Abstract
Endogenous nitric oxide (NO) is known to modulate post-ischemic inflammatory response in various organs. However, the role of nitric oxide synthase isoforms (NOS) in mediating pulmonary post-ischemic inflammatory response is poorly understood. We therefore studied post-ischemic endothelial adhesion molecule expression and leukocyte migration in endothelial NOS knockout (eNOS-KO) mice subjected to pulmonary ischemia and reperfusion in vivo. Under anesthesia and mechanical ventilation, the left pulmonary hilum in wild-type (WT) and eNOS-KO mice was clamped for 1 hour, followed by reperfusion for up to 24 hours. In WT mice, we observed a selective up-regulation of both eNOS mRNA and protein in lung tissue, while inducible NOS (iNOS) and neuronal NOS (nNOS) remained unchanged. Survival in eNOS-KO mice was reduced due to severe pulmonary edema, underlining an increased susceptibility to ischemia-reperfusion (I/R) injury. Interstitial tissue infiltration by CD18- and CD11a-positive white blood cells as well as lung tissue water content peaked at 5 hours of reperfusion and were found significantly higher than in WT mice. Enhanced leukocyte-endothelial interaction was associated with pronounced up-regulation of vascular cell adhesion molecule (VCAM) in eNOS-KO mice during post-ischemic reperfusion. We conclude that eNOS attenuates post-ischemic inflammatory injury to the lung most probably via inhibition of endothelial adhesion molecule expression.
Impaired lung function following temporary interruption of the pulmonary blood supply is described in a variety of clinical settings, such as lung transplantation, cardiac surgery involving cardiopulmonary bypass, or pulmonary embolism that has been treated by thrombectomy or thrombolysis. Recently, the role of cellular adhesion molecules (CAM) such as selectins and integrins in the development of pulmonary tissue damage following intra- and extrapulmonary I/R injury has been appreciated with leukocytes interacting with the endothelial lining and significantly contributing to tissue injury.1–3 Nitric oxide (NO) is a potent vasodilator and is mainly involved in the vasomotor regulation after I/R. Furthermore, NO is believed to modulate post-ischemic tissue damage, exhibiting both pro- and anti-inflammatory actions.4 The mechanisms, however, by which NO mediates the inflammatory process following I/R remain unclear. Enzymes of the NO-synthase family (NOS) include constituent (eNOS and nNOS) and inducible (iNOS) isoforms, synthesizing NO via oxidation of its precursor arginine.5 While the expression of NOS isoforms in the lung has been described to be mainly located in the vascular endothelium and in the bronchial epithelium,6–9 little is known about their pathophysiologic relevance in I/R-associated inflammatory processes. Pathways by which NO may mediate cytotoxic effects are frequent consequences of the ability to form peroxynitrite and thereby the impairment of mitochondrial function, the inhibition of Na(+)/K(+) ATP-ase activity and other oxidative protein modifications, or the initialization of DNA strand breakage.10 On the other hand, NO is believed to inhibit vascular remodeling and to protect against I/R injury via its scavenging capacity for reactive oxygen species and inhibition of leukocyte adhesion to the microvascular endothelium.11–14
Herein, our goal was to investigate the isoform-specific changes of NOS mRNA and protein expression in pulmonary I/R injury in vivo and to determine their functional relevance regarding inflammatory leukocyte tissue infiltration in eNOS-KO mice.
Materials and Methods
Animal Model
All animal experiments were carried out in accordance with the guidelines of the local regulatory agencies and conform with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Six-week-old female WT mice (C57BL/6NCrl; 20 to 23 g; Charles River, Sulzfeld, Germany) and eNOS-KO mice (B6.129P2-Nos3<tm1Unc>; Jackson Laboratory, Durham, NH) were anesthetized by intraperitoneal injection of 75 mg/kg pentobarbital and continuously monitored by electrocardiography. The depth of anesthesia was adjusted in accordance to heart rate and hindpaw withdrawal on standardized tail clamping. Orotracheal intubation was performed, and mechanical ventilation was initiated with an inspiratory oxygen fraction of 30% at a rate of 150 breaths per minute, a maximum airway pressure of 18 to 20 cm H2O and a positive-end expiratory pressure of 2 cm H2O (small animal ventilator, SAR-830P, IITC-Life Science, Woodland Hills, CA). Mice were anticoagulated with 500 I.U. heparin/kg intravenously, followed by a left thoracotomy. The left pulmonary hilum including bronchus, pulmonary artery, and pulmonary veins was occluded using a microsurgical clamp for 1 hour of normothermic ischemia. With removal of the clamp, the left lung was reperfused and reventilated. In control animals without pulmonary ischemia, a thoracotomy was performed and the chest was closed after an equivalent period of time (sham operation).
At the end of the respective reperfusion period, animals were sacrificed, the left lungs were excised, and processed for further analysis. One specimen of native tissue was shock-frozen in liquid nitrogen for isolation of mRNA and protein. Via the main bronchus, the remaining left lung was gently filled with Tissue Tek OCT compound (Sakura Finetek, Torrance, CA) (<15 cm H2O by gravity), embedded in Tissue Tek and shock-frozen in liquid nitrogen for subsequent cryosectioning and immunohistochemistry. For determination of pulmonary wet-to-dry weight ratio, separate experiments were performed with excision of whole left lungs for assessment of tissue water content.
