Abstract
Transcription profile microarray analysis in Escherichia coli was performed to identify the member genes of the Mg2+ stimulon that respond to the availability of external Mg2+ in a PhoP/PhoQ two-component system-dependent manner. The mRNA levels of W3110 in the presence of 30 mM MgCl2, WP3022 (phoP defective), and WQ3007 (phoQ defective) were compared with those of W3110 in the absence of MgCl2. The expression ratios of a total of 232 genes were <0.75 in all three strains (the supplemental data are shown at http://www.nara.kindai.ac.jp/nogei/seiken/array.html), suggesting that the PhoP/PhoQ system is involved directly or indirectly in the transcription of these genes. Of those, 26 contained the PhoP box-like sequences with the direct repeats of (T/G)GTTTA within 500 bp upstream of the initiation codon. Furthermore, S1 nuclease assays of 26 promoters were performed to verify six new Mg2+ stimulon genes, hemL, nagA, rstAB, slyB, vboR, and yrbL, in addition to the phoPQ, mgrB, and mgtA genes reported previously. In gel shift and DNase I footprinting assays, all of these genes were found to be regulated directly by PhoP. Thus, we concluded that the phoPQ, mgrB, mgtA, hemL, nagA, rstAB, slyB, vboR, and yrbL genes make up the Mg2+ stimulon in E. coli.
The two-component system is the most prevalent signal transduction mechanism that mediates bacterial responses to environmental stimuli. The two-component system typically consists of a sensor protein and a regulatory protein. Each sensor monitors a particular environmental signal and responds by modifying the phosphorylated state of its response regulator. The affinity of the response regulator for promoters is controlled by phosphorylation, ultimately leading to transcription activation of a distinct set of the stress response genes. Eubacterial species such as Escherichia coli and Bacillus subtilis harbor >30 two-component systems, each of which responds to a different signal in the environment (2, 9). However, the specific ligand that is recognized by each sensor protein remains unidentified.
The PhoP/PhoQ two-component system was first recognized in Salmonella enterica serovar Typhimurium as a regulatory system that monitors the availability of extracellular Mg2+ (3, 5). The PhoQ protein functions as an Mg2+ sensor (3) and, in the presence of micromolar concentrations of Mg2+, phosphorylates the PhoP regulator. Phosphorylated PhoP activates the transcription of some 30 different genes (13, 14). The PhoP/PhoQ system is present in many nonpathogenic, gram-negative bacteria, suggesting that it plays a fundamental physiological role in the response to Mg2+ starvation (4, 5). However, the mechanism by which the PhoP transcription factor regulates the Mg2+ response genes remains poorly understood. Previously, we identified the tandem direct repeats of the sequence (T/G)GTTTA, which we designated the PhoP box, in promoters of the Mg2+-responsive phoPQ, mgtA, and mgrB genes of E. coli K-12 (7). A search of the entire E. coli genome sequence for the (T/G)GTTTA-5bp-(T/G)GTTTA or TAGTTA-5bp-(T/G)GTTTA motif detected four additional genes, vboR, ydcD, an fimD homolog, and yrbL (7). In order to identify the member genes of the Mg2+ stimulon in E. coli, we carried out in this study a genomewide transcription profile analysis in the presence or absence of MgCl2 by using a DNA microarray. The dependency on the PhoP/PhoQ two-component system was also examined by using E. coli mutants lacking phoP or phoQ. The sequence-activity relationship was analyzed for all of the Mg2+ response promoters herein identified.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this work were E. coli K-12 derivatives (Table 1). WP3022 (W3110 phoP2146::Tn10dCamr) and WQ3007 (W3110 phoQ608::Tn10dCamr) were constructed by P1 transduction from the donors MP4022 and MQ4007 (7) to the recipient W3110, respectively. They were cultured at 37°C in Luria-Bertani (LB) medium in the presence or absence of 30 mM MgCl2.
TABLE 1.
