Abstract
Diminished production of vascular endothelial growth factor (VEGF) and decreased angiogenesis are thought to contribute to impaired tissue repair in diabetic patients. We examined whether recombinant human VEGF165 protein would reverse the impaired wound healing phenotype in genetically diabetic mice. Paired full-thickness skin wounds on the dorsum of db/db mice received 20 μg of VEGF every other day for five doses to one wound and vehicle (phosphate-buffered saline) to the other. We demonstrate significantly accelerated repair in VEGF-treated wounds with an average time to resurfacing of 12 days versus 25 days in untreated mice. VEGF-treated wounds were characterized by an early leaky, malformed vasculature followed by abundant granulation tissue deposition. The VEGF-treated wounds demonstrated increased epithelialization, increased matrix deposition, and enhanced cellular proliferation, as assessed by uptake of 5-bromodeoxyuridine. Analysis of gene expression by real-time reverse transcriptase-polymerase chain reaction demonstrates a significant up-regulation of platelet-derived growth factor-B and fibroblast growth factor-2 in VEGF-treated wounds, which corresponds with the increased granulation tissue in these wounds. These experiments also demonstrated an increase in the rate of repair of the contralateral phosphate-buffered saline-treated wound when compared to wounds in diabetic mice never exposed to VEGF (18 days versus 25 days), suggesting that topical VEGF had a systemic effect. We observed increased numbers of circulating VEGFR2+/CD11b− cells in the VEGF-treated mice by fluorescence-activated cell sorting analysis, which likely represent an endothelial precursor population. In diabetic mice with bone marrow replaced by that of tie2/lacZ mice we demonstrate that the local recruitment of bone marrow-derived endothelial lineage lacZ+ cells was augmented by topical VEGF. We conclude that topical VEGF is able to improve wound healing by locally up-regulating growth factors important for tissue repair and by systemically mobilizing bone marrow-derived cells, including a population that contributes to blood vessel formation, and recruiting these cells to the local wound environment where they are able to accelerate repair. Thus, VEGF therapy may be useful in the treatment of diabetic complications characterized by impaired neovascularization.
Diverse processes such as embryonic development, tumor growth, and tissue repair are linked by the absolute requirement for a vascular bed to deliver nutrients and oxygen to metabolically active cells. The clinical importance of controlled vascular growth is illustrated by disease states resulting from imbalances in blood vessel formation, such as diabetes mellitus. Vasculopathies associated with diabetes include excessive blood vessel formation (eg, retinopathy, glomerular nephropathy) and accelerated atherosclerosis leading to coronary artery disease, peripheral vascular disease, and cerebrovascular disease.1 Microvascular dysfunction is also believed to contribute to morbidity in diabetes by impairing collateral formation, resulting in poor outcomes after vascular occlusive events.2,3
Diabetes impairs numerous components of wound healing, including hemostasis and inflammation, matrix deposition, and angiogenesis. These impairments are present in a wide variety of tissues including myocardium, skeletal muscle, nerve, and skin. Cutaneous wounds in diabetics have been shown to have altered blood flow, impaired neutrophil anti-microbial activity, and a dysfunctional inflammatory state associated with abnormal chemokine expression.4 A number of growth factors essential for wound healing, including FGF-2 and platelet-derived growth factor (PDGF)-B, have also been found to be reduced in experimental diabetic wounds.5–8
Vascular endothelial growth factor (VEGF)-A, a member of a family of growth factors with essential roles in vascular and lymphatic growth and patterning, has been shown to be deficient in experimental and clinical diabetic wounds.9 VEGF acts through at least two receptors, expressed primarily on endothelial cells, along with other vascular cytokines [fibroblast growth factor (FGF), PDGF, and the angiopoietins] to induce and maintain the vasculature.10 VEGF-A (referred subsequently as simply VEGF) is the prototype member of this family and has been shown to be absolutely essential for vascular development.11,12 VEGF facilitates tissue repair by both increasing vascular permeability, allowing the efflux of inflammatory cells into the site of injury, and increasing the migration and proliferation of pre-existing endothelial cells. However, exogenous administration of VEGF results in leaky, malformed vessels which has raised concerns regarding its therapeutic usefulness.13,14
Recent studies have established that angiogenesis is not the sole mechanism by which new vessels are formed. It is now apparent that bone marrow-derived cells, including endothelial progenitor cells (EPCs), are mobilized in response to trauma or ischemia and are able to contribute to tissue repair and new blood vessel formation.15–18 The development of blood vessels from blood-borne endothelial precursors, termed vasculogenesis, was previously thought to be restricted to embryonic development, but is now accepted to play a role in postnatal processes including tissue repair.18–20 We have recently demonstrated that EPCs isolated from diabetic patients are functionally impaired,21 but the contribution of EPC-mediated vasculogenesis on impaired diabetic wound healing remains poorly understood.
In this study, we demonstrate that topical VEGF accelerates wound healing in diabetic mice to nearly the rate observed in nondiabetic mice. Unexpectedly, we found that VEGF also accelerated healing in untreated (control) wounds. This correlated with the systemic mobilization and recruitment of putative endothelial precursors from the bone marrow. Despite the disordered vasculature induced by VEGF administration, repair was accelerated and persisted after cessation of VEGF treatment, suggesting that these vessels are functional during wound healing. This may be explained by an up-regulation of PDGF and basic FGF present in VEGF-treated diabetic wounds. This supports the rational clinical use of VEGF in the treatment of diabetic vascular complications. Because the mechanism partly involves the mobilization and recruitment of bone marrow-derived progenitors, VEGF may be useful for injuries not amenable to topical therapy.
Materials and Methods
Mice
This study was approved by the New York University Medical Center Animal Care Committee. All mice were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in an approved animal care center with 12-hour light cycles and provided standard rodent chow and water ad libitum. For most experiments, the db/db mouse (BKS.Cg-m +/+ Leprdb, Jackson Laboratories stock no. 000642) was used. It is a model of type II diabetes with documented impairments in wound healing.22 Female mice 10 to 12 weeks of age were used. At this age the mice are all diabetic, with blood glucose levels >350 g/dL. For bone marrow transplantation experiments, the FVB/NJ strain of mice was used (Jackson Laboratories stock no. 01800) along with tie2/lacZ transgenic donor mice of the same genetic background [strain FVB/N-TgN(TIE2-lacZ)182Sato, stock no. 002856].
Experimental Wound Model and Gross Wound Measurements
A novel model of wound analysis was used (Galiano et al, submitted for publication). Under sterile conditions, paired 6-mm circular, full-thickness wounds were made on the dorsal skin of the mice after depilation. A donut-shaped 12-mm splint made of 0.5-mm-thick silicone sheeting (Grace Bio-Labs, Bend, OR) was then placed around the wounds and adhered to the skin with cyanoacrylate glue and interrupted 6-0 nylon sutures. Recombinant human VEGF165 protein (supplied by Genentech, South San Francisco, CA) or phosphate-buffered saline (PBS) vehicle was placed into the wound bed at a dose of 20 μg per wound. A transparent sterile occlusive dressing was then placed over the wound and the splint. The dressing and the splint were maintained on the wound throughout the entire course of the experiments.
