Abstract
Genistein is a tyrosine kinase inhibitor which interferes with the activity of several ionic channels either by altering modulatory phosphorylating processes or by direct binding. In whole-cell conditions, genistein induces a partial inhibition of the pacemaker (If) current recorded in cardiac sinoatrial and ventricular myocytes.
We investigated the mechanism of action of genistein (50 μM) on the If current in whole-cell, cell-attached, and inside-out configurations, and the measured fractional inhibitions were similar: 26.6, 27.2, and 33.6%, respectively.
When ATP was removed from the whole-cell pipette solution no differences were revealed in the effect of the drug when compared to metabolically active cells. Genistein fully maintained its blocking ability even when herbimycin, a tyrosine kinase inhibitor, was added to the whole-cell ATP-free pipette solution.
Genistein-induced block was independent of the gating state of the channel and did not display voltage or current dependence; this independence distinguishes genistein from all other f-channel blockers.
When inside-out experiments were performed to test for a direct interaction with the channel, genistein, superfused on the intracellular side of the membrane, decreased the maximal If conductance, and slightly shifted the current–activation curve to the left. Furthermore, the effect of genistein was independent of cAMP modulation.
We conclude that, in addition to its tyrosine kinase-inhibitory properties, genistein also blocks If by directly interacting with the channel, and thus cannot be considered a valuable pharmacological tool to investigate phosphorylation-dependent modulatory pathways of the If current and of cardiac rhythm.
Keywords: Genistein, If, sinoatrial node, herbimycin
Introduction
The hyperpolarization-activated current (If) is essential to the initiation of cardiac spontaneous activity in sinoatrial node (SAN) myocytes, where it provides an inward current that sustains the pacemaker depolarization phase (DiFrancesco, 1993; Robinson & Siegelbaum, 2003; Baruscotti & DiFrancesco, 2004). Due to this functional role, any pathway that modulates the sinoatrial If availability also modifies the slope of the diastolic depolarization, and thus heart rate. Two main mechanisms involved in the modulation of cardiac If current have extensively been investigated by several groups: while one mechanism is a direct binding of the second messenger cAMP to the f-channel (DiFrancesco & Tortora, 1991), a second indirect mechanism is mediated by phosphorylation-dependent processes (Chang et al., 1991; Wu et al., 2000). The action of the cAMP-dependent modulation relies on the allosteric binding of four cAMP molecules to the channel and results in a shift of the activation curve with no change in maximal or single-channel conductance (DiFrancesco, 1999). The structural identification of the site of interaction between the channel and the second messenger has been possible after the cloning of the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel family (Santoro & Tibbs, 1999) and the crystallography of the cyclic nucleotide domain (Zagotta et al., 2003). The importance of phosphorylation-mediated modulations on If has been demonstrated by several experiments. Calyculin A, a type I and II phosphatase inhibitor, increases If by increasing its maximal conductance, with no significant changes in the voltage dependence of the activation curve (Accili et al., 1997). Although a change in single-channel conductance cannot be ruled out, the most likely interpretation of these results is that phosphorylation-dependent processes are an important step in the enhancement of the density of functional proteins in the plasma membrane (Corey et al., 1994; Accili et al., 1997). Further evidence in support of a specific tyrosine-kinase phosphorylation-dependent pathway comes from experiments conducted on SAN myocytes, where the activity of tyrosine kinases was either inhibited (Wu & Cohen, 1997) or induced (Wu et al., 2000), and these actions resulted in the modulation of the If current.
Genistein is an isoflavone compound originally isolated from the fermentation broth of Pseudomonas sp and characterized as a tyrosine kinase inhibitor, which competes for the ATP-binding site with an IC50 of 20.4–111 μM (Akiyama et al., 1987; Akiyama & Ogawara, 1991). Several authors have exploited this property to show that genistein reduces maximal conductance in native If current (Wu & Cohen, 1997) and inhibits HCN2 and HCN4 isoforms, although with different effects (Yu et al., 2004). In agreement with a functionally relevant If block are the results by Ma et al. (2002) showing that, when tested in intact SAN tissue preparations, genistein (50 μM) reduced the rate of contraction via a reduction of the slope of the diastolic depolarization. Additional studies indicate that genistein can modify the activity of several types of channels by mechanisms not involving inhibition of tyrosine-kinase activity. For example, genistein has been shown to directly block neuronal voltage-dependent Na+ channels (Paillart et al., 1997), and similarly reduces the L-type calcium current both in rat (Yokoshiki et al., 1996) and in guinea-pig ventricular myocytes, where it also potentiates the cAMP-dependent chloride current (Chiang et al., 1996). A direct interaction with the channel has also been suggested for cystic fibrosis transmembrane conductance regulator (CFTR) chloride channels (Weinreich et al., 1997). The concentration–response curves for these effects are largely overlapping with the one of tyrosine kinase inhibition: hence, a separation between the latter effect and channel block is not possible by adjusting the concentration of the drug. Preliminary suggestions that genistein could modulate the If current through a phosphorylation-independent mechanism have come from whole-cell studies by Shibata et al. (1999), but a thorough investigation and a definitive evidence is still missing. We have therefore addressed the possibility of a direct interaction between the molecule and the f-channel by comparing the effect of genistein in whole-cell, cell-attached, and inside-out experiments. Our experiments indicate that genistein is a voltage-independent inhibitor of the If current that acts from the intracellular side of the membrane and fully maintains its ability to block f-channels even when metabolic activity is uncoupled from channel activity. We therefore conclude that a direct channel–drug interaction must take place in the cytoplasmic-facing region of the channel. Therefore, genistein cannot be utilized to selectively identify phosphorylation-mediated modulatory processes of the pacemaker If current.
Methods
Animal protocols conformed to the guidelines of the care and use of laboratory animals established by Italian (DL. 116/1992) and European directives (86/609/CEE).
