Abstract
Adenosine 5′-triphosphate (ATP) activated two sequential responses in freshly isolated mouse aortic smooth muscle cells. In the first phase, ATP activated Ca2+-dependent K+ or Cl− currents and the second phase was the activation of a delayed outward current with a reversal potential of −75.9±1.4 mV.
A high concentration of extracellular K+ (130 mM) shifted the reversal potential of the delayed ATP-elicited current to −3.5±1.3 mV. The known K+-channel blockers, iberiotoxin, charybdotoxin, glibenclamide, apamin, 4-aminopyridine, Ba2+ and tetraethylammonium chloride all failed to inhibit the delayed ATP-elicited K+ current. Removal of ATP did not decrease the amplitude of the ATP-elicited current back to the control values.
The simultaneous recording of cytosolic free Ca2+ and membrane currents revealed that the first phase of the ATP-elicited response is associated with an increase in intracellular Ca2+, while the second delayed phase develops after the return of cytosolic free Ca2+ to control levels.
ATP did not activate Ca2+-dependent K+ currents, but did elicit Ca2+-independent K+ currents, in cells dialyzed with ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA). The delay of activation of Ca2+-independent currents decreased from 10.5+3.4 to 1.27±0.33 min in the cells dialyzed with 2 mM EGTA.
Adenosine alone failed to elicit a Ca2+-independent K+ current but simultaneous application of ATP and adenosine activated the delayed K+ current. Intracellular dialysis of cells with guanosine 5′-O-(2-thiodiphosphate) transformed the Ca2+-independent ATP-elicited response from a sustained to a transient one. A phospholipase C inhibitor, U73122 (1 μM), was shown to abolish the delayed ATP-elicited response.
These results indicate that the second phase of the ATP-elicited response was a delayed Ca2+-independent K+ current activated by exogenous ATP. This phase might represent a new vasoregulatory pathway in vascular smooth muscle cells.
Keywords: Aortic smooth muscle cells; intracellular Ca2+; ATP, GDPβs; Ca2+-independent K+ current; purine receptors; PLC blocker
Introduction
It is well documented that adenosine 5′-triphosphate (ATP)-elicited responses occur in almost every organ and tissue system studied to date (Bakhramov et al., 1996; Yamamoto et al., 2000; Ralevic, 2002; Kawamura et al., 2003; Pankratov et al., 2003; Oike et al., 2004). Both the capacitance and the resistance vessels were found to be ATP-sensitive in the vascular system. However, ATP-elicited responses are not uniform. Multiphasic ATP-elicited responses that include vasodilation and vasoconstriction have been reported in vessels from the arterial and venous branches (Ralevic et al., 1998; Wangensteen et al., 2000; Ralevic, 2002; Dietrich et al., 2002).
One important factor in multiphasic ATP-elicited responses is the catabolism of extracellular ATP to adenosine by membrane ectonucleotidases. In addition, nonhomogeneous expression of ATP receptor subtypes in endothelial and smooth muscle cells causes a diverse range of ATP-elicited responses.
Extracellular ATP itself works through two different transduction mechanisms, namely intrinsic ion channels such as P2X receptors, and G protein-coupled P2Y receptors. To date, seven P2X receptors, P2X1–7, and seven P2Y receptors have been cloned, characterized pharmacologically and accepted as valid members of the P2 receptor family in mammals (Ralevic & Burnstock, 1998; North, 2002). Most tissues express more than one subtype of P2X and/ or P2Y receptor.
It is generally assumed that ATP-elicited constriction is a result of stimulation of purinergic receptors expressed on the vascular smooth muscle cells (Loirand & Pacaud, 1995; Steinmetz et al., 2003; Takeuchi et al., 2003). In contrast, ATP-dependent relaxation of intact blood vessels might result from the activation of purinergic receptors expressed on endothelial cells (Boeynaems & Pearson, 1990; Knight et al., 2003; Wihlborg et al., 2003; Liu et al., 2004).
However, P2Y-like receptors expressed on the smooth muscle of a number of blood vessels might also mediate vasodilation (Kennedy & Burnstock, 1985; Mathieson & Burnstock, 1985; Burnstock & Warland, 1987; Brizzolara & Burnstock, 1991; Corr & Burnstock, 1994; Qasabian et al., 1997; Simonsen et al., 1997; Liu et al., 2004). Recently it was suggested that prolonged activation of K+ currents maintain prolonged endothelium-independent vasodilation in the isolated rat mesenteric bed (Ralevic, 2001; 2002). The mechanism underlying endothelium-independent relaxation of smooth muscle by P2Y receptors is not well understood. One potential target of the purinergic receptors could be the variety of K+-channels expressed in the cellular membrane of vascular smooth muscle cells. Multiple signal transduction pathways converge on K+-channels and modulate vascular tone via the regulation of their open probability (Bakhramov et al., 1996; Strobaek et al., 1996; Kamouchi et al., 1998). In this context, our aim was to examine the effects of exogenous ATP on potassium currents that may well be implicated in endothelium-independent vasodilation seen in freshly isolated smooth muscle cells from mouse aorta.
Methods
Mouse aorta preparation and isolation of single smooth muscle cells
All animal handling was in accordance with institutional guidelines established by the ‘Swiss Academy of Medical Sciences' and the ‘Helvetic Society of Natural Sciences': animal experimentation authorization 31.1.1008/2129/0.
Male C57BL/6 mice that were 3–4 weeks old were anesthetized with 2-bromo-2-chloro-1,1,1-trifluoroethane. Smooth muscle cells were isolated as described previously (Bychkov et al., 1997). The thoracic aorta was removed, cleaned of fat and connective tissue and placed in Ca2+-free Hanks solution containing (in mM): NaCl 137, KCl 5.4, K2HPO4 0.44, NaH2PO4 0.42, MgCl2·6H2O 2, NaHCO3 4.17, CaCl2·2H2O 0.05, glucose 11 and HEPES 10; pH was adjusted to 7.4 with NaOH. The aorta was incubated for 40 min at 37°C in Hanks solution containing 2 mg ml−1 elastase (type IV) and 1 mg ml−1 collagenase (type IA-S). Vascular smooth muscle cells were isolated by careful shaking of the tissue, then placed on coverslips and stored at 4°C.
