Abstract
Loss of sympathetic input due to intestinal denervation results in hypersensitivity and increased intestinal secretion. It is unknown whether denervation-induced alterations in intestinal epithelial physiology are the result of changes in adrenoceptors on enterocytes (ENTs).
The purpose of this study was to examine adrenoceptor distribution and pharmacology on small intestinal ENTs following acute intestinal denervation.
Lewis rats underwent small bowel transplantation (SBT) or sham operation and proximal small intestinal segments were harvested 1, 2 and 4 weeks postoperatively. Intestinal electrolyte movement was assessed using short-circuit current (Isc) measurements of stripped epithelial sheets following stimulation with phenylephrine (PE), an α1-adrenoceptor agonist. The presence of adrenoceptor subtypes on separated villus and crypt ENTs was assessed using flow cytometry.
α1-Adrenoceptors were found on approximately 27% of jejunal villus ENTs, but not crypt ENTs, following acute extrinsic denervation. ENTs from the Lewis rat have few β-adrenoceptors.
α1-Adrenoceptor stimulation of acutely denervated intestinal epithelial sheets decreased Isc by −13.45%. This effect was mediated by a reduction in chloride (Cl−) secretion; the absence of Cl− reversed the Isc to +13.79%.
In conclusion, loss of sympathetic innervation to the gastrointestinal epithelium causes acute upregulation of α1-adrenoceptors on villus ENTs, leading to inhibition of Cl− secretion at the villus tip. The increase in adrenoceptors may reflect a compensatory mechanism to combat the increased secretory state of the bowel due to the loss of the sympathetic innervation and tonic control over intestinal secretion.
Keywords: Enterocyte, adrenoceptors, denervation, small bowel transplantation, flow cytometry, short-circuit current
Introduction
Under physiological conditions, the intestine is able to maintain homeostatic control of absorption and secretion through input from epithelial cells, intestinal smooth muscle, mesenchymal and immune cells and the enteric and autonomic nervous systems (Hubel, 1989; Chang & Rao, 1994). Sympathetic innervation from the autonomic nervous system to the small intestine is completely extrinsic (Keast et al., 1984; Cooke & Reddix, 1994) and regulates fluid and electrolyte transport by providing tonic inhibition of secretory fluxes and mediating an absorptive response via adrenoceptors (Oishi & Sarr, 1995).
Sympathetic regulation of intestinal function is crucial when this innervation to the gut is disrupted. Sympathetic denervation has adverse consequences on the ability of the small intestine to regulate absorptive physiology. We and others have demonstrated that loss of sympathetic innervation, such as following small bowel transplantation (SBT), is associated with intestinal complications such as changes in motility and disturbances in intestinal secretion and/or absorption (Deltz et al., 1987; Sigalet et al., 1992a, 1992b; Shibata et al., 1998). The transplant process extrinsically denervates the small intestine without major changes in glucose transport (Sigalet et al., 1992a, 1992b) or the absorption of fat or carbohydrates (Sigalet et al., 1996). However, the extrinsically denervated small intestine exhibits a marked reduction in in vivo water and electrolyte absorption, an increase in chloride (Cl−) secretion and hypersensitivity to catecholamines (Watson et al., 1988; Herkes et al., 1994; Oishi & Sarr, 1995). Application of norepinephrine or clonidine, an α2-adrenoceptor agonist, to ileal epithelial sheets in the extrinsically denervated rat causes a significant increase in the intestinal response and can reverse secretion to absorption (Chang et al., 1986), possibly due to a compensatory increase in adrenoceptors (Chang et al., 1986; Oishi & Sarr, 1995).
However, the epithelial distribution of adrenoceptors following extrinsic denervation is currently unknown and the relationship between sympathetic stimulation and ion movement across the intestinal mucosa remains vague. Therefore, we sought to test the hypothesis that denervation-induced alterations in Cl− secretion are mediated through an increase in adrenoceptors on intestinal epithelial cells.
Methods
Chemicals
BODIPY FL prazosin and BODIPY FL CGP 12177 were obtained from Molecular Probes Inc. (Eugene, OR, U.S.A.) and dissolved in dimethylsulfoxide. All other chemicals were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.) unless otherwise indicated.
Animals
Syngeneic male Lewis rats weighing 225–250 g (Charles River Canada, St Constant, Quebec, Canada) were used for the transplant (denervation) procedure to avoid confounding immunological complications or the need for immunosuppressive therapy. All animals were acclimatized for 7 days in individual Plexiglas cages prior to surgeries, fed Purina standard rat chow (Purina Co., St Louis, MO, U.S.A.) and given free access to water. Animals were fasted overnight before any surgical procedures, all of which were conducted under the guidelines established by the Canadian Council on Animal Care (Canadian Council on Animal Care, 1984) and with the approval of the Animal Welfare Committee at the University of Calgary.
Surgeries
Sham operation
Rats were anesthetized with halothane (1–2% inhalation by mask) and a midline laparotomy was performed. The bowel was divided 1 cm distal to the ligament of Treitz and 5 cm proximal to the ileocecal valve. With the aid of an operating microscope, anastomoses were performed using interrupted sutures of 6–0 silk and the abdomen was closed with 4–0 Vicryl. Animals were given buprenorphine 0.1 mg kg−1 subcutaneously twice daily (b.i.d.) for 48 h for pain, allowed water immediately postoperatively and food was returned on the second postoperative day. In these sham-operated controls, all innervation remained intact.
Transplant procedure
The transplant procedure was performed as a one-stage procedure. Briefly, animals were anesthesized and the donor small intestine (jejuno-ileum) was isolated. The small intestine was quickly removed and stored in ice-cold lactated Ringer's solution until placed in the recipient. Lactated Ringer's solution is a balanced and isotonic solution containing sodium, Cl−, calcium, potassium and lactate, which provide electrolytes in the same composition to that of extracellular fluid. The transplanted bowel was revascularized based on the infrarenal aorta and vena cava using standard microsurgical techniques. Closure and postoperative care of the transplanted animals was identical to that of the sham-operated control group. The small intestine was retrieved 1, 2 or 4 weeks postoperative following euthanization by intraperitoneal injection of Somnotol.
