Abstract
The use of human prokinetic drugs in colic horses leads to inconsistent results. This might be related to differences in gastrointestinal receptor populations. The motor effects of 5-hydroxytryptamine (5-HT; serotonin) on the equine mid-jejunum were therefore studied. Longitudinal muscle preparations were set up for isotonic measurement.
5-HT induced tonic contractions with superimposed phasic activity; these responses were not influenced by tetrodotoxin and atropine, suggesting a non-neurogenic, non-cholinergic pathway.
The 5-HT receptor antagonists GR 127935 (5-HT1B,D), ketanserin (5-HT2A), SB 204741 (5-HT2B), RS 102221 (5-HT2C), granisetron (5-HT3), GR 113808 (5-HT4) and SB 269970 (5-HT7) had no influence on the 5-HT-induced response; the 5-HT1A receptor antagonists NAN 190 (pKb=8.13±0.06) and WAY 100635 (pKb=8.69±0.07), and the 5-HT1,2,5,6,7 receptor antagonist methysergide concentration-dependently inhibited the 5-HT-induced contractile response.
The 5-HT1,7 receptor agonist 5-carboxamidotryptamine (5-CT) induced a contractile response similar to that of 5-HT; its effect was not influenced by tetrodotoxin and atropine, and SB 269970, but antagonised by WAY 100635. 8-OHDPAT, buspiron and flesinoxan, which are active at rat and human 5-HT1A receptors, had no contractile influence.
These results suggest that the contractile effect of 5-HT in equine jejunal longitudinal muscle is due to interaction with muscular 5-HT receptors, which cannot be characterised between the actually known classes of 5-HT receptors.
Keywords: 5-HT, 5-CT, 5-HT1A, equine small intestine, ileus, colic, serotonin, horse
Introduction
Postoperative ileus is a notorious complication in horses that is predominantly seen after surgical intervention for small intestinal colic. Ileus in horses is characterised by a loss of adequate and coordinated intestinal motility and propulsion leading to the production of large amounts of gastric reflux and small intestinal distention. This complication is responsible for as many as 86% of equine deaths following abdominal surgery (Roussel et al., 2001). The pathogenic mechanisms which have been implicated as possible causes are sympathetic inhibitory reflexes, parasympathetic hypoactivity, dopaminergic hyperactivity and inhibitory mediators of the inflammatory response (Gerring & Hunt, 1986; Morris, 1991).
The goals of postoperative treatment are maintenance of adequate hydration, correction of electrolyte imbalance, pain relief, control of infection and last but not least, restoration of normal intestinal propulsion. The latter however often poses a real therapeutic challenge. The mainstays of currently used prokinetic treatments are extrapolated from human medicine by use of cisapride, metoclopramide, domperidone and erythromycin (Van Hoogmoed et al., 2004). Also, postoperative intravenous (i.v.) administration of lidocaine is inspired by human use (Brianceau et al., 2002). Up until now, however, application of these prokinetic treatments is invariably associated with inconsistent to poor results in horses with ileus.
An overview of the literature shows the lack of fundamental in vitro research on the equine intestine to justify the routine use of these human prokinetic drugs in colic horses. There is insufficient scientific evidence that the already established enteral receptor populations that serve as pharmacological target to induce intestinal propulsion in humans are equally important in horses. A possible discrepancy in these receptor populations between humans and horses could partially explain the inconsistent clinical efficacy of human prokinetic agents in equine colic cases.
Recently, increasing scientific interest in the role of serotonin (5-hydroxytryptamin; 5-HT) in human gastrointestinal motility has led to the development of several compounds of potential interest for the treatment of functional gastrointestinal tract disorders. The gastroprokinetic effect of the recently introduced tegaserod in humans is, as for cisapride, related to the activation of 5-HT4 receptors on cholinergic neurons, facilitating release of the contractile neurotransmitter acetylcholine (Talley, 2001). In healthy horses, tegaserod administered intravenously was shown to accelerate gastrocolonic transit of barium-filled particles given via a stomach tube and identified radiographically in the collected faeces; it increased the frequency of defaecation and the gut sounds at the caecal base (Lippold et al., 2004). Little information is available on the in vitro characterization of the 5-HT receptor population in the equine gut. In equine jejunum circular muscle, Nieto et al. (2000) reported that the stimulatory effect of 5-HT was antagonised by a 5-HT2 and a 5-HT3 receptor antagonist, but not by a 5-HT4 receptor antagonist. Both atropine and tetrodotoxin (TTX) had no effect on the 5-HT-induced contractions, which suggests that in this part of the intestine 5-HT mediates its effect through 5-HT2 and 5-HT3 receptors, active via a non-neurogenic, noncholinergic pathway. This is very peculiar, since up until now a solely neuronal localisation has been ascribed to the 5-HT3 receptor. The stimulatory effect of cisapride, which was less pronounced than that of 5-HT, was not influenced by atropine plus TTX and was attributed to 5-HT2 receptor activation, based on the antagonistic effects of the specific 5-HT2 receptor antagonist ketanserin. Again this observation is surprising, since cisapride has only been characterised as a 5-HT2 receptor antagonist. For equine ileum and pelvic flexure circular and longitudinal muscle, Weiss et al. (2002) reported stimulatory effects of 5-HT that were reduced by 5-HT4 receptor antagonism, but still more by 5-HT3 receptor antagonism, so that an interaction with 5-HT3 and 5-HT4 receptors was proposed; tegaserod had a stimulatory effect that was less pronounced than that of 5-HT.
The aim of this study was to identify the contractile serotonergic receptor population in the small intestine of the horse, taking into account all serotonergic receptor-type possibilities, and thus not limiting the study to the testing of the presence of 5-HT receptor populations identified in human intestine, being mainly 5-HT2, 5-HT3 and 5-HT4 receptors. The rationale to investigate primarily small intestine is the fact that postoperative ileus is predominantly located in this intestinal segment. The jejunal longitudinal smooth muscle was elected because up until now no 5-HT receptor population characterization has been performed in this muscle layer.
Methods
Tissue collection and smooth muscle strip preparation
The study population was comprised of horses of various breeds and either sex, with an age range of 2–20 years. Ponies, foals and draft horses were excluded from the study.
Segments of the middle part of the equine jejunum were collected at the slaughterhouse, using the ileum as point of orientation. Shortly after stunning, the gastrointestinal tract was removed from the carcasses and a jejunal segment of 20 cm was dissected at a distance of 8 m proximal to the jejunoileal junction. The segments were then rinsed with oxygenated Krebs–Henseleit solution (composition in mM: glucose 11.1, CaCl2 2.51, NaHCO3 25, MgSO4 1.18, KH2PO4 1.18, KCl 4.69 and NaCl 118) at 4°C, to remove bowel contents and were subsequently immersed in the same oxygenated solution during transportation to the laboratory.
Within 1 h after tissue collection, the intestinal segments were opened along the mesenteric border and were carefully cleared of mucosa, submucosa and mesenterium. Strips (maximum 32 per horse) of approximately 1.5 cm length and 4–5 mm width were then prepared in the direction of the longitudinal muscle layer and mounted onto tissue holders. These were placed in a set-up of 16 organ baths, containing Krebs–Henseleit solution (20 ml) at 37°C, continuously gassed with 95% O2 and 5% CO2. The mechanical activity of the preparations was recorded via isotonic transducers (Harvard apparatus) coupled to a 16-channel PowerLab (ADInstruments, Melbourne, Australia), under a load of 2 g. The load of 2 g was determined as optimal by preliminary testing on strips of 10 horses, measuring maximal carbachol-induced contraction under loads ranging from 1 up to 10 g.
A 1-h stabilisation period was allowed before the start of the experiment, during which the organ baths were flushed with Krebs–Henseleit solution at 30 and 60 min. After this period, regular spontaneous activity was observed in all preparations. Subsequently, the tissue was challenged twice with 1 μM carbachol at an interval of 30 min. This induced in all preparations two tonic contractions of similar size, illustrating complete equilibration of the tissue.
Experimental protocols
Preliminary experiments with 5-HT
In preliminary experiments, the responses to cumulative administration of 5-HT (0.1 nM to 1 μM) within the same tissue were compared with those to administration of eight increasing concentrations of 5-HT (0.1 nM to 3 μM) in eight parallel jejunal strips of the same horse (one concentration per tissue). This learned that the cumulative concentration–response curve to 5-HT was clearly depressed at the higher concentrations of 5-HT in comparison to the isolated one (see Results), so that only isolated concentration–response curves were obtained in further experiments with 5-HT and other 5-HT receptor agonists. Preliminary experiments also indicated that repeated administration of 0.1 μM 5-HT at 15 min interval (with washout once the contractile response was obtained) led to a decreasing response to 5-HT already at the second administration. When the interval was increased to 30 min, the response to repetitive administration of 0.1 μM 5-HT (up to seven times) remained stable.