Experimental Groups
In both WT (n = 30) and eNOS-KO mice (n = 27), the following groups with differing reperfusion intervals were studied: group A, animals with lung reperfusion for 30 minutes (n = 9 to 10); group B, animals with lung reperfusion for 5 hours (n = 9 to 10); group C, animals with lung reperfusion for 24 hours (WT, n = 10; eNOS-KO, n = 9). Time-matched sham-operated animals (sham) that did not undergo pulmonary I/R served as controls (WT, n = 10; eNOS-KO, n = 9). Additional experiments in animals that underwent sham-operation as well as 5 hours and 24 hours of reperfusion were performed for assessment of eNOS mRNA, myeloperoxidase activity, and vascular cell adhesion molecule (VCAM) protein expression in pulmonary post-ischemic tissue.
Tissue Water Content
Wet-to-dry weight ratio served as an indicator of lung tissue edema. For this purpose, total lung weight was measured before (wet weight) and after a 24-hour drying process at an oven temperature of 105°C (dry weight).
Immunohistochemistry
Frozen 5-μm sections of lung tissue were fixed in cold acetone for 10 minutes, air-dried, and treated for 10 minutes with a blocking reagent (DAKO, Hamburg, Germany) for inhibition of endogenous peroxidases. Sections were incubated with a monoclonal rat IgG antibody for CD18 (1:1000; BD PharMingen, Heidelberg, Germany), CD11a (1:5000; BD PharMingen), eNOS (1:100; Chemicon) and iNOS (1:500; Chemicon) for 60 minutes at room temperature (CD18, CD11a), or overnight at 4°C (eNOS, iNOS). For CD11a and CD18 staining, slides were incubated for 30 minutes with an unconjugated secondary rabbit anti-rat antibody (1:100 in 5% mouse serum; DAKO) at room temperature, followed by incubation of horseradish peroxidase (HRP)-conjugated polyclonal tertiary sheep anti-rabbit IgG antibody (1:100 in 5% mouse serum; Silenius) for 30 minutes at room temperature. For eNOS and iNOS staining, the slides were incubated for 30 minutes at room temperature with HRP-conjugated ready-for-use secondary antibody system (EnVision, DAKO). All antibodies were diluted in background reducing buffer (DAKO). Counterstaining was performed with Mayer‘s hematoxylin (Merck). For quantification of CD18/CD11a-positive cells in the alveolar interstitium, positively labeled cells were counted by a blinded investigator three times over 30 high-power fields (HPF; magnification, ×400). For quantification of immunohistochemical expression of eNOS protein in lung tissue, a semi-quantitative scale was used with grade 0, no staining; grade 1, faint staining; grade 2, moderate staining; and grade 3, intense staining. In three different tissue sections of each mouse, eNOS expression was assessed three times by a blinded investigator. For quantitative analysis of tissue sections, a light microscope (Biomed; Leitz, Wetzlar, Germany) was used.
Photometric Assessment of Myeloperoxidase Activity (MPO)
Left lung tissue was weighed and washed with K2HPO4 buffer after centrifugation (10000 × g for 15 minutes). Then, the resuspended pellet was incubated at 60°C to exclude the non-thermoresistent peroxidases, followed by ultrasound homogenization for 10 seconds in 1 ml of warm hexadecyl-trimethyl-ammoniumbromide (HTAB-buffer) and three freeze-and-thaw cycles. Then, the homogenisate was centrifuged (10000 × g) and 3,3–5,5 tetramethylbenzidine (TMB Ready to Use Kit, Sigma T-8665, Deisenhofen, Germany) was added. Changes in extinction at a wavelength of 655 nm were measured by photometer and given as mU per gram wet weight of lung tissue.
Northern Blot Analysis of eNOS
Total RNA from lung samples was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany). For preparation of gel, electrophoresis, and hybridization, a formaldehyde-based system for Northern Blots (Northern Max; Ambion, Austin, TX) was used according to the manufacturer’s instruction. After electrophoresis, RNA was blotted to a positively charged modified nylon membrane (Nytran Plus; Schleicher and Schuell, Dassel, Germany) by capillary transfer with 10X SSC (standard saline citrate) and subsequently cross-linked by UV-irradiation. eNOS DNA probe was then labeled (North2South Biotin Random Prime Kit; Pierce Biotechnology, Rockford, IL) and detected (North2South Chemiluminescent Hybridization and Detection Kit; Pierce Biotechnology) according to the manufacturer’s instruction with exposure to autoradiography films (Kodak Xomat XAR 5; Eastman Kodak Co., Rochester, NY). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize the expression signal in Northern blot analysis.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis of NOS-Isoforms, VCAM, and ICAM-1
Total RNA from lung samples was isolated using the RNeasy mini kit (Qiagen). Of the total RNA, 3 μg was used for RT reaction with Superscript II reverse transcriptase (Invitrogen, Karlsruhe, Germany), according to the manufacturer’s instructions. To amplify specific gene products, the following intron-spanning primers were used: eNOS sense: 5′-AAGACAAGGCAGCGGTGGAA-3′, antisense: 5′-GCAGGGGACAGGAAATAGTT-3′, 292 bp; iNOS sense: 5′-ACGCTTGGGTCTTGTTCACT-3′, antisense: 5′-GTCTCTGGGTCCTCTG-GTCA-3′, 468 bp; nNOS sense: 5′-AATGGAGACCCCCCAGAGAAT-3′, antisense: 5′-TCCA-GGAGAGTGTCCACTGC-3′, 281 bp; ICAM-1 sense: 5′-CAACTGGAAGCTGTTTGAGCTG-3′, antisense: 5′-TAGCTGGAAGATCGAAAGTCCG-3′, 437 bp; VCAM sense: 5′-CCTCACTTGCAGCACTACGGGCT-3′, antisense: 5′-TTTTCCAATATCCTCAATGACGGG-3′, 404 bp. Mouse GAPDH was coamplified as an internal control, using the following primer sequences: GAPDH sense: 5′-CATCACCATCTTCCAGGAGCG-3′, antisense: 5′-GAGGGGCCATCCACAGTCTTC-3′, 357 bp. Then, 3 μl of the cDNA product was used for PCR in a 50-μl reaction mix consisting of 2.5U of TaqDNA polymerase (GeneCraft, Munster, Germany), each primer at 0.5 μmol/L molarity, 0.2 mmol/L deoxynucleotide triphosphate mix, and 3 mmol/L MgCl2. Amplification was performed using a profile with 94°C for 1 minute (denaturation), 94°C for 30 seconds (short denaturation), 61°C for 30 seconds (primer annealing), 72°C for 45 seconds (elongation) for a total of 27 cycles (GAPDH and β-actin) or 35 cycles (NOS-isoforms, VCAM and ICAM-1) followed at the end by 72°C for 7 minutes (extension). The number of cycles used was confirmed for each PCR product to be in the linear range of amplification. Negative controls without RT were carried out in parallel for every PCR reaction to exclude amplification of contaminating DNA. In parallel, controls with H2O instead of DNA were carried out for every PCR reaction. The amplified products were separated on a 1.6% agarose gel and were stained with ethidium bromide. Gels were digitalized by using a computer imaging analysis system (Molecular Analyst 1.5, Biorad). Ratios between GAPDH and NOS-isoforms were determined using Scion Image 4.0.2 software.
