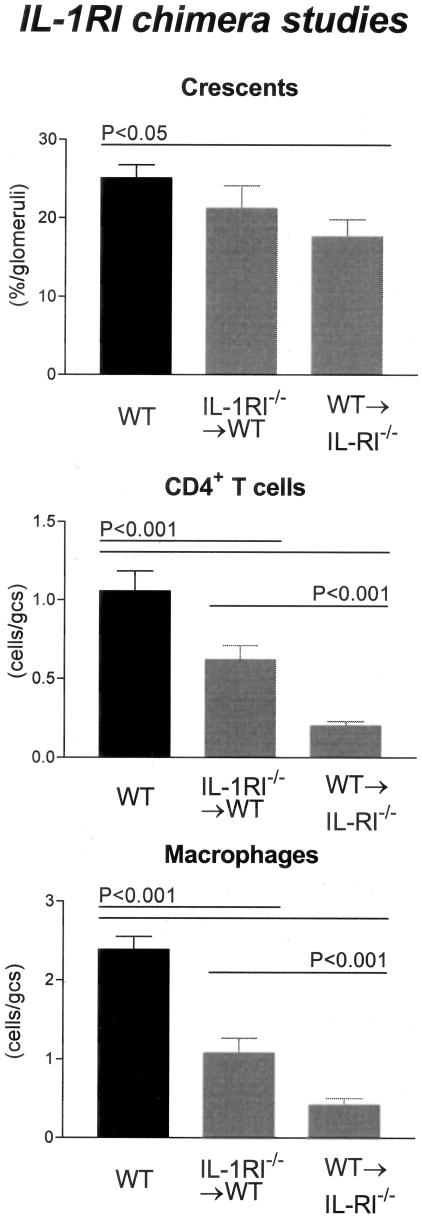
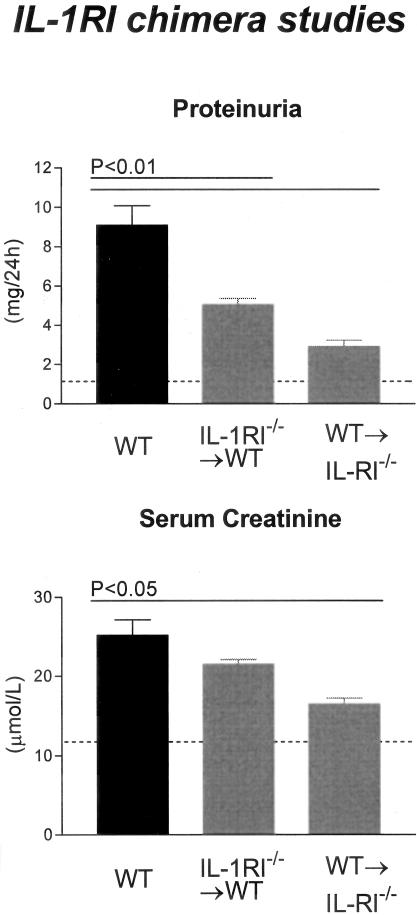
Crescent formation was significantly reduced in WT→IL-1RI−/− chimeras (17.7 ± 2.2% of glomeruli) compared to WT mice (P < 0.05, Figure 6). This trend continued in leukocyte recruitment, with glomerular accumulation of CD4+ T cells (WT→IL-1RI−/− chimeras, 0.2 ± 0.03 c/gcs) and macrophages (WT→IL-1RI−/− chimeras, 0.4 ± 0.09 c/gcs) significantly reduced compared to WT mice (P < 0.001) and IL-1RI−/−→WT chimeras (CD4+ T cells, P < 0.001; macrophages, P < 0.01; Figure 6). Preservation of renal function paralleled the minimal histological changes observed with significantly lower proteinuria (2.9 ± 0.3 mg/24 hours; P < 0.001; compare WT) and lower serum creatinine levels (16.5 ± 0.8 μmol/L; P < 0.001; compare WT; Figure 7) measured in WT→IL-1RI−/− chimeras.
Figure 6.