Influence of TTX and atropine, NG-nitro-L-arginine (L-NNA) and 5-HT receptor antagonists on the response to 5-HT
TTX (0.3 μM) plus atropine (0.3 μM), and L-NNA (100 μM) were tested versus 5-HT as follows. An isolated concentration–response curve to 5-HT was constructed by administering eight increasing concentrations of 5-HT to eight jejunal strips of a horse (thus each preparation only receiving one concentration of 5-HT), and a parallel curve to 5-HT was obtained after incubation for 20 min with TTX plus atropine, or L-NNA in eight strips of the same horse. A series of 5-HT receptor antagonists was tested versus 5-HT in the same way: ketanserin (5-HT2A; 0.3 μM), granisetron (5-HT3; 0.3 μM); GR 113808 ([1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl-1-methyl-1H-indole-3-carboxylate, 5-HT4; 0.1 μM); SB 269970 ((R)-3-(2-(2-(4-methylpiperidin-1-yl) ethyl)pyrrolidine-1-sulphonyl) phenol, 5-HT7; 0.3 μM); methysergide (5-HT1,2,5,6,7; 1, 10 and 100 nM), NAN 190 (5-HT1A; 0.1, 0.3 and 1 μM) and WAY 100635 (N-2-4-(2-methoxyphenyl)-1-piperazinylethyl-N-(2-pyridinyl)cyclohexane carboxamide trihydro-chloride, 5-HT1A; 3, 30 and 300 nM).
TTX (3 μM) and atropine (1 μM) were also tested separately versus 1 μM 5-HT. 5-HT was added twice at 30 min interval; 20 min before the second administration, TTX (3 μM) and/or atropine (1 μM) were added to the organ bath; a third tissue was used as a control. The following 5-HT receptor antagonists were also tested in the same way: GR 127935 (2-methyl-4-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid [4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]amide HCl, 5-HT1B,D; 0.1 μM), ketanserin (5-HT2A, 0.3 μM), SB 204741 (N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea, 5-HT2B; 0.3 μM), RS 102221 (8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulphon-amido)phenyl-5-oxopentyl)]-1, 3, 8-triazaspiro[4.5]decane-2,4-dione hydrochloride, 5-HT2C; 0.3 μM), granisetron (5-HT3; 0.3 μM) and GR 113808 (5-HT4; 0.1 μM).
The above-described experiments showed that WAY 100635, NAN 190 and methysergide were the only 5-HT receptor antagonists, with a clearcut influence on the effect of 5-HT. Therefore, they were also tested in the following way. 5-HT (0.1 μM) was added seven times at 30 min interval with washout after the contractile response was obtained in four tissues of the same horse; 20 min before the second to sixth administration of 5-HT, increasing concentrations of WAY 100635, NAN 190 or methysergide were added; the seventh administration of 5-HT was carried out after washout of the antagonists. The fourth tissue of the same horse was used as a control.
Influence of other 5-HT receptor agonists
Isolated concentration–response curves were also constructed for the 5-HT1A receptor agonists flesinoxan, 8-OH-DPAT (8-hydroxy-2-(di-n-propylamino) tetralin) and buspiron, and for 5-carboxamidotryptamine (5-CT; 5-HT1,7). TTX (0.3 μM) plus atropine (0.3 μM), SB 269970 (0.3 μM) and WAY 100635 (3, 30 and 300 nM) were tested versus isolated concentration–response curves of 5-CT, as described for 5-HT. The influence of TTX (3 μM) and atropine (1 μM) was also tested separately versus 1 μM 5-CT, as described versus 1 μM 5-HT. The possible antagonistic effect of flesinoxan (0.1 μM), 8-OH-DPAT (0.1 μM) and buspiron (1 μM) versus 5-HT was tested by adding 0.1 μM 5-HT twice at 30 min interval; 20 min before the second administration, flesinoxan, 8-OH-DPAT or buspiron was added. The concentrations of flesinoxan, 8-OH-DPAT and buspiron in these experiments were chosen to be at least 100 times higher than their affinity values determined from competition binding with [3H]8-OH-DPAT in CHO cells expressing the human 5-HT1A receptor (Newman-Tancredi et al., 2001).
Drugs
The following drugs were used (abbreviations and respective suppliers in parentheses): carbachol (Merck, Germany), 5-hydroxytryptamine (5-HT; Janssen Research foundation, Belgium), atropine sulphate (Merck, Germany), methysergide maleate, ketanserin tartrate, 1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine HCl (NAN-190), SB204741, GR 113808, GR 127935, L-NNA, granisetron HCl, RS 102221, SB 269970 (Janssen Research foundation, Belgium), TTX (Serva, Germany), 5-CT (Tocris Cookson, UK), 8-OH-DPAT, flesinoxan, buspiron (Janssen Research foundation, Belgium); WAY 100635 (Tocris Cookson, UK). All compounds were dissolved in distilled water, except for NAN 190 that was dissolved in distilled water with 10% cyclodextrin, and SB 204741 that was dissolved in distilled water with 20% cyclodextrin. The solvents had no effect on the muscle strips per se and did not affect the agonist and antagonist concentration–response curves. All stock solutions were prepared freshly on the day of the experiment and dilutions were prepared using distilled water.
Data analysis
Data collection was performed using Chart for Windows (v4.12, ADInstruments, Oxfordshire, UK).
The amplitude of contractions induced by 5-HT and 5-HT receptor agonists is expressed as % of the second carbachol-induced contraction. In the experiments where increasing concentrations of the antagonists methysergide, WAY 100635 and NAN 190 were tested versus 0.1 μM 5-HT, the amplitude of contractions is normalised by expressing them as % of the blanco 5-HT-induced contraction before administration of these antagonists, used as reference.
Concentration–response curves to 5-HT and other agonists were individually fitted to the Hill equation using a computerised iterative nonlinear curve fitting procedure, obtaining curve parameter estimates for upper asymptote Emax, midpoint location pEC50 and Hill slope nH. Curve parameters in the presence of an antagonist were compared to those in its absence by unpaired t-test, accepting competitive antagonism when the pEC50 was significantly decreased but Emax and slope were not significantly altered. In case of competitive antagonism, the pKb of the antagonist was calculated according to log Kb=log B−log (DR-1). When the influence of a single concentration of antagonist was tested versus a single concentration of 5-HT (1 μM), within the same tissue, the responses to 5-HT in the absence and presence of the antagonist were compared by a paired t-test. When several concentrations of a 5-HT receptor antagonist (NAN 190, WAY 100635) were tested in one single strip, versus a fixed dose of 5-HT (0.1 μ), Kb values of the antagonists were calculated using the logistic function described by Lazareno & Birdsall (1993), which represents a modification of the Cheng–Prusoff equation for analysing antagonist inhibition curves in functional experiments:
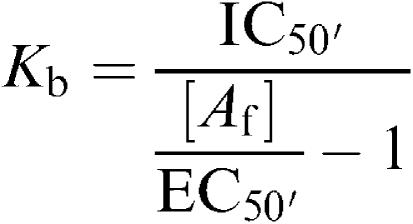 |
where Kb is the antagonist dissociation constant and [Af] is the fixed agonist concentration (in this case 5-HT 0.1 μM). For reasons of accuracy and convenience, when using this method, it is necessary to constrain the agonist and antagonist concentration–effect curves to have the same maximum (in this case 0.1 μM). So, in the above-described logistic function IC50′ is derived from the antagonist inhibition curves, which were constructed by nonlinear regression. The EC50′ value was obtained by fitting the control concentration–response curve to 5-HT in 24 horses (Figure 1b) to a maximum of 0.1 μM 5-HT and constraining the Hill slope to 1.
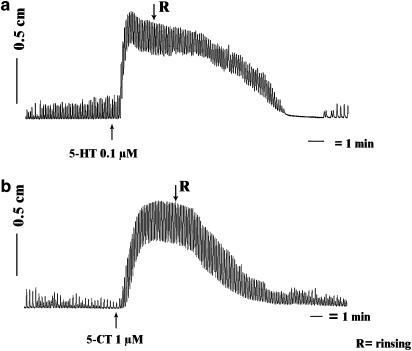
Figure 1.
Representative tracings of the response to 0.1 μM 5-HT (a) and 1 μM 5-CT (b) in isolated equine jejunal longitudinal muscle strips.
All values are expressed as mean±s.e.m.; n denotes the number of tissues obtained from different horses. Significance was set at a value of P<0.05.
Results
Concentration–response curves to 5-HT
The equine jejunal longitudinal muscle strips showed spontaneous phasic activity. 5-HT induced mainly a tonic contraction with superimposed phasic activity (Figure 1a). The amplitude and the frequency of these phasic contractions tended to be increased in comparison to the spontaneous activity before the administration of 5-HT, but this effect did not show concentration-dependency. Only the tonic response was therefore measured for calculation.
