Abstract
The possible role of retinoblastoma protein (Rb) in the pathogenesis of anaplastic large-cell lymphoma (ALCL) is unknown. We investigated Rb protein expression, both total (phosphorylated and underphosphorylated) and active (underphosphorylated), in four anaplastic lymphoma kinase (ALK)-positive ALCL cell lines (Karpas 299, JB-6, SU-DHL1, and SR-786) by Western blot analysis, and in 67 ALCL tumors (30 ALK-positive, 37 ALK-negative) using immunohistochemical methods. We also used fluorescence in situ hybridization and polymerase chain reaction methods to assess for loss of heterozygosity of the rb gene. The findings were correlated with apoptotic rate assessed by the terminal dUTP nick-end labeling assay. Immunoblots showed high total Rb levels in Karpas 299, SU-DHL1 and SR-786 and relatively lower levels in and JB-6. Underphosphorylated Rb was negative or expressed at low levels in all cell lines. In ALCL tumors, total Rb was detected in 44 (66%) and absent in 23 (34%). The mean apoptotic rate was 3.2% in Rb-negative tumors compared with 2.7%, 2.2%, and 1.2% in tumors with <10%, 10 to 50%, and >50% Rb-positive cells, respectively (P = 0.2, Kruskall-Wallis test). In a subset of 25 total Rb-positive tumors we assessed for underphosphorylated Rb, which was detected in 12 tumors. The detection of only total Rb in the remaining 13 tumors suggests that Rb was phosphorylated. Fluorescence in situ hybridization showed allelic loss of the rb gene in 10 (40%) of 25 tumors analyzed and was significantly associated with absence of Rb expression (P = 0.003). Similar results were obtained for loss of heterozygosity of the 13q14 locus. Five-year progression-free survival for patients with Rb-negative ALCL was 89.4% compared with 47.7% for patients with total Rb-positive ALCL (P = 0.006, log-rank test). Similar trends for progression-free survival held true for patients with ALK-positive and ALK-negative tumors analyzed separately. In conclusion, Rb is absent or phosphorylated in most ALCL cell lines and tumors and absence of Rb expression is associated with better clinical outcome in patients with ALCL.
The retinoblastoma (rb) gene, located at 13q14, was the first tumor suppressor gene identified by its involvement in hereditary retinoblastoma.1–3 Accumulating evidence from in vitro and in vivo studies suggests that the rb gene product, a 105-kd protein, is implicated in many cellular functions including cell proliferation, apoptosis, and differentiation.4 The role of Rb protein as a major cell-cycle inhibitor is mediated through its interaction with the E2F transcription factors.5,6 Rb directly binds and inhibits the transcriptional activity of E2F family members by recruitment of several chromatin-remodeling complexes to promoter regions, resulting in chromatin condensation and inhibition of transcription.7–9 As a result, cells undergo arrest in the G1 phase of the cell cycle.10 Although Rb is normally expressed throughout the entire cell cycle, its function depends on its phosphorylation status.11 Underphosphorylated Rb is the active, growth suppressive form of the protein, and phosphorylated Rb is inactive, incapable of binding to E2F proteins. Progressive phosphorylation of Rb in mid to late G1 phase of the cell cycle, by cyclin/cyclin-dependent kinase (CDK) complexes, results in release of E2F proteins, transcription of numerous target genes, and entry of the cell cycle into S phase. CDKs, in turn, are negatively regulated by their inhibitors including the INK4 (p14ARF, p15, p16, p18) and Cip/Kip (p21, p27, p57) families.12 Rb is also involved in apoptotic mechanisms. Loss of Rb function is known to trigger the p53-dependent apoptotic pathway,13 possibly by release of free E2F proteins, which in turn activate p14ARF and other apoptosis-related proteins.14 However, recent studies suggest that loss of Rb function can activate apoptotic pathways that are independent of p53 as well.15
Rb may be inactivated by gene mutation, or by protein changes including hyperphosphorylation, sequestration by viral oncoproteins, and degradation by a caspase-dependent proteolytic pathway.10,16 In many tumor types, rb gene alterations may result in complete loss or low levels of Rb expression.4 Homozygous deletions of the 13q14 locus have been detected in a small subset of lymphoid malignancies,17–19 but these studies have failed to show a correlation between 13q14 deletions and Rb expression in these tumors.
A number of studies have investigated Rb protein expression in various types of non-Hodgkin’s lymphomas with conflicting results.19–24 Because the methods used in these studies did not assess Rb phosphorylation status, it is not known whether the Rb protein detected in these tumors was active (underphosphorylated) or inactive (phosphorylated).
In this study, we assessed Rb expression in anaplastic large cell lymphoma (ALCL), a distinct lymphoma type that frequently carries the t(2;5)(p23;q35) or variant abnormalities involving the 2p23 locus, resulting in overexpression of anaplastic lymphoma kinase (ALK).25–27 We hypothesized that the Rb pathway may be altered in ALCL because previous studies of these tumors have shown deregulation of other cell-cycle-controlling proteins.28–31 To investigate the functional status of Rb in ALCL, we assessed a subset of these tumors for expression of underphosphorylated Rb. We also used fluorescence in situ hybridization (FISH) and polymerase chain reaction (PCR) methods to assess for possible loss of rb alleles. We correlated these results with tumor apoptotic rate (AR), proliferation index, and clinical outcome.
Materials and Methods
Cell Lines and Western Blot Analysis
Four ALK-positive ALCL cell lines, all known to carry the t(2;5) were used, including Karpas 299 (a gift from Dr. M. Kadin, Beth-Israel-Deaconess Medical Center, Boston, MA), SR-786, SU-DHL-1 (both from DSMZ, Braunschweig, Germany), and JB-6 (a gift from Dr. D. Jones, M.D. Anderson Cancer Center, Houston, TX). The cell lines were maintained in RPMI 1640 medium supplemented with 1% nonessential amino acids, 10% fetal calf serum (Invitrogen Corp., Grand Island, NY), and 1% streptomycin-penicillin. Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. For the serum starvation experiments, Karpas 299 and SU-DHL-1 cells were maintained in RPMI 1640 medium supplemented with only 1% fetal calf serum (Invitrogen Corp.), and 1% streptomycin-penicillin. Lysates from serum-starved cells were prepared before the experiment (control or time point 0 hours) and after 48 and 72 hours.
