Abstract
The Fas (CD95)/Fas ligand (CD178) system plays an important role in epithelial damage during the acute respiratory distress syndrome. The goal of this study was to determine whether proximal and distal human lung epithelial cells differ in their sensitivity to Fas ligand (rh-sFasL), and whether the response of lung epithelium to Fas ligation is modulated by proinflammatory cytokines. Although the expression of both Fas message and protein was similar in proximal and distal lung epithelial cells, only distal cells became apoptotic when exposed to serial dilutions of rh-sFasL. Stimulation with tumor necrosis factor-α, interleukin-1β, or interferon-γ significantly increased the sensitivity of proximal cells to rh-sFasL, and exposure to either tumor necrosis factor-α or interferon-γ enhanced the sensitivity of distal cells to Fas ligation. These findings suggest that in normal human lungs, the responses of the epithelium to Fas ligation become more pronounced from proximal to distal locations. Furthermore, proinflammatory cytokines sensitize lung epithelium to Fas-induced death. These findings are relevant for understanding the role of the Fas/FasL system in acute lung injury, in which epithelial damage occurs primarily in distal airway and alveolar epithelium, whereas sFasL is present throughout the airspaces.
The acute respiratory distress syndrome (ARDS) is characterized by a neutrophilic inflammatory response and destruction of the alveolar epithelium.1,2 Proximal lung epithelial cells and distal lung epithelial cells (DLEC) express functional Fas (CD95), and activation of Fas in vivo and in vitro can induce epithelial cell apoptosis.3–8 The natural ligand of Fas, FasL (CD178) is present in the airspaces of patients with ARDS at concentrations capable of inducing apoptosis of DLEC.5 It has been hypothesized that Fas-mediated induction of apoptosis in the distal lung epithelium plays a key role in the pathogenesis of ARDS, by causing loss of alveolar epithelial barrier function. Yet it remains unclear why such destruction would occur primarily in the alveolar epithelium, as it does in ARDS, rather than throughout the entire lung epithelium, because FasL is present in both the alveoli and the airways.9 The goal of this study was to determine whether proximal and distal lung epithelial cells differ in their sensitivity to Fas ligation, and whether Fas-sensitivity can be modulated by proinflammatory cytokines commonly found in the lungs of patients with ARDS.
Fas is a 45-kd type I membrane surface receptor protein belonging to the tumor necrosis factor (TNF) receptor family.10 The natural ligand of Fas is Fas ligand (FasL) (CD178), a type II membrane protein existing as a 40-kd membrane-bound form and as a soluble form (sFasL).11–13 Ligation and clustering of Fas receptors by either the membrane-bound or the soluble forms of FasL result in the assembly of a cytoplasmic protein complex that activates caspase-8, the first in a cascade of proteases whose full activation culminates in apoptosis.10,14,15 Activation of Fas can also lead to nuclear factor-κB translocation and release of proinflammatory cytokines, including interleukin-8 (IL-8), in some cells.6,16–18
In the lungs, Fas and FasL expression is localized to the alveolar and airway epithelium, fibroblasts, and resident alveolar macrophages.3,7,19–25 Several studies suggest that the alveolar epithelium responds with apoptosis to Fas ligation, both in vivo and in vitro,5,7,24,26 and that long-term activation of the Fas/FasL system results in the development of pulmonary fibrosis.24,26 In contrast to lymphocytic-mediated acquired immune responses, in which the membrane-bound form of FasL appears to be a stronger activator of Fas than soluble Fas ligand,27 in humans with ARDS the soluble form of FasL is a biologically relevant inducer of epithelial cell apoptosis.5,28 The biological relevance of sFasL has been confirmed in animal experiments demonstrating that sFasL induces acute lung injury in rabbit lungs.7 Proinflammatory cytokines such as TNF-α, IL-1β, and interferon (IFN)-γ can modulate apoptosis by regulating the expression of cell surface Fas and intracellular apoptosis-related proteins.4,6,29–35 The mechanisms that regulate apoptosis in lung epithelial cells and the factors that modulate the responses of the lung epithelium at different levels of the airways to Fas activation remain unclear.
In this study, we investigated the expression of Fas and TNF receptors in proximal lung epithelial cells and DLEC and the relative sensitivity of the epithelial cells to sFasL and TNF-α. Next, we examined whether the proinflammatory cytokines, TNF-α, IL-1β, and IFN-γ, can modulate the response of lung epithelial cells to Fas activation. We found that Fas ligation results in apoptosis of DLEC, but not of proximal epithelial cells. Fas ligation did not lead to the release of proinflammatory cytokines in either cell type. Exogenous proinflammatory cytokines acted as modulators of the response to Fas ligation, enhancing the sensitivity of epithelial cells to Fas-mediated apoptosis.
Materials and Methods
Reagents
Flow cytometry reagents included phycoerythrin (PE)-conjugated mouse anti-human Fas monoclonal antibody (mAb) Dx2 (BD PharMingen, San Diego, CA), PE-conjugated mouse anti-human TNF receptor (TNFR) I mAb, PE-conjugated mouse anti-human TNFR II mAb (R&D Systems, Minneapolis, MN), and PE-conjugated mouse IgG1 (BD PharMingen) as isotype control mAb. Recombinant human soluble Fas ligand (rh-sFasL) (no. 522-020-C005; Alexis, San Diego, CA) and TNF-α (R&D Systems) were used to study cell death. TNF-α, IL-1β (R&D Systems), and IFN-γ (Genzyme, Cambridge, MA) were used to stimulate cells. The mAb Jo2 (BD PharMingen) was used to activate Fas in mouse lungs in vivo.
Cell Culture
Proximal lung epithelial cells (normal human bronchial epithelial cells, NHBE) and DLEC (small airway epithelial cells) were purchased from Clonetics (San Diego, CA). The NHBE are primary lung epithelial cells isolated from the large proximal airways of normal human lungs. After thawing, NHBE were seeded in 25-cm2 cell culture flasks (Costar, Cambridge, MA) at a density of 1 × 104 cells/cm2, and grown in bronchial epithelial cell growth medium (Clonetics) supplemented with bovine pituitary extract (52 μg/ml), hydrocortisone (0.5 μg/ml), epidermal growth factor (0.5 ng/ml), epinephrine (0.5 μg/ml), transferrin (10 μg/ml), insulin (5 μg/ml), retinoic acid (0.1 ng/ml), triiodothyronine (6.5 ng/ml), gentamicin (50 μg/ml), and amphotericin B (50 ng/ml). The cells were incubated at 37°C, 5% CO2 in a humidified atmosphere and split when they reached 70% confluency. The cells were used before passage 3.
