Abstract
Two novel sugars, 2-acetamido-1,3,6-tri-O-acetyl-2,4-dideoxy-α- and β-d-xylo-hexopyranoses, have been synthesized and their effects on heparan sulfate biosynthesis using primary mouse hepatocytes in tissue culture have been assessed. At concentrations of 0.1 and 1.0 mmol/L a mixture of both anomers significantly inhibited the biosynthesis of heparan sulfate by 60% and 99%, respectively. At 1.0 mmol/L the average molecular weight of the heparan sulfate synthesized is reduced from 77 kd to 40 kd. The biosynthetic inhibition is apparent within 1 hour (the earliest time point examined) of exposure of the hepatocytes to the analogues and appears virtually complete throughout a 24-hour incubation period. Using a radiolabeled version of the β-anomer we demonstrate that the analogue is incorporated into growing heparan sulfate chains. The nature of the analogue, the quantity of analogue isotope incorporated, and the reduction in the size of the heparan sulfate polysaccharide are consistent with UDP activation and incorporation of the analogue and truncation of the growing heparan sulfate chain. At 0.1 mmol/L, and in the presence of a constant concentration of serum amyloid A (the precursor to AA amyloid), each analogue inhibited amyloid deposition (by 95 to 99%) in a tissue culture model of AA amyloidogenesis. At 6 mg/dose twice daily each analogue inhibited in vivo splenic AA amyloid deposition by 65 to 70% when using a rapid induction model of mouse AA amyloidogenesis. These data indicate that polysaccharides, such as heparan sulfate, play an integral part in the pathogenesis of AA amyloid deposition, and potentially other forms of amyloid. These data support our previous work that demonstrated that agents that mimic aspects of heparan sulfate structure and that interfere with heparan sulfate:amyloid protein binding inhibit AA amyloid deposition. They emphasize that heparan sulfate likely plays a critical role in amyloidogenesis, and compounds that interfere with heparan sulfate biosynthesis may provide leads for the development of anti-amyloid therapeutic agents.
For centuries it has been known that white waxy deposits can accumulate in tissues of victims of persistent inflammatory diseases such as tuberculosis, osteomyelitis, and lung abscesses. Some 150 years ago Rudolf Virchow1 coined the term “amyloid” (meaning starch-like) to describe such deposits. He found that their original appearance, suggestive of the surface of a cut raw potato, altered to dark blue or black when an amyloid deposit was allowed to react with iodine in the presence of sulfuric acid, the same reaction as is found with starch or cellulose. The inference was that in situ such deposits are characterized by the presence of a carbohydrate. A century and a half later, we can add that in situ amyloid deposits also possess further unique structural and staining features.2 Some dyes with affinity for protein stain amyloid pink, and when viewed microscopically in natural light appears amorphous. But amyloid deposits have a particular affinity for Congo Red and when so stained exhibit a characteristic red-green birefringence in polarized light. This property is useful diagnostically and indicates an underlying substructure in amyloid.
Electron microscopy eventually revealed the fibrillar nature of this substructure. Amyloid fibrils are 7 to 10 nm in diameter, vary considerably in length, and were found to be either randomly disposed or organized into parallel arrays. Data obtained from X-ray diffraction and infrared spectroscopy examination of fibrils extracted from tissue further indicated that the protein in such fibrils is organized as a crossed β-pleated sheet. From such a unique set of structural and staining properties it was originally natural to infer that the protein component of amyloid was always the same, regardless of the clinical context in which it was found. Data collected since the 1970’s, however, has shown that there are in fact at least 24 different proteins capable of forming amyloid fibrils in living tissue.3 The type of protein deposited [eg, Aβ associated with Alzheimer’s disease, islet amyloid polypeptide associated with type II diabetes, or serum amyloid A (SAA) in persistent inflammatory disorders] is a function of the disease or pathological process with which the specific protein is associated.
The disease/pathological process-specific proteins of amyloids have received the lion’s share of investigational attention. But there is also substantial evidence that additional molecular components in amyloids play critical roles in amyloidogenesis in vivo. A common set of such constituents has been identified, regardless of the amyloid protein involved.4,5 These include serum amyloid P, laminin, collagen IV, entactin, heparan sulfate proteoglycan (HSPG), and apolipoprotein E (apo E). Apart from apo E, these are all building blocks of tissue basement membranes, the usual initial anatomical site of amyloid deposition.6,7
It has been found that mice lacking the gene for either apo E or serum amyloid P are still capable of forming amyloid,8–11 although the time-lag before the onset of deposition, and the subsequent rate of accumulation is slower than in mice possessing these genes. These gene knockout studies thus indicate that, although serum amyloid P and apo E are not absolute requirements for amyloidogenesis, they do play a role in the rate of initiation and subsequent progression of this process. Of the other components only HSPG has received significant attention.
Because it was recently shown that gene knockouts of the enzymes responsible for HS elongation are lethal during embryonic development,12,13 it does not currently seem possible to match, in the case of HS, the investigation of the effects of apo E or serum amyloid P knockouts on amyloid deposition. The purpose of the present work has therefore been to design and synthesize compounds that might instead effect a chemical knockout of HS biosynthesis, and then test such compounds for their possible anti-AA amyloid effects during amyloid induction.
Materials and Methods
Synthesis and Structural Characterization of Glucosamine Analogues
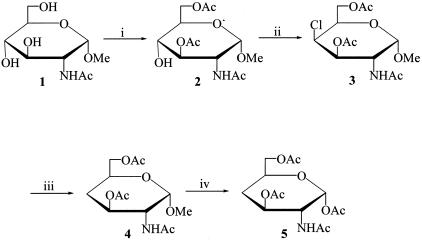
The desired glucosamine analogues were synthesized as shown in Figures 1 and 2.
Figure 1.
Synthesis of 2-acetamido-1,3,6-tri-O-acetyl-2,4-dideoxy-α-d-xylo-hexopyranose (compound 5). Reagents: (i) AcCl, pyr; (ii) SO2Cl2, pyr; (iii) Bu3SnH, VAZO, toluene; (iv) Ac2O, H2SO4.
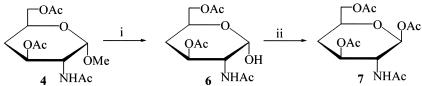
Figure 2.
Synthesis of 2-acetamido-1,3,6-tri-O-acetyl-2,4-dideoxy-β-d-xylo-hexopyranose (compound 7). Reagents: (i) H2SO4, Ac2O; (ii) Ac2O, ZnCl2.
General Methods
Melting points were determined on a Fisher-Johns apparatus and are uncorrected. Optical rotations were measured with a Perkin-Elmer 241 polarimeter for solutions in a 1-dm cell at room temperature. Thin-layer chromatography was performed on Brinkmann precoated aluminum-backed sheets of Silica Gel 60 F254 (Merck). 1H and 13C NMR spectra were recorded at room temperature on a Bruker 400 AVANCE spectrometer at 400.1 and 100.6 MHz, respectively. The signals arising from residual protons in the deuterated solvents were used as internal standards. Chemical shifts (δ) are reported in ppm downfield from tetramethylsilane for 1H and 13C spectra.
Methyl 2-Acetamido-3,6-Di-O-Acetyl-2-Deoxy-α-d-Glucopyranoside (Compound 2)
Acetyl chloride (2.1 equivalents, 6.35 ml) was added dropwise for 45 minutes to a solution of methyl 2-acetamido-2-deoxy-α-d-glucopyranoside (compound 1)14 (10.0 g, 42.5 mmol) in anhydrous pyridine (250 ml) maintained at −45°C under an argon atmosphere. The reaction mixture was allowed to warm to room temperature overnight. MeOH (20 ml) was then added and the mixture was concentrated under reduced pressure and co-evaporated with toluene to dryness. The crude oil was partitioned between CHCl3 (250 ml) and 1 N HCl (150 ml). The organic layer was then washed twice with 1 N HCl (100 ml), dried over anhydrous Na2SO4, and filtered. The solvent was evaporated under reduced pressure. The resultant crude, amber oil was triturated with hot EtOAc to afford compound 2 as a white powder (6.64 g, 48.0%): Rf 0.66 (1:9 MeOH-EtOAc); mp 179 to 180°C; [∝]D +52.0° (c 0.05, CHCl3); 1H NMR (CDCl3): δ 1.93 (s, 3 H, Ac), 2.06 (s, 3 H, Ac), 2.10 (s, 3 H, Ac), 3.38 (s, 3 H, OMe), 3.58 (apparent t, 1 H, J3,4 = J4,5 9.5 Hz, H-4), 3.76 (m, 1 H, J5,6 2.2, J5,6′ 4.3 Hz, H-5), 4.21 (apparent td, 1 H, J2,3 10.2 Hz, H-2), 4.29 (dd, 1 H, J6,6′ 12.1 Hz, H-6), 4.37 (dd, 1 H, J6,6′ 12.1 Hz, H-6′), 4.67 (d, 1 H, J1,2 3.6 Hz, H-1), 5.05 (apparent d, 1 H, J2,NH 9.6 Hz, NH); 13C NMR (CDCl3): δ 20.8 (COCH3), 20.9 (COCH3), 23.1 (COCH3), 51.7 (C-2), 55.2 (OMe), 62.9 (C-6), 68.3 (C-4), 69.8 (C-5), 73.7 (C-3), 98.4 (C-1), 170.2 (C = O), 171.5 (C = O), 172.1 (C = O). Anal. Calcd for C13H21NO8: C, 48.90; H, 6.63; N, 4.39. Found: C, 48.81; H, 6.43; N, 4.15.