Cell isolation
The methods employed in this study to isolate SAN myocytes from rabbit hearts have been previously described in detail (DiFrancesco et al., 1986). New Zealand rabbits (0.8–1.2 kg) were anaesthetized by i.m. injection of a mixture of xilazine (4.6 mg kg−1) and ketamine (60 mg kg−1), killed by cervical dislocation, and exsanguinated. After removal, hearts were placed in prewarmed (37°C) normal Tyrode solution (mM: NaCl, 140; KCl, 5.4; CaCl2, 1.8; MgCl2, 1; D-glucose, 5.5; Hepes-NaOH, 5; pH 7.4) containing 0.5 ml heparin (1000 U ml−1), and the SAN region exposed. The isolated SAN tissue was then cut into strips which were initially rinsed for 3 min in a Ca2+-free solution (mM: NaCl, 140; KCl, 5.4; MgCl2, 0.5; KH2PO4, 1.2; D-glucose, 5.5; taurine, 50; Hepes-NaOH, 5; pH 6.9) to loosen intercellular interactions. Following this initial treatment, type I collagenase (224 U ml−1), elastase (1.9 U ml−1), protease (0.6 U ml−1), bovine serum albumin (BSA, 1 mg ml−1), and CaCl2 (200 μM) were added to the solution to induce enzymatic dissociation that normally lasted 12–25 min at 37°C. The strips were then rinsed in a Ca2+-free solution (mM: KCl, 20; glutamic acid, 70; β-hydroxybutyric acid (sodium salt), 10; KH2PO4, 10; taurine, 10; Hepes-KOH, 5; KOH, 80; BSA 1 mg ml−1; pH 7.4) to remove traces of enzymes, and final cell dispersion was obtained by mechanically shaking the strips in a similar solution. Ca2+, K+, and Na+ concentrations were then gradually adjusted to their final level. Cells were kept alive and in optimal conditions by storing at 4°C for the experimental day.
Experimental conditions
Current recordings were obtained in three different configurations of the patch-clamp technique: whole-cell, cell-attached, and inside-out. The external solution used during whole-cell experiments was a normal Tyrode solution (see Cell isolation), save for BaCl2 (1 mM) and MnCl2 (2 mM) that were added to block contaminating K+ and Ca2+ currents. The intracellular pipette solution contained (mM): NaCl, 10; K-aspartate, 130; ATP (sodium salt), 2; MgCl2, 2; CaCl2 (pCa 7), 2; EGTA-KOH, 5; Hepes-KOH, 10; pH 7.2. When SAN myocytes were voltage clamped by the perforated-patch method (Horn & Marty, 1988), amphotericin B (260 μM) was dissolved in the following intracellular pipette solution (mM): NaCl, 10; K-aspartate, 130; MgCl2, 2; CaCl2, 0.4; EGTA-KOH, 1; Hepes-KOH, 10; pH 7.2. In cell-attached macropatch experiments, the external solution superfusing the cell was (mM): KCl, 130; NaCl, 10; Hepes-NaOH, 5; (pH 7.4), while in inside-out experiments the control solution superfusing the intracellular side of the excised patches contained (mM): NaCl, 10; potassium aspartate, 130; CaCl2, 2 (pCa 7); EGTA, 5; Hepes-KOH, 10; pH 7.2. The patch-pipette solution used in both configurations (cell-attached and inside-out) was a modified external solution previously used by DiFrancesco & Tortora (1991) to increase the size of the If current (mM): NaCl, 70; KCl, 70; CaCl2, 1.8; MgCl2, 1; BaCl2, 1; MnCl2, 2; Hepes-KOH, 5; pH 7.4. Genistein (Sigma Aldrich, Italy), a tyrosine kinase inhibitor that competes for ATP-binding site (Akiyama et al., 1987), was dissolved in dimethylsulfoxide (DMSO) and diluted in test solutions to the desired concentrations; herbimycin A (Sigma Aldrich, Italy), a specific tyrosine kinase inhibitor, was dissolved in DMSO and diluted in intracellular pipette solution at a final concentration of 35 μM (Wu & Cohen, 1997). DMSO was added to control solutions in the same concentration used in test solutions (lower than 0.1%). Control and test solutions were delivered to the cells through a fast superfusion system. Currents were recorded at room temperature (22–25°C), and drug effects were measured at steady state. When filled with internal solution, pipette resistance measured was 3–5 MΩ. Macropatches containing hundreds of f-channels were formed using large-tipped pipettes (0.5–2 MΩ) as described previously (DiFrancesco & Tortora, 1991). The data were acquired and analyzed with a custom-made patch-clamp amplifier and pClamp software (version 7, Axon U.S.A.).
Data analysis
The voltage dependence of If at steady state can be described by the equation If(V)=gf(V)*(V−Vf)=gf,max*y∞(V)*(V−Vf) where gf(V) is the conductance, gf,max is the maximal conductance, y∞(V) is the steady-state activation parameter, and Vf is the reversal potential. Steady-state I–V curves were measured by applying 60 s long hyperpolarizing voltage ramps (−110 mV min−1) from a holding potential of −35 mV. Conductance-voltage (gf(V)) relations were then obtained from the above equation as ratios between steady-state I–V curves (If(V)) and V–Vf, where Vf was set to −12.24 mV (DiFrancesco & Mangoni, 1994). Conductance curves were fitted to the Boltzmann equation gf(V)=gf,max*y∞(V)=gf,max*(1+exp((V−V1/2)/s)), where V1/2 is the half-maximal voltage and s is the slope factor. When comparing different sets of data, statistical analysis was performed with either the Student's t-tests or analysis of variance (ANOVA); significance was set to P<0.05. Results are given as mean±s.e.m. values.
Results
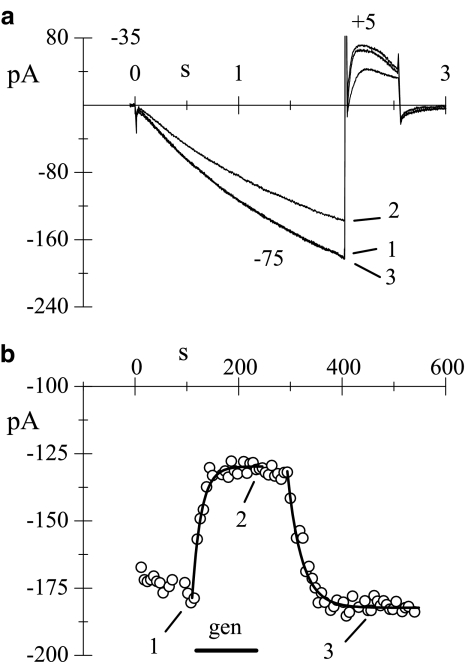
Representative whole-cell If current traces recorded in isolated rabbit SAN myocytes during hyperpolarizing steps to −75 mV (holding potential, −35 mV) in the presence and absence of externally applied genistein (50 μM) are shown in Figure 1a, and the complete time course of genistein-induced block and recovery is presented in Figure 1b. Similar experiments were repeated both in ruptured (n=6) and in perforated patch (n=5) conditions; since no significant differences in the block amplitudes were revealed, the two groups were pooled together. In a total of n=11 cells, the steady-state current reduction was 26.6±2.5%. Single exponential fitting of the time course of block and unblock yielded time constant values of 20.3±2.4 and 25.4±2.7 s, respectively.