The DLEC are nonciliated primary lung epithelial cells isolated from distal airways of less than 1.0 mm in diameter. These cells grow as flat monolayers, stain positive for cytokeratin and surfactant protein A, and show lamellar body-like structures on electron microscopy. They do not contain neurosecretory granules by electron microscopy.5 DLEC were subcultured in small airway epithelial cell growth medium (SAGM, Clonetics) supplemented with bovine pituitary extract (30 μg/ml), hydrocortisone (0.5 μg/ml), epidermal growth factor (0.5 ng/ml), epinephrine (0.5 μg/ml), transferrin (10 μg/ml), insulin (5 μg/ml), retinoic acid (0.1 ng/ml), triiodothyronine (6.5 ng/ml), gentamicin (50 μg/ml), amphotericin B (50 ng/ml), and 0.05% bovine serum albumin. The cells were incubated at 37°C, 5% CO2, split when they reached 70% confluence, and were used before passage 3. In separate experiments, we determined that the pituitary extract has no effect on the Fas sensitivity of either DLEC or NHBE. Morphologically, using both light and electron microscopy, DLEC and NHBE are pure cell populations (> 99% pure). This has been true for each lot that we have examined in detail.
Expression of Fas, FasL, TNFR I, and TNFR II, and Intracellular Proapoptotic Proteins
The expression of mRNA for Fas, FasL, TNFR I, TNFR II, Fas-associated death domain (FADD), TNF receptor-associated death domain (TRADD), and caspase-8 in NHBE and DLEC was determined using reverse transcriptase-polymerase chain reaction (RT-PCR). Total cellular RNA was isolated and treated with DNase I using the Absolutely RNA RT-PCR miniprep kit (Stratagen, La Jolla, CA), according to the instructions of the manufacturer. The RNA was quantified by measuring absorbance at 260 nm. Randomly primed, first-strand cDNA was prepared from 600 ng of total cellular RNA in 20 μl of reaction volume containing 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 5 mmol/L MgCl2, 1 mmol/L deoxynucleotide triphosphates, 1.6 μg of oligo (dT) primer, 40 U of RNase inhibitor, and 40 U of Moloney murine leukemia virus reverse transcriptase (Roche Molecular Biochemicals, Mannheim, Germany). PCR primers for Fas, FasL, TNFR I, TNFR II, FADD, TRADD, caspase-8, and β-actin were designed based on the GenBank sequence nos. M67454, X89102, M63121, M55994, NM012026, NM003789, XM054988, and M10277, respectively, using MacVector software (Oxford Molecular, Madison, WI) (Table 1). The PCR amplifications were performed in a reaction volume of 25 μl, containing 1 μl of cDNA, 0.6 μmol/L primers, 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.2 mmol/L deoxynucleotide triphosphates, and 1.5 U of TaqDNA polymerase (Roche). An initial denaturation at 95°C for 5 minutes was followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 54°C (Fas, β-actin), 55°C (TRADD), 58°C (FasL, FADD, caspase-8), or 52°C (TNFR I, TNFR II) for 30 seconds, and extension at 72°C for 1 minute, using the GeneAmp PCR System 9700 (Perkin-Elmer, Foster City, CA). Control samples not subjected to RT were used to check for the presence of contaminating DNA. The negative control for the PCR amplification step consisted of PCR mix without cDNA. PCR products were separated in 1.5% agarose gels (Promega, Madison, WI) containing ethidium bromide in Tris-acetate/ethylenediaminetetraacetic acid (EDTA). Identity of amplified bands was confirmed by sequence analysis of the amplified fragments (data not shown).
Table 1.
Oligonucleotide Primers for RT-PCR
| Product size | ||
|---|---|---|
| Fas | ||
| Forward | 5′-TCGGAGGATTGCTCAACAACC-3′ | |
| Backward | 5′-AAGAAGAAGACAAAGCCACCCC-3′ | 564 bp |
| FasL | ||
| Forward | 5′-AGGCAAGTCCAACTCAAGGTCC-3′ | |
| Backward | 5′-CATCTTCCCCTCCATCATCACC-3′ | 238 bp |
| TNFR I | ||
| Forward | 5′-CGCTTCAGAAAACCACCTCAGAC-3′ | |
| Backward | 5′-CCAAAGAAAATGACCAGGGGC-3′ | 393 bp |
| TNFR II | ||
| Forward | 5′-GCTCTGACCAGGTGGAAACTCAAG-3′ | |
| Backward | 5′-GGATGAAGTCGTGTTGGAGAACG-3′ | 224 bp |
| FADD | ||
| Forward | 5′-AAGATTGGAGAAGGCTGGCTCG-3′ | |
| Backward | 5′-TCGGAGGTAGATGCGTCTGAGTTC-3′ | 289 bp |
| TRADD | ||
| Forward | 5′-CTTTTGGAGAACCTGGATGGC-3′ | |
| Backward | 5′-AACTGTAAGGGCTGGCTGTAAGC-3′ | 274 bp |
| Caspase-8 | ||
| Forward | 5′-TTATTCAGGCTTGTCAGGGGG-3′ | |
| Backward | 5′-GCACCATCAATCAGAAGGGAAG-3′ | 380 bp |
| β-actin | ||
| Forward | 5′-AACACCCCAGCCATGTACGTTG-3′ | |
| Backward | 5′-ATGTCACGCACGATTTCCCG-3′ | 254 bp |
Surface Expression of Fas, TNFR I, and TNFR II
Surface expression of Fas on NHBE and DLECs was analyzed by flow cytometry. Briefly, cells were grown on 10-cm diameter culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ). After reaching 70 to 80% confluency, the cells were detached with 0.025% trypsin/0.01% EDTA and washed with phosphate-buffered saline (PBS) (Sigma, St. Louis, MO). The cell pellet was resuspended at 106 cells/ml in PBS with 0.1% bovine serum albumin (Roche), and incubated at 4°C in the dark for 45 minutes, in the presence of 2.5 μg/ml of PE-conjugated mouse anti-human Fas, or control mAb. After incubation, the cells were washed twice with PBS and analyzed by flow cytometry (FACScan; Becton Dickinson, Mountain View, CA). The trypsinization procedure did not affect the expression of Fas on either cell type.
