Abstract
A molecular probe was developed to monitor caspase activity in living cells by flow cytometry. It consists of CFP and YFP with a peptide linker containing two caspase-cleavage sites (LEVD). Its expression resulted in intense fluorescence resonance energy transfer (FRET), whereas cleavage of this linker by caspases eliminated FRET because of physical separation of the CFP and YFP moieties. Using flow cytometry, cells expressing this probe exhibited two patterns, strong FRET and diminished or absent FRET. The appearance of diminished FRET was inhibited by a pan-caspase inhibitor z-VAD or D->A mutations in the LEVD sequence and was markedly increased by apoptosis-inducing agents, etoposide and camptothecin, or overexpression of a caspase 8-red fluorescent protein fusion protein. Importantly, this probe’s ability to monitor caspase activity was comparable with results obtained with fluorogenic substrates or fluorochrome-labeled inhibitors of caspases. Specific caspase inhibitors indicated the probe was highly sensitive to cleavage by caspase 6 and 8, less sensitive to caspase 4, and resistant to other caspases. Activation of caspase 8 by Fas engagement markedly increased the probe’s cleavage, whereas treatment of caspase 8-deficient cells with anti-Fas did not increase cleavage. However, staurosporine induced cleavage of the probe in caspase 8-deficient cells by a mechanism that was inhibited by overexpression of bcl-x. Taken together, the data indicate that this caspase-sensitive probe can be used to monitor the basal and apoptosis-related activities of caspases, including an initiator caspase, caspase 8, and effector caspases, such as caspase 6.
Apoptosis is characterized by a distinct series of morphological and biochemical changes that eventually result in the programmed death of cells. It plays an essential role in morphogenesis, host defense and homeostasis of all tissues, including the immune system.1–4 In addition, the degree of apoptosis induced plays a prognostic factor for therapy of a number of malignancies.3 Among the key biochemical mediators of apoptosis are a group of cysteine proteases, designated as the caspases.5–9 The caspases can be classified into three groups according to their substrate specificities.10,11 Group I (caspase-1, 4, and 5) prefers the tetrapeptide sequence WEHD, whereas Group II (caspase-2, 3, and 7), the primary apoptotic effector caspases, prefers the peptide recognition motif DEXD, while the caspases in Group III (6, 8, and 9) prefer the sequence (L/V)EXD.
Given their central role as apoptosis mediators, a great deal of interest has been focused on the potential of using caspases as therapeutic targets for various diseases.3,12 The chemical substrates, including fluorogenic substrates and fluorochrome-labeled inhibitors of caspases (FLICAs), thus have been developed to monitor caspase activity in living cells.13–15 However, a genetic approach for directly monitoring caspase activity within living cells may provide a more rapid and accurate method and also afford the possibility of separating cells based on their caspase activity. With the advent of the GFP variants, CFP and YFP,16–19 the use of an in vivo CFP->YFP fluorescence resonance energy transfer (FRET) assay has become feasible to monitor many distance-sensitive cellular activities, such as protein-protein interaction, enzymatic activity, protein conformational change, kinase activity, etc.20–24 One application of this technology has been the development of assays to measure caspase activity in living cells. These methods make use of constructs of CFP and YFP with a linker between them.20,25–29 The linker harbors at least one caspase target sequence (LEVD, DEVD, or other caspase-sensitive cleavage sequence). If the length of the linker is sufficiently short, FRET will occur between CFP and YFP. However, if the cell has been stimulated to express caspase activity, the linker will be cleaved resulting in two physically separated CFP and YFP molecules and a marked decrease in FRET activity. The utility of GFP-based flurochromes (CFP and YFP or BFP and GFP) as in vitro and in vivo substrates for protease assays has been demonstrated.20,26,29 However, the measurement of FRET signals has largely been achieved using fluorescence microscopy.20,25–29 This is time consuming and permits analysis of only a limited number of cells. Recently we have developed an in vivo flow cytometric FRET method for measuring the molecular distance between CFP and YFP flurochromes.21 Importantly, flow cytometry measures the FRET signals in a high-throughput manner and analyzes viable cells without any subjective selection of cells of interest. Additionally, when examined by flow cytometry, FRET-positive cells can be subsequently sorted to perform additional experiments.
Here we describe a flow cytometric FRET method to analyze caspase-activity in living cells using a caspase-sensitive probe that has two caspase-cleavage sites between CFP and YFP (CFP-LEVD-YFP). Activation of caspases is detected by the appearance of a transfected population with diminished FRET, resulting from cleavage of the fusion protein and physical separation of the CFP and YFP moieties. The responses of this probe to chemical stimuli, different inhibitors of specific caspase activity and over- or underexpression of caspase 8 were examined to validate its activity. The disappearance of the population with diminished FRET was achieved by adding the universal caspase inhibitor z-VAD to the culture or by mutating the cleavage site. With specific inhibitors to each individual caspase, cleavage of this probe was found to be highly sensitive to caspase 6 and 8, to be less sensitive to caspase 4, and to be resistant to caspase 1,2,3, 5, and 9. Notably, the appearance of the population with diminished FRET was markedly increased by overexpression of caspase 8. Stimulation of wild-type Jurkat cells by anti-Fas antibody triggered markedly increased cleavage of the probe that was not observed in Jurkat cells deficient in caspase 8. However, staurosporine could still induce cleavage of the probe in the caspase 8-deficient cells, indicating that caspases besides caspase 8 could cleave this probe in living cells. Of interest, cells that cleaved the probe bound annexin V very weakly, unless stimulated by strong apoptosis inducers, indicating that caspase activation was not dependent on apoptosis. Finally, the CFP-LEVD-YFP caspase-sensitive probe was found to yield results similar to other methods, such as the use of fluorogenic substrates and FLICAs, to monitor caspase activity in living cells. These results document the utility of this probe and flow cytometric evaluation to monitor basal and apoptosis-related caspase activity in living cells.
Materials and Methods
Materials
The universal protease inhibitor z-VAD-fmk and caspase 4 inhibitor z-LEVD-fmk were purchased from ICN Biomedicals, Inc. (Aurora, OH). Topoisomerase inhibitors etoposide, camptothecin, and other specific caspase inhibitors (z-YVAD-fmk for caspase 1 and 4; z-VDVAD-fmk for caspase 2; z-DEVE-fmk for caspase 3; z-WEHD-fmk for caspase 5; z-VEID-fmk for caspase 6; z-IETD-fmk for caspase 8; and z-LEHD-fmk for caspase 9) were purchased from CalBiochem (La Jolla, CA). The red fluorescence FLICAs, SR-VAD-FMK and SR-DEVD-FMK, were purchased from Chemicon International (Temecula, CA). The red fluorescence fluorogenic substrate PhiPhiLux-G2D2 (DEVDase) was purchased from Oncolmmunin, Inc. (Gaithersburg, MD). Dulbecco’s modified Eagle’s medium (DMEM), fetal calf serum (FCS), Optimal medium, phosphate-buffered saline (PBS), and LipofectAmine Plus were obtained from Invitrogen (Carlsbad, CA). Staurosporine was purchased from Sigma (St. Louis, MO). HRP directly conjugated goat anti-GFP antibody was purchased from ABCam (England). The vectors for pECFP and pEYFP and dsRFP were purchased from Clontech (Palo Alto, CA). The APC-conjugated annexin V for identifying early apoptotic cells was purchased from BD PharMingen (La Jolla, CA). Anti-Fas monoclonal antibody was purchased from Kamiya Biomedical (Thousand Oaks, CA).
Plasmids Constructs
The caspase-sensitive FRET probe CFP-LEVD-YFP was prepared by inserting the annealed oligos 5′ CC GGA GCA CTG GAG GTC GAT GCC CTG GAG GTC GAT C 3′ and 5′ CC GGG ATC GAC CTC CAG GGC ATC GAC CTC CAG TGC T 3′ directly into the BspE I site of the CFP-YFP FRET-positive control. The CFP-YFP FRET-positive control plasmid was described previously.21,30 A plasmid with D->A mutations (LEVD->LEVA) in the caspase cleavage sites of the spacer of the CFP-LEVD-YFP plasmid, designated as CFP-14m-YFP, was made by inserting the annealed oligos 5′ CC GGA GCA CTG GAG GTC GCA GCC CTG GAG GTC GCA C 3′ and 5′ CC GGG TGC GAC CTC CAG GGC TGC GAC CTC CAG TGC T 3′ directly into the BspE I site of the CFP-YFP FRET-positive control. The human caspase 8 cDNA inserted into the pEGFP-N1 vector was described previously.31 The Hand III-SacII digested fragments of human caspase 8 were cloned into the same sites in the pRed2-N1 vector (Clontech; Palto Alto, CA) to make the caspase 8-RFP plasmid.