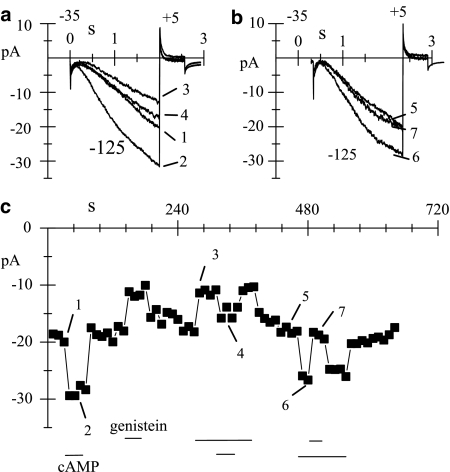
Figure 1.
Genistein reduces the whole-cell If current. (a) If current was elicited every 6 s by a 2-s hyperpolarizing step to −75 mV from a holding potential of −35 mV. Sample traces recorded in control solution (1), during superfusion of genistein (50 μM, 2) and after washout (3) are shown. (b) Time course of If amplitude at −75 mV; the horizontal bar indicates the time of genistein superfusion (gen); the calculated inhibition was 24.2%. Continuous line represents the single exponential fitting of the onset (τ=17.3 s) and removal (τ=27.4 s) of the block.
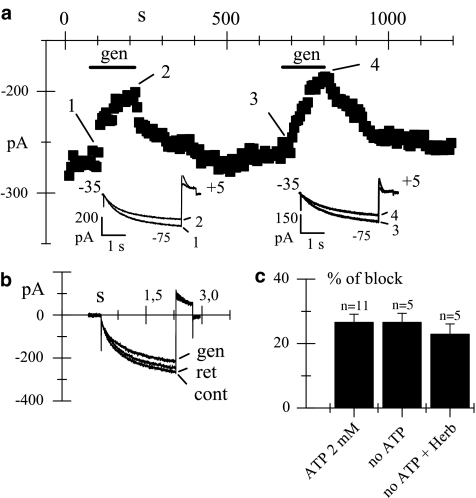
Experiments such as those presented in Figure 1 confirm that genistein (50 μM) partially blocks the If current; however, they do not help to identify the pathways involved. Indeed, the action of the drug could be fully mediated by the inhibition of tyrosine kinase-dependent phosphorylation processes, but other inhibitory pathways cannot be excluded. To evaluate this latter possibility, we first proceeded with whole-cell experiments in which ATP was removed from the intracellular pipette solution, and the efficacy of genistein (50 μM) was tested by two repetitive superfusions separated by 10 min (Figure 2a). Although this interval should be long enough to allow for substantial washout of intracellular ATP, genistein maintained its blocking ability even during the second exposure, and the degree of block was similar during the first (26.9%) and second exposures (28.8%). In Figure 2a, the time course of the If amplitude and representative whole-cell If traces (insets) are shown. Similar experiments yielded a genistein-induced mean current decrease of 22.4±2.5% (n=9) upon the first application, and of 26.6±2.8% (n=5) when it was superfused a second time. Kinetic evaluation of block and unblock reactions also confirmed a similar behavior between first (τon 24.2±5.7 s, n=7; τoff 23.8±5.4 s, n=5) and second (τon 26.8±8.9 s, n=4; τoff 27.1±5.1 s, n=3) genistein exposures, and with whole-cell experiments in the presence of ATP.
Figure 2.
Action of genistein on whole-cell If in the absence of intracellular ATP and PTK activity. (a) Time course of steady-state If amplitudes at −75 mV (holding potential, −35 mV); horizontal bars indicate duration of genistein superfusion (gen); genistein was applied twice at an interval of 10 min. Drug-induced If reductions were 26.9 and 28.8% during the first and second exposures, respectively. Sample If traces in control conditions (1,3) and during superfusion of genistein (50 μM; 2,4) are shown in the insets. (b) Current traces recorded at −95 mV in control condition (cont) was started (gen) 9 min after superfusion of genistein (50 μM) and after washout (ret). The pipette solution contained regular intracellular solution plus herbimycin (35 μM) and no ATP. (c) Graph bar showing the genistein-induced mean (±s.e.m.) If reductions obtained with a regular intracellular pipette solution containing ATP 2 mM, no ATP, and no ATP plus herbimycin 35 μM; blocks were 26.6±2.5% (n=11), 26.6±2.8% (n=5), and 22.9±3.2% (n=5), for the three conditions, respectively. All recordings started at least 6 min after establishing the whole-cell configuration. Blocks were not significantly different.
To further support the finding of a metabolic independent block observed during the second exposure to genistein, herbimycin (35 μM), a potent tyrosine kinase inhibitor (Fukazawa et al., 1991; Wu & Cohen, 1997), was added to the ATP-free pipette solution, and the effect of genistein on the If current was tested several minutes after establishing the whole-cell condition, and after the current had reached a stable level. Sample traces presented in Figure 2b were recorded 9 min after the equilibration of the cytoplasm with the pipette solution, and show that in the cell tested genistein was still able to induce a 22.7% block. The same protocol was repeated in several cells and drug effects were tested at least 6 min after the beginning of the experiments; the mean block observed was 22.9±3.2% (n=5). In Figure 2c the mean If blocks induced by genistein with the three pipette solutions tested are summarized: ATP 2 mM (26.6±2.5%, n=11), ATP-free (26.6±2.8%, n=5), and ATP-free plus herbimycin 35 μM (22.9±3.2%, n=5); statistical analysis did not reveal significative differences.
We next investigated whether genistein-induced block of f-currents depended on the gating state of the channel (Figures 3 and 4).
Figure 3.
Genistein-induced block of closed f-channels. A train of activating/deactivating steps (−105 mV*3 s/+5 mV*0.75 s; 1/6 Hz) was delivered to the cell under study and stable If traces were recorded (left). Current stimulation was then stopped for 72 s by clamping the cell at the holding potential (−35 mV). Genistein (50 μM) superfusion started at the beginning of this silent period, and was maintained throughout the whole time of the experiment. Train stimulation was then resumed (right). When traces recorded just after the interruption (b) and at the end of the experiment (c) were compared to the trace recorded just before the interruption (a), the calculated ratios b/a and c/a were 0.63 and 0.64, respectively, while the c/b ratio yielded a value of 1.01.
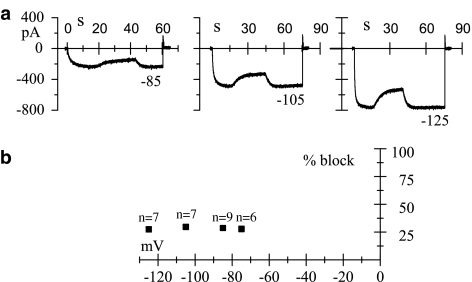
Figure 4.
