Abstract
The aim of this study was to identify human colonic resident cells able to initiate an inflammatory response in postischemic injury.
Postischemic colonic injury, a condition relevant to various clinical settings, involves an inflammatory cascade in intestinal tissues through the recruitment of circulating inflammatory cells. However, there is no information on the nature of resident cells of the different intestinal layers able to initiate a postischemic inflammatory response. It is however an important issue in the context of a pharmacological approach of the early phase of intestinal ischemia.
We reasoned that maintaining the different colonic layers as explant cultures in an oxygenated medium immediately after colonic resection, that is, after an ischemic period, would allow one to identify the resident cells able to initiate an inflammatory cascade, without interference of recruited inflammatory/immune cells. To this end, we designed an explant culture system that operationally defines three compartments in surgical specimens of the human colon, based on the microdissected layers, that is, mucosa, submucosa (containing muscularis mucosae) and muscularis propria. To validate the results obtained in explant cultures in the clinical setting of ischemic colitis, eight cases of sigmoid volvulus were examined.
Only the myocytes-containing explants produced tumor necrosis factor alpha (TNFα), via an ADAM17 (a disintegrin and metalloproteinase-17)-dependent pathway, as shown by the abrogation of TNFα production by the inhibitor Tapi-2. Immunofluorescence studies identified nonvascular and vascular myocytes as resident cells coexpressing TNFα and ADAM17, both in our postischemic explant system and in surgical specimens from ischemic colitis patients. Finally, time-course experiments on explanted tissues showed that TNFα production by myocytes was an early event triggered by a postischemic oxidative stress involving nuclear factor kappa B (NF-κB).
In conclusion, this study identifies human intestinal myocytes as resident cells able to initiate an inflammatory reaction through TNFα production in postischemic conditions, and delineates two points of control in TNFα production, NF-κB and ADAM17, which can be targeted by pharmacological manipulation.
Keywords: ADAM17, TNFα, human colonic myocytes, postischemic inflammation, explant culture, ischemic colitis, NF-κB, oxidative stress
Introduction
Large intestinal ischemia exhibits a spectrum of lesions ranging from fatal infarctions with gangrene to reversible ischemia. Reversible ischemia is characterized by inflammatory changes, the so-called postischemic colitis, obliterating the normal tissue architecture, the severity of the lesions depending on the cause, duration of ischemia and reperfusion. Postischemic colitis affects patients with low flow states. Occlusive causes include arterial or venous compression during volvulus, for example (Fenoglio-Preiser, 1999). Finally, postischemic colitis also complicates major surgery (Fenoglio-Preiser, 1999). Although numerous studies based on experimental models have dealt with the potential mechanisms causing the development and progression of postischemic injury, including recruitment and activation of circulating inflammatory cells, release of proinflammatory cytokines and reactive oxygen species (ROS) (Grisham et al., 1986; Zimmerman & Granger, 1990; Kurtel et al., 1992; Anup et al., 1999; Mallick et al., 2004), there is only limited information about the participation of resident cells of the different intestinal layers in the initiation of the inflammatory response. In addition, in humans, it is difficult to design an experimental setting allowing one to address this important issue.
We reasoned that maintaining the different colonic layers as explant cultures in an oxygenated medium immediately after surgical colonic resection, that is, after an ischemic period, would allow one to identify the resident cells able to initiate an inflammatory cascade, without interference of recruited inflammatory/immune cells. To this end, we designed an explant culture system that operationally defines three compartments in surgical specimens of the human colon, that is, mucosa (devoid of muscularis mucosa), muscularis mucosae-containing submucosa and muscularis propria, based on the microdissected layers. Since the explanted tissues cannot recruit circulating inflammatory cells, they form an ideal system to identify the resident cells that initiate an inflammation in postischemic conditions. Attention has been focused in this work on tumor necrosis factor alpha (TNFα)-producing cells, since TNFα has been shown to orchestrate the development of inflammation and tissue disruption in the intestine (Neurath et al., 1997; D'Hauteville et al., 2002). In addition, a blocking antibody against TNFα was found to attenuate postischemic intestinal changes in an animal model (Russell et al., 2000). Finally, consistent with its key role, TNFα production is tightly regulated at several levels, that is, transcriptional and post-transcriptional (Collart et al., 1990; Gueydan et al., 1999) that can be considered as points of control in inflammation (Kontoyiannis et al., 1999). Of particular importance is the TNFα-converting enzyme or ADAM17, which belongs to the ADAM (a disintegrin and metalloproteinase) family of transmembrane multidomain zinc metalloproteinases (Blobel, 2000; Primakoff & Myles, 2000). ADAM17 has been identified as the main secretase responsible for the proteolytic release or shedding of the precursor, membrane-bound form of TNFα (Black et al., 1997; Moss et al., 1997).
In this work, we identified the intestinal nonvascular and vascular myocytes as resident cells able to initiate an inflammatory reaction via TNFα production, both in our ex vivo system of postischemic oxidative stress, and in the clinical setting of ischemic colitis.