Histological features of glomerular injury in WT and IL-1RI chimeric mice with GN: glomerular crescent formation, glomerular CD4+ T cell accumulation, and glomerular macrophage accumulation. Control WT mice with GN developed severe GN with a high incidence of glomerular crescent formation. Similarly WT→IL-1RI−/− chimeric mice formed numerous crescents with a high incidence of glomeruli affected. In contrast WT→IL-1RI−/− chimeric mice had a significant reduction in the proportion of crescentic glomeruli compared to WT mice. Glomerular accumulation of CD4+ T cells and macrophages was significantly reduced in both chimeric groups, with a trend to a greater reduction observed in WT→IL-1RI−/− chimeras because inflammatory cellular infiltrate is significantly reduced compared to IL-1RI−/−→WT chimeras.
Figure 7.
Functional indices of injury in IL-1RI chimeric mice with GN. IL-1RI chimeric mice developed significantly less proteinuria compared with WT mice with GN. Serum creatinine demonstrated impaired renal function in WT and IL-1RI−/−→WT chimeric mice, whereas WT→IL-1RI−/− chimeric mice had significantly lower serum creatinine than WT mice. The dotted line indicates the urinary protein excretion and serum creatinine of normal WT mice without GN.
WT and IL-1RI−/−→WT Chimeras Develop Similar Patterns of Renal Injury
In comparison to the WT→IL-1RI−/− chimeras, mice with IL-1RI expression on intrinsic renal cells but absent expression on BM-derived cells (IL-1RI−/−→WT chimeras) exhibited similar disease severity as WT mice (Figure 3E). Crescent formation (21.2 ± 2.9% of glomeruli), glomerular CD4+ T cell (0.6 ± 0.09 c/gcs), and glomerular macrophage (1.1 ± 0.18 c/gcs) accumulation was significantly higher than WT→IL-1RI−/− chimeric mice (CD4+ T cells, P < 0.001; macrophages, P < 0.01; Figure 6). In addition, renal function was not protected to the same extent as WT→IL-1RI−/− chimeric mice with elevated proteinuria (5.0 ± 0.3 mg/24 hours) and serum creatinine levels (21.5 ± 0.6 μmol/L) observed in IL-1RI−/−→WT chimeric mice (Figure 7).
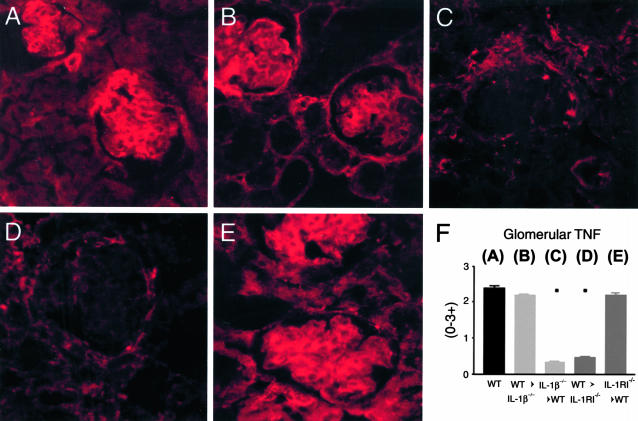
TNF Expression in IL-1β and IL-1RI Chimeric Mice (Figure 8)
Figure 8.
Immunofluorescence assessment of renal TNF expression. A: In WT mice, prominent glomerular TNF was observed on intraglomerular cells, particularly in the glomerular stalk. TNF expression was also observed on tubular cells. This prominent pattern of TNF expression was also observed in WT→IL-1β−/− (B) and IL-1RI−/−→WT (E) chimeras. In contrast, TNF expression was significantly reduced in IL-1β−/−→WT (C) and WT→IL-RI−/− (D) chimeric mice, with minimal TNF expression on intraglomerular cells and in interstitial area. F: Semiquantitative analysis showed significant reduction in renal TNF expression in IL-1β−/−→WT and IL-1RI−/−→WT chimeras compared to other groups (▪, P < 0.001; see WT) Confocal immunofluorescence, under oil immersion. Original magnifications, ×600.
