Abstract
Neuronal intermediate filament (IF) inclusion disease (NIFID) is a novel neurological disease of early onset with a variable clinical phenotype including frontotemporal dementia, pyramidal, and extrapyramidal signs. Pathologically, in affected areas, there is neuronal loss, astrocytosis, and neuronal intracytoplasmic aggregates of abnormal neuronal IFs that contain neither tau nor α-synuclein. Thus, to characterize the neuronal IF protein profile of inclusions in NIFID, immunohistochemistry (IHC) was performed on 10 cases of NIFID, four normal aged controls (NL), and two cases of Alzheimer’s disease (AD) using a panel of anti-neuronal IF proteins. Immunoelectron microscopy was performed on selected cases and frozen tissue from the frontal lobe of four cases was used for biochemical studies including sequential extractions and Western blotting. Based on these studies, we report here for the first time that α-internexin, a neuronal IF protein, is present within the inclusions of NIFID as are all three neurofilament subunits: heavy, medium, and light. Thus, all class IV neuronal IF proteins are present within the pathological inclusions of this disease. Biochemistry revealed that IF aggregates were soluble in sodium dodecyl sulfate (SDS) and no post-translational modification was detected when compared with Alzheimer’s disease or aged control brains. Hence, we conclude that NIFID is characterized by the pathological cytoplasmic aggregation of all class IV neuronal IF proteins in brain. The discovery of α-internexin in the cytoplasmic inclusions implicates novel mechanisms of pathogenesis in NIFID and other neurological diseases with pathological accumulations of IFs.
Many chronic progressive neurodegenerative disorders are characterized by the presence of abnormal protein aggregates in neurons and glia of the central nervous system.1–4 The identification of disease-specific abnormal protein inclusions has illuminated mechanisms of pathogenesis as well as facilitating the molecular classification of the neurodegenerative diseases. Neuronal intermediate filament (IF) inclusion disease (NIFID) is a novel neurological disease with a clinically heterogeneous phenotype including progressive early-onset dementia, pyramidal, and extrapyramidal signs. Grossly there is focal atrophy of the frontal lobes, and to a lesser degree the temporal and parietal lobes, and microscopically there are intraneuronal, cytoplasmic, neurofilament inclusions which are variably ubiquitinated but contain neither tau nor α-synuclein.5–10 The inclusions are present in both neocortex where clusters of inclusions have been reported11 and subcortical nuclei and spinal cord.
Neurofilaments (NFs) are abundant IFs of the neuronal cytoskeleton and they are composed of light (NF-L), medium (NF-M), and heavy (NF-H) subunits of approximately 68 kd, 145 kd, and 200 kd, respectively.3,12 All three subunits are phosphorylated and most of the phosphorylation sites are located in the tail domain of NF-H.13,14 The use of phosphorylation-dependent and -independent antibodies to NF epitopes has enabled the immunohistochemical dissection of these proteins and has revealed that NFs within the perikaryon and proximal segments of axons and dendrites are normally hypophosphorylated while NFs in axons are heavily phosphorylated. In neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), dementia with Lewy bodies (DLB) and motor neuron disease (MND), abnormal accumulations of phosphorylated NF proteins in the cell body have been reported,15–19 although the significance of the phosphorylation of NF proteins within the cytoplasm is unclear. However, abnormal phosphorylation may impede axonal transport and contributes to neuronal dysfunction, while constitutive phosphorylation of NFs may protect them against proteolysis.19 Mutations in NF-H and NF-L genes in MND have been associated with abnormal accumulations of NF proteins3 and transgenic mice that overexpress NF proteins, such as the NFH/lacZ mouse, have selective degeneration of Purkinje cells with Lewy body-like inclusions.20
In addition to the three NF triplet proteins, a fourth neuronal IF protein in the brain, α-internexin, has been classified as a type IV IF.21 The gene for α-internexin is located on chromosome 10 and its transcript is a 499 amino acid protein with a molecular weight of 55.4 kd and an apparent molecular weight of 66 kd on Western blots. The protein is expressed by most, if not all, neurons as they commence differentiation and precedes the expression of the NF triplet proteins.22 In the adult brain, α-internexin is expressed at relatively low levels in comparison to the NF proteins and there is selective anatomical expression with greater immunoreactivity being seen in the cerebellar granule cells, the source of thin-caliber parallel fibers,23 and in the neuron cell bodies and processes of cortical layer II neurons. α-Internexin also co-assembles with the NF triplet proteins.24 A transgenic mouse model with overexpression of rat α-internexin has been shown to cause abnormal neurofilamentous accumulations and motor coordination deficits,25 but α-internexin has not previously been identified as a major component of the pathological inclusions of any neurodegenerative disease.
Materials and Methods
Tissue Collection and Processing
Brain tissues from 10 cases of NIFID were obtained from Canada, France, Norway, Spain, Japan (one case from each), and from France, the United Kingdom and the United States (two cases from each) and all displayed the pathological features of this disease previously described (Table 1).5–8,10 Normal aged controls (four cases) and two cases of AD, a neurodegenerative disease control, were obtained from the Center for Neurodegenerative Disease Research, University of Pennsylvania School of Medicine, Philadelphia, PA. After death, the consent of the next of kin was obtained for brain removal, following Local Ethics Committee procedures. Brain tissue was preserved in buffered 10% formal saline and when available, tissue was frozen at −70°C for biochemistry.