Total protein was extracted from these cell lines and Western blot analysis performed using methods described previously.31 Primary antibodies specific for total Rb (clone Rb1; DAKO, Carpinteria, CA) and underphosphorylated Rb (clone G99-549; BD Biosciences Pharmingen, San Diego, CA) were used. We further tested the specificity of the underphosphorylated Rb antibody using Western blot analysis and lysates of previously serum-starved Karpas 299 cells. Because the underphosphorylated Rb migrates faster in 6% polyacrylamide gels than the phosphorylated form of the protein (higher molecular weight), immunoblots using the underphosphorylated Rb antibody revealed the presence of a band corresponding only to a lower molecular weight (underphosphorylated) protein product. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Ambion, Austin, TX) was used as a control for protein load and integrity.
ALCL Tumors
This group included 67 cases of systemic ALCL obtained from previously untreated patients accessioned at The University of Texas M.D. Anderson Cancer Center and the University of Freiburg, Germany from 1982 to 2001. The diagnosis of ALCL was based on morphological and immunohistological criteria as specified by the World Health Organization classification.25 ALK-positive lymphomas, regardless of morphological features (ie, classical, monomorphous, lymphohistiocytic), were diagnosed as ALCL. ALK-negative ALCL tumors were composed predominantly of large anaplastic cells. Some investigators, as acknowledged in the World Health Organization classification, believe that ALK-negative ALCLs are better classified as high-grade peripheral T-cell lymphoma unspecified. All ALCL tumors were uniformly positive for CD30 and negative for B-cell antigens, including CD20, CD79a, and PAX-5. Fifty-three (79%) tumors were T cell and 14 (21%) were null cell. ALK was assessed using the ALK-1 antibody (1:30, DAKO) and was positive in 30 (45%) cases.
All patients were treated with doxorubicin-based chemotherapy. The clinicopathological features of most of these patients have been reported previously.32 The median age of patients with ALK-positive tumors was 29 years compared with 52 years for patients with ALK-negative tumors (P < 0.0001 by Mann-Whitney U-test). Patients with ALK-negative tumors were more frequently associated with anemia (P = 0.03, Fisher’s exact test). All other clinicopathological parameters between the two groups were comparable.
Design and Construction of the Tissue Array
Tissue sections of ALCL tumors, 5 μm thick, were cut from whole paraffin blocks (19 tumors) or a tissue microarray (48 tumors). The tissue microarray included triplicate or quadruplet tumor cores from all 48 tumors and two reactive lymph nodes and was constructed using a manual tissue arrayer (Beecher Instruments, Silver Springs, MD) as described previously.33
Immunohistochemical Methods and Terminal dUTP Nick-End Labeling (TUNEL) Assay
The immunohistochemical methods used in this study have been described previously.32 For all antibodies, heat-induced epitope retrieval was performed. Rb was assessed using monoclonal antibodies specific for total Rb (clone Rb1, dilution 1:50; DAKO) and underphosphorylated Rb (clone G99-549, dilution 1:25; BD Biosciences). The latter antibody recognizes an epitope between amino acids 514 to 610 of human Rb and does not recognize the phosphorylated forms of the protein. Proliferation rate was assessed using the MIB-1 (Ki-67) antibody (dilution 1:120; Immunotech, Westbrook, ME). The slides were incubated with the total and underphosphorylated Rb monoclonal antibodies at 4°C overnight, and with MIB-1 at room temperature for 1 hour. Detection of the immunoreaction was performed using the LSAB+ kit (DAKO). Reactive small lymphocytes in all tissue sections served as internal positive controls for total Rb and underphosphorylated Rb. Slides stained with normal rabbit serum (DAKO) without primary antibody were used as negative controls.
Any nuclear staining of ALCL cells was considered positive, irrespective of intensity. Expression levels for Rb were determined by counting the percentage of positive tumor cells and, for the purpose of statistical analysis, the tumors were divided into five groups as follows: negative, <10% positive, 10 to 50% positive, 50 to 90% positive, and >90% positive. Proliferation index was designated as the percentage of MIB1-positive tumor nuclei. AR was assessed using a modified TUNEL assay and designated as the percentage of TUNEL-positive tumor nuclei as described elsewhere.32
FISH
FISH was performed on a 5-μm paraffin section from the tissue microarray used in this study after appropriate deparaffinization in fresh xylene. Our FISH methods have been described in detail elsewhere.34,35 We used the Spectrum Orange LSI 13/RB-1 and the CEP 8 probes purchased from Vysis (Downers Grove, IL) to assess the rb gene and the centromeric region (8p11.1-q11.1) of chromosome 8 (control), respectively. The tissue microarray slide was counterstained with 4,6-diamidino-2-phenylindole in an anti-fade mounting fluid before it completely dried. The signal was viewed at ×1000 magnification with a Zeiss fluorescence microscope (Carl Zeiss, Thornwood, NY) using Vysis FISH filters specific for 4,6-diamidino-2-phenylindole/Spectrum Orange. We analyzed at least 100 evaluable nuclei of each tumor. Only isolated cells with nonoverlapping nuclei were counted. To avoid overestimation of tumors with allelic loss of rb because of sectioning of the tumor cells, we defined allelic loss of rb as being present using a conservative cutoff of 50% or more cells that had only one signal with the RB1 probe. Most small reactive lymphocytes cut in full cross-section in tumor cores showed two signals and served as internal positive controls for the presence of both rb alleles.
Loss of Heterozygosity (LOH) Studies
After histological examination of 37 ALCL tumors (12 ALK-positive, 25 ALK-negative), areas of tumor infiltration were delineated microscopically, and most of the uninvolved lymph node or other normal tissue in the paraffin blocks was removed with sterile surgical blades. Subsequently, 20-μm sections were cut from the trimmed blocks and genomic DNA was extracted using the QIAamp DNA extraction kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions.
To determine LOH, genomic DNA was amplified using a PCR method and two microsatellite markers, D13S153 and D13S263, spanning the 13q14 locus. Both microsatellite markers are known to have a high frequency of heterozygosity (82% and 84%, respectively). The D13S153 and D13S263 markers were labeled with the fluorescent dyes TET and HEX, respectively, and were purchased from Research Genetics (Huntsville, AL). In addition, a control microsatellite marker derived from the 13q32 locus and labeled with HEX (Research Genetics) was used. The 50-μl reaction mixture contained 500 ng of DNA (5 μl), 5 μl of 10× PCR buffer (Invitrogen Corp.), 1.5 mmol/L MgCl2, 200 μmol/L each dNTP, 200 nmol/L each primer pair (Research Genetics) and 2.5 U of TaqDNA polymerase (Invitrogen Corp.). The TaqDNA polymerase was added to the reaction mixture after the denaturation step (hot start). The thermal cycler was programmed as follows: 95°C for 10 minutes (denaturation); 30 cycles of 45 seconds at 94°C, 45 seconds at 54°C, and 60 seconds at 72°C; and finally 10 minutes at 72°C. The presence of the PCR products was confirmed by electrophoresis on a 1.5% agarose gel. Amplified products were subsequently analyzed by high-resolution capillary electrophoresis using the 310-Genetic Analyzer (PE/Applied Biosystems, Foster City, CA), as previously described.36 Fluorescence data were analyzed using GeneScan and Genotyper software (PE/Applied Biosystems). Informative cases were heterozygous for the RB1 locus. Cases homozygous for this locus were considered unevaluable for LOH. In heterozygous cases, a decrease in signal intensity of more than 50% between the two alleles was required to establish the presence of LOH.