Prominent TNF expression was observed in glomeruli, tubules, and interstitial areas of WT mice (Figure 8A), and was unaffected by absence of intrinsic renal cell IL-1β (WT→IL-1β−/− chimeras, Figure 8B) or leukocyte IL-RI expression (IL-1RI−/−→WT chimeras, Figure 8E). However, in IL-1β−/−→WT and WT→IL-1RI−/− chimeras (Figure 8, C and D, respectively), glomerular TNF expression was markedly attenuated, in the absence of leukocyte IL-1β production (IL-1β−/−→WT) or intrinsic renal cell IL-1RI (WT→IL-RI−/−). Sparse TNF expression was observed on some periglomerular cells, interstitial cells, and occasional tubular cells in these mice. This pattern of expression was semiquantitatively scored and glomerular TNF expression did not differ between WT (2.4 ± 0.07), WT→IL-1β−/− chimeric (2.2 ± 0.05), and IL-1RI−/−→WT chimeric mice (2.2 ± 0.06), but was significantly reduced in IL-1β−/−→WT (0.3 ± 0.06) and WT→IL-1RI−/− (0.5 ± 0.04) mice (refer to graph). These results were confirmed by ELISA analysis of kidney extracts. A significant reduction of renal TNF was observed in IL-1β−/−→WT and WT→IL-1RI−/− chimeric mice, whereas WT, WT→IL-1β−/−, and IL-1RI−/−→WT mice had similar levels of renal TNF. Serum TNF levels were equivalent in all groups of mice (WT, 0.04 ± 0.004 ng/ml; WT→IL-1β−/−, 0.02 ± 0.002 ng/ml; IL-1β−/−→WT, 0.03 ± 0.002 ng/ml; WT→IL-1RI−/−, 0.03 ± 0.005 ng/ml; and IL-1RI−/−→WT, 0.03 ± 0.003 ng/ml).
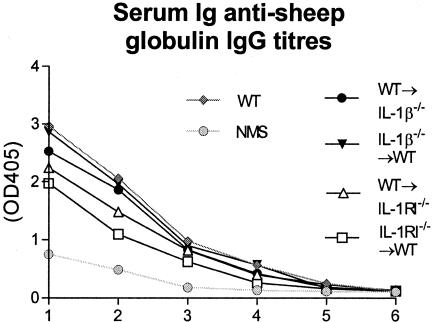
Serum Ab Titers to Sheep Globulin (Figure 9)
Figure 9.
Circulating mouse anti-sheep globulin titers in chimeric mice with crescentic GN demonstrating similar levels of serum anti-sheep globulin antibody in WT, IL-1β−/−→WT, and WT→IL-1β−/− groups. Nonsignificant reductions of serum anti-sheep globulin antibody titers in WT→IL-1RI−/− and IL-1RI−/−→WT chimeric groups were observed. Bottom dotted line represents the circulating serum anti-sheep globulin antibody levels observed in normal WT mice without GN.
No significant differences were observed in the serum titers of mouse anti-sheep globulin antibody in any groups.
Discussion
Several studies have assessed the primary source of IL-1 in GN and have established a link between glomerular macrophage infiltration and increased glomerular expression of IL-1β in experimental GN.25,34–37 Studies of human GN have also demonstrated significant glomerular IL-1β mRNA and protein expression within glomerular crescents and have attributed this expression to infiltrating macrophages rather than intrinsic renal cells.21,38–41 Intrinsic renal cells are capable of producing IL-1 in vitro, and histological studies have suggested intrinsic renal cells may be the major source of IL-1 during GN.28,29,42
In the present study, the relative contribution of leukocytes and intrinsic renal cells to IL-1β-dependent injury in crescentic nephritis was addressed by creating chimeric mice. This technique has been previously demonstrated to result in >90% replacement of recipient BM, splenic, and circulating leukocytes with those of the donor phenotype.13 An important role for leukocyte IL-1β production was demonstrated in WT→IL-1β−/− chimeric mice with restricted expression of IL-1β to BM-derived cells. These mice developed crescentic nephritis in a similar pattern to WT controls, particularly the incidence of crescent formation and serum creatinine elevation. However it should be noted that cellular recruitment (CD4+ T cell and macrophage accumulation) and proteinuria are decreased with the absence of renal IL-1, suggesting a role for renal-derived IL-1β in a feedback loop for leukocyte recruitment that may involve up-regulation of chemokine expression.43–48
In contrast, crescentic GN was markedly attenuated in the absence of leukocyte IL-1β, despite the intact ability of intrinsic renal cells to produce IL-1β. Crescent formation, CD4+ T cell and macrophage glomerular accumulation were all significantly reduced compared to WT controls and WT→IL-1β−/− chimeric mice, as were functional indices of renal injury. These studies demonstrate that although IL-1β production by intrinsic renal cells contributes to some aspects of crescentic GN, leukocyte IL-1β alone is sufficient for induction of crescent formation in this model. Absent leukocyte IL-1β production provides equivalent protection to total IL-1β deficiency, indicating that IL-1β production by intrinsic renal cells cannot compensate for absent leukocyte IL-1β.