To obtain positive controls for detection of NOS isoforms by Western blot and RT-PCR techniques, WT mice (n = 5) were treated with lipopolysaccharide from E. coli (serotype O26:B6; 2 mg/kg ip; Sigma) with harvest of lung tissue after 24 hours.
Western Blot Analysis of NOS Isoforms
Frozen lung tissue was weighed and homogenized in ice-cold 300 μl radioimmunoprecipitation assay (RIPA) buffer (50 mmol/L Tris at pH 7.5, 0.1% Triton, 0.1% SDS, 0.5% sodium deoxycholate, 10% glycerol, 140 mmol/L NaCl, 25 mmol/L β-glycerolphosphat, 1 mmol/L PMSF, 1 mmol/L sodium-vanadate, 5 mmol/L EDTA at pH 8.0, and a mixture of protease inhibitors including aprotinin). Samples were sonicated for 2 minutes, and centrifuged at 4°C and 16000 × g for 15 minutes. The protein concentration was determined using the Bradford method (Bio-Rad Laboratories, München, Germany). Immunoprecipitation was performed by incubation of 50 to 100 μl of protein extract with a concentration of 430 mg/ml (eNOS) or 860 mg/ml (iNOS and nNOS) with 2 μg primary antibody (anti-eNOS/anti-iNOS/anti-nNOS; BD Transduction Laboratories) for 1 hour at 4°C, followed by incubation with 20 μl protein-A agarose (Santa Cruz Biotechnology, Heidelberg, Germany) for another 1 to 2 hours at 4°C. Samples were washed three times in RIPA buffer and centrifuged at 4°C and 16000 × g for 1 minute. After removal of the supernatant, 15 μl 2X concentrated electrophoresis sample buffer (125 mmol/L Tris (pH 6.8), 175 mmol/L 5% SDS, 0.2% glycerin, 0.002% bromphenol blue, 5% β-mercaptoethanol) was added, boiled for 10 minutes at 95°C and centrifuged at 16000 × g for 1 minute. The supernantant was separated by SDS-PAGE using 8% gels. Equal loading of gel was verified by Coomassie blue staining. Gels were transferred to polyvinylidene difluoride membrane (PVDF) (NEN Life Science Products, Boston, MA). Blots were blocked (3% bovine serum albumin (BSA), 1% Triton X in phosphate-buffered saline (PBS)) and incubated with primary antibody overnight at 4°C (1:1000 anti-eNOS; 1:500 anti-iNOS; 1:1000 anti-nNOS), followed by washing and incubation with secondary antibody (1:3000) HRP-conjugated anti-mouse Ig (Amersham Pharmacia Biotech, Freiburg, Germany) for 60 minutes at 37°C. Blots were developed with enhanced chemiluminescence (Amersham Pharmacia Biotech).
Western Blot Analysis of VCAM
For whole protein extracts and Western blot analysis of VCAM, lung tissue was homogenized in lysis buffer (10 mmol/L Tris (pH 7.5), 10 mmol/L NaCl, 0.1 mmol/L EDTA, 0.5% Triton-X 100, 0.02% NaN3, 0.2 mmol/L PMSF), incubated for 30 minutes on ice and centrifuged for 15 minutes at 10000 × g. Before use, all buffers received a protease inhibitor cocktail (1:100 v/v; Sigma). Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay (Sigma) with BSA. Twenty μg protein/lane were separated discontinuously by SDS-PAGE using 12% gels and transferred to a polyvinyldifluoride membrane (Immobilon-P, Millipore, Eschborn, Germany). After blockade of non-specific binding sites, membranes were incubated for 2 hours at room temperature with goat polyclonal anti-VCAM (1:100; Santa Cruz Biotechnology, Heidelberg, Germany) followed by peroxidase-conjugated donkey anti-goat IgG antibody (1:40000; Santa Cruz Biotechnology) as secondary antibody. Blots were further processed as described above.
Statistical Analysis
Data are presented as mean ± SD. Statistical analysis between groups was performed using analysis of variance followed by the appropriate post-hoc comparison test. Correlation was performed using linear regression analysis (SPSS statistical software, version 11.0, SPSS Inc., Chicago, IL). A P value <0.05 was considered as statistically significant.