Table 1.
Summary of Demographic Information of Cases
| Case | Sex | Age at onset (years) | Duration (years) | Age at death (years) | Brain weight (g) | PMI (hours) | Reference |
|---|---|---|---|---|---|---|---|
| NIFID-1 | F | 52 | 3 | 54 | 813 | 24 | 5 |
| NIFID-2 | F | 38 | 3 | 41 | 904 | 15 | 5 |
| NIFID-3 | F | 23 | 5 | 28 | 860 | n/a | 5 |
| NIFID-4 | M | 47 | 3 | 50 | 1,200 | n/a | 5 |
| NIFID-5 | M | 39 | 4 | 43 | 950 | n/a | 6 |
| NIFID-6 | M | 28 | 4 | 32 | n/a | n/a | 6 |
| NIFID-7 | M | 56 | 4 | 60 | 1,250 | 24 | 7 |
| NIFID-8 | M | 48 | 4 | 52 | 1,310 | 24 | 8 |
| NIFID-9 | M | 48 | 13 | 61 | 850 | 2 | 10 |
| NIFID-10 | F | 25 | 4 | 29 | 710 | n/a | — |
| Mean (range) | — | 40.4 (23–56) | 4.7 (3–13) | 45.0 (28–61) | 983 (710–1,310) | 17.8 (2–24) | — |
| NL-1 | M | — | — | 43 | 1,545 | 31 | — |
| NL-2 | M | — | — | 49 | 1,300 | 5 | — |
| NL-3 | M | — | — | 62 | 1,360 | 5 | — |
| NL-4 | M | — | — | 65 | 1,346 | 26 | — |
| Mean (range) | — | — | — | 57 (43–65) | 1,388 (1,300–1,545) | 17 (5–31) | — |
| AD-1 | F | 62 | 11 | 73 | 947 | 11 | — |
| AD-2 | F | 43 | 9 | 52 | 1,213 | 7 | — |
| Mean (range) | — | 53 (43–62) | 10 (9–11) | 63 (52–73) | 1,080 (1,080–1,213) | 9 (7–11) | — |
NIFID, AD, and neuropathologically normal (NL) cases used in this study are numbered in the left-most column.
Abbreviations: PMI, post-mortem interval; n/a, not available; M, male; F, female.
Histology and Immunohistochemistry
Tissue blocks were taken when available from representative areas including: the frontal, temporal, parietal, and occipital lobes, hippocampus, basal ganglia including the nucleus basalis of Meynert, thalamus, midbrain, pons, medulla oblongata, cerebellum, and spinal cord. Histological stains included: hematoxylin and eosin, Klüver-Barrera, thioflavine S, and a modified Bielschowsky silver impregnation. Antigen retrieval was performed by heating sections in a solution of 0.5% ethylenediaminetetraacetic acid (EDTA) in 100 mmol/L Tris (pH 7.6) at 100°C for 10 minutes. Immunohistochemistry (IHC) was undertaken on 6- to 10-μm thick sections prepared from formalin- (cases NIFID-1–7, 9–10) or 4% paraformaldehyde- (case NIFID-8) fixed, paraffin wax-embedded tissue blocks using the avidin-biotin complex detection system (Vector Laboratories, Burlingame, CA) and the chromogen 3,3′-diaminobenzidine (DAB) and sections were then counterstained with hematoxylin as previously described.26
Antibodies used included those that recognized epitopes of all class IV neuronal intermediate filament proteins: phosphorylation-dependent neurofilament heavy subunit (pNF-H, mouse monoclonal antibody (mAb) RMO 2416), non-phosphorylation-dependent (npNF-H, mAb RMd 0916), phosphorylation-independent (pind) NF-M (RMO 18915), phosphorylation-independent NF-L (rabbit polyclonal Ab NFL27), and α-internexin (mAb 2E3, Zymed Laboratories, Inc., San Francisco, CA). In addition, the following antibodies were used: α-synuclein (mAb Syn30328), phosphorylation-dependent tau (Ser202/Thr205, mAb AT8, Innogenetics, Belgium) and ubiquitin (mAb 1510) purchased from Chemicon International Inc., Temecula, CA.