To address the cellular targets for IL-1 in this model, we studied the requirement for IL-1RI expression on leukocytes and intrinsic renal cells. Absence of intrinsic renal cell IL-1RI provided similar protection to complete IL-1RI deficiency, indicating that intrinsic renal cells are the major targets for IL-1. Crescent formation, CD4+ T cell and macrophage accumulation, proteinuria, and serum creatinine were all significantly reduced compared to WT animals. In contrast, chimeric mice with absent IL-1RI expression on BM-derived cells but with renal cells expressing the IL-1 receptor developed crescentic GN similar to WT mice. Therefore, intrinsic renal cells need to express the IL-1 type I receptor for the full development of injury in crescentic GN.
Together with IL-1, TNF is a key cytokine in the pathogenesis of crescentic GN. It recently has been demonstrated that intrinsic renal cells are the major source of TNF that is responsible for a substantial component of injury and crescent formation in this model of crescentic GN.14 TNF is produced by intrinsic renal cells, including mesangial,49–51 glomerular,52,53 and tubular epithelial cells.54,55 Macrovascular endothelial cells also produce TNF56,57 but TNF production by glomerular endothelial cells has not been reported.
The current study demonstrates that leukocyte production of IL-1β and expression of IL-1RI on intrinsic renal cells is required for intraglomerular production of TNF. IL-1β has been reported to induce TNF expression in vitro and in vivo.58 By confocal and ELISA analysis, we have demonstrated equivalent TNF expression in the kidneys of WT→IL-1β−/− chimeric, and IL-1RI−/−→WT chimeric mice with crescentic GN to that seen in the WT mice. In these mice, IL-1β expression was predominantly cellular associated, whereas TNF expression was prominently expressed in glomeruli and on tubules. In comparison, IL-1β−/−→WT chimeric mice, which had absent leukocyte IL-1β and limited renal expression of IL-1β (primarily observed on tubular cells), had a reduction in renal IL-1β measured by ELISA. TNF expression by glomerular cells was similarly attenuated in these chimeric mice, indicating that leukocyte-derived IL-1β is required for glomerular TNF expression. Furthermore, in the absence of intrinsic renal cell IL-1RI expression in WT→IL-1RI−/− chimeras, glomerular production of TNF was markedly reduced despite equivalent renal IL-1β production to WT mice. These studies outline a pathway of renal injury, in which infiltrating BM-derived cells by their production of IL-1, interact with the IL-1RI on intrinsic renal cells, causing the release of TNF and subsequent renal injury.
In summary, these studies demonstrate that leukocyte production of IL-1β induces intrinsic glomerular cells to produce TNF via the IL-1 type I receptor. These studies demonstrate a possible pathological pathway of the perpetuation of inflammation, and indicate that both BM-derived cells and local cells are of equal importance in their contributions in organ-specific inflammatory injury.
Acknowledgments
We thank Ms. Alice Wright, Ms. Janelle Sharkey, Ms. Christine Vaculik, D. Calleci, and T. Niklou for technical assistance; and Dr. Abhay Satoskar for the gift of the IL-1RI−/− mice.