Cell Culture, Transfection, Chemical or Anti-FAS Treatment and Immunoblot
Hela and Jurkat cells were cultured in DMEM and RPMI 1640 medium (Invitrogen), respectively, supplemented with penicillin (100 units/ml), streptomycin (100 μg/ml), and 10% fetal calf serum. Wild-type Jurkat A3 and caspase-8-deficient Jurkat I9.2 cells32 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). V-FLIP and Bcl-x Jurkat transfectants33 were a gift from Dr. Diane Bolton, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health. Hela cells were transfected with 500 ng of each plasmid as indicated with the LipofectAMINE reagents in 6-well plates according to the instructions provided by Invitrogen. The universal caspase activity inhibitor z-VAD-fmk and a caspase 4-inhibitor z-LEVD-fmk at a final concentration of 20 μmol/L were added to the culture during transfection. Other caspase inhibitors were added at a final concentration of 2 μmol/L. Three hours after transfection, either 100 μmol/L etoposide or 2 μmol/L camptothecin was added where indicated. Sixteen hours after transfection, the cells were detached from the wells by incubation with 2 mmol/L ethylenediamine tetraacetic acid (EDTA) in PBS, washed once in optimal medium, and resuspended in PBS/1% FCS. Where indicated, samples were analyzed for membrane changes associated with apoptosis using APC-conjugated annexin V according to the protocol provided by the company (BD PharMingen).
One to two × 107 Jurkat cells were transfected with 20 μg of the CFP-LEVD-YFP construct using a BTX electroporator as previously described.34 Eighteen to twenty-four hours later, cells were treated with 1 μg/ml staurosporine or 300 ng/ml anti-Fas APO1–3 mAb with 30 ng/ml protein A for maximal receptor cross-linking. Four to six hours later, cells were analyzed for apoptosis and FRET by staining with 5 μl annexin-V-APC and propidium iodide. The GFP and PARP Western blots were performed as previously described.21
Flow Cytometric Detection of FRET and Apoptosis
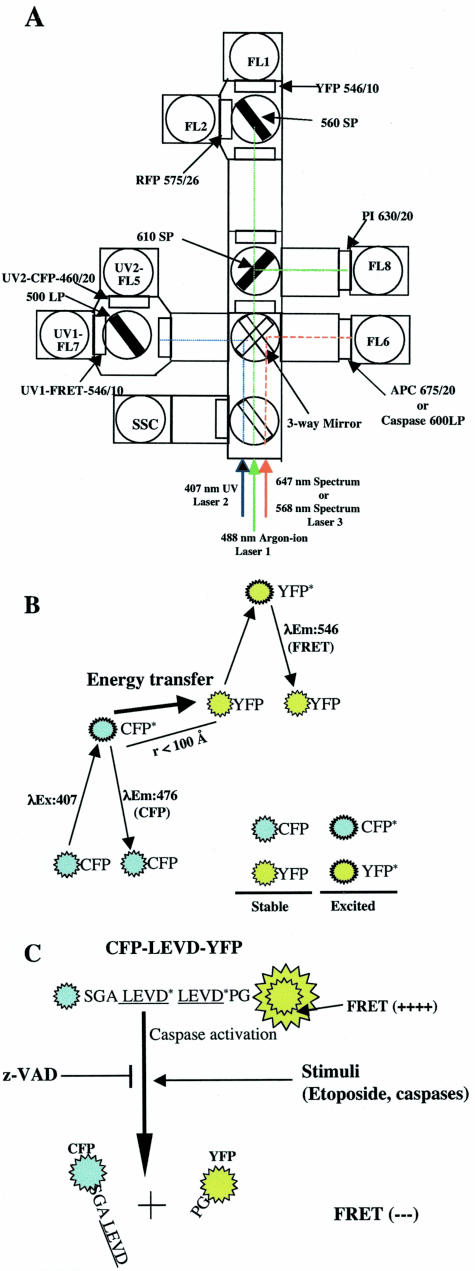
All flow cytometric data were collected using a BD FACS Vantage SE with digital signal processor (FACS DiVa) or using a DakoCytomation MoFlo (Fort Collins, CO). The argon-ion 488 nm at 150 mW, the krypton-ion UV 407-nm laser lines at 50 mW and the spectrum 647-nm laser lines at 50 mW were used to excite YFP/RFP, CFP, and annexin V-APC, respectively. The optical configuration has been described previously with a slight modification to detect red fluorescent protein signals (Figure 1).21,35,36 Briefly, the YFP and RFP signals were collected using 546/10, 575/26 band-pass filters, respectively, in the primary laser pathway (laser 1). The CFP and FRET signals were collected using 460/20 and 546/10 band-pass filters, respectively, with a 500 long-pass dichroic splitter filter inserted into the krypton-ion laser pathway (laser 2). To distinguish apoptotic cells from necrotic cells, cellular staining using APC-conjugated annexin V and PI (propidium iodide) was carried out according to the protocol provided by PharMingen. APC signals were collected with a 675/20-nm band-pass filter in the spectrum laser pathway (laser 3) whereas PI signals were collected with a 630/20-nm band-pass filter in the primary laser pathway (laser 1).
Figure 1.
FRET and its use for measurement of caspase activity by flow cytometry. A: Optical configuration of the BD FACSDiVa triple-laser system (488-nm, 407-nm, and 647-nm laser lines) and DakoCymation MoFlo used to measure spatially separated FRET, CFP, YFP, RFP, PI, and APC emission signals as well as red fluorescence that are emitted from a caspase-sensitive substrate or FLICAs. A 488-nm line emitted from the argon-ion laser was used to excite YFP (FL1), RFP (FL2), and PI (FL8). A 546/10 band-pass filter was used with a 560 short-pass dichroic filter to detect the acceptor YFP signal (FL1). A 575/26 band-pass filter and a 630/20 band-pass filter were used to detect RFP (FL2) and PI (FL8) signals, respectively. RFP, PI, and YFP signals were detected in the first laser pathway. A 460/20 and a 546/10 band-pass filter in the second laser pathway was used with a 500 long-pass dichroic filter to detect the UV-excited CFP signal and the FRET or “sensitized” YFP signal, respectively (FL5 and FL7). A 675/20 band-pass filter was used to detect the 647-nm line-excited APC signals in spectrum laser pathway (laser 3). When caspase activities in living cells were determined by using the fluorogenic substrate PhiPhiLux-G2D2 or the FLICAs, SR-VAD-FMK, or SR-DEVD-FMK, the substrate or FLICA was excited by 568-nm line emitted from the tunable Spectrum laser (the third laser) and its red emission was collected by a 600 long-pass filter (FL6). B: FRET between CFP and YFP when they are physically in close proximity. CFP molecules are excited by the 407-nm line emitted from the krypton laser. These excited CFP molecules revert to the resting state by either directly emitting energy or transferring energy to closely approximated acceptor YFP molecules. The latter process leads to direct excitation of YFP molecules and emission of YFP photons as FRET signals. C: Diagram showing that FRET can be used to monitor cellular caspase activity. A 14-amino acid spacer that has two caspase cleavage sites (LEVD) was inserted between CFP and YFP. In cells with caspase activity, there is a loss of FRET signals because of the cleavage of the probe and the physical separation of the CFP and YFP moieties. Several agents that induce or inhibit caspase activity were examined using this caspase-sensitive probe and flow cytometry.
