Abstract
Angiotensin II receptor blockers (AIIRBs) have been shown to prevent atrial fibrillation. The pulmonary veins (PVs) are the most important focus for the generation of atrial fibrillation. The aim of this study was to evaluate whether angiotensin II or AIIRB may change the arrhythmogenic activity of the PVs.
Conventional microelectrodes and whole-cell patch clamps were used to investigate the action potentials (APs) and ionic currents in isolated rabbit PV tissue and single cardiomyocytes before and after administering angiotensin II or losartan (AIIRB).
In the tissue preparations, angiotensin II induced delayed after-depolarizations (1, 10, and 100 nM) and accelerated the automatic rhythm (10 and 100 nM). Angiotensin II (100 nM) prolonged the AP duration and increased the contractile force (10 and 100 nM). Losartan (1 and 10 μM) inhibited the automatic rhythm. Losartan (10 μM) prolonged the AP duration and reduced the contractile force (1 and 10 μM).
Angiotensin II reduced the transient outward potassium current (Ito) but increased the L-type calcium, delayed rectifier potassium (IK), transient inward (Iti), pacemaker, and Na+–Ca2+ exchanger (NCX) currents in the PV cardiomyocytes. Losartan decreased the Ito, IK, Iti, and NCX currents.
In conclusion, angiotensin II and AIIRB modulate the PV electrical activity, which may play a role in the pathophysiology of atrial fibrillation.
Keywords: Angiotensin II, atrial fibrillation, ionic currents, pulmonary vein
Introduction
Atrial fibrillation is the most common sustained arrhythmia in clinical medicine. Hypertension and heart failure are important risk factors for atrial fibrillation (Feinberg et al., 1995). Activation of the atrial angiotensin system has been suggested to play a role in the mechanisms of atrial fibrillation. In humans, chronic atrial fibrillation and paroxysmal atrial fibrillation are associated with significant changes in the atrial expression of angiotensin receptors (Goette et al., 2000a). In addition, patients with atrial fibrillation showed an increased expression of the extracellular signal-regulated kinases and angiotensin-converting enzyme in interstitial cells and marked atrial fibrosis (Goette et al., 2000b; Boldt et al., 2003). Angiotensin II, the major active component of the renin–angiotensin system, has various effects on ionic currents. Angiotensin II increases the intracellular free calcium, alters the potassium conductance, and induces the genesis of transient inward currents (Daleau & Turgeon, 1994; Enous & Opie, 1994; Boston et al., 1998; Yu et al., 2000). It has been shown to increase spontaneous firing activity and change the action potential (AP) duration of cardiomyocytes (Allen et al., 1988; Chen et al., 1991). These findings suggest that angiotensin II has a high arrhythmogenic activity and may play an important role in the pathophysiology of atrial fibrillation. However, our knowledge of the mechanisms of angiotensin II-induced atrial fibrillation is limited.
Angiotensin II receptor blockers (AIIRBs) are medications frequently used in the treatment of hypertension and congestive heart failure and have several cardiac electrophysiological effects. Nakashima et al. (2000) demonstrated that the shortening of the atrial refractory period during rapid atrial pacing was prevented by treatment with AIIRB. Another AIIRB, irbesartan, also was found to contribute to the maintenance of sinus rhythm after successful electrical cardioversion (Madrid et al., 2002). These findings suggest a role for AIIRBs in the treatment of atrial fibrillation. Nevertheless, it is not clear how AIIRBs prevent attacks of atrial fibrillation.