Methods
Explant culture
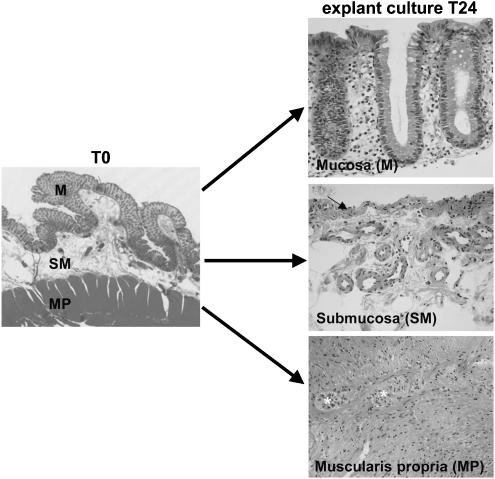
Colon specimens were obtained from 19 patients undergoing surgery for colon carcinoma (16 men, three women, mean age 69±3 years, range: 53–86 years). Fragments of the human normal colon, mainly distal, were taken at about 10 cm downstream to the tumor. These tissues were processed according to the Guidelines of the French Ethics Committee for Research on human tissues. Data included in this report concern only those patients who did not undergo radiotherapy or chemotherapy. The mean duration of ischemia after surgical ligation of vessels was about 45 min. A sample, taken adjacent to the explants, was systematically submitted to histological analysis and subsequently reported as normal by the pathologists. Immediately after removal, the tissues were placed in 4°C oxygenated Krebs as described previously (Neunlist et al., 2003; Rouet-Benzineb et al., 2004). As shown in Figure 1, microdissection under microscopic control in a Sylgard-containing Petri dish led to the identification of three different compartments, that is, mucosa without muscularis mucosae, submucosa including muscularis mucosae, and muscularis propria. These compartments are referred to throughout the text as mucosa, muscularis mucosae-containing submucosa and muscularis, respectively. The muscularis (longitudinal and circular muscle layers) was first removed and then placed in Krebs solution. Then, the mucosa was carefully stripped from the underlying compartment made of muscularis mucosae and submucosa. Fragments of 20–30 mg were cut out and pinned in Sylgard-coated Petri dishes and maintained in culture in 2 ml of RPMI 1640 (InVitrogen, Cergy Pontoise, France) containing BSA (0.01%; Sigma, Saint Quentin Fallavier, France), antibiotics (200 μg ml−1 penicillin, 200 U ml−1 streptomycin; InVitrogen) and fungizone (1%; InVitrogen). The explants were maintained at 37°C in 95% oxygen, 5% carbon dioxide humid atmosphere on a rocking platform at 30 r.p.m. At least three explants of each layer in a given condition were cultured. Before culture (T0), some dissected fragments were frozen before further processing with the different assays performed (morphology, cell viability and RNA extraction, n=3 for each). Most explants were maintained in culture for 24 h. Kinetic studies (5, 9 and 24 h) were also performed (one experiment at time points 5, 9 and 24 h, n=4 each).
Figure 1.
Experimental setup and morphological assessment of the explants integrity after a 24 h culture. Histology (HE staining) of the human colon before microdissection (left) and of the three microdissected layers after a 24 h explant culture (right). The mucosa explants (M) contain the epithelial lining and the underlying lamina propria. The submucosa explants (SM) contain the muscularis mucosae (arrow) and the submucosa. The muscularis propria explants (MP) contain the two muscle layers and myenteric plexuses (*). Original magnification × 200.
Some muscularis mucosae-containing submucosa explants were treated with Tapi-2 (50 μM; Calbiochem, VWR, France), an ADAM17 inhibitor (Mohler et al., 1994), for 24 h. Some muscularis mucosae-containing submucosa explants were treated with the nuclear factor kappa B (NF-κB) inhibitors curcumin (25 μM; Sigma) or triptolide (55 and 550 nM; Sigma) and with N-acetylcysteine (NAC, 20 mM; Sigma), a well-known antioxidant and scavenger of ROS (Zafarullah et al., 2003), for 5 h.
At the end of the culture, the culture medium was centrifuged, aliquoted, and stored at −80°C for further analysis. The cultured tissue specimens were cut out into three fragments: the largest was used for RNA extraction, and two smaller fragments were used for intracellular ATP measurement, and for histological examination after formalin fixation/paraffin embedding, respectively (Rouet-Benzineb et al., 2004).
Tissue integrity and cell viability of the explants were assessed by standard morphological analysis, that is, by hematoxylin–eosin (HE) staining on paraffin sections. Cell viability was also assessed by two assays: measurement of the % of LDH released and of the intracellular ATP level. LDH was measured with the Enzyline LDH Kit (Biomérieux, France) both in the supernatant after the 24 h incubation (extracellular LDH or LDHe) and in a small fragment of the tissue (intracellular LDH or LDHi). Percent toxicity, represented by % LDH released, was calculated as LDHe/(LDHe+LDHi) × 100, as described previously (Le Goffe et al., 2002; Rouet-Benzineb et al., 2004), taking into account the weight of the tissue samples. ATP was measured in tissue lysates with a luminometric assay (ATPlite kit, Perkin-Elmer, Wellesley, MA, U.S.A.) according to the manufacturer's instructions. Proteins were measured by the Bradford method. Results were expressed as pmol ATP per μg proteins. Results show that cultured explants maintained a good morphology as assessed on HE staining. LDH release was limited in the mucosa and muscularis mucosae-containing submucosa explants (13.4±1.4 and 17.3±2.7%, respectively, n=17 explant cultures from five different experiments), but significantly higher in the muscularis explants (25±3%, n=17, P<0.01 compared with mucosa and submucosa explants). Accordingly, ATP levels after a 24 h culture (T24) was not significantly different from that at T0 in the mucosa and submucosa explants (mucosa at T24: 128±15% relative to T0; submucosa at T24: 89±19% relative to T0; n=15 from five different experiments), but was significantly lower in the muscularis explants (T24: 67±16% relative to T0, P=0.02; n=15 from five different experiments). The inhibitors used in muscularis mucosae-containing submucosa explant cultures (Tapi-2, curcumin, triptolide and NAC) did not significantly modify LDH release or intracellular ATP levels, and did not alter tissue morphology.
Patients with ischemic colitis
Surgical specimens from eight patients undergoing volvulus were studied (three men and five women, mean age 71±6 years, range: 39–89 years). These tissues were processed according to the Guidelines of the French Ethics Committee for Research on human tissues. The lesions were located in the sigmoid colon in five cases, and in the ascending colon in three cases. Histologically, early ischemic lesions were observed, consisting in mucosal and submucosal edema, with a mild inflammatory infiltrate. Only minor architectural alterations of the colonic crypts were noted in some cases, with no sign of necrosis. Paraffin sections from six cases were immunostained for NF-κB p65 (see below). Frozen tissue was available in three cases, and processed for ADAM17 and TNFα double immunofluorescence staining followed by confocal microscopy, as described below.