Statistical Analysis
The chi-square and Fisher’s exact tests were used to compare total and underphosphorylated Rb expression (positive versus negative) with various clinicopathological parameters. The Mann-Whitney U-test and the Kruskal-Wallis test were chosen for the nonparametric correlation of proliferation index and AR between various Rb expression levels. Progression-free survival (PFS) was defined as time from initiation of therapy to last clinical follow-up, primary treatment failure, or relapse. Univariate survival analysis was based on the method of Kaplan and Meier using the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. All computations were performed using the StatView statistical program (Abacus Concepts, Inc., Berkeley, CA).
Results
Cell Lines
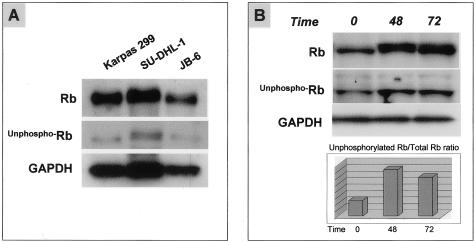
Total Rb was expressed at high levels in Karpas 299, SU-DHL1, and SR-786 cells and at lower levels in JB-6 cells (Figure 1A). Immunoblots showed a thick band in positive cell lines, consistent with phosphorylated and hyperphosphorylated forms of Rb.19 Underphosphorylated Rb was detected at low levels in all four ALCL cell lines (Figure 1A). Serum starvation of SU-DHL1 and Karpas 299 cells resulted in an increased underphosphorylated Rb/total Rb ratio indicating that a subset of these cells underwent cell-cycle arrest through activation of Rb protein (Figure 1B).
Figure 1.
Expression of Rb in ALCL cell lines. A: Immunoblot showing three cell lines that express total Rb, with Karpas 299 and SU-DHL-1 cells having higher levels compared with JB-6 cells. Underphosphorylated Rb was detected at low levels in all three ALCL cell lines. Similar results were obtained for the SR-786 cell line (data not shown). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included as a control for protein load and integrity, and its levels are similar among all three ALCL cell lines. B: Immunoblot showing the levels of total and underphosphorylated Rb protein after serum starvation of SU-DHL-1 cells. Densitometry showed that the underphosphorylated Rb/total Rb ratio was higher in cells treated with RPMI and only 1% of FBS (serum starvation) at 48 and 72 hours compared with control cells.
Expression of Total Rb in ALCL Tumors
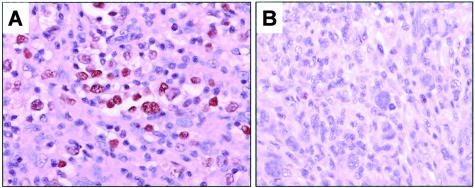
In reactive lymph nodes, total Rb was detected predominantly in germinal center cells as described previously.37 Total Rb was detected in 44 (66%) ALCL, including 18 of 30 (60%) ALK-positive and 26 of 37 (70%) ALK-negative tumors (P = 0.4, Fisher’s exact test). Rb immunoreactivity was localized in the tumor nuclei with variable staining intensity (Figure 2). Three tumors had >90% cells positive, 11 tumors had >50 to 90% cells positive, 15 tumors had 10 to 50% cells positive, and 15 tumors had <10% cells positive. Twenty-three (34%) ALCLs showed complete absence of Rb (Figure 2) including 12 ALK-positive and 11 ALK-negative. Rb expression in ALCL did not significantly correlate with clinical and laboratory features in these patients.
Figure 2.
Total Rb expression in ALCL tumors. A: A Rb-positive ALCL case with strong nuclear immunoreactivity in most tumor cells. B: A Rb-negative ALCL case. Occasional small reactive lymphocytes were Rb-positive and served as internal positive controls. DAB, hematoxylin counterstain, for all panels.
Correlation of Total Rb Expression with AR and Proliferation Index
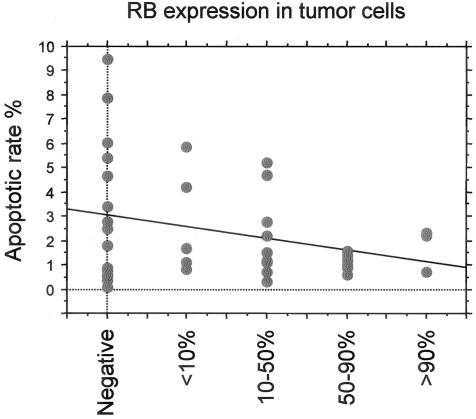
AR was assessed previously for 43 of these ALCL tumors.32 In this group, higher AR was commonly associated with absence or lower numbers of total Rb-positive cells as shown in Figure 3. The mean AR was 3.2% for Rb-negative ALCLs and 1.9% for Rb-positive ALCLs. In Rb-positive tumors, the percentage of positive cells also appeared to be inversely correlated with AR although this was not statistically significant (P = 0.2, Kruskal-Wallis test; Figure 3). More specifically, the mean AR was 2.7%, 2.2%, and 1.2% in tumors with <10%, 10 to 50%, and >50% Rb-positive cells, respectively. Correlation between AR and functional status of Rb, showed that the mean AR was 3.2% for Rb-negative tumors, compared with 1.9% for underphosphorylated Rb-positive tumors and 1.6% for phosphorylated Rb-positive tumors (P = not significant, Kruskal-Wallis test). Proliferation index was evaluated in 51 ALCL tumors and ranged from 20 to 99%, with a mean of 75 ± 17%. No correlation between proliferation index and total Rb expression was observed.
Figure 3.
Association of total Rb expression with apoptotic rate (AR) in ALCL tumors. AR appears to correlate inversely with percentage of Rb-positive cells.