Bacterial strains used in this study
| Strain | Descriptiona | Source or reference |
|---|---|---|
| MC4100 | F− Δ(argF-lac)U169 araD139 rpsL150 | 2a |
| MP4022 | MC4100 phoP2146::Tn10dCamr | 7 |
| MQ4007 | MC4100 phoQ608::Tn10 dCamr | 7 |
| W3110 | Wild type | Laboratory stock |
| WP3022 | W3110 phoP2146::Tn10dCamr | W3110 × P1(MP4022)→Camr |
| WQ3007 | W3110 phoQ608::Tn10dCamr | W3110 × P1(MQ4007)→Camr |
Camr, chloramphenicol resistance.
RNA isolation, cDNA labeling, and hybridization to DNA microarrays.
To prepare total RNA for DNA microarray analysis, overnight cultures were diluted 100-fold in 30 ml of LB medium in the presence or absence of 30 mM MgCl2 and grown to an optical density at 600 nm (OD600) of 0.3 to 0.4 (approximately 8 × 107cells/ml) at 37°C. Subsequent purification steps were carried out as described previously (7). The resulting total RNA preparations were treated with RNase-free DNase I (Takara Co.) in accordance with the manufacturer's protocol. The RNA preparations from each sample were used as the template for cDNA preparations, labeled with Cy3-dUTP or Cy5-dUTP (Amersham Pharmacia), and hybridized to E. coli DNA microarrays (IntelliGene E. coli CHIP Version beta; Takara Shuzo Company, Ohtsu, Japan). The corrected intensities of duplicate spots were averaged as described previously (11, 12).
Preparation of the labeled promoter fragments for S1 nuclease and gel shift assays.
The 32P-labeled probes were generated by PCR amplification with the primer pairs shown in Table 2. Primers PH3, MGR4, BGR2, HEM2, NAG2, RST2, SLY2, YRL-R, and BOR3 were labeled with 10 μCi of [γ-32P]ATP (5,000 Ci/mmol) by T4 polynucleotide kinase (Toyobo). E. coli W3110 genome DNA (100 ng) was used as the template for Ex Taq DNA polymerase (Takara). The PCR product with 32P at its termini was recovered from a polyacrylamide gel and then used for S1 nuclease and gel shift assays.
TABLE 2.
PCR primers used in this study
| Gene | Primer name and sequence | Position | GenBank no. |
|---|---|---|---|
| phoPQ | PH6: 5′-CCG GCT AAC TAT ATT GGT CG-3′ | 4957-4977 | AE000213 |
| PH3: 5′-CTG CGT CGT CGA CCT GAT GAC CAG-3′ | 4667-4691 | ||
| mgtA | MGF6: 5′-AAC TGT AGA TTT CCC CAC GC-3′ | 7826-7846 | AE000495 |
| MGR4: 5′-GTT TTA ATC TCC GTC GAG GG-3′ | 8096-8116 | ||
| mgrB | BGF2: 5′-TCC ATA CCA GTG CTA TCA GC-3′ | 395-415 | AE000277 |
| BGR2: 5′-CCT GAT CGC ACA TCA TGT TG-3′ | 120-140 | ||
| hemL | HEM1: 5′-ATC CAA TGA TCA CTT ATT GG-3′ | 1595-1615 | AE000125 |
| HEM2: 5′-ACG CCA GTA AAG GCG CGA AC-3′ | 1303-1323 | ||
| nagA | NAG1: 5′-GCT ACT ACA GCT TTA TGC AC-3′ | 6048-6068 | AE000171 |
| NAG2: 5′-GAC AGT CAG GGC ATA TTT TG-3′ | 5694-5714 | ||
| rstA | RST1: 5′-TCG GGA AAA GTG GAA TCA GC-3′ | 3864-3884 | AE000256 |
| RST2: 5′-AAC CTG CAT ATC ATG TTT TG-3′ | 4165-4185 | ||
| slyB | SLY1: 5′-AAA GCG GCC CGA TTT CAT AG-3′ | 7192-7214 | AE000259 |
| SLY2: 5′-ATT GAA ACA ACC AAT ACG CG-3′ | 7492-7512 | ||
| vboR | BOR1: 5′-GAG AAT TCC GAT GGA TTA CAA ATA-3′ | 2164-2188 | AE000161 |
| BOR3: 5′-GTG CTA CTG CTG TCT GTT TG-3′ | 1685-1705 | ||
| yrbL | YRL-F: 5′-CCG AGG CTG AAG CCA ACA GC-3′ | 1620-1640 | AE000400 |
| YRL-R: 5′-GCC CAG GGG ACT TTG TTC AG-3′ | 1995-2015 |
S1 nuclease assay.