Patch clamp recording
Membrane currents were recorded at room temperature (20°C) using nystatin perforated patch and whole-cell configuration with a patch amplifier (EPC9; HEKA Instruments (Germany) and Axopclamp 200B). The patch electrodes were pulled from borosilicate capillary glass using a Sutter instrument (Model P-2000, U.S.A.). These had a resistance of 5–7 MΩ. Patch pipettes were filled with (in mM): KCl 130, HEPES 10, and ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) 2 (pH=7.4) for the whole-cell experiments. The same solution without EGTA was used for the perforated patch clamp experiments. Nystatin (Sigma, Deisenhofen, FRG) was dissolved in DMSO and diluted into the pipette solution to give a final concentration range of 50–100 μg ml−1. The aortic smooth muscle cells were bathed in a solution containing (in mM) NaCl 130, KCl 5.6, MgCl2·6H2O 1, CaCl2·2H2O 2, HEPES 8 and glucose 10 (pH 7.5). ATP and other chemicals were added to the bath solution. The linear voltage ramps were applied from a holding potential of −60 mV for 500 ms duration and at voltages ranging from −100 to 100 mV. Voltage step pulses were applied from the holding potential of −60 mV with 10 mV increments between −100 and 100 mV. A holding potential of −30 mV was used for the steady-state recordings. Conductivity plots were obtained from the currents evoked by ramps in individual cells. The mean conductivity plot was obtained and fitted with a first- or second-order Boltzmann function. The dose response data set was fitted with logistic function
where Y is the amplitude of the ATP-elicited response (pA), A1 and A2 are the initial and final values (pA), X is the concentration of the ATP and p is the slope of the curve.
Fluo 3 was used for the simultaneous study of the calcium response and membrane current. The loading solution contained 2% pluronic acid F-127 solution and Fluo 3 (2 μM). All dissociated smooth muscle cells were incubated in this solution for 30 min at room temperature. The fluorescence microscope Nikon Diaphot, equipped with a 100-W xenon light source and a CCD camera (Photonic Science, XTI), was used for simultaneous recording of [Ca2+]i and membrane currents. Images of the cells were taken at regular intervals (4 images s−1) and 40 and 60 × Nikon microscope objectives were used.
Chemicals
ATP, U73122, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethylester) (BAPTA-AM), iberiotoxin, charybdotoxin, tetraethylammonium chloride (TEA), 4-aminopyridine (4AP), glibenclamide, apamin, EGTA, elastase (type IV from porcine pancreas) and collagenase (type IA-S) were obtained from Sigma Chemical (Buchs, Switzerland). Fluo 3 AM was purchased from Molecular Probes (Juro, Switzerland). Pluronic acid F-127 and guanosine 5′-O-(2-thiodiphosphate) (GDPβs) were obtained from Calbiochem (San Diego, CA, U.S.A.).
Statistics
Each set of data was expressed as mean±s.e.m. and all represented experiments were repeated at least five times. We employed the two-sample t-test or the nonparametric Wilcoxon two-sample test to compare data sets. Pairs of data sets represented measurements of the current amplitude, the slope values and the time duration in both control conditions and after ATP addition or application of blockers.
Results
ATP elicits sequential responses in freshly isolated aortic smooth muscle cells
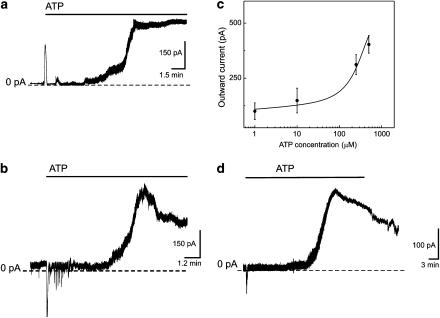
Ion currents were recorded from freshly isolated smooth muscle cells in the perforated patch clamp configuration. ATP elicited two sequential responses. The first phase was generated immediately after ATP application and this was followed by a second phase that was delayed by several minutes. The first phase consisted of fast inactivating transient currents (n=7) or an oscillatory response (n=5) (Figure 1a and b). Ca2+-dependent inward Cl− and outward K+ currents were elicited by ATP during the first phase of the response. These responses appeared briefly after ATP administration to the bath solution and are well documented (Ralevic & Burnstock, 1998). We characterized these responses in freshly isolated mouse aortic smooth muscle cells in a previous publication (Fanchaouy et al., 2005). Longer exposure of smooth muscle cells to extracellular ATP evoked a delayed activation of an outward current (Figure 1a, b and d). The amplitude of the current recorded at a steady-state holding potential of −30 mV increased from 82±9 pA in control conditions to 536±66 pA under ATP administration (n=19). The second response was delayed by 13.8±1.9 min (n=31). This delay is the time taken to reach the second peak. The common features of the delayed ATP-elicited response in the second phase were the slow activation followed by a sustained increase in the current amplitude that lasted for at least 10 min. The delayed sustained effect of ATP was not abolished after the removal of the ATP from the bath solution (n=5) (Figure 1d). The amplitude was unstable and slowly decreased when the ATP-containing solution was replaced by ATP-free solution. However, the amplitude of the delayed ATP-elicited current did not return to the control level during the time available for recordings.
Figure 1.
Sequential activation of ATP-elicited responses in freshly isolated mouse aortic smooth muscle cells. Experiments were performed in a perforated patch clamp configuration and membrane potential was held at −30 mV. Application of 250 μM ATP is indicated by a solid line. (a) ATP transiently increased the amplitude of the outward current followed by a delayed increase of the amplitude of the outward current. (b) ATP elicited a burst of short oscillations followed by a delayed increase of the amplitude of the outward current. (c) Mean dose–response relation shows the effect of ATP on delayed outward K+ current. Current amplitude was measured at −30 mV. The effect of ATP was plotted against mean current amplitude. Data points of the graph were fitted with logistic function. (d) Effect of ATP removal from the extracellular solution. The time of the ATP application is indicated by the line bar.
ATP increased the amplitude of the delayed outward current in a dose-dependent manner (Figure 1c). Four concentrations of ATP were tested. Each concentration was tested on at least five smooth muscle cells. The apparent IC50 found for our data set was 235±36 μM.
Delayed ATP-elicited response in the nonhomogeneous population of freshly isolated smooth muscle cells from the mouse aorta
Smooth muscle cells isolated from the same vessel might differ in electrophysiological properties (Gollasch et al., 1996). Two types of current voltage relations were recorded in perforated patch clamp configuration in control conditions. Was this ATP-elicited response linked to one particular type of smooth muscle cell?