Naïve controls
Segments of proximal small intestine were harvested from naïve animals that had not undergone any surgical procedure. The small intestine was retrieved following euthanization by intraperitoneal injection of Somnotol and experiments using these intestinal segments were conducted immediately upon removal from the animal.
Intestinal epithelial cell isolation
Epithelial cell isolation was performed as previously described for guinea-pig and rat intestinal epithelial cells (Weiser, 1973; Lang et al., 1996; Baglole et al., 2005). Briefly, segments of proximal intestine were immersed in warm (37°C) phosphate-buffered saline (PBS). The intestinal segment was cannulated and filled with oxygenated citrate buffer to a pressure of 50 cm of H2O; this intraluminal pressure is within a physiological range for the small intestine (Hirokawa et al., 1997). The segment was immersed in 0.15 M sodium chloride (NaCl) at 37°C for 15 min, the luminal fluid discarded, refilled in the same manner with oxygenated buffer containing EDTA and again immersed in 0.15 M NaCl for 5 min. The luminal contents, containing the eluted fractions of villus epithelial cells, were sequentially collected by drainage for a total of four times. Following this, the intestinal segment was refilled with EDTA buffer, incubated in 0.15 M NaCl at 37°C for one 7-min and two 10-min intervals and the luminal fluid discarded. Crypt epithelial cells were collected by refilling the segment with EDTA buffer and incubating in 0.15 M NaCl at 37°C for 10 min and eluting the fluid. These fractions have previously been determined to represent villus tip and crypt epithelial cells (Lang et al., 1996; Baglole et al., 2005).
Once isolated, the villus and crypt cell fractions were pelleted by centrifugation (1200 r.p.m. for 5 min) and rinsed twice with ice-cold Krebs buffer. After the second wash, villus and crypt cells were reconstituted in 1 ml of Krebs buffer and incubated with 50 μl of DNAse for 30 min at room temperature to minimize cell clumping. The cells were rinsed and filtered twice, first through 40 μM Falcon® cell strainers (Becton Dickinson Labware, Franklin Lakes, NJ, U.S.A.) and then through 30 μM nylon mesh (Small Parts Inc., Miami Lakes, FL, U.S.A.). Upon filtering, the cells were rinsed repeatedly with Krebs buffer to reduce mucus and brought to a final volume of 500 μl at which time they were distributed into 5 ml polystyrene Falcon® tubes (Becton Dickinson Labware, Franklin Lakes, NJ, U.S.A.) for the binding experiments.
Receptor binding
Total binding was determined by incubating whole-cell suspensions with increasing concentrations of the fluorescent probes for α1- and β-adrenoceptors (BODIPY FL prazosin and BODIPY FL CGP12177, respectively) in a light-protected environment for 45 min at room temperature. The lowest concentration that gave maximal binding was used for all subsequent experiments (α1-adrenoceptors: 4 μM, sham; 2 and 4 μM, transplant villus and crypt, respectively. β-Adrenoceptors: 4 μM, transplant; 6 and 4 μM, villus and crypt, respectively).
To examine specific binding sites, we evaluated the ability of specific receptor antagonists to prevent binding of the fluorescent probes by preincubating the cells with excess (1 mM) unlabelled prazosin (α1-adrenoceptor antagonist) (Molecular Probes Inc., Eugene, OR, U.S.A.) or propranolol (β-adrenoceptor antagonist) followed by incubation with the fluorescent probe. Both prazosin and propranolol are well established and widely referenced antagonists for their respective receptors (Reid & Vincent, 1986; Fukuda et al., 2001). Cells were preincubated with the unlabelled ligand for 30 min at room temperature followed by incubation with the fluorescent probe for 45 min. Specific (displaceable) binding was determined as the difference between total binding and nonspecific binding and taken to indicate the presence of receptor binding sites. Negative controls included incubation of cells with buffer or unlabelled ligand.
Once binding experiments were complete, cells were washed with Krebs buffer, brought to a final volume of 500 μl and 10,000 cells/sample were acquired and analyzed using a Becton-Dickinson FACScan and Cell Quest software V3.1f (Becton Dickinson Immunocytometry Systems, San Jose, CA, U.S.A.).
Sucrase assay
Sucrase is a brush border enzyme that is high within the villus region but has little enzymatic activity in the crypts (Traber, 1999). Sucrase activity was therefore assessed in villus and crypt epithelial cells isolated from the small bowel to ensure adequately pure separation between the cell fractions according to the colorimetric assay described by Dahlqvist (1964) and previously used by us to assess villus and crypt intestinal epithelial cell maturity (Lang et al., 1996). Briefly, villus and crypt epithelial cells were isolated, protein content was determined and the cells diluted in PBS to 0.4 mg ml−1 protein. Samples were incubated with sucrose substrate (0.056 M) (100 μl of each) and TGO reagent (0.3 ml of glucose oxidase in 50 ml Tris buffer, 0.5 ml peroxidase, 0.5 ml o-diansidine, 1 ml Triton; diluted to 100 ml Tris buffer) and incubated at 37°C for 60 min. Negative controls (blanks) consisted of adding the sample to the sucrose substrate and immediately placing them in boiling water for 2 min followed by addition of the TGO reagent. As an estimate of sucrase activity, the production of glucose was measured using a spectrophotometer at a wavelength of 420 nm and expressed per gram of protein. Results are expressed as unit of activity per gram of protein.