RNA was prepared as described previously (7). The labeled promoter fragment was incubated with 100 μg of total RNA in hybridization buffer (80% formamide,0.4 M NaCl, 20 mM HEPES [pH 6.4]) at 75°C for 10 min, followed by further incubation at 37°C overnight and then digested with S1 nuclease. The undigested DNA was precipitated by ethanol, dissolved in formamide dye solution (95% formamide, 0.05% bromophenol blue, 0.05% xylene cyanol), and analyzed by electrophoresis on a 6% polyacrylamide gel containing 8 M urea.
Purification of PhoP.
His-tagged PhoP for gel shift and DNA footprinting assays was purified as described previously (16).
Gel shift assay.
The 32P-labeled probes for the S1 nuclease assay were also used in the gel shift assay. The probe was incubated at 37°C for 10 min with the purified His-tagged PhoP (0 to 20 pmol) and bovine serum albumin (BSA; 100 pmol) in binding buffer (5 mM CaCl2, 3 mM MgCl2). After addition of the DNA dye solution (40% glycerol, 0.025% bromophenol blue, 0.025% xylene cyanol), the mixtures were directly subjected to 6% polyacrylamide gel electrophoresis (pH 6.0).
DNase I footprinting assay.
The 32P-labeled probe was incubated at 37°C for 10 min with purified, His-tagged PhoP in 25 μl of 50 mM Tris-HCl (pH 7.8)-50 mM NaCl-3 mM magnesium acetate-5 mM CaCl2-0.1 mM EDTA-0.1 mM dithiothreitol-25 μg of BSA per ml. After incubation for 10 min, DNA digestion was initiated by the addition of 5 ng of DNase I (Takara). After digestion for 30 s at 25°C, the reaction was terminated by the addition of 45 μl of DNase I stop solution (20 mM EDTA, 200 mM NaCl, 1% sodium dodecyl sulfate, 250 μg of yeast tRNA per ml). Digested products were precipitated by ethanol, dissolved in formamide dye solution, and analyzed by electrophoresis on a 6% polyacrylamide gel containing 8 M urea.
Computer search for the PhoP box.
The DNA sequences 500 bp upstream of the initiation codon of 232 genes, which were estimated by DNA microarray analysis, were obtained from GenoBase (http://ecoli.aist-nara.ac.jp). The PhoP box (TGTTTANNNNN TGTTTA) was searched for within their sequences with GENETYX-MAC (Software Development Co., Ltd.) set up with a 2-bp mismatch.
RESULTS AND DISCUSSION
DNA microarray-based identification of Mg2+-responsive genes controlled by the PhoP/PhoQ two-component system.
To search for the member genes of the Mg2+stimulon of E. coli, wild-type strain W3110 was grown in LB medium in the presence or absence of 30 mM MgCl2 and genomewide transcription profiles were analyzed with DNA microarrays. On addition of external Mg2+, the mRNA levels for a number of genes were markedly reduced. To identify whether these genes are under the control of the PhoP/PhoQ two-component system, the microarray assay was also performed for both WP3022 (phoP disruptant) and WQ3007 (phoQ disruptant) in the absence of MgCl2 and the transcription profiles were compared with that of W3110. A total of 232 genes, whose expression ratios were <0.75, were found to be repressed by the addition of external MgCl2 and in a PhoP/PhoQ-dependent manner (for details, see the supplemental data at http://www.nara.kindai.ac.jp/nogei/seiken/array.html). After sequence analysis of 500-bp-long promoter regions upstream from the ATG initiation codon of all of these genes, a total of 26 genes, including the previously identified phoPQ, mgtA, and mgrB genes (7), were found to possess a PhoP box with the consensus sequence (T/G)GTTTA-N5-(T/G)GTTTA (Table 3).