Genistein blocks If in a voltage independent way. (a) The If current was elicited by hyperpolarizing pulses to the voltages indicated and genistein (50 μM) was applied only after steady-state activation was reached. Evaluation of steady-state blocks yielded values of 28.5, 29.4, and 27.8% for currents recorded at −85, −105, and −125 mV, respectively. (b) Mean±s.e.m.% block obtained at different voltages (−75, −85, −105, and −125 mV) are plotted against voltage; statistical analysis did not reveal any voltage dependence of the block.
Figure 3 shows an experiment specifically devised to test whether genistein can block f-channels when they are maintained in the closed state. An initial brief period of current–activation elicited by a train of activation/deactivation steps (−105 mV*3 s/+5 mV*0.75 s; 1/6 Hz) in control condition was followed by a prolonged clamp of the cell at the holding potential (−35 mV*72 s) to force all the f-channels in the closed state. Genistein (50 μM) superfusion started at the beginning of this steady-state clamp. After this period, and still in the presence of the drug, the pulsing protocol was resumed. When the first current trace recorded upon pulse resuming (b) was compared to the last trace obtained in control conditions (a), a significant decrease of current amplitude was evident (b/a ratio of 0.63). No further reduction was observed during the following pulses (c/b ratio of 1.01). This experimental paradigm was applied to four cells, and the analysis yielded values of 0.68±0.03 and of 0.99±0.01 for b/a and c/b ratios, respectively. These results clearly indicate that genistein has full access to its binding site even when channels are closed.
We then evaluated whether genistein can still block f-channels when they are open and an inward current is flowing.
In Figure 4a, sample current traces activated by long hyperpolarizing pulses at different potentials are shown; genistein (50 μM) was applied at steady-state current–activation and it always induced an upward deflection of the trace, indicating the development of the block. We then plotted the steady-state drug-induced reductions obtained at different membrane potentials and observed that values were not significantly different, indicating a lack of voltage dependence of the block (Figure 4b). The mean fractional block obtained by averaging block values independently from the voltages was 27.9±0.7% (n=29). This value is not different from that obtained for closed channels (32.3±3.0%, n=4), thus indicating that genistein-induced block is not influenced by the channel state.
The experiments presented so far show that genistein fully exerts its If-blocking ability even when tyrosine kinase-dependent phosphorylation was abolished or at least severely limited (Figure 2), and that block was independent of channel state (Figures 3 and 4). We then verified the possibility of alternative inhibitory pathways by the next series of experiments.
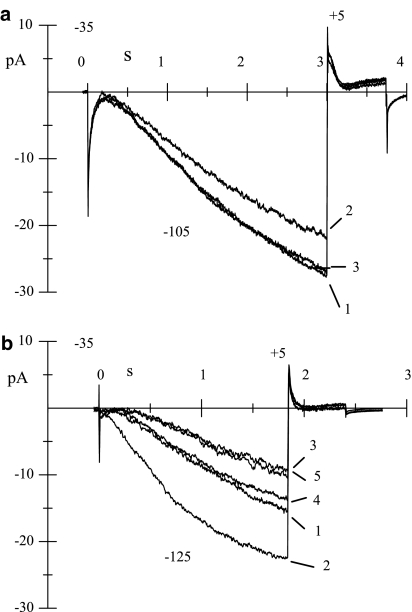
We first investigated whether genistein exerts its action from the extracellular environment or from the cytoplasmic side; experiments were carried out in macropatch cell-attached conditions to verify that genistein, dissolved in the external superfusing solution, could still block the channel (Figure 5a). The degree of genistein-induced macro-patch If reduction (27.2±7.6%, n=8) and time constants of block and recovery (21.1±4.8 s, n=6, and 21.2±1.1 s, n=3) in cell-attached recordings were not significantly different from those observed in whole-cell recordings. Since externally applied genistein maintained its blocking efficacy also in cell-attached experiments, we excluded that the blocking molecule could enter the channel and access its binding site from the external vestibule. We then evaluated the effects of genistein on If recorded in inside-out membrane patches, since this recording configuration allowed the complete control of the superfusing solution which did not contain ATP. After patch excision, If was recorded every 10 s by stepping to −125 mV from a holding potential of −35 mV (Figure 5b); the very negative value of the activating step was used to compensate for the well-known hyperpolarized shift of the If activation curve observed in excised membrane patches (DiFrancesco & Mangoni, 1994). After testing for the integrity of the inside-out configuration by exposure to cAMP (10 μM, 2), genistein (50 μM) was applied for the first time to the cytoplasmic side of the patch, and a rapid, fully reversible If block was elicited (3). The same patch was exposed to genistein two more times and comparable blocks were obtained; traces 4 and 5 (third exposure) were recorded in control condition (4) and upon genistein superfusion (5) 300 s after the first exposure. Similar experiments (n=6 patches) yielded a mean block of 33.6±1.7% (n=6), not different from whole-cell results. Block and unblock rates were fast when compared both to pulse frequency (0.1 Hz) and drug delivery; therefore, no quantification of the time course of these processes was attempted as it was done for external drug superfusion. The permanence of genistein-induced inhibition of the If current and the fast kinetic of block onset and removal in inside-out conditions clearly indicate that a direct interaction between the channel and the drug must take place.
Figure 5.
Action of genistein on If in cell-attached and inside-out macropatches. (a) If recordings obtained in cell-attached conditions during hyperpolarizations to −105 mV in control solution (1), superfusion of genistein (50 μM; 2), and after washout (3); the steady-state block was 24.3%. (b) If traces recorded in inside-out conditions during hyperpolarizations to −125 mV; the recordings were taken at various times in control conditions (1, 4), during superfusions of cAMP (10 μM; 2) and genistein (50 μM; 3, 5); traces 4 and 5 were recorded 300 s after traces 1, 2, and 3, and correspond to the third exposure to the drug (second exposure not shown). The holding potential was set for all recordings to −35 mV and activating steps delivered at 0.1 Hz.
f-Channels belong to the HCN channel family and, among other properties, are characterized by the presence of a cAMP-binding site at the intracellular C-terminus. Inside-out experiments were devised to test for possible functional interactions between cAMP and genistein. Representative current traces (a, b) and the time course (c) of an excised inside-out membrane patch sequentially exposed to different combinations of genistein (50 μM) and cAMP (0.2 μM) are shown in Figure 6. Upon cAMP superfusion the current increased by 47.2% in the absence (1, 2) and by 44.9% in the presence (3, 4) of genistein-induced inhibition. When genistein was tested upon previous cAMP activation (6, 7), it induced a fast and reversible inhibition of the current (28.4%), which was comparable to that obtained in the absence (1, 3) of cAMP prestimulation (32.5%). Summary data relative to three patches yielded a cAMP-induced current increase of If by 33.5±8.2 and 40.1±3.2% in the absence and presence of genistein (not significantly different), and a genistein-induced current decrease of 32.8±6.4 and 28.3±3.6% before and during superfusion with cAMP (not significantly different).