Ussing chamber analysis
Ussing chambers were used to assess changes in electrogenic ion movement in intestinal tissue. In all, 10-cm segments immediately distal to the segment used for the cell isolation were flushed with oxygenated (5% CO2–95% O2) ice-cold Krebs buffer. This portion of the jejunum was placed over a glass rod, partially scored along the mesenteric border and the external muscle layers stripped away with fine forceps. The remaining tissue was opened and the epithelial sheets were rinsed in ice-cold Krebs and mounted in standard Ussing chambers. Once mounted, the tissue was bathed in 4 ml of buffer 1 (in mM: NaCl, 107; KCl, 4.5, NaHCO3, 25; CaCl2, 1.25; Na2HPO4, 1.8; NaH2PO4, 0.2; glucose, 12; pH 7.4) or low Cl− buffer. For the low Cl− buffer, the composition was the same as for buffer 1, except NaCl was replaced with sodium gluconate and the Ca2+ was elevated to 5.8 mM (Diener et al., 1996). The chambers were maintained at 37°C and buffers were aerated and mixed using a gas lift system (5% CO2–95% O2).
Once the tissue was mounted and the buffer added, the tissue was allowed to stabilize for 20–25 min before further manipulation. Tissue responses were measured by clamping the potential voltage (PD) to 0 mV by applying a short-circuit current (Isc) with a voltage clamp apparatus (EVC 4000, World Precision Instruments, Sarasota, FL, U.S.A.) (Vergnolle et al., 1998). To determine the Isc response to adrenoceptor stimulation, phenylephrine (PE) was dissolved in buffer 1 or low Cl− buffer and added to both sides of the tissue at a final concentration of 10 mM. Forskolin, which causes cAMP-induced Cl−secretion (10 mM stock dissolved in ethanol) was added to the serosal surface at the end of these experiments to ensure tissue viability. Tissue that did not exhibit at least a 20% change in Isc due to the addition of forskolin was not used. In other experiments, 20 mM glucose was also added to the mucosa surface. Once drugs were added to the tissue, the change in Isc was monitored and taken as an indicator of net active electrolyte transport (Montrose et al., 1999). The Isc was recorded and analyzed with Acq-Knowledge software (version 3.1.3, BioPac).
Statistical analysis
All data are presented as the mean±s.e.m. Statistics and graphs were generated using GraphPad Prism, version 3.00 (GraphPad Software Inc., San Diego, CA, U.S.A.). Statistical analysis between two samples was performed using a Student's t-test. Statistical comparison of more than two groups was performed using one-way ANOVA with Tukey's multiple comparison post-test. In all cases, a P-value of <0.05 was considered to be significant.
Results
High sucrase activity in isolated villus, but not crypt, intestinal epithelial cells
Since sucrase is high within the villus region but not the crypt (Traber, 1999), sucrase activity was measured in villus and crypt cells isolated from the jejunum of control and denervated animals to confirm the purity of the cellular fractions. Intestinal epithelial cells from control jejunum exhibited high levels of sucrase activity only in cells from the villus fraction and nearly undetectable levels in the crypt cells (22.0±11.1 versus 3.48±1 U g−1 protein, respectively). Similar results were obtained for the cell fractions isolated from the jejunum of transplanted animals where there was higher sucrase activity in the villus as compared to the crypt fraction (9.4±3.5 versus 1.5±0.28 U g−1 protein, villus and crypt, respectively). There was no significant difference in sucrase activity between control and denervated villus and crypt epithelial cells. Therefore, the cell isolation technique used in this study allows for the separation of distinct villus and crypt cell fractions.
Flow cytometric identification of enterocytes (ENTs) and intraepithelial lymphocytes (IEL)
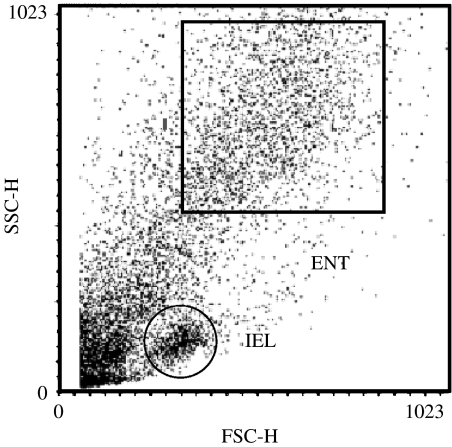
Intestinal epithelial cells are a heterogeneous mixture of cells. The majority of intestinal epithelial cells are ENTs and we have previously used an intestinal epithelial cell isolation technique (Weiser, 1973) in conjunction with flow cytometry to identify receptors on ENTs from the small intestine of guinea-pigs (Lang et al., 1996; Baglole et al., 2005). We have previously characterized the other major population of intestinal epithelial cells as IELs (Figure 1) based on known immunological markers (Lang et al., 1996); this cell fraction was not analyzed further in this study. Therefore, all binding studies were performed using the population of epithelial cells previously identified as ENTs. Flow cytometry dot plots of epithelial cells from the small intestine of sham-operated controls or transplant recipients were similar and gating within the ENT region identical.
Figure 1.
Representative dot-plot profile of villus intestinal epithelial cells isolated from the proximal small intestine of the Lewis rat. The forward scatter of light (FSC-H) is a measure of increasing cell size and the side scatter of light (SSC-H) is a measure of increasing cell granularity (values are in arbitrary units). The box, ENT, represents enterocytes and all binding experiments were conducted from cells within this region. IEL: intraepithelial lymphocytes.
Acute extrinsic denervation increases α1-adreneroceptors on villus ENTs
To determine which adrenoceptor subtype may mediate a physiological response, we examined isolated villus and crypt ENTs for the presence of α1- and β-adrenoceptors. Although binding of BOPDIPY FL CGP 12177 occurred on villus and crypt ENTs from both sham-operated controls and transplant recipients, attempts to displace this binding by propranolol was unsuccessful, suggesting that there are very few β-adrenoceptors on ENTs from the Lewis rat.