Pulmonary veins (PVs) are important sources of ectopic beats for the initiation of paroxysmal atrial fibrillation (Haissaguerre et al., 1998; Chen et al., 1999) and the maintenance of atrial fibrillation (Pappone et al., 2000). PVs are known to contain cardiomyocytes with electrical activity and are suggested to be subsidiary pacemakers (Blom et al., 1999; Perez-Lugones et al., 2003). Through several mechanisms, PVs have been found to have a high arrhythmogenic potential to induce atrial arrhythmias (Chen et al., 2000, 2001, 2002; Hocini et al., 2002; Zhou et al., 2002). Studies of single cells have shown that PVs have cardiomyocytes with and without pacemaker activity (Chen et al., 2001, 2002). Long-term (6–8 weeks) rapid atrial pacing (780 b.p.m.) and the administration of thyroid hormone or isoproterenol were demonstrated to increase PV arrhythmogenic activity (Chen et al., 2000, 2001, 2002). Angiotensin II is known to induce structural changes with increasing cellular uncoupling (Sun et al., 1997), which may further enhance the ectopic firing from PVs. Therefore, it is possible that angiotensin II may provoke atrial fibrillation by enhancing the ectopic electrical activity from the PVs, and AIIRB may prevent atrial fibrillation through suppression of the PV arrhythmogenic activity. The purpose of the present study was to investigate the effects of angiotensin II and AIIRB on the electrophysiological characteristics and electrical activity of the PVs.
Methods
Tissue preparations and the electropharmacological study in isolated PVs
The investigation conformed to the institutional Guide for the Care and Use of Laboratory Animals. Rabbits (weight, 1–2 kg) were anesthetized with an intraperitoneal injection of sodium pentobarbital (40 mg kg−1) and the heart with lung was removed after mid-line thoracotomy. Then, the PVs were separated from the atria at the left atrium–PV junction and separated from the lungs at the end of the PV myocardial sleeves in normal Tyrode's solution with a composition (in mM) of 137 NaCl; 4 KCl; 15 NaHCO3; 0.5 NaH2PO4; 0.5 MgCl2; 2.7 CaCl2, and 11 dextrose. One end of the preparation was pinned to the bottom of a tissue bath and the other end was connected to a Grass FT03C force transducer with an initial tension of 250 mg. The adventitia of the PVs faced upwards.
Transmembrane APs of the PVs were recorded by means of machine-pulled glass capillary microelectrodes filled with 3 mol l−1 of KCl and connected to a WPI Duo 773 electrometer. The electrical and mechanical events were displayed simultaneously on a Gould 4072 oscilloscope and a Gould TA11 recorder. The AP duration at repolarization of 90% of the AP amplitude (APD90) was measured during electrical stimuli (1 Hz) with a 2-ms duration and suprathreshold strength (30% above the threshold) provided using a Grass S88 stimulator through a Grass SIU5B stimulus isolation unit (Chen et al., 2000). Angiotensin II (1, 10 and 100 nM) or losartan (1 and 10 μM, Merck & Co., Inc., NJ, U.S.A.) were superfused to test the pharmacological responses.
Electropharmacological studies in single PV cardiomyocytes
PV cardiomyocytes from rabbits were enzymatically dissociated through the same procedure as described previously (Chen et al., 2002). PV cardiomyocytes with pacemaker activity were identified by the presence of constantly spontaneous beating or an AP that maintained a spontaneous diastolic depolarization during perfusion with normal Tyrode's solution.
Whole-cell patch clamp was performed in the PV cardiomyocytes with and without the administration of angiotensin II (100 nM) and losartan (10 μM) by an Axopatch 1D amplifier (Axon Instruments, CA, U.S.A.) at 35±1°C (n=210). This concentration was chosen because a previous study showed that 10 μM losartan exerted protective effects (Louch et al., 2000). A small hyperpolarizing step from a holding potential of −50 mV to a testing potential of −55 mV for 80 ms was delivered at the beginning of each experiment. The area under the capacitative currents was divided by the applied voltage step to obtain the total cell capacitance. Normally, 60–80% series resistance (Rs) was electronically compensated. The APs were recorded in the current–clamp mode and ionic currents in the voltage–clamp mode (Chen et al., 2001, 2002). Micropipettes were filled with a solution containing (in mM) CsCl 130, MgCl2 1, Mg2ATP 5, HEPES 10, EGTA 10, NaGTP 0.1, and Na2 phosphocreatine 5, titrated to a pH of 7.2 with CsOH for the experiments on the L-type calcium current (ICa-L), containing (in mM) NaCl 20, CsCl 110, MgCl2 0.4, CaCl2 1.75, tetraethylammonium 20, BAPTA 5, glucose 5, Mg2ATP 5, and HEPES 10, titrated to a pH of 7.25 for the experiments on the Na+–Ca2+ exchanger (NCX) current, and containing (in mM) KCl 20, K aspartate 110, MgCl2 1, Mg2ATP 5, HEPES 10, EGTA 0.5, LiGTP 0.1, and Na2 phosphocreatine 5, titrated to a pH of 7.2 with KOH for the experiments on the other currents.