Electron Microscopy
Ultrastructural studies were performed on both formalin-fixed and paraffin wax-embedded tissue. The superior frontal gyrus, an area rich in NF-positive inclusions, was identified by immunohistochemistry. Specimens were post-fixed in 1% osmium tetroxide and embedded in epoxy resin. Ultra-thin sections were cut and stained with uranyl acetate and lead citrate following standard procedures.29
Sequential Biochemical Fractionation
Gray and underlying white matter was dissected from the superior frontal gyrus and weighed. Tissue was homogenized in Hi-Salt (HS) (50 mmol/L Tris) buffer containing 10 mmol/L EDTA, 5 mmol/L MgSO4, 0.75 mol/L NaCl, 0.02 mol/L NaF, 0.5 mmol/L phenylmethyl sulfonyl fluoride (PMSF), and a cocktail of protease inhibitors, then centrifuged at 25,000 × g for 30 minutes at 4°C. Supernatants were saved as the HS fraction and pellets were washed by re-extraction in HS buffer. Resulting pellets were subjected to two sequential extractions in 10 ml/g of Triton-X (TX) buffer containing Hi-Salt, 1% Triton X-100, and protease inhibitors and centrifuged as for the HS fraction. Supernatants were saved as the TX fraction. Pellets were homogenized in buffer containing 150 mmol/L NaCL, 0.1% sodium dodecylsulfate (SDS), 0.5% sodium deoxoycholate, 1% Nonidet P-40, 50 mmol/L Tris, pH 8.0 (RIPA), and centrifuged and pelleted as above. The supernatants were saved as the RIPA fraction. Pellets were resuspended in 2% SDS in 50 mmol/L Tris with protease inhibitors, and centrifuged as above but at 15°C. Supernatants were preserved as the SDS fraction. Pellets were resuspended by sonication in 70% formic acid (FA) and centrifuged at 25,000 × g for 1 hour at 4°C. Myelin precipitate was removed before the samples were vacuum dried and saved as the FA fraction. Protein concentration was determined using the Coomassie protein assay (Pierce, Rockford, IL) and bovine serum albumin as a standard. SDS sample buffer (10 mmol/L Tris, pH 6.8, 1 mmol/L EDTA, 40 mmol/L dithiothreitol, 1% SDS, 10% sucrose) was added to samples of HS, TX, RIPA, and FA, and sample buffer without SDS (10 mmol/L Tris (pH 6.8), 1 mmol/L EDTA, 40 mmol/L dithiothreitol, 10% sucrose) was added to SDS-soluble samples, followed by heating at 100°C for 5 minutes.
Western Blot Analysis
Proteins were separated by 7.5% SDS-polyacrylamide gel electrophoresis and subsequently transferred electrophoretically to nitrocellulose membrane (Schleicher & Schuell, Keene, NH) in buffer containing 25 mmol/L Tris, 190 mmol/L glycine, and 10% methanol. Membranes were blocked with a 5% solution of powdered skimmed milk dissolved in Tris-buffered saline (50 mmol/L Tris pH 7.6; 150 mmol/L NaCl), incubated with primary antibody. Monoclonal antibody against α-internexin (mAB 2E3) was used as a primary, and mouse IgG conjugated to horse radish peroxidase was used as secondary antibodies. Enhanced Chemoluminescent reagent (ECL, Perkin Elmer LIfe Sciences, Inc., Boston) was used for the detection, and each nitrocellulose replica was exposed onto X-Omat Blue XB-1 films (Kodak, Rochester, NY). For quantitative immunoblot analysis, 125I-labeled rabbit polyclonal antibody and whole mouse IgG were used as the detection antibody. The quantitative data were generated using ImageQuant analysis software where the signal intensity correlates with pixel number as previously described.30,31
Results
Pathological Inclusions in NIFID Contain α-Internexin
Accumulations of α-internexin were observed in the cytoplasm of affected neurons and axons in the neocortex and underlying white matter of all lobes with the highest density being found in the frontal lobe (Figure 1) and only rare neuronal inclusions in the occipital lobe. Neuronal loss was neither a prominent feature of the pyramidal neurons of the hippocampus nor the granule cells of the dentate gyrus, but neuronal cytoplasmic inclusions and axonal swellings could clearly be seen in the CA1 subfield (Figure 2a) and in the granule neurons of the dentate gyrus (Figure 2b) as well as in affected gray and underlying white matter. Inclusions were also present in the basal ganglia, thalamus, and nuclei of the midbrain, pons, medulla, and, in one case, the gray matter of the spinal cord was also affected. The most abundant aggregates of α-internexin were seen in areas that had only mild or no neuronal loss. In areas of the most pronounced neuronal loss, no or few IF inclusions were seen. No aggregates of α-internexin were observed by IHC in the neuronal cytoplasm of normal aged controls, although occasional neurofibrillary tangles and dystrophic neurites of neuritic plaques in superficial layers in AD were stained (data not shown). Neurofibrillary tangles were unstained although occasional dystrophic neurites in superficial layers in AD were stained (data not shown).
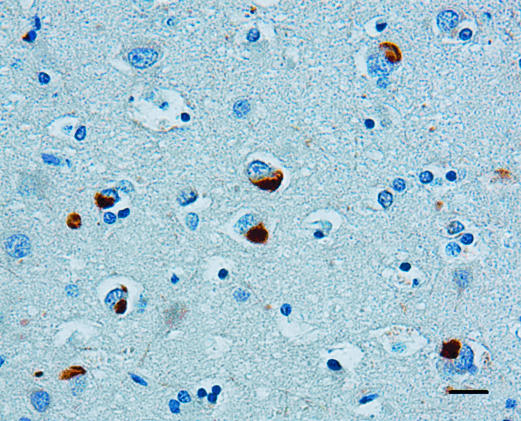
Figure 1.
Low-power photomicrograph showing neuronal intracytoplasmic inclusions in layer III of the middle frontal gyrus of a case of NIFID. The inclusions are compact and intensely stained and the nucleus is in an eccentric position. α-Internexin immunohistochemistry. Bar, 10 μm.