Footnotes
Address reprint requests to Dr. Jennifer Timoshanko, Monash University, Department of Medicine, Monash Medical Center, 246 Clayton Rd., Clayton, 3168, Victoria, Australia. E-mail: jennifer.timoshanko@med.monash.edu.au.
Supported by a National Health and Medical Research Council of Australia program grant.
P.G.T. is a National Health and Medical Research Council of Australia Principal Research Fellow.
References
- Holdsworth SR, Kitching AR, Tipping PG. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 1999;55:1198–1216. doi: 10.1046/j.1523-1755.1999.00369.x. [DOI] [PubMed] [Google Scholar]
- Huang XR, Holdsworth SR, Tipping PG. Evidence for delayed-type hypersensitivity mechanisms in glomerular crescent formation. Kidney Int. 1994;46:69–78. doi: 10.1038/ki.1994.245. [DOI] [PubMed] [Google Scholar]
- Huang XR, Tipping PG, Shuo L, Holdsworth SR. Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int. 1997;51:94–103. doi: 10.1038/ki.1997.12. [DOI] [PubMed] [Google Scholar]
- Kitching AR, Tipping PG, Holdsworth SR. IL-12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur J Immunol. 1999;29:1–10. doi: 10.1002/(SICI)1521-4141(199901)29:01<1::AID-IMMU1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kitching AR, Holdsworth SR, Tipping PG. IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol. 1999;10:752–759. doi: 10.1681/ASN.V104752. [DOI] [PubMed] [Google Scholar]
- Tipping PG, Kitching AR, Huang XR, Mutch DA, Holdsworth SR. Immune modulation with interleukin-4 and interleukin-10 prevents crescent formation and glomerular injury in experimental glomerulonephritis. Eur J Immunol. 1997;27:530–537. doi: 10.1002/eji.1830270226. [DOI] [PubMed] [Google Scholar]
- Abbott F, Ryan JJ, Ceska M, Matsushima K, Sarraf CE, Rees AJ. Interleukin-1 beta stimulates human mesangial cells to synthesize and release interleukins-6 and -8. Kidney Int. 1991;40:597–605. doi: 10.1038/ki.1991.250. [DOI] [PubMed] [Google Scholar]
- Funk JL, Cordaro LA, Wei H, Benjamin JB, Yocum DE. Synovium as a source of increased amino-terminal parathyroid hormone-related protein expression in rheumatoid arthritis. A possible role for locally produced parathyroid hormone-related protein in the pathogenesis of rheumatoid arthritis. J Clin Invest. 1998;101:1362–1371. doi: 10.1172/JCI484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Inflammation in EAE: role of chemokine/cytokine expression by resident and infiltrating cells. Neurochem Res. 1996;21:511–525. doi: 10.1007/BF02527717. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Li J, Ventura E, Rostami A. Parenchymal microglia of naive adult C57BL/6J mice express high levels of B7.1, B7.2, and MHC class II. Exp Mol Pathol. 2002;73:35–45. doi: 10.1006/exmp.2002.2441. [DOI] [PubMed] [Google Scholar]
- Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J Exp Med. 2001;193:967–974. doi: 10.1084/jem.193.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoshanko JR, Kitching AR, Holdsworth SR, Tipping PG. Interleukin-12 from intrinsic cells is an effector of renal injury in crescentic glomerulonephritis. J Am Soc Nephrol. 2001;12:464–471. doi: 10.1681/ASN.V123464. [DOI] [PubMed] [Google Scholar]
- Timoshanko JR, Holdsworth SR, Kitching AR, Tipping PG. IFN-gamma production by intrinsic renal cells and bone marrow-derived cells is required for full expression of crescentic glomerulonephritis in mice. J Immunol. 2002;168:4135–4141. doi: 10.4049/jimmunol.168.8.4135. [DOI] [PubMed] [Google Scholar]