Methyl 2-Acetamido-3,6-Di-O-Acetyl-4-Chloro-2,4-Dideoxy-α-d-Galactopyranoside (Compound 3)
To a solution of compound 2 (10.0 g, 31.3 mmol) in anhydrous pyridine (150 ml) at 0°C under an argon atmosphere was added SO2Cl2 dropwise for 10 minutes. The bright-yellow solution was kept in a refrigerator overnight and then at room temperature for 3 hours. The dark-brown mixture was concentrated under reduced pressure and co-evaporated with toluene to dryness. The brown sludge was partitioned between CHCl3 (250 ml) and 1 N HCl (150 ml). The organic layer was washed twice with 1 N HCl (100 ml), dried over anhydrous Na2SO4, and filtered. The solvent was evaporated under reduced pressure. The resultant amber oil was crystallized from EtOH to afford compound 3 (11.4 g, 83.1%) as fine white needles: Rf 0.55 (1:9 MeOH-EtOAc); [∝]D +148.7° (c 0.05, MeOH); mp 122 to 123°C; 1H NMR (CDCl3): δ 1.97 (s, 3 H, Ac), 2.08 (s, 3 H, Ac), 2.10 (s, 3 H, Ac), 3.40 (s, 3 H, OMe), 4.19 to 4.31 (m, 3 H, H-5, H-6, H-6′), 4.40 (d, 1 H, J3,4 = J4,5 3.5 Hz, H-4), 4.69 (apparent td, 1 H, J2,3 10.8 Hz, H-2), 4.75 (d, 1 H, J1,2 3.7 Hz, H-1), 5.18 (dd, 1 H, J2,3 10.8, J3,4 3.5 Hz, H-3), 5.62 (d, 1 H, J2,NH 9.6 Hz, NH); 13C NMR (CDCl3): δ 20.7 (COCH3), 20.8 (COCH3), 23.3 (COCH3), 47.3 (C-2), 55.4 (OMe), 58.7 (C-4), 63.5 (C-6), 66.8 (C-5), 69.3 (C-3), 98.6 (C-1), 169.8 (C = O), 170.4 (C = O), 171.0 (C = O). Anal. Calcd for C13H21ClNO7: C, 46.23; H, 5.97; Cl, 10.50; N, 4.15. Found: C, 46.19; H, 5.87; Cl, 10.33; N, 4.08.
Methyl 2-Acetamido-3,6-Di-O-Acetyl-2,4-Dideoxy-α-d-Xylo-Hexopyranoside (Compound 4)
A solution of compound 3 (5.0 g, 14.8 mmol), tri-n-butyltin hydride (2 equivalents, 7.96 ml) and a catalytic amount of 1,1′-azobis(cyclohexanecarbonitrile) (VAZO, 50 mg) in anhydrous toluene under an argon atmosphere was heated to reflux temperature; after 2 hours the solution was cooled to room temperature. The resultant precipitate was collected by filtration to afford compound 4 (3.9 g, 87.6%) as feathery white needles: Rf 0.58 (1:9 MeOH-EtOAc); [∝]D + 88.4° (c 0.05, CHCl3), lit. 88.8° (c 1, CHCl3);15 mp 159 to 160° C, lit. mp 158 to 160°C;15 1H NMR (CDCl3): δ 1.65 to 1.72 (apparent q, 1 H, J3, 4ax = J4ax,4eq = J4ax,5 12.0 Hz, H-4ax), 1.98 (s, 3 H, Ac), 2.00 to 2.08 (m, 4 H, H-4eq, Ac), 2.11 (s, 3 H, Ac), 3.39 (s, 3 H, OMe), 4.02 to 4.04 (m, 1 H, H-5), 4.15 (apparent d, 2 H, J 4.8 Hz, H-6, H-6′), 4.20 (apparent td, 1 H, H-2), 4.75 (d, 1 H, J1,2 3.4 Hz, H-1), 5.15 (apparent td, 1 H, J2,3 = J3,4ax 10.9 Hz, H-3), 5.70 (d, 1 H, J2,NH 9.4 Hz, NH); 13C NMR (CDCl3): δ 20.7 (COCH3), 20.9 (COCH3), 23.3 (COCH3), 32.8 (C-4), 52.4 (C-2), 55.1 (OMe), 65.6 (C-5), 65.7 (C-6), 68.5 (C-3), 99.1 (C-1), 169.9 (C = O), 170.6 (C = O), 171.1 (C = O). Anal. Calcd for C13H21NO7: C, 51.48; H, 6.98; N, 4.62. Found: C, 51.27; H, 7.12; N, 4.56.
2-Acetamido-1,3,6-Tri-O-Acetyl-2,4-Dideoxy-α-d-Xylo-Hexopyranose (Compound 5)
To a suspension of compound 4 (2.5 g, 8.2 mmol) in acetic anhydride (35 ml) at 0°C was added H2SO4 (0.5 ml). The clear solution was warmed to room temperature and after 48 hours was poured into an ice-distilled water (250 ml) mixture. The aqueous solution was neutralized with NaHCO3 and then extracted five times with CHCl3 (150 ml). The organic layers were combined, dried over anhydrous Na2SO4, and filtered. The resultant amber oil was recrystallized from EtOAc to afford the byproduct compound 6 (0.6 g, 23.9%) as a white solid. Recrystallization of the residue from the mother liquor from EtOAc-Et2O afforded compound 5 (1.2 g, 45.0%) as white crystals: Rf 0.54 (1:9 MeOH-EtOAc); [∝]D +96.7° (c 0.05, CHCl3); mp 111 to 112°C; 1H NMR (CDCl3): δ 1.64 to 1.80 (apparent q, 1 H, J3, 4ax = J4ax,4eq = J4ax,5 12.2 Hz, H-4ax), 1.94 (s, 3 H, OAc), 2.01 to 2.08 (m, 7 H, H-4eq, 2 Ac), 2.16 (s, 3 H, Ac), 4.08 to 4.15 (m, 3 H, H-5, H-6, H-6′), 4.30 (apparent td, 1 H, J2,NH 9.2, J1,2 3.5 Hz, H-2), 5.18 (apparent td, 1 H, J2,3 = J3,4ax 10.9, J3,4eq 4.8 Hz, H-3), 5.58 (d, 1 H, J2,NH 9.2 Hz, NH), 6.18 (d, 1 H, J1,2 3.5 Hz, H-1); 13C NMR (CDCl3): δ 20.7, 21.0, and 23.1 (3 OCOCH3 and NCOCH3), 32.5 (C-4), 51.7 (C-2), 65.3 (C-6), 67.6 (C-5), 67.7 (C-3), 91.8 (C-1), 168.9, 170.1, 170.7, and 171.5 (3 OCOCH3, NC = O). Anal. Calcd for C14H21NO7: C, 50.75; H, 6.93; N, 4.23. Found: C, 50.62; H, 6.30; N, 4.17.