Figure 6.
Action of genistein on inside-out If in the presence of cAMP. (a, b) If currents recorded during hyperpolarizations to −125 mV applied every 10 s from a holding potential of −35 mV; the traces correspond to various times in control conditions and during superfusion of genistein (50 μM) either alone or in combination with cAMP (0.2 μM), as shown in (c). (c) Time course of If amplitude at −125 mV; horizontal bars indicate superfusion of the different solutions.
Yu et al. (2004) have recently shown that genistein reduces the currents induced by heterologous expression of both HCN2 and HCN4 clones, although with different mechanisms; no effects were observed for HCN1. We therefore investigated the mechanism responsible for the If reduction, and in particular whether it arises from a negative shift of the current–activation curve, as in the case of HCN2, or from a reduction of maximal conductance, as in the case of HCN4.
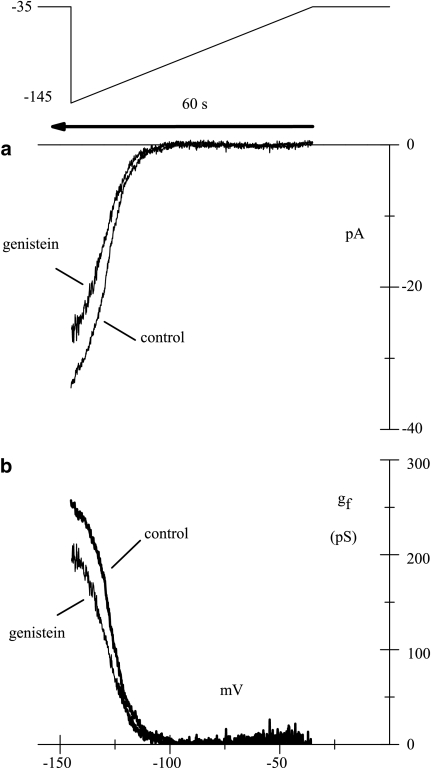
A ramp protocol (see Methods) was used for a detailed quantification of the effect of genistein on the gf(V) relation. As shown in Figure 7a, the current recorded in inside-out patches at −145 mV was reduced by genistein, indicating a decrease in the maximal conductance of If. The conductances curves (Figure 7b) obtained in control and in the presence of the drug were fitted to a Boltzmann relation (see Methods), and the following parameters were obtained: V1/2=−127.0 mV, s=4.9 for control conditions, and −128.8 mV, s=5.5 mV in drug superfusion. In n=6 experiments the maximal conductance decreased by 16.7±1.4%, while the mid-points of activation (V1/2) and the slope factors (s) increased from −122.7±2.3 and 6.1±0.6 mV to −124.1±2.4 and 6.8±0.5 mV in control and in the presence of genistein; all parameters were significantly modified by the drug treatment. These data confirm that the drug inhibits the If current mainly by decreasing the maximal conductance, with only a minor change in its voltage dependence.
Figure 7.
Action of genistein on current– and conductance–voltage relations. (a, top) Hyperpolarizing ramps from −35 to −145 mV were applied at a rate of −110 mV min−1 to an inside-out membrane patch. Note that time runs backward. (a, bottom) Steady-state I–V relations in control solution and during superfusion of genistein (50 μM) as indicated. (b) gf(V) curves obtained as detailed in Methods in control and in the presence of genistein.
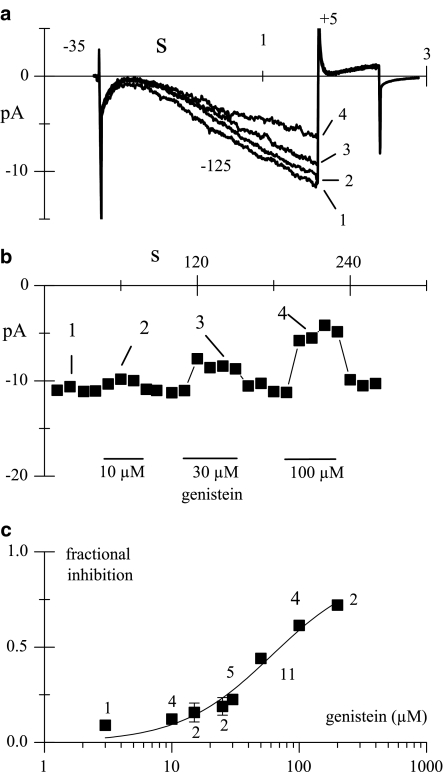
Genistein concentration–response curve was then determined in the range 3–200 μM in inside-out macropatches as shown in Figure 8. The time course of steady-state If current amplitudes recorded in control condition and in the presence of different genistein concentrations is plotted in Figure 8b. Sample traces extracted from these experiments are shown in Figure 8a. The inhibitory effect of the drug was calculated for each concentration and the data were plotted on a semilog scale (Figure 8c). To avoid cumulative effects, increasing concentrations of genistein were applied only after full recovery of the current to control values, and each patch was exposed to no more than three genistein concentrations. Hill function fitting yielded a half-maximal effective concentration (IC50) of 60.9 μM, a maximal fractional inhibition (ymax) of 0.90, and a Hill coefficient (nH) of 1.2.
Figure 8.
Concentration–response curve of the If current inhibition by genistein. If was recorded in inside-out conditions during hyperpolarizing steps to −125 mV applied every 10 s (holding potential −35 mV) in control solution and during superfusion of increasing concentrations of genistein (10, 30, and 100 μM). (b) Time course of If amplitude at −125 mV; horizontal bars indicate the period of drug superfusion at each concentration used. (c) Concentration–response curve. Fitting of data points with the Hill equation y=ymax*(1/(1+(IC50/x)nH)) yielded a maximal fractional inhibition (ymax) of 0.90, a half-block concentration (IC50) of 60.9 μM and a Hill coefficient (nH) of 1.2. The numbers of patches tested at the various concentrations are indicated near data points.
Discussion
Genistein is a phytoestrogenic molecule of particular importance because of its cancer-preventive and cardioprotective properties (Zielonka et al., 2003; Altavilla et al., 2004). Due to its inhibitory action on the activity of tyrosine kinases, genistein has been widely used to dissect modulatory pathways based on phosphorylating events. In order to investigate whether, in addition to knock down phosphorylation-mediated effects, genistein can also affect If through an alternative pathway, we have combined whole-cell, cell-attached, and inside-out experiments.