- Timoshanko JR, Sedgwick JD, Holdsworth SR, Tipping PG. Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:1785–1793. doi: 10.1097/01.asn.0000073902.38428.33. [DOI] [PubMed] [Google Scholar]
- Li S, Kurts C, Kontgen F, Holdsworth SR, Tipping PG. Major histocompatibility complex class II expression by intrinsic renal cells is required for crescentic glomerulonephritis. J Exp Med. 1998;188:597–602. doi: 10.1084/jem.188.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Rinner WA, Bauer J, Schmidts M, Lassmann H, Hickey WF. Resident microglia and hematogenous macrophages as phagocytes in adoptively transferred experimental autoimmune encephalomyelitis: an investigation using rat radiation bone marrow chimeras. Glia. 1995;14:257–266. doi: 10.1002/glia.440140403. [DOI] [PubMed] [Google Scholar]
- Myers KJ, Dougherty JP, Ron Y. In vivo antigen presentation by both brain parenchymal cells and hematopoietically derived cells during the induction of experimental autoimmune encephalomyelitis. J Immunol. 1993;151:2252–2260. [PubMed] [Google Scholar]
- Murphy CA, Hoek RM, Wiekowski MT, Lira SA, Sedgwick JD. Interactions between hemopoietically derived TNF and central nervous system-resident glial chemokines underlie initiation of autoimmune inflammation in the brain. J Immunol. 2002;169:7054–7062. doi: 10.4049/jimmunol.169.12.7054. [DOI] [PubMed] [Google Scholar]
- Ruth AJ, Kitching AR, Semple TJ, Tipping PG, Holdsworth SR. Intrinsic renal cell expression of CD40 directs Th1 effectors inducing experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2003;14:2813–2822. doi: 10.1097/01.asn.0000091381.60059.fb. [DOI] [PubMed] [Google Scholar]
- Noronha IL, Kruger C, Andrassy K, Ritz E, Waldherr R. In situ production of TNF-alpha, IL-1 beta and IL-2R in ANCA-positive glomerulonephritis. Kidney Int. 1993;43:682–692. doi: 10.1038/ki.1993.98. [DOI] [PubMed] [Google Scholar]
- Karkar AM, Smith J, Pusey CD. Prevention and treatment of experimental crescentic glomerulonephritis by blocking tumour necrosis factor-alpha. Nephrol Dial Transplant. 2001;16:518–524. doi: 10.1093/ndt/16.3.518. [DOI] [PubMed] [Google Scholar]
- Karkar AM, Tam FW, Steinkasserer A, Kurrle R, Langner K, Scallon BJ, Meager A, Rees AJ. Modulation of antibody-mediated glomerular injury in vivo by IL-1ra, soluble IL-1 receptor, and soluble TNF receptor. Kidney Int. 1995;48:1738–1746. doi: 10.1038/ki.1995.472. [DOI] [PubMed] [Google Scholar]
- Karkar AM, Koshino Y, Cashman SJ, Dash AC, Bonnefoy J, Meager A, Rees AJ. Passive immunization against tumour necrosis factor-alpha (TNF-alpha) and IL-1 beta protects from LPS enhancing glomerular injury in nephrotoxic nephritis in rats. Clin Exp Immunol. 1992;90:312–318. doi: 10.1111/j.1365-2249.1992.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping PG, Lowe MG, Holdsworth SR. Glomerular interleukin 1 production is dependent on macrophage infiltration in anti-GBM glomerulonephritis. Kidney Int. 1991;39:103–110. doi: 10.1038/ki.1991.13. [DOI] [PubMed] [Google Scholar]
- Cook HT, Avey C, Taylor GM. Interleukin-1 beta gene expression in experimental glomerulonephritis in the rat: an in-situ hybridization study. Int J Exp Pathol. 1994;75:157–163. [PMC free article] [PubMed] [Google Scholar]
- Diamond JR, Pesek I. Glomerular tumor necrosis factor and interleukin 1 during acute aminonucleoside nephrosis. An immunohistochemical study. Lab Invest. 1991;64:21–28. [PubMed] [Google Scholar]