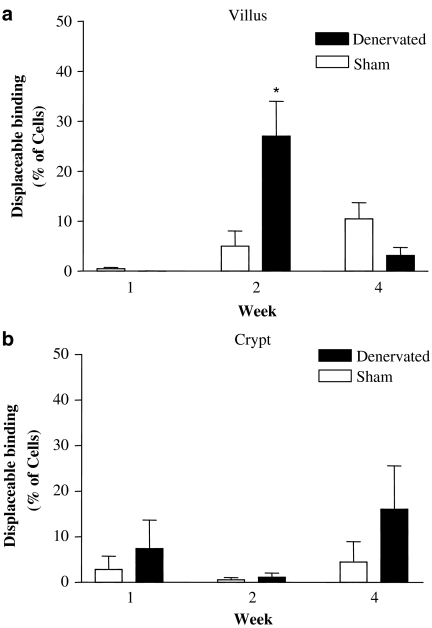
We next examined the distribution of α1-adrenoceptors on villus and crypt ENTs following SBT. Competition experiments revealed that binding of the fluorescent α1-adrenoceptor probe BODIPY FL prazosin to villus ENTs in the denervated gut at week 2 could be substantially reduced with unlabelled prazosin (Figure 2). At the highest concentration of prazosin used, 1 mM, displaceable binding was observed on a subset of these villus ENTs, 27±6.7% of the cells (Figure 2a), indicating that extrinsic denervation augments α1-adrenoceptor expression on a specific subset of intestinal epithelial cells. The amount of displaceable binding on villus ENTs from the denervated intestine at weeks 1 and 4 on the other hand was very low, occurring on only 0.0 (week 1) and 3.14±1.6% (week 4) of cells. Displaceable binding on villus ENTs from the innervated controls was also very low; here specific binding was between 0.45±0.27% (week 1) and 10.4±3.2% (week 4) of the cells.
Figure 2.
Percentage of (a) villus and (b) crypt ENTs isolated from the small intestine of Lewis rats exhibiting specific (displaceable) binding for α1-adrenoceptor. (a) Villus ENTs from the denervated gut have a higher proportion of cells with α1-adrenoceptors at week 2 compared with weeks 1 and 4 and at any time point in the sham (*P<0.05). (b) Displaceable binding of the α1-adrenoceptor probe BODIPY FL prazosin from crypt ENTs was low and not significantly different between the experimental groups at any time point. Results are expressed as mean±s.e.m., n=3–5 animals.
Prazosin was unable to displace the fluorescent α1-adrenoceptor probe on crypt ENTs (Figure 2b). Here, displaceable binding to crypt ENTs from the proximal small bowel of both experimental groups was negligible, ranging between 0.45±0.27 and 16.08 ±9.46% of cells, indicating that acute extrinsic denervation does not alter the proportion of crypt ENTs with α1-adrenoceptors.
Acute extrinsic denervation alters the epithelial response to α1-adrenoceptor stimulation
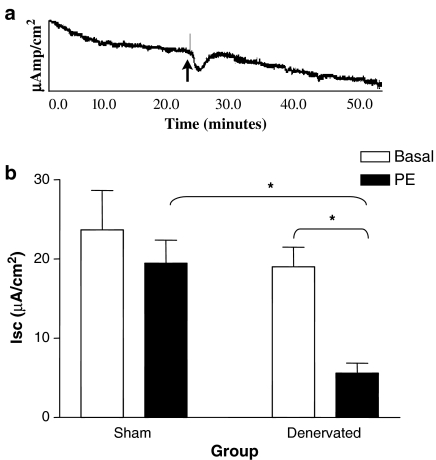
Sympathetic denervation induces a profound change in electrolyte movement across the epithelium (Chang et al., 1986; Oishi & Sarr, 1995) and decreases Isc (Field & McColl, 1973; Hildebrand & Brown, 1992). To determine if acute denervation altered the epithelial response to α1-adrenoceptor stimulation, we mounted intestinal epithelial sheets from control and denervated animals in Ussing chambers and assessed alterations in electrogenic ion movement. Administration of PE, a specific α1-adrenoceptor agonist, to the apical and basolateral surfaces of epithelial sheets from the denervated jejunum 2 weeks postoperatively induced a rapid and significant decrease in Isc (Figure 3a). This decrease was not observed in jejunum from sham-operated animals (Figure 3b). Note that the baseline Isc values were not significantly different between denervated and sham-operated (innervated) controls. Further, there was no significant change in Isc at weeks 1 and 4 in either experimental group (data not shown). Clearly, at the time point where there is an increased presence of α1-adrenoceptors on villus ENTs, there is a corresponding alteration in intestinal epithelial physiology.
Figure 3.
Phenylephrine induced α1-adrenoceptor stimulation induces a significant decrease in Isc in denervated, but not sham-operated, jejunum at week 2. (a) Representative trace of the Isc response (μamp/cm2) to α1-adrenoceptor stimulation by phenylephrine (PE) of epithelial sheets from denervated jejunum 2 weeks postoperative. Arrow indicates the time at which PE was added to the apical and basolateral surfaces. (b) Basal Isc values were not significantly different between control and denervated intestine. There was a significant decrease in Isc after administration of PE in the denervated gut at 2 weeks postoperative that was not observed in the innervated controls (ΔIsc=−4.2±2.75). *P<0.05 for both the difference from baseline at week 2 and for the change in Isc between the sham and transplant intestine at week 2. Results are expressed as mean±s.e.m., n=4.