Comparison of the CFP-LEVD-YFP Caspase-Sensitive Probe with Other Approaches Using Fluorogenic Substrate and FLICAs to Monitor Caspase Activity
The use of FLICAs and fluorogenic substrates to monitor caspase activity has been described in detail previously.13–15 Because of the difficulties in the commercial availability of reagents and in the spectral overlay between YFP and the green fluorescence emitted from the most extensively used fluorescence substrates and FLICAs, the red fluorescence emitting substrate (PhiPhiLux-G2D2) and the FLICAs (SR-VDA-FMK and SR-DEVD-FMK) that are used to determine the activities of pan-caspase (z-VAD) and caspase 3 (EDVD), but not those that are specifically used for monitoring caspase 8 (IETD), were chosen to compare with the method using the CFP-LEVD-YFP probe to monitor caspase activity in living cells. When used to detect caspase activity in living cells, the fluorescent substrate PhiPhiLux-G2D2 and the FLICAs were excited with 568-nm lines emitted from the tunable Spectrum laser and their emissions were collected with a 600 long-pass filter (FL6) in the third laser pathway. Hela cells were transfected with the CFP-LEVD-YFP probe and beginning 3 hours after transfection, the cells were treated with 100 μmol/L etoposide (VP16) to induce apoptosis. After overnight incubation, the cells were harvested by trypsinization, suspended at a concentration of 107 cells/ml, and then were loaded with the fluorogenic substrate or FLICAs for an additional 1 hour to assess caspase activity as described by the manufacturer. Finally the cells were analyzed for CFP, YFP, FRET, and red fluorescence (FL6, caspase activity) emitted from the fluorogenic substrate or FLICAs using flow cytometry.
Calculation of FRET-Based Caspase Activity by Flow Cytometry
With the caspase-sensitive FRET probe, caspase activity in living cells is represented by a population with diminished FRET, that is reflected by two parameters: the percentage of this population within all transfected cells and a comparison of this population to the strongly FRET-positive one. The latter analysis involves comparison of the mean FRET in the cells with diminished FRET and the strongly FRET-positive cells. Taken together, caspase activity (CAf) can be calculated by flow cytometry using a formula:
CAf = [(FRETbright − FRETdim)/FRETbright] × 100 × %FRETdim, where FRETbright and FRETdim are the mean fluorescence intensities of FRET in the strongly FRET-positive cells and cells with diminished FRET, respectively; %FRETdim is the percentage of all transfected cells with diminished FRET and CAf is caspase activity determined by flow cytometric data.
Results
The Loss of FRET in Cells Transfected with the Caspase-Sensitive Probe, CFP-LEVD-YFP, Is Prevented by the Caspase Inhibitor, z-VAD
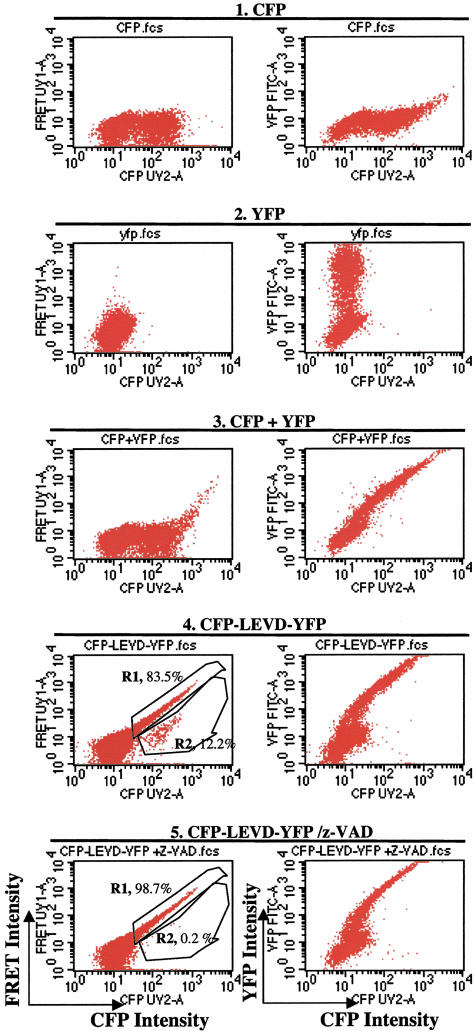
Although in vivo FRET-based caspase activity can be successfully measured using fluorescence microscopy,20,25,26,30,37 the capacity to carry out this analysis in a high-throughput manner by flow cytometry has been reported infrequently in the literature, and then using only BFP and GFP.38,39 These fluorophores are not optimal because BFP suffers from being easily photobleached and having a low quantum yield. Therefore, a FRET assay using CFP and YFP, which have better spectral properties for measuring caspase activity by flow cytometry, is highly desirable. With the optical configuration shown in Figure 1A, CFP and FRET signals can be detected in the second laser pathway, whereas YFP and RFP signals are measured in the pathway of the first laser. FRET signals directly result from emission of excited YFP moieties that only occurs when excited CFP molecules are within 100 Å of YFP molecules (Figure 1B). Because of its sensitivity to molecular distance, FRET can be used to determine in vivo caspase activity by its decrease because of the physical separation between CFP and YFP after cleavage of the fusion protein by caspase (Figure 1C). Because it is a putative target for caspases-3, -6, -8, and -10,40 two LEVD sequences were inserted in a 14 amino acid linker between CFP and YFP to create a caspase-sensitive FRET probe (CFP-LEVD-YFP) (Figure 1C). Hela cells or 293 cells were transfected either with this probe or control plasmids, CFP and YFP alone, or co-transfected with CFP and YFP, and then analyzed for FRET signals using flow cytometry. Transfection of either CFP or YFP alone did not generate any FRET signal (Figure 2, rows 1 and 2). When cells were co-transfected with CFP and YFP, FRET was negative in the cells with the relatively lower expression of the fluorescent proteins, but was weakly positive in those with higher expression (Figure 2, row 3). This has been reported as resulting from a pseudo-interaction between the highly expressed CFP and YFP proteins.30 In contrast, cells transfected with the caspase-sensitive probe showed easily detectable FRET signals (Figure 2, row 4). Interestingly, two different populations were noted, those with strong FRET (R1, 83.5% of all transfected cells) and those with diminished FRET signals (R2, 12.2% of all transfected cells) (Figure 2, row 4). The finding that the population with diminished FRET represented cells with spontaneous activation of caspases was documented by its disappearance in the presence of z-VAD (Figure 2, row 5).
Figure 2.
Flow cytometric profiles showing FRET measurement and its disappearance as an indicator of caspase activation. CFP, YFP, and FRET signals in the cells transfected with either CFP (1) or YFP (2) alone, or co-transfected with CFP and YFP (3), or in the cells transfected with the caspase-sensitive FRET probe CFP-LEVD-YFP alone in the absence (4), or presence (5) of z-VAD, a caspase inhibitor. The cells transfected with the caspase-sensitive FRET probe showed strong positive FRET. However, in the absence of z-VAD, there was a population with bright FRET and one with diminished FRET, denoted as R1 and R2, respectively. The population with diminished FRET disappeared with the addition of z-VAD to the culture.
The Population with Diminished FRET Exhibits Minimal Evidence of Apoptosis
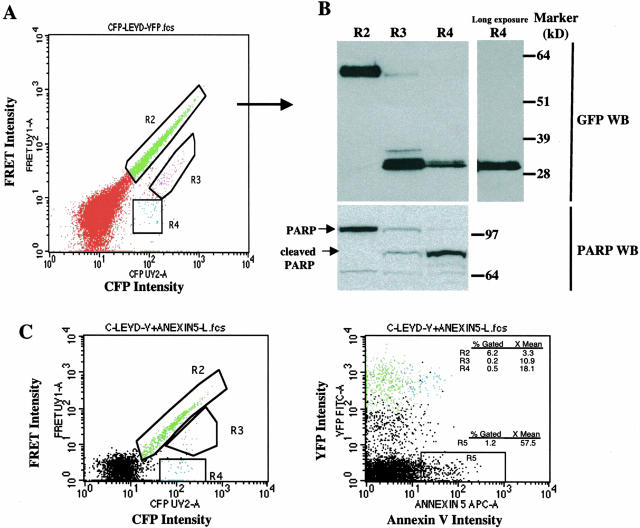
Careful scrutiny of the profile of FRET versus CFP intensity indicated that there were two populations within the cells with diminished FRET (R2, as defined in Figure 2): a weakly FRET-positive population (R3) and a completely FRET-negative population (R4) (Figure 3A). The presence of these two populations implied that the caspase-sensitive probe protein might have been partially cleaved in R3 and completely cleaved in R4. To examine this possibility, the cells within these three different regions were sorted and then assayed by GFP immunoblotting for cleavage of the fusion protein. As can be seen in Figure 3B, Western blotting showed that the fusion protein was completely intact in R2 (lane 1) and completely cleaved in R4 (lanes 3 and 4). The presence of both cleaved and uncleavage fusion protein was detected in R3. The partial cleavage of the probe in R3 suggested there was a heterogeneous response of this caspase-sensitive probe to caspase activity, even in individual cells in which caspase activity had been upregulated. To examine whether this cleavage activity is correlated with programmed cell death, the same membrane was re-probed with antibody against PARP, an endogenous substrate for caspases in apoptotic cells. It was found that PARP was completely cleaved in R4 and partially cleaved in R3, but not cleaved in R2 (Figure 2B). Cleavage of the CFP-LEVD-YFP probe completely correlated with that of endogenous caspase-sensitive substrate PARP, demonstrating that the construct was a sensitive sensor of endogenous caspase activity.