2-Acetamido-3,6-Di-O-Acetyl-2,4-Dideoxy-α-d-Xylo-Hexopyranose (Compound 6)
Compound 6 was obtained as either a byproduct of the synthesis of compound 5 or directly, in a separate experiment, by treating the acetic anhydride-H2SO4 solution of compound 4 (1.00 g, 3.3 mmol) after the 48-hour reaction period with distilled water (10 ml), which was added dropwise throughout a 0.5-hour period, and then pouring the solution into an ice-distilled water mixture. The mixture was then processed as described for the preparation of compound 5. Crystallization from EtOAc afforded compound 6 (0.59, 62.5%) as a white solid: Rf 0.55 (1:9 MeOH-EtOAc); [∝]D +67.1° (c 0.01, MeOH); mp 174 to 175 °C; 1H NMR (CDCl3): δ 1.63 to 1.72 (apparent q, 1 H, J3, 4ax = J4ax,4eq = J4ax,5 12.0 Hz, H-4ax), 1.97 (s, 3 H, Ac), 2.03 to 2.05 (m, 4 H, H-4eq, Ac), 2.09 (s, 3 H, Ac), 4.08 to 4.17 (m, 3 H, H-2, H-6, H-6′), 4.26 to 4.32 (m, 1 H, H-5), 5.23 (apparent td, 1 H, J2,3 = J3,4ax 11.0 Hz, J3,4eq 4.9 Hz, H-3), 5.29 (d, 1 H, J1,2 3.2 Hz, H-1), 5.85 (d, 1 H, J2,NH 9.2 Hz, NH); 13C NMR (CDCl3): δ 20.8 (COCH3), 21.0 (COCH3), 23.2 (COCH3), 33.0 (C-4), 52.8 (C-2), 65.7 (C-5), 65.9 (C-6), 68.1 (C-3), 92.6 (C-1), 170.4 (C = O), 170.8 (C = O), 171.3 (C = O). Anal. Calcd for C12H19NO7: C, 49.82; H, 6.62; N, 4.84. Found: C, 49.77; H, 6.72; N, 4.74.
2-Acetamido-1,3,6-Tri-O-Acetyl-2,4-Dideoxy-β-d-Xylo-Hexopyranose (Compound 7)
A solution of compound 6 (0.5 g, 1.7 mmol) and ZnCl2 (100 mg) in acetic anhydride was kept at 50°C for 1 hour and then NaOAc (0.57 g, 5 equivalents) was added to the warm mixture. After a further hour the mixture was poured into an ice-distilled water solution (200 ml) and processed as described for compound 5. The crude oil mixture of α- and β-anomers was crystallized from EtOAc to afford pure compound 7 (0.27 g, 47.3%) as a white solid: Rf 0.54 (1:9 MeOH-EtOAc); [∝]D +0.2° (c 0.05, CHCl3); mp 149 to 150°C; 1H NMR (CDCl3): δ 1.59 to 1.66 (apparent q, 1 H, J3, 4ax = J4ax,4eq = J4ax,5 12.0 Hz, H-4ax), 1.86 (s, 3 H, Ac), 1.97 to 2.08 (m, 10 H, H-4eq, 3 Ac), 3.79 to 3.81 (m, 1 H, H-5), 4.01 (apparent q, 1 H, J2,NH 9.5 Hz, H-2), 4.08 (apparent d, 2 H, J 4.6 Hz, H-6, H-6′), 4.93 (apparent td, 1 H, J2,3 = J3,4ax 10.8, J3,4eq 5.0 Hz, H-3), 5.54 (d, 1 H, J1,2 8.7 Hz, H-1), 5.62 (d, 1 H, J2,NH 9.5 Hz, NH); 13C NMR (CDCl3): δ 20.7, 20.9, and 23.2 (3 OCOCH3 and NCOCH3), 32.5 (C-4), 53.7 (C-2), 65.2 (C-6), 70.1 (C-5), 70.6 (C-3), 93.2 (C-1), 169.6, 170.2, 170.6, and 171.0 (3 OCOCH3, NC = O). Anal. Calcd for C14H21NO7: C, 50.75; H, 6.93; N, 4.23. Found: C, 50.61; H, 6.14; N, 4.06.
Animals
Swiss-white CD1 8- to 10-week-old female mice were purchased from Charles River Canada, St. Constant, Quebec. Mice were kept in a temperature-controlled room on a 12-hour light/dark cycle and were fed with Purina Lab Chow pellets and water ad libitum.
Chemicals
All chemicals for biochemical or biological studies were purchased from Fisher Scientific Co. (Nepean, Ontario, Canada), Sigma Chemical Co. (St. Louis, MO), ICN (Aurora, OH), or Bio-Rad (Hercules, CA). Culture media were purchased from Life Technologies (Burlington, Ontario, Canada). Radiolabeled Na235SO4 (43 Ci/mmol), d-[3H]glucosamine hydrochloride (20.3 Ci/mmol), and l-[3H]leucine (110 Ci/mmol) were purchased from ICN.
Cell Cultures
Primary mouse hepatocytes were isolated as described previously.16,17 The viability of the cells was assessed by Trypan blue exclusion and those preparations with greater than 85% viable cells were established in culture in Williams-E medium supplemented with 1% fetal calf serum, and a 1% antibiotic/anti-mycotic mixture (penicillin, streptomycin, and amphotericin). After 24 hours the medium was replaced with fresh medium either containing or lacking varying concentrations of the novel sugar analogues as published previously.18 [3H]Glucosamine and [35S]SO4 were used to monitor the synthesis and sulfation of hepatocyte-associated HS, and [3H]leucine was used to monitor protein biosynthesis as described previously.15,19 Chondroitin sulfate (CS) biosynthesis was assessed by the degree of chondroitinase ABCase-sensitive [35S]SO4 incorporation into glycoaminoglycans in the hepatocytes.
The size of the GAG chains formed in the absence or presence of a mixture of the anomers (1.0 mmol/L) was investigated using GAGs synthesized in the presence of radiolabeled precursors. The labeled GAGs were isolated as described previously,15,18,19 and examined by gel chromatography on a Sephadex G-100 (Sigma) column (0.7 cm × 100 cm) by eluting with 1 mol/L NaCl at a flow rate of 3.9 ml/h (gravity). Aliquots (0.3 ml) of each fraction were analyzed for radioactivity by liquid scintillation spectroscopy. The column was calibrated by monitoring the elution volumes of various-sized dextrans having molecular weights of 4.7, 11, 19.5, 44, and 77 kd. The void volume (V0) and total bed volume (Vt) were determined using Blue dextran and uridine, respectively.
Induction of AA Amyloid
In Culture
AA amyloid was induced in J774 mouse macrophage tissue cultures using a modification of the method described previously by Kluve-Beckerman and colleagues.20,21 Briefly J774 were grown to 75 to 80% confluence in RPMI medium for 24 hours after which the medium was changed to include 20 μg of amyloid-enhancing factor as AA amyloid fibrils.22,23 After an additional 24 hours the medium was changed to include HDL/SAA (the AA amyloid precursor) ± an individual anomer in appropriate concentrations. Cultures were maintained in this manner for 6 days, replenishing the medium every 48 hours, after which they were fixed and stained with Congo Red.
In Vivo
AA amyloid was induced in 8- to 10-week-old CD1 mice with amyloid-enhancing factor and AgNO3, as an inflammatory stimulus, as described previously.24 Twenty-four hours after induction groups of five animals were treated either with the α- or β-anomer at 6 mg/dose administered intravenously via the lateral tail vein every 12 hours, and sacrificed by CO2 narcosis 5 days after the commencement of therapy. The quantity of amyloid/unit area of tissue (spleen and liver) was determined by image analysis as described previously.25 Total splenic amyloid was corrected for changing spleen weight as indicated in Table 2.
Table 2.
The Percent AA Amyloid per Unit Area of Tissue in Mice Treated with Either of the 4-Deoxy Analogues of Peracetylated 2-Acetamido-2-Deoxy-α- and β-d-Glucose and Normalized to Untreated Controls
| Anomer | Group | Liver | Spleen | Spleen Wt | Spleen (corrected) |
|---|---|---|---|---|---|
| Untreated | Control | 100 | 100 | 177 mg | 100 |
| Compound 5 | 6 mg/bid | 35.7 | 35.7 | 123 mg | 24.8 |
| Untreated | Control | 100 | 100 | 192 mg | 100 |
| Compound 7 | 6 mg/bid | 70.3 | 57.6 | 111 mg | 33.3 |
The results are the means of five animals per group expressed as a percent of the untreated amyloid group. The corrected spleen amyloid accounts for the changes in spleen weight relative to the controls which occur because of the treatment with either compounds 5 or 7. Normal spleen weights in mice 8 to 10 weeks of age are ∼80 mg.
Statistical Analysis
Data were analyzed either by a one- or two-way analysis of variance.