Expression of Underphosphorylated Rb in ALCL Tumors
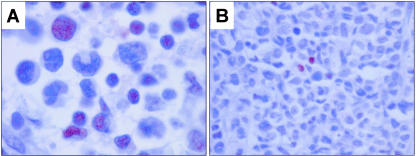
In reactive lymph nodes, underphosphorylated Rb was detected in a small subset of germinal center cells and small reactive lymphocytes. Expression of underphosphorylated Rb was assessed in a subset of 40 ALCL, including 25 total Rb-positive and 15 total Rb-negative. As shown in Table 1, 12 (30%) of these 40 tumors, all total Rb-positive, expressed underphosphorylated Rb (Figure 4). In the group of 12 total Rb-positive tumors, 5 tumors had >50 to 90% cells positive, 5 tumors had 10 to 50% cells positive, and 2 tumors had <10% cells positive. In four tumors, the number of underphosphorylated Rb-positive cells approximated the number of total Rb-positive cells. Hence, in eight tumors the number of underphosphorylated Rb-positive cells was clearly less than the number of total Rb-positive cells, suggesting that Rb in these tumors is predominantly phosphorylated (inactive) (Table 1). However, our methods do not allow us to quantify the amounts of total Rb and underphosphorylated Rb in individual tumor cells. Expression of underphosphorylated Rb did not correlate with ALK expression (Table 2).
Table 1.
Total Rb and Underphosphorylated Rb Expression in 40 ALCL Tumors
| Underphosphorylated Rb expression
|
||
|---|---|---|
| Positive | Negative | |
| Total Rb expression | ||
| Positive | 12 | 13 |
| Negative | 0 | 15 |
| P value* | P = 0.001 | |
By Fisher’s exact test.
Figure 4.
Expression of underphosphorylated Rb in ALCL tumors. A: An ALK-positive ALCL case positive for underphosphorylated Rb in a small number (<10%) of tumor cells. Intensity of underphosphorylated Rb immunostaining was generally weaker compared with total Rb. B: An ALK-positive ALCL case negative for underphosphorylated Rb. Occasional small reactive lymphocytes show nuclear immunoreactivity and are considered as internal positive controls. DAB, hematoxylin counterstain, for all panels.
Table 2.
Total Rb Expression, Underphosphorylated Rb Expression, and Allelic Loss of the rb Locus in ALK-positive and ALK-negative ALCL
| Total Rb expression (n = 67) | Underphosphorylated Rb expression (n = 40) | Allelic loss of rb locus
|
||
|---|---|---|---|---|
| FISH (n = 25) | LOH (n = 20) | |||
| ALK-positive | 18/30 (60%) | 4/17 (24%) | 5/11 (45%) | 2/6 (33%) |
| ALK-negative | 26/37 (70%) | 8/23 (35%) | 5/14 (36%) | 7/14 (50%) |
| P value* | P = 0.4 | P = 0.5 | P = 0.7 | P = 0.6 |
By Fisher’s exact test.
FISH Analysis of the rb Gene
To investigate the mechanism of relatively low level of Rb expression in JB-6 cells, we performed FISH analysis using cytospin preparations, a probe specific for the RB1 locus, and a control chromosome 8 centromeric probe. FISH analysis revealed the presence of an equal number of alleles for the RB1 and control probes suggesting no deletion of the rb gene locus (data not shown).
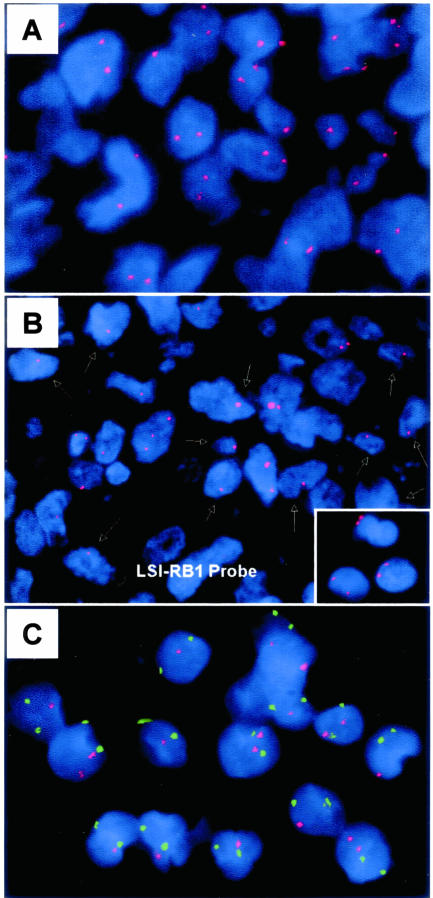
The rb gene was assessed for allelic loss in 25 ALCL (11 ALK-positive, 14 ALK-negative). Deletion of at least one rb allele was found in 10 (40%) tumors (Figure 5), 5 ALK-positive and 5 ALK-negative (Table 2). Nine of these tumors had hemizygous allelic loss at rb and one ALK-negative tumor had homozygous deletion of rb. Loss of rb correlated significantly with absence of total Rb protein. Eight of ten (80%) tumors with rb allelic loss were negative for Rb and two tumors expressed Rb in less than 50% of cells. In contrast, only 1 of 15 (7%) tumors that retained both rb alleles were negative for Rb (P = 0.003, Fisher’s exact test).
Figure 5.
FISH analysis for allelic loss at the rb locus using the Spectrum Orange LSI 13/RB-1 probe (Vysis). A: An ALK-positive ALCL case with most tumor cells showing two signals indicating the presence of both rb alleles. B: An ALK-negative ALCL case with hemizygous allelic loss of rb. Most tumor nuclei show a single signal indicated by arrows. Inset: Small reactive lymphocytes present in all tumor samples show two distinct signals for rb gene locus. C: An ALK-positive ALCL case with deletion of the rb locus. The red and green signals indicate the RB1 and centromeric (chromosome 8) probes, respectively.