Figure 3.
Analysis of PARP cleavage and annexin V binding in cells transfected with the CFP-LEVD-YFP probe. A: Flow cytometric profile showing a population with bright FRET (R2, intact CFP-LEVD-YFP molecules) and a population with diminished FRET that could be further classified into R3 (incomplete cleavage of the probe), and R4 (complete cleavage of the probe). B: The GFP and PARP immunoblots confirmed the cleavage of the probe and of the endogenous caspase-sensitive substrate PARP in the regions indicated in (A). C: Apoptosis revealed by annexin V staining demonstrated annexin V binding by the population with diminished FRET. Hela cells transfected with the caspase-sensitive probe were stained with APC-conjugated annexin V and then were analyzed for FRET and annexin V binding. Three different regions as indicated in (A) in a plot of CFP versus FRET intensity were gated and further visualized in a plot of annexin V versus YFP intensity. The blue cells in R4, but not those in R2, bound annexin V weakly. Spontaneous caspase activity was documented in 1.2 percentages of all cultured Hela cells (R5).
To explore whether the cells with diminished FRET have evidence of early apoptosis, the cells transfected with the caspase-sensitive CFP-LEVD-YFP probe were stained with APC-conjugated annexin V and then analyzed by flow cytometry for both FRET and annexin V binding. Three different regions (R2, R3, and R4) in the plot of CFP versus FRET were gated as before and then analyzed for binding of annexin V versus YFP fluorescence (Figure 3C). Annexin V binding was weakly positive in the FRET-negative cells (blue, R4), less positive in the cells with diminished, but positive, FRET (magenta, R3), but nearly negative for the cells with strong FRET activity (green cells, R2). These data strongly suggest that the CFP-LEVD-YFP probe can detect increased caspase activity even in cells with minimal evidence of apoptosis.
Responses of the Caspase-Sensitive FRET CFP-LEVD-YFP Probe to Apoptosis Inducers
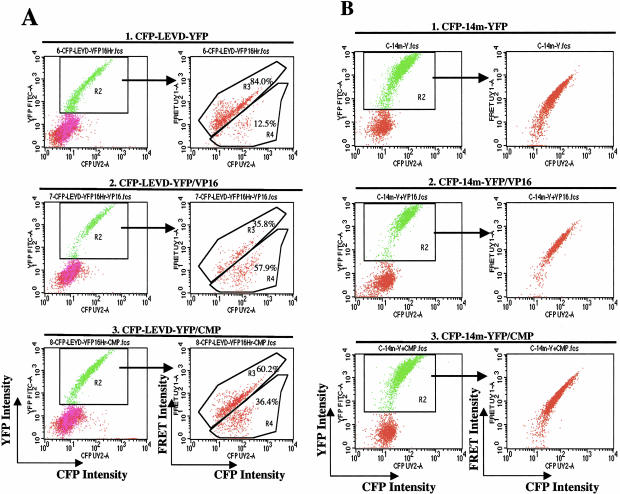
The transfected cells were treated with two different topoisomerase inhibitors, 100 μmol/L etoposide (VP16) and 2 μmol/L camptothecin (CMP) and analyzed for FRET.41–43 The percentages of the populations with strong FRET and diminished FRET were analyzed in the cells gated for the expression of both CFP and YFP. As described above, spontaneous caspase activity resulted in nearly 12.5% (R4) of all transfected cells having diminished FRET (Figure 4A, row 1). When transfected cells were treated with etoposide (VP16), the percentage of the cells expressing diminished-FRET increased to nearly 58% of all transfected cells (Figure 4A, row 2). Camptothecin also induced caspase activity, but of lesser magnitude (36% of all transfected cells in R4) (Figure 4A, row 3). Of note, some cells in R4 still had positive FRET signals, similar to the data noted in Figure 3.
Figure 4.
Flow cytometric profiles showing the increased fraction of cells with diminished FRET after stimulation by apoptosis-inducing agents. A: The appearance of the population with diminished FRET, along with the FRET-bright population, after cells were transfected with the caspase-sensitive probe alone (1). The transfected cells were then treated with two different topoisomerase inhibitors, VP16 (2) and campthothecin (3) for an additional 16 hours and then assessed for FRET by flow cytometry. Both chemical treatments induced caspase activation, as evidenced by the increased proportion of cells with diminished FRET compared to all gated CFP/YFP double-positive cells in R2. B: No FRET-negative population was observed in the cells transfected with a plasmid in which D->A (LEVD->LEVA) substitutions were introduced into the linker of the caspase-sensitive probe (CFP-14m-YFP), despite treatment with either etoposide (1) or camptothecin (2).
To examine the role of caspase in the generation of the population with diminished FRET, additional experiments examined the activity of the probe with a D->A mutation in the linker that prevented caspase-mediated cleavage. Expression of this probe resulted in the appearance of FRET-positive cells but no population with diminished FRET (Figure 4B). Stimulation of cells expressing the D->A mutant probe with etoposide (VP16) or camptothecin (CMP) had no effect on the FRET-positive cells but failed to induce a population with diminished FRET. The data indicated that the induction of apoptosis did not alter the behavior of the probe that lacked caspase cleavage sites.
The CFP-LEVD-YFP Probe Is Sensitive to Cleavage by Caspase 4, 6, and 8, but not Caspase 1, 2, 3, 5, and 9
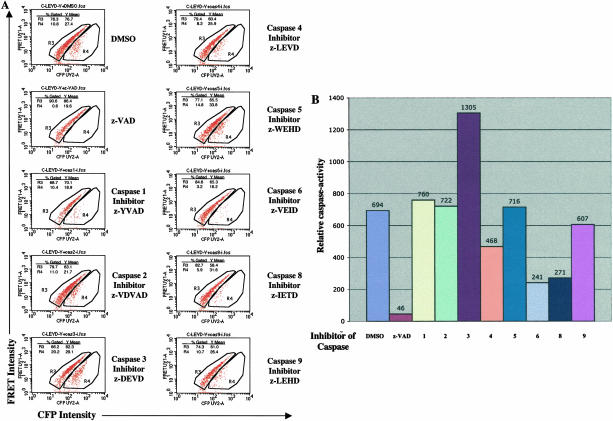
To determine the specificity of the CFP-LEVD-YFP probe, Hela cells were pretreated with different specific caspase inhibitors and then transfected with the probe. These transfected samples were analyzed by flow cytometry for the fraction of cells with diminished FRET. In the DMSO-pretreated control, 10.8% of the cells exhibited diminished FRET (Figure 5A). In contrast, nearly no cells were found in this population in the cells pretreated with the universal caspase inhibitor, z-VAD, and a smaller fraction of cells with diminished FRET was found in the population pretreated with the caspase 6 inhibitor, z-VEID, the caspase 8 inhibitor, z-IETD, or the caspase 4 inhibitor, z-LEVD. Using these inhibitors, the index of caspase activity (CAf) was calculated. As can be seen in Figure 5B, inhibitors of caspase 6 and 8 inhibited cleavage of the CFP-LEVD-YFP probe most effectively, whereas the inhibitor of caspase 4 was less effective but still active, and inhibitors of caspase 1, 2, 3, 5, and 9 had no effect.
Figure 5.