TABLE 3.
DNA microarray analysis resultsa
| Gene | ΔphoP (−)/wild (−)
|
ΔphoQ (−)/wild (−)
|
Wild (+)/wild (−)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Ratio | Avg | Ratio | Ratio | Avg | Ratio | Ratio | Avg | |
| ais | 0.51 | NDd | 0.51 | ND | ND | ND | ND | ND | ND |
| argD | 0.55 | ND | 0.55 | ND | ND | ND | ND | ND | ND |
| carA | 0.67 | 0.61 | 0.64 | 0.42 | 0.40 | 0.41 | 0.21 | ND | 0.21 |
| crcA | 0.47 | 0.77 | 0.62 | 0.46 | ND | 0.46 | ND | ND | ND |
| dfp | 0.64 | 0.53 | 0.58 | 0.56 | 0.55 | 0.56 | 0.27 | 0.26 | 0.26 |
| dinG | 0.69 | 0.81 | 0.75 | 0.56 | 0.90 | 0.73 | 0.46 | ND | 0.46 |
| gcvT | 0.77 | 0.68 | 0.73 | 0.60 | 0.55 | 0.58 | 0.33 | 0.37 | 0.35 |
| gppA | 0.68 | 0.64 | 0.66 | 0.61 | 0.66 | 0.63 | 0.49 | 0.44 | 0.46 |
| hemL | 0.32 | 0.41 | 0.37 | 0.35 | 0.35 | 0.35 | 0.41 | 0.44 | 0.43 |
| mgrB | 0.21b | 0.17b | 0.19b | —c | — | — | — | — | — |
| nagA | 0.55 | 0.53 | 0.54 | 0.59 | 0.70 | 0.64 | 0.52 | 0.68 | 0.60 |
| phoP | 0.22 | 0.21 | 0.21 | 0.67 | 0.50 | 0.59 | 0.34 | 0.45 | 0.40 |
| rstA | 0.54 | 0.46 | 0.50 | 0.47 | 0.29 | 0.38 | 0.13 | ND | 0.13 |
| sfcA | 0.38 | 0.35 | 0.36 | 0.41 | 0.27 | 0.34 | 0.26 | 0.21 | 0.24 |
| slyB | 0.29b | 0.27b | 0.28b | — | — | — | — | — | — |
| srlD | 0.71 | 0.50 | 0.60 | 0.77 | 0.66 | 0.71 | 0.43 | 0.31 | 0.37 |
| sucB | 0.31 | 0.25 | 0.28 | 0.75 | 0.56 | 0.66 | 0.10 | ND | 0.10 |
| vboR | 0.21 | 0.12 | 0.17 | ND | ND | ND | ND | ND | ND |
| xylA | 0.69 | ND | 0.69 | ND | ND | ND | ND | ND | ND |
| 411#13 b2362 | 0.63 | ND | 0.63 | ND | ND | ND | ND | ND | ND |
| ybjX | 0.12 | 0.10 | 0.11 | 0.13 | 0.09 | 0.11 | 0.10 | 0.18 | 0.14 |
| yfcS | 0.46 | 0.94 | 0.70 | ND | ND | ND | ND | ND | ND |
| yfdC | 0.57 | ND | 0.57 | ND | ND | ND | ND | ND | ND |
| ygeW | 0.23 | ND | 0.23 | ND | ND | ND | ND | ND | ND |
| yijF | 0.43 | ND | 0.43 | ND | ND | ND | ND | ND | ND |
| yrbL | 0.12 | 0.10 | 0.11 | 0.15 | 0.14 | 0.15 | 0.55 | 0.60 | 0.58 |
mgtA was not spotted on the DNA microarray used in this experiment. The symbols + and − mean presence and absence of MgCl2, respectively. ΔphoP, WP3022; ΔphoQ, WQ3007; wild, W3110. The underlined genes were identified as parts of the Mg2+ stimulon. The values shown are individual intensities of duplicate spots and the average of the two preceding values.