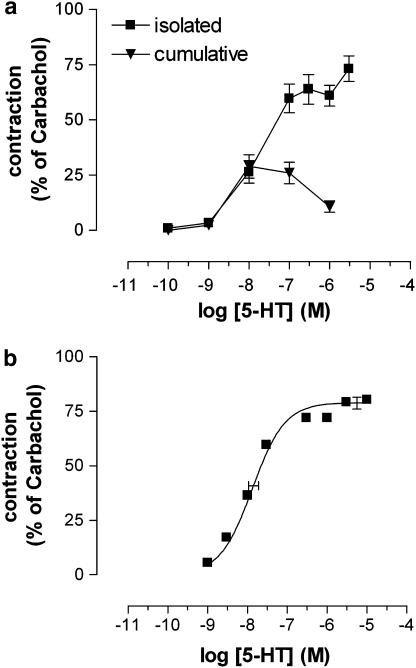
Figure 2a shows the concentration–response curves obtained by cumulative administration of 5-HT in the same strip and by administration of eight increasing concentrations of 5-HT to eight parallel strips. The cumulative concentration–response curve was bell shaped and the maximal effect was clearly decreased compared to that of the isolated curve. Accordingly, the cumulative administration protocol was not used to investigate the effect of 5-HT in equine jejunum longitudinal muscle.
Figure 2.
(a) Mean (±s.e.m) concentration–response curves to 5-HT, when added cumulatively or in an isolated way (eight increasing concentrations in eight different tissues) in equine jejunal longitudinal muscle (n=6). (b) Mean isolated concentration–response curve to 5-HT in equine jejunal longitudinal smooth muscle strips (n=24). The curve shown represents a simulation using the Hill equation; the estimates of Emax (with vertical error bars) and pEC50 (with horizontal error bars) are shown.
Figure 2b shows the constructed mean isolated 5-HT (1 nM–10 μM) concentration–response curve of 24 horses. It has the features of a monophasic sigmoidal concentration–response curve, consistent with a single-site interaction. The iterative fitting procedure of the individual curves yields a mean upper asymptote Emax of 79.04±2.47%, a mean midpoint location pEC50 of 7.88±0.07 and a mean Hill slope of 1.07±0.08.
Effect of TTX, atropine and L-NNA on the response to 5-HT
Addition of TTX and atropine to the organ baths had no effect on frequency or amplitude of spontaneous activity or base-line tonus. The midpoint location, slope and upper asymptotes of the isolated concentration–response curves to 5-HT that served as a control were not significantly influenced by the combination of TTX (0.3 μM) and atropine (0.3 μM), (Table 1). The contractile response to 1 μM 5-HT in the presence of TTX (3 μM) or atropine (1 μM) or the combination of both was also not changed in comparison to the response induced by 1 μM 5-HT before adding TTX and/or atropine (Table 2).
Table 1.
Curve parameters for the isolated concentration–response curves to 5-HT and 5-CT in the absence and presence of the antagonists indicated
| Agonist | In the absence of antagonist | In the presence of antagonist | ||||
|---|---|---|---|---|---|---|
| Emax (%) | pEC50 | nH | Emax (%) | pEC50 | nH | |
| 5-HT | ||||||
| TTX plus atropine (n=4) (both 0.3 μM) | 90.44±2.31 | 7.56±0.23 | 0.88±0.14 | 89.29±2.88 | 7.66±0.11 | 0.83±0.19 |
| L-NNA (n=4) (100 μM) | 65.80±6.00 | 7.85±0.13 | 0.90±0.05 | 73.00±7.82 | 7.52±0.26 | 0.90±0.05 |
| Ketanserin (n=4) (0.3 μM) | 80.64±3.55 | 7.83±0.05 | 0.81±0.06 | 86.91±4.46 | 7.63±0.14 | 0.70±0.12 |
| Granisetron (n=4) (0.3 μM) | 85.40±2.57 | 8.26±0.08 | 1.30±0.15 | 82.26±0.84 | 8.15±0.11 | 1.04±0.13 |
| GR 113808 (n=4) (0.1 μM) | 75.46±4.12 | 7.92±0.08 | 1.01±0.16 | 76.98±4.16 | 7.87±0.10 | 0.84±0.07 |
| SB 269970 (n=4) (0.3 μM) | 70.94±7.60 | 7.72±0.11 | 0.91±0.15 | 72.22±8.18 | 7.52±0.07 | 0.74±0.07 |
| 5-CT | ||||||
| TTX plus atropine (n=4) (both 0.3 μM) | 86.90±7.48 | 6.19±0.26 | 0.74±0.06 | 86.16±7.71 | 6.34±0.31 | 0.73±0.09 |
| SB 269970 (n=4) (0.3 μM) | 86.90±7.48 | 6.19±0.26 | 0.74±0.06 | 83.99±3.19 | 6.31±0.27 | 0.69±0.06 |
Values are expressed as mean±s.e.m.
Table 2.
Contractile responses to 1 μM 5-HT and 1 μM 5-CT before and in the presence of the antagonists indicated
| Antagonist | Response to 1 μM 5-HT | |
|---|---|---|
| Before | In the presence | |
| TTX (n=4) (3 μM) | 73.32±1.02 | 72.44±1.75 |
| Atropine (n=4) (1 μM) | 74.03±2.37 | 74.93±1.81 |
| TTX plus atropine (n=4) (3 and 1 μM) | 73.33±1.64 | 74.38±1.34 |
| GR 127935 (n=5) (0.1 μM) | 49.26±2.07 | 49.65±2.16 |
| Ketanserin (n=6) (0.3 μM) | 49.35±6.44 | 39.08±5.63 |
| SB 204741 (n=4) (0.3 μM) | 52.38±2.18 | 41.33±6.27 |
| RS 102221 (n=6) (0.3 μM) | 52.29±2.88 | 51.79±3.32 |
| Granisetron (n=6) (0.3 μM) | 57.72±3.16 | 52.94±4.17 |
| GR 113808 (n=6) (0.1 μM) | 58.08±3.91 | 56.99±5.15 |
| Response to 1 μM 5-CT | ||
| TTX (n=4) (3 μM) | 68.42±3.71 | 68.54±2.71 |
| Atropine (n=4) (1 μM) | 66.98±0.35 | 69.61±1.58 |
| TTX plus atropine (n=4) (3 and 1 μM) | 65.91±2.68 | 65.97±2.16 |
Values are expressed as mean±s.e.m.
L-NNA (100 μM), a nitric oxide synthase inhibitor, did not influence spontaneous activity or base-line tonus of the tissues. There was no significant effect on the concentration–response curve to 5-HT (Table 1).
Effect of 5-HT receptor antagonists on the response to 5-HT
Neither the selective 5-HT2A-receptor antagonist ketanserin (0.3 μM; Hoyer et al., 1994), nor the selective 5-HT3 receptor antagonist granisetron (0.3 μM; Sanger & Nelson, 1989) altered the concentration–response curve to 5-HT (Table 1). The same observation was made for the highly selective 5-HT4 receptor antagonist GR 113808 (0.1 μM; Johnson et al., 1993) and the 5-HT7 receptor antagonist SB 269970 (0.3 μM; Hagan et al., 2000).
In further experiments, antagonists were tested by studying the response to 1 μM 5-HT before and in the presence of a given antagonist within the same tissue. These experiments confirmed that the 5-HT2A receptor antagonist ketanserin (0.3 μM), the 5-HT3 receptor antagonist granisetron (0.3 μM) and the 5-HT4 receptor antagonist GR 113808 (0.1 μM) had no significant influence on the response to 5-HT; they further showed that also the 5-HT1B,D receptor antagonist GR 127935 (0.1 μM; Terron, 1996), the 5-HT2B receptor antagonist SB 204741 (0.3 μM; Forbes et al., 1995) and the 5-HT2C receptor antagonist RS 102221 (0.3 μM; Bonhaus et al., 1997) did not significantly influence the response to 5-HT (Table 2).
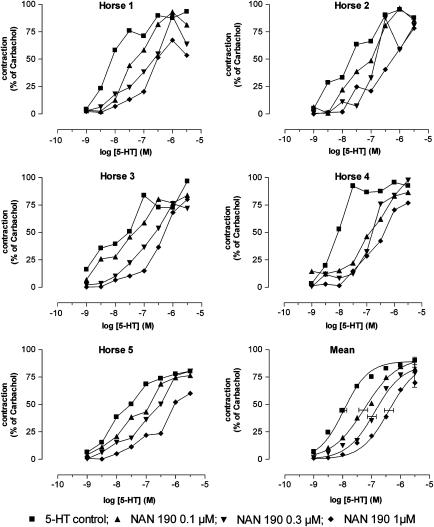
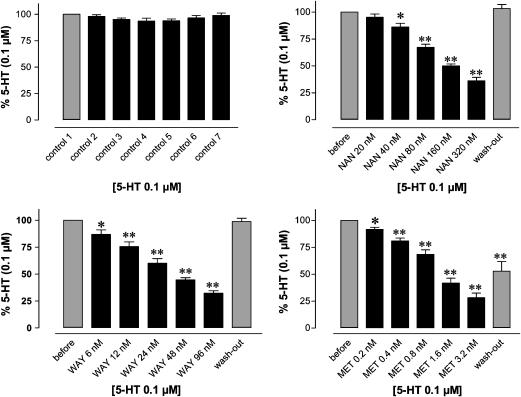
The 5-HT1A receptor antagonists NAN 190 (0.1, 0.3 and 1 μM; Cao & Rodgers, 1997) and WAY 100635 (3, 30 and 300 nM; Khawaja et al., 1995) inhibited the contractions to 5-HT in a concentration-dependent fashion. Figure 3 shows the results for NAN 190. Although the concentration–response curves of 5-HT in an individual horse showed a somewhat capricious shape, the mean results illustrate a parallel rightward shift of the concentration–response curve to 5-HT in the presence of increasing concentrations of NAN 190. The slopes and upper asymptotes of the concentration–response curves to 5-HT in the presence and the absence of NAN 190 were indeed not significantly different, while the pEC50 significantly decreased (Table 3). The pKb values calculated on the basis of the results with 0.1, 0.3 and 1 μM NAN 190 were 7.58±0.51, 7.54±0.24 and 7.55±0.19, respectively. WAY 100635 (3 nM) shifted the concentration–response curve to 5-HT to the right in a parallel way without a change in Emax, but the higher concentrations of WAY 100635 (30 and 300 nM) significantly depressed the Emax of 5-HT (Figure 4, Table 3). Apparently, WAY 100635 behaves as a noncompetitive antagonist in these higher concentration ranges. The pKb value calculated for the lowest concentration of WAY 100635 was 8.83±0.44.