LOH at 13q14
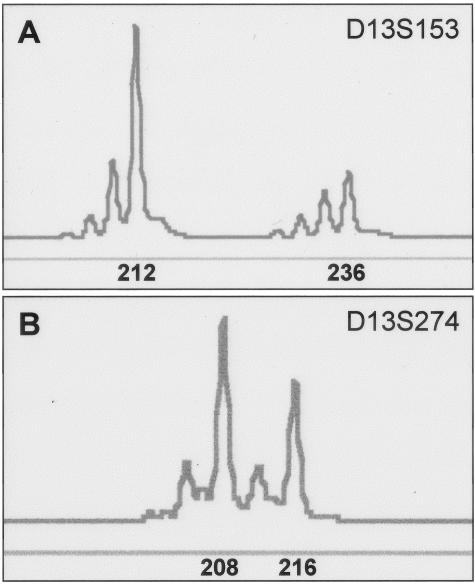
Using PCR and two microsatellite markers specific for the 13q14 locus, LOH was found in 9 (45%) of 20 informative ALCL tumors; 2 ALK-positive and 7 ALK-negative (Figure 6, Table 2). All 11 ALCL tumors with no evidence of LOH were total Rb-positive whereas 6 of 9 (67%) ALCL tumors with LOH were total Rb-negative (P = 0.002, Fisher’s exact test).
Figure 6.
Representative cases of ALCL tumors analyzed for LOH using the GeneScan and Genotyper software. A: An ALK-positive ALCL tumor with LOH of the 13q14 locus assessed with the D13S153 microsatellite marker. The expected size of the alleles (212 and 236) is shown. B: An ALK-negative ALCL tumor with heterozygous 13q32 locus assessed with the D13S274 microsatellite marker that served as a control. The expected size of the alleles (208 and 216) is shown.
Correlation of rb Allelic Loss and RB Phosphorylation Status with p27 Labeling Index
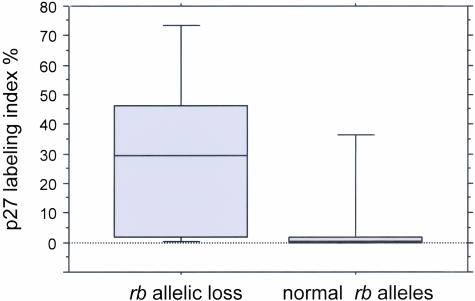
RB1 allelic loss as shown by FISH correlated with p27 levels assessed previously in this ALCL series.31 The mean percentage of p27-positive tumor cells was 30% for ALCL tumors with rb allelic loss (either hemizygous or homozygous) compared with 9% for tumors without rb deletion (P = 0.09, Mann-Whitney test; Figure 7). A correlation between underphosphorylated Rb expression and p27 labeling index was also suggested but this did not reach statistical significance (P = 0.2 by Mann-Whitney test).
Figure 7.
Association of rb allelic loss with p27 labeling index. Box plot showing higher levels of p27 expression in ALCL tumors with loss of the rb gene locus compared to tumors with normal rb alleles.
Clinical Outcome
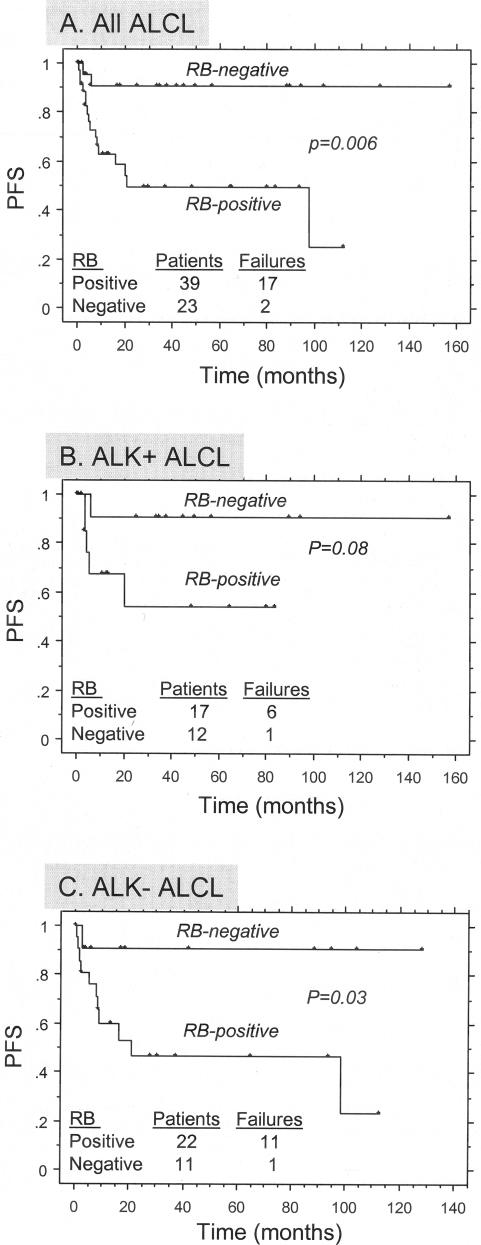
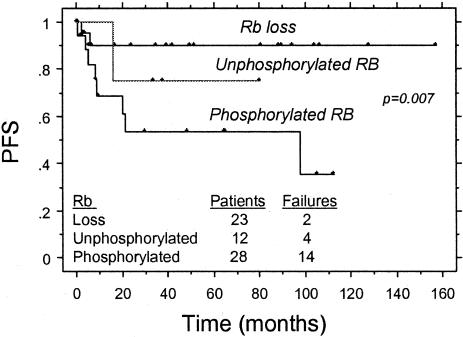
Complete follow-up data were available for 62 patients analyzed in this study. The median follow-up period was 46 months (1 to 129 months). For the entire group, 5-year PFS was 89.4% for patients with total Rb-negative tumors compared with 47.7% for patients with total Rb-positive tumors (P = 0.006 by log rank, Figure 8A). Survival analysis was also performed separately for the ALK-positive and ALK-negative groups. For 29 patients with ALK-positive ALCL, 5-year PFS was 88.9% for patients with total Rb-negative tumors compared with 48.6% for patients with total Rb-positive tumors (P = 0.08 by log rank, Figure 8B). For 33 patients with ALK-negative ALCL, 5-year PFS was 90.9% for patients with total Rb-negative tumors versus 46.5% for patients with total Rb-positive tumors (P = 0.03 by log rank, Figure 8C). In 53 patients with available data, functional loss of Rb protein was correlated with clinical outcome. Patients with total Rb-negative tumors because of allelic loss of rb locus were associated with a significantly higher PFS compared to those patients with underphosphorylated Rb expression or phosphorylated (functionally inactive) Rb (P = 0.007, log rank; Figure 9). Multivariate analysis for PFS using the Cox proportional hazards model demonstrated the independent prognostic value of total Rb expression in this series of ALCL patients, along with other known clinical prognostic factors such as Ann Arbor stage IV, elevated serum lactate dehydrogenase (LDH) levels, and age >60 years (Table 3).
Figure 8.