The data were obtained with E. coli DNA microarrays fabricated by Mori et al., in which N-minimal medium (3) was used.
—, no experiments were done.
ND, fluorescent signal too weak for detection.
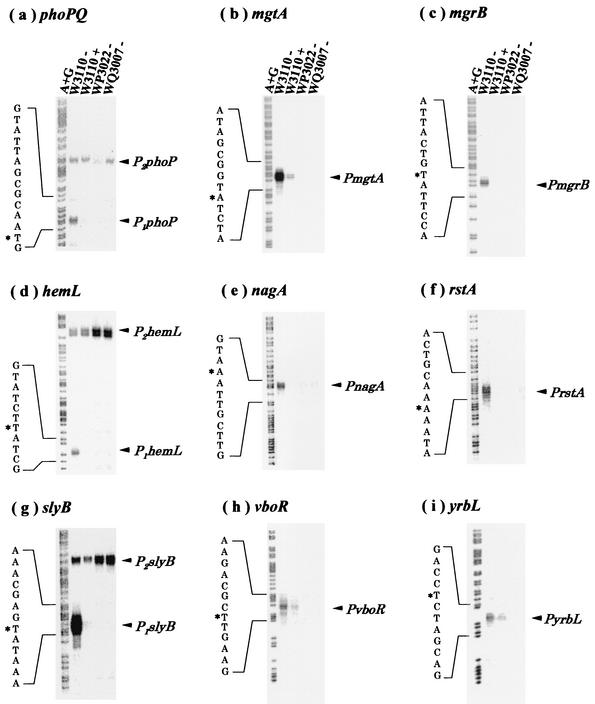
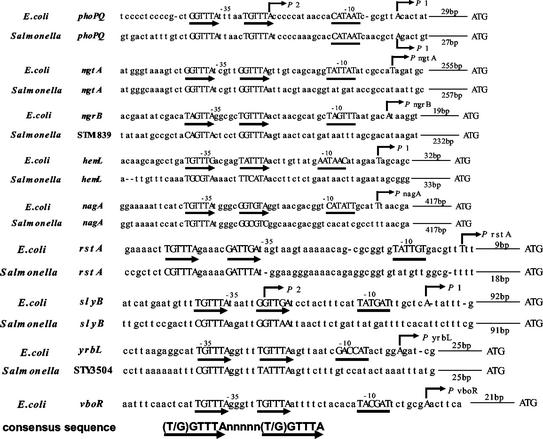
To validate these putative Mg2+-responsive genes regulated by PhoP/PhoQ, an S1 nuclease assay was performed for all 26 genes. In addition to the previously identified phoPQ, mgtA, and mgrB genes (7), six other genes (hemL, nagA, rstAB, slyB, vboR, and yrbL) were found to be repressed in the presence of high concentrations of extracellular Mg2+ (Fig. 1). mRNAs for these genes were detected in W3110 but not in WP3022 or WQ3007. In each case, Mg2+-dependent repression required the presence of both PhoP and PhoQ (Fig. 1). In the promoter regions of all of these Mg2+-responsive genes, the consensus PhoP box was identified (Fig. 2). The corresponding genes of S. enterica serovar Typhimurium (phoPQ, mgtA, STM1839, hemL, nagA, rstAB, slyB, and STM35040) also carry the PhoP box sequence in the respective promoter regions (Fig. 2).
FIG. 1.
S1 nuclease assays. E. coli W3110 (wild type), WP3022 (phoP defective), and WQ3007 (phoQ defective) were grown in LB medium to early exponential phase (OD600, 0.3 to 0.4) in the presence (+) or absence (−) of 30 mM MgCl2. S1 nuclease mapping was carried out to determine the transcription start sites of phoPQ (a), mgtA (b), mgrB (c), hemL (d), nagA (e), rstAB (f), slyB (g), yrbL (h), and vboR (i). Electrophoresis was performed with a 6% acrylamide sequencing gel. Lanes A+G represent Maxam-Gilbert sequencing reactions. Arrowheads show the transcripts protected from nuclease digestion. Transcription start sites are marked with asterisks.