VEGF protein was applied sterilely to the wounds on days 0, 2, 4, 6, and 8 after wounding. Wounds were covered with an occlusive dressing after VEGF or PBS administration. Digital photographs were taken every 2 days. Time to closure was defined as the time until the wound bed was completely resurfaced with new tissue, and was determined by a blinded observer. Wound area was calculated as a percent area of the original wound size; because the splint has a constant area, it was used to normalize the wound sizes, even at different focal distances. Three to five mice were analyzed at each time point.
Throughout this article, wounds were classified into three groups. The first group was the VEGF-treated wound group, and consisted of wounds that received topical VEGF using the dosing regimen given above. The contralateral PBS-treated wound group consisted of the paired wounds in these VEGF-treated mice; these wounds received PBS instead of VEGF. The final group consisted of vehicle-treated wounds in a separate group of mice. These mice did not receive VEGF in either wound, and are referred to as control wounds in mice not treated with VEGF.
Histology and Wound Analysis
At time intervals ranging from 5 days through 21 days after wounding, wounds were excised with a 2-mm rim of surrounding tissue and placed either in Bouin’s fixative overnight or snap-frozen in liquid nitrogen. The wounds were then bisected down the center, and 8-μm sections were processed for routine hematoxylin and eosin (H&E) staining. Digital imaging software (SigmaScan; SPSS Science, Chicago, IL) was used to measure granulation tissue area and epithelial gap histomorphometrically. Granulation tissue area is measured in pixels, and epithelialization is presented as the gap between the leading epithelial edges, as measured from the wound edges. This distance (epithelial gap) is presented in pixels. For these measurements, H&E-stained wound sections were analyzed at ×40 magnification. Three mice were analyzed at each time point.
Proliferation
A subset of animals was injected with 100 mg of bromodeoxyuridine (BrdU) (Sigma Chemicals, St. Louis, MO) intraperitoneally 3 hours before sacrifice and wound harvest. BrdU incorporation into proliferating cells was detected with a biotinylated anti-BrdU antibody (Zymed, South Francisco, CA) after brief trypsin digestion of the paraffin sections. The number of proliferating cells was determined by manually scoring the number of positive staining cells at ×200 magnification. A total of six random fields from either the leading wound margin or the wound center (for closed wounds) were counted in each wound by a blinded observer.
Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Wounds were harvested on day 12 and from unwounded skin from control and experimental mice by excising the wound and ∼1 mm of surrounding skin. The samples were immediately homogenized and purified using the RNeasy kit (Qiagen, Valencia, CA). Purified RNA was quantified by absorbance spectroscopy at 260/280 nm. After extraction and purification, RNA was converted to cDNA using the RNA PCR Core kit (Applied Biosystems, Foster City, CA) containing MuLV reverse transcriptase and stored at −20°C until use.
Complementary DNA of murine GAPDH, PDGF-B, and FGF-2 was amplified for use in real-time PCR standardization by PCR of mouse genomic DNA, and purified using PCR product purification columns (Qiagen, Valencia, CA). The specificity of the primers used and product size was confirmed by electrophoresis on a 2% agarose gel. The number of copies of cDNA/μl of purified template was determined by UV spectroscopy. Serial dilutions of each purified product were made and standard curves encompassing from 10−8 to 102 copies were obtained on a real-time PCR cycler (Cepheid Smartcycler, Sunnyvale, CA) using Platinum Sybr Green Supermix as per the manufacturer’s instructions. The amplification protocol for each gene was as follows: an initial denaturation step at 95°C for 2 minutes, followed by 40 cycles of 95°C for 15 seconds, 60°C annealing for 30 seconds, and 72°C extension for 30 seconds. The data acquisition was performed at 80°C for 10 seconds (optics ON), and a melting curve was designed at 0.2-degree increments. This four-step protocol was chosen to minimize primer-dimer formation. For each experiment, a negative control was included by placing water in place of cDNA.
The following primers were used: GAPDH forward, (5′-ACCACAGTCCATGCCATCAC-3′) and reverse, (5′-TCCACCACCCTGTTGCTGTA-3′); connective tissue growth factor forward, (5′-TATCCCACCAAAGTGAGAACG-3′) and reverse, (5′-TGGAATCAGAATGGTCAGAGC-3′); insulin-like growth factor-I forward, (5′-ATGTACTGTGCCCCACTGAAG-3′) and reverse, (5′-GTGTTTCGATGTTTTGCAGGT-3′); transforming growth factor-β1 forward, (5′-AACAATTCCTGGCGTTACCTT-3′) and reverse, (5′-TTTGCTGTCACAAGAGCAGTG-3′); FGF-2 forward, (5′-GCTGCTGGCTTCTAAGTGTGT-3′) and reverse, (5′-CCAACTGGAGTATTTCCGTGA-3′); PDGF-B forward, (5′-GGTCAAACCTCTGAGGAAAGG-3′) and reverse, (5′-AGTACCATGGGCTCATTTCTGA-3′). After acquisition of a standard curve for each gene, gene expression was then determined by real-time RT-PCR and the absolute number of gene copies was quantified using each representative standard curve.
CD31 Immunohistochemistry
Frozen sections were placed on glass slides, dried at 50°C for 1 hour and then rinsed in PBS. Cytokeratin 6 was detected with an anti-mouse keratin-specific antibody (MK6; Covance, Berkeley, CA) at a 1:500 dilution. The secondary antibody was an Alexa-Fluor 488-linked anti-mouse IgG antibody (Molecular Probes, Eugene, OR) used at a 1:100 dilution. Murine CD31 was detected with a rat monoclonal antibody (clone MEC 13.3; BD Biosciences, San Diego, CA). The secondary antibody was an Alexa-Fluor 594-linked anti-rat IgG antibody (Molecular Probes) used at a 1:100 dilution. All blocking steps were performed with SuperBlock reagent (Biogenex, San Ramon, CA). Processed sections were mounted in mounting media (VectaShield; Vector Laboratories, Burlingame, CA) and viewed on an Olympus BX51 epifluorescent microscope. For quantification of CD31-positive cells, wound edges or wound centers were analyzed under ×200 magnification, and total positive cells per high-power field (hpf) were counted by a blinded observer. The keratin staining was used to delineate the wound edges under immunofluorescence.
Quantitative Wound CD31 Assay
For the in vivo measurement of wound vascular density the rat monoclonal anti-CD31 antibody used above was radiolabeled with 125I (Dupont NEN, Boston, MA); a nonspecific isotype control antibody (rat anti-mouse CD 35, clone 8C12; Pharmingen, San Diego, CA) was radiolabeled with 131I/125I (Dupont NEN). The control antibody was used to account for any nonspecific antibody binding, vascular leakage, or any blood left in the tissue. All antibodies were iodinated using the iodogen method in a ratio of 1 μg of antibody to 1 μCi of either 125I or 131I as described.23
Mice were anesthetized and the left carotid artery and right jugular vein were isolated and cannulated. The animals were heparinized with 40 U of sodium heparin (Elkins-Sinn, Cherry Hill, NJ). To measure CD31 antibody binding, a mixture of 125I-CD31 monoclonal antibody (10 μg) and 131I-nonbinding monoclonal antibody (equivalent to 500,000 cpm) was diluted with PBS to a volume of 200 μl. Initial radioactivity was counted in a 2-μl sample using a Wallac Wizard 3“ gamma counter (model 1480; Perkin Elmer, Gaithersburg, MD). Thirty μg of unlabeled CD31 monoclonal antibody was added to the solution. The mixture was injected through the jugular vein catheter and allowed to circulate for 5 minutes. At the end of 5 minutes a blood sample was obtained from the carotid catheter to measure the circulating radiolabeled antibody level. The animal was then exsanguinated by perfusion with bicarbonate-buffered saline through the jugular catheter with simultaneous blood withdrawal from the carotid catheter. This was followed by perfusion of bicarbonate-buffered saline through the carotid catheter (15 ml) after severing the inferior vena cava at the thoracic level. Wounds were collected, weighed, and radioactivity measured with a gamma counter. Results are presented as μg antibody per g of tissue.