Figure 3.
Influence of increasing concentrations of NAN 190 on the 5-HT-induced contraction of equine jejunal longitudinal muscle strips. The individual responses in five different horses (horse 1–5) are shown, as well as the mean curve simulations using the Hill equation; in the latter panel, the estimates for Emax (with vertical error bars) and pEC50 (with horizontal error bars) are given.
Table 3.
Curve parameters for the isolated concentration–response curves to 5-HT and 5-CT in the absence and presence of increasing concentrations of NAN 190 (5-HT) or WAY 100635 (5-HT, 5-CT)
| Emax | pEC50 | nH | |
|---|---|---|---|
| 5-HT control | 89.32±3.00 | 7.96±0.09 | 1.02±0.23 |
| NAN 190 (0.1 μM) | 92.74±4.74 | 7.24±0.16* | 0.76±0.11 |
| NAN 190 (0.3 μM) | 86.41±5.39 | 6.89±0.08** | 0.90±0.11 |
| NAN 190 (1 μM) | 88.92±9.18 | 6.40±0.17** | 0.90±0.16 |
| 5-HT control | 83.55±3.04 | 7.53±0.17 | 0.83±0.10 |
| WAY 100635 (3 nM) | 80.96±6.28 | 7.13±0.08* | 0.99±0.21 |
| WAY 100635 (30 nM) | 73.00±3.53* | 6.60±0.10** | 0.72±0.12 |
| WAY 100635 (300 nM) | 56.10±10.47* | 6.35±0.21** | 1.22±0.16* |
| 5-CT control | 85.86±3.78 | 6.20±0.13 | 0.63±0.09 |
| WAY 100635 (3 nM) | 84.22±1.90 | 5.81±0.09* | 0.76±0.11 |
| WAY 100635 (30 nM) | 66.22±5.44* | 5.78±0.14** | 1.10±0.07* |
| WAY 100635 (300 nM) | 33.35±6.85** | 5.48±0.21** | 1.30±0.13* |
Values are expressed as mean±s.e.m (n=5–6).
P<0.05,
P<0.001: significantly different versus 5-HT or 5-CT in the absence of antagonist.
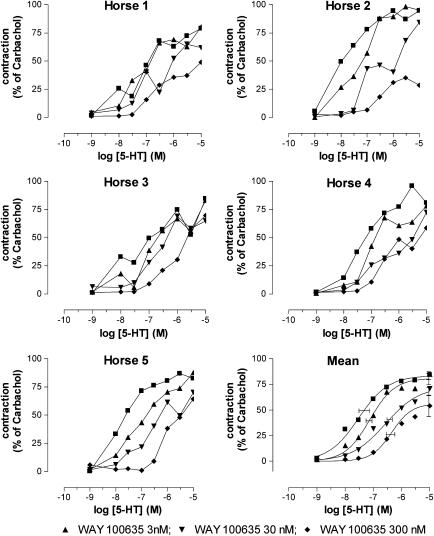
Figure 4.
Influence of increasing concentrations of WAY 100635 on the 5-HT-induced contraction of equine jejunal longitudinal muscle strips. The individual responses in five different horses (horse 1–5) are shown, as well as the mean curve simulations using the Hill equation; in the latter panel, the estimates for Emax (with vertical error bars) and pEC50 (with horizontal error bars) are given.
In view of the capricious shape of the isolated concentration–response curves to 5-HT in the individual horses in Figures 3 and 4, and the fact that different antagonist concentrations were tested in different tissues in these experiments, the antagonists NAN 190 and WAY 100635 were also tested in different concentrations versus 0.1 μM 5-HT within the same tissue (Figures 5 and 6). Both antagonists concentration-dependently reduced the response to 0.1 μM 5-HT; their effect was easily rinsed out. From these experiments, a pKb value of 8.13±0.06 was estimated for NAN 190 and a pKb value of 8.69±0.07 for WAY 100635, using the ‘functional' version of the Cheng–Prusoff equation proposed by Lazareno & Birdsall (1993).
Figure 5.
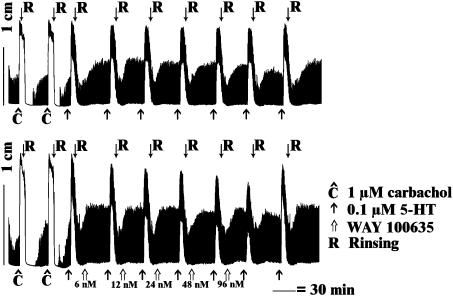
Representative traces showing the influence of 1 μM carbachol and 0.1 μM 5-HT in two equine jejunum longitudinal muscle strips. In the upper panel, 5-HT was studied seven times consecutively without adding an antagonist (control); in the lower panel, the response to 5-HT was studied in the presence of increasing concentrations of WAY 100635.
Figure 6.
Influence of increasing concentrations of NAN 190 (NAN), WAY 100635 (WAY) and methysergide (MET) on the 5-HT-induced (0.1 μM) response of equine jejunal longitudinal muscle strips. 5-HT was administered seven times at 30 min interval; antagonists were added 20 min before the second to sixth administration. Tissues were rinsed after each 5-HT-induced contraction; the seventh administration was performed to check for wash out of the antagonists. In control strips, 5-HT was tested seven times without adding antagonist (left upper panel). Data are expressed as mean values±s.e.m (n=8). *P<0.05; **P<0.001 versus 5-HT before antagonist.
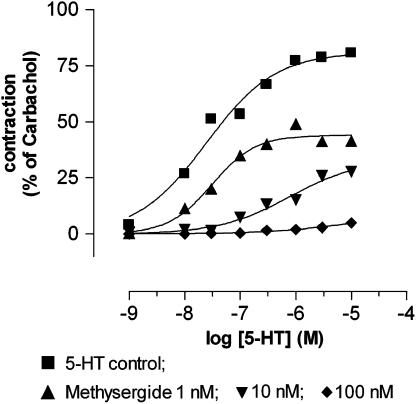
The 5-HT1, 5-HT2, 5-HT5, 5-HT6 and 5-HT7 receptor antagonist methysergide (1, 10 and 100 nM; Gommeren et al., 1998) antagonised nonsurmountably the 5-HT-induced concentration–response curve as shown in Figure 7. When tested in different concentrations (0.2–3.2 nM) versus 0.1 μM 5-HT in the same tissues, methysergide induced a concentration-dependent reduction of the response to 5-HT; this effect was only partially washed out (Figure 6).
Figure 7.
Concentration–response curves to 5-HT in the absence and the presence of increasing concentrations of methysergide in equine jejunal longitudinal muscle strips (n=5). The curves shown represent simulations using the Hill equation.
Influence of other 5-HT receptor agonists
In accordance with the 5-HT response, it was shown in preliminary experiments that the cumulative concentration–response curve to the 5-HT1, 5-HT7 receptor agonist 5-CT (Hoyer et al., 1994) was clearly depressed at the higher concentrations of 5-CT in comparison to the isolated one, so that only isolated concentration–response curves were obtained in further experiments with 5-CT.
Addition of 5-CT to the organ baths elicited a response similar to that of 5-HT (Figure 1b). The contractile responses to 5-CT are concentration-dependent, yielding curve parameters for Emax of 85.86±3.78%, pEC50 of 6.02±0.18 and a mean Hill slope of 0.76±0.09.
In contrast, the 5-HT1A receptor agonists 8-OH-DPAT (Sanger & Schoemaker, 1992) and flesinoxan (Hadrava et al., 1995), and the partial 5-HT1A receptor agonist buspiron (Sharif et al., 2004) had no effect in the muscle strips (tested concentrations: 1 nM to 3 μM). When 5-HT (1 μM) was added on top of 8-OH-DPAT, flesinoxan or buspiron, this immediately induced muscle strip contraction. 8-OH-DPAT, flesinoxan and buspiron did not antagonise the response to 5-HT. The contractile response to 0.1 μM 5-HT was 66.16±6.20% before and 70.99±8.69% in the presence of 0.1 μM 8-OH-DPAT, 64.43±5.56% before and 68.70±5.14% in the presence of 0.1 μM flesinoxan, and 68.38±4.88% before and 63.15±7.47% in the presence of 1 μM buspiron (n=6 for each series); the response to 5-HT in the parallel control tissues not receiving antagonist was 72.23±3.24 and 79.57±4.68% (n=6).