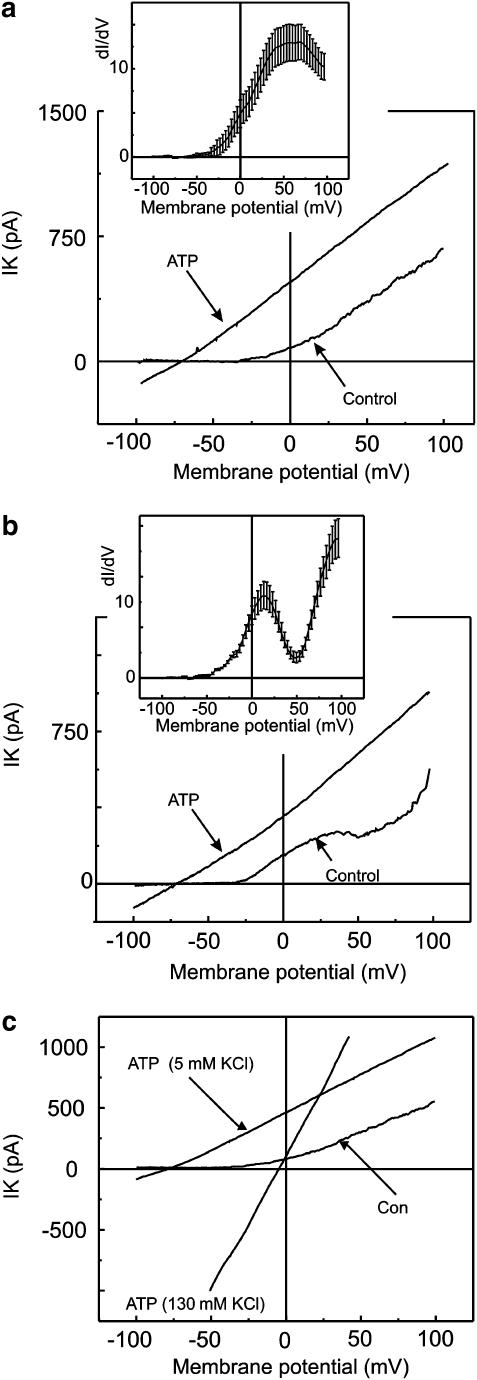
The first type of freshly isolated smooth muscle cells had an exponential-like current voltage relation, named S-type, and this represented 60% of investigated cells (Figure 2a). The fit revealed a peak at 43±4 mV, indicating the presence of a single type of dominating current in the current voltage relation. The amplitude of the control current was measured at 50 mV and it was shown to decrease by 71.5±2.3% after the application of iberiotoxin (100 nM) (n=5). Secondly, smooth muscle cells that had a current voltage relation with a biphasic increase of the current amplitude under control conditions were named N-type and represented 40% of investigated cells (Figure 2b). The fit revealed two peaks at 18.4±1.6 and 79.2±3.5 mV, suggesting the presence of two types of current with different kinetics. The amplitude of the control current in the N-type cells was measured at 50 mV. The amplitude decreased by 32.6±4.7% after the application of iberiotoxin (100 nM) (n=5). The rest of the outward current was insensitive to iberiotoxin in N-type cells but decreased by 74.1.±6.3% (n=6) after 4-AP application (2 mM).
Figure 2.
Effect of ATP on two types of identified mouse aortic smooth muscle cells. Membrane currents were evoked by linear voltage ramps varying from −100 mV to 100 mV from a holding potential of −60 mV. (a, b) Two types of cells under control conditions that differ one from another by their current voltage relations and their conductivity plots. (a) Represents S-type cells. Conductivity plots were obtained from the currents evoked by ramps in 10 individual cells in control conditions. Then the mean conductivity plot was produced and fitted with a single-order Boltzmann function. (b) Represents N-type cells. Conductivity plots were obtained from the currents evoked by ramps in 10 individual cells in control conditions. The mean conductivity plot was obtained from the data set and was fitted with a second-order Boltzmann function. Conductivity plots, the derivative of the current to voltage, are presented in the insets of (a) and (b) and represent the mean±s.e.m. (see text). (c) Represents current voltage relations of the delayed ATP-elicited current at the plato of the sustained activation recorded in asymmetrical K+ concentration and after the reperfusion of the smooth muscle cell with 130 mM KCl.
ATP evoked a delayed activation of the outward noninactivating current regardless of the type of current voltage relation found in control conditions. In the next series of experiments, the low extracellular K+ concentration (5.6 mM) was increased to a high K+ (130 mM) concentration. The reversal potential of the delayed ATP-elicited current shifted from −75.9±1.4 to −3.5±1.3 mV (n=7) with a high extracellular K+ (Figure 2c). The shift in the reversal potential of the ATP-elicited current was close to the theoretical shift in the reversal potential of a K+ conductance predicted by the Nernst equation. These results suggest that the K+ ion is the major charge carrier of the delayed ATP-elicited response.
Pharmacological characterization of ATP-elicited K+ currents: role of intracellular Ca2+
The pharmacological properties of the delayed activating ATP-elicited current were first studied in the perforated patch clamp configuration. Current voltage relations were recorded under control conditions, at the sustained phase of the delayed ATP-elicited response and after application of K+-channel blockers.
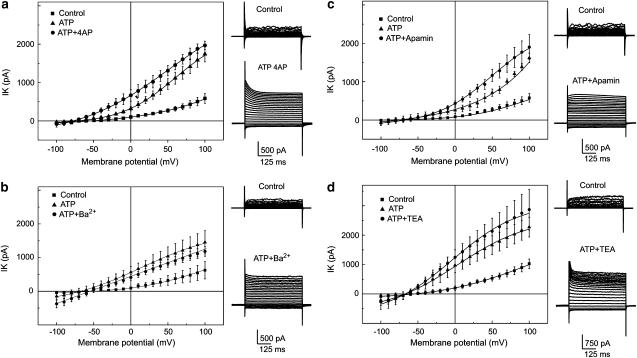
The non-specific K+-channel blockers, Ba2+ (100 μM, n=8), 4-AP (2mM, n=9) and TEA (2 mM, n=6), as well as the inhibitor of small conductance Ca2+-dependent K+-channels, apamin (1 μM, n=10), were tested to determine their effect on the delayed ATP-elicited current. Data sets are represented as the amplitudes of the delayed ATP-elicited current voltage relations after the application of the K+-channel blockers, compared to control values. The data, summarized in the plots (Figure 3), demonstrate that none of the tested K+-channel blockers decreased the amplitude of the delayed ATP-elicited current back to the control level (P-values: 0.0007, 0.0002, 0.0004, 0.0003).