Laser Doppler Flow Measurements
Mice were sedated and positioned such that the wound was 25 cm from the reflection mirror. A 1 cm by 1 cm square of tissue was imaged in triplicate, with the wound centered in the square, using a MoorLDI laser Doppler imager (Moor Instruments Limited, Devon, UK). Flow is reported in relative units.
In Vitro Culture and Quantification of EPCs
In a subset of mice, peripheral blood (500 μl per animal) was drawn before sacrifice by intracardiac puncture. Mononuclear cells were separated by density centrifugation with Histopaque 1083 (Sigma) and plated on fibronectin-coated four-well glass slides. This EPC culture assay used has been described elsewhere.20 In brief, mononuclear cells were cultured in media supplemented with endothelial cell growth medium microvascular SingleQuots (EGM-2-MV; Cambrex BioProducts, East Rutherford, NJ). After 4 days, fluorescence staining with fluorescein isothiocyanate-conjugated BS1-lectin (Vector Laboratories) and the uptake of DiI-labeled acetylated LDL (ac-LDL) (Biomedical Technologies, Inc., Stoughton, MA) were used to detect EPCs (dual-staining cells). This has previously been shown to be representative of circulating EPCs.15,24 The number of dual-staining cells (EPCs) per hpf was determined by an independent reviewer analyzing 10 random fields. EPCs from three mice were analyzed for each group.
Fluorescence-Activated Cell Sorting (FACS) Analysis and Antibodies
To further quantify the effects of VEGF on mobilizing EPCS to the circulation, we performed FACS analysis on freshly isolated peripheral blood mononuclear cells. Peripheral blood mononuclear cells from VEGF-treated and nontreated animals were stained with a phycoerythrin-labeled anti-flk-1/VEGFR-2 (clone Avas 12α1, BD Biosciences) antibody (2 μg/ml) to delineate those circulating cells expressing VEGF receptor-2. Two other cell surface markers were used to further define the circulating endothelial progenitor population. One group of samples was co-incubated with a fluorescein isothiocyanate-labeled CD31 antibody (clone MEC 13.3, BD Biosciences) at 5 μg/ml. Because some monocytes also express VEGFR-2, we excluded cells of the myeloid/monocyte lineage by also staining another group of samples with a fluorescein isothiocyanate-labeled CD11b (clone M1/70, BD Biosciences) antibody (0.5 μg/ml). Circulating VEGFR-2+/CD11b− cells have previously been shown to represent an EPC population.16,25 Quantitative analysis was performed on a FACStar flow cytometer (BD Biosciences). Each experiment was repeated with peripheral blood mononuclear cells from four different mice.
Murine Bone Marrow Transplantation
Bone marrow cells were collected from the tibia and femurs of transgenic mice that express lacZ under the control of the endothelial-specific tie2 promoter (tie2/lacZ mice) (Jackson Laboratories). Bone marrow cells were purified by density centrifugation (Histopaque 1083) and 2 × 106 cells were systemically transplanted to FVB/NJ wild-type mice that had been lethally irradiated (12 Gy). Four weeks after bone marrow transplantation, diabetes was induced in the transplanted mice by administering intraperitoneal injections of streptozotocin (40 mg/kg) (Sigma) for 5 consecutive days. Two weeks after streptozotocin treatment, blood glucose levels were assessed with a glucometer (Roche Bioproducts, Indianapolis IN) to confirm successful induction of diabetes by streptozotocin. Only mice with blood glucose levels >300 mg/dL were used for these experiments. Six weeks later, diabetic animals were wounded and treated with VEGF in an identical manner as described above.
Tissue Staining and Enumeration of lacZ-Positive Cells in Wounds of tie2/lacZ Bone Marrow-Transplanted Mice
After surgery, the wounds from four different mice were harvested at days 14 and 21. They were fixed in 1% paraformaldehyde/0.5% gluteraldehyde in PBS for 4 hours, then washed (in PBS with 2 mmol/L MgCl2, 5 mmol/L ethylenediaminetetraacetic acid, 0.001% sodium deoxycholate, and 0.02% Nonidet P-40) and stained for β-galactosidase activity overnight at 37°C (β-gal staining kit, Roche). Before further processing, each skin sample was placed under a dissecting microscope to visualize and document foci of lacZ-positive cells. A blinded observer quantified the number of positive foci. The tissue was then embedded in paraffin, and 10 μmol/L sections were cut and counterstained with eosin.
Statistical Analyses
Statistical analyses were performed using the SigmaStat program (SPSS Science, Chicago IL). A two-tailed unpaired Student’s t-test or an analysis of variance was used to analyze differences between groups. All data are presented as mean ± SEM. A P value less than 0.05 is considered significant.
Results
VEGF Accelerates Healing of Cutaneous Wounds in Diabetic Mice
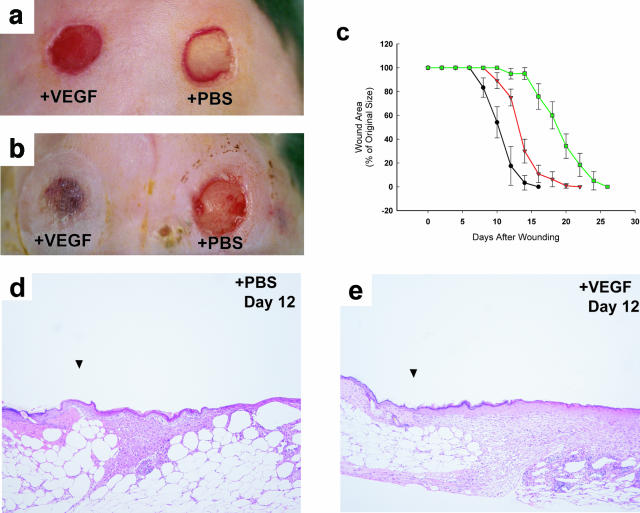
To accurately quantitate the rate of healing, we developed a novel wound healing model that preserves sterility of the wound bed, prevents wound contraction, and minimizes eschar formation (Galiano et al, submitted for publication). Using this model, we determined that untreated excisional wounds in the db/db mouse require 25 days for complete closure (green line in Figure 1c). The serial application of 20 μg of rhVEGF165 had a dramatic effect on wound healing, with resurfacing occurring by 12 days (black line in Figure 1c), nearly identical to the rate of closure in nondiabetic mice (10 days, data not shown). Grossly, VEGF-treated wounds became hyperemic by day 3, and by day 5 a rich, red granulation tissue bed was present (Figure 1a). The wounds began to visually close by days 7 to 8, with complete resurfacing occurring in most of the VEGF-treated wounds by days 12 to 13 (Figure 1, b and c). A copious amount of edema fluid was regularly noted in the VEGF-treated wound beds during days 3 to 7, consistent with the permeability-inducing effects of VEGF on endothelium. Differences in mean wound sizes between the different groups were statistically significant (P < 0.05) at all time points after day 5 until complete healing.