The specificity of the cleavage of the caspase-sensitive CFP-LEVD-YFP probe by individual caspases was determined using specific caspase inhibitors. A: Hela cells were incubated with various caspase inhibitors as indicated, then were transfected with the caspase-sensitive probe, and analyzed for FRET assay by flow cytometry. A FRET-positive population and a population with diminished FRET were gated in R3 and R4, respectively. The mean fluorescence intensities (MFI) and percentage of each population compared to all gated CFP/YFP double-positive cells are shown in each panel. To quantitate caspase activity (CAf) by flow cytometry, caspase activity was calculated by the formula: CAf = (FRETbright − FRETdim)/FRETbright × 100 × FRETdim, where FRETbright and FRETdim are the mean fluorescence intensities of FRET in the FRETbright cells and the cells with diminished FRET, respectively; and where %FRETdim is the percentage of cells with diminished FRET among all transfected cells. B: The FRET-based caspase activity in living cells when incubated with specific caspase inhibitors.
Response of the Caspase-Sensitive CFP-LEVD-YFP Probe to Expression of Human Caspase 8
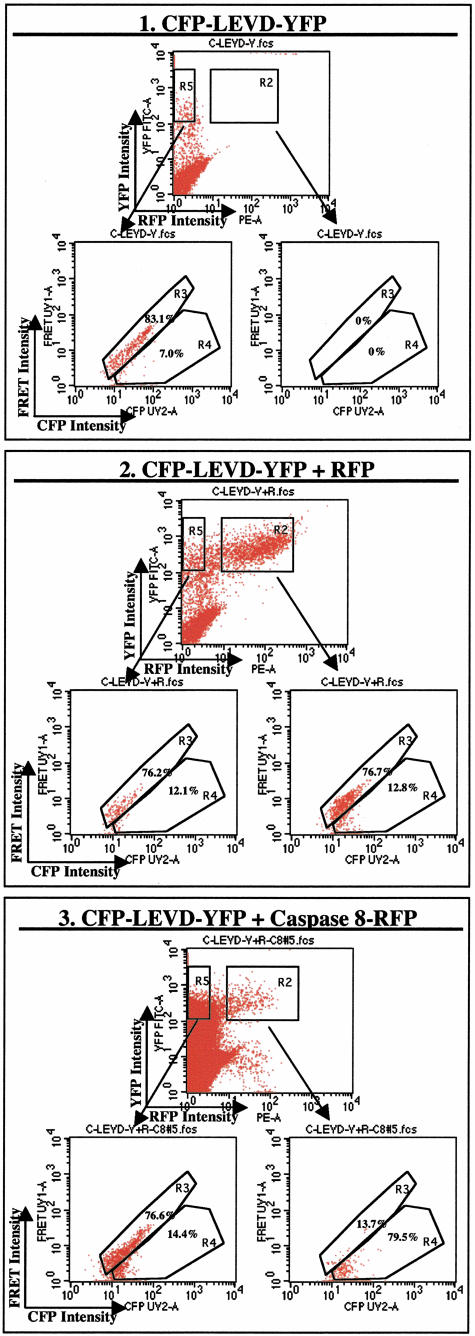
As suggested by the previous data as well as other biochemical evidence,10,11 caspase 8 should have been capable of cleaving the caspase-sensitive probe in living cells. Therefore, additional experiments directly examined the impact of expression of human caspase 8 on the FRET activity of the caspase-sensitive probe. To accomplish this, Hela cells were co-transfected with the caspase-sensitive CFP-LEVD-YFP probe and a caspase 8-RFP plasmid and then analyzed by four-color flow cytometry. Transfected cells were gated for either triple-positive (YFP, CFP, and RFP) cells (region R2) or double-positive (YFP, CFP) cells (region R5) in a plot of RFP versus YFP. The percentages of FRET-positive cells and cells with diminished FRET were then determined in each gated region (Figure 6). Overexpression of human caspase 8 led to a significantly increased fraction of cells with diminished FRET (79.5%) in the R2-gated population (Figure 6, panel 3). In contrast, the population in R5 of the same sample had a significantly smaller fraction of cells with diminished FRET (14.4% of all R5-gated cells). In contrast, overexpression of RFP itself did not increase the fraction of cells with diminished FRET (Figure 6, panel 2). There were nearly no events in the R2 gate of cells transfected with only the caspase-sensitive probe without RFP (Figure 6, panel 1), indicating that the R2 gate was exclusively restricted to RFP-positive cells. These data confirm that caspase 8 is one of the specific caspases capable of cleaving the caspase-sensitive CFP-LEVD-YFP probe.
Figure 6.
The caspase-sensitive CFP-LEVD-YFP probe is cleaved in Hela cells overexpressing human caspase 8. A fusion protein consisting of human caspase 8 and red fluorescent protein (RFP) was used for these experiments. With the four-color two-laser optical configuration shown in Figure 1A, CFP, FRET, YFP, and RFP signals could be easily distinguished. For these experiments, the red fluorescent protein signal was assessed with the FL2 detector. Cells were transfected with the caspase-sensitive CFP-LEVD-YFP probe with or without caspase 8-RFP or RFP. Transfected cells expressing YFP (and CFP) along with RFP are indicated in R2, whereas the cells expressing YFP (and CFP) but not RFP are gated in R5. FRET was then assessed for each population and the FRET-positive cells and cells with diminished FRET in R2 and R5 were analyzed. (1). Flow cytometric profiles showing cells transfected with the caspase-sensitive CFP-LEVD-YFP probe only. There were nearly no events gated in R2. The cells within R5 had a similar pattern in a plot of CFP versus FRET signals as shown in Figure 2 with a large population of FRET-positive cells and fewer cells with diminished FRET. (2). Flow cytometric profiles showing cells co-transfected with the caspase-sensitive CFP-LEVD-YFP probe and RFP. The patterns of FRET-positive and cells with diminished FRET in R2 and R5 were similar. (3). Flow cytometric profiles showing that caspase 8 cleaved the caspase-sensitive CFP-LEVD-YFP probe in vivo. The cells expressing caspase 8-RFP (R2) manifested a markedly increased fraction of cells with diminished FRET (79.5%) compared to cells in R5 that did not express caspase 8-RFP (14.4%) or in cells transfected with CFP-LEVD-YFP alone (7.0%) or cells transfected with CFP-LEVD-YFP plus RFP (12.1 to 12.8%).
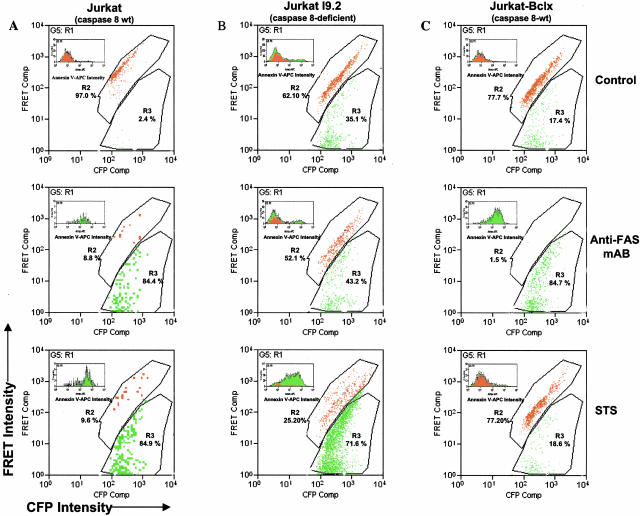
To determine whether the CFP-LEVD-YFP probe requires caspase 8 for cleavage, wild-type and caspase 8-deficient Jurkat I9.2 cell lines were transfected with the CFP-LEVD-YFP and were examined for the capacity to cleave the probe. Cells were stimulated with a monoclonal antibody against Fas to induce caspase 8 activity (Figure 7). Anti-Fas antibody triggered dramatic cleavage of the CFP-LEVD-YFP probe as evidenced by a significant increase in cells with diminished FRET activity (from 2.4% in control to 84.4%) (Figure 7A, top two panels). In contrast, there was no significant additional increase in cleavage of the probe induced by anti-Fas antibody in Jurkat I9.2 cells that were deficient in caspase 8 (35.1% versus 43.2%) (Figure 7B, top two panels). It should be noted, however, that there was considerable constitutive cleavage of the CFP-LEVD-YFP probe in the caspase 8-deficient cells without stimulation with anti-Fas antibody. Notably, cleavage of the CFP-LEVD-YFP probe by caspase 8-deficient cells was dramatically increased by treatment with staurosporine, which activates the mitochondria-dependent and caspase 8-independent apoptotic pathway (Figure 7A, bottom panel). In caspase 8-deficient Jurkat cells, cleavage of the CFP-LEVD-YFP probe induced by staurosporine but not anti-Fas antibody was blocked by overexpression of bcl-x that inhibits the mitochrondrial pathway of apoptosis. Combined with the specificity demonstrated in Figure 5, these data strongly suggest that this caspase-sensitive probe could be used to monitor activities of the initiator caspases 8, 4, and possibly the effector caspase 6.