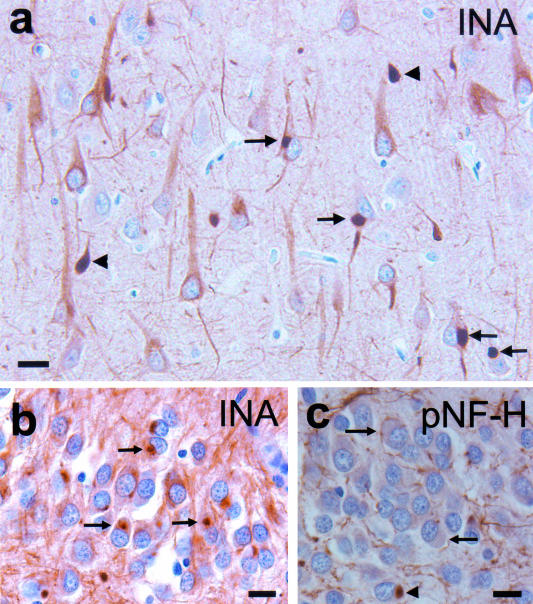
Figure 2.
Neuronal intracytoplasmic inclusions (arrows) and swollen neuronal processes (arrowheads) in the pyramidal neurons of the CA1 subfield of the hippocampus (a). Normal pale staining of α-internexin filaments can be seen in neuronal apical dendrites and axons. Intracytoplasmic inclusions in the granule cells of the dentate gyrus (b). α-Internexin immunohistochemistry (INA). Inclusions in the dentate granule cells are unstained or weakly stained (arrows) and occasionally stained (arrowhead) by an antibody recognizing epitopes of pNF-H (RMO 24) (c). Bar, 10 μm (a–c).
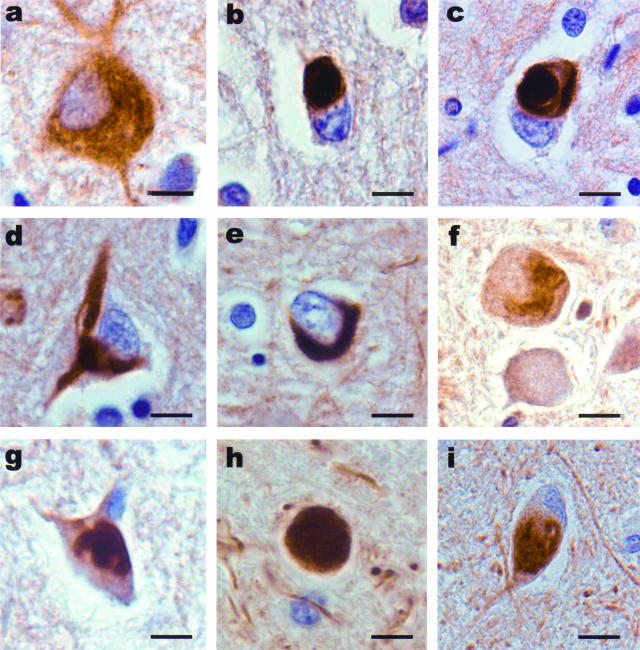
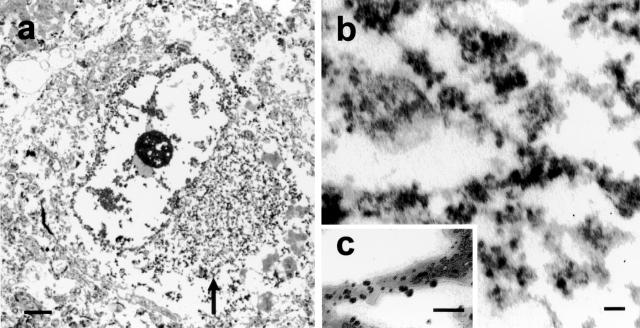
The morphology of α-internexin aggregates was extremely variable throughout the neuraxis. Achromatic neurons contained diffuse staining of presumably loosely assembled α-internexin filaments (Figure 3a). Pick body-like inclusions were the most abundant morphological type and could be seen in cortical laminae with the highest densities in layers V/VI (Figure 3b). Other morphological forms of neuronal cytoplasmic inclusions were seen in cortical and subcortical nuclei and spinal cord (Figure 3, a to i). Swollen axons and axonal swellings, similar to those found in amyotrophic lateral sclerosis and normal aging, which are not specific to any neurodegenerative disease, were also seen in affected areas and in underlying white matter, corticospinal tracts, and other white matter tracts (Figure 3h). Ultrastructural study of the neuronal cytoplasmic inclusions in NIFID revealed aggregates of granular filamentous material with no apparent limiting membrane (Figure 4a). The granular material resembled the morphology of ribosomes and the filaments had an apparent diameter of 10 to 25 nm (Figure 4b). Immunoelectron microscopy demonstrated that the filaments of the inclusions contain epitopes of NF proteins and α-internexin (Figure 4c).
Figure 3.