FIG. 2.
Mg2+-responsive promoters. The E. coli Mg2+-responsive promoters, identified in Fig. 1, are aligned in parallel with the corresponding promoters from S. enterica serovar Typhimurium. The following DNA sequences of E. coli and S. enterica serovar Typhimurium are from the GenBank database (the percentages in parentheses are the homologies of the proteins): phoPQ, AE000213 and AE008753 (PhoP, 89%; PhoQ, 79%); mgtA, AE000495 and AE008909 (MgtA, 87%); mgrB, AE000277 and AE008782 (MgrB, 63%); hemL, AE000125 and AE008704 (HemL, 93%); nagA, AE000171 and AE008727 (NagA, 92%); rstAB, AE000256 and AE008764 (RstA, 80%; RstB, 78%); slyB, AE000259 and AE008762 (SlyB, 63%); yrbL, AE000400, STY3504, and AE008853 (YrbL, 72%); vboR, AE000161. Thin and bold arrows indicate starts and directions of transcription and direct repeats (PhoP box), respectively. The −10 region of each promoter is underlined. In S. enterica serovar Typhimurium, no promoters except phoPQ have been clarified. The −10 hexamer, start site, and direct repeats are all capitalized.
Recently, Oshima et al. (11) published the transcription profile for an E. coli mutant defective in the PhoP/PhoQ two-component system as determined by DNA microarray analysis. The expression of at least 28 genes, including rstAB, slyB, vboR, and yrbL, was decreased in the phoP/phoQ mutant, but it remained to be determined whether these genes respond to the availability of Mg2+ in the external environment. In an S1 nuclease assay, the mRNA levels of three genes, sfcA, gppA, and srlD, decreased in the presence of Mg2+, but this reduction was not observed even with the phoP and phoQ mutants (data not shown). These genes are not under direct control of the PhoP/PhoQ system. In fact, the PhoP box sequence is not present in these promoters. These results, together, indicate that the presence of a PhoP box is important for PhoP/PhoQ-dependent transcription of the Mg2+ stimulon genes.
Gel shift assay of PhoP-binding activity for the Mg2+-responsive gene promoters.
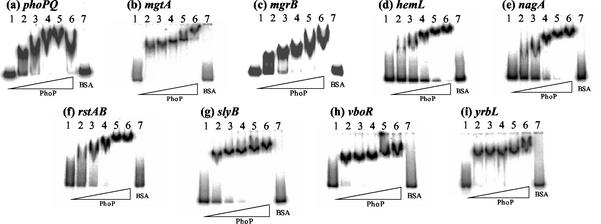
Recently, we found that the purified PhoP protein specifically binds in vitro to the PhoP box located within the mgtA promoter region (16). To check whether the PhoP protein can also bind to the PhoP box associated with the newly identified Mg2+-responsive genes, we carried out gel shift assays with DNA fragments from the promoters from these nine genes (phoPQ, mgtA, mgrB, hemL, nagA, rstA, slyB, vboR, and yrbL). All nine of the DNA probes used were retarded to form a single band of the probe DNA-PhoP protein complex (Fig. 3). The minimum concentration of PhoP protein required to convert all of the input probes to PhoP complexes was, however, different among the nine promoters, suggesting differences in their affinity to PhoP. We quantified the amount of PhoP needed to bind half of the probe amount used in gel shift assay (Table 4). PhoP binds the promoters of two genes (mgtA and vboR) with the highest affinity. Among the remaining six promoters, probes of hemL and nagA showed the lowest affinity.
FIG. 3.
Gel shift assays. The probes used in Fig. 1 were incubated at 37°C for 10 min with purified PhoP (BSA amounts: lane 1, 0 pmol; lane 2, 1.25 pmol; lane 3, 2.5 pmol; lane 4, 5 pmol; lane 5, 10 pmol; lane 6, 20 pmol; lane 7, 100 pmol). The mixtures were directly subjected to polyacrylamide gel electrophoresis. The sequences of the DNA probes used are described in Fig. 1.