Figure 1.
Topical VEGF accelerates wound healing in db/db mice. Depicted are the gross appearance of wounds treated with either VEGF (left wounds) or PBS vehicle (right wounds). A: Wounds at day 5. The wound on the left treated with VEGF has developed a hyperemic wound bed that contains abundantly vascularized newly formed granulating tissue. It has not yet begun to significantly close at this time. The wound on the right treated with PBS exhibits scant healing. B: Wounds at day 12 after wounding. The VEGF-treated wound (left) has completely resurfaced, while the wound treated with PBS (right) is only beginning to heal. There is a granulation tissue forming in the PBS-treated wound, but unlike the granulation bed present in VEGF-treated wounds in A, there is no evidence of excessive hyperemia. C: This graph demonstrates the kinetics of closure in VEGF-treated wounds (•), PBS-treated contralateral wounds (▾), and wounds made in control db/db mice in which neither wound was treated with VEGF (▪). VEGF normalizes the impairment in repair seen in this model of diabetic wound healing. Note that wounds made in animals in which neither wound was treated with VEGF have the most delayed healing. Each point represents the mean of the percentage in area of the original wound size ± the SEM of wounds harvested from three to five mice. D and E: Histological comparison of wounds treated with VEGF and control wounds. Wounds at day 12 after wounding: The PBS-treated wound (D) shows early histological evidence of granulation tissue deposition; epithelialization of the wound bed is apparent. The VEGF-treated wound (E) has abundant granulation tissue completely covering the wound; the epithelial layer is multilayered and the wound is completely epithelialized. All wounds were stained with H&E. Original magnifications, ×100.
Histologically, more rapid epithelialization of the wound was observed in VEGF-treated wounds, with full re-epithelialization of VEGF-treated wounds noted by day 9 (versus day 17 in the untreated wounds). Granulation tissue deposition was more abundant in wounds receiving topical VEGF at all time points, with a 225% increase in granulation tissue area in VEGF-treated wounds over PBS-treated control wounds at day 12 and a 20% increase at day 21 (P < 0.05) (Figure 1, d and e; and Table 1). The contralateral wound never achieved the same amount of granulation tissue deposition as VEGF-treated wounds (Table 1).
Table 1.
Wound Histomorphometric Analysis
| Days after wounding | Epithelial gap (pixels)
|
Granulation tissue area (pixels)
|
||
|---|---|---|---|---|
| + VEGF-treated wound | + PBS-treated wound | + VEGF-treated wound | + PBS-treated wound | |
| 7 | 675 ± 264 | 2295 ± 195 | 3224 ± 805 | 155 ± 74 |
| 12 | 0 | 427 ± 335 | 74580 ± 11070 | 33754 ± 9068 |
| 21 | 0 | 0 | 45132 ± 9940 | 38652 ± 8637 |
Proliferation in VEGF-Treated Wounds Persists until Wound Closure
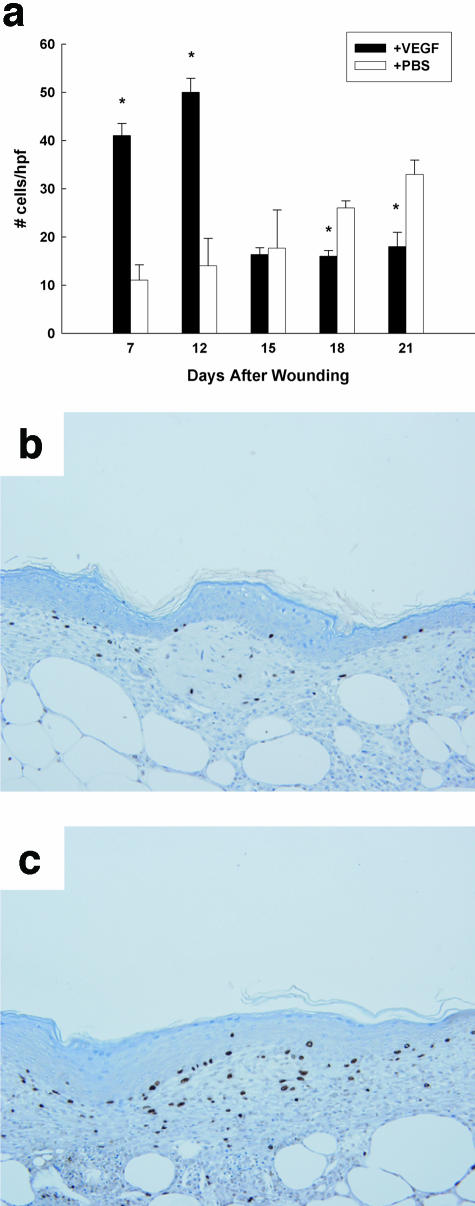
VEGF-treated wounds in the db/db mice were characterized by a significantly greater proliferative index during VEGF treatment (Figure 2). At day 7, there were four times as many proliferating cells in the VEGF-treated wound as in the contralateral PBS-treated control wound (41 ± 3 BrdU-positive cells/hpf versus 11 ± 2 BrdU-positive cells/hpf, P < 0.01). After closure, however, the number of proliferating cells rapidly decreased, and by days 14 and 21 the number of proliferating cells in the PBS-treated wounds (that were still healing at these time points) exceeded the number of proliferating cells in the healed VEGF-treated wounds (33 ± 4 BrdU-positive cells/hpf versus 18 ± 3 BrdU-positive cells/hpf; P < 0.05, day 21 wounds). The PBS-treated contralateral wound never achieved the maximal proliferative rate seen in VEGF-treated wounds, suggesting that exogenous VEGF induces supraphysiological levels of cellular proliferation. However, the rapid decrease in cellular proliferation after closure suggests that the normal homeostatic mechanisms of cellular regression are not circumvented by VEGF therapy.
Figure 2.
VEGF-treated wounds have increased proliferation in the granulation tissue during the phases of active healing. a: The mitotic index is graphed as a function of time in both VEGF-treated as well as control wounds. The graph depicts the number of BrdU-positive cells per hpf at the time points listed. The solid bars represent the wounds treated with VEGF; open bars represent the contralateral wounds treated with PBS vehicle. Six fields per wound from a total of three mice were analyzed at each time point. Only cells in the granulation tissue were enumerated. There is a large increase in proliferating cells early during the course of wound healing in VEGF-treated wounds; this proliferative response diminishes by day 15. b: The center of a day 12 wound treated with PBS is shown. c: The central area of a VEGF-treated wound at day 12 is shown. Note the greater number of BrdU-positive cells in the VEGF-treated wound. *, P value <0.05. All sections are stained with hematoxylin. Original magnification, ×200 (a).