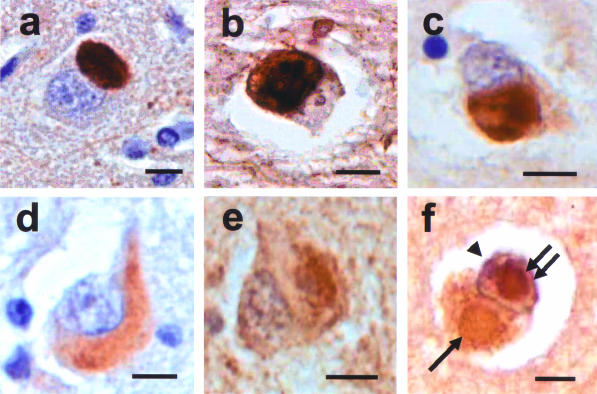
Neuronal inclusions in NIFID are pleomorphic. α-Internexin immunohistochemistry. A swollen achromatic neuron in the frontal lobe has diffuse and pale staining (a). A Pick body-like inclusion in the frontal lobe (b). A globose neurofibrillary tangle-like inclusion in layer V of the frontal lobe (c). A neurofibrillary tangle-like inclusion in a pyramidal neuron of layer III of the frontal lobe (d). A crescentic inclusion in the temporal lobe (e). A serpiginous neuronal inclusion in the inferior olivary nucleus (f). An inclusion in a neuron in the cervical spinal cord (g). Axonal spheroid in the spinal cord (h). A neuronal inclusion in the medial dorsal thalamus (i). Bars, 10 μm.
Figure 4.
An electron micrograph of a neuronal cytoplasmic inclusion (arrow) in the frontal lobe of a case of NIFID (a). The inclusion is not membrane bound and contains granular filamentous material with a diameter of 10 to 25 nm (b). Immunoelectron microscopy reveals that the filaments of the inclusion contain epitopes of α-internexin (c). Bars, 1 μm (a); 100 nm (b and c).
Pathological Inclusions in NIFID Contain All Class IV Intermediate Filament Proteins
To determine the IF protein composition of the cytoplasmic inclusions, a panel of antibodies that recognize epitopes of all class IV intermediate filaments was used. Immunohistochemistry demonstrated the presence within the inclusions of epitopes of pNF-H (RMO 24), npNF-H (RMd 09), pindNF-M (RMO 189), NF-L, and α-internexin (Figure 5, a to e). Although epitopes of all NF proteins and α-internexin were present in most neurons, some inclusions were labeled by α-internexin (Figure 2b) and only weakly, or not at all, by antibodies recognizing the NF triplet proteins (Figure 2c). The cytoplasmic inclusions were variably ubiquitinated as demonstrated by immunohistochemistry and IF and ubiquitinated inclusions were most abundant in the youngest cases (NIFID-3 and -10, data not shown). Generally, the more compact Pick body-like inclusions were ubiquitinated (Figure 5f) and variation between cases may reflect variations in tissue preservation between centers because antigen retrieval enhanced staining in some cases. Rare intranuclear inclusions were observed in neurons containing cytoplasmic inclusions and these were present in 4 of 10 cases. These intranuclear inclusions were compact and round and contained ubiquitin epitopes (Figure 5f).
Figure 5.
Neuronal cytoplasmic inclusions in NIFID contain epitopes of all class IV intermediate filament proteins. Intracytoplasmic inclusions are labeled by: α-internexin (a), pNF-H (RMO 24) (b), npNF-H (RMd 09) (c), pindNF-M (RMO 189) (d), NF-L (e), and ubiquitin (f). Intracytoplasmic inclusions are variably ubiquitinated (Figure 5f, single arrow) and rarely ubiquitinated intranuclear inclusions are present within the same neuron (double arrows), nucleolus (arrowhead). Ubiquitin immunohistochemistry. Bars, 10 μm.
Intermediate Filament Inclusions Are Soluble in SDS
To determine whether the IF proteins in the inclusions were insoluble, sequential extraction of proteins in buffers of increasing protein solubilization strengths were performed on samples from diseased brains (see Materials and Methods). Western blot analysis of biochemical fractions from NIFID frontal cortex revealed the presence of α-internexin in the SDS-soluble fraction, but not in more soluble fractions (HS, TX and RIPA, data not shown).
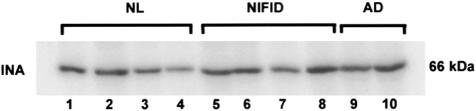
Neuronal loss in neurodegenerative disease is associated with relative loss of neuronal proteins and an increase in astrocytic, microglial, and inflammatory proteins. Therefore, to determine the relative loss of IF proteins we used quantitative Western blot analysis to determine and compare the protein levels of neuronal IF proteins in SDS-soluble fractions of the frontal lobe of four cases of NIFID, four normal aged controls, and two AD cases. The relative mobility of α-internexin on Western blots was similar to that of control and AD brain tissue (Figure 6) indicating that this protein is not post-translationally modified as a consequence of abnormal aggregation. Similarly, the mobilities of the NF proteins were indistinguishable from those of aged matched controls and AD (data not shown).
Figure 6.