TABLE 4.
Differential affinity of PhoP to bind Mg2+ stimulon genesa
| Gene | Amt (pmol) of PhoP |
|---|---|
| phoPQ | 1.54 |
| mgtA | 0.88 |
| mgrB | 2.25 |
| hemL | 9.66 |
| nagA | 4.32 |
| rstAB | 2.37 |
| slyB | 2.30 |
| vboR | 0.94 |
| yrbL | 1.0 |
The data were estimated by measuring the density of the probe band (lanes 1 to 6) in Fig. 3.
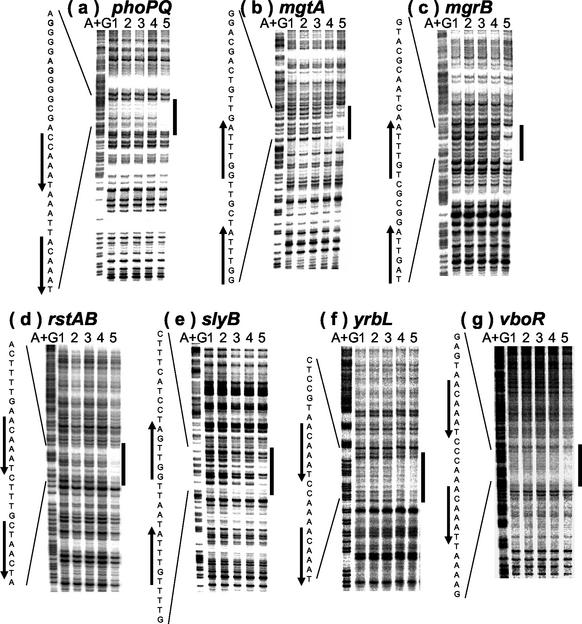
DNase I footprinting of PhoP-binding sites within the Mg2+-responsive gene promoters.
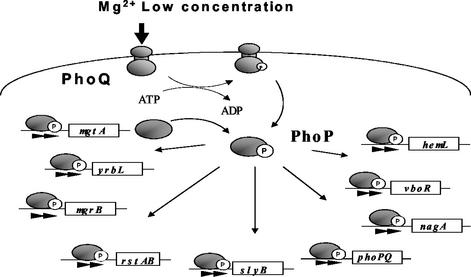
Next, we tried to identify the precise site of PhoP binding by using a DNase I footprinting assay. As expected, the purified PhoP protein was found to bind the PhoP box region of phoPQ, mgtA, mgrB, rstAB, slyB, vboR, and yrbL (Fig. 4). In the nagA, phoPQ, mgtA, mgrB, rstAB, slyB, and vboR promoters, PhoP also bound to the PhoP box in the simultaneous presence of RNA polymerase (data not shown). However, we failed to detect PhoP binding to the PhoP box of the hemL promoter. The lack of stable binding of PhoP alone to the PhoP box of the hemL and nagA promoters coincides with the results of the gel shift assays and supports the assumption that the affinity of PhoP differs among the nine promoters tested. Taking all of these observations together, we concluded that PhoP is a global transcriptional activator that directly binds to the PhoP box in the promoters of the Mg2+ stimulon of E. coli (Fig. 5).
FIG. 4.
DNase I footprinting assays. Coding (b, mgtA; c, mgrB; e, slyB) and noncoding (a, phoPQ; d, rstAB; f, yrbL; g, vboR) strands containing the PhoP box region were labeled with 32P at the 5′ end, incubated with various amounts of purified PhoP (lanes 1, 2, 3, 4, and 5 contain 0, 10, 20, 40, and 80 pmol, respectively), and subjected to DNase I footprinting assays. The DNA probes described in Fig. 1 were used, except that MGF6, BGF2, and SLY1 were labeled with [γ-32P]ATP. Lanes A+G represent the Maxam-Gilbert sequence reaction. DNA sequences from the bottom (5′) to the top (3′) are shown. Black boxes and bold arrows indicate PhoP-binding regions and PhoP boxes, respectively.
FIG. 5.
Proposed Mg2+ stimulon of E. coli.