Determination of Cell Death
Cell death was assessed by the Alamar Blue fluorescence assay and DNA nick-end labeling assay.
Alamar Blue Assay
Alamar Blue (BioSource International, Camarillo CA) is a redox-sensitive indicator that fluoresces red in response to reducing equivalents generated during cellular metabolism. After incubation with experimental media and additives as described below, the experimental media was completely removed and fresh media supplemented with 10% Alamar Blue was added to the wells. The cells were then incubated for 4 hours at 37°C in 5% CO2. After incubation, fluorescence was measured at 530-nm excitation and 590-nm emission using a CytoFlour II fluorometer (PerSeptive Biosystems, Framingham, MA).
DNA Nick-End Labeling Assay
DNA nick-end labeling was performed using the TACS 1 Klenow-DAB in situ apoptosis detection kit (Trevigen, Gaithersburg, MD) according to the instructions from the manufacturer. Briefly, the cells were fixed in 3.7% buffered formaldehyde for 10 minutes, dehydrated in 70% ethanol for 5 minutes, and rehydrated in PBS (Sigma) for 10 minutes. After rehydration, the cells were treated with 0.02 mg/ml Proteinase K in double-distilled water for 15 minutes at room temperature. Endogenous peroxidase was quenched with 2% hydrogen peroxide (Sigma) in double-distilled water for 5 minutes. The slides were treated with Klenow labeling buffer for 1 minute, then incubated with Klenow enzyme and Klenow dNTP mix in Klenow labeling buffer for 60 minutes at 37°C. Negative control samples were incubated with the labeling mixture without the Klenow enzyme. After incubation, the slides were completely immersed in Klenow stop buffer for 5 minutes at room temperature and washed in PBS for 2 minutes, then treated with streptavidin-horseradish peroxidase detection solution for 15 minutes, washed twice for 2 minutes in PBS, and incubated for 7 minutes at room temperature in diaminobenzidine (Sigma) to develop color. Lastly, the slides were rinsed twice in double-distilled water and counterstained with 1% methyl green in 0.1 mol/L sodium acetate, pH 4.0, for 5 minutes, then quickly dehydrated in 95% and 100% ethanol, cleared in xylene, and mounted with Permount (Fisher Scientific, Pittsburgh, PA) on glass slides.
Effects of FasL and TNF-α on NHBE and DLEC Apoptosis
NHBE and DLEC were grown on 96-well tissue culture plates (Costar) until reaching 60 to 70% confluence, and incubated with media supplemented with serial concentrations of either sFasL or TNF-α. Control cells were incubated in untreated medium. After incubation for 18 hours at 37°C, 5% CO2, cell survival was determined using the Alamar Blue assay. Cell supernatants were collected to measure cytokine concentrations. To assess DNA fragmentation, the cells were grown on 16-well chamber slides (Nalge Nunc, Naperville, IL) until reaching 60 to 70% confluence, then incubated with media containing 250 ng/ml or 500 ng/ml of either sFasL, TNF-α, or untreated medium. The cells were incubated for 18 hours at 37°C in 5% CO2, then DNA fragmentation was determined using the DNA nick-end labeling assay. A minimum of 150 cells was counted in three high-power fields (×400), and the percentage of positive cells calculated. Positive cells were defined as cells staining with the brown diaminobenzidine reaction product.
Measurement of Proinflammatory Cytokines
The cytokines, IL-8, and monocyte chemoattractant protein-1 (MCP-1), were measured in cell culture supernatants by immunoassay. IL-8 was detected using anti-human IL-8 mAb (MAB208, R&D Systems) as the capture mAb and biotinylated anti-human IL-8 mAb (BAF208, R&D Systems) as the detection antibody. MCP-1 was measured using anti-human MCP-1 mAb (MAB679, R&D Systems) for capture and biotinylated anti-human MCP-1 mAb (BAF279, R&D Systems) for detection. The lower limit of detection for each of these assays was 15.5 pg/ml.
Modulation of Fas-Mediated Apoptosis by Cytokines
Preincubation Experiments
Cells were grown to 60 to 70% confluence in 96-well tissue culture plates, then incubated in fresh media supplemented with either 10 ng/ml TNF-α, 10 ng/ml IL-1β, 100 U/ml IFN-γ, or untreated medium for 18 hours at 37°C, 5% CO2. The concentrations of these cytokines were chosen according to previous studies in vivo and in vitro.4,32,34–37 The culture supernatants were then removed and cytokine-free fresh media containing serial concentrations of sFasL were added to the wells. After incubation for 18 hours at 37°C in 5% CO2, cell survival was assessed using the Alamar Blue assay.
Co-Incubation Experiments
Cells grown in 96-well tissue culture plates were incubated for 2 hours at 37°C, 5% CO2 in media containing either 10 ng/ml TNF-α, 10 ng/ml IL-1β, 100 U/ml IFN-γ, or untreated medium. The supernatants were removed and the cells were incubated with fresh media containing serial concentrations of sFasL plus either TNF-α, IL-1β, IFN-γ, or media only. After an additional 16 hours of incubation cell survival was measured by the Alamar Blue assay.
Effect of Proinflammatory Cytokines on Membrane Fas Expression
NHBE and DLEC were incubated in media in 10-cm diameter cell culture dishes (Becton Dickinson Labware) until reaching 60 to 70% confluence. The media was then replaced with media supplemented with either 10 ng/ml TNF-α, 10 ng/ml IL-1β, 100 U/ml IFN-γ, or untreated medium, and the cells were incubated for 18 hours at 37°C in 5% CO2. Surface expression of Fas was determined by flow cytometry.