Figure 3.
Effect of 4-AP, Ba2+, TEA and apamin on delayed ATP-elicited outward current. Experiments were performed in a perforated patch clamp configuration. Current voltage relations represent mean±s.e.m. (obtained from six to 10 individual cells) amplitude of the control current (filled squares), delayed ATP-elicited outward current (filled circles) and tested blockers (filled triangles). K+ currents were evoked by voltage step pulses from −100 mV with a step increment of 10 mV. Holding potential was −60 mV. Families of superimposed current traces represent examples of the recording obtained under control conditions and after the application of ATP plus an inhibitor. (a) Mean±s.e.m. current voltage relations obtained at control conditions, under the plateau phase of the ATP-elicited response (250 μM) and after the application of 4AP (2 mM). (b) Mean±s.e.m. current voltage relations obtained at control conditions, under the plateau phase of the ATP-elicited response (250 μM) and after the application of Ba2+ (200 μM). (c) Mean±s.e.m. current voltage relations obtained at control conditions, under the plateau phase of the ATP-elicited response (250 μM) and after the application of apamin (2 μM). (d) Mean±s.e.m. current voltage relations obtained at control conditions, under the plateau phase of the ATP-elicited response (250 μM) and after the application of TEA (2 mM).
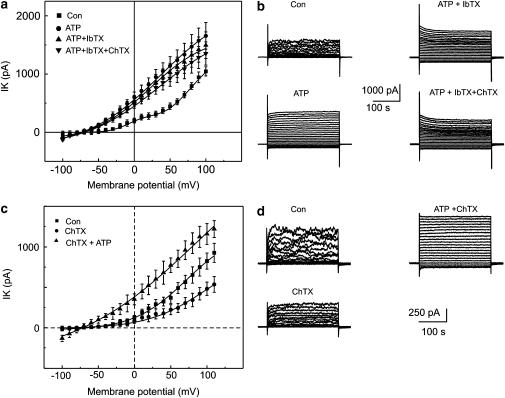
Next, iberiotoxin (100 nM) and charybdotoxin (100 nM) were applied consecutively. Both blockers failed to decrease the amplitude of the delayed ATP-elicited K+ current (Figure 4a and b) (n=10) from −60 to 80 mV (P-value 0.0008).
Figure 4.
Effect of charybdotoxin and iberiotoxin on the delayed ATP-elicited current. Experiments were performed in a perforated patch clamp configuration and shown in (a) and (b). (a) Mean±s.e.m. amplitude of the currents in control conditions (filled squares), and after extracellular administration of ATP (250 μM) (filled circles), was plotted against the corresponding voltage. The effect of the cumulative application of iberiotoxin (100 nM) is demonstrated by filled right side up triangles and of charybdotoxin by filled upside down triangles, respectively. (b) Superimposed traces of the currents evoked during voltage step pulses applied from a holding potential of −60 mV with 10 mV increments between −100 and 100 mV. Experiments and recordings presented in (c) and (d) were performed in a whole-cell configuration. Cells were dialyzed and bathed in solutions containing 2 mM EGTA (0 mM Ca2+). (c) Mean±s.e.m. current amplitude in control conditions (filled squares), and after the application of charybdotoxin (100 nM) (filled circles), was plotted against the corresponding voltage. The effect of the cumulative application of ATP (250 μM) is illustrated by filled triangles. (d) Superimposed traces of currents evoked by voltage step pulses applied from a holding potential of −60 mV with 10 mV increments between −100 and 100 mV.
The following set of experiments examined the role of intracellular free Ca2+. Experiments were performed in the whole-cell configuration. In control conditions, cells were bathed and dialyzed with Ca2+-free solution containing 2 mM EGTA. Charybdotoxin added to the bath solution 2 min after the rupture of the membrane decreased the amplitude of the outward K+ currents only at positive potentials to 30 mV (Figure 4c and d). The amplitude of the control current decreased from 370.8±69.1 to 223.9±61.7 at 50 mV (n=7) and this inhibition was statistically different (P=0.0007). However, the inhibition was not statistically different at −30 mV (n=7), with the amplitude of the control current decreasing from 46.9±6.5 to 37.2±10 pA. ATP added cumulatively to the charybdotoxin in these conditions elicited the generation of a linear, weakly voltage-dependent current. Thus, ATP evoked the generation of a delayed Ca2+-independent K+ current. The delay in the activation of Ca2+-independent currents decreased from 10.5±3.4 min in the perforated patch clamp configuration to 1.27±0.33 min in the cells dialyzed with 2 mM EGTA (n=9). In the next series of experiments, ATP was applied to cells pretreated with BAPTA-AM (10 μM). Current measurements were performed in the perforated patch clamp configuration. The delay in the activation of delayed ATP-elicited current decreased from 12.3±2.1 min in control cells to 2.4±0.6 min in the cells pretreated with BAPTA AM (n=6) (P=0.008).
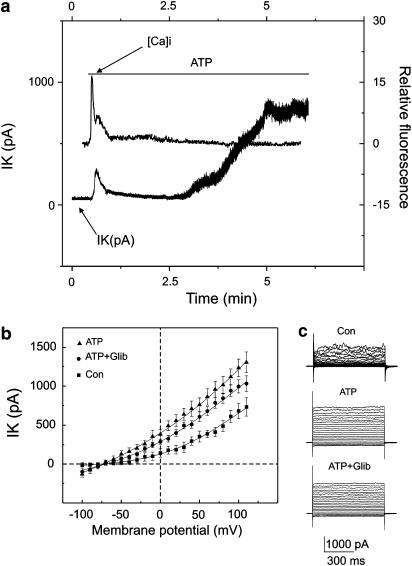
To confirm these observations, simultaneous measurements of the intracellular free Ca2+ and membrane currents were performed in the perforated patch clamp configuration. Cells were loaded with Fluo 3 and recordings were taken at a holding potential of −30 mV. The increase in intracellular free Ca2+ elicited by extracellular application of ATP correlated with the increase in outward net currents (Figure 5a) (n=7). This transient increase in current and intracellular Ca2+ was followed by a return to control levels. The continuous presence of ATP in the bath solution delayed activation of the outward K+ current. However, intracellular Ca2+ remained unchanged under these conditions (Figure 5a).