α-Internexin in NIFID is soluble in SDS. Ten μl of each fraction was loaded in each lane of a 7.5% polyacrylamide gel. 125I-quantitative Western blot analysis of α-internexin (INA) in NIFID (lanes 5 to 8, cases NIFID-1, 2, 7, and 8), AD (lanes 9 and 10, cases AD-1 and 2), and normal control brains (lanes 1 to 4, cases NL-1 to 4).
Discussion
NIFID is a recently described neurological disorder5–9 and this study demonstrates for the first time that α-internexin, a neuronal class IV IF in the brain, is also a major component of the inclusions of NIFID. In addition, IHC indicates that α-internexin is present in some inclusions that are not labeled by antibodies to NF proteins. This observation may be explained, in part, by the differential expression of α-internexin in discrete neuronal populations: higher levels of expression have been reported in neurons with small caliber axons such as layer II neurons, cerebellar granule, and basket cells.21,22 However, α-internexin is expressed at relatively low levels in the adult brain when compared to the NF triplet proteins and the distribution of inclusions in our cases did not coincide with the populations of neurons that have generally higher levels of expression of this protein. The only correspondence we observed between neurons expressing higher levels of α-internexin and the density of cytoplasmic inclusions was in neocortical layer II neurons in some but not all cases. Neuronal loss and microvacuolation in the superficial layers, especially layer II, were seen to varying degrees in all cases of NIFID. As α-internexin is a neurodevelopmentally regulated protein and precedes the NF triplet proteins in axon growth,22 increased expression of α-internexin in vulnerable neurons may suggest a regenerative response. Increased expression or failure of axonal transport of α-internexin in vulnerable and degenerating neurons may contribute to the formation of pathological intracytoplasmic inclusions in this disease.25
The most abundant aggregates of α-internexin were seen in areas that had only mild or no neuronal loss. In areas of the most pronounced neuronal loss, no or few IF inclusions were seen. These observations may indicate that α-internexin aggregation is an early event in the pathogenesis of the disease and that after neurons degenerate, the inclusions are released into the extracellular space where they are rapidly cleared, unlike the extracellular “ghost” tangles formed by tau filaments that remain following degeneration of the neuron in which the tangle is located.
α-Internexin and NF immunohistochemistry, electron microscopy, and biochemistry link the neuropathology of NIFID most closely to MND. In both diseases, phosphorylated epitopes of NFs are found in chromatolytic neurons and as fibrillary inclusions in affected neurons as well as in their axons, and can be readily identified by ubiquitin and NF IHC. In both MND and MND with dementia, chromatolytic neurons, cytoplasmic fibrillary inclusions, axonal enlargements (5 to 25 μm diameter), and large swellings called spheroids (>25 mm diameter) are found most frequently in the spinal cord and they contain predominantly phosphorylated accumulations of NFs, although some inclusions also contain α-internexin. Axonal swellings and spheroids are found in normal aged spinal cords but the number of spheroids/perykarya is significantly increased in the anterior horns of spinal cords in both moderate and severe MND.16,18,32 This study demonstrates that another IF protein, α-internexin, is a major component of axonal swellings and spheroids in NIFID. While IFs are important cytoskeletal proteins, constitutive IF phosphorylation has been proposed as a mechanism protecting against proteolysis19 and the abnormal phosphorylation of NFs within the perikaryon has been proposed as a pathological mechanism in MND.18 However, using biochemical methods, one study found no difference in the physicochemical properties of NF-H extracted from MND versus the normal spinal cord suggesting that the appearance of highly phosphorylated NFs in MND neurons reflects the aberrant somatotopic localization of normally phosphorylated NF-H.32 Conversely, the distribution and number of phosphorylated neurofilamentous aggregates in NIFID was quite different from that of MND and MND with dementia. In particular, the frontal lobes and basal ganglia were preferentially affected in NIFID, and the density of intraneuronal neurofilament inclusions was unlike that seen in any case of MND or MND with dementia.
IHC studies of NFs in MND and MND with dementia have demonstrated a somatodendritic distribution of NF phosphorylation. Neurofilamentous aggregates, including cytoplasmic inclusions in the anterior horn cells, globules, and spheroids, show a pattern of NF phosphorylation that reflects a gradient of phosphorylation such that NF subunits in the perikaryon are in a primitive state of phosphorylation and become increasingly phosphorylated as they migrate to the synapse. Thus, normal dendrites and axons show the strongest NF immunoreactivity with pNF antibodies and neuronal perikarya expressing predominantly hypophosphorylated epitopes of all NF subunits. In NIFID, the neuronal cytoplasmic pleomorphic inclusions contained predominantly α-internexin and phosphorylated NFs indicating abnormal cellular localization of these proteins. Thus, the cytoplasmic location of pNF aggregates in NIFID contrasts with the constitutive axonal distribution of these proteins and their presence in axonal swellings and spheroids in aging and MND.
NF accumulations have been reported in several human neurological diseases including MND, PD, DLB, progressive supranuclear palsy, Charcot-Marie-Tooth (CMT) disease, diabetic neuropathy, and giant axonal neuropathy.3 We report for the first time the presence of another IF, α-internexin, in the pathological inclusions of a novel neurological disease, NIFID, with features of dementia and MND. Although mutations in NF genes have been associated with CMT disease, PD, and MND, none of our cases had a family history of neurological or psychiatric disease. On the other hand, the early age at onset might signify that NIFID is a recessive genetic disorder; this idea remains to be examined in more detail.