Immunofluorescence and immunoperoxidase studies
For ADAM17 and TNFα immunostaining followed by confocal microscopy, 7 μm cryostat sections were performed, fixed in 4% paraformaldehyde in PBS buffer, pH 7.4, for 15 min on ice, and processed for immunofluorescence as reported previously (Jarry et al., 2004). Briefly, tissue sections were preincubated for 30 min with PBS/4% horse serum, and exposed for 1 h at room temperature with a mixture of antibodies directed to TNFα (monoclonal, 1 : 100; Diaclone, Besançon, France) and ADAM17 (polyclonal, 1 : 200; Chemicon, Temacula, CA, U.S.A.). After washing, sections were incubated for 30 min with a mixture of Alexa Fluor 488-conjugated goat anti-rabbit and Alexa Fluor 568-conjugated goat anti-mouse antibodies (1 : 200; Molecular Probes, Eugene, OR, U.S.A.). Nuclear staining was performed with TOPRO-3 (1 μM; Molecular Probes). Double immunostaining with anti-desmin (monoclonal, 1 : 50; Immunotech, Marseille, France) and anti-TNFα (polyclonal, 1 : 150; Abcam, Cambridge, U.K.) antibodies was also performed as described above. Sections were then mounted using Prolong antifade medium (Molecular Probes). Imaging was performed on a Leica TCS-SP confocal laser-scanning microscope (Leica, Heidelberg, Germany) equipped with an argon–krypton laser. Sections were visualized with a × 63/1.4 oil objective lens. Image processing was performed using TCS-NT software (Leica). Quantification of the overlay of labeling was carried out using a MetaMorph 4.6.5 program (Universal Imaging Corp., Downingtown, PA, U.S.A.). Results are expressed as the mean±s.e. of at least 10 different regions analyzed per tissue section.
Cells exhibiting NF-κB activation were identified by examining the nuclear translocation of the p65 subunit that contains a strong transcriptional activation domain, using immunohistochemistry. A standard immunoperoxidase staining was performed on 4 μm paraffin sections after antigen retrieval and endogeneous peroxidase quenching with 3% H2O2, using a monoclonal anti-p65 antibody (1 : 300; Santa Cruz, CA, U.S.A.), and a streptavidin–biotin–peroxidase method (LSAB–peroxidase–DAB kit; DakoCytomation, Trappes, France).
RNA extraction and real-time RT–PCR analysis
RNA isolation and cDNA synthesis
Total RNA was extracted from the explanted colonic layers (T0 and T24) from 10 patients, using the Qiamp Total RNA kit (Qiagen, Valencia, CA, U.S.A.) and the Fast Prep cell disrupter (Bio 101). RNA (5 μg) was denaturated at 72°C for 3 min and then reverse transcribed for 60 min at 42°C in a 20 μl reaction volume (50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 10 mM DTT) containing 0.5 μg of random hexamers (Promega, Madison, WI, U.S.A.), dNTPs (1 mM each), RNasin (50 U) and RnaseH− MMLV reverse transcriptase (200 U).
Quantitative PCR
Real-time PCR was performed according to previous reports with some modifications (Renaudin et al., 2004; Toumi et al., 2004). The amplification conditions of the TNFα and β-actin templates were optimized for the Rotorgene 2000 instrument (Corbett Research). Primers were designed from the sequence of the human cDNAs using the GeneJockey software. They were selected for binding to separate exons to avoid false-positive results arising from amplification of contaminating genomic DNA. The sequences of these primers are as follows: 5′-CCTTCCTGGGCATGGAGTCCTG-3′ and 5′-GGAGCAATGATCTTGATCTTC-3′ for β-actin; 5′-AGGCGGTGCTTGTTCCTCA-3′ and 5′-GTTCGAGAAGATGATCTGACTGCC-3′ for TNFα. PCR amplifications were performed using Titanium Taq DNA polymerase (Clontech, Palo Alto, CA, U.S.A.). The reaction mixture contained 2 μl of the supplied 10 × Titanium Taq PCR buffer (containing magnesium chloride), 2 μl of a 1/1000 dilution of SYBR Green I (Roche Molecular Biochemicals, Meylan, France), 1 μl of each primer (0.4 μM each), 0.2 μl of Titanium Taq DNA polymerase, 0.5 μl of dNTPs (10 mM each) and PCR-grade water to a volume of 10 μl. Microtubes (0.2 ml) were loaded with 10 μl of this master mix and 10 μl of the template (cDNA diluted 1 : 50) and the run was initiated. The cycling conditions were as follows: denaturation for 5 min at 95°C; amplification for 35 cycles, with denaturation for 5 s at 95°C, annealing for 5 s (at 63°C for β-actin, 67°C for TNFα) and extension for 5 s at 72°C. To exclude primer–dimer artifacts, fluorescence was not measured at the end of the extension step, but a separate detection step was added (10 s) at a temperature above the melting point of primer–dimers and below the melting point of the specific PCR product (87°C for β-actin, 88°C for TNFα). After completion of the cycling process, samples were subjected to a temperature ramp from 63°C to 99°C, with continuous fluorescence monitoring for melting curve analysis. For each amplification, apart from primer–dimers, a single narrow peak was obtained at the expected melting temperature, indicating specific amplification without significant by-products. An external standard curve was generated with serial five-fold dilutions of pooled cDNAs samples. The reference curve was constructed by plotting the relative amounts of these dilutions vs the corresponding Ct (threshold cycle) values. The correlation coefficient of these curves was always greater than 0.99. The amount of TNFα and β-actin transcript was calculated from these standard curves using the RotorGene software. Samples were tested in triplicate and the average values were used for quantification. For each sample, the ratio between the relative amount of each specific transcript and β-actin was then calculated to compensate for variations in quantity or quality of starting mRNA as well as for differences in reverse transcriptase efficiency.
ADAM17 mRNA was quantified using a commercially available kit (TaqMan Gene Expression Assay Hs00234224, Applied Biosystems, Foster City, CA, U.S.A.) according to the manufacturer's recommendations. Amplifications were performed in a 7700 thermocycler (Applied Biosystems), using the ABI Prism 7000 SDS software.
TNFα assay
Soluble TNFα released from tissue to the incubation solution was determined by ELISA (OptEIA™ Set Human TNFα; BD Biosciences, San Diego, CA, U.S.A.) in samples collected at the end of the experiment, according to the manufacturer's protocol.