Figure 7.
Cleavage of the CFP-LEVD-YFP FRET probe and its relationship to annexin V binding in Jurkat cells, including wild-type (A), caspase 8-deficient (B) or those stably transfected with bcl-x (C). Cells were transfected with the CFP-LEVD-YFP probe and then stimulated with anti-Fas antibody or staurosporine (STS). Distinct regions R2 (in red) and R3 (in green) were gated for the FRET-positive population and a population with diminished FRET, respectively. The gated annexin V-APC histogram of each population (green, R3; red, R2) is shown in each panel.
The CFP-LEVD-YFP Probe Can Be Used to Detect Basal and Apoptosis-Related Caspase Activities
Annexin V binding by the various Jurkat populations was examined to examine in greater detail the relationship between apoptosis and cleavage of the CFP-LEVD-YFP probe. To accomplish this, Jurkat cell lines were transfected with the caspase-sensitive CFP-LEVD-YFP probe, cultured overnight, and stained with APC-conjugatedannexin V and finally analyzed for APC, CFP, YFP, and FRETsignals by flow cytometry. FRET-positive and FRET-diminished populations were identified as R2 (red) and R3 (green), respectively. The capacity of these populations to bind annexin V-APC is shown as an inset in each panel. Interestingly, in unstimulated Jurkat cells, a majority of cells with diminished FRET activity failed to bind annexin V (Figure 7, top row), indicating that Jurkat cells expressed basal caspase activity in the absence of apoptosis. However, when apoptosis was induced by anti-Fas antibody or staurosporine in wild-type Jurkat cells, those that cleaved the probe (R3) also bound annexin V (Figure 7A). In contrast, annexin V binding in the R3 population with diminished FRET was only observed in the caspase 8-deficient Jurkat cells when treated with staurosporine, but not with anti-Fas antibody (Figure 7B). Conversely, annexin V binding by cells that had cleaved the probe (R3) was only seen in the Jurkat cells stably transfected with bcl-x when treated with anti-Fas antibody, but not with staurosporine (Figure 7C). Taken together, these data indicate that the CFP-LEVD-YFP caspase-sensitive probe could be used to monitor both basal and apoptosis-related caspase activities.
The Direct Comparison of the Detection of Cellular Caspase Activity Using the CFP-LEVD-YFP Probe, a Fluorogenic Substrate, and FLICAs
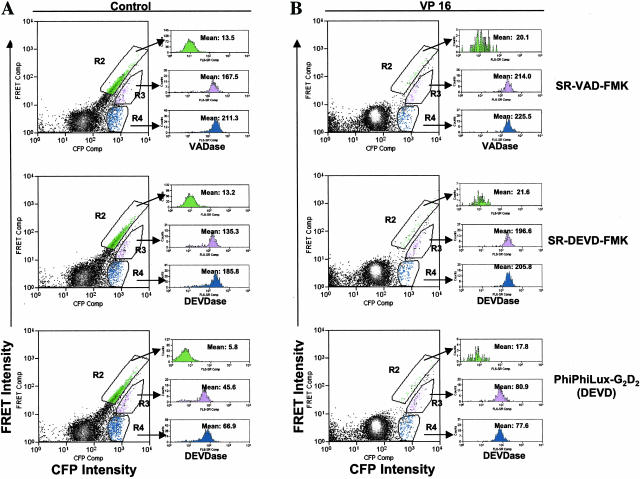
To document the comparability between the detection of caspase activity using the caspase-sensitive FRET probe and other means, Hela cells transfected with CFP-LEVD-YFP probe were loaded with the fluorogenic DEVD-mimetic substrate (PhiPhiLux-G2D2) or FLICAs (SR-DEVD-FMK and SR-VAD-FMK). As can be seen from Figure 8, three different regions R2 (green), R3 (purple) and R4 (blue) were gated, which contained the population with strong FRET, the population with diminished FRET and FRET-negative population, respectively. The cells within the gated population were assessed for caspase activity using the fluorogenic substrate or FLICAs and depicted in the FL6 histogram (VADase or DEVDase) (Figure 8, right panels). In control cells, caspase activity (VADase, DEVDase) revealed by FLICAs (SR-VAD-FMK or SR-DEVD-FMK) or the fluorogenic substrate (PhiPhiLux-G2D2) was inversely related to their FRET activity (Figure 8A). For example, the population with strong FRET displayed minimal VADase or DEVDase activity, whereas the FRET-negative population displayed the highest activity of VADase or DEVDase. Consistently, VP16 treatment triggered a significant increase in the proportion of cells with diminished or negative FRET and both of these populations manifested increased VADase or DEVDase activity (Figure 8B). Taken together, the data strongly indicate that the caspase-sensitive FRET probe CFP-LEVD-YFP has a similar capability to detect cellular apoptosis-related caspase activity as other approaches using a fluorogenic substrate or FLICAs.
Figure 8.
The comparison of detecting caspase activity using the CFP-LEVD-YFP FRET probe, a fluorogenic substrate and FLICAs in living cells. Both control (A) and VP-16-treated cells (B) that were previously transfected with CFP-LEVD-YFP probe were loaded with the fluorogenic substrate or FLICAs and their CFP, YFP, FRET, and caspase activity (VADase and DEVDase) (FL6) intensities were determined by using the DakoCytomation MoFlo. R2 (green), R3 (purple) and R4 (blue) were gated to contain the population with strong FRET, the population with diminished FRET and the FRET-negative population, respectively. Their caspase activities determined by the fluorogenic substrate or FLICAs) are depicted as the red fluorescence intensities in the FL6 histograms (right).
Discussion
In the present study we describe a CFP->YFP FRET probe (CFP-LEVD-YFP) that has been successfully used to monitor caspase activity in living cells using flow cytometry. The ability of this probe to monitor in vivo cellular caspase activity was confirmed by its expected responses to caspase inhibitors, chemical inducers of caspase activity, and overexpression or deficiency of caspase 8. This probe’s capacity to detect caspase activity in living cells was also found to be comparable with other approaches using FLICAs and a fluorogenic substrate.
Cells transfected with the probe manifested populations with both strong- and diminished-FRET activity (Figure 2, row 4). The occurrence of the latter indicated spontaneous activity of caspase in the cells. That the diminished FRET population contained active caspase was further demonstrated by its disappearance when z-VAD, a universal caspase inhibitor, was added to the culture (Figure 2, row 5). Cleavage of the probe was directly shown in the FRET-diminished population (Figure 3, A and B). Moreover, cleavage of other caspase sensitive proteins, such as PARP, was noted in the population with diminished FRET but not in the FRET-positive population (Figure 3B). This conclusion was further supported by the observation that there was an additional increase in the FRET-diminished cells when the cells were treated with topoisomerase inhibitors, etoposide and camptothecin, which are known to activate caspases41,42(Figures 4 and 8). Furthermore, the FRET-diminished population was lost when the LEVD cleavage sites were mutated, confirming the specificity of the probe for caspase activity. Finally, the population of cells with diminished FRET activity was markedly increased when caspase 8 was expressed (Figure 6) or when cells were activated by anti-Fas antibody (Figure 7A), directly indicating that CFP-LEVD-YFP probe is a substrate of caspase 8 cleavage in vivo. This conclusion is also consistent with previous biochemical evidence that a tetra-peptide LEVD was determined to be a target of caspase 8 in vitro.10
However, caspase 8 is not the only member of the caspase family that can cleave the CFP-LEVD-YFP probe. By using specific inhibitors of each individual caspase, caspases 4 and 6 were determined to be capable of cleaving the probe (Figure 5). Furthermore, the occurrence of a constitutive population with diminished FRET as well as the induction of an increased population with diminished FRET by treatment with staurosporine in caspase 8-deficient Jurkat cells strongly supports the conclusion that caspase 8 is not the only caspase that cleaves this caspase-sensitive FRET probe. It should be noted that the cleavage of the probe in Jurkat cells is not likely to be related to caspase 10 activity, since Jurkat cells were found to have low and functionally insignificant quantities of caspase 10.44 Finally, the data indicate that this LEVD FRET probe is not sensitive to caspase 3 and therefore is not useful to monitor the activity of this major effector caspase.