Animal Protocol
The protocols were approved by the Animal Research Committee of the Puget Sound Health Care System. Briefly, mice were weighed and anesthetized with ketamine/xylazine (70 mg/kg ketamine, 10 mg/kg xylazine). The mice were placed on a 45° incline board, a fiberoptic light source was placed just above the thoracic inlet (IntraLux 6000–1; Volpi Manufacturing, Auburn, NY), and the vocal cords were visualized directly using a small catheter introducer (BD Biosciences, Rutherford, NJ). The trachea was cannulated with a 22-gauge gavage feeding needle (Kent Scientific, Litchfield, CT) connected to a tuberculin syringe. The plunger was removed from the tuberculin syringe and 100 μl of saline was placed at the distal end before its use. The correct position of the gavage needle in the trachea was verified by movement of the saline column in the tuberculin syringe. The anti-Fas mAb, Jo2 (1 μg/ml), or PBS were instilled directly into the trachea through the gavage needle, at a volume of 2.5 μl/g. Six hours later, the mice were euthanized with 120 mg/kg of pentobarbital. The mice were exsanguinated by direct cardiac puncture and the thoracic cavity was opened by midline incision. The trachea was exposed and cannulated with a 24-gauge catheter, which was secured with a 2-0 silk suture. The lungs were fixed overnight with 4% paraformaldehyde at an instillation pressure of 15-cm H2O, dehydrated with serial alcohol baths, and embedded in paraffin.
Caspase-3 Immunohistochemistry
Immunohistochemistry was performed using the TSA biotin labeling kit (NEN Life Science Products, Boston, MA). Briefly, the slides were deparaffinized by heating at 57°C for 60 minutes, and rehydrated by washing twice in Clear Rite (Richard Allan Scientific, Kalamazoo, MI), then twice in 100% ethanol for 3 minutes, twice in 95% ethanol for 3 minutes, and once in dH2O for 5 minutes. The slides were rinsed twice with PBS for 5 minutes and endogenous peroxidase was quenched with 3% H2O2 in MeOH for 60 minutes at room temperature. The samples were digested with citric buffer (Vector Laboratories, Burlingame CA) in a microwave for 15 minutes at a setting of 50. After digestion the slides were cooled to room temperature for 10 minutes, rinsed twice with PBS for 5 minutes and blocked with 3% normal goat serum in nonfat milk for 60 minutes at room temperature. Then, the samples were labeled with rabbit anti-active caspase-3 overnight in a moist chamber at 4°C. Next, the slides were rinsed twice with PBS and labeled with goat anti-rabbit biotinylated antibody for 2 hours at room temperature. The slides were rinsed twice with PBS and labeled with streptavidin-horseradish peroxidase, then incubated in a moist chamber at room temperature for 30 minutes. After rinsing twice with PBS, the slides were incubated in TSA-biotin at room temperature for 8.5 minutes, washed twice in PBS, and incubated again with 1 μmol/L To-Pro-3 and Alexa 568 (1:200) for 15 to 60 minutes at room temperature. The slides were washed twice in PBS, shaked dry, and mounted with an aqueous medium (Vectashield Hard, Vector Laboratories). The slides were examined using a confocal microscope.
Data Analysis
Data are shown as mean ± SEM. Comparisons between two groups were performed with Student’s unpaired t-test. Comparisons between multiple groups were performed using one-way analysis of variance followed by Fisher’s least significant difference test. A P value of less than 0.05 was considered significant.
Results
Fas Expression Is Similar in Proximal and Distal Lung Epithelial Cells
First, RT-PCR was used to determine whether mRNA transcripts coding for Fas, FasL, TNFR I, TNFR II, FADD, TRADD, and caspase-8 were present in total cellular RNA isolated from NHBE and DLEC. Products with the expected size of the Fas (564 bp) and TNFR I (393 bp) target sequences were amplified from both NHBE and DLEC (Figure 1A). Direct sequencing of these PCR products confirmed the expected Fas and TNFR I sequences. Neither NHBE nor DLEC expressed mRNA for FasL or TNFR II. The densitometric ratios of Fas and TNFR I were similar in NHBE and DLEC (Figure 1B). The expression of FADD, TRADD, and caspase-8 mRNA was similar in both cell types using similar methods (data not shown).
Figure 1.
A: Expression of mRNA for Fas, FasL, TNFR I, and TNFR II in NHBE and DLEC as determined by RT-PCR. Lanes 1 to 5 show results with NHBE and lanes 6 to 10 show results with DLEC. Lanes 1 and 6, Fas; lanes 2 and 7, FasL; lanes 3 and 8, TNFR I; lanes 4 and 9, TNFR II; lanes 5 and 10, β-actin. Results are representative of four separate studies. B: Densitometric ratio of mRNA expression of Fas and TNFR I against β-actin mRNA in NHBE and DLEC (n = 4). The expressions of RT-PCR products are expressed as the ratio against β-actin mRNA expression by densitometric analysis. Data are shown as means ± SEM. P = N.S.
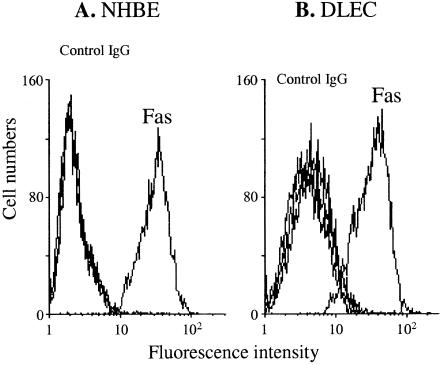
Second, the cell surface expression of Fas on NHBE and DLEC was determined by flow cytometry. Both NHBE and DLEC constitutively expressed Fas on their cell surface (Figure 2). The relative mean fluorescence intensity (rMFI) of Fas was calculated by dividing the mean fluorescence intensity of Fas by that of the control IgG as a marker of surface expression. The rMFI of surface Fas on NHBE was similar to that of DLEC (10.07 ± 1.82 versus 10.45 ± 2.48 for NHBE versus DLEC, n = 4).