Statistical analyses
Statistical analyses were performed with Statview F-4.5 (Abacus Concepts, Berkeley, CA, U.S.A.). Mann–Withney U-test was used to compare mRNA and protein levels between mucosa, muscularis mucosae-containing submucosa and muscularis explants. For each type of sample, Wilcoxon's paired test was used to compare protein and mRNA levels before (T0) and after 24 h culture (T24). For all analyses, a probability value (P) of less than 0.05 was considered as statistically significant.
Results
TNFα production by myocytes-containing compartments of the human colonic wall
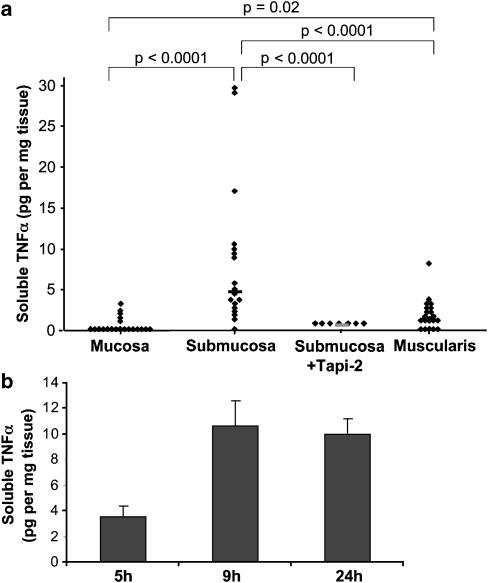
We first evaluated TNFα production in explant cultures of the three compartments dissected from the human colonic wall, that is, mucosa, muscularis mucosae-containing submucosa and muscularis propria. These explants were maintained in organ culture for 24 h in good viability conditions as mentioned in Methods. TNFα concentration was determined in culture medium by ELISA. TNFα production was restricted to muscularis mucosae-containing submucosa explant cultures where it reached up to 29 pg mg−1 wet tissue, and to a lower rate to muscularis explants (Figure 2a). No significant TNFα production was observed in mucosa explants from the same colon samples (Figure 2a). To investigate the functional connection between ADAM17 and TNFα, muscularis mucosae-containing submucosa explants, releasing the highest TNFα levels, were treated with Tapi-2 (50 μM), an ADAM17 inhibitor. As shown in Figure 2a, Tapi-2 abolished TNFα production. Altogether, these data show that TNFα production by muscularis mucosae-containing submucosa explants is an active, ADAM17-dependent process.
Figure 2.
TNFα secretion by explanted cultures of the different layers of the human colon. (a) TNFα levels were analyzed by ELISA in the culture medium after a 24 h incubation. Each point indicates individual explant culture (mucosa, muscularis mucosae-containing submucosa, treated or not with the ADAM17 inhibitor Tapi-2 (50 μM) and muscularis propria). Data are expressed as pg mg−1 of wet tissue. Horizontal lines represent median values of 19–20 determinations from six different experiments for mucosa, submucosa and muscularis, and of seven determinations from two experiments for submucosa+Tapi-2. (b) Time course of TNFα secretion by the muscularis mucosae-containing submucosa explants. TNFα levels were analyzed by ELISA in the culture medium at the indicated time points. Data, expressed as pg mg−1 of wet tissue, represent the mean±s.e.m. of four determinations from one experiment. P=0.02 at time points 9 and 24 h vs 5 h.
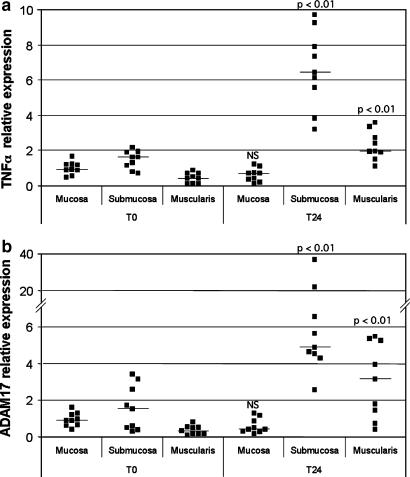
We then analyzed TNFα and ADAM17 expression at the mRNA level in the explant cultures. Real-time PCR allowed us to detect both TNFα and ADAM17 mRNAs, at a very low level in the mucosa, submucosa and muscularis explants before culture (T0) (Figure 3a and b). Interestingly, both genes were significantly upregulated (six- to eight-folds) in the submucosa and muscularis explants after a 24 h culture (T24) (Figure 3a and b). In contrast, in the mucosa explants, neither TNFα nor ADAM17 mRNA levels were modified after a 24 h culture (Figure 3a and b).
Figure 3.
Relative expression of TNFα (a) and ADAM17 (b) in the mucosa, muscularis mucosae-containing submucosa and muscularis propria explants before (T0) and after a 24 h explant culture (T24). Total RNA was isolated from each sample, cDNA was synthesized and TNFα, ADAM17 and β-actin mRNAs were quantified as described in Methods. The ratio TNFα/β-actin and ADAM17/β-actin were calculated and the results are expressed considering 1 as the ratio for mucosa before culture. Each point represents the mean of duplicate measurements obtained for one sample. Horizontal lines represent median values (nine determinations from three different experiments).
Morphological evidence for ADAM17 and TNFα coexpression by vascular and nonvascular myocytes, in postischemic explant cultures and ischemic colitis patients
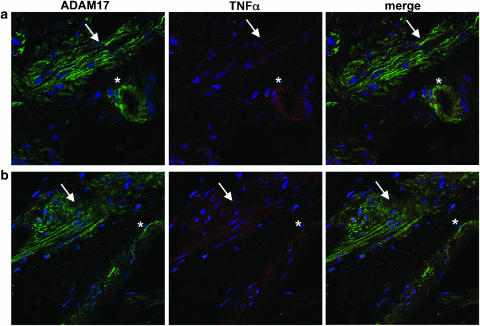
Immunofluorescence followed by confocal microscopy showed that ADAM17 was expressed in the muscularis mucosae-containing submucosa (Figure 4a) and muscularis propria (not shown) both before and after a 24 h culture (Figure 4a). It was mainly expressed by muscularis mucosae and muscularis propria myocytes, as confirmed by double staining with an anti-desmin antibody. The other structures expressing ADAM17 were endothelial cells, vascular smooth muscle cells and a few immune cells (Figure 4a). Double immunostaining showed that these ADAM17-positive cells scored negative with an anti-TNFα antibody before culture (data not shown). However, after a 24 h culture, ADAM17-positive cells also expressed TNFα (Figure 4a). Quantification of the overlay of labeling with the image-processing Metamorph showed that the majority of TNFα colocalized with ADAM17 (74±5%). Finally, the demonstration that myocytes produced TNFα was provided by double immunofluorescence using anti-desmin and anti-TNFα antibodies (Figure 5a).