VEGF Treatment Induces a Robust Neovascularization in Diabetic Wounds
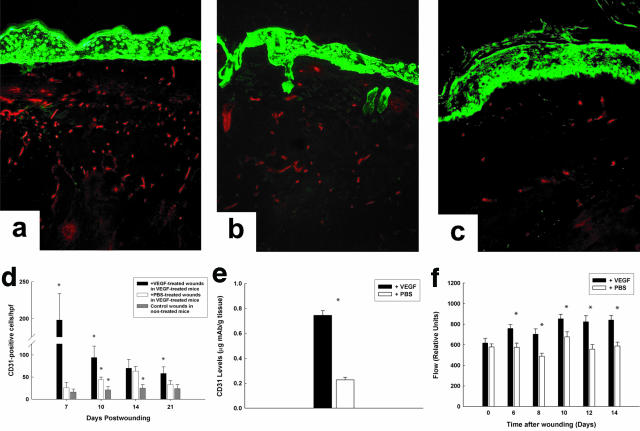
Neovascularization was significantly increased during active topical VEGF treatment (days 3 to 7). When examined histologically, large vacuolar-like vessels were seen in the wound bed at these time points. This correlated with edema formation, which persisted until VEGF therapy was withdrawn. Interestingly, most granulation tissue deposition occurred after termination of VEGF treatment, suggesting that tissue repair stimulated by exogenous VEGF persisted even after VEGF withdrawal. By day 7, wounds receiving VEGF had 10-fold more CD31-positive cells than the contralateral PBS-treated wound (198 ± 36 versus 26 ± 12 CD31-positive cells per hpf, P < 0.001; Figure 3d). By day 10 vessels started to decline in the VEGF-treated wounds (94 ± 26 cells/hpf), and by day 21 wound vascularity approached that in control animals not exposed to VEGF (Figure 3; a to c). Although the number of CD31-positive cells in the contralateral PBS-treated wounds was consistently less than that in the VEGF-treated wounds, the levels were significantly higher than control wounds made in db/db mice never exposed to exogenous VEGF (Figure 3d), suggesting that VEGF exerted a systemic effect.
Figure 3.
VEGF-treated wounds demonstrate an increase in endothelial cells in the wound. a–c: Representative wounds at day 21. The keratinocytes are stained with a green fluorescent antibody to better define the wound architecture. a: A VEGF-treated wound. b: A PBS-treated wound in a VEGF-treated mouse. c: A PBS-treated wound in a control mouse that did not receive any exogenous VEGF. d: Graph demonstrating the number of CD31-positive cells per hpf at either the wound edge (for healing wounds) or in the center of the wound (for closed wounds). Note that VEGF-treated wounds consistently demonstrate greater numbers of CD31-positive cells at all time points. Statistical significance was determined by analysis of variance. e: Wounds treated with VEGF have 2.3-fold greater number of CD31 cells, as measured with a radiolabeled quantitative CD31 assay. f: VEGF-treated wounds consistently demonstrate greater blood flow than wounds not treated with VEGF, as measured with a laser Doppler flowmeter. An asterisk above a group signifies a P value <0.05 when compared to the other groups. Original magnification, ×200 (d).
A quantitative CD31 wound-binding assay (Figure 4e), also demonstrated that at day 10 there was 3.4-fold more CD31 antigen in wounds treated with VEGF (0.744 ± 0.036 versus 0.226 ± 0.021, P < 0.001). Laser Doppler flow analysis demonstrates that these vessels were functional, and flow was significantly augmented at all time points through day 14 in wounds treated with VEGF (Figure 3f).
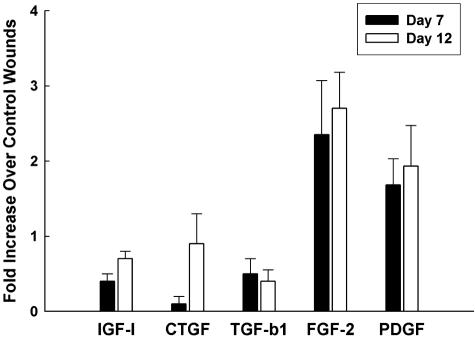
Figure 4.
VEGF up-regulates the expression of PDGF and FGF-2 in treated wounds. VEGF treatment up-regulated the mRNA levels of PDGF-B and FGF-2 at days 7 and 12 in db/db mice. Insulin-like growth factor-I, transforming growth factor-β1, and connective tissue growth factor are not significantly up-regulated compared to control PBS-treated wounds. The graph depicts the up-regulation of PDGF and FGF-2 as assayed by real-time RT-PCR in VEGF-treated wounds as compared to PBS-treated wounds. Each experiment was repeated three times.
VEGF Administration Up-Regulates Expression of PDGF and Basic FGF in Diabetic Wounds
The increase in blood vessels and granulation tissue seen in wounds treated with VEGF is likely not solely because of the effects of VEGF because VEGF is mainly an endothelial-specific mitogen. To obtain an insight into how VEGF induces the granulation tissue seen, we assayed the wounds for the expression of several growth factors that might be candidates for producing the increased granulation tissue seen. We examined a panel of growth factors that are important in both angiogenesis as well as matrix production, including PDGF-B, insulin-like growth factor-I, transforming growth factor-β1 (TGF-β1), FGF-2, and connective tissue growth factor. Of these growth factors, PDGF-B and FGF-2 had the strongest up-regulation of RNA levels in VEGF-treated wounds, as assayed by real-time RT-PCR (Figure 4). Wounds treated with VEGF had 2.3-fold more FGF-2 and 1.7-fold more PDGF-B than wounds treated with PBS at day 7; this up-regulation in the levels of these two cytokines persisted even at day 12, well beyond the last administration of VEGF.
Topical VEGF Treatment Mobilizes a Large Number of Circulating VEGFR2+ Cells in Diabetic Mice
Although the contralateral PBS-treated wound never displayed the exaggerated vascularization observed in the wound topically receiving VEGF, these wounds did exhibit accelerated healing when compared to wounds in db/db mice never receiving VEGF. This increase in healing was associated with an increase in blood vessel density compared to wounds in mice not treated with VEGF at days 14 and 21 (Figure 3). Because systemic VEGF has been shown to mobilize bone marrow-derived endothelial progenitors that may contribute to new blood vessel growth, we postulated that this effect was because of the systemic mobilization of bone marrow-derived precursors, including EPCs.