The ICa-L was measured as an inward current during depolarization from a holding potential of −50 mV to testing potentials ranging from −40 to +60 mV in 10-mV steps for 300 ms at a frequency of 0.1 Hz by means of perforated patch clamp with amphotericin B. The NaCl and KCl in the external solution were replaced by tetraethylammonium chloride and CsCl, respectively.
The transient outward current (Ito) was studied with a double-pulse protocol. A 30-ms prepulse from −80 to −40 mV was used to inactivate the sodium channels, followed by a 300-ms test pulse to +60 mV in 10-mV steps at a frequency of 0.1 Hz. CdCl2 (200 μM) was added to the bath solution to inhibit ICa-L. Ito was measured as the difference between the peak outward current and steady-state current. The delayed rectified outward potassium current (IK) was measured from the peak outward current at the end of 500 ms of the depolarization from −40 to +60 mV in 10-mV steps at a frequency of 0.1 Hz during the infusion of CdCl2 (200 μM) and 4-aminopyridine (2 mM) in the bath solution.
A transient inward current (Iti) was induced at clamped potentials from −40 to +40 mV for a duration of 3 s and then repolarized to −40 mV. The amplitude of the Iti was measured as the difference between the peak of the transient current and the mean of the current just before and after the transient current (Chen et al., 2001, 2002).
The inward rectifier potassium current (IK1) was activated from −40 mV to test potentials ranging from −20 to −120 mV in 10-mV steps for 1 s at a frequency of 0.1 Hz under the infusion of CdCl2 (200 μM) and 4-aminopyridine (2 mM) in the bath solution. The amplitudes of the IK1 were measured as 1 mM barium-sensitive currents. After the infusion of barium (1 mM) to inhibit IK1, the pacemaker current (If) was activated during hyperpolarization from a holding potential of −40 mV to test potentials ranging from −20 to −120 mV in 10-mV steps for 1 s at 0.1 Hz.
The NCX current was elicited by depolarizing pulses between −100 to +100 mV from a holding potential of −40 mV for 300 ms at a frequency of 0.1 Hz. The amplitudes of the NCX current were measured as 10 mM nickel-sensitive currents. The external solution (in mM) consisted of NaCl 140, CaCl2 2, MgCl2 1, HEPES 5, and glucose 10 with a pH of 7.4, and contained strophanthidin (10 μM), nitrendipine (10 μM), and niflumic acid (100 μM).
Statistical methods
All quantitative data are expressed as mean±s.e. A paired t-test or two-way repeated ANOVA were used to compare the differences before and after the drug administration in the PV specimens or PV cardiomyocytes with and without pacemaker activity. Multiple comparisons were analyzed with the Fisher's least significant difference test. A P-value lower than 0.05 was considered statistically significant.