It was interesting that this probe could be used to monitor constitutive caspase activity in cells that were not undergoing apoptosis. In Jurkat cell lines that were either deficient in caspase 8 or stably transfected with bcl-x, the population of cells with diminished FRET largely failed to bind annexin V, a marker of early apoptosis. When these cells were stimulated to undergo apoptosis, the number of cells with diminished FRET increased and all bound annexin V. In Hela cells, the population with diminished FRET bound annexin V weakly, indicating they might be at a pre-apoptotic stage. The discrepancy between the ability of cells to cleave the CFP-LEVD-YFP probe and bind annexin V suggests strongly that this probe can be used to assess caspase activity in cells that are not undergoing apoptosis. Moreover, this probe was confirmed to have a similar ability as other methods, such as the use of FLICAs and fluorogenic substrates, to detect apoptosis-related caspase activity (Figure 8).
Importantly the fourth color, red fluorescent protein (RFP), was successfully introduced into the flow cytometric system to examine whether the caspase-sensitive probe can be cleaved by ectopic expression of proteins that are fused with RFP. The success of this approach was documented by showing that only cells overexpressing caspase 8 (linked to RFP) but not in those overexpressing RFP alone manifested increased cleavage of the CFP-LEVD-YFP probe. This four-color optical configuration of the flow cytometer will make this technique more widely useful when determining whether caspase activation is regulated by the expression of additional genes in living cells.
Finally, it must be emphasized that flow cytometry has been used in all of the experiments to achieve successful measurement of FRET in living cells in a high-throughput manner. Currently, the FRET signal is most often measured by fluorescence microscopy. Recently, we developed a confocal microscopic method to measure FRET using acceptor photobleaching.21,30 However, this method requires the fixation of samples and it is extremely time consuming to analyze the multiple samples necessary to achieve statistical significance. Others have used wide-field fluorescence microscopy to measure FRET signals by YFP/CFP ratio change.25–27,45–47 However, this approach suffers from complications caused by bleed-through of CFP fluorescence into the YFP channel. The correction for signal cross-talk must be carried out first, and therefore FRET cannot be analyzed easily. Moreover, for all experiments using microscopy, selection of the field of interest is always a limiting factor. Thus, a method with the ability to analyze FRET in viable cells in a high-throughput manner objectively is highly desirable. Flow cytometry overcomes these limitations because it measures signals (CFP, YFP, RFP, and FRET) in viable cells at a speed of 6000 to 25,000 events per second, and because it includes the capacity to carry out electronic compensation that deletes cross-talk signals. Moreover, this flow cytometric measurement does not involve sample-analysis selection and can detect FRET signals more sensitively than confocal microscopy.48 Although CFP->YFP FRET signals have been successfully measured in the previous publications using flow cytometry,21,35,36 no previous reports have used flow cytometry employing CFP->YFP FRET for the measurement of caspase activity in vivo. There has only been a limited report of the use of BFP->GFP-paired FRET analyzed by flow cytometry to detect caspase activity.29 However, the heterogeneity of FRET, typically represented by the dual-plot of flow cytometric data, was not shown in the previous report. Here we used flow cytometry to detect the CFP->YFP FRET signal and its decrease as an accurate and reproducible indicator of caspase activation in individual cells.
In summary, we have established a FRET-based probe to monitor cellular caspase activity in living cells directly using flow cytometry. Of importance, this approach should be useful to assess the physiological role of caspases in vivo and also to screen potential drugs that target this pathway.
Footnotes
Address reprint requests to Liusheng He, Flow Cytometry Section, Office of Science and Technology, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, Maryland 20892. E-mail: Lihe@mail.nih.gov.
References
- Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Turk B, Stoka V, Rozman-Pungercar J, Cirman T, Droga-Mazovec G, Oreic K, Turk V. Apoptotic pathways: involvement of lysosomal proteases. Biol Chem. 2002;383:1035–1044. doi: 10.1515/BC.2002.112. [DOI] [PubMed] [Google Scholar]
- Katoch B, Sebastian S, Sahdev S, Padh H, Hasnain SE, Begum R. Programmed cell death and its clinical implications. Indian J Exp Biol. 2002;40:513–524. [PubMed] [Google Scholar]
- Jaattela M. Programmed cell death: many ways for cells to die decently. Ann Med. 2002;34:480–488. doi: 10.1080/078538902321012423. [DOI] [PubMed] [Google Scholar]
- Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- Bratton SB, Cohen GM. Caspase cascades in chemically-induced apoptosis. Adv Exp Med Biol. 2001;500:407–420. doi: 10.1007/978-1-4615-0667-6_63. [DOI] [PubMed] [Google Scholar]
- Richter BW, Duckett CS. The IAP proteins: caspase inhibitors and beyond. Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.44.pe1. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Hengartner MO. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526–534. doi: 10.1016/s0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- Creagh EM, Martin SJ. Caspases: cellular demolition experts. Biochem Soc Trans. 2001;29:696–702. doi: 10.1042/0300-5127:0290696. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B: functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Thornberry NA. The caspase family of cysteine proteases. Br Med Bull. 1997;53:478–490. doi: 10.1093/oxfordjournals.bmb.a011625. [DOI] [PubMed] [Google Scholar]
- Nicholson DW. ICE/CED3-like proteases as therapeutic targets for the control of inappropriate apoptosis. Nature Biotechnol. 1996;14:297–301. doi: 10.1038/nbt0396-297. [DOI] [PubMed] [Google Scholar]
- Komoriya A, Packard BZ, Brown MJ, Wu ML, Henkart PA. Assessment of caspase activities in intact apoptotic thymocytes using cell-permeable fluorogenic caspase substrates. J Exp Med. 2000;191:1819–1828. doi: 10.1084/jem.191.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloc F, Belaud-Rotureau MA, Lavignolle V, Bascans E, Braz-Pereira E, Durrieu F, Lacombe F. Flow cytometry detection of caspase 3 activation in preapoptotic leukemic cells. Cytometry. 2000;40:151–160. doi: 10.1002/(sici)1097-0320(20000601)40:2<151::aid-cyto9>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Smolewski P, Bedner E, Du L, Hsieh TC, Wu JM, Phelps DJ, Darzynkiewicz Z. Detection of caspases activation by fluorochrome-labeled inhibitors: multiparameter analysis by laser scanning cytometry. Cytometry. 2001;44:73–82. doi: 10.1002/1097-0320(20010501)44:1<73::aid-cyto1084>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Yang F, Moss LG, Phillips JR GN. The molecular structure of green fluorescent protein. Nature Biotechnol. 1996;14:1246–1251. doi: 10.1038/nbt1096-1246. [DOI] [PubMed] [Google Scholar]
- Wachter RM, Elsliger MA, Kallio K, Hanson GT, Remington SJ. Structural basis of spectral shifts in the yellow-emission variants of green fluorescent protein. Structure. 1998;6:1267–1277. doi: 10.1016/s0969-2126(98)00127-0. [DOI] [PubMed] [Google Scholar]
- Battistutta R, Negro A, Zanotti G. Crystal structure and refolding properties of the mutant F99S/M153T/V163A of the green fluorescent protein. Proteins. 2000;41:429–437. doi: 10.1002/1097-0134(20001201)41:4<429::aid-prot10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Rekas A, Alattia JR, Nagai T, Miyawaki A, Ikura M. Crystal structure of venus, a yellow fluorescent protein with improved maturation and reduced environmental sensitivity. J Biol Chem. 2002;277:50573–50578. doi: 10.1074/jbc.M209524200. [DOI] [PubMed] [Google Scholar]