Acute extrinsic denervation does not alter sodium absorption
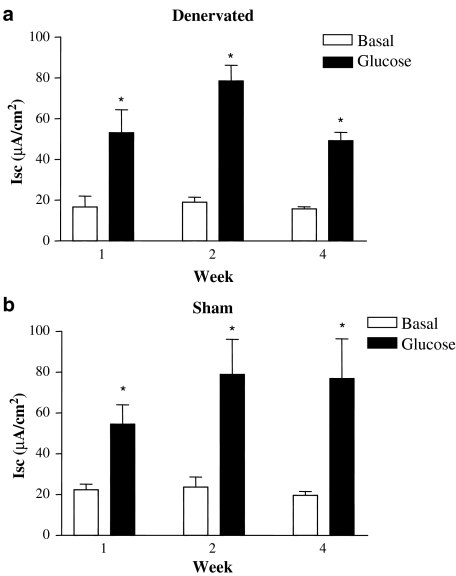
To elucidate the ionic basic for the deflection in Isc, we first determined if an increase in sodium absorption could account for the decrease in Isc by the addition of glucose. The physiological response to apical addition of glucose is electrogenic absorption of sodium (and glucose) through the sodium/glucose-linked transporter-1. Addition of glucose to the apical surface prompted a significant increase in the Isc response in both experimental groups (Figure 4). However, there was no significant difference in the magnitude of this response at any time point or between experimental groups, indicating that acute denervation does not alter the uptake of sodium ions.
Figure 4.
Intestinal denervation does not alter sodium absorption. There was a significant increase in the Isc response upon addition of apical glucose to epithelial sheets from both (a) denervated and (b) sham-operated animals. *P<0.05 represents the difference from baseline. There was no significant difference between the two experimental groups at any time point. Results are expressed as mean±s.e.m., n=4–5.
α1-Adrenoceptor stimulation in the acutely denervated small intestine alters Cl− secretion
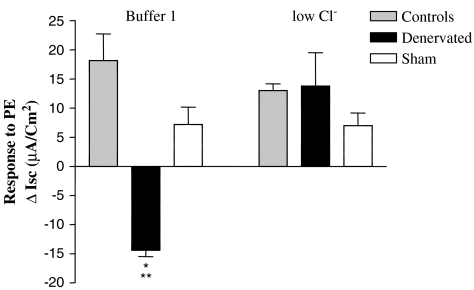
To determine whether the observed change in Isc following α1-adrenoceptor stimulation was the result of Cl− movement, we next compared the Isc response to PE using a physiological standard buffer (buffer 1) or low Cl− buffer. Addition of 10 mM PE to tissue segments from the denervated gut at week 2 bathed in buffer 1 again caused a significant decrease in Isc (Isc=−14.39±1.08 for six separate experiments). However, this PE-induced decrease in Isc in the denervated gut was reversed when the tissue was bathed in low Cl− buffer (Figure 5).
Figure 5.
Phenylephrine-induced α1-adrenoceptor activation inhibits Cl− secretion in the denervated small intestine. The PE-induced decrease in Isc of denervated gut is significantly reversed in low Cl− buffer (**P<0.001). The decrease in Isc in the denervated gut bathed in buffer 1 was significantly different than both controls groups bathed in either buffer (*P<0.05). The PE-induced change in Isc of tissue from either naïve (controls) or sham-operated (Sham) controls was the same, regardless of the buffer. Results are expressed as mean±s.e.m., n=3–10.
To be sure that the alterations in Cl− movement were in fact the result of denervation, we also examined the response to α1-adrenoceptor stimulation of intestinal segments from both naïve and sham-operated controls in either buffer 1 or low Cl− buffer 1. The response to PE in these experimental controls remained unchanged in low Cl− buffer (Figure 5). Collectively, these data strongly indicate that acute extrinsic denervation causes a transient and compensatory increase in α1-adrenoceptors only on villus ENTs, resulting in a decrease in Cl− movement across the villus tip.
Discussion
Sympathetic regulation of intestinal function is critical in maintaining a balance between absorption and secretion. Few studies have directly addressed the effect that sympathetic denervation has on intestinal epithelial function. Through a unique combination of receptor–ligand interactions and in vitro pharmacological characterization of intestinal secretion, we have demonstrated for the first time that sympathetic denervation alters the intestinal epithelial response to α1-adrenoceptor stimulation through increased α1-adrenoceptors located on villus ENTs. Activation of these ENT α1-adrenoceptors significantly altered Cl− movement across the mucosa of the denervated jejunum, a novel finding.
An increase in adrenoceptor numbers on smooth muscle cells was proposed to explain the hypersensitivity of norepinephrine on intestinal smooth muscle contractility (Shibata et al., 1997; Ohtani et al., 2000). Following intestinal denervation, there is also a decrease in the muscarinic M1 receptor and an increase in the muscarinic M3 receptor subtype (Stadelmann et al., 1998). As transmural homogenates were used in that study, the presence of muscarinic receptors within the epithelium could not be determined. Difficulty in assessing receptors in the intestinal mucosa is due to the heterogeneous mixture of epithelial cells. We have modified an intestinal epithelial cell isolation technique (Lang et al., 1996; Baglole et al., 2005) and, in combination with flow cytometry, were able to show that there is a significant increase in α1-adrenoceptors on villus ENTs 2 weeks postdenervation. The results presented herein are the first to address a significant gap in the literature by examining changes in receptor distribution within specific populations of intestinal epithelial cells.
We have demonstrated that extrinsic sympathetic denervation increases α1-adrenoceptors on villus ENTs and that stimulation of these receptors alters Cl− movement at 2 weeks but not at 4 weeks. This transient expression of α1-adrenoceptors may be the result of sympathetic reinnervation to the transplanted gut. Kiyochi et al. (1994) have demonstrated that sympathetic nerve fibers are present as early as 3 weeks post-transplantation in the Lewis rat, indicating that the reinnervation process has already begun. Further, a response to sympathetic nerve stimulation can reappear 3 weeks after denervation even when the reinnervation is minimal (Hill et al., 1985). The decrease in α1-adrenoceptors by 4 weeks postdenervation suggests that the transient alterations in α1-adrenoceptor distribution may be the result of reinnervation to the small intestine.