Figure 5.
Delayed ATP-elicited response and intracellular free calcium. (a) Simultaneous recordings of intracellular free Ca2+ and outward ATP-elicited K+ current. Membrane potential was held at −30 mV. ATP application is indicated with a solid line. (b) Mean±s.e.m. plots of the current voltage relations recorded under normal conditions, after 3 min of ATP (250 μM) application, and after the application of glibenclamide (10 μM). Membrane currents were recorded in a whole-cell configuration. K+ currents were evoked by voltage step pulses applied from a holding potential of −60 mV with the 10 mV increment between −100 and 100 mV. (c) Superimposed family of outward K+ currents under control conditions (Con), after ATP application (ATP) and after the cumulative application of ATP and glibenclamide (10 μM) (ATP+Glib).
This delayed activation of the Ca2+-independent K+ current might be due to the depletion of intracellular ATP responsible for the activation of the KATP currents. To examine this further, experiments were performed in the whole-cell configuration. Cells were bathed and dialyzed with Ca2+-free solution containing 2 mM EGTA. Glibenclamide (10 μM), a KATP channel blocker, did not inhibit ATP-elicited Ca2+-independent K+ currents (Figure 5b and c).
Action of adenosine on the ion currents recorded from mouse aortic smooth muscle cells
Smooth muscle cells possess ectonucleotidases that could be responsible for the continuous conversion of ATP to adenosine. Since ATP was in the bath solution for more than 10 min, ATP could degrade to adenosine that would in turn activate the delayed ATP-elicited K+ current.
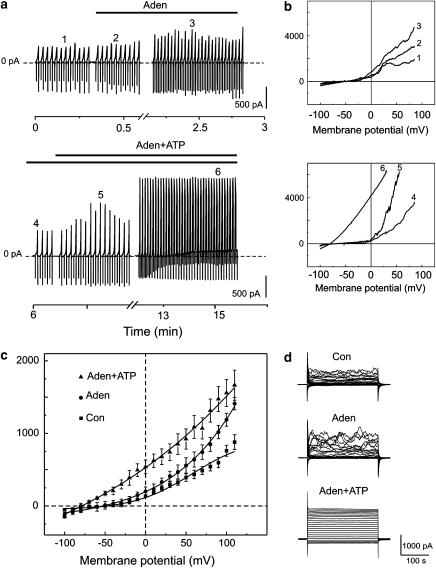
To test whether adenosine had an effect on membrane currents, we performed further experiments using the perforated patch clamp configuration. Adenosine (250 μM) evoked a brief increase (32.5±6.2 s) in the amplitude of membrane currents (n=7). The continuous recordings of the time series of ramp-evoked currents are shown superimposed in Figure 5a. The reversal potential of the current voltage relation in the presence of adenosine (250 μM) shifted from −75±1.5 to −44.4±2.7 mV (n=7) (Figure 6b). Adenosine alone did not elicit an activation of the delayed Ca2+-independent K+ current (Figure 6a) but ATP applied together with adenosine elicited a delayed activation of the K+ current, suggesting that the delayed response depends exclusively on ATP (Figure 6c and d). The reversal potential shifted from −65.1±2.5 to −77.8±1.3 mV (n=5).
Figure 6.
Effect of adenosine (250 μM) on the K+ currents in freshly isolated smooth muscle cells. K+ currents were recorded in the perforated patch clamp configuration. (a) Continuous recording of the ion currents at a holding potential of −60 mV. Superimposed current voltage relations were obtained by voltage ramps varying from −100 to 100 mV. Application of adenosine (250 μM) and ATP (250 μM) is indicated by solid lines. Numbers on the continuous traces indicate current voltage relations superimposed in (b). (b) Upper panel represents current voltage relations recorded at different time points after the application of adenosine (traces 2 and 3) compared to control (trace 1). Lower panel represents current voltage relations recorded at different time points after the application of ATP (traces 5 and 6) compared to the trace recorded under adenosine administration (trace 4). (c) Mean±s.e.m. amplitude of currents plotted against the corresponding voltage: in the control conditions (squares), after application of adenosine (circles) and after application of ATP plus adenosine (point-up triangles). K+ currents were evoked by voltage step pulses applied from the holding potential of −60 with 10 mV increments between −100 and 100 mV. (d) Superimposed currents evoked by voltage step pulses recorded in the control (Con), under adenosine administration (Aden) and under ATP administration (ATP).
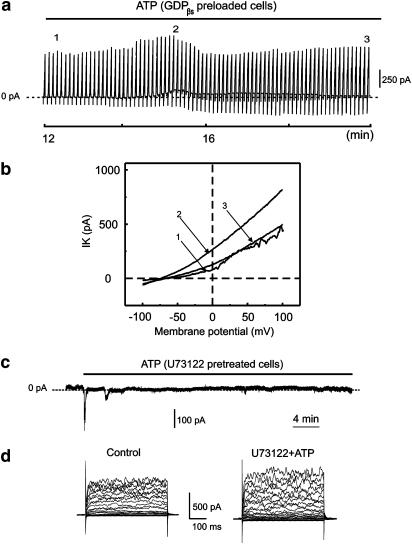
In the next series of experiments, aortic smooth muscle cells were dialyzed with 1 mM GDPβs in order to inhibit the activity of G-proteins and clarify their role in the generation of the delayed ATP-elicited response. Addition of GDPβs to the intracellular solution transformed the ATP-elicited response from a sustained to a transient one (Figure 7). However, the current was not completely abolished. The amplitude was measured at −30 mV and two data sets of the current amplitude were compared using the two-sample paired t-test. One data set measured the amplitudes of the control currents (130±34 pA, n=6) and the second measured the amplitudes of the ATP-elicited currents 3 min after the delayed ATP-elicited peak, current amplitude (196±45 pA, n=6), in GDPβs dialyzed cells. At P<0.05, the difference in the population means was significantly different.