Results
Differential NOS Isoform Expression in WT Mice following I/R
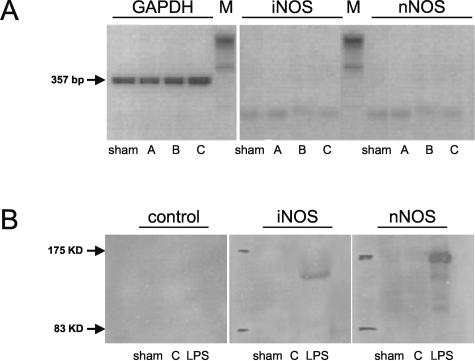
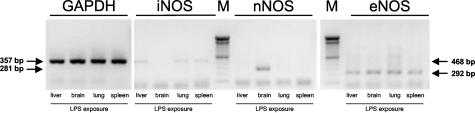
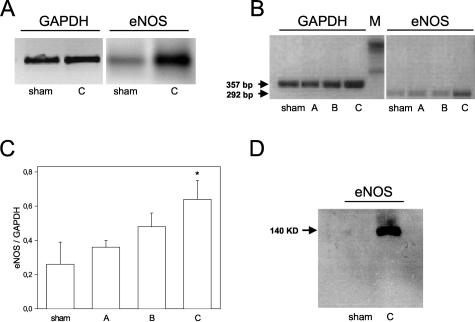
In WT mice, neither iNOS or nNOS mRNA nor iNOS or nNOS protein up-regulation on pulmonary I/R was detected in any of the groups by RT-PCR or Western blot, respectively (Figure 1, A and B). False-negative results were excluded by running positive controls of pulmonary iNOS and brain nNOS mRNA and protein after challenge of animals with LPS (Figure 1B and Figure 2). The post-ischemic eNOS expression pattern, however, showed a distinct response to pulmonary I/R (Figure 3, A to D). As assessed by RT-PCR, there was a mild increase in eNOS mRNA after 30 minutes of reperfusion (group A-WT), followed by a more pronounced increase after 5 hours of reperfusion (group B-WT; Figure 3, B and C). After 24 hours of reperfusion, induction of pulmonary eNOS mRNA synthesis had reached significantly increased levels (eNOS/GAPDH, group C-WT: 0.64 ± 0.11, P = 0.038 versus sham-WT: 0.26 ± 0.13; Figure 3, B and C). RT-PCR data are underlined by marked up-regulation of eNOS mRNA assessed by Northern blot technique (Figure 3A). Protein analysis of eNOS using immunoprecipitation and Western blot techniques further confirmed the up-regulation of this protein at 24 hours of reperfusion (Figure 3D).
Figure 1.
A: Detection of GAPDH (357 bp), iNOS (468 bp) and nNOS transcripts (281 bp) by RT-PCR in RNA extracted from sham-operated lungs (sham) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A), 5 hours of reperfusion (group B), or 24 hours of reperfusion (group C). Experiments were performed in WT animals. B: Western blot analysis of iNOS and nNOS protein in sham-operated control lung tissue (sham) and in lung tissue exposed to 1 hour of ischemia followed by 24 hours of reperfusion (group C). Protein from lung tissue (iNOS) and brain tissue (nNOS) harvested from LPS-exposed animals served as positive control (LPS). To assess unspecific background, controls without primary antibody were performed. Experiments were performed in WT animals.
Figure 2.
Detection of GAPDH (357 bp), iNOS (468 bp), nNOS (281 bp), and eNOS (292 bp) transcripts by RT-PCR in RNA extracted from liver, brain, lung, and spleen of LPS-exposed WT animals.
Figure 3.
A: Northern blot analysis of GAPDH and eNOS mRNA in sham-operated control lung tissue (sham) and in lung tissue exposed to 1 hour of ischemia followed by 24 hours of reperfusion (group C). Experiments were performed in WT animals. B: Detection of GAPDH (357 bp) and eNOS (292 bp) transcripts by RT-PCR in RNA extracted from sham-operated control lungs (sham) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A), 5 hours of reperfusion (group B), or 24 hours of reperfusion (group C). Experiments were performed in WT animals. C: Densitometric assessment of pulmonary eNOS to GAPDH transcript ratio in sham-operated control lungs (sham) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A), 5 hours of reperfusion (group B), or 24 hours of reperfusion (group C). Experiments were performed in WT animals. Mean ± SD, * P < 0.05 versus sham. D: Western blot analysis of eNOS protein in sham-operated control lung tissue (sham) and in lung tissue exposed to 1 hour of ischemia followed by 24 hours of reperfusion (group C). Experiments were performed in WT animals.
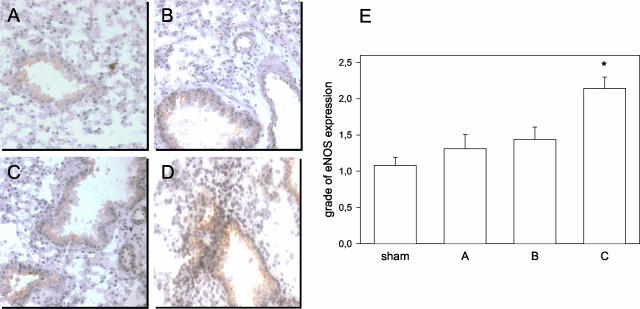
In addition, immunohistochemistry of lung tissue specimen displayed eNOS protein expression in both bronchial epithelium and vascular endothelium of lung tissue after sham operation (Figure 4A) as well as after I/R (Figure 4, B to D). Semi-quantitative scoring revealed a slight increase of eNOS expression within the first hours of reperfusion (group A-WT and B-WT; Figure 4E). At 24 hours of reperfusion, a significantly elevated grade in eNOS expression could be detected (group C-WT; Figure 4E).
Figure 4.