Results
Effects of angiotensin II and losartan on PV tissue preparations
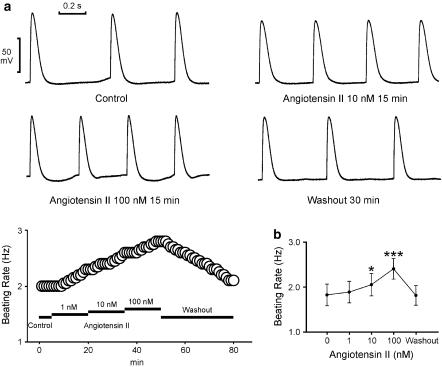
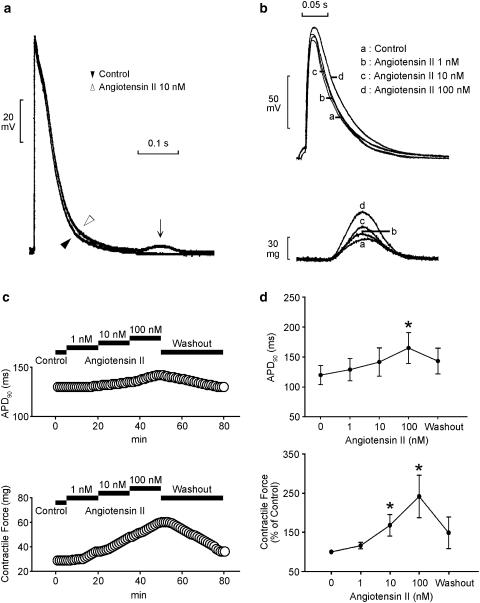
The superfusion with angiotensin II (10 and 100 nM) increased the automatic rhythm in the spontaneously active PV tissue preparations (n=7; Figure 1a). Figure 1b summarizes the effects of different concentrations of angiotensin II on the PV automaticity. This effect was reversible after the washout of angiotensin II. In addition, angiotensin II induced the occurrence of delayed after-depolarizations in two PVs (1 nM) and three PVs (10 and 100 nM) during electrical stimulation (Figure 2a). Angiotensin II (100 nM) prolonged the APD90 and angiotensin II (10 and 100 nM) increased the contractile force in the PVs without spontaneous activity (n=7, Figure 2b). Figure 2c summarizes the effects of different concentrations of angiotensin II on the APD90 and contractile force of the PV tissue preparations.
Figure 1.
Effects of angiotensin II on PV spontaneous activity. (a) The tracings and time course show an example that angiotensin II (10 and 100 nM) increased the PV spontaneous activity. The action potentials without a significant phase 4 depolarization suggested that these recordings were not directly from the cardiomyocytes with pacemaker activity. (b) The concentration–response curve of angiotensin II's effect on the automatic rhythm (n=7). *P<0.05, **P<0.01 versus before drugs.
Figure 2.
Effect of angiotensin II on the AP configuration and contractile force. (a) Superimposed tracings of a delayed after-depolarization (arrow) during a 1 Hz electrical stimulation induced by 10 nM angiotensin II. (b and c) Superimposed tracings and time course show the effects of angiotensin II (1, 10, and 100 nM) on the AP duration and contractile force. (d) The concentration–response curve for angiotensin II's effect on the 90% of the AP duration (APD90) and contractile force (n=7). *P<0.05 versus before drugs.
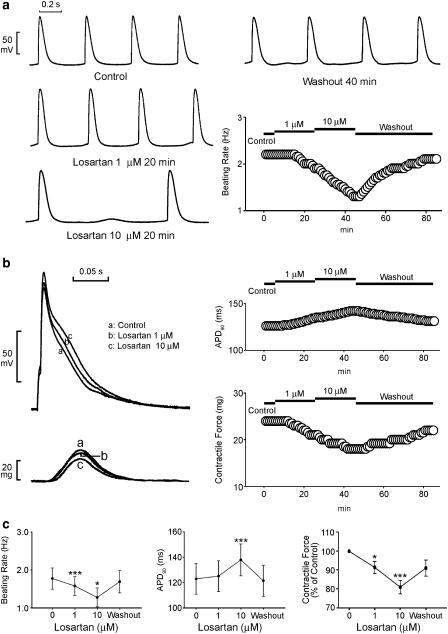
Losartan (1 and 10 μM) inhibited the automatic rhythm (Figure 3a) in the spontaneously active PV tissue preparations (n=7). This effect was reversible after the washout of losartan. Losartan (10 μM) prolonged the APD90 and losartan (1 and 10 μM) decreased the contractile force (Figure 3b) in the PVs without spontaneous activity (n=7). Figure 3c summarizes the effects of different concentrations of losartan on the PV automaticity, APD90, and contractile force.