Figure 2.
Surface expression of Fas on NHBE (A) and DLECs (B), as determined by flow cytometry. The curves for control IgG are superimposed in both panels. Results are representative of four separate studies.
Proximal and Distal Airway Epithelia Differ in Sensitivity for Fas Ligation
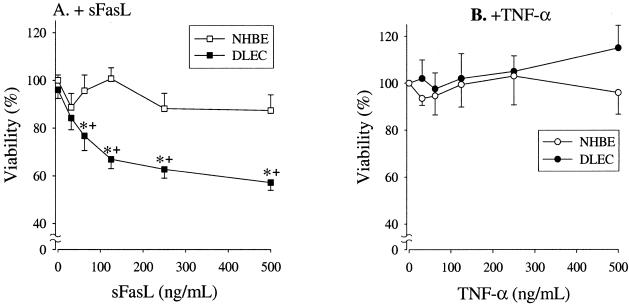
To ascertain the functional significance of cellular Fas, we investigated whether sFasL induces cell death in NHBE and DLEC. In addition, because TNFR I mRNA was detected in both NHBE and DLECs (Figure 1), we also investigated the effects of TNF-α on cell survival. Incubation of DLEC with rh-sFasL at concentrations ranging from 0 to 500 ng/ml resulted in a dose-dependent decrease in cell survival, as determined by Alamar Blue (Figure 3A). In contrast, sFasL had no effect on NHBE, even at high concentrations. Incubation in media supplemented with TNF-α at concentrations ranging from 0 to 500 ng/ml had no significant effect on the viability of either NHBE or DLEC (Figure 3B).
Figure 3.
Viability of NHBE and DLECs incubated with sFasL (A, n = 7) and TNF-α (B, n = 4). Data are shown as the percentage of control, which is the value of cells incubated with media only. Data are shown as means ± SEM. *, P < 0.05 versus NHBE; +, P < 0.005 versus control (incubated with media only).
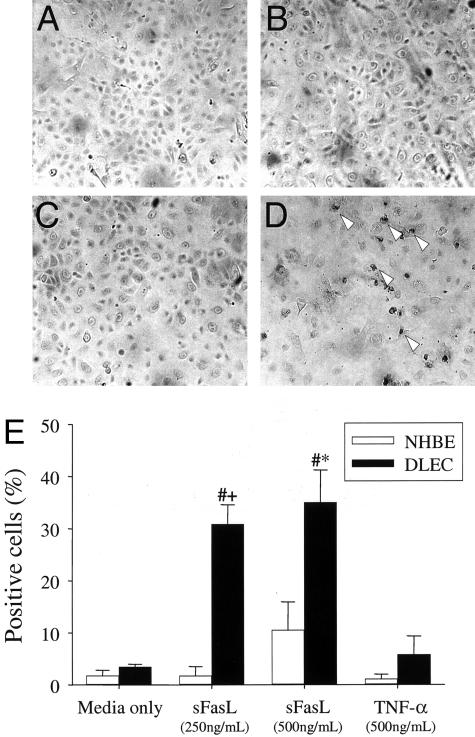
The DNA nick-end labeling assay showed DNA fragmentation in DLEC incubated for 18 hours in media supplemented with sFasL at 250 or 500 ng/ml, suggesting an apoptotic form of death (Figure 4; A to D). The percentage of DLEC with DNA fragmentation was significantly higher than that of DLEC incubated in media only (30.9 ± 3.7% and 35.1 ± 6.1% after incubation with sFasL, 250 and 500 ng/ml, respectively, as compared with 3.5 ± 0.5% for DLEC incubated in media only, n = 3, P < 0.005) (Figure 4E). A small number of NHBE contained DNA fragmentation after exposure to sFasL, 500 ng/ml, but the percentage of positive cells was not significantly different from that of NHBE incubated in media only (1.8 ± 1.0% versus 10.5 ± 5.4%, n = 3, P = N.S.). No significant DNA fragmentation was detected in either NHBE or DLEC after 18 hours of incubation in media containing 500 ng/ml TNF-α (Figure 4, D and H).
Figure 4.
DNA nick-end labeling assay findings in NHBE (A and B) and DLECs (C and D) incubated with media only (A and C) or sFasL 250 ng/ml (B and D). Arrowheads show some representative positive cells. The percentage of positive cells is shown in E (means ± SEM of three separate experiments). #, P < 0.05 compared to DLEC, media only; *, P < 0.05 compared to NHBE.
To confirm the biological relevance of the in vitro experiments, C57BL/6 mice were treated with intratracheal instillations of the Fas-activating mAb Jo2, and were euthanized 6 hours later (n = 3). Control mice were treated with PBS. Serial lung tissue sections were processed for activated (cleaved) caspase-3 by immunohistochemistry. Activated caspase-3 was detected in the epithelia covering the alveolar spaces and in some intra-alveolar cells, but was absent in the cells of the airway epithelium (Figure 5).
Figure 5.
Serial sections from the lungs of a mouse, 6 hours after intratracheal instillation of the Fas-activating mAb Jo2. The sections were treated with either normal rabbit serum (A) or rabbit anti-mouse active caspase-3 mAb (B). The nuclei are counterstained with DAPI (blue), the green signal is autofluorescence, and the red signal corresponds to anti-active caspase-3. Note the strong red signal generated in the walls of the alveolar spaces, but not in the airway epithelium. Original magnifications, ×400.
Fas Ligation Does Not Induce the Release of Proinflammatory Cytokines in Proximal or Distal Lung Epithelial Cells
Exposure to rh-sFasL at concentrations ranging from 0 to 500 ng/ml in vitro did not cause release of IL-8 in either NHBE or DLEC (data not shown). MCP-1 was not detectable in any of the supernatants.