We next sought to determine if there was a corresponding functional significance to the increase in villus enterocyte α1-adrenoceptors. We initially determined that α1-adrenoceptor stimulation significantly decreased Isc following acute denervation. Multiple transport pathways involved in ion movement exist in the proximal small intestine and a decrease in Isc could be due to a decrease in Cl− (Vieira-Coelho & Soares-da-Silva, 1998). We examined the α1-adrenoceptor response to epithelial tissue that was bathed in low Cl− buffer. The reduction of Cl− reversed the PE-induced Isc response only in the group of animals that had undergone extrinsic denervation at the 2-week time point, indicative of an ephemeral decrease in Cl− absorption. Transient alterations in the intestinal physiology following extrinsic denervation have been observed in the canine jejunum (Herkes et al., 1994), ileum (Tsiotos et al., 2001) and colon (Kendrick et al., 2001, 2002) as well as the rat jejunum (Balsiger & Sarr, 2003). The resolution of the absorptive capacity in the small and large intestine may reflect adaptation (Kendrick et al., 2001), and serve as a compensatory mechanism to combat the heightened secretory state of the small bowel. Sympathetic innervation regulates fluid and electrolyte transport by providing tonic inhibition of secretory fluxes and mediates a proabsorptive response via adrenoceptors (Carey & Zafirova, 1990). Although denervation would ablate neuronal sources of epinephrine and norepinephrine, the augmented adrenoceptors on the villus ENTs at 2 weeks postdenervation may be responding to catecholamines produced by nonneuronal cell bodies within the gastrointestinal tract (Eisenhofer et al., 1997).
A major finding of this study is that an inhibition of Cl− secretion can occur at the villus tip, a novel and controversial finding. Cl− secretion in the intestine has long been thought to occur through one major pathway: the cAMP/cGMP-regulated CFTR Cl− channel (Grubb, 1995), which is localized primarily to the apical membrane of crypt ENTs, with little expression found in the villus epithelium (Ameen et al., 2000). However, both the villus and crypt regions may be involved in the secretory response (Gunter-Smith & White, 1979; Donowitz & Madara, 1982; Stewart & Turnberg, 1989; Butt et al., 1998). It has been recently demonstrated that apical ClC-2 Cl− channels are expressed by the villus epithelium in the murine ileum (Gyomorey et al., 2000) and are capable of contributing to Cl− secretion (Gyomorey et al., 2000; Mohammad-Panah et al., 2001). ClC-2 Cl− channels may be responsible for the α1-adrenoceptor-induced inhibition of Cl− secretion at the villus tip following acute extrinsic denervation; this pathway is the subject of current investigation.
In summary, the results presented herein are the first to demonstrate that denervation hypersensitivity increases ENT α1-adrenoceptors. We have shown that acute denervation upregulates α1-adrenoceptors only on villus ENTs. Second, we were able to characterize functionally the ionic basis behind the in vitro secretory response following acute denervation and demonstrate that Cl− movement across the mucosa is altered. Collectively, these data suggest that the loss of sympathetic innervation to the intestinal epithelium causes acute upregulation of α1-adrenoceptors only on villus ENTs, ultimately leading to an inhibition of Cl− secretion at the villus tip. The increase in adrenoceptors in the intestinal epithelium may reflect the development of a compensatory mechanism to combat the increased secretory state of the bowel due to the loss of the sympathetic innervation and tonic control over intestinal secretion. A heightened understanding of intestinal epithelial physiology and its local regulatory mechanisms may eventually lead to new and novel pharmacological agents to combat intestinal secretory diseases.
Acknowledgments
We like to acknowledge the technical assistance of D. Kirk. CJB was supported by a Natural Sciences and Engineering Council of Canada postgraduate scholarship. This work was supported by the CIHR.
Abbreviations
- b.i.d
twice daily
- Cl−
chloride
- ENT
enterocyte
- IEL
intraepithelial lymphocyte
- Isc
short-circuit current
- NaCl
sodium chloride
- PBS
phosphate-buffered saline
- PD
potential voltage
- PE
phenylephrine
- SBT
small bowel transplantation
References
- AMEEN N., ALEXIS J., SALAS P. Cellular localization of the cystic fibrosis transmembrane conductance regulator in mouse intestinal tract. Histochem. Cell Biol. 2000;114:69–75. doi: 10.1007/s004180000164. [DOI] [PubMed] [Google Scholar]
- BAGLOLE C.J., DAVISON J.S., MEDDINGS J.B. Epithelial distribution of neural receptors in the guinea pig small intestine. Can. J. Physiol. Pharmacol. 2005;83:389–395. doi: 10.1139/y05-024. [DOI] [PubMed] [Google Scholar]
- BALSIGER B.M., SARR M.G. Chronic extrinsic denervation of the small bowel: effect on adrenergic and cholinergic contractile mechanisms in canine ileal circular muscle. Surgery. 2003;134:783–790. doi: 10.1016/s0039-6060(03)00255-1. [DOI] [PubMed] [Google Scholar]