The mechanisms leading to neuronal IF aggregation in the cytoplasm and proximal axons of NIFID are unknown. This study shows that α-internexin is a component of the pathological inclusions in NIFID. Although NFs aggregate in neurodegenerative diseases, the role of α-internexin in this process is currently unknown. It is possible that a failure of axonal transport may contribute to the abnormal cytoplasmic accumulation, and transgenic mouse models have demonstrated abnormal NF accumulations indicating that this is a primary cause of accumulation. Toxic insults, including elevated levels of the neurotransmitter glutamate, may also contribute to decreased axonal transport and NF accumulation.3,33 The role of α-internexin in the pathogenesis of NFID and other neurodegenerative disorders characterized by pathological aggregates of IF remains to be elucidated.
Acknowledgments
We thank the staff of the Center for Neurodegenerative Disease Research for technical support and the Biomedical Core Facility of the University of Pennsylvania for assistance with the electron microscopic studies. We also thank the families of patients whose generosity made this research possible.
Footnotes
Address reprint requests to Nigel J. Cairns, Ph.D., MRCPath, Center for Neurodegenerative Disease Research; University of Pennsylvania School of Medicine; 3600 Spruce Street, Philadelphia, PA 19104-4283 USA. E-mail: cairns@mail.med.upenn.edu.
Support for this work was provided by grants from the National Institute on Aging of the National Institutes of Health (AG-09215, AG-10124, AG-17586 to V.M.-Y.L. and J.Q.T., and AG-10130 and ES12068 to M.G.), and from the Wellcome Trust (GR066166AIA) to N.J.C. V.M.-Y.L. is the John H. Ware, 3rd Professor of Alzheimer’s Research and J.Q.T. is the William Maul Measey-Truman G. Schnabel, Jr. M.D., Professor of Geriatric Medicine and Gerontology of the Center for Neurodegenerative Disease Research; University of Pennsylvania School of Medicine.
References
- Lee VM-Y, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Serpell LC, Berriman J, Smith MJ, Jakes R, Crowther RA. From genetics to pathology: tau and α-synuclein assemblies in neurodegerative diseases. Philos Trans R Soc Lond B Biol Sci. 2001:213–227. doi: 10.1098/rstb.2000.0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Chalabi A, Miller CCJ. Neurofilaments and neurological disease. BioEssays. 2003;25:346–365. doi: 10.1002/bies.10251. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Dickson D. Update on the neuropathological diagnosis of frontotemporal dementia. J Neuropathol Exp Neurol. 2001;60:1123–1126. doi: 10.1093/jnen/60.12.1123. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Perry RH, Jaros E, Burn D, McKeith IG, Lowe JS, Holton J, Rossor MN, Skullerud K, Duyckaerts C, Cruz-Sanchez FF, Lantos PL. Patients with a novel neurofilamantopathy: dementia with neurofilament inclusions. Neurosci Lett. 2003;341:177–180. doi: 10.1016/s0304-3940(03)00100-9. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Mokhtari K, Fontaine B, Hauw J-J. Maladie de Pick généralisée: une démence mal nommée caractérisée par des inclusions neurofilamentaires. Rev Neurol (Paris) 2003;159:219–219. [Google Scholar]
- Bigio EH, Lipton AM, White CL, III, Dickson DW, Hirano A. Frontotemporal dementia and motor neurone degeneration with neurofilament inclusion bodies: additional evidence for overlap between FTD and ALS. Neuropathol Appl Neurobiol. 2003;29:239–253. doi: 10.1046/j.1365-2990.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- Gearing M, Castellano AA, Hunter SB, Brat DJ, Glass JD. Unusual neuropathologic findings in a case of primary lateral sclerosis. J Neuropathol Exp Neurol. 2003;62:555–555. [Google Scholar]
- Josephs KA, Holton JL, Rossor MN, Braendgaard H, Ozawa T, Fox NC, Petersen RC, Pearl GS, Ganguly M, Rosa P, Laursen H, Parisi JE, Waldemar G, Quinn NP, Dickson DW, Revesz T. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126:2291–2303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- Yokoo H, Oyama T, Hirato J, Sasaki A, Nakazato Y. A case of Pick’s disease with unusual neuronal inclusions. Acta Neuropathol (Berl) 1994;88:267–272. doi: 10.1007/BF00293404. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Armstrong RA. Clustering of neurofilament inclusions in the temporal lobe in dementia with neurofilament inclusions. Acta Neuropathol (Berl) 2003;106:125–128. doi: 10.1007/s00401-003-0710-5. [DOI] [PubMed] [Google Scholar]