- Niemir ZI, Stein H, Dworacki G, Mundel P, Koehl N, Koch B, Autschbach F, Andrassy K, Ritz E, Waldherr R, Otto HF. Podocytes are the major source of IL-1 alpha and IL-1 beta in human glomerulonephritides. Kidney Int. 1997;52:393–403. doi: 10.1038/ki.1997.346. [DOI] [PubMed] [Google Scholar]
- Tesch GH, Yang N, Yu H, Lan HY, Foti R, Chadban SJ, Atkins RC, Nikolic-Paterson DJ. Intrinsic renal cells are the major source of interleukin-1 beta synthesis in normal and diseased rat kidney. Nephrol Dial Transplant. 1997;12:1109–1115. doi: 10.1093/ndt/12.6.1109. [DOI] [PubMed] [Google Scholar]
- Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow M, Shuster D, Zetterstrom M, Nunes P, Terry R, Cullinan EB, Bartfai T, Solorzano C, Moldawer LL, Chizzonite R, McIntyre KW. Absence of IL-1 signaling and reduced inflammatory response in IL-1 type I receptor-deficient mice. J Immunol. 1997;159:2452–2461. [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Timoshanko JR, Kitching AR, Iwakura Y, Holdsworth SR, Tipping PG. Contributions of IL-1beta and IL-1 alpha to crescentic glomerulonephritis in mice. J Am Soc Nephrol. 2004;15:910–918. doi: 10.1097/01.asn.0000115704.86897.f4. [DOI] [PubMed] [Google Scholar]
- Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141:3050–3054. [PubMed] [Google Scholar]
- Boswell JM, Yui MA, Endres S, Burt DW, Kelley VE. Novel and enhanced IL-1 gene expression in autoimmune mice with lupus. J Immunol. 1988;141:118–124. [PubMed] [Google Scholar]
- Matsumoto K. Production of interleukin-1 by glomerular macrophages in nephrotoxic serum nephritis. Am J Nephrol. 1990;10:502–506. doi: 10.1159/000168176. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Dowling J, Atkins RC. Production of interleukin 1 in glomerular cell cultures from patients with rapidly progressive crescentic glomerulonephritis. Am J Nephrol. 1988;8:463–470. doi: 10.1159/000167656. [DOI] [PubMed] [Google Scholar]
- Jenkins DA, Wojtacha DR, Swan P, Fleming S, Cumming AD. Intrarenal localization of interleukin-1 beta mRNA in crescentic glomerulonephritis. Nephrol Dial Transplant. 1994;9:1228–1233. [PubMed] [Google Scholar]
- Noronha IL, Hartley B, Cameron JS, Waldherr R. Detection of IL-1 beta and TNF-alpha message and protein in renal allograft biopsies. Transplantation. 1993;56:1026–1029. [PubMed] [Google Scholar]
- Takemura T, Yoshioka K, Murakami K, Akano N, Okada M, Aya N, Maki S. Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch. 1994;424:459–464. doi: 10.1007/BF00191429. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Takemura T, Murakami K, Okada M, Yagi K, Miyazato H, Matsushima K, Maki S. In situ expression of cytokines in IgA nephritis. Kidney Int. 1993;44:825–833. doi: 10.1038/ki.1993.317. [DOI] [PubMed] [Google Scholar]
- Zoja C, Rambaldi A, Remuzzi G. Interleukin-1 and glomerular mesangial cells. Renal Physiol Biochem. 1993;16:89–92. doi: 10.1159/000173754. [DOI] [PubMed] [Google Scholar]
- Rovin BH, Lu L, Marsh CB. Lymphocytes induce monocyte chemoattractant protein-1 production by renal cells after Fc gamma receptor cross-linking: role of IL-1beta. J Leukoc Biol. 2001;69:435–439. [PubMed] [Google Scholar]
- Ikeda M, Ikeda U, Shimada K, Minota S, Kano S. Regulation of ICAM-1 expression by inflammatory cytokines in rat mesangial cells. Cytokine. 1996;8:109–114. doi: 10.1006/cyto.1996.0015. [DOI] [PubMed] [Google Scholar]