Results
Chemical Synthesis of 4-Deoxy Analogues of Peracetylated 2-Acetamido-2-Deoxy-α- and β-d-Glucose (Compounds 5 and 7, Respectively)
The α-anomer of the peracetylated 4-deoxy analogue of 2-acetamido-2-deoxy-d-glucose (2-acetamido-1,3,6,-tri-O-acetyl-2,4-dideoxy-α-d-xylo-hexopyranose; compound 5) was synthesized as shown in Figure 1. Methyl 2-acetamido-2-deoxy-α-d-glucopyranoside (compound 1)14 was selectively acetylated using acetyl chloride (2.1 equivalents) in pyridine at −45°C to afford the 3,6-di-O-acetyl derivative compound 2 in 48% yield. The hydroxyl group at C-4 in compound 2 was removed by way of a reductive dechlorination procedure. Thus, treatment of compound 2 with sulfuryl chloride in pyridine at 0°C and isolation of the product at room temperature afforded methyl 2-acetamido-3,6-di-O-acetyl-4-chloro-2,4-dideoxy-α-d-galactopyranoside (compound 3) in 83.1% yield. Compound 3 was reductively dechlorinated by treatment with tri-n-butyltin hydride and a catalytic amount of 1,1′-azobis(cyclohexanecarbonitrile) in anhydrous toluene to give the key intermediate methyl 2-acetamido-3,6-di-O-acetyl-2,4-dideoxy-α-d-xylo-hexopyranoside (compound 4) in 87.6% yield. Acetolysis of compound 4 in the presence of acetic anhydride and sulfuric acid afforded the target compound, namely 2-acetamido-1,3,6-tri-O-acetyl-2,4-dideoxy-α-d-xylo-hexopyranose (compound 5) in 45% yield.
The key intermediate compound 4 also was converted easily into the desired β-anomer, namely 2-acetamido-1,3,6-tri-O-acetyl-2,4-dideoxy-β-d-xylo-hexopyranose (compound 7) (Figure 2). The free sugar compound 6 could be obtained by treatment, in a separate experiment, of the acetolysis reaction mixture containing compound 4 (see above) with water for 30 minutes. Finally, the target β-anomer compound 7 was afforded in 47.3% yield by treatment of compound 6 with acetic anhydride and zinc chloride and fractional crystallization of the mixture of a α- and β-anomers.
Effects of Compounds 5 and 7 on Hepatocyte HS, CS, and Protein Synthesis in Tissue Culture
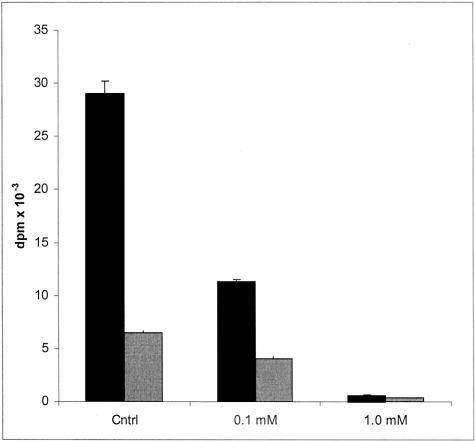
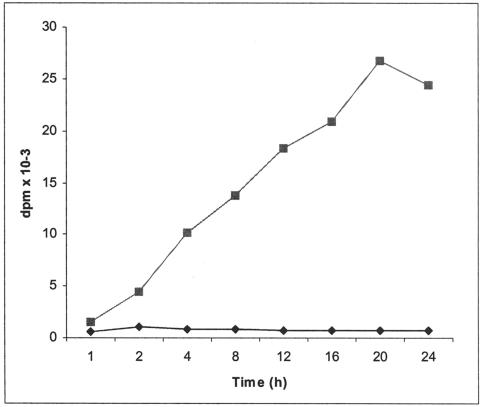
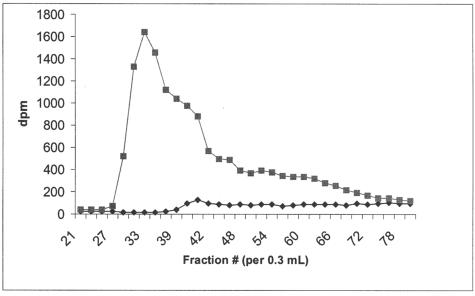
The effects of a mixture15 of the two analogues, compounds 5 and 7 (2:1, respectively, as shown by 1H NMR spectroscopy), on HS biosynthesis are exemplified in Figure 3. At 0.1 mmol/L the mixture inhibited the incorporation of [3H]glucosamine and [35S]SO4 into cell-associated HS to 39% and 63% of control values, respectively. At 1.0 mmol/L the incorporation of the two labeled precursors was negligible. The onset of the inhibitory effect on HS synthesis is illustrated in Figure 4. Within the first hour there is an abrupt cessation of [3H]glucosamine incorporation into cell-associated HS that persists for the remaining period of incubation. A similar result is apparent with [35S]SO4 (data not shown). Assessment of the size of the cell-associated HS synthesized in control cultures with those synthesized in the presence of 1.0 mmol/L of the analogues (Figure 5) indicated a 50% reduction in length. The average sizes of HS from control cells and those from analogue-treated cells were 77 kd and 40 kd, respectively. When a tritiated form of these analogues, at concentrations of 0, 1, 10, and 20 mmol/L, was incubated with hepatocytes for 24 hours the HS isolated from such cells contained small quantities of the radiolabeled analogue, as would be expected if each incorporated molecule of analogue truncated further elongation of the polysaccharide chain (Table 1).
Figure 3.
The effect of increasing concentrations of a mixture of 4-deoxy analogues of peracetylated 2-acetamido-2-deoxy-α- and β-d-glucose on hepatocyte cellular HS biosynthesis. The cells were incubated with d-[3H]glucosamine (black shading) and [35S]sulfate (gray shading) for 24 hours in the absence or presence of varying concentrations of the compounds. The values represent the mean ± SD of triplicate cultures. Statistical analyses revealed that the inhibition of HS synthesis was significant at P < 0.01 at both concentrations.
Figure 4.
The effect of a mixture of 4-deoxy analogues of peracetylated 2-acetamido-2-deoxy-α- and β-d-glucose on the time course of d-[3H]glucosamine incorporation into cellular HS. Hepatocytes were incubated with d-[3H]glucosamine as described in Materials and Methods in the absence (squares) or presence (diamonds) of the compounds at 1.0 mmol/L and the incubations were terminated at various times. The glycosaminglycans from the cells of each culture were then isolated and the counts determined. The values represent the mean of triplicate analyses.
Figure 5.
Sephadex G-100 elution profiles of d-[3H]glucosamine-labeled glycosaminoglycans from control hepatocytes (squares) and those treated with a mixture of 4-deoxy analogues of peracetylated 2-acetamido-2-deoxy-α- and β-d-glucose at 1.0 mmol/L (diamonds). Fraction 21 corresponds to the Vo as determined by the elution of Blue dextran. Vt corresponds to fraction 60 as determined by the elution of uridine. The peak of radioactivity in control cultures (fraction 35) corresponds to 77 kd and that of the treated culture (fraction 42) to 40 kd.
Table 1.
The Incorporation of Radiolabel into Hepatocyte Heparan Sulfate with Increasing Concentrations of Methyl 2-Acetamido-2,4-Dideoxy-β-d-xylo-Hexopyranoside-6-Tritium
| Concentration (mmol/L) | dpm +/− SD |
|---|---|
| 0 | 21 +/− 5 (background) |
| 1 | 102 +/− 14 |
| 10 | 387 +/− 68 |
| 20 | 403 +/− 15 |
Hepatocytes were incubated for 24 hours as described in Materials and Methods in the absence or presence of methyl 2-acetamido-2,4-dideoxy-β-d-xylo-hexopyranoside-6-tritium at concentrations of 1, 10, and 20 mmol/L. The values, +/− the standard deviation of triplicate cultures, represent the quantity of label incorporated per culture into cell-associated heparan sulfate. Statistical analyses revealed that the quantities incorporated at all concentrations were significantly greater than background, with P < 0.001.
Our early work in vivo characterizing the effects of the 4-deoxy compounds was done with the mixture described above. To be sure that the observed in vivo inhibitory effect on amyloidogenesis was not because of one or the other of these anomers procedures were developed to produce each of them separately (Figures 1 and 2) so that each could be tested separately in culture and in vivo. As shown below, each is equally effective separately.