Correlation of total Rb expression in ALCL with progression-free survival (PFS). A: All patients; B: ALK-positive ALCL patients; C: ALK-negative ALCL patients.
Figure 9.
Correlation of functional loss of Rb in ALCL with PFS. Patients with ALCL tumors exhibiting loss of rb locus have a higher PFS compared to patients with phosphorylated or unphosphorylated Rb expression.
Table 3.
Multivariate Analysis for Progression-Free Survival in Patients with Anaplastic Large-Cell Lymphoma
| Prognostic factor | Relative risk of failure
|
||
|---|---|---|---|
| Relative risk | Standard error | P value* | |
| Total Rb-positive | 2.6 | 1.1 | 0.019 |
| Performance status = >2 | 1.9 | 0.8 | 0.013 |
| Secondary skin involvement | 1.1 | 0.5 | 0.039 |
| Age >60 years | 1.1 | 0.5 | 0.044 |
Cox’s proportional hazards model.
Discussion
In this report, we have shown that complete absence of Rb expression, as shown by immunohistochemistry, is found in 40% of ALCL tumors. Absence of Rb expression has been reported in many tumor types including non-Hodgkin’s lymphomas.18,37,38 Complete loss of Rb may be because of chromosomal alterations involving the rb gene at 13q14. Homozygous deletions of 13q14 have been detected in a subset of non-Hodgkin’s lymphomas,18,19 but these studies failed to show a correlation between 13q14 chromosomal alterations and Rb expression. In our study we assessed a subset of 25 ALCL tumors for deletions of the rb gene using FISH methods. Ten (40%) of these tumors showed loss of one or both rb alleles and this finding significantly correlated with absence of Rb expression. In addition, we performed LOH studies that showed allelic imbalances of the 13q14 locus in 45% of the informative ALCL cases, confirming the FISH results. Using similar FISH methods, Wada and co-workers39 also detected deletions of the 13q locus in 40% of aggressive lymphomas including one case of ALCL of T-cell lineage. Rosenwald and colleagues40 also used FISH methods and reported allelic loss at 13q14 in 3 of 13 peripheral T-cell lymphomas.
Absence of Rb protein expression may be associated with a higher AR in ALCL tumors (Figure 3) because the data in this series seem to show a trend, however, this association did not reach statistical significance. Accumulating evidence suggests that Rb is an inhibitor, not only of cell growth, but also of cell death. This was first revealed in rb-null mice that developed phenotypes with an increased rate of apoptosis.16 Because inactivation of Rb stimulates both cell proliferation and apoptosis, it seems likely that cells respond to mitogenic or apoptotic signals, respectively, by activating different molecular pathways.
For the entire study group of ALCL, absence of total Rb expression significantly correlated with favorable PFS (Figure 8). This was also true when patients with ALK-positive or ALK-negative ALCL were analyzed separately. Multivariate survival analysis confirmed the independent prognostic significance of Rb expression in ALCL. Loss of Rb at the DNA level also correlated with clinical outcome. Patients with Rb-negative tumors because of allelic loss of the rb locus were associated with a significantly higher PFS compared to patients whose tumors expressed underphosphorylated or phosphorylated Rb (Figure 9). There is little precedent for this finding in the literature except in chronic lymphocytic leukemia. Dohner and colleagues41 have reported that patients with chronic lymphocytic leukemia and deletion of the 13q14 locus are associated with the longest median survival times. The explanation of these findings is uncertain. However, the possibility of loss of other unknown genes within the region of deletion cannot be excluded.
Rb is expressed in approximately two thirds of ALCL tumors at a variable level, but its active (underphosphorylated) form is immunohistochemically undetectable in almost half of these tumors. Thus, where Rb is present in ALCL cells, it is often phosphorylated and therefore cannot bind and inhibit E2F family proteins. This was also true in the ALK-positive ALCL cell lines we studied. As a result, E2F transcriptional activity is uncontrolled leading to expression of a number of target genes that promote cell cycle progression from G1 to S phase.10 These results suggest that deregulation of Rb function may contribute to tumor cell proliferation and have a pathogenetic role in ALCL. This is also supported by the fact that ALCL cells may alter the phosphorylation status of Rb, as a response to growth inhibition stimuli.42
It is well established that Rb function depends on its phosphorylation by multiple CDKs, which in turn are regulated by CDK inhibitors, including p27Kip1. Because p27 is not expressed in most ALCL tumors,31 it seems reasonable to speculate that low expression levels of p27 lead to increased kinase activity of cyclin/CDK complexes that directly phosphorylate and inactivate Rb in ALCL, resulting in cell proliferation. This is further supported by our findings here, because the presence of rb alleles correlated with significantly lower levels of p27 (Figure 7). The high proliferation index in ALCL tumors supports this concept. Cooperation of Rb and p27 proteins to suppress tumor growth, through different regulatory signals, also has been proposed.43
It is of interest that phosphorylated Rb, but not its underphosphorylated counterpart, has been shown to translocate to the nucleolus through direct binding with nucleophosmin in normal human fibroblasts in late S or G2 phase of the cell cycle.44 Nucleophosmin is a component of the nucleophosmin-ALK fusion protein resulting from the t(2;5), the most frequent translocation in ALK-positive ALCL tumors. Nucleophosmin is a nonribosomal RNA-binding protein involved in bi-directional shuttling of proteins between the nucleolus and the cytoplasm45 and it has the ability to oligomerize. Whether nucleophosmin-ALK chimeric protein (and not only nucleophosmin) also can interact with phosphorylated Rb is unknown and the potential biological significance of this interaction in ALCL needs to be explored in future functional studies.
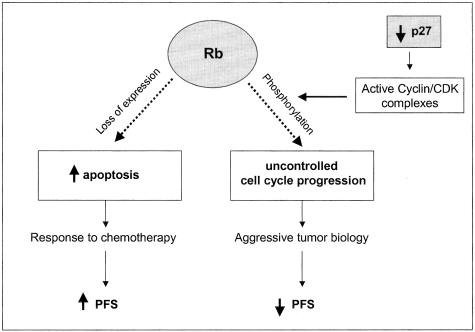
In conclusion, loss of Rb expression, most likely because of allelic loss of the rb gene, is a common event in ALCL tumors and independently predicts a better prognosis in these patients. When Rb is present in ALCL, the protein is commonly phosphorylated, perhaps the result of low levels of p2731 (Figure 10). Experimental therapeutic approaches targeting the Rb pathway may be useful in treating patients with ALCL.
Figure 10.