Immunohistochemical staining of eNOS expression in lung tissue of a sham-operated control lung (A) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (B), 5 hours of reperfusion (C), or 24 hours of reperfusion (D). Note the increase in eNOS expression on I/R in both bronchial epithelium and vascular endothelium of lung tissue (B–D), while there is only faint immunostaining for eNOS under sham conditions. Magnification, ×200. E: Average immuno-histochemical expression of pulmonary eNOS protein is displayed in sham-operated control lungs (sham) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A), 5 hours of reperfusion (group B), or 24 hours of reperfusion (group C). Experiments were performed in WT animals. For semi-quantitative scoring, see the Materials and Methods section. Mean ± SD; * P < 0.05 versus sham.
Post-Ischemic Mortality
All WT and eNOS-KO mice survived the first 5 hours of post-ischemic reperfusion. However, during later reperfusion, eNOS-KO mice developed clinical signs of dyspnoe with 25% of the animals dying in severe pulmonary edema (4 of 16). In contrast, no mortality (0 of 16) and no clinical signs of dyspnoe were observed in the WT animals.
Leukocyte Tissue Infiltration after I/R
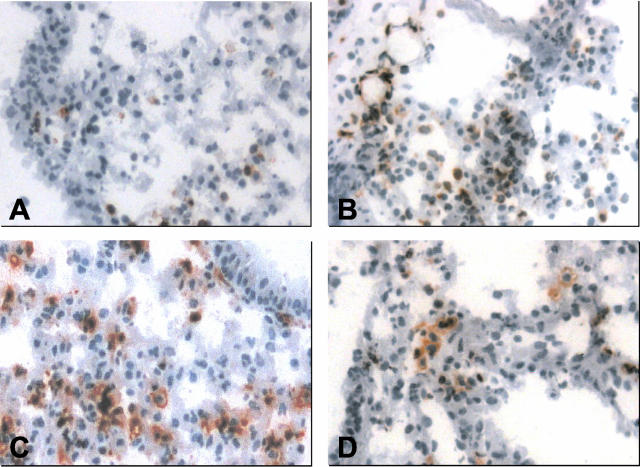
Post-ischemic reperfusion of the lung was characterized by an infiltration of CD18- and CD11a-positive leukocytes into the alveolar interstitium (Figure 5, A to D, Figure 6A). Quantitative analysis of cellular tissue infiltration revealed a 1.5-fold and almost fourfold increase at 30 minutes (Figure 5B) and 5 hours of reperfusion (Figure 5C), which significantly differed (P < 0.01) when compared with that of sham-operated control animals (Figure 5A) with approximately 200 cells/30 HPF (Figure 6A). At 24 hours of reperfusion, leukocytic tissue infiltration had abated back to values of sham-operated control animals (Figure 5D and Figure 6A). The proportion of CD18- to CD11a-positive leukocytes in WT mice did not change during reperfusion (sham-WT, 1.45 ± 0.15; group A-WT, 1.49 ± 0.21; group B-WT, 1.10 ± 0.13; group C-WT, 1.47 ± 0.18).
Figure 5.
Immunohistochemical staining of CD18-positive leukocytes in pulmonary tissue from sham-operated lungs (sham, A) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A, B), 5 hours of reperfusion (group B, C), or 24 hours of reperfusion (group C, D). Magnification, ×200.
Figure 6.
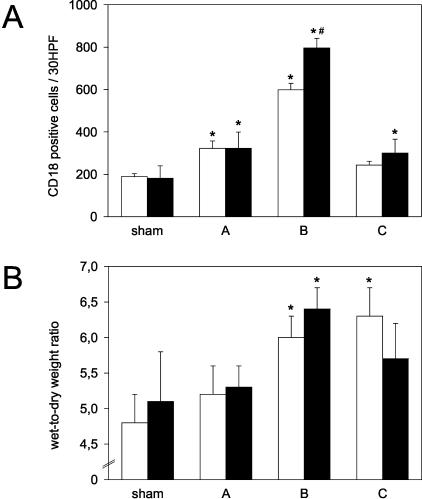
A: Quantitative assessment of the number of CD18-positive cells in alveolar interstitium of pulmonary tissue from sham-operated lungs (sham) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A), 5 hours of reperfusion (group B), or 24 hours of reperfusion (group C). Open bars indicate data from WT mice, while filled bars indicate data from eNOS-KO mice. Mean ± SD; * P < 0.05 versus sham, # P < 0.05 versus WT mice. B: Quantitative assessment of wet-to-dry weight ratio of pulmonary tissue from sham-operated lungs (sham) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A), 5 hours of reperfusion (group B), or 24 hours of reperfusion (group C). Open bars indicate data from WT mice, while filled bars indicate data from eNOS-KO mice. Mean ± SD; * P < 0.05 versus sham.
Of interest, while leukocyte tissue infiltration in WT and eNOS-KO mice did not differ at the very early reperfusion time point (groups A), eNOS-KO mice revealed a significantly higher number of infiltrating leukocytes at 5 hours of reperfusion (group B-KO, 796 ± 45 cells/30 HPF, P = 0.001 versus group B-WT, 599 ± 30 cells/30 HPF; Figure 6A). Accordingly, assessment of MPO activity revealed markedly increased levels at 5 hours of reperfusion in the eNOS-KO mice (29.5 ± 4.9 mU/g versus WT mice: 14.5 ± 7.7 mU/g), while at 24 hours of reperfusion groups did not greatly differ, reaching almost those levels found in sham-WT animals (<10 mU/g).
Lung Tissue Water Content after I/R
Wet-to-dry weight ratios showed a continuous increase during reperfusion up to markedly raised values at both 5 hours and 24 hours of reperfusion (Figure 6B). In the case of comparison of tissue water content of WT and surviving eNOS-KO mice, there was a slight tendency to higher values of wet-to-dry weight ratios in eNOS-KO mice at 5 hours of reperfusion (Figure 6B). Including the data of tissue water content of non-surviving eNOS-KO mice (6.99 ± 0.40), statistical analysis revealed a significantly higher value than in WT mice (6.03 ± 0.29, P = 0.032).