The mixture of compounds 5 and 7, at the concentrations tested, as well as others in similar series, failed to exert any inhibitory effect on the incorporation of [3H]leucine into hepatocyte proteins in tissue culture.15 Similarly, as measured by the degree of chondroitinase ABCase-sensitive [35S]SO4 incorporation into glycoaminoglycans in the hepatocyte there was little effect of these analogues on chondroitin sulfate biosynthesis. This was examined in the following way. After 24 hours in primary culture and labeling with 35S-sulfate 92 to 95% of hepatocyte-associated glycosaminoglycans (GAG) were sensitive to nitrous acid digestion. That is, the predominant GAG is HS because this is the only GAG sensitive to nitrous acid digestion.18 The remaining 5 to 10% was sensitive to chondroitinase ABCase digestion. Incubations in the presence of 0.1 mmol/L of the anomeric mixture reduced the 35S-sulfate incorporation into GAGs by 50% (Figure 3) and the chondroitinase ABCase sensitive fraction increased to 20 to 25% indicating that the compounds had little effect on chondroitin sulfate biosynthesis.
Effects of Compounds 5 and 7 on AA Amyloid Induction
In Culture
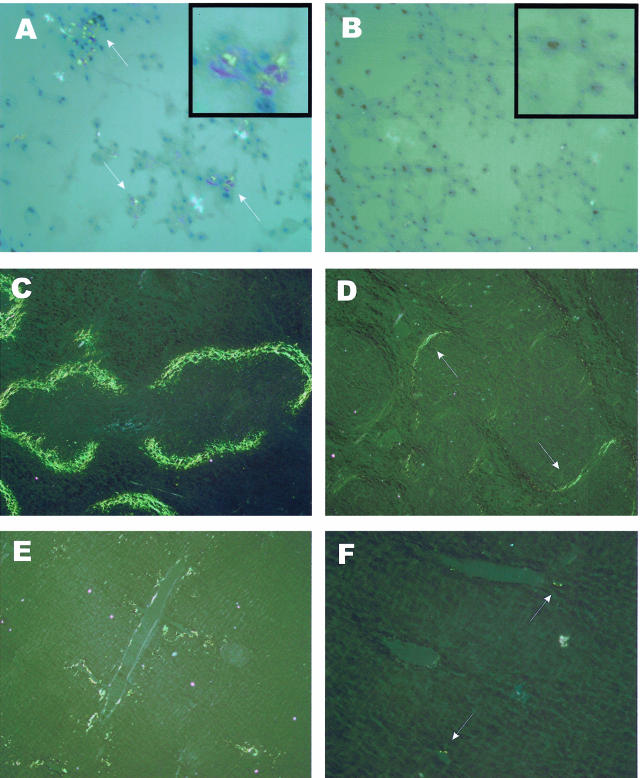
The effects of the individual analogues in a macrophage tissue culture model of AA amyloidogenesis were comparable and are exemplified in Figure 6, A and B. In the absence of the analogue easily demonstrable birefringent amyloid deposits could be identified on Congo Red staining (Figure 6A). The presence of the β-anomer (compound 7, 0.1 mmol/L) appeared to completely inhibit AA amyloid deposition (Figure 6B).
Figure 6.
Congo Red-stained tissue cultures (A and B), spleens (C and D), and livers (E and F) of control (A, C, and E) and glucosamine analogue-treated cells or mice (B, D, and F). A: A macrophage culture primed to deposit AA amyloid, as described in the Materials and Methods in the absence of analogue. Congo Red-positive red-green birefringent amyloid deposits are easily identified (white arrows) among the clusters of cells. B: A macrophage culture primed to deposit AA amyloid in the presence of 0.1 mmol/L of the β-anomer compound 7. Congo Red staining is absent. C: Congo Red-stained section of spleen from a mouse 6 days after being primed to deposit AA amyloid with amyloid enhancing factor and silver nitrate as the inflammatory stimulus without being treated with the analogues. The usual perifollicular deposition of substantial quantities of amyloid are present. D: An equivalently primed mouse that has been treated for the last 5 days with 6 mg of the α-anomer compound 5 twice daily intravenously. The quantity of amyloid in the perifollicular zones (white arrows) is substantially reduced. E and F: Corresponding comparisons from sections of liver from an untreated mouse and one treated with 6 mg of the α-anomer compound 5 twice daily intravenously. Original magnifications: ×135 (A, B); ×70 (C–F); ×350 (insets in A, B).
In Vivo
The effect of the individual anomeric forms of the analogues on AA amyloidogenesis in vivo are illustrated in Figure 6, C to F, and in Table 2. Figure 6, C and D, are a comparison of the effects of 5 days of treatment with compound 5 (the α-anomer) versus untreated animals on splenic amyloid deposition. Figure 6, E and F, are an equivalent comparison of the effects of 5 days of treatment with compound 5 on liver amyloid deposition. Note the reduction in the quantity of perifollicular birefringent amyloid stained with Congo Red in Figure 6D and the quantity of liver amyloid in Figure 6F.
The data in Table 2 illustrate the effects of compounds 5 and 7 on the induction of AA amyloid in spleen and liver. The percent tissue area occupied by amyloid was determined by image analysis,25 and the results were then normalized to the data in the untreated controls. Compounds 5 and 7 reduced the tissue area occupied by AA amyloid in spleen by 65% and 43%, respectively. Throughout the 6 days of amyloid induction spleen weights in untreated animals increased from ∼80 mg (unmanipulated animals) to 175 to 200 mg, whereas those in the treated groups increase only to 110 to 125 mg. When corrected for this smaller spleen size, total spleen amyloid in the treated groups (compounds 5 and 7) is only 25% and 33% of their respective controls. The inhibitory effects of compounds 5 and 7 on the induction of liver AA amyloid is similar to that in the spleen for compound 5 (65%) but less pronounced for compound 7 (30%).
Toxicity
During the 24-hour hepatocyte cultures, 7- to 8-day macrophage amyloid induction cultures and the 5-day in vivo treatment no toxicity was evident. However, it is not known whether longer exposure of cells or animals to these compounds would exhibit any toxicity.
Discussion
In situ amyloids consist not only of the disease- or pathological process-specific proteins, that form the basis of their classification, but also a common set of components identified in all in vivo amyloids so far examined.4,26 During the rapid in vivo induction of AA amyloidogenesis (the form associated with persistent inflammation) the basement membrane form of HSPG, perlecan, is deposited as part of the amyloid fibril coincidentally with the SAA protein.27–29 The spleen is the first organ affected during AA amyloid induction,24 and a threefold to fivefold increase in spleen perlecan mRNA appears to precede splenic amyloid deposition.30 A similar increase in entactin, α1-IV collagen chain, laminin β and γ chain (but not laminin α chain) mRNAs precedes spleen amyloid deposition, indicating a coordinated up-regulation of genes for basement membrane protein expression during the earliest phases of amyloidogenesis.31 Such an up-regulation is however restricted to those animals receiving the full amyloid induction protocol. It is not seen in untreated animals or in those receiving individual components of the protocol. Furthermore, SAA possesses HS-binding domains.32 Unlike other forms of glycosaminoglycans, HS, when interacting with the SAA isoform responsible for AA amyloid, imparts to this isoform a marked increase in β-sheet structure,33 which is the characteristic conformation of protein in amyloid fibrils. No such conformational change is seen when using nonamyloidogenic SAA isoforms.33,34 It has also been found that compounds that interfere with HS:SAA binding, when administered to mice receiving the rapid AA amyloid induction protocol, inhibit in vivo AA amyloid deposition,25 and promote regression of already induced deposits.25,35 It is thus difficult to avoid the conclusion that basement membrane protein gene expression, particularly as regards HSPG and the HS moiety of HSPG, is intimately involved in the initiation and progress of amyloid deposition. Similar but less extensive data have been obtained in relation to several other forms of amyloid, such as Aβ.4,36
These observations, not surprisingly, have suggested that the development of agents that interfere with the biosynthesis of HS may prove to have anti-amyloid properties in vivo, just as agents that interfere with HS:SAA binding inhibit in vivo AA amyloid deposition,25 and promote regression of already induced deposits.25,35 The strategy chosen to design and synthesize the desired agents is based on the natural biosynthetic steps known to be required for elongation of the HS polysaccharide chain.
Details of HS biosynthesis are fairly well established.37–39 This process takes place in the Golgi where a serine residue(s) in the protein moiety of the proteoglycan serves as an attachment point for the synthesis of a tetrasaccharide linkage region composed of xylose-galactose-galactose-glucuronate, the xylose being linked to the serine. This is followed by alternating and sequential additions of multiple N-acetylglucosamine and glucuronate units that are then sulfated to varying degrees at the N and 6-O positions of the glucosamine, and the 2-O position of the uronate. The uronate may be either d-glucuronate or l-iduronate. The epimerization of the glucuronate to iduronate requires the successful N-deacetylation and sulfation of the adjacent glucosamine on the nonreducing side of the growing polysaccharide chain.