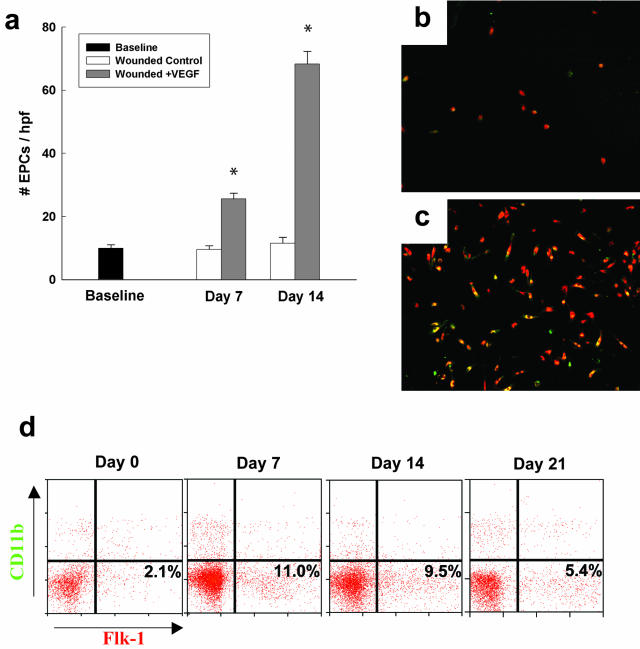
To address this question, we examined whether the topical application of VEGF to wounds is able to mobilize bone marrow-derived precursor cells in diabetic mice. We focused our analyses on a population of cells that is likely to contribute to blood vessel growth. We found that topical VEGF administration induced a profound increase in putative EPC mobilization (Figure 5) as measured by both an in vitro assay and flow cytometry. Using the in vitro proliferation assay, there was a sixfold increase in the number of Ulex+/AcLDL-uptaking cells in VEGF-treated mice by day 14 after wounding (P < 0.001; Figure 5; a to c). Flow cytometry using a CD11b marker to exclude myeloid/monocyte lineage cells demonstrated similar increases in the proportion of VEGFR2+/CD11b− cells in the circulation, with an increase of 5.2-fold by day 7, with levels remaining significantly elevated through day 21 (Figure 5). FACS analysis using VEGFR-2 and CD31 co-staining demonstrated similar levels of circulating VEGFR2+/CD31+ cells in VEGF-treated mice, with a sixfold increase by day 7 and elevated levels persisting to day 21 (data not shown). Both the in vitro assay and FACS demonstrated a significant mobilization of bone marrow-derived angiogenic precursors into the circulation of VEGF-treated mice that persisted after VEGF withdrawal, consistent with other reports on EPC mobilization after cytokine treatment.25
Figure 5.
Topical VEGF application induces significant mobilization of circulating endothelial precursors in diabetic mice. a: Depicted is the increase in EPCs after a 4-day in vitro culture assay. The number of ac-LDL/lectin-positive cells per hpf are depicted. Representative data from four different experiments is shown. b: Depicted are cells cultured from the monocyte layer after 4 days of culture that are positive for both ac-LDL (red) and BS-I lectin (green). This sample was isolated from a diabetic mouse that was wounded and never received VEGF. c: This represents the EPCs that grew from the blood of a mouse in which one wound was treated with VEGF. Note the greatly increased number of EPCs compared to b. d: Representative histograms showing the proportions of VEGFR-2+/CD11b− cells (EPCs) in the mononuclear population isolated from peripheral blood in VEGF-treated db/db mice.
Tie2-lacZ Staining Bone Marrow-Derived Cells Are Preferentially Recruited to Wounds Treated with Topical VEGF
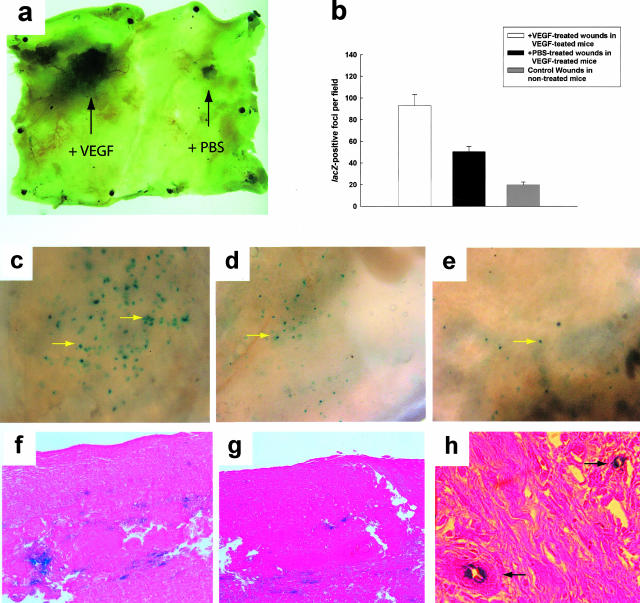
It remained unclear whether the accelerated closure seen in the topical VEGF-treated wound resulted solely from local stimulation of angiogenesis or included a contribution from the recruitment of mobilized circulating precursor cells. There are no EPC-specific markers in mice that can be used for immunohistochemical identification of these cells within tissues. Therefore, to address this question we performed a bone marrow transplant from tie2/lacZ mice into irradiated FVB/NJ mice as previously described.20 Diabetes was induced via streptozotocin treatment. This model permitted an assessment of endothelial progenitor recruitment to the wound bed, because most cells expressing β-galactosidase must be endothelial lineage cells derived from the bone marrow. We found that tie-2/lacZ-positive cells were preferentially recruited to the topical VEGF-treated wounds. In situ staining of a specimen shows grossly more lacZ staining in VEGF-treated wounds at day 21 (Figure 6a) and the number of lacZ-positive foci (defined as a cluster of cells that stained positive for β-galactosidase activity in the wound when the whole mount specimen was viewed under ×20 magnification) was significantly higher in the VEGF-treated wound bed than the PBS-treated contralateral wound (93 lacZ+ foci per field versus 50 lacZ+ foci per field, P < 0.001) (Figure 6b). When histological sections were examined, a significant proportion of these cells were incorporated into blood vessels (Figure 6h), confirming that these cells were most likely marrow-derived EPCs that were contributing to new vessel growth. This suggests that topical VEGF administration recruits endothelial precursors into the wound, either directly or through an intermediary. In addition, more tie2/lacZ-positive cells were observed in the contralateral PBS-treated wound than in wounds in non-VEGF-treated db/db mice (50 lacZ+ foci per field versus 21 lacZ+ foci per field, P < 0.01), which may reflect the effect of increased numbers of circulating bone marrow-derived precursor cells mobilized after topical VEGF therapy.
Figure 6.
Increased EPCs are recruited to VEGF-treated wounds. a: This is a whole-mount specimen encompassing the entire back skin of a streptozotocin-diabetic FVB/NJ mouse transplanted with bone marrow cells from a tie2/lacZ donor as described. It has been stained for β-galactosidase activity (blue areas). Note the much greater staining in the VEGF-treated wound. This specimen was harvested when both wounds were healed, at day 21. b: Graph depicting the number of lacZ+-staining foci per field from the wounds as depicted in c–e. c–e: Close up pictures of the wound whole mounts in the diabetic tie2/lacZ marrow-transplanted mice, viewed under a dissecting microscope. The blue dots represent a cluster of cells within the wound that stain positive for lacZ. f–h: Histological sections of the wounds. Note that there are greater numbers of lacZ-positive cells in the wound treated with VEGF (c, f). There is an intermediate amount of staining in the contralateral PBS-treated wound in VEGF-treated mice (d, g). Note that animals in which neither wound was treated with VEGF exhibit a minimal amount of EPC recruitment to the wound site (e). h: A high-power magnified view showing lacZ-staining cells in two blood vessels from a VEGF-treated wound. All sections are stained with eosin. Original magnifications: ×20 (c–e); ×500 (h).