Schematic showing the possible role of Rb expression in the biology and prognosis of ALCL. As previously reported,31 expression levels of the CDK inhibitor p27 are low in ALCL, thus allowing phosphorylation of Rb by cyclin/CDK complexes. When phosphorylated, Rb is not capable of binding to E2F transcriptional factors leading to uncontrolled cell proliferation and possibly more aggressive tumor biology. Loss of Rb expression, commonly by deletion of the rb gene, results in increased tumor apoptosis and may sensitize tumor cells to chemotherapy. This is a possible explanation for the better PFS observed in patients with total Rb-negative ALCL.
Footnotes
Address reprint requests to L. Jeffrey Medeiros, M.D., Department of Hematopathology, Box 72, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030. E-mail: jmedeiro@mail.mdanderson.org.
Supported by The University of Texas M.D. Anderson Cancer Center (an institutional research grant to L.J.M.) and the Alexander S. Onassis Foundation (scholarship to G.Z.R.).
This study was presented in part at the 91st United States and Canadian Academy of Pathology meeting, Chicago, IL, February 23 to 28, 2002.
References
- Lee WH, Bookstein R, Hong F, Young LJ, Shew JY, Lee EY. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987;235:1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Fung YK, Murphree AL, T’Ang A, Qian J, Hinrichs SH, Benedict WF. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987;236:1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Knudson AG., Jr Retinoblastoma: a prototypic hereditary neoplasm. Semin Oncol. 1978;5:57–60. [PubMed] [Google Scholar]
- Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910–917. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- Helin K, Lees JA, Vidal M, Dyson N, Harlow E, Fattaey A. A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell. 1992;70:337–350. doi: 10.1016/0092-8674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Krek W, Sellers WR, DeCaprio JA, Ajchenbaum F, Fuchs CS, Chittenden T, Li Y, Farnham PJ, Blanar MA, Livingston DM, Flemington EK. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992;70:351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Johnson DG, Schwarz JK, Cress WD, Nevins JR. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- Harbour JW, Dean DC. Corepressors and retinoblastoma protein function. Curr Top Microbiol Immunol. 2001;254:137–144. doi: 10.1007/978-3-662-10595-5_7. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Goodrich DW, Wang NP, Qian YW, Lee EY, Lee WH. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991;67:293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Morgenbesser SD, Williams BO, Jacks T, DePinho RA. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 1994;371:72–74. doi: 10.1038/371072a0. [DOI] [PubMed] [Google Scholar]
- Bates S, Phillips AC, Clark PA, Stott F, Peters G, Ludwig RL, Vousden KH. p14ARF links the tumour suppressors RB and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- Hsieh JK, Fredersdorf S, Kouzarides T, Martin K, Lu X. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–1852. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- Chau BN, Wang JY. Coordinated regulation of life and death by RB. Nat Rev Cancer. 2003;3:130–138. doi: 10.1038/nrc993. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hermanson M, Grander D, Merup M, Wu X, Heyman M, Rasool O, Juliusson G, Gahrton G, Detlofsson R, Nikiforova N, Buys C, Soderhall S, Yankowsky N, Zabarovsky E, Einhorn S. 13q deletions in lymphoid malignancies. Blood. 1995;86:1911–1915. [PubMed] [Google Scholar]
- Hangaishi A, Ogawa S, Imamura N, Miyawaki S, Miura Y, Uike N, Shimazaki C, Emi N, Takeyama K, Hirosawa S, Kamada N, Kobayashi Y, Takemoto Y, Kitani T, Toyama K, Ohtake S, Yazaki Y, Ueda R, Hirai H. Inactivation of multiple tumor-suppressor genes involved in negative regulation of the cell cycle, MTS1/p16INK4A/CDKN2, MTS2/p15INK4B, p53, and Rb genes in primary lymphoid malignancies. Blood. 1996;87:4949–4958. [PubMed] [Google Scholar]
- Galteland E, Smedshammer L, Suo Z, DeAngelis P, Stokke T. Proliferation-dependent expression and phosphorylation of pRB in B cell non-Hodgkin’s lymphomas: dependence on RB1 copy number. Leukemia. 2002;16:1549–1555. doi: 10.1038/sj.leu.2402644. [DOI] [PubMed] [Google Scholar]
- Jares P, Campo E, Pinyol M, Bosch F, Miquel R, Fernandez PL, Sanchez-Beato M, Soler F, Perez-Losada A, Nayach I, Mallofre C, Piris MA, Montserrat E, Cardesa A. Expression of retinoblastoma gene product (pRb) in mantle cell lymphomas. Correlation with cyclin D1 (PRAD1/CCND1) mRNA levels and proliferative activity. Am J Pathol. 1996;148:1591–1600. [PMC free article] [PubMed] [Google Scholar]
- Nguyen PL, Zukerberg LR, Benedict WF, Harris NL. Immunohistochemical detection of p53, bcl-2, and retinoblastoma proteins in follicular lymphoma. Am J Clin Pathol. 1996;105:538–543. doi: 10.1093/ajcp/105.5.538. [DOI] [PubMed] [Google Scholar]
- Geradts J, Andriko JW, Abbondanzo SL. Loss of tumor suppressor gene expression in high-grade but not low-grade non-Hodgkin’s lymphomas. Am J Clin Pathol. 1998;109:669–674. doi: 10.1093/ajcp/109.6.669. [DOI] [PubMed] [Google Scholar]
- Moller MB, Kania PW, Ino Y, Gerdes AM, Nielsen O, Louis DN, Skjodt K, Pedersen NT. Frequent disruption of the RB1 pathway in diffuse large B cell lymphoma: prognostic significance of E2F-1 and p16INK4A. Leukemia. 2000;14:898–904. doi: 10.1038/sj.leu.2401761. [DOI] [PubMed] [Google Scholar]
- Bai M, Vlachonikolis J, Agnantis NJ, Tsanou E, Dimou S, Nicolaides C, Stefanaki S, Pavlidis N, Kanavaros P. Low expression of p27 protein combined with altered p53 and Rb/p16 expression status is associated with increased expression of cyclin A and cyclin B1 in diffuse large B-cell lymphomas. Mod Pathol. 2001;14:1105–1113. doi: 10.1038/modpathol.3880444. [DOI] [PubMed] [Google Scholar]