The enzymatic addition of the N-acetylglucosamine to the glucuronate at the growing end of the polysaccharide chain takes place through an appropriate transferase that utilizes the UDP activated form of N-acetylglucosamine linking its C-1 hydroxyl to the C-4 hydroxyl of the glucuronate. Similarly, the enzymatic addition of the glucuronate to the N-acetylglucosamine at the growing end of the polysaccharide chain takes place through an appropriate transferase that utilizes the UDP-activated form of glucuronate linking its C-1 hydroxyl to the C-4 hydroxyl of the N-acetylglucosamine. Thus an N-acetylglucosamine that contains a C-1 hydroxyl (the site of UDP activation) but lacks a C-4 hydroxyl, if incorporated into the growing polysaccharide chain, would terminate chain elongation. It was these features that served as the focal points for the design and synthesis of the two anomers of 4-deoxy analogues of N-acetylglucosamine used in the present study. The acetylation of the hydroxyl groups at the 1,3 and 6 positions was to facilitate the entry of these agents into the cell where esterases would remove these substituents thus generating the 4-deoxy-free sugar analogues of N-acetylglucosamine.40 This free sugar in solution would exist in both anomeric configurations regardless of the starting anomer of the acetylated form. It is as free sugars that the compounds are activated with UTP to be incorporated into the growing HS polymer. The synthetic processes shown in Figures 1 and 2 provided the desired compounds in very good yield.
The assessment of these compounds regarding their effect on HS biosynthesis was performed with mouse primary hepatocytes in tissue culture. Hepatocyte-associated glycosaminoglycans consist almost entirely (92 to 95%) of HS.18 Thus any significant effect of compounds 5 and 7 on the incorporation of [3H]glucosamine and [35S]SO4 into cell-associated HS would be a clear indication of their influence on HS biosynthesis. As demonstrated, the biosynthetic inhibition of HS is apparent within 1 hour (the earliest time point examined) of exposure of hepatocytes to these compounds. At 1.0 mmol/L the incorporation of both [3H]glucosamine and [35S]SO4 into HS is almost completely inhibited and the average size of the HS chain is reduced from 77 to 40 kd. This is not because of a dilution of the specific activity of the intracellular [3H]-N-acetylglucosamine pool by compounds 5 and 7 because 1.0 mmol/L of unmodified N-acetylglucosamine fails to exert this effect nor does it have an effect on [35S]SO4 incorporation (data not shown). Furthermore, using a radiolabeled version of compounds 5 and 7, namely methyl 2-acetamido-2,4-dideoxy-β-d-xylo-hexopyranoside-6-tritium, we demonstrated that the analogue is incorporated into the growing HS chain. The nature of the analogue, its incorporation into the HS chain, the quantity of analogue isotope incorporated (only one such molecule can be incorporated per growing glycosaminoglycan chain), and the reduction in the average size of the HS polysaccharide are consistent with UDP activation of the sugar analogue, its incorporation, and the consequent truncation of the growing HS chain.
As described above, HS has been implicated in the structure and stability of amyloid fibrils and in the process of amyloid fibril assembly in vivo. The effects of compounds 5 and 7 were therefore examined in a tissue culture model and a rapid in vivo induction model of murine AA amyloidogenesis. Both compounds exerted profound inhibitory effects on amyloid deposition in each of the models studied. In tissue culture, at 0.1 mmol/L, both compounds 5 and 7 virtually abolished amyloid formation. This result was seen in the presence of a constant concentration of HDL-SAA and therefore is not because of a lack of AA amyloid precursor. Previous studies with this tissue culture model have indicated that it recapitulates most of the in vivo characteristics of AA amyloidogenesis (manuscript submitted). In this model the speed of induction of AA amyloid is a function of the presence of amyloid enhancing factor, as it is in vivo, and amyloid deposition is dependent on the availability of HDL-SAA. Of the two acute-phase forms of SAA (SAA1.1 and SAA2.1) only SAA1.1 is deposited as AA amyloid, and the size spectrum of SAA1.1 peptides in the amyloid matches that seen in vivo. Furthermore, HS proteoglycan is part of the amyloid deposit, as it is in vivo. Moreover, poly(vinylsulfonate sodium salt), a compound that inhibits the binding of SAA to HS inhibits AA amyloidogenesis virtually completely in tissue culture as it does in vivo.25 In addition, an AA peptide that contains the specific HS-binding domain,32 when added to our culture model, eliminates AA amyloidogenesis in culture at a concentration of 50 nmol/L (E. Elimova, J.B. Ancsin, and R. Kisilevsky, manuscript submitted). Other anti-amyloid peptides cited in the literature are effective only at concentrations that are three orders of magnitude higher. And, strikingly, transgenic mice overexpressing heparanase fail to deposit AA amyloid but only in those tissues that overexpress the gene/mRNA/enzyme. In the identical mice tissues that fail to express the heparanase gene deposit AA amyloid equally well in control and transgenic mice (J.P. Li, U. Lindahl, I. Vlodavsky, and R. Kisilevsky, manuscript in preparation). Taken in toto there is substantial evidence that in vivo HS plays a very significant, and likely direct, role in AA amyloidogenesis. The results with compounds 5 and 7 in tissue culture thus help to interpret the observations made with compounds 5 and 7 in vivo.
Compounds 5 and 7 also exert a profound inhibitory effect on in vivo splenic AA amyloid induction (65 to 70%) but it is not as complete as seen in tissue culture. Mouse liver amyloid is also inhibited substantially (30 to 65%). In culture the concentration of the novel sugars can be maintained approximately at a constant level throughout the entire period of incubation. This is not possible in vivo. Glucosamine is known to be rapidly cleared from the circulation.41 Because the analogues are so similar in structure to the parent compound they too are likely cleared rapidly. The actual effective concentration in vivo is not known and may be reached only when the compounds are first injected. The injected dose, 6 mg, distributed throughout the entire body volume of a 30 g mouse would create a concentration of ∼0.6 mmol/L that would rapidly decay as the compound is cleared. Such a concentration would not be reached again until 12 hours later on the injection of a subsequent dose. Nevertheless, the anti-amyloid effects of compounds 5 and 7 in vivo are clearly apparent.
Although the plasma precursor levels (SAA) were not examined in vivo the tissue culture studies indicate clearly that the anti-amyloid effects of compounds 5 and 7 are not because of a lack of SAA. Furthermore, these compounds were not administered until 24 hours after the induction of acute inflammation, by which time the plasma SAA concentration is of the order of 1000 μg/ml.42,43 Moreover, our past studies with agents that interfere with HS:SAA interactions have shown that such agents are effective anti-amyloid compounds even in the presence of SAA concentrations ranging between 500 to 1000 μg/ml.25
N-Acetylglucosamine is a biosynthetic precursor not only for HS, but also keratan sulfate (KS), hyaluronan, and complex polysaccharide side chains on glycoproteins, and compounds 5 and 7 could therefore also interfere with their biosynthesis. Although KS has been reported to be present in Aβ amyloid deposits,44,45 neither hyaluronan nor KS have been found in AA amyloid. Furthermore, the acute-phase forms of SAA (SAA1.1 and SAA2.1) are not glycosylated.42,46,47 If inhibition of glycoprotein glycosylation is playing an anti-amyloid role in our present tissue culture and in vivo studies it would have to do so in an indirect manner. Our present data, taken in isolation, would at a minimum indicate that inhibition of polysaccharide biosynthesis (HS, KS, hyaluronan, and/or glycoprotein glycosylation) can inhibit AA amyloidogenesis. However, taken in the context of HSs previously demonstrated role in the fibrillogenesis of many different amyloidogenic proteins,33,48–53 and the demonstration that agents that interfere with HS:amyloidogenic protein binding also inhibit amyloid deposition in vivo,25,54 it is highly likely that compounds 5 and 7 interfere with AA amyloidogenesis primarily through their effects on HS biosynthesis. These observations with compounds 5 and 7 not only identify agents that may interfere with amyloidogenesis, but in doing so they add further support to the concept that polysaccharide biosynthesis, likely of HS, is closely linked to amyloidogenesis. Furthermore, they suggest that inhibitors of HS biosynthesis, or agents that modify HS structure, may provide lead compounds or avenues for possible therapeutic attack on disorders in which amyloids play an important pathogenetic role, such as Alzheimer’s disease. These considerations raise the question whether compounds such as described herein are able to cross the blood brain barrier. Experiments addressing this issue have not as yet been performed. However, a perusal of the available literature indicates that d-glucosamine, N-acetylglucosamine, and d-galactosamine readily penetrate the brain,55 as do some such sugars with more extensive modifications. Furthermore, the enzymes for the activation of these sugars to their UDP form and the transferase enzymes for glycosaminoglycan biosynthesis are also present in brain indicating that the brain is prepared to use such sugars for biosynthetic purposes.56–60 Given the minor modifications we made to N-acetylglucosamine it is highly likely that the analogues described in our present work may be able to cross the blood brain barrier.