- Luo KQ, Yu VC, Pu Y, Chang DC. Measuring dynamics of caspase-8 activation in a single living HeLa cell during TNFα-induced apoptosis. Biochem Biophys Res Commun. 2003;304:217–222. doi: 10.1016/s0006-291x(03)00559-x. [DOI] [PubMed] [Google Scholar]
- He L, Bradrick TD, Karpova TS, Wu X, Fox MH, Fischer R, McNally JG, Knutson JR, Grammer AC, Lipsky PE. Flow cytometric measurement of fluorescence (Forster) resonance energy transfer from cyan fluorescent protein to yellow fluorescent protein using single-laser excitation at 458 nm. Cytometry. 2003;53A:39–54. doi: 10.1002/cyto.a.10037. [DOI] [PubMed] [Google Scholar]
- Calleja V, Ameer-Beg SM, Vojnovic B, Woscholski R, Downward J, Larijani B. Monitoring conformational changes of proteins in cells by fluorescence lifetime imaging microscopy. Biochem J. 2003 doi: 10.1042/BJ20030358. Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AY, Kain KH, Klemke RL, Tsien RY. Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc Natl Acad Sci USA. 2001;98:15003–15008. doi: 10.1073/pnas.211564598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tertoolen LG, Blanchetot C, Jiang G, Overvoorde J, Gadella TW, Jr, Hunter T, den Hertog J. Dimerization of receptor protein-tyrosine phosphatase α in living cells. BMC Cell Biol. 2001;2:8. doi: 10.1186/1471-2121-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K, Nagai T, Miyawaki A, Miura M. Spatio-temporal activation of caspase revealed by indicator that is insensitive to environmental effects. J Cell Biol. 2003;160:235–243. doi: 10.1083/jcb.200207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo KQ, Yu VC, Pu Y, Chang DC. Application of the fluorescence resonance energy transfer method for studying the dynamics of caspase-3 activation during UV-induced apoptosis in living HeLa cells. Biochem Biophys Res Commun. 2001;283:1054–1060. doi: 10.1006/bbrc.2001.4896. [DOI] [PubMed] [Google Scholar]
- Rehm M, Dussmann H, Janicke RU, Tavare JM, Kogel D, Prehn JH. Single-cell fluorescence resonance energy transfer analysis demonstrates that caspase activation during apoptosis is a rapid process: role of caspase-3. J Biol Chem. 2002;277:24506–24514. doi: 10.1074/jbc.M110789200. [DOI] [PubMed] [Google Scholar]
- Jones J, Heim R, Hare E, Stack J, Pollok BA. Development and application of a GFP-FRET intracellular caspase assay for drug screening. J Biomol Screen. 2000;5:307–318. doi: 10.1177/108705710000500502. [DOI] [PubMed] [Google Scholar]
- Xu X, Gerard AL, Huang BC, Anderson DC, Payan DG, Luo Y. Detection of programmed cell death using fluorescence energy transfer. Nucleic Acids Res. 1998;26:2034–2035. doi: 10.1093/nar/26.8.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova TS, Baumann CT, He L, Wu X, Grammer A, Lipsky P, Hager GL, McNally JG. Fluorescence resonance energy transfer from cyan to yellow fluorescent protein detected by acceptor photobleaching using confocal microscopy and a single laser. J Microsc. 2003;209:56–70. doi: 10.1046/j.1365-2818.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- Siegel RM, Martin DA, Zheng L, Ng SY, Bertin J, Cohen J, Lenardo MJ. Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J Cell Biol. 1998;141:1243–1253. doi: 10.1083/jcb.141.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juo P, Kuo CJ, Yuan J, Blenis J. Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol. 1998;8:1001–1008. doi: 10.1016/s0960-9822(07)00420-4. [DOI] [PubMed] [Google Scholar]
- Bolton DL, Hahn BI, Park EA, Lehnhoff LL, Hornung F, Lenardo MJ. Death of CD4(+) T-cell lines caused by human immunodeficiency virus type 1 does not depend on caspases or apoptosis. J Virol. 2002;76:5094–5107. doi: 10.1128/JVI.76.10.5094-5107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Zheng L, Siegel RM, Huang B, Fisher GH, Wang J, Jackson CE, Puck JM, Dale J, Straus SE, Peter ME, Krammer PH, Fesik S, Lenardo MJ. Defective CD95/APO-1/Fas signal complex formation in the human autoimmune lymphoproliferative syndrome, type Ia. Proc Natl Acad Sci USA. 1999;96:4552–4557. doi: 10.1073/pnas.96.8.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan FK, Siegel RM, Zacharias D, Swofford R, Holmes KL, Tsien RY, Lenardo MJ. Fluorescence resonance energy transfer analysis of cell surface receptor interactions and signaling using spectral variants of the green fluorescent protein. Cytometry. 2001;44:361–368. doi: 10.1002/1097-0320(20010801)44:4<361::aid-cyto1128>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Siegel RM, Chan FK, Zacharias DA, Swofford R, Holmes KL, Tsien RY, Lenardo MJ. Measurement of molecular interactions in living cells by fluorescence resonance energy transfer between variants of the green fluorescent protein. Sci STKE. 2000;2000:PL1. doi: 10.1126/stke.2000.38.pl1. [DOI] [PubMed] [Google Scholar]
- Harpur AG, Wouters FS, Bastiaens PI. Imaging FRET between spectrally similar GFP molecules in single cells. Nature Biotechnol. 2001;19:167–169. doi: 10.1038/84443. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Thorburn J, Thomas L, Maxwell T, Brothman AR, Thorburn A. An apoptosis signaling pathway induced by the death domain of FADD selectively kills normal but not cancerous prostate epithelial cells. Cell Death Differ. 2001;8:696–705. doi: 10.1038/sj.cdd.4400866. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Thorburn A. Measurement of caspase activity in individual cells reveals differences in the kinetics of caspase activation between cells. Cell Death Differ. 2001;8:38–43. doi: 10.1038/sj.cdd.4400800. [DOI] [PubMed] [Google Scholar]
- Leo E, Deveraux QL, Buchholtz C, Welsh K, Matsuzawa S, Stennicke HR, Salvesen GS, Reed JC. TRAF1 is a substrate of caspases activated during tumor necrosis factor receptor-α-induced apoptosis. J Biol Chem. 2001;276:8087–8093. doi: 10.1074/jbc.M009450200. [DOI] [PubMed] [Google Scholar]
- Wright SC, Schellenberger U, Wang H, Wang Y, Kinder DH. Chemotherapeutic drug activation of the AP24 protease in apoptosis: requirement for caspase 3-like-proteases. Biochem Biophys Res Commun. 1998;245:797–803. doi: 10.1006/bbrc.1998.8508. [DOI] [PubMed] [Google Scholar]
- Oizumi S, Isobe H, Ogura S, Ishida T, Yamazaki K, Nishimura M, Kawakami Y, Dosaka-Akita H. Topoisomerase inhibitor-induced apoptosis accompanied by down-regulation of Bcl-2 in human lung cancer cells. Anticancer Res. 2002;22:4029–4037. [PubMed] [Google Scholar]
- Kim JS, Amorino GP, Pyo H, Cao Q, Choy H. Radiation enhancement by the combined use of topoisomerase I inhibitors, RFS-2000 or CPT-11, and topoisomerase II inhibitor etoposide in human lung cancer cells. Radiother Oncol. 2002;62:61–67. doi: 10.1016/s0167-8140(01)00465-0. [DOI] [PubMed] [Google Scholar]
- Wang J, Chun HJ, Wong W, Spencer DM, Lenardo MJ. Caspase-10 is an initiator caspase in death receptor signaling. Proc Natl Acad Sci USA. 2001;98:13884–13888. doi: 10.1073/pnas.241358198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, McClure M, Huang F, Carter R. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr Biol. 2000;10:1395–1398. doi: 10.1016/s0960-9822(00)00785-5. [DOI] [PubMed] [Google Scholar]
- Sorkin A. Internalization of the epidermal growth factor receptor: role in signaling. Biochem Soc Trans. 2001;29:480–484. doi: 10.1042/bst0290480. [DOI] [PubMed] [Google Scholar]
- Witherow DS, Tovey SC, Wang Q, Willars GB, Slepak VZ. G beta 5. RGS7 inhibits G α q-mediated signaling via a direct protein-protein interaction. J Biol Chem. 2003;278:21307–21313. doi: 10.1074/jbc.M212884200. [DOI] [PubMed] [Google Scholar]
- He L, Olson DP, Wu X, Karpova TS, McNally JG, Lipsky PE. A flow cytometric method to detect protein-protein interaction in living cells by directly visualizing donor fluorophore quenching during CFP->YFP fluorescence resonance energy transfer (FRET). Cytometry. 2003;55A:71–85. doi: 10.1002/cyto.a.10073. [DOI] [PubMed] [Google Scholar]