Effect of antagonists on the response to 5-CT
Curve parameters of the concentration–response curves to 5-CT were not influenced by application of TTX plus atropine (both 0.3 μM), nor by the selective 5-HT7 receptor antagonist SB 269970 (0.3 μM) (Table 1). Likewise, the contractile response to 1 μM 5-CT in the presence of TTX (3 μM) or atropine (1 μM) or the combination of both was also not changed in comparison to the response induced by 1 μM 5-CT before adding TTX and/or atropine (Table 2).
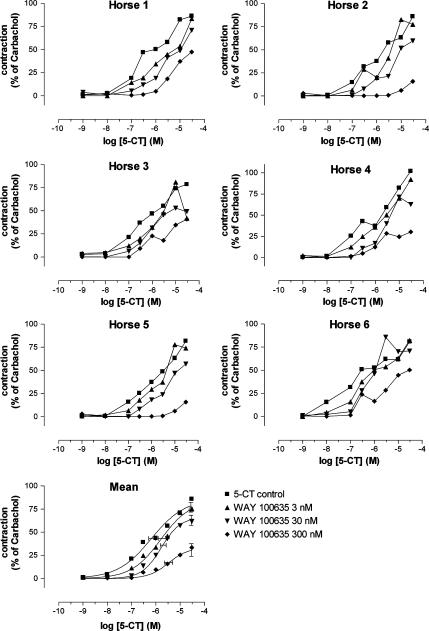
The specific 5-HT1A receptor antagonist WAY 100635 in its lowest concentration (3 nM) produced a parallel rightward shift of the concentration–contraction curve to 5-CT, without influence on the maximum response (Figure 8; Table 3). When WAY 100635 was applied at a concentration of 30 nM and 0.3 μM, the concentration–response curve to 5-CT was further shifted to the right but there was a clear concomitant suppression of the maximum effect elicited by 5-CT. The pKb value calculated for the lowest concentration of WAY 100635 was 8.63±0.34.
Figure 8.
Influence of increasing concentrations of WAY 100635 on the 5-CT-induced contraction of equine jejunal longitudinal muscle strips. The individual responses in six different horses (horse 1–6) are shown, as well as the mean curve simulations using the Hill equation; in the latter panel, the estimates for Emax (with vertical error bars) and pEC50 (with horizontal error bars) are given.
Discussion
Interaction of 5-HT with muscular 5-HT receptors, antagonised by the 5-HT1A receptor antagonists NAN 190 and WAY 100635
The inability of TTX and atropine, even in the higher concentrations tested, to affect the 5-HT-induced contractile response in equine jejunal longitudinal smooth muscle suggests that 5-HT mediates its effects through non-neurogenic, nonholinergic pathways. A similar mechanism of action was observed in the circular smooth muscle of the equine jejunum (Nieto et al., 2000). Also a possible interference of NO release by 5-HT was excluded by the lack of effect of the NO synthase inhibitor L-NNA on the 5-HT-induced contractile response. From the experiments in which several antagonists were tested versus a full 5-HT concentration–response curve, or versus a single nearly maximal concentration of 5-HT, the participation of 5-HT1B,1D, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT4 and 5-HT7 receptors in the 5-HT-induced contractile response can be excluded. These findings on longitudinal muscle are in contrast with those on 5-HT-induced responses in equine jejunal circular smooth muscle, where interaction with 5-HT2 and 5-HT3 receptors has been proposed (Nieto et al., 2000).
Of all tested antagonists, only the 5-HT1A receptor antagonists NAN 190 and WAY 100635, and the 5-HT1,2,5,6,7 receptor antagonist methysergide elicited a clearcut inhibitory effect on the 5-HT-induced contractile response of equine jejunum longitudinal smooth muscle. The specific 5-HT1A receptor antagonist NAN 190 fulfiled all requirements of pure competitive antagonism. The pKb calculated from the experiments, where increasing concentrations of NAN 190 were tested versus a fixed concentration of 5-HT (8.13±0.06), is in good accordance with the affinity of NAN 190 for the 5-HT1A receptor, reported in the literature (Ahlers et al., 1992: pigeon brain, pKb=8.12; Sharif et al., 2004: human cloned 5-HT1A receptors, pKb=8.5). The pKb calculated from the experiments with concentration–response curves of 5-HT was more than a half unit lower (7.54–7.58). We have no explanation for this difference.
The second 5-HT1A receptor antagonist WAY 100635 (3, 30 and 300 nM) also concentration-dependently antagonised the contractile responses to 5-HT but from 30 nM on, it behaved as a noncompetitive antagonist, decreasing the maximal effect of 5-HT. A pKb estimate was calculated from the experiments where five concentrations of WAY 100635 were tested versus 0.1 μM 5-HT. It should be realised that the pKb calculated in this way can be to some extent an overestimation of the antagonising effect of WAY 100635 as the decrease in the 5-HT-induced response by WAY 100635 is not solely determined by competitive antagonism. Still the pKb estimate obtained (8.69±0.07) was similar to that calculated for the lowest concentration of WAY 100635 versus the concentration–response curve of 5-HT (8.83±0.44); these values correspond to pKb values reported before for WAY 100635 at 5-HT1A receptors (Fletcher et al., 1994: rat hippocampal 5-HT1A receptors, pIC50=8.87±0.14; Khawaja et al., 1997: CHO cell line transfected with human recombinant 5-HT1A receptors, pIC50=8.39±0.12; Hall et al., 1997: human brain, pKb=8.60). All these cited in vitro studies were performed in brain tissue, the principal location of 5-HT1A receptors. In these tissues, WAY 100635 behaves as a pure competitive antagonist. However, in one study on a gastrointestinal myenterically localised 5-HT1A receptor, WAY 100635 behaved as a competitive antagonist of 5-CT when tested in electrically stimulated guinea-pig ileum up to a concentration of 0.3 nM, but showed insurmountable antagonism at higher concentrations (Forster et al., 1995). The results with NAN 190 and WAY 100635 thus seem to point to an interaction of 5-HT with 5-HT1A receptors in equine jejunal longitudinal smooth muscle. This seems corroborated by the results with the 5-HT1,7 receptor agonist 5-CT.
As for the 5-HT-induced contractile response, it was observed that TTX and atropine did not influence the effect of 5-CT. Owing to the lack of effect of the 5-HT7 receptor antagonist SB 269970, the 5-CT-induced motor effects point to activation of 5-HT1 receptors, located directly on the smooth muscle cells. The influence of WAY 100635 on the concentration–response curve of 5-CT was similar to its effect on 5-HT and the pKb calculated for the lowest concentration of WAY 100635, which influenced the concentration–response curve of 5-CT in a competitive way, was similar to that obtained for 5-HT (8.63 versus 8.83), supporting the interaction of 5-CT and 5-HT with the same receptor.
The presence of a gastrointestinal muscular 5-HT1A receptor would be exceptional. The 5-HT1A receptor is found predominantly in the central nervous system, the hippocampus and neocortex (Pazos & Palacios, 1985; Moller et al., 2004). 5-HT1A receptors are only occasionally described in the gastrointestinal tract and when a gastrointestinal localisation was identified, they reside in neuronal tissue where they mediate inhibitory functions. In the myenteric plexus of the isolated guinea-pig ileum and stomach, the neuronally localised 5-HT1A receptors mediate inhibition of electrically evoked twitch contractions (Bill et al., 1990; Buchheit & Buhl, 1994; Lepard & Galligan, 2004). In situ hybridisation reveals that many submucosal and myenteric neurons of the rat and guinea-pig small intestine express mRNA encoding the 5-HT1A receptor (Kirchgessner et al., 1993, 1996). The response of enteric neurons to 5-HT that has been attributed to 5-HT1A receptors is a hyperpolarisation, accompanied by an increase in input resistance caused by an increase in K+ conductance (Galligan et al., 1988). Inhibitory enteric 5-HT1A receptors have also been located on nerve terminals releasing the mediators of fast and slow excitatory postsynaptic potentials. Inhibition of synaptic transmission in the myenteric plexus is likely to account for 5-HT-induced inhibition of the peristaltic reflex in some studies (Galligan, 1996). Indeed, 5-HT1A receptor activation has been found to induce inhibition of acetylcholine release from the guinea-pig myenteric plexus (Dietrich & Kilbinger, 1996). In contrast, a gastrointestinal muscular 5-HT1A receptor is expected to induce an excitatory contractile response, when coupled to inhibition of adenylate cyclase. This is indeed the primary coupling mechanism of this receptor, although also other coupling mechanisms are described (Raymond et al., 1999).