Inflammatory Cytokines Increase the Sensitivity of NHBE and DLEC to Fas-Mediated Apoptosis
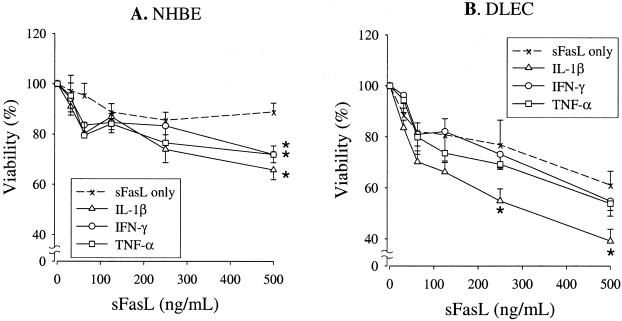
To determine whether the response of NHBE and DLEC to Fas ligation is modulated by TNF-α, IL1-β, or IFN-γ, we performed a series of preincubation experiments (exposure to cytokines before exposure to sFasL) and co-incubation experiments (simultaneous exposure to cytokines and sFasL). None of these cytokines when used alone induced death of either NHBE or DLEC (data not shown). NHBE preincubated for 18 hours in media supplemented with either TNF-α (10 ng/ml), IL-1β (10 ng/ml), or IFN-γ (100 U/ml) became sensitive to subsequent exposure to sFasL, whereas NHBE preincubated with media only remained insensitive to Fas ligation (n = 7) (Figure 6A). In contrast, when NHBE were simultaneously incubated with cytokines and FasL, the cytokines failed to increase the sensitivity to Fas ligation (n = 7) (Figure 7A). The responses of DLEC were slightly different from those of NHBE. Preincubation with TNF-α and IFN-γ, but not with IL-1β, increased the sensitivity of DLEC to subsequent exposure to sFasL (n = 7) (Figure 6B). In contrast, the sensitivity of DLEC to Fas ligation was not affected by simultaneous incubation with any of the cytokines tested (n = 7) (Figure 7B).
Figure 6.
Viability of NHBE (A) and DLEC (B) incubated with sFasL after stimulation with either TNF-α (10 ng/ml), IL-1β (10 ng/ml), or IFN-γ (100 U/ml) (prestimulation experiments.). Data are shown as the percentage of control, which is the value of cells incubated with media only after cytokine stimulation (n = 7). Data are shown as means ± SEM. *, P < 0.05 versus sFasL only (incubated with sFasL after incubation with media only).
Figure 7.
Viability of NHBE (A) and DLEC (B) co-incubated with sFasL and either TNF-α (10 ng/ml), IL-1β (10 ng/ml), or IFN-γ (100 U/ml) (co-incubation experiments.). Data are shown as the percentage of control, which is the value of cells incubated with cytokines only (n = 7). Data are shown as means ± SEM.
Cell Surface Fas Expression
We investigated whether the enhanced response of NHBE and DLEC to Fas ligation induced by inflammatory cytokines is associated with increased expression of surface Fas. NHBE and DLEC were incubated for 18 hours in media supplemented with either 10 ng/ml TNF-α, 10 ng/ml IL-1β, or 100 U/ml IFN-γ, then treated with either anti-Fas mAb or a control mAb and analyzed by flow cytometry. The surface expression of Fas was similar in cells incubated in media supplemented with cytokines, as compared to that of cells incubated in media only (n = 4). The rMFI of Fas in NHBE incubated with TNF-α, IL-1β, IFN-γ, and media alone was 8.57 ± 2.75, 9.45 ± 3.55, 11.28 ± 4.74, and 11.28 ± 2.67, respectively (n = 4, P = N.S.). The rMFI of Fas in DLEC incubated with TNF-α, IL-1β, IFN-γ, and media alone was 9.96 ± 1.87, 7.97 ± 1.88, 9.60 ± 1.37, and 10.39 ± 2.06, respectively (n = 4, P = N.S.). Exposure of both types of cells to these cytokines did not increase Fas expression on the cell surface. Therefore, cytokine exposure did not change surface Fas expression in either NHBE or DLEC.
Discussion
The main goal of this study was to investigate the responses of proximal and distal human lung epithelial cells to Fas ligation in vitro and in vivo, and the factors that modulate these responses. We found that both NHBE and DLEC constitutively expressed Fas, but that NHBE did not become apoptotic in response to Fas ligation unless stimulated by inflammatory cytokines. In contrast, DLEC were sensitive to Fas ligation at baseline, and the sensitivity increased significantly after stimulation with proinflammatory cytokines. Fas ligation did not lead to the release of proinflammatory cytokines from either NHBE or DLEC. In normal mice, the intranasal administration of the Fas-activating mAb Jo2 was followed by immunohistochemical evidence of caspase-3 activation in cells of the alveolar walls, but not in cells of the airway epithelium.
It has been postulated that alveolar epithelial injury during ARDS is associated, in part, with Fas-mediated apoptosis of alveolar epithelial cells. Because in ARDS epithelial injury is localized primarily to the distal airspaces,7,8 whereas Fas is expressed in both the distal and proximal epithelium,3,20,21,23,25 the hypothesis that the Fas/FasL system is a major inducer of alveolar epithelial cell apoptosis requires an explanation that accounts for the lack of injury seen in the airway epithelium. The differential sensitivity of lung airway epithelial cells to FasL seen in vitro and in vivo in this study suggests the existence of an apoptosis susceptibility gradient along the bronchial tree, with the sensitivity to Fas-mediated apoptosis increasing toward the distal regions of the lung. Although the shape of the dose response curves is somewhat different in Figures 3, 6, and 7, the overall sensitivity is the same in these three figures, with viability of DLEC in response to 500 ng/ml of FasL of 58% in Figure 3, 55% in Figure 6, and 55% in Figure 7. Thus, experimental variability exists in these experiments between different lots of cells, however the differential sensitivity is present in all of the experiments. This further supports our interpretation that DLEC are more sensitive to sFasL-mediated apoptosis than NHBE.