As previously shown by other groups (Wu & Cohen, 1997; Shibata et al., 1999), we have initially confirmed with whole-cell experiments that genistein induces a partial and reversible inhibition of If in SAN cells (Figure 1). Similar results were also obtained by Wu et al. (2000), who have shown that the stimulatory effect of epidermal growth factor (EGF) on If was inhibited by the presence of genistein. The proposed mechanism of action was based on an EGF-dependent activation of protein tyrosine kinases (PTKs) that can phosphorylate and therefore activate If channels. This cascade of events is interrupted by the inhibitory action of genistein on PTKs. Along with this possibility, it should also be considered that genistein could have additional inhibitory actions independent of their block of PTK activity. This hypothesis has been tested by the experiments described in Figure 2, which show that genistein retained its ability to block the f-channels also when tyrosine phosphorylation was severely impaired by removing ATP and adding herbimycin, a strong inhibitor of PTKs, to the whole-cell pipette solution.
The time course of genistein action in our whole-cell experiments is comparable to that observed for the genistein-induced Na+ current block in neurons, which was attributed to direct channel block (complete effect in ∼20 s; Paillart et al., 1997). A relatively rapid and reversible inhibitory effect of genistein was also observed on the L-type Ca2+ current, on the delayed K+ current, and on the inwardly rectifying K+ current in guinea-pig ventricular myocytes (Chiang et al., 1996; 2002; Hool et al., 1998). Since calyculin A, a phosphatase inhibitor, requires longer times to increase If (τ=466 s; Accili et al., 1997), the time course of the action of genistein on If found in our experiments is more compatible with a direct blocking action than with de-phosphorylation via tyrosine kinase inhibition. The noncatalytic dependent effect of genistein on f-channels found in our experiments can be compared to the genistein-induced inhibition of rod cyclic nucleotide gated (CNG) channels, which was attributed by Molokanova et al. (1999) to channel block independent of phosphorylating reactions. These authors conclude that when PTKs are bound to genistein, they can allosterically influence gating of the rod channel, independent of their role in catalyzing phosphorylation. However, the same authors have observed that ATP decreases the effectiveness of genistein-induced channel inhibition. In contrast, we did not notice a dependence of the If block by genistein upon intracellular ATP.
Our whole-cell experiments strongly point to the existence of a phosphorylation-independent blockade of the If current, as was also observed by Shibata et al. (1999), who reported the permanence of genistein block when PTK activity was inhibited by tyrphostin 25. The whole-cell approach, though, relies on the assumption that, after several minutes from the formation of the whole-cell condition, intracellular ATP and tyrosine kinase activity should be negligible, but it cannot be fully excluded that partially inaccessible subcellular microenvironments favor the persistence of localized phosphorylating pathways. Our experiments presented in Figure 5 make use of a more direct approach (cell-attached and inside-out) to demonstrate a direct interaction of genistein with f-channels at the intracellular level. Indeed genistein acts on f-channels in cell-attached experiments (Figure 5a), and steady-state block occurs in less than 10 s (i.e. the interpulse interval), a value comparable to that required for cAMP action in similar conditions (∼8 s; DiFrancesco & Tortora, 1991). Genistein exerts a wide spectrum of actions which include tyrosine kinase inhibition, direct block of ionic channels, and NO synthase activity enhancement (Rathel et al., 2005); for this reason, the dissection of single effects is extremely difficult. This situation is further complicated by the fact that most of the concentration–response curves obtained for these genistein actions yielded similar IC50 values (range 17.5–111 μM; Akiyama & Ogawara, 1991; Chiang et al., 1996; Paillart et al., 1997), and therefore a dissection of the effects based on drug concentration is nearly impossible. The concentration–response curve shown in our Figure 8 yielded an IC50 value of 60.9 μM that agrees well with those just mentioned and with that obtained by Shibata et al. (1999) for the whole-cell If current (62.3 μM). In our inside-out experiments, though, the extremely simplified and controlled situation allows to conclude that genistein directly acts on the f-channel. Interestingly, when a similar concentration (50 μM) was tested on spontaneously beating intact SAN preparations (Ma et al., 2002), it significantly slowed the spontaneous rate of contraction by a selective decrease of the slope of phase 4 with no modification of the repolarization phase (Table 1 from Ma et al., 2002). A significant decrease of the maximal upstroke velocity (Vmax) was also observed. Although the authors did not thoroughly investigate the molecular reasons for these effects, these data are consistent with the inhibition of both If and ICaL currents.
Direct activation by cyclic nucleotides is a property shared by f- and CNG channels. Since genistein is an inhibitory agonist for the nucleotide-binding sites of PTKs (Akiyama & Ogawara, 1991), we also hypothesized a possible interaction of genistein with the cAMP-binding site of If channels. However, we found that If modulation by cAMP was not modified by genistein and that cAMP did not prevent the blocking effect of the drug (Figure 6), and this suggests different sites of action. Genistein is an unspecific ion channel blocker; indeed, in the literature there are several reports that highlight the broad range of blocking mechanisms and these include direct blockade both at extracellular and intracellular sites. For example, genistein inhibits cardiac L-type calcium currents in guinea-pig ventricular myocytes (Chiang et al., 1996), and single-channel recordings in neonatal rat ventricular myocytes show that genistein decreases the open probability of the calcium channels without affecting the mean open time and the conductance (Katsube et al., 1998). These data suggest that there is a direct binding of the drug to the channel. Furthermore, genistein (100 μM) abolishes the opening of ATP-sensitive K+ channels of the rabbit portal vein smooth muscle cells by a mechanism that may involve a direct inhibition (Ogata et al., 1997). Our data point to the involvement of intracellular channel structures as sites for drug binding. Several f-channel blockers such as zatebradine, ZD7288, cilobradine, caesium, and ivabradine have been described in the literature and their mechanisms of action can be very complex (for a complete review, see Baruscotti et al., 2005). For all these drugs, the affinities for f-channels are variously influenced by several parameters such as voltage, gating state, and current flow. For example, ivabradine, a novel heart-rate-reducing agent, can access its binding site in SAN native f-channels only when the channels are open, but the inward current flow destabilizes the drug–channel interaction, thus revealing a strong current dependence of the block (Bucchi et al., 2002). Our data indicate that genistein represents a case of a molecule that directly blocks f-channels, whose affinity is independent of the functional state of the channel. A similar result was found for alinidine (Van Bogaert & Goethals, 1987), although a direct interaction was not demonstrated.