Differences between the receptor mediating the contractile effect of 5-HT in equine jejunum and the 5-HT1A receptor
Although the results with NAN 190 and WAY 100635 versus 5-HT and 5-CT suggest the presence of a 5-HT1A receptor in equine jejunum, several observations do not fit with this conclusion.
5-CT is expected to be equipotent with 5-HT or even more potent than 5-HT at 5-HT1A receptors (Newman-Tancredi et al., 1998; Cowen et al., 2005). However, in equine jejunum longitudinal muscle, 5-CT was at least 10-fold less potent than 5-HT.
Methysergide has been shown to possess agonist activity (Pauwels et al., 1993; Hoyer et al., 1994) and to have a low affinity (Kilpatrick et al., 1989) at 5-HT1A receptors. However, in equine jejunum, methysergide had no contractile effect per se and seemed to have a high affinity at the receptor involved, having a pronounced antagonising effect at 1 nM. It can be mentioned that methysergide was shown to antagonise the inhibitory effect of 5-HT via 5-HT1A receptors on electrically induced GABA release from GABAergic neurones in the guinea-pig ileum, but in a concentration of 300 nM (Shirakawa et al., 1989).
Three specific 5-HT1A receptor agonists, that is, 8-OH-DPAT, buspiron and flesinoxan, did not elicit any contractile effect in the equine jejunum. They also did not antagonise the effect of 5-HT. In a system with low efficacy reserve, a partial 5-HT1A receptor agonist such as buspiron (Pauwels et al., 1993; Sharif et al., 2004) might stay without effect per se, but it should antagonise the effect of the full agonist 5-HT, which was not the case.
It is thus clear that the receptor involved in the contractile effect of 5-HT and 5-CT in equine jejunal longitudinal muscle does not correspond with a classic 5-HT1A receptor. This might be related to the presence of another 5-HT receptor subtype, not yet described. Alternatively, a possible explanation could be found in interspecies differences in the specific structure of the 5-HT1A receptor. As a member of the 5-HT1 family of serotonin receptors, the 5-HT1A receptor is a seven-transmembrane spanning receptor, composed of 422 amino acids. The rat and human 5-HT1A receptor nucleic acid sequences are 88% homologous with each other and accordingly there appears to be a similar pharmacological profile observed between these species (Raymond et al., 1999). The 5-HT1A receptor has one antagonist-binding site and five different agonist-binding sites (Raymond et al., 1999). Restricted mutations can lead to very important changes in the effect of a given substance. When Guan et al. (1992) mutated Asn386 in the seventh transmembrane domain of the human 5-HT1A receptor, this caused a 100-fold decline in the affinity of the antagonist pindolol binding to the 5-HT1A receptor. Ho et al. (1992) rendered the 5-HT1A receptor refractory to 5-HT stimulation in several ways by introducing various point mutations. The substitution of a conserved asparagine at position 396 (localised in the seventh transmembrane region) with either alanine, phenylalanine or valine results in a 5-HT1A receptor that is refractory to 8-OH-DPAT activation (Chanda et al., 1993).
It can be concluded that the muscular contractile 5-HT receptor in equine jejunal longitudinal muscle cannot be characterised between the actually known classes of 5-HT receptors with the experimental data provided, but is sensitive to the 5-HT1A receptor antagonists NAN 190 and WAY 100635.
Desensitization of the equine muscular 5-HT receptor
In the former studies concerning in vitro characterization of 5-HT-induced responses in the equine gut, it is not mentioned whether it was tested that the applied cumulative administration protocol of 5-HT yielded the same contractile responses as isolated administration (Nieto et al., 2000; Weiss et al., 2002). In our study, apparently a fast desensitisation of the muscular 5-HT receptors takes place. It can be mentioned that desensitisation is a typical feature of the 5-HT1A receptor (Raymond et al., 1999; Serres et al., 2000; Hensler & Durgam, 2001). Acute treatment with 5-HT1A agonists leads to rapid desensitisation of central 5-HT1A autoreceptors (Beer et al., 1990; Seth et al., 1997; Riad et al., 2001). Rapid desensitisation of 5-HT1A receptors by agonists has also been described in various transfected cell lines (Nebigil et al., 1995; Rotondo et al., 1997; Della Rocca et al., 1999). Whether we are dealing with an ‘equine' 5-HT1A receptor or another not yet characterised 5-HT receptor, our observation of a rapidly desensitising muscular 5-HT receptor in the equine jejunum opens interesting considerations concerning the possible role of this receptor in the complex pathophysiology of ileus in colic horses, where several factors can serve as a possible source of 5-HT overload. Bailey et al. (2003) already identified the presence of bioactive amines formed by bacterial decarboxylation of amino acids in the caecum and colon of healthy and colic horses. It is known that the permeability of intestinal mucosa in horses is increased during intestinal ischaemia, which promotes translocation of endotoxins and possibly dietary amines, among which 5-HT, from the chyme into the systemic circulation (Snyder, 1989; Morris, 1991; Bailey et al., 2000, 2004; Vatistas et al., 2003). Within the scope of research into the ethiopathogenesis of laminitis in horses, it was shown that during i.v. administration of Escherichia coli lipopolysacharids for experimental induction of endotoxemia, a clear increase in plasma 5-HT and thromboxane beta 2 levels is seen. Both substances are released during activation of blood platelets (Elliott et al., 2003; Vatistas et al., 2003; Menzies-Gow et al., 2004). Therefore, important amounts of 5-HT can be released into the blood stream in colic horses with ischaemic or necrotic intestinal segments. These increased 5-HT levels in ileus horses might lead to desensitisation of the muscular 5-HT receptor, meaning that 5-HT can no longer stimulate the smooth muscle cells via these receptors. In how far the muscular contractile 5-HT receptor might contribute to hypomotility in ileus has to be further investigated.
Conclusion
This study shows the presence of muscular 5-HT receptors, inducing contraction in equine jejunal longitudinal muscle. The receptor does not belong to the 5-HT1B,1D, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT4 and 5-HT7 receptor class. Although blocked by the 5-HT1A receptor antagonists NAN 190 and WAY 100635, the receptor cannot be classified as a classic 5-HT1A receptor since the 5-HT1A receptor agonists 8-OH-DPAT, flesinoxan and buspiron were not active. Whether a horse-specific 5-HT1A receptor or a not yet described 5-HT receptor subtype is involved needs further investigation. More research is also needed to clarify whether these muscular contractile 5-HT receptors play a role in the pathophysiology of ileus and/or can serve as pharmacological target for possible prokinetic medication in horses.
Acknowledgments
This study was financially supported by an Interuniversity Attraction Poles Programme-Belgian Science Policy (P5/20). We thank Dr P. Janssen, Dr P. Claes, J. De Maeyer, W. De Ridder and L. Hoskens for technical support and help in the first series of experiments.
Abbreviations
- 5-CT
5-carboxamidotryptamine
- 5-HT
5-hydroxytryptamine
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino) tetralin
- GABA
gamma-aminobutyric acid
- GR 127935
2-methyl-4-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid [4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]amide HCl
- GR 113808
[1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl-1-methyl-1H-indole-3-carboxylate
- i.v.