- BUTT A.G., BOWLER J.M., MCLAUGHLIN C.W. Villus and crypt cell composition in the secreting mouse jejunum measured with X-ray microanalysis. J. Membr. Biol. 1998;162:17–29. doi: 10.1007/s002329900338. [DOI] [PubMed] [Google Scholar]
- CANADIAN COUNCIL ON CARE . Guide to the Care and Use of Experimental Animals. Ottawa: Canadian Council on Animal Care; 1984. [Google Scholar]
- CAREY H.V., ZAFIROVA M. Adrenergic inhibition of neurally evoked secretion in ground squirrel intestine. Eur. J. Pharmacol. 1990;181:43–50. doi: 10.1016/0014-2999(90)90243-y. [DOI] [PubMed] [Google Scholar]
- CHANG E.B., FEDORAK R.N., FIELD M. Experimental diabetic diarrhea in rats. Intestinal mucosal denervation hypersensitivity and treatment with clonidine. Gastroenterology. 1986;91:564–569. [PubMed] [Google Scholar]
- CHANG E.B., RAO M.C.Intestinal water and electrolyte transport: mechanisms of physiological and adaptive responses Physiology of the Gastrointestinal Tract 1994New York: Raven Press; 2027–2081.ed. Johnson, L.R., Alpers, D.H., Christensen, J., Jacobson, E.D. & Walsh, J.H. pp [Google Scholar]
- COOKE H.J., REDDIX R.A.Neural regulation of intestinal electrolyte transport Physiology of the Gasotrintestinal Tract 1994New York: Raven Press; 2083–2132.ed. Johnson, L.R., Alpers, D.H., Christensen, J., Jacobson, E.D. & Walsh, J.H. pp [Google Scholar]
- DAHLQVIST A. Method for assay of intestinal disaccharidases. Anal. Biochem. 1964;57:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- DELTZ E., GEBHARDT J.H., PREISSNER C., SCHROEDER P., HANSMANN M.L., KAISERLING E., MULLER-HERMELINK H.K., THIEDE A. Distribution of gastrointestinal hormones in the adaptive response after small bowel transplantation. Gut. 1987;28 (Suppl):217–220. doi: 10.1136/gut.28.suppl.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIENER M., BERTOG M., FROMM M., SCHARRER E. Segmental heterogeneity of swelling-induced Cl− transport in rat small intestine. Pflugers Arch. 1996;432:293–300. doi: 10.1007/s004240050136. [DOI] [PubMed] [Google Scholar]
- DONOWITZ M., MADARA J.L. Effect of extracellular calcium depletion on epithelial structure and function in rabbit ileum: a model for selective crypt or villus epithelial cell damage and suggestion of secretion by villus epithelial cells. Gastroenterology. 1982;83:1231–1243. [PubMed] [Google Scholar]
- EISENHOFER G., ANEMAN A., FRIBERG P., HOOPER D., FANDRIKS L., LONROTH H., HUNYADY B., MEZEY E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 1997;82:3864–3871. doi: 10.1210/jcem.82.11.4339. [DOI] [PubMed] [Google Scholar]
- FIELD M., MCCOLL I. Ion transport in rabbit ileal mucosa. 3. Effects of catecholamines. Am. J. Physiol. 1973;225:852–857. doi: 10.1152/ajplegacy.1973.225.4.852. [DOI] [PubMed] [Google Scholar]
- FUKUDA N., JAYR C., LAZRAK A., WANG Y., LUCAS R., MATALON S., MATTHAY M.A. Mechanisms of TNF-alpha stimulation of amiloride-sensitive sodium transport across alveolar epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L1258–L1265. doi: 10.1152/ajplung.2001.280.6.L1258. [DOI] [PubMed] [Google Scholar]
- GRUBB B.R. Ion transport across the jejunum in normal and cystic fibrosis mice. Am. J. Physiol. 1995;268:G505–G513. doi: 10.1152/ajpgi.1995.268.3.G505. [DOI] [PubMed] [Google Scholar]
- GUNTER-SMITH P.J., WHITE J.F. Contribution of villus and intervillus epithelium to intestinal transmural potential difference and response to theophylline and sugar. Biochim. Biophys. Acta. 1979;557:425–435. doi: 10.1016/0005-2736(79)90340-7. [DOI] [PubMed] [Google Scholar]
- GYOMOREY K., YEGER H., ACKERLEY C., GARAMI E., BEAR C.E. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am. J. Physiol. Cell. Physiol. 2000;279:C1787–C1794. doi: 10.1152/ajpcell.2000.279.6.C1787. [DOI] [PubMed] [Google Scholar]
- HERKES S.M., SMITH C.D., SARR M.G. Jejunal responses to absorptive and secretory stimuli in the neurally isolated jejunum in vivo. Surgery. 1994;116:576–586. [PubMed] [Google Scholar]
- HILDEBRAND K.R., BROWN D.R. Norepinephrine and alpha-2 adrenoceptors modulate active ion transport in porcine small intestine. J. Pharmacol. Exp. Ther. 1992;263:510–519. [PubMed] [Google Scholar]
- HILL C.E., HIRST G.D., NGU M.C., VAN HELDEN D.F. Sympathetic postganglionic reinnervation of mesenteric arteries and enteric neurones of the ileum of the rat. J. Auton Nerv. Syst. 1985;14:317–334. doi: 10.1016/0165-1838(85)90079-7. [DOI] [PubMed] [Google Scholar]
- HIROKAWA M., MIURA S., SHIGEMATSU T., YOSHIDA H., HOKARI R., HIGUCHI H., KUROSE I., KIMURA H., SAITO H., NAKAKI T., ISHII H. Pressure stimulates proliferation and DNA synthesis in rat intestinal epithelial cells. Life Sci. 1997;61:667–672. doi: 10.1016/s0024-3205(97)00531-6. [DOI] [PubMed] [Google Scholar]
- HUBEL K.A.Control of intestinal secretion Gastrointestinal Secretion 1989London: Butterworth & Co; 178–201.ed. Davison, J.S. pp [Google Scholar]
- KEAST J.R., FURNESS J.B., COSTA M. Origins of peptide and norepinephrine nerves in the mucosa of the guinea pig small intestine. Gastroenterology. 1984;86:637–644. [PubMed] [Google Scholar]