Expression of Endothelial Adhesion Molecules
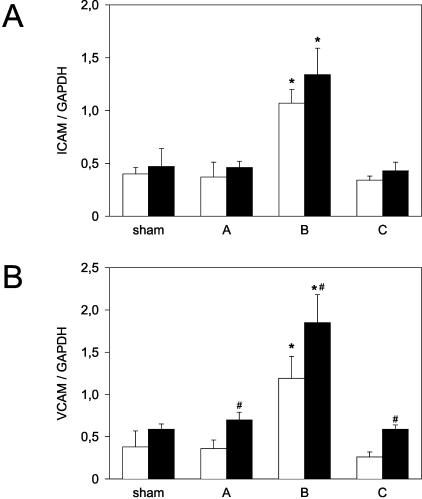
To elucidate the role of endothelial adhesion molecule expression in the eNOS-mediated inflammatory response to pulmonary I/R, we compared the post-ischemic gene transcription of ICAM-1 and VCAM in WT (Figure 7A) and eNOS-KO mice (Figure 7B). The expression of ICAM-1 mRNA in sham-operated eNOS-KO lung tissue was no different from that assessed in WT mice (Figure 8A). During post-ischemic reperfusion, we noted an increase in ICAM-1 mRNA that peaked at 5 hours and had vanished at 24 hours of reperfusion (Figure 8A). The temporal ICAM-1 transcription pattern was virtually identical in WT and eNOS-KO mice (Figure 8A).
Figure 7.
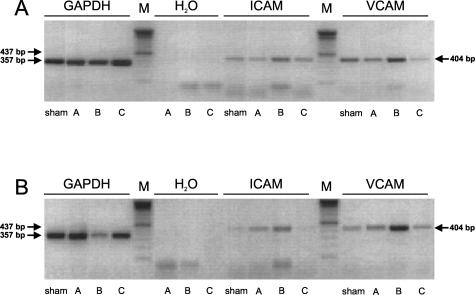
A: Detection of GAPDH (357 bp), ICAM-1 (437 bp) and VCAM (404 bp) transcripts by RT-PCR in RNA extracted from sham-operated lungs (sham) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A), 5 hours of reperfusion (group B), or 24 hours of reperfusion (group C). Samples with H2O instead of DNA template were run to exclude DNA contamination. Experiments were performed in WT animals. B: Detection of GAPDH (357 bp), ICAM-1 (437 bp), and VCAM (404 bp) transcripts by RT-PCR in RNA extracted from sham-operated lungs (sham) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A), 5 hours of reperfusion (group B), or 24 hours of reperfusion (group C). Samples with H2O instead of DNA template were run to exclude DNA contamination. Experiments were performed in eNOS-KO animals.
Figure 8.
Densitometric assessment of pulmonary (A) ICAM-1 to GAPDH transcript ratio and (B) VCAM to GAPDH transcript ratio in sham-operated control lungs (sham) and left lungs exposed to 1 hour of ischemia, followed by either 0.5 hours of reperfusion (group A), 5 hours of reperfusion (group B), or 24 hours of reperfusion (group C). Open bars indicate data from WT mice, while filled bars indicate data from eNOS-KO mice. Mean ± SD; * P < 0.05 versus sham, # P < 0.05 versus WT mice.
While baseline expression of VCAM mRNA was similar in both WT and eNOS-KO mice, the post-ischemic VCAM mRNA expression differed distinctly with a significant up-regulation at 30 minutes (0.70 ± 0.09), 5 hours (1.85 ± 0.33), and 24 hours (0.59 ± 0.05) of reperfusion when compared with adhesion molecule expression in WT mice (0.36 ± 0.10, 1.19 ± 0.26, and 0.26 ± 0.06; P < 0.05; Figure 8B), and was further underlined by a corresponding pattern of Western blot VCAM protein expression (data not shown). Linear regression analysis revealed a significant correlation between leukocyte infiltration and VCAM mRNA up-regulation (r2 = 0.941).
Discussion
In the present study, we identified eNOS as the predominant NOS isoform that is up-regulated during pulmonary I/R. Post-ischemic eNOS up-regulation appears to limit the local cellular inflammatory response, as evidenced by the increased leukocyte infiltration and enhanced MPO activities in eNOS-KO mice. The functional relevance of this observation is indicated by both the increased lung tissue water content and the higher postoperative mortality the KO animals. The mechanism linking eNOS expression and post-ischemic leukocyte infiltration seems to involve endothelial VCAM transcription, as evidenced by the higher VCAM mRNA and protein levels in post-ischemic eNOS-KO mice. It has been well established that activation and migration of polymorphonuclear leukocytes into lung tissue contributes to inflammatory tissue injury and pathological remodeling of the tissue architecture. Cellular adhesion molecules and their ligands, ie, ICAM-1, LFA-1, or selectins play an incremental role for leukocyte adhesion to vascular endothelium and transendothelial migration, and respond to a variety of pathophysiologic stimuli including I/R.1,2,15,16
In the present study, we investigated the role of endogenous NO-producing enzymes eNOS, nNOS, and iNOS in pulmonary post-ischemic inflammatory response, because NO has been implicated in the modulation of cellular trafficking into tissue.16 For instance, Lu et al11 have shown that in isolated, blood-perfused rat lungs that endogenously produced NO is protective against I/R lung injury in both normal as well as endotoxemic rats. The differential regulation of NOS isoforms has long been acknowledged, but the isoform-specific activation pattern in pulmonary I/R remains controversial. In our WT mouse model, eNOS was mainly expressed in bronchial epithelium and vascular endothelium under baseline conditions, while nNOS and iNOS were not up-regulated in pulmonary tissue unless animals were challenged by LPS. This finding is supported by studies of others, describing the LPS-dependent iNOS-expression in mouse models and isolated blood vessels.4,17 In response to I/R, however, the differential NOS isoform expression pattern seems to be different, as we detected exclusive up-regulation of eNOS both on the translational and transcriptional level. The view that eNOS is the key isoform in the non-septic pulmonary stress response is further supported by Fagan et al, 18 who demonstrated predominant up-regulation of eNOS in a mouse model of chronic severe hypoxia.