intravenous
- L-NNA
NG-nitro-L-arginine
- NAN-190
1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]piperazine HCl
- RS 102221
8-[5-(2,4-dimethoxy-5-(4-trifluoromethylphenylsulphon-amido)phenyl-5-oxopentyl)]-1,3,8-triazaspiro[4.5]decane-2,4-dione hydrochloride
- SB204741
N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea
- SB 269970
(R)-3-(2-(2-(4-methylpiperidin-1-yl) ethyl)pyrrolidine-1-sulphonyl) phenol
- TTX
tetrodotoxin
- WAY 100635
N-2-4-(2-methoxyphenyl)-1-piperazinylethyl-N-(2-pyridinyl)cyclohexane carboxamide trihydro-chloride
References
- AHLERS S.T., WEISSMAN B.A., BARRETT J.E. Antagonism studies with BMY-7378 and NAN 190 – effects on 8-hydroxy-2-(di-normal-propylamino)tetralin-induced increases in punished responding of pigeons. J. Pharmacol. Exp. Ther. 1992;260:474–481. [PubMed] [Google Scholar]
- BAILEY S.R., CUNNINGHAM F.M., ELLIOTT J. Endotoxin and dietary amines may increase plasma 5-hydroxytryptamine in the horse. Eq. Vet. J. 2000;32:497–504. doi: 10.2746/042516400777584730. [DOI] [PubMed] [Google Scholar]
- BAILEY S.R., MARR C.M., ELLIOTT J. Identification and quantification of amines in the equine caecum. Res. Vet. Sci. 2003;74:113–118. doi: 10.1016/s0034-5288(02)00175-3. [DOI] [PubMed] [Google Scholar]
- BAILEY S.R., MENZIES-GOW N.J., MARR C.M., ELLIOTT J. The effects of vasoactive amines found in the equine hindgut on digital blood flow in the normal horse. Eq. Vet. J. 2004;36:267–272. doi: 10.2746/0425164044877297. [DOI] [PubMed] [Google Scholar]
- BEER M., KENNETH G.A., CURZON G. A single dose of 8-OH-DPAT reduces raphe binding of [3H]8-OH-DPAT and increases the effect of raphe stimulation on 5-HT metabolism. Eur. J. Pharmacol. 1990;178:179–187. doi: 10.1016/0014-2999(90)90473-j. [DOI] [PubMed] [Google Scholar]
- BILL S.J., DOVER G.M., RHODES K.F. Demonstration of 5-HT1A agonist actions of 5-carboxamidotryptamine in the isolated transmurally stimulated ileum of the guinea-pig. Br. J. Pharmacol. 1990;100:483. [Google Scholar]
- BONHAUS D.W., WEINHARDT K.K., TAYLOR M., DESOUZA A., McNEELEY P.M. RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- BRIANCEAU P., CHEVALIER H., KARAS A., COURT M.H., BASSAGE L., KIRKER-HEAD C. Intravenous Lidocaine and small-intestinal size, abdominal fluid, and outcome after colic surgery in horses. J. Vet. Intern. Med. 2002;16:736–741. doi: 10.1892/0891-6640(2002)016<0736:ilassa>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- BUCHHEIT K.H., BUHL T. Stimulant effects of 5-hydroxytryptamine on guinea-pig stomach preparations in-vitro. Eur. J. Pharmacol. 1994;262:91–97. doi: 10.1016/0014-2999(94)90031-0. [DOI] [PubMed] [Google Scholar]
- CAO B.J., RODGERS R.J. Influence of 5-HT1A receptor antagonism on plus-maze behaviour in mice. II. WAY 100635, SDZ 216-525 and NAN-190. Pharmacol. Biochem. Behav. 1997;58:593–603. doi: 10.1016/s0091-3057(97)00279-7. [DOI] [PubMed] [Google Scholar]
- CHANDA P.K., MINCHIN M.C., DAVIS A.R., GREENBERG L., REILLY Y. Identification of residues important for ligand binding to the human 5-hydroxytryptamine1A serotonin receptor. J. Exp. Pharmacol. Ther. 1993;43:516–520. [PubMed] [Google Scholar]
- COWEN D.S., JOHNSON-FARLEY N.N., TRAVKINA T. 5-HT1A receptors couple to activation of Akt, but not extracellular-regulated kinase (ERK), in cultured hippocampal neurons. J. Neurochem. 2005;93:910–917. doi: 10.1111/j.1471-4159.2005.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELLA ROCCA G.J., MUKHIN Y.V., GARNOVSKAYA M.N., DAAKA Y., CLARK G.J., LUTTRELL L.M., LEFKOWITZ R.J., RAYMOND J.R. Serotonin 5-HT1A receptor-mediated Erk activation requires calcium/calmodulin-dependent receptor endocytosis. J. Biol. Chem. 1999;274:4749–4753. doi: 10.1074/jbc.274.8.4749. [DOI] [PubMed] [Google Scholar]
- DIETRICH C., KILBINGER H. 5-HT1A receptor mediated inhibition of of acetylcholine release from guinea-pig myenteric plexus: potential mechanisms. Neuropharmacology. 1996;35:483–488. doi: 10.1016/0028-3908(95)00197-2. [DOI] [PubMed] [Google Scholar]
- ELLIOTT J., BERHANE Y., BAILEY S.R. Effects of monoamines formed in the cecum of horses on equine digital blood vessels and platelets. Am. J. Vet. Res. 2003;64:1124–1131. doi: 10.2460/ajvr.2003.64.1124. [DOI] [PubMed] [Google Scholar]
- FLETCHER A., BILL D.J., CLIFFE I.A., FORSTER E.A., JONES D., REILLY Y. A pharmacological profile of WAY-100635, a potent and selective 5-HT1A receptor antagonist. Br. J. Pharmacol. 1994;122:91. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- FORBES I.T., JONES G.E., MURPHY O.E. N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea: a novel, high affinity 5-HT2B receptor antagonist. J. Med. Chem. 1995;38:855–857. doi: 10.1021/jm00006a001. [DOI] [PubMed] [Google Scholar]
- FORSTER E.A., CLIFFE I.A., BILL D.J., DOVER G.M., JONES D., REILLY Y., FLETCHER A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist WAY 100635. Eur. J. Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- GALLIGAN J.J. Electrophysiological studies of 5-hydroxytryptamine receptors on enteric neurones. Behav. Brain Res. 1996;73:199–201. doi: 10.1016/0166-4328(96)00096-4. [DOI] [PubMed] [Google Scholar]
- GALLIGAN J.J., SUPRENANT A., TONINI M., NORTH A. Differential localization of 5-HT1 receptors on myenteric and submucosal neurons. Am. J. Physiol. 1988;255:603–611. doi: 10.1152/ajpgi.1988.255.5.G603. [DOI] [PubMed] [Google Scholar]
- GERRING E.E.L., HUNT J.M. Pathophysiology of equine postoperative ileus: effects of adrenergic blockade, parasympathetic stimulation and metoclopramide in an experimental model. Eq. Vet. J. 1986;18:249–255. doi: 10.1111/j.2042-3306.1986.tb03618.x. [DOI] [PubMed] [Google Scholar]
- GOMMEREN W., RENDERS J., VAN GOMPEL P., LESAGE A., LEYSEN J.E., JURZAK MT. Extensive pharmacological study of the G-protein coupled fraction of human 5-HT receptors using agonist radioligand binding. Naunyn Schmiedebergh's Arch. Pharmacol. 1998;358:8–42. [Google Scholar]
- GUAN X.M., PERTOUKA S.J., KOBILKA B.K. Identification of a single amino acid residue responsible for the binding of a class of β-adrenergic receptor antagonists to 5-hydroxytryptamine1A receptors. Mol. Pharmacol. 1992;41:695–698. [PubMed] [Google Scholar]
- HADRAVA V., BLIER P., DENNIS T., ORTEMANN C., DE MONTIGNY C. Characterization of 5-hydroxytryptamine(1A) properties of flesinoxan-in-vivo electrophysiology and hypothermia study. Neuropharmacology. 1995;34:1311–1326. doi: 10.1016/0028-3908(95)00098-q. [DOI] [PubMed] [Google Scholar]
- HAGAN J.J., PRICE G.W., JEFFREY P., DEEKS N.J., STEAN T., PIPER D., SMITH M.I., UPTON N., MEDHURST A.D., MIDDLEMISS D.N., RILEY G.J., LOVELL P.J., BROMIDGE S.M., THOMAS D.R. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL H., LUNDKVIST C., HALLDIN C., FARDE L., PIKE V.W., McCARRON J.A., FlETCHER A., CLIFFE I.A., BARF T., WIKSTRÖM H., SEDVALL G. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [H-3]WAY-100635 and [C-11]WAY-100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- HENSLER J.G., DURGAM H. Regulation of 5-HT1A receptor-stimulated [S-35]-GTP gamma S binding as measured by quantitative autoradiography following chronic agonist administration. Br. J. Pharmacol. 2001;132:605–611. doi: 10.1038/sj.bjp.0703855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO B.Y., KARSCHIN A., BRANCHEK T., DAVIDSON N., LESTER H.A. The role of conserved aspartate and serine residues in ligand binding and in function of the 5-HT1A receptor: a site-directed mutagenesis study. FEBS Lett. 1992;312:259–262. doi: 10.1016/0014-5793(92)80948-g. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- JOHNSON M.P., AUDIA J.E., NISSEN J.S., NELSON D.L. N(1)-substituted ergolines and tryptamines show species differences for the agonist-labeled 5-HT2 receptor. Eur. J. Pharmacol. 1993;239:111–118. doi: 10.1016/0014-2999(93)90983-o. [DOI] [PubMed] [Google Scholar]
- KHAWAJA X., ENNIS C., MINCHIN M.C.W. Pharmacological characterization of recombinant human 5-HT1A receptors using a novel antagonist radioligand, [3H] WAY 100635. Life Sci. 1997;60:653–665. doi: 10.1016/s0024-3205(96)00701-1. [DOI] [PubMed] [Google Scholar]