- KENDRICK M.L., MEILE T., ZYROMSKI N.J., TANAKA T., LIBSCH K.D., SARR M.G. Extrinsic denervation causes a transient proabsorptive adrenergic hypersensitivity in the canine proximal colon. Dig. Dis. Sci. 2002;47:1752–1757. doi: 10.1023/a:1016436310180. [DOI] [PubMed] [Google Scholar]
- KENDRICK M.L., MEILE T., ZYROMSKI N.J., TANAKA T., SARR M.G. Extrinsic neural innervation mediates absorption of water and electrolytes in canine proximal colon in vivo. J. Surg. Res. 2001;97:76–80. doi: 10.1006/jsre.2001.6115. [DOI] [PubMed] [Google Scholar]
- KIYOCHI H., ONO A., SHIMAHARA Y., KOBAYASHI N. Extrinsic reinnervation after intestinal transplantation in rats. Transplant Proc. 1994;26:951–952. [PubMed] [Google Scholar]
- LANG M.E., DAVISON J.S., BATES S.L., MEDDINGS J.B. Opioid receptors on guinea-pig intestinal crypt epithelial cells. J. Physiol. 1996;497 (Part 1):161–174. doi: 10.1113/jphysiol.1996.sp021757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOHAMMAD-PANAH R., GYOMOREY K., ROMMENS J., CHOUDHURY M., LI C., WANG Y., BEAR C.E. ClC-2 contributes to native chloride secretion by a human intestinal cell line, Caco-2. J. Biol. Chem. 2001;276:8306–8313. doi: 10.1074/jbc.M006764200. [DOI] [PubMed] [Google Scholar]
- MONTROSE M.H., KEELY S.J., BARRETT K.E.Electrolyte secretion and absorption: small intestine and colon Textbook of Gastroenterology 1999Philadelphia: Lippincott Williams & Wilkins Publishers; 320–355.ed. Yamada, T., Alpers, D.H., Laine, L., Owyang, C. & Powell, D.W. pp [Google Scholar]
- OHTANI N., BALSIGER B.M., ANDING W.J., DUENES J.A., SARR M.G. Small bowel transplantation induces adrenergic hypersensitivity in ileal longitudinal smooth muscle in rats. J. Gastrointest. Surg. 2000;4:77–85. doi: 10.1016/s1091-255x(00)80036-0. [DOI] [PubMed] [Google Scholar]
- OISHI A.J., SARR M.G. Intestinal transplantation: effects on ileal enteric absorptive physiology. Surgery. 1995;117:545–553. doi: 10.1016/s0039-6060(05)80254-5. [DOI] [PubMed] [Google Scholar]
- REID J.L., VINCENT J. Clinical pharmacology and therapeutic role of prazosin and related alpha-adrenoceptor antagonists. Cardiology. 1986;73:164–174. doi: 10.1159/000174002. [DOI] [PubMed] [Google Scholar]
- SHIBATA C., BALSIGER B.M., ANDING W.J., DUENES J.A., MILLER V.M., SARR M.G. Functional changes in nonadrenergic, noncholinergic inhibitory neurons in ileal circular smooth muscle after small bowel transplantation in rats. Dig. Dis. Sci. 1998;43:2446–2454. doi: 10.1023/a:1026630115009. [DOI] [PubMed] [Google Scholar]
- SHIBATA C., BALSIGER B.M., ANDING W.J., SARR M.G. Adrenergic denervation hypersensitivity in ileal circular smooth muscle after small bowel transplantation in rats. Dig. Dis. Sci. 1997;42:2213–2221. doi: 10.1023/a:1018850214119. [DOI] [PubMed] [Google Scholar]
- SIGALET D.L., KNETEMAN N.M., FEDORAK R.N., KIZILISIK A.T., THOMSON A.B. Intestinal function following allogeneic small intestinal transplantation in the rat. Transplantation. 1992a;53:264–271. doi: 10.1097/00007890-199202010-00003. [DOI] [PubMed] [Google Scholar]
- SIGALET D.L., KNETEMAN N.N., FEDORAK R.N., KIZILISIK T., MADSEN K.E., THOMSON A.B. Small intestinal function following syngeneic transplantation in the rat. J. Surg. Res. 1996;61:379–384. doi: 10.1006/jsre.1996.0133. [DOI] [PubMed] [Google Scholar]
- SIGALET D., KNETEMAN N.M., THOMSON A.B. Small bowel transplantation: past, present and future. Dig. Dis. 1992b;10:258–273. doi: 10.1159/000171364. [DOI] [PubMed] [Google Scholar]
- STADELMANN A.M., WALGENBACH-TELFORD S., TELFORD G.L., KOCH T.R. Distribution of muscarinic receptor subtypes in rat small intestine. J. Surg. Res. 1998;80:320–325. doi: 10.1006/jsre.1998.5431. [DOI] [PubMed] [Google Scholar]
- STEWART C.P., TURNBERG L.A. A microelectrode study of responses to secretagogues by epithelial cells on villus and crypt of rat small intestine. Am. J. Physiol. 1989;257:G334–G343. doi: 10.1152/ajpgi.1989.257.3.G334. [DOI] [PubMed] [Google Scholar]
- TRABER P.G.Carbohydrate assimilation Textbook of Gastroenterology 1999Philadelphia: Lippincott Williams & Wilkins; 404–428.ed. Yamada, T., Alpers, D.H., Laine, L., Owyang, C. & Powell, D.W. pp [Google Scholar]
- TSIOTOS G.G., KENDRICK M.L., LIBSCH K., BIERENS K., LANKISCH P., DUENES J.A., SARR M.G. Ileal absorptive adaptation to jejunal resection and extrinsic denervation: implications for living-related small bowel transplantation. J. Gastrointest. Surg. 2001;5:517–524. doi: 10.1016/s1091-255x(01)80090-1. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., MACNAUGHTON W.K., AL-ANI B., SAIFEDDINE M., WALLACE J.L., HOLLENBERG M.D. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIEIRA-COELHO M.A., SOARES-DA-SILVA P. Alpha2-adrenoceptors mediate the effect of dopamine on adult rat jejunal electrolyte transport. Eur. J. Pharmacol. 1998;356:59–65. doi: 10.1016/s0014-2999(98)00500-7. [DOI] [PubMed] [Google Scholar]
- WATSON A.J., LEAR P.A., MONTGOMERY A., ELLIOTT E., DACRE J., FARTHING M.J., WOOD R.F. Water, electrolyte, glucose, and glycine absorption in rat small intestinal transplants. Gastroenterology. 1988;94:863–869. doi: 10.1016/0016-5085(88)90540-9. [DOI] [PubMed] [Google Scholar]
- WEISER M.M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J. Biol. Chem. 1973;248:2536–2541. [PubMed] [Google Scholar]