- Largen PJ, Tam FW, Rees AJ, Cattell V. Rat mesangial cells have a selective role in macrophage recruitment and activation. Exp Nephrol. 1995;3:34–39. [PubMed] [Google Scholar]
- Feng L, Xia Y, Kreisberg JI, Wilson CB. Interleukin-1 alpha stimulates KC synthesis in rat mesangial cells: glucocorticoids inhibit KC induction by IL-1. Am J Physiol. 1994;266:F713–F722. doi: 10.1152/ajprenal.1994.266.5.F713. [DOI] [PubMed] [Google Scholar]
- Rovin BH, Yoshiumura T, Tan L. Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J Immunol. 1992;148:2148–2153. [PubMed] [Google Scholar]
- Zoja C, Wang JM, Bettoni S, Sironi M, Renzi D, Chiaffarino F, Abboud HE, Van Damme J, Mantovani A, Remuzzi G. Interleukin-1 beta and tumor necrosis factor-alpha induce gene expression and production of leukocyte chemotactic factors, colony-stimulating factors, and interleukin-6 in human mesangial cells. Am J Pathol. 1991;138:991–1003. [PMC free article] [PubMed] [Google Scholar]
- Gomez-Guerrero C, Lopez-Armada MJ, Gonzalez E, Egido J. Soluble IgA and IgG aggregates are catabolized by cultured rat mesangial cells and induce production of TNF-alpha and IL-6, and proliferation. J Immunol. 1994;153:5247–5255. [PubMed] [Google Scholar]
- Nakamura A, Johns EJ, Imaizumi A, Niimi R, Yanagawa Y, Kohsaka T. Role of angiotensin II-induced cAMP in mesangial TNF-alpha production. Cytokine. 2002;19:47–51. doi: 10.1006/cyto.2002.1040. [DOI] [PubMed] [Google Scholar]
- Baud L, Oudinet JP, Bens M, Noe L, Peraldi MN, Rondeau E, Etienne J, Ardaillou R. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989;35:1111–1118. doi: 10.1038/ki.1989.98. [DOI] [PubMed] [Google Scholar]
- Neale TJ, Ruger BM, Macaulay H, Dunbar PR, Hasan Q, Bourke A, Murray-McIntosh RP, Kitching AR. Tumor necrosis factor-alpha is expressed by glomerular visceral epithelial cells in human membranous nephropathy. Am J Pathol. 1995;146:1444–1454. [PMC free article] [PubMed] [Google Scholar]
- Noiri E, Kuwata S, Nosaka K, Tokunaga K, Juji T, Shibata Y, Kurokawa K. Tumor necrosis factor-alpha mRNA expression in lipopolysaccharide-stimulated rat kidney. Chronological analysis of localization. Am J Pathol. 1994;144:1159–1166. [PMC free article] [PubMed] [Google Scholar]
- Jevnikar AM, Brennan DC, Singer GG, Heng JE, Maslinski W, Wuthrich RP, Glimcher LH, Kelley VE. Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF alpha. Kidney Int. 1991;40:203–211. doi: 10.1038/ki.1991.201. [DOI] [PubMed] [Google Scholar]
- Wuthrich RP, Glimcher LH, Yui MA, Jevnikar AM, Dumas SE, Kelley VE. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 1990;37:783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]
- Albaugh G, Kann B, Strande L, Vemulapalli P, Hewitt C, Alexander JB. Nicotine induces endothelial TNF-alpha expression, which mediates growth retardation in vitro. J Surg Res. 2001;99:381–384. doi: 10.1006/jsre.2001.6215. [DOI] [PubMed] [Google Scholar]
- Eissner G, Lindner H, Konur A, Kreutz M, Andreesen R, Holler E. Naive monocytes can trigger transendothelial migration of peripheral blood cells through the induction of endothelial tumour necrosis factor-alpha. Scand J Immunol. 2000;51:251–261. doi: 10.1046/j.1365-3083.2000.00677.x. [DOI] [PubMed] [Google Scholar]
- Ikejima T, Okusawa S, Ghezzi P, van der Meer JW, Dinarello CA. Interleukin-1 induces tumor necrosis factor (TNF) in human peripheral blood mononuclear cells in vitro and a circulating TNF-like activity in rabbits. J Infect Dis. 1990;162:215–223. doi: 10.1093/infdis/162.1.215. [DOI] [PubMed] [Google Scholar]