Acknowledgments
We thank Mrs. Ruth Tan and Mr. Lee Boudreau for their able technical assistance; and Professor Keith Brown, University of Oslo, Norway, for his helpful input in the preparation of parts of this manuscript.
Footnotes
Address reprint requests to Dr. R. Kisilevsky, Department of Pathology, Queen’s University, Kingston, Ontario, Canada K7L 3N6. E-mail: kisilevsky@cliff.path.queensu.ca.
Supported by the Canadian Institutes for Health Research (formerly the Medical Research Council of Canada) (grant MOP-3153 to R.K.), the Royal College of Physicians and Surgeons of Canada (Detweiler Traveling Fellowship to R.K.), the Natural Science and Engineering Research Council of Canada (to W.A.S.), and the Institute for the Study of Aging, New York, New York (to R.K. and W.A.S.).
References
- Virchow R. Zur Cellulosefrage. Virchows Arch Pathol Anat Physiol. 1854;6:416–426. [Google Scholar]
- Kisilevsky R. Amyloidosis. Rubin E, Farber J, editors. Philadelphia: Lippincott-Raven; Pathology. 1999:pp 1220–1234. [Google Scholar]
- Westermark P. Classification of amyloid fibril proteins and their precursors: an ongoing discussion. Amyloid. 1997;4:216–218. [Google Scholar]
- Kisilevsky R, Fraser PE. Aβ amyloidogenesis: unique or variation on a systemic theme? Crit Rev Biochem Mol Biol. 1997;32:361–404. doi: 10.3109/10409239709082674. [DOI] [PubMed] [Google Scholar]
- Kisilevsky R. Review: amyloidogenesis—unquestioned answers and unanswered questions. J Struct Biol. 2000;130:99–108. doi: 10.1006/jsbi.2000.4222. [DOI] [PubMed] [Google Scholar]
- Horiguchi Y, Fine JD, Leigh IM, Yoshiki T, Ueda M, Imamura S. Lamina densa malformation involved in histogenesis of primary localized cutaneous amyloidosis. J Invest Dermatol. 1992;99:12–18. doi: 10.1111/1523-1747.ep12611384. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Yamazaki T, Lemere CA, Frosch MP, Selkoe DJ. Beta-amyloid is focally deposited within the outer basement membrane in the amyloid angiopathy of Alzheimer’s disease—an immunoelectron microscopic study. Am J Pathol. 1992;141:249–259. [PMC free article] [PubMed] [Google Scholar]
- Botto M, Hawkins PN, Bickerstaff MCM, Herbert J, Bygrave AE, Mcbride A, Hutchinson WL, Tennent GA, Walport MJ, Pepys MB. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- Elliott-Bryant R, Cathcart ES. Apolipoprotein E and apolipoprotein A-1 knock-out mice readily develop amyloid A protein amyloidosis. Clin Immunol Immunopathol. 1997;85:104–108. doi: 10.1006/clin.1997.4397. [DOI] [PubMed] [Google Scholar]
- Bales KR, Verina T, Dodel RC, Du YS, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- Kindy MS, Rader DJ. Reduction in amyloid A amyloid formation in apolipoprotein-E-deficient mice. Am J Pathol. 1998;152:1387–1395. [PMC free article] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- Ringvall M, Ledin J, Holmborn K, Vankuppevelt T, Ellin F, Eriksson I, Olofsson AM, Kjellen L, Forsberg E. Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem. 2000;275:25926–25930. doi: 10.1074/jbc.C000359200. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Kamiguchi T, Ikeda Y, Kitagawa I. Chemical transformation of uronic acids leading to aminocyclitols. IV. Synthesis of hexaacetyl-streptamine from N-acetyl-d-glucosamine by means of electrolytic decarboxylation. Chem Pharm Bull. 1981;29:2582–2586. [Google Scholar]
- Berkin A, Szarek MA, Plenkiewicz J, Szarek WA, Kisilevsky R. Synthesis of 4-deoxy analogues of 2-acetamido2-deoxy-d-glucose and 2-acetamido-2-deoxy-d-xylose and their effects on glycoconjugate biosynthesis. Carbohydr Res. 2000;325:30–45. doi: 10.1016/s0008-6215(99)00314-6. [DOI] [PubMed] [Google Scholar]
- Subrahmanyan L, Kisilevsky R. Effects of culture substrates and normal hepatic sinusoidal cells on in-vitro hepatocyte synthesis of apo-SAA. Scand J Immunol. 1988;27:251–260. doi: 10.1111/j.1365-3083.1988.tb02345.x. [DOI] [PubMed] [Google Scholar]
- Kisilevsky R, Subrahmanyan L. Serum amyloid A changes high density lipoprotein’s cellular affinity: a clue to serum amyloid A’s principal function. Lab Invest. 1992;66:778–785. [PubMed] [Google Scholar]
- Thomas SS, Plenkiewicz J, Ison ER, Bols M, Zou W, Szarek WA, Kisilevsky R. Influence of monosaccharide derivatives on liver cell glycosaminoglycan synthesis: 3-deoxy-d-xylo-hexose (3-deoxy-d-galactose) and methyl(methyl 4-chloro-4-deoxy-beta-d-galactopyranosid)uronate. Biochim Biophys Acta. 1995;1272:37–48. doi: 10.1016/0925-4439(95)00065-c. [DOI] [PubMed] [Google Scholar]
- Berkin A, Szarek WA, Kisilevsky R. Synthesis and biological evaluation of a radiolabeled analog of methyl 2-acetamido-2,4-dideoxy-beta-d-xylo-hexopyranoside directed towards influencing cellular glycosaminoglycan biosynthesis. Carbohydr Res. 2002;337:37–44. doi: 10.1016/s0008-6215(01)00285-3. [DOI] [PubMed] [Google Scholar]
- Kluve-Beckerman B, Liepnieks JJ, Wang LS, Benson MD. A cell culture system for the study of amyloid pathogenesis—amyloid formation by peritoneal macrophages cultured with recombinant serum amyloid A. Am J Pathol. 1999;155:123–133. doi: 10.1016/S0002-9440(10)65107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluve-Beckerman B, Manaloor J, Leipnieks JJ. Binding, trafficking and accumulation of serum amyloid A in peritoneal macrophages. Scand J Immunol. 2001;53:393–400. doi: 10.1046/j.1365-3083.2001.00879.x. [DOI] [PubMed] [Google Scholar]
- Baltz ML, Caspi D, Hind CRK, Feinstein A, Pepys MB. Isolation and characterization of amyloid enhancing factor (AEF). Glenner GG, Osserman EF, Benditt EP, Calkins E, Cohen AS, Zucker-Franklin D, editors. New York: New York, Plenum Press; Amyloidosis. 1986:pp 115–121. [Google Scholar]
- Kisilevsky R, Gruys E, Shirahama T. Does amyloid enhancing factor (AEF) exist? Is AEF a single biological entity? Amyloid. 1995;2:128–133. [Google Scholar]
- Kisilevsky R, Boudreau L. The kinetics of amyloid deposition: I. The effect of amyloid enhancing factor and splenectomy. Lab Invest. 1983;48:53–59. [PubMed] [Google Scholar]
- Kisilevsky R, Lemieux LJ, Fraser PE, Kong XQ, Hultin PG, Szarek WA. Arresting amyloidosis in vivo using small-molecule anionic sulphonates or sulphates: implications for Alzheimer’s disease. Nat Med. 1995;1:143–148. doi: 10.1038/nm0295-143. [DOI] [PubMed] [Google Scholar]
- Lyon AW, Narindrasorasak S, Young ID, Anastassiades T, Couchman JR, McCarthy K, Kisilevsky R. Co-deposition of basement membrane components during the induction of murine splenic AA amyloid. Lab Invest. 1991;64:785–790. [PubMed] [Google Scholar]
- Snow AD, Kisilevsky R. Temporal relationship between glycosaminoglycan accumulation and amyloid deposition during experimental amyloidosis: a histochemical study. Lab Invest. 1985;53:37–44. [PubMed] [Google Scholar]
- Snow AD, Kisilevsky R. A close ultrastructural relationship between sulphated proteoglycans and AA amyloid fibrils. Lab Invest. 1988;57:687–698. [PubMed] [Google Scholar]