- Lee K, Cleveland DW. Neuronal intermediate filaments. Annu Rev Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and non-phosphorylated forms of neurofilaments. Proc Natl Acad Sci USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM-Y, Carden MJ, Schlaepfer WW, Trojanowski JQ. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987;7:3478–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ML, Murray J, Lee VM-Y, Hill WD, Wertkin A, Trojanowski JQ. Epitope map of neurofilament protein domains in cortical and peripheral nervous system Lewy bodies. Am J Pathol. 1991;139:53–65. [PMC free article] [PubMed] [Google Scholar]
- Schmidt ML, Carden MJ, Lee VM-Y, Trojanowski JQ. Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest. 1987;56:282–294. [PubMed] [Google Scholar]
- Manetto V, Sternberger NH, Perry G, Sternberger LA, Gambetti P. Phosphorylation of neurofilaments is altered in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1988;47:642–653. doi: 10.1097/00005072-198811000-00007. [DOI] [PubMed] [Google Scholar]
- Leigh PN, Dodson A, Swash M, Brion J-P, Anderton BH. Cytoskeletal abnormalities in motor neuron disease: an immunohistochemical study. Brain. 1989;112:521–535. doi: 10.1093/brain/112.2.521. [DOI] [PubMed] [Google Scholar]
- Goldstein E, Sternberger NM, Sternberger LA. Phosphorylation protects neurofilaments against proteolysis. J Neuroimmunol. 1987;14:149–160. doi: 10.1016/0165-5728(87)90049-x. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Nakamura M, McIntosh TK, Saatman KE, Sampathu D, Raghupathi R, Lee VM-Y, Trojanowski JQ. Neurofilament-rich intraneuronal inclusions exacerbate neurodegenerative sequelae of brain trauma in NFH/LacZ transgenic mice. Exp Neurol. 2000;165:77–89. doi: 10.1006/exnr.2000.7461. [DOI] [PubMed] [Google Scholar]
- Ching GY, Liem RKH. Structure of the gene for the neuronal intermediate filament protein α-internexin and functional analysis of its promoter. J Biol Chem. 1991;29:19459–19468. [PubMed] [Google Scholar]
- Kaplan MP, Chin SSM, Fliegner KH, Liem RKH. α-Internexin, a novel neuronal intermediate filament protein, precedes the low molecular weight neurofilament protein (NF-L) in the developing brain. J Neurosci. 1990;10:2735–2748. doi: 10.1523/JNEUROSCI.10-08-02735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegner KH, Kaplan MP, Wood TL, Pintar JE, Liem RKH. Expression of the gene for the neuronal intermediate filament protein α-internexin coincides with the onset of neuronal differentiation in the developing rat nervous system. J Comp Neurol. 1994;342:161–173. doi: 10.1002/cne.903420202. [DOI] [PubMed] [Google Scholar]
- Ching GY, Liem RKH. Roles of head and tail domains in α-internexin’s self-assembly and coassembly with the neurofilament triplet proteins. J Cell Sci. 1998;111:321–333. doi: 10.1242/jcs.111.3.321. [DOI] [PubMed] [Google Scholar]
- Ching GY, Chien C-L, Flores R, Liem RKH. Overexpression of α-internexin causes abnormal neurofilamentous accumulations and motor coordination deficits in transgenic mice. J Neurosci. 1999;19:2974–2986. doi: 10.1523/JNEUROSCI.19-08-02974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos PL, Cairns NJ, Khan MN, King A, Revesz T, Janssen JC, Morris H, Rossor MN. Neuropathologic variation in frontotemporal dementia due to the intronic tau 10(+16) mutation. Neurology. 2002;58:1169–1175. doi: 10.1212/wnl.58.8.1169. [DOI] [PubMed] [Google Scholar]
- Carden MJ, Trojanowski JQ, Schlaepfer WW, Lee VM-Y. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987;7:3499–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Hill WD, Lee VM-Y, Hurtig HI, Murray JM, Trojanowski JQ. Epitopes located in spatially separate domains of each neurofilament subunit are present in Parkinson’s disease Lewy body. J Comp Neurol. 1991;309:150–160. doi: 10.1002/cne.903090111. [DOI] [PubMed] [Google Scholar]
- Lee VM-Y, Wang J, Trojanowski JQ. Purification of paired helical filament tau and normal tau from human brain tissue. Methods Enzymol. 1999;309:81–89. doi: 10.1016/s0076-6879(99)09008-4. [DOI] [PubMed] [Google Scholar]
- Zhukareva V, Vogelsberg-Ragaglia V, Van Deerlin VM, Bruce J, Shuck T, Grossman M, Clark CM, Arnold SE, Masliah E, Galasko D, Trojanowski JQ, Lee VM. Loss of brain tau defines novel sporadic and familial tauopathies with frontotemporal dementia. Ann Neurol. 2001;49:165–175. doi: 10.1002/1531-8249(20010201)49:2<165::aid-ana36>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Strong WL, Jaffe H, Traggert B, Sopper MM, Pant HC. Phosphorylation state of the native high-molecular-weight neurofilament subunit protein from cervical spinal cord in sporadic amyotrophic lateral sclerosis. J Neurochem. 2001;76:1315–1325. doi: 10.1046/j.1471-4159.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- Durham HD. Aggregation of intermediate filaments by 2,5-hexanedione: comparison of effects on neurofilaments, GFAP filaments, and vimentin filaments in dissociated cultures of mouse spinal cord dorsal root ganglia. J Neuropathol Exp Neurol. 1988;47:432–442. doi: 10.1097/00005072-198807000-00004. [DOI] [PubMed] [Google Scholar]