The finding that cytokines modulate the epithelial responses to Fas ligand has additional implications for acute lung injury. Proinflammatory cytokines, including TNF-α and IL-1β are present in bronchoalveolar lavage fluids from patients with ARDS.36–40 The bronchoalveolar lavage concentrations of TNF-α, IL-1β, and IL-8 correlate with indices of alveolar-epithelial permeability in humans with ARDS.36,37 The present results suggest that the local inflammatory milieu changes the response of the lung epithelium to sFasL. Thus, in ARDS, increases in TNF-α and IL-1β could result in important increases in the epithelial susceptibility to FasL, allowing relatively low concentrations of FasL to contribute to epithelial injury.5 Moreover, the observation that stimulation with TNF-α or IL-1β enhances the susceptibility of the bronchial epithelium to Fas-mediated apoptosis extends the findings in previous reports suggesting that TNF-α and IL-1β modulate the responses to sFasL,32 and provides an explanation for previous conflicting reports regarding the role of the Fas/FasL system in epithelial apoptosis.
The current findings also suggest that the effects of activation of the Fas/FasL system on lung epithelial cells result primarily in the activation of proapoptotic pathways, rather than activation of proinflammatory pathways. Previous investigators found that Fas ligation stimulates nuclear factor-κB activation and IL-8 production by DLEC.6 In that study, an agonistic antibody was used to aggregate membrane Fas, whereas we used the naturally occurring ligand (rh-sFasL), which may in part explain the experimental differences. Our previous study suggests that sFasL also has a proinflammatory role in vivo, through direct effects on alveolar macrophages and other nonepithelial cells.7
We investigated the mechanism whereby distal and proximal lung epithelial cells differ in their baseline susceptibility to Fas ligation, despite similar expression of cell membrane Fas and intracellular Fas mRNA. The difference in sensitivity was not associated with differential expression of FADD, TRADD, or caspase-8 mRNA, as detected by RT-PCR. Therefore, the factors that modulate the differential responses to Fas ligation remain unknown. One explanation for the differential sensitivity of lung epithelial cells to Fas ligation could involve major differences in the oligomerization of the intracellular components of the Fas receptor, which is necessary for recruiting docking proteins to form the caspase activation complex.41 As an alternative explanation, there may be important differences in the three-dimensional structure of the intracellular receptor cluster formed by the interaction of Fas and FasL.41
The mechanisms whereby TNF-α and IL-1β modulate the response to FasL also remain unclear. In other types of cells, such as the lung adenoma cell line LA-4, both TNF-α and IL-1β increased the sensitivity to Fas-mediated apoptosis.32 Similarly, Hagimoto and colleagues6 found that changes in surface expression of Fas represent a mechanism whereby the combination of TNF-α and IFN-γ modifies the cellular response to Fas ligation. However, in the present study, neither TNF-α nor IL-1β modified surface expression of Fas on NHBE or DLEC. It remains to be determined whether induction of proapoptotic proteins and/or reduction of anti-apoptotic proteins underlie the modulation of responses to Fas ligation by inflammatory cytokines. Alternatively, structural changes in cell surface Fas (clustering versus aggregation) might be associated with cytokine stimulation.
In summary, Fas (CD95) is constitutively expressed in both proximal and distal airway epithelial cells, yet these cells respond differently to Fas ligation in vitro and in vivo. The responses of the epithelium to Fas ligation become more pronounced from proximal to distal locations. In both types of epithelial cells, activation of Fas leads primarily to activation of proapoptotic pathways, rather than activation of proinflammatory pathways. This segment-specific susceptibility to Fas/FasL-dependent apoptosis may be an important mechanism in the pathogenesis of lung diseases. Inflammatory cytokines increase the sensitivity of both proximal and distal epithelial cells to Fas-mediated apoptosis, suggesting that the local inflammatory milieu may modulate the response of pulmonary epithelial cells to sFasL. Fas-induced death of lung epithelial cells could be an explanation for the disruption of the distal airway epithelium in acute lung injury.
Acknowledgments
We thank Amy Selk and Kimberly Ballman for expert technical assistance.
Footnotes
Address reprint requests to Thomas R. Martin, M.D, Seattle Veterans Affairs Medial Center, 151L, 1660 South Columbian Way, Seattle, WA 98108-1597. E-mail: trmartin@u.washington.edu.
Supported by the National Institutes of Health (grants HL 70840-01 to G.M.B., HL62995 to W.C.L., and HL30542 to T.R.M.) and the Medical Research Service of the Department of Veterans Affairs.
References
- Bachofen H, Schurch S, Michel RP, Weibel ER. Experimental hydrostatic pulmonary edema in rabbit lungs. Morphology. Am Rev Respir Dis. 1993;147:989–996. doi: 10.1164/ajrccm/147.4.989. [DOI] [PubMed] [Google Scholar]
- Bachofen H, Schurch S, Weibel ER. Experimental hydrostatic pulmonary edema in rabbit lungs. Barrier lesions. Am Rev Respir Dis. 1993;147:997–1004. doi: 10.1164/ajrccm/147.4.997. [DOI] [PubMed] [Google Scholar]
- Fine A, Anderson NL, Rothstein TL, Williams MC, Gochuico BR. Fas expression in pulmonary alveolar type II cells. Am J Physiol. 1997;273:L64–L71. doi: 10.1152/ajplung.1997.273.1.L64. [DOI] [PubMed] [Google Scholar]
- Wen LP, Madani K, Fahrni JA, Duncan SR, Rosen GD. Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN-gamma and Fas. Am J Physiol. 1997;273:L921–L929. doi: 10.1152/ajplung.1997.273.5.L921. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- Hagimoto N, Kuwano K, Kawasaki M, Yoshimi M, Kaneko Y, Kunitake R, Maeyama T, Tanaka T, Hara N. Induction of interleukin-8 secretion and apoptosis in bronchiolar epithelial cells by Fas ligation. Am J Respir Cell Mol Biol. 1999;21:436–445. doi: 10.1165/ajrcmb.21.3.3397. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Liles WC, Frevert CW, Nakamura M, Ballman K, Vathanaprida C, Kiener PA, Martin TR. Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am J Physiol. 2001;281:L328–L335. doi: 10.1152/ajplung.2001.281.2.L328. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol. 2001;158:153–161. doi: 10.1016/S0002-9440(10)63953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochuico BR, Miranda KM, Hessel EM, De Bie JJ, Van Oosterhout AJ, Cruikshank WW, Fine A. Airway epithelial Fas ligand expression: potential role in modulating bronchial inflammation. Am J Physiol. 1998;274:L444–L449. doi: 10.1152/ajplung.1998.274.3.L444. [DOI] [PubMed] [Google Scholar]