Figure 7.
Effect of GDPβs on the ATP-elicited delayed activation of K+ currents. Freshly dissociated aortic smooth muscle cells were dialyzed with 1 mM GDPβs. Current voltage relations were evoked by voltage ramps varied from −100 to 100 mV and applied from the holding potential of −60 mV. (a) Recording of K+ current at a −60 mV holding potential with superimposed current voltage relations under prolonged exposure to extracellular ATP (250 μM). The presence of ATP is indicated by a solid line. The numbers indicate the current voltage relations taken at different time points and superimposed in (b). Smooth muscle cells were pretreated with the PLC inhibitor U73122 (1 μM) for 20 min. (c) Action of ATP on U73122 (1 μM) pretreated cells. Currents were recorded at a holding potential of −30 mV. The application of ATP is indicated by a line bar. (d) Superimposed traces of currents elicited by voltage steps under control conditions and after application of U73122 (1 μM) with ATP (250 μM).
In the next series of experiments, smooth muscle cells were pretreated with the known phospholipase C (PLC) inhibitor, U73122 (1 μM), for 20 min. This PLC inhibitor was shown to abolish the delayed ATP-elicited response (Figure 7c and d). The kinetics of the currents elicited by voltage pulses remained unchanged in the control conditions and following application of U73122 (1 μM) with ATP (250 μM). The amplitude of the current was measured at 50 mV and was 494±64 pA under control conditions compared to 557±62 pA in the presence of U73122 and ATP (n=9). The difference of the population means was not significantly different.
Discussion
In our preparation cells under continuous exposure to extracellular ATP elicited two types of response that were separated in time and differing in pharmacological and biophysical properties. The initial response that occurred rapidly after ATP application to the bath solution was named the first phase. The responses recorded in the first phase have been reported previously in different preparations of vascular smooth muscle, endothelial, epithelia and neuronal cells (Kumari et al., 2003; Brockhaus et al., 2004). Indeed, the first phase represents a complex event involving several types of ATP-elicited intracellular Ca2+ transients accompanied with changes in the Ca2+-dependent membrane currents (Duchene & Takeda, 1997).
In our experiments the patched vascular smooth muscle cells were held for longer than usual after ATP application. Continuous exposure of the aortic smooth muscle cells to extracellular ATP evoked a delayed second phase, suggesting the presence of a different ATP-dependent mechanism. The second phase consisted of a slowly developed outward current that reached a sustained level. To our knowledge, K+ currents underlying the second phase of the response have not been described previously during patch clamp investigations in vascular smooth muscle cells. The reversal potential of the delayed ATP-elicited outward current was found to be about −80 mV. The shift of the reversal potential to 0 mV evoked by the substitution of the physiological K+ concentration to symmetrical K+ solutions has confirmed the K+ selectivity of the delayed ATP-elicited outward current.
Vascular smooth muscle cells express several types of K+-channel. The quantities of the types of channels expressed differ from one cell to another. Thus, it was found with whole-cell currents that there were two types of current voltage relations generated that have different conductivity plots. A delayed ATP-elicited response was recorded in both cell types.
This response was not inhibited by iberiotoxin, charybdotoxin or glibenclamide, suggesting that BKCa, IKCa, SKCa and KATP are not involved in the delayed ATP-elicited response and KV and KIR are probably not implicated either. However, one should not exclude the possibility of K+-channel modification. Activation of P2 receptors could lead to the binding of unknown intracellular factors to the channel that change its biophysical and pharmacological properties.
It is well established that ATP in the vascular smooth muscle cells causes an increase in intracellular Ca2+ (Ralevic & Burnstock, 1998; Bergner & Sanderson, 2002; Lamont & Wier, 2002; Matsuura et al., 2004). The rise in intracellular Ca2+ is accompanied by changes in membrane currents during the first phase of the ATP-elicited response. In vascular smooth muscle cells, two types of Ca2+-dependent current have been reported, a Ca2+-dependent Cl− current and a K+ current (von der Weid et al., 1993, Bakhramov et al., 1996). Both currents are activated in the first phase of the ATP-elicited response (Bakhramov et al., 1996; Strobaek et al., 1996). The mechanisms underlying the generation of this first phase of ATP-elicited responses were out of the scope of the current study and have been reviewed previously (Ralevic & Burnstock, 1998; Fanchaouy et al., 2005).
Simultaneous measurement of ATP-elicited Ca2+ transients and ion currents demonstrated that generation of the second phase current was not accompanied by a rise in intracellular Ca2+. Further evidence for this came from whole-cell experiments in which cells were dialyzed with EGTA. The first phase of the response could not be detected in a Ca2+-free solution. However, the second phase of the delayed current did occur, so therefore the second phase of the ATP-elicited response appears to be Ca2+ independent.
However, this calcium independency can be explained differently. On the one hand, it may well be that intracellular Ca2+ is not simply not correlated with the second phase ATP-elicited current, but that it regulates ATP-elicited currents in an inverse manner. In order to prove this ‘reversed' calcium dependency a direct experiment is needed. This experiment should demonstrate that calcium ions bind to the channel and decrease the channel's open probability. A second possible explanation is based on the hypothesis that intracellular calcium could switch off and on an unknown membrane or cytoplasmic regulatory factor that might in its turn change the open probability of the delayed ATP-activated channel.
ATP present in the bath solution for 30 min could also degrade continuously to adenosine. That membrane-bound ectonucleotidases have been reported in vascular smooth muscle cells (Zimmermann, 1996) supports this suggestion. However, adenosine alone did not elicit the delayed outward current, but ATP added cumulatively with adenosine still elicited a delayed K+ current. Thus adenosine receptors were not involved in the delayed ATP-elicited response.
The question of what type of purinergic receptor was mediating the second phase of the response remains open. Nevertheless, some preliminary conclusions can be drawn. P2X receptors most likely were not involved in the second phase of the response in aortic smooth muscle cells. P2X receptor channels are known to be nonselective cation channels (Liu & Adams, 2001; Fujiwara & Kubo, 2004). Our experiments indicate that the K+ ion was the major charge carrier. However, one cannot exclude the possibility that there is a new type of K+ selective P2X receptor (Faria et al., 2005). P2Y receptors could mediate the delayed ATP-elicited Ca2+-independent K+ currents. The fact that GDPβs only modified but did not inhibit the delayed ATP-elicited response supports this hypothesis. On the other hand, smooth muscle cells are known to express several types of G-protein and GDPβs could partially inhibit them. The fact that in the smooth muscle cells pretreated with a PLC inhibitor U73122, ATP failed to elicit the delayed response, strongly supports the hypothesis that activation of P2Y receptors brings about the activation of the delayed ATP-elicited current.