Under physiological conditions genistein exists both in the neutral and monoanionic forms, since the pKa value for the first deprotonation reaction is 7.2 (Zielonka et al., 2003). The lack of voltage dependence observed in our experiments (Figure 4b) could be explained by assuming that genistein interacts with the f-channels in the neutral form. More intriguing is the lack of interference of the gating mechanism on genistein block. Indeed, molecules such as ZD7288 or ivabradine that directly block pacemaker channels from the intracellular environment can be trapped by the channel gating structures, thus suggesting the existence of a wide inner hydrophilic vestibule near the inner mouth of the pore (Shin et al., 2001; Bucchi et al., 2002). Genistein does not conform to this type of behavior, thus suggesting that the gating structures do not limit the access of the drug to the pore.
One additional hypothesis on the action of genistein on ionic channels has been proposed by Hwang et al. (2003) these authors provide evidence that genistein can alter the mechanical properties of bilayers, and this would in turn influence the gramicidin A channel function. Although this is an interesting hypothesis, it still has to be confirmed in native cell membrane and ionic channels.
Inhibitory drugs can modify whole-cell currents either by shifting the voltage dependence of gating and/or by reducing the maximal conductance. Experiments such as those presented in Figure 7 clearly indicate that genistein acts on f-channels by a strong reduction of the maximal conductance and a minor, but still significant, modification of the voltage dependence of the activation curve. Recently, Yu et al. (2004) have shown that HCN clones are differently affected by genistein; while genistein does not have any effect on HCN1, it modifies HCN4 by selectively reducing maximal conductance, and inhibits HCN2 by shifting its voltage dependence towards more negative values. Since in rabbit SAN cells If is mostly composed by HCN4 isoform (Shi et al., 1999; Altomare et al., 2003), it is thus not surprising that the most evident inhibitory mechanism shown in Figure 7 is a maximal conductance reduction. Partially unexpected are the observed modifications of the voltage-dependent parameters (V1/2 and s), that could be linked to a limited presence of HCN2 isoforms. Although HCN2 contribution to native sinoatrial f-current has been excluded based on RNA detection methods (Shi et al., 1999), a careful analysis of the presence and distribution of HCN protein in the SA node is still missing. Thus, the presence of a limited, yet relevant, functional participation of HCN2 to native f-currents cannot be excluded.
The analysis of the primary sequences of HCN isoforms has allowed Yu et al. (2004) to identify several tyrosine residues that could be the target for the activity of tyrosine kinases. Future experiments should therefore study the effect of genistein and of phosphorylation on channels in which single and/or combined mutation of these residues will be induced. Such an approach is likely to shed light on the importance of phosphorylating mechanism on the basal activity of HCN and therefore of native f-channels.
In conclusion, our data provide a direct demonstration that genistein inhibits f-channel activity by a direct interaction with the intracellular side of the channel, and binding stability is not influenced by the voltage and by the gating state of the channel. The direct inhibition of If implies that genistein does not represent a useful pharmacological tool to study the implication of tyrosine kinase activity in the possible control of f-channels by phosphorylating processes.
Acknowledgments
We should like to thank Dr DiFrancesco, Dr Bois, and Dr Viscomi for productive discussions and encouragements.
Abbreviations
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- PTKs
protein tyrosine kinases
- SAN
sinoatrial node
References
- ACCILI E.A., REDAELLI G., DIFRANCESCO D. Differential control of the hyperpolarization-activated (if) current by cAMP and phosphatase inhibition in rabbit sino-atrial node myocytes. J. Physiol. (London) 1997;500.3:643–651. doi: 10.1113/jphysiol.1997.sp022049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKIYAMA T., OGAWARA H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S., ITOH N., SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- ALTAVILLA D., CRISAFULLI A., MARINI H., ESPOSITO M., D'ANNA R., CORRADO F., BITTO A., SQUADRITO F. Cardiovascular effects of the phytoestrogen genistein. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2004;2:179–186. doi: 10.2174/1568016043477297. [DOI] [PubMed] [Google Scholar]
- ALTOMARE C., TERRAGNI B., BRIOSCHI C., MILANESI R., PAGLIUCA C., VISCOMI C., MORONI A., BARUSCOTTI M., DIFRANCESCO D. Heteromeric HCN1–HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J. Physiol. (London) 2003;549:347–359. doi: 10.1113/jphysiol.2002.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARUSCOTTI M., DIFRANCESCO D. Pacemaker channels. Ann. NY. Acad. Sci. 2004;1015:111–121. doi: 10.1196/annals.1302.009. [DOI] [PubMed] [Google Scholar]
- BARUSCOTTI M., BUCCHI A., DIFRANCESCO D. Physiology and pharmacology of the cardiac pacemaker (‘funny') current. Pharmacol. and Therap. 2005;107:59–79. doi: 10.1016/j.pharmthera.2005.01.005. [DOI] [PubMed] [Google Scholar]
- BUCCHI A., BARUSCOTTI M., DIFRANCESCO D. Current-dependent block of rabbit sino-atrial node If channels by ivabradine. J. Gen. Physiol. 2002;120:1–13. doi: 10.1085/jgp.20028593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG F., COHEN I.S., DIFRANCESCO D., ROSEN M.R., TROMBA C. Effects of protein kinase inhibitors on canine Purkinje fibre pacemaker depolarization and the pacemaker current i(f) J. Physiol. (London) 1991;440:367–384. doi: 10.1113/jphysiol.1991.sp018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIANG C.E., CHEN S.A., CHANG M.S., LIN C.I., LUK H.N. Genistein directly inhibits L-type calcium currents but potentiates cAMP-dependent chloride currents in cardiomyocytes. Biochem. Biophys. Res. Commun. 1996;223:598–603. doi: 10.1006/bbrc.1996.0941. [DOI] [PubMed] [Google Scholar]
- CHIANG C.E., LUK H.N., CHEN L.L., WANG T.M., DING P.Y. Genistein inhibits the inward rectifying potassium current in guinea pig ventricular myocytes. J. Biomed. Sci. 2002;9:321–326. doi: 10.1007/BF02256587. [DOI] [PubMed] [Google Scholar]