- Delsol G, Ralfkiaer E, Stein H, Wright D, Jaffe ES. Anaplastic large cell lymphoma. Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Lyon: France, IARC Press,; Pathology and Genetics of Tumors of Haematopoietic and Lymphoid Tissues. 2001:pp 230–235. [Google Scholar]
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene ALK, to a nucleolar protein gene NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- Morris SW, Naeve C, Mathew P, James PL, Kirstein MN, Cui X, Witte DP. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK). Oncogene. 1997;14:2175–2188. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- Inghirami G, Macri L, Cesarman E, Chadburn A, Zhong J, Knowles DM. Molecular characterization of CD30+ anaplastic large-cell lymphoma: high frequency of c-myc proto-oncogene activation. Blood. 1994;83:3581–3590. [PubMed] [Google Scholar]
- Chilosi M, Doglioni C, Magalini A, Inghirami G, Krampera M, Nadali G, Rahal D, Pedron S, Benedetti A, Scardoni M, Macri E, Lestani M, Menestrina F, Pizzolo G, Scarpa A. p21/WAF1 cyclin-kinase inhibitor expression in non-Hodgkin’s lymphomas: a potential marker of p53 tumor-suppressor gene function. Blood. 1996;88:4012–4020. [PubMed] [Google Scholar]
- Chilosi M, Doglioni C, Yan Z, Lestani M, Menestrina F, Sorio C, Benedetti A, Vinante F, Pizzolo G, Inghirami G. Differential expression of cyclin-dependent kinase 6 in cortical thymocytes and T-cell lymphoblastic lymphoma/leukemia. Am J Pathol. 1998;152:209–217. [PMC free article] [PubMed] [Google Scholar]
- Rassidakis GZ, Claret FX, Lai R, Zhang Q, Sarris AH, McDonnell TJ, Medeiros LJ. Expression of p27(Kip1) and c-Jun activation binding protein 1 are inversely correlated in systemic anaplastic large cell lymphoma. Clin Cancer Res. 2003;9:1121–1128. [PubMed] [Google Scholar]
- Rassidakis GZ, Sarris AH, Herling M, Ford RJ, Cabanillas F, McDonnell TJ, Medeiros LJ. Differential expression of BCL-2 family proteins in ALK-positive and ALK-negative anaplastic large cell lymphoma of T/null-cell lineage. Am J Pathol. 2001;159:527–535. doi: 10.1016/S0002-9440(10)61724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassidakis GZ, Jones D, Thomaides A, Sen F, Lai R, Cabanillas F, McDonnell TJ, Medeiros LJ. Apoptotic rate in peripheral T-cell lymphomas: a study using a tissue microarray with validation on full tissue sections. Am J Clin Pathol. 2002;118:328–334. doi: 10.1309/HKMV-VMPP-0CH8-3DPQ. [DOI] [PubMed] [Google Scholar]
- Katz RL, Caraway NP, Gu J, Jiang F, Pasco-Miller LA, Glassman AB, Luthra R, Hayes KJ, Romaguera JE, Cabanillas FF, Medeiros LJ. Detection of chromosome 11q13 breakpoints by interphase fluorescence in situ hybridization. A useful ancillary method for the diagnosis of mantle cell lymphoma. Am J Clin Pathol. 2000;114:248–257. doi: 10.1309/69EJ-RFM5-E976-BUTP. [DOI] [PubMed] [Google Scholar]
- Jiang F, Lin F, Price R, Gu J, Medeiros LJ, Zhang HZ, Xie SS, Caraway NP, Katz RL. Rapid detection of IgH/BCL2 rearrangement in follicular lymphoma by interphase fluorescence in situ hybridization with bacterial artificial chromosome probes. J Mol Diagn. 2002;4:144–149. doi: 10.1016/S1525-1578(10)60695-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega F, Medeiros LJ, Jones D, Abruzzo LV, Lai R, Manning J, Dunmire V, Luthra R. A novel four-color PCR assay to assess T-cell receptor gamma gene rearrangements in lymphoproliferative lesions. Am J Clin Pathol. 2001;116:17–24. doi: 10.1309/5WFQ-N12E-DT05-UX1T. [DOI] [PubMed] [Google Scholar]
- Martinez JC, Piris MA, Sanchez-Beato M, Villuendas R, Orradre JL, Algara P, Sanchez-Verde L, Martinez P. Retinoblastoma (Rb) gene product expression in lymphomas. Correlation with Ki67 growth fraction. J Pathol. 1993;169:405–412. doi: 10.1002/path.1711690404. [DOI] [PubMed] [Google Scholar]
- Weide R, Tiemann M, Pfluger KH, Koppler H, Parvizl B, Wacker HH, Kreipe HH, Havemann K, Parwaresch MR. Altered expression of the retinoblastoma gene product in human high grade non-Hodgkin’s lymphomas. Leukemia. 1994;8:97–101. [PubMed] [Google Scholar]
- Wada M, Okamura T, Okada M, Teramura M, Masuda M, Motoji T, Mizoguchi H. Frequent chromosome arm 13q deletion in aggressive non-Hodgkin’s lymphoma. Leukemia. 1999;13:792–798. doi: 10.1038/sj.leu.2401395. [DOI] [PubMed] [Google Scholar]
- Rosenwald A, Ott G, Krumdiek AK, Dreyling MH, Katzenberger T, Kalla J, Roth S, Ott MM, Muller-Hermelink HK. A biological role for deletions in chromosomal band 13q14 in mantle cell and peripheral T-cell lymphomas? Genes Chromosom Cancer. 1999;26:210–214. doi: 10.1002/(sici)1098-2264(199911)26:3<210::aid-gcc4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Dohner H, Stilgenbauer S, Benner A, Leupolt E, Krober A, Bullinger L, Dohner K, Bentz M, Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- Levi E, Pfeifer WM, Kadin ME. CD30-activation-mediated growth inhibition of anaplastic large-cell lymphoma cell lines: apoptosis or cell-cycle arrest? Blood. 2001;98:1630–1632. doi: 10.1182/blood.v98.5.1630. [DOI] [PubMed] [Google Scholar]
- Park MS, Rosai J, Nguyen HT, Capodieci P, Cordon-Cardo C, Koff A. p27 and Rb are on overlapping pathways suppressing tumorigenesis in mice. Proc Natl Acad Sci USA. 1999;96:6382–6387. doi: 10.1073/pnas.96.11.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura M, Ohoka F, Perpelescu M, Ogawa M, Matsushita H, Takaba T, Akiyama T, Umekawa H, Furuichi Y, Cook PR, Yoshida S. Phosphorylation-dependent migration of retinoblastoma protein into the nucleolus triggered by binding to nucleophosmin/B23. Exp Cell Res. 2002;276:233–241. doi: 10.1006/excr.2002.5523. [DOI] [PubMed] [Google Scholar]
- Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–390. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]