Figure 4.
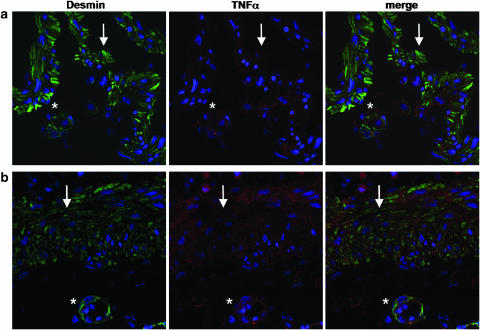
Coexpression of TNFα and ADAM17 in postischemic conditions. (a) Explant culture of the muscularis mucosae-containing submucosa (24 h culture) and (b) muscularis mucosae and submucosa from a patient with ischemic colitis (sigmoid volvulus). Double immunostaining of ADAM17 (green) and TNFα (red) was performed on frozen sections as described in Methods. Nuclei appear in blue. Myocytes of the muscularis mucosae (arrow) and of blood vessels (*) scored positive with both ADAM17 and TNFα antibodies. Original magnification × 630.
Figure 5.
Coexpression of TNFα and a myocyte marker (desmin) in postischemic conditions. (a) Explant culture of the muscularis mucosae-containing submucosa (24 h culture) and (b) muscularis mucosae and submucosa from a patient with ischemic colitis (sigmoid volvulus). Double immunostaining of desmin (green) and TNFα (red) was performed on frozen sections as described in Methods. Nuclei appear in blue. Myocytes of the muscularis mucosae (arrow) and of blood vessels (*), expressing the cytoskeletal marker desmin, produced TNFα. Original magnification × 630.
In order to validate these results, that is, TNFα-expressing myocytes in postischemic conditions, in the clinical setting of ischemic colitis, we examined TNFα and ADAM17 expression on frozen tissue sections of three cases of ischemic colitis (sigmoid volvulus). Double immunofluorescence (ADAM17/TNFα and desmin/TNFα) showed results paralleling those obtained on submucosa explants, that is, the coexpression of TNFα and ADAM17 (Figure 4b; 62±3% of TNFα colocalized with ADAM17), by nonvascular and vascular myocytes, that were labeled with the specific myocyte marker desmin (Figure 5b).
TNFα production by human colonic myocytes is an early event mediated by oxidative stress and which involves NF-κB activation
Time-course experiments of TNFα production by muscularis mucosae-containing submucosa explants showed that TNFα was released as early as 5 h culture into the medium, peaked at 9 h and remained constant thereafter (Figure 2b).
To test whether a redox state imbalance resulting from the production of ROS could account for this early TNFα production, we used the antioxidant NAC (20 mM). As shown in Figure 6a, NAC significantly inhibited TNFα production by muscularis mucosae-containing submucosa explants cultured for 5 h.
Figure 6.
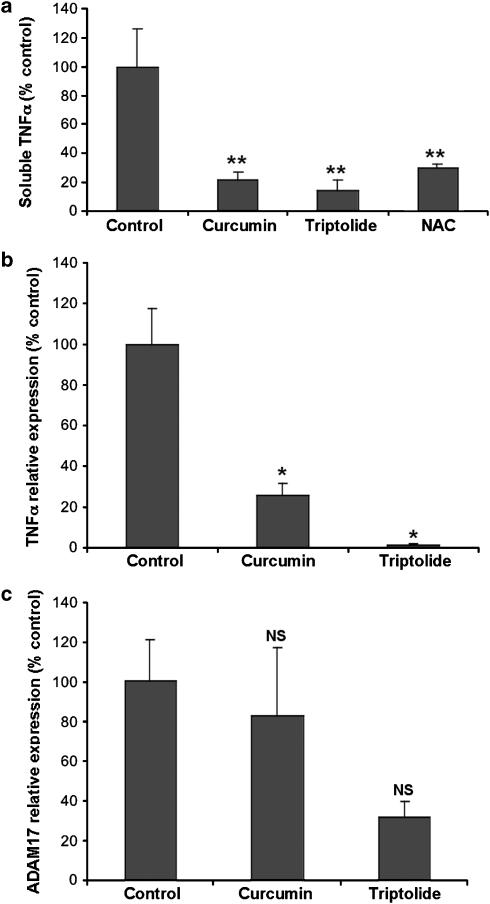
Effect of a scavenger of ROS, N-acetylcysteine (NAC) and of NF-κB inhibitors on TNFα production and synthesis by the muscularis mucosae-containing submucosa explants. (a) TNFα levels were analyzed by ELISA in the culture medium after a 5 h culture in the presence or absence of NAC (20 mM), or the NF-κB inhibitors curcumin (25 μM) or triptolide (550 nM). Data are expressed relative to the control value, considered as 100%. Mean±s.e. of seven determinations from two experiments. **P=0.0005 for each inhibitor vs control. (b and c) Relative expression of TNFα (b) and ADAM17 (c) mRNAs in the submucosa explants after a 5 h culture. Quantification was performed relative to β-actin, as reported in the legend of Figure 3. Results are expressed relative to the control value, considered as 100%. Mean±s.e. of six determinations from two experiments. *P=0.02 vs control.