Discussion
Here we demonstrate that topical VEGF application accelerates cutaneous repair in murine models of diabetes in part by mobilizing and recruiting vascular progenitors. VEGF is one of many cytokines released during tissue repair,26–28 but while other growth factors serve redundant, overlapping functions (eg, epidermal growth factor, transforming growth factor-β, and the FGFs), the role of VEGF is thought to be important solely for stimulating angiogenesis. Previous studies have demonstrated that modulation of oxidative damage29 or inhibition of the receptor for advanced glycation end products30 improved wound healing in diabetic animals and was associated with the up-regulation of endogenous VEGF. Moreover, VEGF administration improves healing in nondiabetic ischemic wounds31 and blocking VEGF with neutralizing antibodies impedes tissue repair.32 These experimental studies have been clinically validated by the demonstration of reduced VEGF activity in chronic wounds in humans.33 Previous data from our laboratory suggested that the decreased VEGF tissue levels in diabetes resulted from an inability of diabetics to appropriately up-regulate VEGF expression in response to hypoxia.34 Together, these observations support the notion that VEGF is critical for repair in impaired healing states and that targeted VEGF supplementation may be useful.9,34
In the present study, a short dosing interval of every other day administration for five doses total (a regimen that could easily be reproduced clinically) was used. The dose used in this study (20 μg) was chosen to exaggerate any effects of VEGF on repair and stem cell recruitment, and is consistent with other preclinical studies using recombinant growth factors in similarly sized wounds.31 Studies with smaller doses show similar effects; however, a single dose was not sufficient to accelerate healing (data not shown). The potential clinical use of VEGF to enhance repair is therefore supported by this study, especially in situations of impaired healing.
An unexpected finding in this study was that contralateral PBS-treated wounds in VEGF-treated mice healed in an expedited manner when compared to wounds made in control animals never receiving VEGF (Figure 1c). Because the wounds in this model are not in direct communication with each other physically and because no edema was observed in the contralateral wound (a significant bridge of skin prevents spillover of VEGF protein from one wound to the other) this must be because of a systemic effect. This suggests that the effects of VEGF on tissue repair extend beyond local augmentation of endothelial cell proliferation, and highlight the caution that must be exercised when using proangiogenic therapies, particularly in diabetic patients who are predisposed to aberrant angiogenesis in distant sites such as the retina and kidney.
Because VEGF is known to mobilize bone marrow-derived cells (including EPCs) from the bone marrow, and directs vasculogenesis and angioblast behavior prenatally as well as postnatally,20,24,35–42 we examined the impact of VEGF on a circulating population of bone marrow-derived progenitor cells that have previously been reported to participate in vasculogenesis. We demonstrate in this report that the application of topical VEGF is able to mobilize VEGFR2+/CD11b− cells into the circulation. There are, however, some limitations to these observations. As there are no specific markers for EPCs in mice (analogous to, for example, AC133 antigen in human EPCs) we have relied on FACS analyses of a VEGF receptor-positive population that does not stain for the myeloid/monocytic marker CD11b to identify EPCs, as previously described.16,25 This was corroborated with the experiments using tie2-lacZ marrow-transplanted mice, which showed an increased trafficking of lacZ+ cells from the bone marrow into the wound bed in both wounds in mice receiving VEGF. The endothelial nature of these cells is supported by the observation that they incorporate within the vasculature. In previous studies, the proportional contributions of local angiogenesis compared to blood vessel growth mediated by circulating vascular progenitors have not been quantified. Although these experiments by necessity used a streptozotocin model of diabetes, the acceleration of repair was similar to that seen in db/db mice (50% reduction in time to closure, data not shown).
Most studies evaluating the effects of VEGF on wound healing have been limited to descriptive observations of local angiogenesis. Our model of wound healing allowed us to distinguish the local from systemic effects of VEGF on wound repair in diabetic mice. Because the contralateral PBS-treated wounds healed on day 18 (as opposed to day 25 for controls), we can estimate that ∼50% of the accelerated wound repair is secondary to VEGF-mobilized bone marrow-derived cells. These mobilized bone marrow-derived cells thus seem to contribute significantly to reversing the impaired tissue repair in the db/db mouse. The importance of VEGF on both local angiogenesis and vasculogenesis may explain why tissue repair in the db/db mouse model, which has abnormally low levels of endogenous VEGF expression after wounding,9 is so profoundly disturbed. It is interesting that the increased healing seen in the contralateral, PBS-treated wounds occurred without any evidence of edema despite the increase in blood vessel density. This suggests that the systemic contributions of VEGF to repair by means of progenitor cell mobilization and recruitment avoid some of the local stigmata of VEGF therapy.
Because we used pharmacological doses of VEGF, it is still unclear whether the smaller amounts of endogenously expressed VEGF present after tissue injury exert a significant effect on mobilizing EPCs and other bone marrow-derived precursors. However, because EPCs have been found in wounds and other sites of injury where VEGF is normally expressed19,24 it is likely that endogenous expression of VEGF during normal tissue repair plays a role in the mobilization of EPCs.
We have recently shown that EPCs from human diabetics are functionally impaired.21 This impairment in EPC function may provide an explanation for the other vascular defects common to diabetics, such as reduced collateral formation or the embryonic vasculopathy seen in the offspring of diabetic mothers. It is unclear whether impairments are present in other stem cells.43 A global impairment in stem cell function seems plausible given that VEGF is an essential survival factor for a broad class of hematopoietic stem cells,25,37,44–47 many of which are important in tissue repair. The strategy outlined in this report, namely, the mobilization of supraphysiological numbers of bone marrow-derived cells via pharmacological therapy, seems sufficient to overcome any existing functional deficit in EPC function and enhance blood vessel growth in diabetics.
It remains unclear how VEGF recruits circulating stem cells to the wound environment. It is conceivable that VEGF directly recruits bone marrow-derived progenitor cells through a chemotactic gradient via VEGF receptors expressed on these cells. In such a scenario, VEGF would act as both a soluble circulating factor signaling EPC release from the marrow (via its soluble isoforms) and also as a local signal mediating recruitment to sites of injury (via its heparin-binding nonsoluble isoforms). An alternative explanation is that VEGF indirectly augments progenitor cell recruitment by altering the expression of other molecules involved in stem cell trafficking. The enhanced EPC recruitment seen in the PBS-treated wounds in VEGF-treated mice suggests that other mechanisms may be important for EPC recruitment. Candidate molecules include SDF-1 and CXCR4,48–51 members of a very restricted cohort of molecules that uniquely affect stem cell mobilization and recruitment. The presence of these molecules in the dermis and on blood vessels is suggestive of a role in stem and progenitor cell recruitment during cutaneous repair.52 Studies are currently underway to determine whether VEGF applied to wounds mobilizes bone marrow cells directly, or whether it also regulates other molecules important for stem cell mobilization and recruitment.
This study demonstrates that pharmacological VEGF therapy in diabetics enhances neovascularization at the site of injury with a clinically significant effect. The mechanism for this effect is through a stimulation of local angiogenesis, enhanced expression of growth factors including PDGF and FGF-2, and a systemic mobilization of bone marrow-derived stem cells. This combination of effects is likely responsible for the increased perfusion, improved peripheral neuropathy, and enhanced collateral formation seen after VEGF therapy in other studies.53–55 Because VEGF is uniquely able to enhance local angiogenesis and mobilize endothelial progenitors into the circulation, this suggests that VEGF therapy may be exploited to promote tissue repair in a wide variety of acute and chronic injuries, particularly in conditions such as diabetes or aging.
Acknowledgments
We thank Mary Armour, Leo Deguzman, Jose Zavala-Solorio, and Scott J. Garza for excellent technical assistance.