- Snow AD, Bramson R, Mar H, Wight TN, Kisilevsky R. A temporal and ultrastructural relationship between heparan sulfate proteoglycans and AA amyloid in experimental amyloidosis. J Histochem Cytochem. 1991;39:1321–1330. doi: 10.1177/39.10.1940305. [DOI] [PubMed] [Google Scholar]
- Ailles L, Kisilevsky R, Young ID. Induction of perlecan gene expression precedes amyloid formation during experimental murine AA amyloidogenesis. Lab Invest. 1993;69:443–448. [PubMed] [Google Scholar]
- Woodrow SI, Stewart RJ, Kisilevsky R, Gore J, Young ID. Experimental AA amyloidogenesis is associated with differential expression of extracellular matrix genes. Amyloid. 1999;6:22–30. doi: 10.3109/13506129908993284. [DOI] [PubMed] [Google Scholar]
- Ancsin JB, Kisilevsky R. The heparin/heparan sulfate-binding site on apo-serum amyloid A: implications for the therapeutic intervention of amyloidosis. J Biol Chem. 1999;274:7172–7181. doi: 10.1074/jbc.274.11.7172. [DOI] [PubMed] [Google Scholar]
- McCubbin WD, Kay CM, Narindrasorasak S, Kisilevsky R. Circular dichroism and fluorescence studies on two murine serum amyloid A proteins. Biochem J. 1988;256:775–783. doi: 10.1042/bj2560775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beer MC, de Beer FC, McCubbin WD, Kay CM, Kindy MS. Structural prerequisites for serum amyloid A fibril formation. J Biol Chem. 1993;268:20606–20612. [PubMed] [Google Scholar]
- Inoue S, Hultin PG, Szarek WA, Kisilevsky R. Effect of poly(vinylsulfonate) on murine AA amyloid: a high-resolution ultrastructural study. Lab Invest. 1996;74:1081–1090. [PubMed] [Google Scholar]
- Yang DS, Serpell LC, Yip CM, Mclaurin J, Christi MA, Horne P, Kisilevsky R, Westaway D, Fraser PE. Assembly of Alzheimer’s amyloid-β fibrils and approaches for therapeutic intervention. Amyloid J Prot Folding Disorders. 2001;8(Suppl 1):10–19. [PubMed] [Google Scholar]
- Salmivirta M, Lidholt K, Lindahl U. Heparan sulfate: a piece of information. EMBO J. 1996;10:1270–1279. doi: 10.1096/fasebj.10.11.8836040. [DOI] [PubMed] [Google Scholar]
- Lindahl U, Kusche-Gullberg M, Kjellen L. Regulated diversity of heparan sulfate. J Biol Chem. 1998;273:24979–24982. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Kitagawa H. Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr Opin Struct Biol. 2000;10:518–527. doi: 10.1016/s0959-440x(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Bernacki RJ, Sharma M, Porter NK, Rustum Y, Paul B, Korytnyk W. Biochemical characteristics, metabolism, antitumor activity of several acetylated hexosamines. J Supramol Struct. 1977;7:235–250. doi: 10.1002/jss.400070208. [DOI] [PubMed] [Google Scholar]
- Aghazadeh-Habashi A, Sattari S, Pasutto F, Jamali F. Single dose pharmacokinetics and the bioavailability of glucosamine in the rat. J Pharm Sci. 2002;5:181–184. [PubMed] [Google Scholar]
- Husby G, Marhaug G, Dowton B, Sletten K, Sipe JD. Serum amyloid A (SAA): biochemistry, genetics and the pathogenesis of AA amyloidosis. Amyloid. 1994;1:118–137. [Google Scholar]
- Sipe JD. Serum amyloid A: from fibril to function. Current status. Amyloid. 2000;7:10–12. doi: 10.3109/13506120009146815. [DOI] [PubMed] [Google Scholar]
- Snow AD, Nochlin D, Sekiguchi R, Carlson SS. Identification and immunolocalization of a new class of proteoglycan (keratan sulfate) to the neuritic plaques of Alzheimer’s disease. Exp Neurol. 1996;138:305–317. doi: 10.1006/exnr.1996.0069. [DOI] [PubMed] [Google Scholar]
- Lindahl B, Eriksson L, Spillmann D, Caterson B, Lindahl U. Selective loss of cerebral keratan sulfate in Alzheimer’s disease. J Biol Chem. 1996;271:16991–16994. doi: 10.1074/jbc.271.29.16991. [DOI] [PubMed] [Google Scholar]
- Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 1998;334:489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Spillantini MG, Hasegawa M, Smith MJ, Crowther RA. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature. 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- Castillo GM, Ngo C, Cummings J, Wight TN, Snow AD. Perlecan binds to the β-amyloid proteins (Aβ) of Alzheimer’s disease, accelerates Aβ fibril formation, and maintains Aβ fibril stability. J Neurochem. 1997;69:2452–2465. doi: 10.1046/j.1471-4159.1997.69062452.x. [DOI] [PubMed] [Google Scholar]
- Castillo GM, Cummings JA, Yang WH, Judge ME, Sheardown MJ, Rimvall K, Hansen JB, Snow AD. Sulfate content and specific glycosaminoglycan backbone of perlecan are critical for perlecan’s enhancement of islet amyloid polypeptide (amylin) fibril formation. Diabetes. 1998;47:612–620. doi: 10.2337/diabetes.47.4.612. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Halfter W, Cole GJ. Agrin binds to β-amyloid (Aβ), accelerates Aβ fibril formation, and is localized to Aβ deposits in Alzheimer’s disease brain. Mol Cell Neurosci. 2000;15:183–198. doi: 10.1006/mcne.1999.0816. [DOI] [PubMed] [Google Scholar]
- Mclaurin J, Franklin T, Zhang XQ, Deng JP, Fraser PE. Interactions of Alzheimer amyloid-β peptides with glycosaminoglycans—effects on fibril nucleation and growth. Eur J Biochem. 1999;266:1101–1110. doi: 10.1046/j.1432-1327.1999.00957.x. [DOI] [PubMed] [Google Scholar]
- Cohlberg JA, Li J, Uversky VN, Fink AL. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from α-synuclein in vitro. Biochemistry. 2002;41:1502–1511. doi: 10.1021/bi011711s. [DOI] [PubMed] [Google Scholar]
- Gervais F, Chalifour R, Garceau D, Kong XQ, Laurin J, McLaughlin R, Morissette C, Paquette J. Glycosaminoglycan mimetics: a therapeutic approach to cerebral amyloid angiopathy. Amyloid J Prot Folding Disorders. 2001;8(Suppl 1):28–35. [PubMed] [Google Scholar]
- Popov N. Effects of D-galactosamine and D-glucosamine on retention performance of a brightness discrimination task in rats. Biomed Biochim Acta. 1985;44:611–622. [PubMed] [Google Scholar]
- Okuyama R, Marshall S. UDP-N-acetylglucosaminyl transferase (OGT) in brain tissue: temperature sensitivity and subcellular distribution of cytosolic and nuclear enzyme. J Neurochem. 2003;86:1271–1280. doi: 10.1046/j.1471-4159.2003.01939.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Yoshida K, Gotoh M, Sugioka S, Kikuchi N, Kwon YD, Tawada A, Maeyama K, Inaba N, Hiruma T, Kimata K, Narimatsu H. Characterization of a heparan sulfate 3-O-sulfotransferase-5, an enzyme synthesizing a tetrasulfated disaccharide. J Biol Chem. 2003;278:26780–26787. doi: 10.1074/jbc.M301861200. [DOI] [PubMed] [Google Scholar]
- Inatani M, Yamaguchi Y. Gene expression of EXT1 and EXT2 during mouse brain development. Brain Res Dev Brain Res. 2003;141:129–136. doi: 10.1016/s0165-3806(03)00010-5. [DOI] [PubMed] [Google Scholar]
- Akimoto Y, Comer FI, Cole RN, Kudo A, Kawakami H, Hirano H, Hart GW. Localization of the O-GlcNAc transferase and O-GlcNAc-modified proteins in rat cerebellar cortex. Brain Res. 2003;966:194–205. doi: 10.1016/s0006-8993(02)04158-6. [DOI] [PubMed] [Google Scholar]
- Grobe K, Esko JD. Regulated translation of heparan sulfate N-acetylglucosamine N-deacetylase/n-sulfotransferase isoenzymes by structured 5′-untranslated regions and internal ribosome entry sites. J Biol Chem. 2002;277:30699–30706. doi: 10.1074/jbc.M111904200. [DOI] [PubMed] [Google Scholar]