Several questions remain. Are the same P2Y receptors that are involved in the first phase of the ATP-elicited response also involved in the second phase? What mechanism is regulating the order of these responses?
The new Ca2+-independent ATP-elicited response reported here represents a new vasoregulatory pathway in mouse aortic smooth muscle cells. It may regulate vasodilation by the hyperpolarization of the membrane potential. However, its precise role remains to be clarified.
Acknowledgments
This work was supported by Swiss National Science Foundation Grants FN 31-61716 and 31-62502 and Fond Claraz. We thank Peter Dudek for a critical reading of the manuscript.
Abbreviations
- 4AP
4-aminopyridine
- ATP
adenosine 5′-triphosphate
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethylester)
- EGTA
ethylene glycol-bis (2-aminoethylether)-N,N,N′,N′-tetraacetic acid
- GDPβs
guanosine 5′-O-(2-thiodiphosphate)
- PLC
phospholipase C
- TEA
tetraethylammonium chloride
References
- BAKHRAMOV A., HARTLEY S.A., SALTER K.J., KOZLOWSKI R.Z. Contractile agonists preferentially activate Cl− over K+ Currents in arterial myocytes. Biochem. Biophys. Res. Commun. 1996;227:168–175. doi: 10.1006/bbrc.1996.1484. [DOI] [PubMed] [Google Scholar]
- BERGNER A., SANDERSON M.J. ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2002;283:L1271–L1279. doi: 10.1152/ajplung.00139.2002. [DOI] [PubMed] [Google Scholar]
- BOEYNAEMS J.M., PEARSON J.D. P2 purinoceptors on vascular endothelial cells: Physiological significance and transduction mechanisms. Trends Pharmacol. Sci. 1990;11:34–37. doi: 10.1016/0165-6147(90)90039-b. [DOI] [PubMed] [Google Scholar]
- BRIZZOLARA A.L., BURNSTOCK G. Endothelium-dependent and endothelium-independent vasodilatation of the hepatic artery of the rabbit. Br. J. Pharmacol. 1991;103:1206–1212. doi: 10.1111/j.1476-5381.1991.tb12325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCKHAUS J., DRESSEL D., HEROLD S., DEITMER J.W. Purinergic modulation of synaptic input to Purkinje neurons in rat cerebellar brain slices. Eur. J. Neurosci. 2004;19:2221–2230. doi: 10.1111/j.0953-816X.2004.03325.x. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., WARLAND J.J. A pharmacological study of the rabbit saphenous artery in vitro: a vessel with a large purinergic contractile response to sympathetic nerve stimulation. Br. J. Pharmacol. 1987;90:111–120. doi: 10.1111/j.1476-5381.1987.tb16830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYCHKOV R., GOLLASCH M., RIED C., LUFT F.C., HALLER H. Regulation of spontaneous transient outward currents in human coronary arteries. Circulation. 1997;95:503–510. doi: 10.1161/01.cir.95.2.503. [DOI] [PubMed] [Google Scholar]
- CORR L., BURNSTOCK G. Analysis of P2-purinoceptor subtypes on the smooth muscle and endothelium of rabbit coronary artery. J. Cardiovasc. Pharmacol. 1994;23:709–715. doi: 10.1097/00005344-199405000-00004. [DOI] [PubMed] [Google Scholar]
- DIETRICH H.H., ELLSWORTH M.L., DACEY-JR R.G. The red blood cell, ATP and integrated vascular responses to neuronal stimulation. Int. Congress Ser. 2002;1235:277–287. [Google Scholar]
- DUCHENE A.D., TAKEDA K. P2Y- and P2U-mediated increases in internal calcium in single bovine aortic endothelial cells in primary culture. Endothelium. 1997;5:277–286. doi: 10.3109/10623329709052592. [DOI] [PubMed] [Google Scholar]
- FANCHAOUY M., SERIR K., MEISTER J.J., BENY J.L., BYCHKOV R. Intercellular communication: role of gap junctions in establishing the pattern of ATP-elicited Ca2+ oscillations and Ca2+-dependent currents in freshly isolated aortic smooth muscle cells. Cell Calcium. 2005;37:25–34. doi: 10.1016/j.ceca.2004.06.008. [DOI] [PubMed] [Google Scholar]
- FARIA R.X., DEFARIAS F.P., ALVES L.A. Are second messengers crucial for opening the pore associated with P2X7 receptor. Am. J. Physiol. Cell. Physiol. 2005;288:C260–C271. doi: 10.1152/ajpcell.00215.2004. [DOI] [PubMed] [Google Scholar]
- FUJIWARA Y., KUBO Y. Density-dependent changes of the pore properties of the P2X2 receptor channel. J. Physiol. 2004;558 (Part 1):31–43. doi: 10.1113/jphysiol.2004.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLLASCH M., RIED C., BYCHKOV R., LUFT F.C., HALLER H. K+ currents in human coronary artery vascular smooth muscle cells. Circ. Res. 1996;78:676–688. doi: 10.1161/01.res.78.4.676. [DOI] [PubMed] [Google Scholar]
- KAMOUCHI M., FUJISHIMA M., ITO Y., KITAMURA K. Simultaneous activation of Ca(2+)-dependent K+ and Cl− currents by various forms of stimulation in the membrane of smooth muscle cells from the rabbit basilar artery. J. Smooth Muscle Res. 1998;34:57–68. doi: 10.1540/jsmr.34.57. [DOI] [PubMed] [Google Scholar]
- KAWAMURA H., SUGIYAMA T., WU D.M., KOBAYASHI M., YAMANISHI S., KATSUMURA K., PURO D.G. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J. Physiol. 2003;551 (Part 3):787–799. doi: 10.1113/jphysiol.2003.047977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY C., BURNSTOCK G. Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur. J. Pharmacol. 1985;111:49–56. doi: 10.1016/0014-2999(85)90112-8. [DOI] [PubMed] [Google Scholar]
- KNIGHT G.E., OLIVER-REDGATE R., BURNSTOCK G. Unusual absence of endothelium-dependent or –independent vasodilatation to purines or pyrimidines in the rat renal artery. Kidney Int. 2003;64:1389–1397. doi: 10.1046/j.1523-1755.2003.00233.x. [DOI] [PubMed] [Google Scholar]