- KHAWAJA X., EVANS N., REILLY Y., ENNIS C., MINCHIN M.C.W. Characterization of the binding of (3H)WAY-100635, a novel 5-hydroxytryptamine1A receptor antagonist, to rat brain. J. Neorochem. 1995;64:2716–2726. doi: 10.1046/j.1471-4159.1995.64062716.x. [DOI] [PubMed] [Google Scholar]
- KILPATRICK A.T., BROWN C.M., MACKINNON A.C., SPEDDING M. The α2-adrenoceptor antagonist SK&F 104078 has high affinity for 5-HT1A and 5-HT2 receptors. Eur. J. Pharmacol. 1989;166:315–318. doi: 10.1016/0014-2999(89)90075-7. [DOI] [PubMed] [Google Scholar]
- KIRCHGESSNER A.L., LIU M.T., HOWARD M.J., GERSHON M.D. Detection of the 5-HT1A and 5-HT1A receptor mRNA in the rat bowel and pancreas: comparison with 5-HT1p receptors. J. Comp. Neurol. 1993;327:233–250. doi: 10.1002/cne.903270206. [DOI] [PubMed] [Google Scholar]
- KIRCHGESSNER A.L., LIU M.T., RAYMOND J.R. Identification of cells that express 5-hydroxytryptamine1A receptors in the nervous systems of the bowel and pancreas. J. Comp. Neurol. 1996;364:439–455. doi: 10.1002/(SICI)1096-9861(19960115)364:3<439::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., BIRDSALL N.J.M. Estimation of antagonist Kb from inhibition curves in functional experiments: alternatives to the Cheng–Prusoff equation. TiPS. 1993;14:237–239. doi: 10.1016/0165-6147(93)90018-f. [DOI] [PubMed] [Google Scholar]
- LEPARD K.J., GALLIGAN J.J. Presynaptic modulation of cholinergic and non-cholinergic fast synaptic transmission in the myenteric plexus of guinea pig ileum. Neurogastroenterol. Motil. 2004;16:355–364. doi: 10.1111/j.1365-2982.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- LIPPOLD B.S., HILDEBRAND J., STRAUB R. Tegaserod (HTF 919) stimulates gut motility in normal horses. Eq. Vet. J. 2004;36:622–627. doi: 10.2746/0425164044864543. [DOI] [PubMed] [Google Scholar]
- MENZIES-GOW N.J., BAILEY S.R., KATZ L.C., MARR C.M., ELLIOTT J. Endotoxin-induced digital vasoconstriction in horses: associated changes in plasma concentrations of vasoconstrictor mediators. Eq. Vet. J. 2004;36:273–278. doi: 10.2746/0425164044877260. [DOI] [PubMed] [Google Scholar]
- MOLLER M., CUMMING P., ANDERSEN G., GJEDDE A. Parametric mapping of serotonin5HT1Areceptors in healthy humanbrain. Neuroimage. 2004;22:T157. [Google Scholar]
- MORRIS D.D. Endotoxemia in horses. A review of cellular and humoral mediators involved in its pathogenesis. J. Vet. Intern. Med. 1991;5:167–181. doi: 10.1111/j.1939-1676.1991.tb00944.x. [DOI] [PubMed] [Google Scholar]
- NEBIGIL C.G., GARNOVSKAYA M.N., CASANAS S.J., MULHERON J.G., PARKER E.M., GETTYS T.W., RAYMOND J.R. Agonist-induced desensitization and phosphorylation of human 5-HT1A receptors expressed in SF9 insect cells. Biochemistry. 1995;34:11954–11962. doi: 10.1021/bi00037a037. [DOI] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., VERRIELE L., MILLAN M.J. Differential modulation by GTP gamma S of agonist and inverse agonist binding to h5-HT1A receptors revealed by [H-3]-WAY100635. Br. J. Pharmacol. 2001;132:518–524. doi: 10.1038/sj.bjp.0703832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., GAVAUDAN S., CONTE C., CHAPUT C., TOUZARD M., VERRIELE L., AUDINOT V., MILLAN M.J. Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [35S] GTPγS binding study. Eur. J. Pharmacol. 1998;355:245–256. doi: 10.1016/s0014-2999(98)00483-x. [DOI] [PubMed] [Google Scholar]
- NIETO J.E., SNYDER J.R., KOLLIAS-BAKER C., STANLEY S. In vitro effects of 5-hydroxytryptamine and cisapride on the circular smooth muscle of the jejunum of horses. Am. J. Vet. Res. 2000;61:1561–1565. doi: 10.2460/ajvr.2000.61.1561. [DOI] [PubMed] [Google Scholar]
- PAUWELS P.J., VAN GOMPEL P., LEYSEN J.E. Activity of serotonin (5-HT) receptor agonists, partial agonists and antagonists at cloned human 5-HT1A receptors that are negatively coupled to adenylate cyclase in permanently transfected HeLa cells. Biochem. Pharmacol. 1993;45:375–383. doi: 10.1016/0006-2952(93)90073-6. [DOI] [PubMed] [Google Scholar]
- PAZOS A., PALACIOS J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- RAYMOND J.R., MUKHIN Y.V., THOMAS W.G., GARNOVSKAYA M.N. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br. J. Pharmacol. 1999;127:1751–1764. doi: 10.1038/sj.bjp.0702723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIAD M., WATKINS K.C., DOUCET E., HAMON M., DESCARRIES L. Agonist-induced internalization of serotonin-1A receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors) J. Neurosci. 2001;21:8378–8386. doi: 10.1523/JNEUROSCI.21-21-08378.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTONDO A., NIELSEN D.A., NAKHAI B., HULIHAN-GIBLIN B., BOLOS A., GOLDMAN D. Agonist-promoted down-regulation and functional desensitization in two naturally occurring variants of the human serotonin(1A) receptor. Neuropsychopharmacology. 1997;17:18–26. doi: 10.1016/S0893-133X(97)00021-3. [DOI] [PubMed] [Google Scholar]
- ROUSSEL A.J., COHEN N.D., HOOPER R.N., RAKESTRAW P.C. Risk factors associated with development of postoperative ileus in horses. J. Am. Vet. Med. Assoc. 2001;219:72–78. doi: 10.2460/javma.2001.219.72. [DOI] [PubMed] [Google Scholar]
- SANGER D.J., SCHOEMAKER H. Discriminative stimulus properties of 8-OH-DPAT: relationship to affinity for 5HT1A receptors. Psychopharmacology. 1992;108:85–92. doi: 10.1007/BF02245290. [DOI] [PubMed] [Google Scholar]
- SANGER G., NELSON D.R. Selective and functional 5-hydroxytryptamine 3 receptor antagonism by BRL 43694 (granisetron) Eur. J. Pharmacol. 1989;159:113–124. doi: 10.1016/0014-2999(89)90695-x. [DOI] [PubMed] [Google Scholar]
- SERRES F., MUMA N.A., RAAP D.K., GARCIA F., BATTAGLIA G., VAN DE KAR L.D. Coadministration of 5-hydroxytryptamine (1A) antagonist WAY-100635 prevents fluoxetine-induced desensitization of postsynaptic 5-hydroxytryptamine (1A) receptors in hypothalamus. J. Pharmacol. Exp. Ther. 2000;294:296–301. [PubMed] [Google Scholar]
- SETH P., GAJENDIRAN M., GANGULY D.K. Desensitization of spinal 5-HT1A receptors to 8-OH-DPAT: an in vivo spinal reflex study. Neuro Rep. 1997;8:2489–2493. doi: 10.1097/00001756-199707280-00015. [DOI] [PubMed] [Google Scholar]
- SHARIF N.A., DRACE C.D., WILLIAMS G.W., CRIDER J.Y. Cloned human 5-HT1A receptor pharmacology determined using agonist binding and measurement of cAMP accumulation. J. Pharm. Pharmacol. 2004;56:1267–1274. doi: 10.1211/0022357044346. [DOI] [PubMed] [Google Scholar]
- SHIRAKAWA J., TAKEDA K., TANIYAMA K., TANAKA C. Dual effects of 5-hydroxytryptamine on the release of G-aminobutyric acid from myenteric neurones of the guinea-pig ileum. Br. J. Pharmacol. 1989;98:339–341. doi: 10.1111/j.1476-5381.1989.tb12601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER J.R. The pathophysiology of intestinal damage: effects of luminal distention and ischemia. Vet. Clin. North. Am. Equine Pract. 1989;5:247–270. doi: 10.1016/s0749-0739(17)30587-4. [DOI] [PubMed] [Google Scholar]
- TALLEY N.J. Serotoninergic neuroenteric modulators. Lancet. 2001;15:2061–2068. doi: 10.1016/S0140-6736(01)07103-3. [DOI] [PubMed] [Google Scholar]
- TERRON J.A. GR 127935 is a potent antagonist of the 5-HT1-like receptor mediating contraction in the canine coronary artery. Eur. J. Pharmacol. 1996;300:109–112. doi: 10.1016/0014-2999(96)00041-6. [DOI] [PubMed] [Google Scholar]
- VAN HOOGMOED L.M., NIETO J.E., SNYDER J.R. Survey of prokinetic use in horses with gastrointestinal injury. Vet. Surg. 2004;33:279–285. doi: 10.1111/j.1532-950X.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- VATISTAS N.J., NIETO J.E., VAN HOOGMOED L., GARDNER I., SNYDER J.R. Use of an isolated intestinal circuit to evaluate the effect of ischemia and reperfusion on mucosal permeability of the equine jejunum. Vet. Surg. 2003;32:52–61. doi: 10.1053/jvet.2003.49999. [DOI] [PubMed] [Google Scholar]
- WEISS R., ABEL D., SSCOLTYSIK G., STRAUB R., MEVISSEN M. 5-Hydroxytryptamine mediated contractions in isolated preparations of equine ileum and pelvic flexure: pharmacological characterization of a new 5-HT4 agonist. J. Vet. Pharmacol. Ther. 2002;25:49–58. doi: 10.1046/j.1365-2885.2002.00380.x. [DOI] [PubMed] [Google Scholar]