Despite the fact that the NOS isoform expression pattern in response to pulmonary I/R has been established, it remains to be determined whether the up-regulation is deleterious or represents a protective mechanism. Our hypothesis that eNOS up-regulation is a protective response to pulmonary ischemia is supported by the increased susceptibility of eNOS-KO mice for post-ischemic reperfusion injury. Those animals that died presented with severe symptoms of pulmonary edema, indicating a disruption of the endothelial-alveolar barrier with acute fluid extravasation. This was quantified and confirmed by a marked increase in wet-to-dry weight ratio in non-surviving animals. Though parameters of lung injury have been found more modest in surviving animals, it is still reasonable to speculate that eNOS deficiency is responsible for aggravation of local inflammation, as indicated by the significantly higher post-ischemic leukocyte infiltration and MPO activities in eNOS-KO versus WT mice.
Beside differences in inflammatory response and defense mechanisms, as demonstrated in the present study, eNOS-KO mice are known to differ from WT mice with respect to pulmonary hemodynamics,19 most supposedly also determining pathophysiology on I/R challenge. The complex scenario of an in vivo survival model as present does not allow clear differentiation between the multiple factors contributing to susceptibility against injury and indeed exceeds the scope of this manuscript.
Of interest, eNOS appears to exert its anti-inflammatory properties in a relatively narrow time window. Postoperative mortality occurred after 5 hours to 12 hours of reperfusion, concomitant with a maximum of histological differences between WT and eNOS-KO mice at 5 hours of reperfusion. This may indicate that eNOS modulates inflammatory cell migration via a process involving local de novo protein expression, rather than by direct chemical interference of rapidly synthesized NO with leukocyte attracting receptors. In line with this, we found marked up-regulation of VCAM mRNA and protein during reperfusion in eNOS-KO mice. Together with the finding that there is little VCAM up-regulation in WT mice with functioning eNOS signaling, this indicates that eNOS may inhibit post-ischemic pulmonary inflammatory cell migration via suppression of endothelial VCAM gene transcription. Lu et al20 described a biphasic effect of I/R on ICAM-1 expression with early down-regulation and late up-regulation after 3 hours of reperfusion in a model of blood-perfused rat lungs. Our findings principally confirm their observation, but, in contrast to VCAM, expression of ICAM-1 appears to be independent from eNOS.
Discrepancies between NOS expression and NO synthesis or classic cGMP-mediated NO effects have been noted by several groups. Vural and Oz21 observed in an in vivo rat model of pulmonary I/R that local nitric oxide synthesis was suppressed and Le Cras and McCurtry22 emphasized that hypoxia limits NO synthesis even when the expression of NOS is increased. In line with this, it has been shown that ischemic lung preservation and reperfusion is associated with increased protein contents of eNOS with, however, inhibited NOS activity.23 Moreover, it has been shown in a model of isolated perfused rat lungs that non-cGMP related mechanisms of NO are involved in maintaining post-ischemic endothelial integrity.24 Based on the apparent dissociation between NO actions and NOS expression in pulmonary I/R, one may speculate that either NOS isoforms exert their anti-inflammatory effects via an entirely NO-independent mechanism, or that NO serves as a nuclear transcription factor (or activator of a transcription factor) in concentrations that are below the detection level.
From our results, we conclude that endogenous up-regulation of eNOS is a protective mechanism against inflammatory pulmonary tissue damage following I/R, most likely involving suppression of endothelial VCAM expression and thus preventing excessive leukocytic tissue infiltration. Therefore, therapeutical strategies to augment eNOS expression may help to improve post-ischemic pulmonary function in the clinical setting.
Acknowledgments
We thank Maren Nerowski and Anna Schmicker for excellent technical assistance and Siegfried Bachmann (Hannover Medical School, Hannover, Germany) for MPO measurements.
Footnotes
Address reprint requests to Prof. Dr. Gustav Steinhoff, Klinik für Herzchirurgie, Universität Rostock, Schillingallee 35, 18057 Rostock, Germany. E-mail: gustav.steinhoff@med.uni-rostock.de.
Supported by the German Research Foundation, STE 495/4-1.