In order to define whether the transcription factor NF-κB may be involved in the TNFα production, submucosa explant cultures were treated or not with 2 NF-κB inhibitors, curcumin (25 μM) and triptolide (55 nM and 550 nM), for 5 h. Curcumin and triptolide significantly inhibited TNFα production (Figure 6a). As expected from two drugs that target the transcription factor NF-κB, both curcumin and triptolide inhibited TNFα mRNA levels (Figure 6b). Interestingly, ADAM17 mRNA levels were not significantly downregulated (Figure 6c).
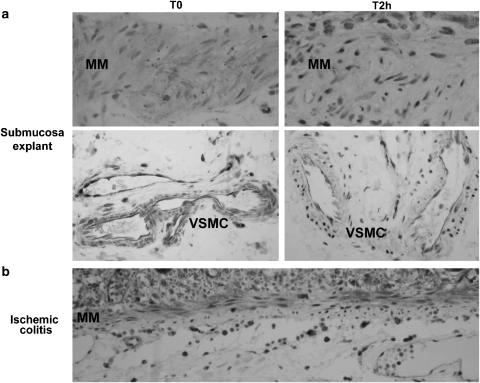
In order to precise the cells exhibiting activated NF-κB, immunohistochemistry was performed on paraffin sections of submucosa explants before and after culture, using a p65 monoclonal antibody. Before culture (T0), vascular and nonvascular myocytes exhibited a weak cytoplasmic staining (Figure 7). Some endothelial cells and immune cells displayed a nuclear staining. As early as 2 h culture, the majority of nonvascular and vascular myocytes showed an NF-κB nuclear translocation (Figure 7). This NF-κB staining pattern was identical after a 5 h culture. In the experiments using NF-κB inhibitors in 5 h cultures, the number of vascular and nonvascular myocytes with a nuclear staining was highly reduced (not shown).
Figure 7.
NF-κB nuclear translocation in human colonic myocytes in postischemic conditions. (a) Explant culture of submucosa before treatment (T0) or after a 2 h culture (T2 h). (b) Ischemic colitis (sigmoid volvulus). NF-κB activation was determined by cytoplasmic-to-nuclear translocation of the NF-κB p65 subunit by immunohistochemistry. In submucosa explants at T0, muscularis mucosae (MM) myocytes as well as vascular myocytes (VSMC) displayed a cytoplasmic NF-κB staining. At T2 h, the majority of nonvascular and vascular myocytes showed an intense nuclear expression of NF-κB. Some endothelial cells exhibited a nuclear expression of NF-κB before and after culture. In ischemic colitis, muscularis mucosae (MM) myocytes and endothelial cells of the submucosa displayed a nuclear expression of NF-κ. Original magnification × 400.
Activation of NF-κB was also examined on paraffin sections of six cases of ischemic colitis, using a p65 monoclonal antibody. Nuclear staining was observed in vascular and nonvascular myocytes, and in endothelial cells (Figure 7).
Discussion
This work brings up novel findings in relation to the initiation by human colonic resident cells of postischemic inflammation.
We identified human intestinal myocytes, both nonvascular (muscularis mucosae and muscularis propria) and vascular, as resident cells that constitutively express the molecular machinery involved in TNFα production, that is, ADAM17. Therefore, we reasoned that these cells could rapidly induce an inflammatory response in postischemic conditions. We tested this hypothesis by using an explant culture system that operationally defines three compartments, one devoid of myocytes (mucosa) and two containing myocytes (muscularis mucosae-containing submucosa and muscularis propria). Interestingly, we found that only the two myocytes-containing compartments are able to produce TNFα upon a 24 h culture, via an ADAM17-dependent pathway, as shown by abrogation of this TNFα production by Tapi-2. This production occurs very early, as soon as 5 h culture. Finally, our demonstration of a TNFα and ADAM17 coexpression by human intestinal myocytes clearly identifies these cells as TNFα-producing cells. Recent studies have shown that human cultured intestinal myocytes are not passive bystanders in an inflammatory reaction, since they can respond to various cytokines by producing proinflammatory mediators (Salinthone et al., 2004). However, up to now, the ability of intestinal myocytes to produce TNFα has not been examined. In light of these findings and of ours, it is now clear that human intestinal myocytes are not only able to contribute to an inflammatory reaction triggered by inflammatory/immune cells, but are also able to initiate an inflammatory cascade through TNFα production following a period of ischemia. Finally, we show here that, in addition to colonic myocytes, some endothelial cells, which are known to express ADAM17 (Blanchot-Jossic et al., 2005), are able to participate in ADAM17-dependent TNFα production.
Finally, our findings, in patients undergoing sigmoid volvulus displaying early lesions of ischemic colitis, show that muscularis mucosae and muscularis propria myocytes as well vascular myocytes strongly coexpressed TNFα and ADAM17.
Based on our experimental system, it was possible to perform both mechanistic and pharmacological studies. Cellular hypoxia and hypoxia-reoxygenation are known to produce ROS, leading to the development of an oxidant stress (Li & Jackson, 2002). In addition, several antioxidants including NAC were found useful to prevent postischemic damage in a rat model (Ferrer et al., 1998). In this context, we were prompted to examine the role of the ROS scavenger NAC on the TNFα production. Interestingly, we found in our ex vivo human model system that NAC abolished the TNFα production.
As we identified a parallel upregulation of TNFα and ADAM17 mRNA levels, we sought for a common transcriptional regulatory pathway. NF-κB activation was a likely candidate to regulate TNFα upon oxidative stress as (1) the TNFα promoter has a number of functional NF-κB sites (Yao et al., 1997; Baer et al., 1998) and (2) NF-κB was found to be involved in hypoxia-reoxygenation injuries in several animal models (Russell et al., 2000; Yeh et al., 2000; Nichols, 2004; Souza et al., 2005). In fact, in our experimental setting, NF-κB activation, as shown by the nuclear translocation in myocytes of the NF-κB p65 subunit, known to have a strong transcriptional activation domain, was found to precede TNFα production. In addition, we examined the effects of two NF-κB inhibitors, that is, curcumin, which blocks inhibitory factor IκBα activity (Jobin et al., 1999), and triptolide, which promotes nuclear inhibition of NF-κB transcriptional activation (Qiu et al., 1999). Our inhibition studies clearly indicate that in our model of postischemic stress, TNFα production was dependent on NF-κB activation. Interestingly, while TNFα mRNA levels were significantly downregulated by NF-κB inhibitors, the downregulation of ADAM17 mRNA levels was not significant, thus indicating a more complicated regulation of ADAM17 mRNAs, which may involve other transcription factors in addition to NF-κB. Interestingly, Hurtado et al. (2001) have shown an upregulation of ADAM17 in a rat model of oxygen–glucose deprivation forebrain slices. Together, their findings and ours strongly suggest that ADAM17 upregulation may be a general mechanism involved in the initial steps of ischemic injury. However, further work is needed to decipher the mechanisms underlying ADAM17 upregulation.