- KUMARI R., GOH G., NG L.L., BOARDER M.R. ATP and UTP responses of cultured rat aortic smooth muscle cells revisited: dominance of P2Y2 receptors. Br. J. Pharmacol. 2003;140:1169–1176. doi: 10.1038/sj.bjp.0705526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMONT C., WIER W.G. Evoked and spontaneous purinergic junctional Ca2+ transients (jCaTs) in rat small arteries. Circ. Res. 2002;91:454–456. doi: 10.1161/01.res.0000035060.98415.4b. [DOI] [PubMed] [Google Scholar]
- LIU C., MATHER S., HUANG Y., GARLAND C.J., YAO X. Extracellular ATP facilitates flow-induced vasodilatation in rat small mesenteric arteries. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1688–H1695. doi: 10.1152/ajpheart.00576.2003. [DOI] [PubMed] [Google Scholar]
- LIU D.M., ADAMS D.J. Ionic selectivity of native ATP-activated (P2X) receptor channels in dissociated neurones from rat parasympathetic ganglia. J. Physiol. 2001;534 (Part 2):423–435. doi: 10.1111/j.1469-7793.2001.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOIRAND G., PACAUD P. Mechanism of the ATP-induced rise in cytosolic Ca2+ in freshly isolated smooth muscle cells from human saphenous vein. Pflugers. Arch. 1995;430:429–436. doi: 10.1007/BF00373919. [DOI] [PubMed] [Google Scholar]
- MATHIESON J.J., BURNSTOCK G. Purine-mediated relaxation and constriction of isolated rabbit mesenteric artery are not endothelium-dependent. Eur. J. Pharmacol. 1985;118:221–229. doi: 10.1016/0014-2999(85)90132-3. [DOI] [PubMed] [Google Scholar]
- MATSUURA M., SAINO T., SATOH Y. Response to ATP is accompanied by a Ca2+ influx via P2X purinoceptors in the coronary arterioles of golden hamsters. Arch. Histol. Cytol. 2004;67:95–105. doi: 10.1679/aohc.67.95. [DOI] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- OIKE M., DROOGMANS G., ITO Y. ATP release pathways in vascular endothelial cells. Folia Pharmacol. Jpn. 2004;123:403–411. doi: 10.1254/fpj.123.403. [DOI] [PubMed] [Google Scholar]
- PANKRATOV Y., LALO U., KRISHTAL O., VERKHRATSKY A. P2X receptor-mediated excitatory synaptic currents in somstosensory cortex. Mol. Cell. Neurosci. 2003;24:842–849. doi: 10.1016/s1044-7431(03)00233-1. [DOI] [PubMed] [Google Scholar]
- QASABIAN R.A., SCHYVENS C., OWE-YOUNG R., KILLEN J.P., MACDONALD P.S., CONIGRAVE A.D., WILLIAMSON D.J. Characterization of the P2 receptors in rabbit pulmonary artery. Br. J. Pharmacol. 1997;120:553–558. doi: 10.1038/sj.bjp.0700924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V. Mechanism of prolonged vasorelaxation to ATP in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 2001;132:685–692. doi: 10.1038/sj.bjp.0703868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V. The involvement of smooth muscle P2X receptors in the prolonged vasorelaxation response to purine nucleotides in the rat mesenteric arterial bed. Br. J. Pharmacol. 2002;135:1988–1994. doi: 10.1038/sj.bjp.0704663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SIMONSEN U., GARCIA-SACRISTAN A., PRIETO D. Involvement of ATP in the non-adrenergic non-cholinergic inhibitory neurotransmission of lamb isolated coronary small arteries. Br. J. Pharmacol. 1997;120:411–420. doi: 10.1038/sj.bjp.0700918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINMETZ M., GABRIELS G., PIECHOTA H.J., RAHN K.H., SCHLATTER E. Vasoactivity of diadenosine polyphosphates in human small renal resistance arteries. Nephrol. Dial. Transplant. 2003;18:2496–2504. doi: 10.1093/ndt/gfg405. [DOI] [PubMed] [Google Scholar]
- STROBAEK D., CHRISTOPHERSEN P., DISSING S., OLESEN S.P. Simultaneous activation of Ca(2+)-dependent K+ and Cl− currents by various forms of stimulation in the membrane of smooth muscle cells from the rabbit basilar artery. Am. J. Physiol. 1996;271 (Part 1):C1463–C1471. doi: 10.1152/ajpcell.1996.271.5.C1463. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI T., KITAYAMA M., KUSHIDA M., FUJITA A., HATA F. Essential role of ATP synthesized by creatine kinase in contraction of – toxin permeabilized preparations of tonic type smooth muscle. J. Pharmacol. Sci. 2003;92:374–380. doi: 10.1254/jphs.92.374. [DOI] [PubMed] [Google Scholar]
- VON DER WEID P.Y., SEREBRYAKOV V.N., ORALLO F., BERGMANN C., SNETKOV V.A., TAKEDA K. Effects of ATP on cultured smooth muscle cells from rat aorta. Br. J. Pharmacol. 1993;108:638–645. doi: 10.1111/j.1476-5381.1993.tb12854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANGENSTEEN R., FERNANDEZ O., SAINZ J., QUESADA A., VARGAS F., OSUNA A. Contribution of endothelium-derived relaxing factors to P2Y-purinoceptor-induced vasodilation in the isolated rat kidney. Gen. Pharmacol.: Vasc. System. 2000;35:129–133. doi: 10.1016/s0306-3623(01)00091-x. [DOI] [PubMed] [Google Scholar]
- WIHLBORG A.K., MALMSJO M., EYJOLFSSON A., GUSTAFSSON R., JACOBSON K., ERLINGE D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br. J. Pharmacol. 2003;138:1451–1458. doi: 10.1038/sj.bjp.0705186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO K., KORENAGA R., KAMIYA A., ZHI Q.I., SOKABE M., ANDO J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN H. Biochemestry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog. Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]