- COREY J.L., DAVIDSON N., LESTER H.A., BRECHA N., QUICK M.W. Protein kinase C modulates the activity of a cloned gamma-aminobutyric acid transporter expressed in Xenopus oocytes via regulated subcellular redistribution of the transporter. J. Biol. Chem. 1994;269:14759–14767. [PubMed] [Google Scholar]
- DIFRANCESCO D. Pacemaker mechanisms in cardiac tissue. Annu. Rev. Physiol. 1993;55:451–467. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- DIFRANCESCO D. Dual allosteric modulation of pacemaker (f) channels by cAMP and voltage in rabbit SA node. J. Physiol. (London) 1999;512:367–376. doi: 10.1111/j.1469-7793.1999.367ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIFRANCESCO D., FERRONI A., MAZZANTI M., TROMBA C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J. Physiol. (London) 1986;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIFRANCESCO D., MANGONI M. Modulation of single hyperpolarization-activated channels (if) by cAMP in the rabbit sino-atrial node. J. Physiol. (London) 1994;474:473–482. doi: 10.1113/jphysiol.1994.sp020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIFRANCESCO D., TORTORA P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- FUKAZAWA H., LI P.M., YAMAMOTO C., MURAKAMI Y., MIZUNO S., UEHARA Y. Specific inhibition of cytoplasmic protein tyrosine kinases by herbimycin A in vitro. Biochem. Pharmacol. 1991;42:1661–1671. doi: 10.1016/0006-2952(91)90500-5. [DOI] [PubMed] [Google Scholar]
- HOOL L.C., MIDDLETON L.M., HARVEY R.D. Genistein increases the sensitivity of cardiac ion channels to beta-adrenergic receptor stimulation. Circ. Res. 1998;83:33–42. doi: 10.1161/01.res.83.1.33. [DOI] [PubMed] [Google Scholar]
- HORN R., MARTY A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG T.C., KOEPPE R.E., ANDERSEN O.S. Genistein can modulate channel function by a phosphorylation-independent mechanism: importance of hydrophobic mismatch and bilayer mechanics. Biochemistry (U.S.A.) 2003;42:13646–13658. doi: 10.1021/bi034887y. [DOI] [PubMed] [Google Scholar]
- KATSUBE Y., YOKOSHIKI H., NGUYEN L., YAMAMOTO M., SPERELAKIS N. Inhibition of Ca2+ current in neonatal and adult rat ventricular myocytes by the tyrosine kinase inhibitor, genistein. Eur. J. Pharmacol. 1998;345:309–314. doi: 10.1016/s0014-2999(98)00010-7. [DOI] [PubMed] [Google Scholar]
- MA T., FAN Z.-Z., HE R-R. Electrophysiological effects of phytoestrogen genistein on pacemaker cells in sinoatrial nodes of rabbits. Acta Pharmacol. Sin. 2002;23:367–370. [PubMed] [Google Scholar]
- MOLOKANOVA E., SAVCHENKO A., KRAMER R.H. Noncatalytic inhibition of cyclic nucleotide-gated channels by tyrosine kinase induced by genistein. J. Gen. Physiol. 1999;113:45–56. doi: 10.1085/jgp.113.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGATA R., KITAMURA K., ITO Y., NAKANO H. Inhibitory effects of genistein on ATP-sensitive K+ channels in rabbit portal vein smooth muscle. Br. J. Pharmacol. 1997;122:1395–1404. doi: 10.1038/sj.bjp.0701532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAILLART C., CARLIER E., GUEDIN D., DARGENT B., COURAUD F. Direct block of voltage-sensitive sodium channels by genistein, a tyrosine kinase inhibitor. J. Pharmacol. Exp. Ther. 1997;280:521–526. [PubMed] [Google Scholar]
- RATHEL T.R., LEIKERT J.F., VOLLMAR A.M., DIRSCH V.M. The soy isoflavone genistein induces a late but sustained activation of the endothelial nitric oxide-synthase system in vitro. Br. J. Pharmacol. 2005;144:394–399. doi: 10.1038/sj.bjp.0706075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON R.B., SIEGELBAUM S.A. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- SANTORO B., TIBBS G.R. The HCN gene family: Molecular basis of the hyperpolarization-activated pacemaker channels. Ann. NY Acad. Sci. 1999;868:741–764. doi: 10.1111/j.1749-6632.1999.tb11353.x. [DOI] [PubMed] [Google Scholar]
- SHI W., WYMORE R., YU H., WU J., WYMORE R.T., PAN Z., ROBINSON R.B., DIXON J.E., MCKINNON D., COHEN I.S. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ. Res. 1999;85:e1–e6. doi: 10.1161/01.res.85.1.e1. [DOI] [PubMed] [Google Scholar]
- SHIBATA S., ONO K., IIJIMA T. Inhibition by genistein of the hyperpolarization-activated cation current in porcine sino-atrial node cells. Br. J. Pharmacol. 1999;128:1284–1290. doi: 10.1038/sj.bjp.0702903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIN K.S., ROTHBERG B., YELLEN G. Blocker state dependence and trapping in hyperpolarization-activated cation channels: evidence for an intracellular activation gate. J. Gen. Physiol. 2001;117:91–101. doi: 10.1085/jgp.117.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BOGAERT P.P., GOETHALS M. Pharmacological influence of specific bradycardic agents on the pacemaker current of sheep cardiac Purkinje fibres. A comparison between three different molecules. Eur. Heart J. 1987;8 (Suppl L):35–42. doi: 10.1093/eurheartj/8.suppl_l.35. [DOI] [PubMed] [Google Scholar]
- WEINREICH F., WOOD P.G., RIORDAN J.R., NAGEL G. Direct action of genistein on CFTR. Pflug. Arch. Eur. J. Phys. 1997;434:484–491. doi: 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- WU J.Y., COHEN I.S. Tyrosine kinase inhibition reduces i(f) in rabbit sinoatrial node myocytes. Pflug. Arch. Eur. J. Phys. 1997;434:509–514. doi: 10.1007/s004240050430. [DOI] [PubMed] [Google Scholar]
- WU J.Y., YU H., COHEN I.S. Epidermal growth factor increases i(f) in rabbit SA node cells by activating a tyrosine kinase. Biochim. Biophys. Acta. 2000;1463:15–19. doi: 10.1016/s0005-2736(99)00233-3. [DOI] [PubMed] [Google Scholar]
- YOKOSHIKI H., SUMII K., SPERELAKIS N. Inhibition of L-type calcium current in rat ventricular cells by the tyrosine kinase inhibitor, genistein and its inactive analog, daidzein. J. Mol. Cell. Cardiol. 1996;28:807–814. doi: 10.1006/jmcc.1996.0075. [DOI] [PubMed] [Google Scholar]
- YU H.G., LU Z., PAN Z., COHEN I.S. Tyrosine kinase inhibition differentially regulates heterologously expressed HCN channels. Pflug. Arch. Eur. J. Phys. 2004;447:392–400. doi: 10.1007/s00424-003-1204-y. [DOI] [PubMed] [Google Scholar]
- ZAGOTTA W.N., OLIVIER N.B., BLACK K.D., YOUNG E.C., OLSON R., GOUAUX E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature. 2003;425:200–205. doi: 10.1038/nature01922. [DOI] [PubMed] [Google Scholar]
- ZIELONKA J., GEBICKI J., GRYNKIWICZ G. Radical scavenging properties of genistein. Free Radic. Biol. Med. 2003;35:958–965. doi: 10.1016/s0891-5849(03)00472-6. [DOI] [PubMed] [Google Scholar]