In conclusion, this experimental setting allows one to study the very initial steps of a postischemic inflammation in the human colon at the cellular and molecular levels, that is, the sequence of events following the postischemic redox imbalance. It identifies human intestinal myocytes as resident cells able to initiate an inflammatory reaction through TNFα production in a context of oxidant stress. In addition, this study delineates two points of control in TNFα production by human intestinal myocytes, transcriptional (NF-κB dependent) and post-trancriptional (ADAM17 dependent), that can be targeted by pharmacological manipulation.
Acknowledgments
This work was supported in part by a grant (ACIM A03038 GS) from the French Ministère Délégué à la Recherche et aux Nouvelles Technologies. We thank Dr Caroline Colombeix for help with the Metamorph software. We also thank Dr Le Forestier and his Staff from the ‘Photologie' Department for their help.
Abbreviations
- ADAM17
a disintegrin and metalloproteinase-17
- HE
hematoxylin–eosin
- NAC
N-acetylcysteine
- NF-κB
nuclear factor kappa B
- ROS
reactive oxygen species
- TNFα
tumor necrosis factor alpha
References
- ANUP R., APARNA V., PULIMOOD A., BALASUBRAMANIAN K.A. Surgical stress and the small intestine: role of oxygen free radicals. Surgery. 1999;125:560–569. [PubMed] [Google Scholar]
- BAER M., DILLNER A., SCHWARTZ R.C., SEDON C., NEDOSPASOV S., JOHNSON P.F. Tumor necrosis factor alpha transcription in macrophages is attenuated by an autocrine factor that preferentially induces NF-kappaB p50. Mol. Cell. Biol. 1998;18:5678–5689. doi: 10.1128/mcb.18.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK R.A., RAUCH C.T., KOZLOSKY C.J., PESCHON J.J., SLACK J.L., WOLFSON M.F., CASTNER B., STOCKING K., REDDY P., SRINIVASAN S., NELSON N., BOIANI N., SCHOOLEY K.A., GERHART M., DAVIS R., FITZNER J.N., JOHNSON R.S., PAXTON R.J., MARCH C., CERRETTI D.P. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- BLANCHOT-JOSSIC F., JARRY A., MASSON D., BACH-NGOHOU K., PAINEAU J., DENIS M.G., LABOISSE C.L., MOSNIER J.F. Up-regulated expression of ADAM17 in human colon carcinoma: co-expression with EGFR in neoplastic and endothelial cells. J. Pathol. 2005;207:156–163. doi: 10.1002/path.1814. [DOI] [PubMed] [Google Scholar]
- BLOBEL C.P. Remarkable roles of proteolysis on and beyond the cell surface. Curr. Opin. Cell Biol. 2000;12:606–612. doi: 10.1016/s0955-0674(00)00139-3. [DOI] [PubMed] [Google Scholar]
- COLLART M.A., BAEUERLE P., VASSALLI P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol. Cell. Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'HAUTEVILLE H., KHAN S., MASKELL D.J., KUSSAK A., WEINTRAUB A., MATHISON J., ULEVITCH R.J., WUSCHER N., PARSOT C., SANSONETTI P.J. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J. Immunol. 2002;168:5240–5251. doi: 10.4049/jimmunol.168.10.5240. [DOI] [PubMed] [Google Scholar]
- FENOGLIO-PREISER C.M.Non neoplastic lesions of the colon Gastrointestinal Pathology. An Atlas and Text 1999Philadelphia: Lippincott–Raven Press; 795–807.2nd edn., ed. Fenoglio-Preiser, C.M. pp [Google Scholar]
- FERRER J.V., ARICETA J., GUERRERO D., GOMIS T., LARREA M.M., BALEN E., LERA J.M. Allopurinol and N-acetylcysteine avoid 60% of intestinal necrosis in an ischemia–reperfusion experimental model. Transplant. Proc. 1998;30:2672. doi: 10.1016/s0041-1345(98)00784-2. [DOI] [PubMed] [Google Scholar]
- GRISHAM M.B., HERNANDEZ L.A., GRANGER D.N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am. J. Physiol. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- GUEYDAN C., DROOGMANS L., CHALON P., HUEZ G., CAPUT D., KRUYS V. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J. Biol. Chem. 1999;274:2322–2326. doi: 10.1074/jbc.274.4.2322. [DOI] [PubMed] [Google Scholar]
- HURTADO O., CARDENAS A., LIZASOAIN I., BOSCA L., LEZA J.C., LORENZO P., MORO M.A. Up-regulation of TNF-alpha convertase (TACE/ADAM17) after oxygen–glucose deprivation in rat forebrain slices. Neuropharmacology. 2001;40:1094–1102. doi: 10.1016/s0028-3908(01)00035-1. [DOI] [PubMed] [Google Scholar]
- JARRY A., CHARRIER L., BOU-HANNA C., DEVILDER M.C., CRUSSAIRE V., DENIS M.G., VALLETTE G., LABOISSE C.L. Position in cell cycle controls the sensitivity of colon cancer cells to nitric oxide-dependent programmed cell death. Cancer Res. 2004;64:4227–4234. doi: 10.1158/0008-5472.CAN-04-0254. [DOI] [PubMed] [Google Scholar]
- JOBIN C., BRADHAM C.A., RUSSO M.P., JUMA B., NARULA A.S., BRENNER D.A., SARTOR R.B. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999;163:3474–3483. [PubMed] [Google Scholar]
- KONTOYIANNIS D., PASPARAKIS M., PIZARRO T.T., COMINELLI F., KOLLIAS G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- KURTEL H., TSO P., GRANGER D.N. Granulocyte accumulation in postischemic intestine: role of leukocyte adhesion glycoprotein CD11/CD18. Am. J. Physiol. 1992;262:G878–G882. doi: 10.1152/ajpgi.1992.262.5.G878. [DOI] [PubMed] [Google Scholar]