References
- Levine AJ, Parkes K, Rooney SJ, Bonser RS. The effect of adhesion molecule blockade on pulmonary reperfusion injury. Ann Thorac Surg. 2002;73:1101–1106. doi: 10.1016/s0003-4975(01)03380-x. [DOI] [PubMed] [Google Scholar]
- Kuzu MA, Koksoy C, Kuzu I, Gurhan I, Ergun H, Demirpence E. Role of integrins and intracellular adhesion molecule-1 in lung injury after intestinal ischemia-reperfusion. Am J Surg. 2002;183:70–74. doi: 10.1016/s0002-9610(01)00846-7. [DOI] [PubMed] [Google Scholar]
- Harris ES, McIntyre TM, Prescott SM, Zimmermann GA. The leukocyte integrins. J Biol Chem. 2000;275:23409–23412. doi: 10.1074/jbc.R000004200. [DOI] [PubMed] [Google Scholar]
- Hickey MJ, Granger DN, Kubes P. Inducible nitric oxide synthase (iNOS) and regulation of leucocyte/endothelial cell interactions: studies in iNOS-deficient mice. Acta Physiol Scand. 2001;173:119–126. doi: 10.1046/j.1365-201X.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- Tapiero H, Mathe G, Couvreur P, Tew KD. I. Arginine. Biomed Pharmacother. 2002;56:439–445. doi: 10.1016/s0753-3322(02)00284-6. [DOI] [PubMed] [Google Scholar]
- Kouyoumdjian C, Adnot S, Levame M, Eddahibi S, Bousbaa H, Raffestin B. Continuous inhalation of nitric oxide protects against development of pulmonary hypertension in chronically hypoxic rats. J Clin Invest. 1994;94:578–584. doi: 10.1172/JCI117372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FH, De Raeve HR, Rice TW, Stuehr DJ, Thunnissen FB, Erzurum SC. Continuous nitric oxide synthesis by inducible nitric oxide synthase in normal human airway epithelium in vivo. Proc Natl Acad Sci USA. 1995;92:7809–7813. doi: 10.1073/pnas.92.17.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbower AC, Tuder RM, Franklin WA, Pollock JS, Forstermann U, Abman SH. Maturation-related changes in endothelial nitric oxide synthase immunolocalization in developing ovine lung. Am J Physiol. 1994;267:L585–L591. doi: 10.1152/ajplung.1994.267.5.L585. [DOI] [PubMed] [Google Scholar]
- Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS. Nitric oxide synthase in human and rat lung: immunocytochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:371–377. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- Szabo C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003;140–141:105–112. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- Lu YT, Liu SF, Mitchell JA, Malik AB, Hellewell PG, Evans TW. The role of endogenous nitric oxide in modulating ischemia-reperfusion injury in the isolated, blood-perfused rat lung. Am J Respir Crit Care Med. 1998;157:273–279. doi: 10.1164/ajrccm.157.1.97-03057. [DOI] [PubMed] [Google Scholar]
- Kurose I, Wolf R, Grisham MB, Granger DN. Modulation of ischemia/reperfusion-induced microvascular dysfunction by nitric oxide. Circ Res. 1994;74:376–382. doi: 10.1161/01.res.74.3.376. [DOI] [PubMed] [Google Scholar]
- Wink DA, Hanbauer I, Krishna MC, DeGraff W, Gamson J, Mitchell JB. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci USA. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HP, Ackland-Berglund CE, Pritchard KA, Jr, Guice KS, Oldham KT. Attenuated expression of inducible nitric oxide synthase in lung microvascular endothelial cells is associated with an increase in ICAM-1 expression. J Pediatr Surg. 2001;36:1136–1142. doi: 10.1053/jpsu.2001.25731. [DOI] [PubMed] [Google Scholar]
- Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–74. [PubMed] [Google Scholar]
- Lefer AM, Lefer DJ. Nitric oxide. II. Nitric oxide protects in intestinal inflammation. Am J Physiol. 1999;276:G572–G575. doi: 10.1152/ajpgi.1999.276.3.G572. [DOI] [PubMed] [Google Scholar]
- Pulido EJ, Shames BD, Fullerton DA, Sheridan BC, Selzman CH, Gamboni-Robertson F, Bensard DD, McIntyre RC., Jr Differential inducible nitric oxide synthase expression in systemic and pulmonary vessels after endotoxin. Am J Physiol. 2000;278:R1232–R1239. doi: 10.1152/ajpregu.2000.278.5.R1232. [DOI] [PubMed] [Google Scholar]
- Fagan KA, Morrissey B, Fouty BW, Sato K, Harral JW, Morris KG, Jr, Hoedt-Miller M, Vidmar S, McMurtry IF, Rodman DM. Up-regulation of nitric oxide synthase in mice with severe hypoxia-induced pulmonary hypertension. Respir Res. 2001;2:306–313. doi: 10.1186/rr74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, Picard MH, Zapol WM. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J Clin Invest. 1998;101:2468–2477. doi: 10.1172/JCI2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YT, Chen PG, Liu SF. Time course of lung ischemia-reperfusion-induced ICAM-1 expression and its role in ischemia-reperfusion lung injury. J Appl Physiol. 2002;93:620–628. doi: 10.1152/japplphysiol.01200.2001. [DOI] [PubMed] [Google Scholar]
- Vural KM, Oz MC. Endothelial adhesivity, pulmonary hemodynamics, and nitric oxide synthesis in ischemia-reperfusion. Eur J Cardiothorac Surg. 2000;18:348–352. doi: 10.1016/s1010-7940(00)00492-9. [DOI] [PubMed] [Google Scholar]
- Le Cras TD, McMurtry IF. Nitric oxide production in the hypoxic lung. Am J Physiol. 2001;280:L575–L582. doi: 10.1152/ajplung.2001.280.4.L575. [DOI] [PubMed] [Google Scholar]
- Liu M, Tremblay L, Cassivi SD, Bai XH, Mourgeon E, Pierre AF, Slutsky AS, Post M, Keshavjee S. Alterations of nitric oxide synthase expression and activity during rat lung transplantation. Am J Physiol. 2000;278:L1071–L1081. doi: 10.1152/ajplung.2000.278.5.L1071. [DOI] [PubMed] [Google Scholar]
- Schutte H, Witzenrath M, Mayer K, Rosseau S, Seeger W, Grimminger F. Short-term “preconditioning” with inhaled nitric oxide protects rabbit lungs against ischemia-reperfusion injury. Transplantation. 2001;72:1363–1370. doi: 10.1097/00007890-200110270-00005. [DOI] [PubMed] [Google Scholar]