The PhoP-PhoQ system responds to changes in the external Ca2+ level, as well as changes in the external Mg2+ level (3). In fact, we found that the phoPQ P1 promoter responds to changes in the extracellular Ca2+ level in a PhoP/PhoQ-dependent manner (data not shown). The response of E. coli to changes in extracellular Fe3+, Cu2+, Ag+, and Ni2+ levels was found to be under the control of the two-component systems PmrB/PmrA (1, 15), CusS/CusR (10), SilS/SilR (6), and NrsS/NrsR (8), respectively. These metal-responsive promoters also contain inverted or direct repeat sequences encompassing 5 bp. The binding of the respective regulatory proteins to these sequences is currently being examined. The signal transduction systems triggered by extracellular metal ions should be good models with which to obtain insights into the global network of bacterial gene expression.
Acknowledgments
This work was supported in part by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and CREST of JST (Japan Science and Technology).
REFERENCES
- 1.Aguirre, A., S. Lejona, E. G. Vescovi, and F. C. Soncini. 2000. Phosphorylated PmrA interacts with the promoter region of ugd in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:3874-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fabret, C., V. A. Feher, and J. A. Hoch. 1999. Two-component signal transduction in Bacillus subtilis: how one organism sees its world. J. Bacteriol. 181:1975-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Casadaban, M. J. 1976. Transcription and fusion of lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 3.Garcia Vescovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 4.Groisman, E. A., F. Heffron, and A. Solomon.1992. Molecular genetic analysis of the Escherichia coli phoP locus. J. Bacteriol. 174:486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta, A., K. Matsui, J. F. Lo, and S. Silver. 1999. Molecular basis for resistance to silver cations in Salmonella. Nat. Med. 5:183-188. [DOI] [PubMed] [Google Scholar]
- 7.Kato, A., H. Tanabe, and R. Utsumi. 1999. Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J. Bacteriol. 181:5516-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Maury, L., M. Garcia-Dominguez, F. J. Florencio, and J. C. Reyes. 2002. A two-component signal transduction system involved in nickel sensing in the cyanobacterium Synechocystis sp. PCC 6803. Mol. Microbiol. 43:247-256. [DOI] [PubMed] [Google Scholar]
- 9.Mizuno, T. 1997. Compilation of all genes encoding two-component phosphotransfer signal transducers in the genome of Escherichia coli. DNA Res. 4:161-168. [DOI] [PubMed] [Google Scholar]
- 10.Munson, G. P., D. L. Lam, F. W. Outten, and T. V. O'Halloran. 2000. Identification of a copper-responsive two-component system on the chromosome of Escherichia coli K-12. J. Bacteriol. 182:5864-5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oshima, T., H. Aiba, Y. Masuda, S. Kanaya, M. Sugiura, B. L. Wanner, H. Mori, and T. Mizuno. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281-291. [DOI] [PubMed] [Google Scholar]
- 12.Oshima, T., C. Wada, Y. Kawagoe, T. Ara, M. Maeda, Y. Masuda, S. Hiraga, and H. Mori. 2002. Genome-wide analysis of deoxyadenosine methyltransferase-mediated control of gene expression in Escherichia coli. Mol. Microbiol. 45:673-695. [DOI] [PubMed] [Google Scholar]
- 13.Soncini, F. C., and E. A. Groisman. 1996. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J. Bacteriol. 177:4364-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soncini, F. C., E. G. Vescovi, and E. A. Groisman. 1996. Two-component regulatory systems can interact to process multiple environmental signals. J. Bacteriol. 178:6796-6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wosten, M. M., L. F. Kox, S. Chamnongpol, F. C. Soncini, and E. A. Groisman. 2000. A signal transduction system that responds to extracellular iron. Cell 103:113-125. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto, K., H. Ogasawara, N. Fujita, R. Utsumi, and A. Ishihama. 2002. Novel mode of transcription regulation of divergently overlapping promoters by PhoP, the regulator of two-component system sensing external magnesium availability. Mol. Microbiol. 45:423-438. [DOI] [PubMed] [Google Scholar]