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzio M, Salvesen GS, Dixit VM. FLICE induced apoptosis in a cell-free system. Cleavage of caspase zymogens. J Biol Chem. 1997;272:2952–2956. doi: 10.1074/jbc.272.5.2952. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- Rensing-Ehl A, Hess S, Ziegler-Heitbrock HW, Riethmuller G, Engelmann H. Fas/Apo-1 activates nuclear factor kappa B and induces interleukin-6 production. J Inflamm. 1995;45:161–174. [PubMed] [Google Scholar]
- Ponton A, Clement MV, Stamenkovic I. The CD95 (APO-1/Fas) receptor activates NF-kappaB independently of its cytotoxic function. J Biol Chem. 1996;271:8991–8995. doi: 10.1074/jbc.271.15.8991. [DOI] [PubMed] [Google Scholar]
- Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- Uhal BD, Joshi I, True AL, Mundle S, Raza A, Pardo A, Selman M. Fibroblasts isolated after fibrotic lung injury induce apoptosis of alveolar epithelial cells in vitro. Am J Physiol. 1995;269:L819–L828. doi: 10.1152/ajplung.1995.269.6.L819. [DOI] [PubMed] [Google Scholar]
- French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J. Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri L, Devilard E, Hassoun J, Mawas C, Birg F. Fas ligand is not only expressed in immune privileged human organs but is also coexpressed with Fas in various epithelial tissues. Mol Pathol. 1997;50:87–91. doi: 10.1136/mp.50.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochuico BR, Miranda KM, Hessel EM, De Bie JJ, Van Oosterhout AJ, Cruikshank WW, Fine A. Airway epithelial Fas ligand expression: potential role in modulating bronchial inflammation. Am J Physiol. 1998;274:L444–L449. doi: 10.1152/ajplung.1998.274.3.L444. [DOI] [PubMed] [Google Scholar]
- Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, White SR. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol. 1998;19:537–542. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]
- Kuwano K, Hagimoto N, Kawasaki M, Yatomi T, Nakamura N, Nagata S, Suda T, Kunitake R, Maeyama T, Miyazaki H, Hara N. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest. 1999;104:13–19. doi: 10.1172/JCI5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe ME, Rubin LP, Jude C, Lesieur-Brooks AM, Mills DR, Luks FI. Fas ligand expression coincides with alveolar cell apoptosis in late-gestation fetal lung development. Am J Physiol. 2000;279:L967–L976. doi: 10.1152/ajplung.2000.279.5.L967. [DOI] [PubMed] [Google Scholar]
- Hagimoto N, Kuwano K, Miyazaki H, Kunitake R, Fujita M, Kawasaki M, Kaneko Y, Hara N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol. 1997;17:272–278. doi: 10.1165/ajrcmb.17.3.2893. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, Matthay MA, Ware LB. Fas and Fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiji M, Adachi Y, Sogo S, Taketani S, Oyaizu N, Than S, Inaba M, Phawa S, Hioki K, Ikehara S. Apoptosis of colorectal adenocarcinoma (COLO 201) by tumour necrosis factor-alpha (TNF-alpha) and/or interferon-gamma (IFN-gamma), resulting from down-modulation of Bcl-2 expression. Clin Exp Immunol. 1998;111:211–218. doi: 10.1046/j.1365-2249.1998.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttmann W, Opfer A, Dauer E, Foerster M, Matthys H, Eibel H, Schulze-Osthoff K, Kroegel C, Virchow JC. Differential regulation of CD95 (Fas/APO-1) expression in human blood eosinophils. Eur J Immunol. 1998;28:2057–2065. doi: 10.1002/(SICI)1521-4141(199807)28:07<2057::AID-IMMU2057>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Tsushima H, Imaizumi Y, Imanishi D, Fuchigami K, Tomonaga M. Fas antigen (CD95) in pure erythroid cell line AS-E2 is induced by interferon-gamma and tumor necrosis factor-alpha and potentiates apoptotic death. Exp Hematol. 1999;27:433–440. doi: 10.1016/s0301-472x(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Trifilieff A, Futjitani Y, Coyle AJ, Bertrand C. Fas-induced death of a murine pulmonary epithelial cell line: modulation by inflammatory cytokines. Fundam Clin Pharmacol. 1999;13:656–661. doi: 10.1111/j.1472-8206.1999.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Ruemmele FM, Russo P, Beaulieu J, Dionne S, Levy E, Lentze MJ, Seidman EG. Susceptibility to FAS-induced apoptosis in human nontumoral enterocytes: role of costimulatory factors. J Cell Physiol. 1999;181:45–54. doi: 10.1002/(SICI)1097-4652(199910)181:1<45::AID-JCP5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Riccioli A, Starace D, D’Alessio A, Starace G, Padula F, De Cesaris P, Filippini A, Ziparo E. TNF-alpha and IFN-gamma regulate expression and function of the Fas system in the seminiferous epithelium. J Immunol. 2000;165:743–749. doi: 10.4049/jimmunol.165.2.743. [DOI] [PubMed] [Google Scholar]
- O’Connell J, Bennett MW, Nally K, O’Sullivan GC, Collins JK, Shanahan F. Interferon-gamma sensitizes colonic epithelial cell lines to physiological and therapeutic inducers of colonocyte apoptosis. J Cell Physiol. 2000;185:331–338. doi: 10.1002/1097-4652(200012)185:3<331::AID-JCP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108:1303–1314. doi: 10.1378/chest.108.5.1303. [DOI] [PubMed] [Google Scholar]
- Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD, Martin TR. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997;155:1187–1205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- Park WY, Goodman RB, Steinberg KP, Ruzinski JT, Radella F, II, Park DR, Pugin J, Skerrett SJ, Hudson LD, Martin TR. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- Thilenius AR, Braun K, Russell JH. Agonist antibody and Fas ligand mediate different sensitivity to death in the signaling pathways of Fas and cytoplasmic mutants. Eur J Immunol. 1997;27:1108–1114. doi: 10.1002/eji.1830270510. [DOI] [PubMed] [Google Scholar]