Footnotes
Address reprint requests to Geoffrey C. Gurtner, M.D., New York University Medical Center, TH-169, 560 First Ave., New York, NY 10016. E-mail: geoffrey.gurtner@med.nyu.edu.
Supported by the Sarnoff Foundation (to G.C.G.) and the Juvenile Diabetes Research Foundation (to O.T.).
References
- Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–145. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Study Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–2242. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- Beer HD, Longaker MT, Werner S. Reduced expression of PDGF and PDGF receptors during impaired wound healing. J Invest Dermatol. 1997;109:132–138. doi: 10.1111/1523-1747.ep12319188. [DOI] [PubMed] [Google Scholar]
- Werner S, Breeden M, Hubner G, Greenhalgh DG, Longaker MT. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J Invest Dermatol. 1994;103:469–473. doi: 10.1111/1523-1747.ep12395564. [DOI] [PubMed] [Google Scholar]
- Bitar MS, Labbad ZN. Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J Surg Res. 1996;61:113–119. doi: 10.1006/jsre.1996.0090. [DOI] [PubMed] [Google Scholar]
- Brown DL, Kane CD, Chernausek SD, Greenhalgh DG. Differential expression and localization of insulin-like growth factors I and II in cutaneous wounds of diabetic and nondiabetic mice. Am J Pathol. 1997;151:715–724. [PMC free article] [PubMed] [Google Scholar]
- Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Little CD. Exogenous vascular endothelial growth factor induces malformed and hyperfused vessels during embryonic neovascularization. Proc Natl Acad Sci USA. 1995;92:7657–7661. doi: 10.1073/pnas.92.17.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- Tsuboi R, Shi CM, Rifkin DB, Ogawa H. A wound healing model using healing-impaired diabetic mice. J Dermatol. 1992;19:673–675. doi: 10.1111/j.1346-8138.1992.tb03757.x. [DOI] [PubMed] [Google Scholar]
- Eppihimer MJ, Russell J, Langley R, Vallien G, Anderson DC, Granger DN. Differential expression of platelet-endothelial cell adhesion molecule-1 (PECAM-1) in murine tissues. Microcirculation. 1998;5:179–188. [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- Howdieshell TR, Riegner C, Gupta V, Callaway D, Grembowicz K, Sathyanarayana, McNeil PL. Normoxic wound fluid contains high levels of vascular endothelial growth factor. Ann Surg. 1998;228:707–715. doi: 10.1097/00000658-199811000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altavilla D, Saitta A, Cucinotta D, Galeano M, Deodato B, Colonna M, Torre V, Russo G, Sardella A, Urna G, Campo GM, Cavallari V, Squadrito G, Squadrito F. Inhibition of lipid peroxidation restores impaired vascular endothelial growth factor expression and stimulates wound healing and angiogenesis in the genetically diabetic mouse. Diabetes. 2001;50:667–674. doi: 10.2337/diabetes.50.3.667. [DOI] [PubMed] [Google Scholar]
- Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, Stern DM, Schmidt AM. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, Mustoe TA. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg. 1999;134:200–205. doi: 10.1001/archsurg.134.2.200. [DOI] [PubMed] [Google Scholar]
- Howdieshell TR, Callaway D, Webb WL, Gaines MD, Procter CD, Jr, Sathyanarayana, Pollock JS, Brock TL, McNeil PL. Antibody neutralization of vascular endothelial growth factor inhibits wound granulation tissue formation. J Surg Res. 2001;96:173–182. doi: 10.1006/jsre.2001.6089. [DOI] [PubMed] [Google Scholar]
- Lauer G, Sollberg S, Cole M, Flamme I, Sturzebecher J, Mann K, Krieg T, Eming SA. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol. 2000;115:12–18. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162:303–312. doi: 10.1016/S0002-9440(10)63821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ, LaRue A, Ferrara N, Little CD. VEGF regulates cell behavior during vasculogenesis. Dev Biol. 2000;224:178–188. doi: 10.1006/dbio.2000.9744. [DOI] [PubMed] [Google Scholar]
- Cho NK, Keyes L, Johnson E, Heller J, Ryner L, Karim F, Krasnow MA. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell. 2002;108:865–876. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Damert A, Miquerol L, Gertsenstein M, Risau W, Nagy A. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development. 2002;129:1881–1892. doi: 10.1242/dev.129.8.1881. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Ho J, Stanford WL, Fischer KD, Schuh AC, Schwartz L, Bernstein A, Rossant J. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Young PP, Hofling AA, Sands MS. VEGF increases engraftment of bone marrow-derived endothelial progenitor cells (EPCs) into vasculature of newborn murine recipients. Proc Natl Acad Sci USA. 2002;99:11951–11956. doi: 10.1073/pnas.182215799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi E, Valtieri M, Coppola S, Botta R, Gabbianelli M, Lulli V, Marziali G, Masella B, Muller R, Sgadari C, Testa U, Bonanno G, Peschle C. Identification of the hemangioblast in postnatal life. Blood. 2002;100:3203–3208. doi: 10.1182/blood-2002-05-1511. [DOI] [PubMed] [Google Scholar]
- Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. Blood-derived angioblasts accelerate blood-flow restoration in diabetic mice. J Clin Invest. 2000;106:571–578. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Luttun A. The emerging role of the bone marrow-derived stem cells in (therapeutic) angiogenesis. Thromb Haemost. 2001;86:289–297. [PubMed] [Google Scholar]
- Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, Hong K, Marsters JC, Ferrara N. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- Bautz F, Rafii S, Kanz L, Mohle R. Expression and secretion of vascular endothelial growth factor-A by cytokine-stimulated hematopoietic progenitor cells. Possible role in the hematopoietic microenvironment. Exp Hematol. 2000;28:700–706. doi: 10.1016/s0301-472x(00)00168-5. [DOI] [PubMed] [Google Scholar]
- Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- Moore MA. Cytokine and chemokine networks influencing stem cell proliferation, differentiation, and marrow homing. J Cell Biochem Suppl. 2002;38:29–38. doi: 10.1002/jcb.10105. [DOI] [PubMed] [Google Scholar]
- Jo DY, Rafii S, Hamada T, Moore MA. Chemotaxis of primitive hematopoietic cells in response to stromal cell-derived factor-1. J Clin Invest. 2000;105:101–111. doi: 10.1172/JCI7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Cheng T, Olszak I, Garcia-Zepeda E, Lu Z, Herrmann S, Fallon R, Luster AD, Scadden DT. CXCR-4 desensitization is associated with tissue localization of hemopoietic progenitor cells. J Immunol. 2001;166:5027–5033. doi: 10.4049/jimmunol.166.8.5027. [DOI] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF and in dermal wound healing. J Immunol. 2001;166:5749–5754. doi: 10.4049/jimmunol.166.9.5749. [DOI] [PubMed] [Google Scholar]
- Isner JM, Ropper A, Hirst K. VEGF gene transfer for diabetic neuropathy. Hum Gene Ther. 2001;12:1593–1594. [PubMed] [Google Scholar]
- Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratzberger P, Walter DH, Rittig K, Bahlmann FH, Pola R, Curry C, Silver M, Krainin JG, Weinberg DH, Ropper AH, Isner JM. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J Clin Invest. 2001;107:1083–1092. doi: 10.1172/JCI12188. [DOI] [PMC free article] [PubMed] [Google Scholar]