- LE GOFFE C., VALLETTE G., CHARRIER L., CANDELON T., BOU-HANNA C., BOUHOURS J.F., LABOISSE C.L. Metabolic control of resistance of human epithelial cells to H2O2 and NO stresses. Biochem. J. 2002;364:349–359. doi: 10.1042/BJ20011856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C., JACKSON R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- MALLICK I.H., YANG W., WINSLET M.C., SEIFALIAN A.M. Ischemia–reperfusion injury of the intestine and protective strategies against injury. Dig. Dis. Sci. 2004;49:1359–1377. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- MOHLER K.M., SLEATH P.R., FITZNER J.N., CERRETTI D.P., ALDERSON M., KERWAR S.S., TORRANCE D.S., OTTEN-EVANS C., GREENSTREET T., WEERAWARNA K., KROHNHEIM S.R., PETERSEN M., GERHART M., KOZLOSKY C.J., MARCH C.J., BLACK R.A. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–220. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- MOSS M.L., JIN S.L., MILLA M.E., BICKETT D.M., BURKHART W., CARTER H.L., CHEN W.J., CLAY W.C., DIDSBURY J.R., HASSLER D., HOFFMAN CR KOST T.A., LAMBERT M.H., LEESNITZER M.A., MCCAULEY P., MCGEEHAN G., MITCHELL J., MOYER M., PAHEL G., ROCQUE W., OVERTON L.K., SCHOENEN F., SEATON T., SU J.L., WARNER J., WILLARD D., BECHERER J.D. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–736. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- NEUNLIST M., TOUMI F., ORESCHKOVA T., DENIS M., LEBORGNE J., LABOISSE C.L., GALMICHE J.P., JARRY A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am. J. Physiol. 2003;285:G1028–G1036. doi: 10.1152/ajpgi.00066.2003. [DOI] [PubMed] [Google Scholar]
- NEURATH M.F., FUSS I., PASPARAKIS M., ALEXOPOULOU L., HARALAMBOUS S., MEYER ZUM BUSCHENFELDE K.H., STROBER W., KOLLIAS G. Predominant pathogenic role of tumor necrosis factor in experimental colitis in mice. Eur. J. Immunol. 1997;27:1743–1750. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- NICHOLS T.C. NF-kappaB and reperfusion injury. Drug News Perspect. 2004;17:99–104. doi: 10.1358/dnp.2004.17.2.829042. [DOI] [PubMed] [Google Scholar]
- PRIMAKOFF P., MYLES D.G. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- QIU D., ZHAO G., AOKI Y., SHI L., UYEI A., NAZARIAN S., NG J.C., KAO P.N. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J. Biol. Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- RENAUDIN K., DENIS M.G., KARAM G., VALLETTE G., BUZELIN F., LABOISSE C.L., JARRY A. Loss of NOS1 expression in high-grade renal cell carcinoma associated with a shift of NO signalling. Br. J. Cancer. 2004;90:2364–2369. doi: 10.1038/sj.bjc.6601809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUET-BENZINEB P., ROUYER-FESSARD C., JARRY A., AVONDO V., POUZET C., YANAGISAWA M., LABOISSE C., LABURTHE M., VOISIN T. Orexins acting at native OX(1) receptor in colon cancer and neuroblastoma cells or at recombinant OX(1) receptor suppress cell growth by inducing apoptosis. J. Biol. Chem. 2004;279:45875–45886. doi: 10.1074/jbc.M404136200. [DOI] [PubMed] [Google Scholar]
- RUSSELL J., EPSTEIN C.J., GRISHAM M.B., ALEXANDER J.S., YEH K.Y., GRANGER D.N. Regulation of E-selectin expression in postischemic intestinal microvasculature. Am. J. Physiol. 2000;278:G878–G885. doi: 10.1152/ajpgi.2000.278.6.G878. [DOI] [PubMed] [Google Scholar]
- SALINTHONE S., SINGER C.A., GERTHOFFER W.T. Inflammatory gene expression by human colonic smooth muscle cells. Am. J. Physiol. 2004;287:G627–G637. doi: 10.1152/ajpgi.00462.2003. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., VIEIRA A.T., PINHO V., SOUSA L.P., ANDRADE A.A., BONJARDIM C.A., MCMILLAN M., KAHN M., TEIXEIRA M.M. NF-kappaB plays a major role during the systemic and local acute inflammatory response following intestinal reperfusion injury. Br. J. Pharmacol. 2005;145:246–254. doi: 10.1038/sj.bjp.0706190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOUMI F., NEUNLIST M., DENIS M.G., ORESHKOVA T., LABOISSE C.L., GALMICHE J.P., JARRY A. Vasoactive intestinal peptide induces IL-8 production in human colonic epithelial cells via MAP kinase-dependent and PKA-independent pathways. Biochem. Biophys. Res. Commun. 2004;317:187–191. doi: 10.1016/j.bbrc.2004.03.033. [DOI] [PubMed] [Google Scholar]
- YAO J., MACKMAN N., EDGINGTON T.S., FAN S.T. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J. Biol. Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- YEH K.Y., YEH M., GLASS J., GRANGER D.N. Rapid activation of NF-kappaB and AP-1 and target gene expression in postischemic rat intestine. Gastroenterology. 2000;118:525–534. doi: 10.1016/s0016-5085(00)70258-7. [DOI] [PubMed] [Google Scholar]
- ZAFARULLAH M., LI W.Q., SYLVESTER J., AHMAD M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol. Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN B.J., GRANGER D.N. Reperfusion-induced leukocyte infiltration: role of elastase. Am. J. Physiol. 1990;259:H390–H394. doi: 10.1152/ajpheart.1990.259.2.H390. [DOI] [PubMed] [Google Scholar]