Figure 3.
Effect of losartan on the PV electrical activity. (a) The tracings and time course show an example that losartan (1 and 10 μM) decreased the PV spontaneous activity. (b) The superimposed tracings and time courses of the effects of losartan (1 and 10 μM) on the AP duration and contractile force. (c) The concentration–response curve for losartan's effect on the automatic rhythm (n=7), APD90, and contractile force (n=7). *P<0.05, **P<0.01, ***P<0.001 versus before drugs.
Effect of angiotensin II and losartan on the membrane currents of PV cardiomyocytes
ICa-L
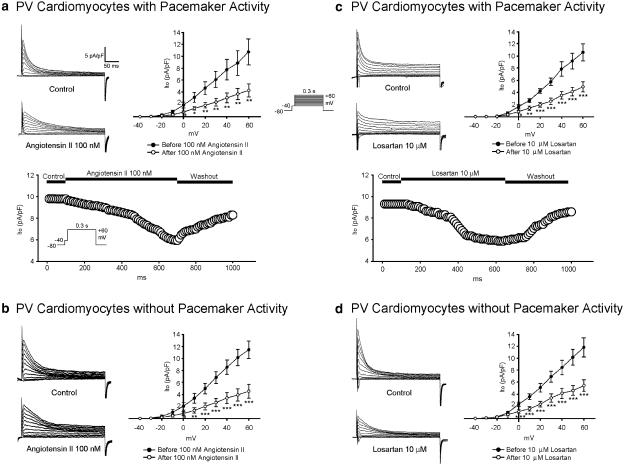
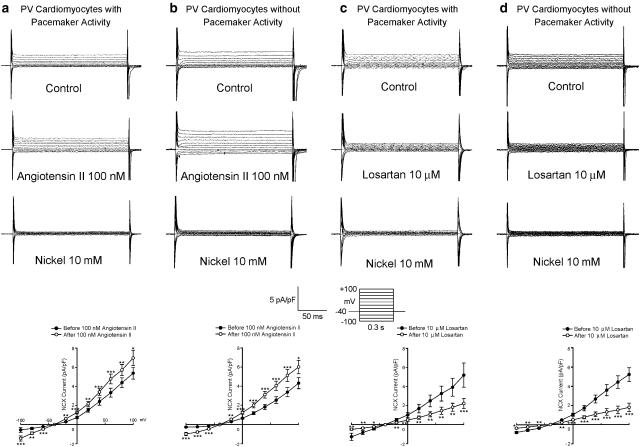
ICa-L was increased after the administration of angiotensin II (100 nM) in the PV cardiomyocytes with and without pacemaker activity (Figures 4a and b). However, losartan (10 μM) had little effect on ICa-L in the PV cardiomyocytes with and without pacemaker activity (Figures 4c and d).
Figure 4.
Effect of angiotensin II and losartan on ICa-L in PV cardiomyocytes. (a and b) The superimposed current traces (depolarization from −50 to +10 mV), time course, and I–V relationship (lower panels) of ICa-L before and after angiotensin II administration in PV cardiomyocytes with (n=10) and without (n=7) pacemaker activity. (c and d) Traces, time course, and I–V relationship before and after the administration of losartan in PV cardiomyocytes with (n=6) and without (n=7) pacemaker activity. *P<0.05 versus before drugs. The insets in the current traces show the various clamp protocols.
Ito, IK
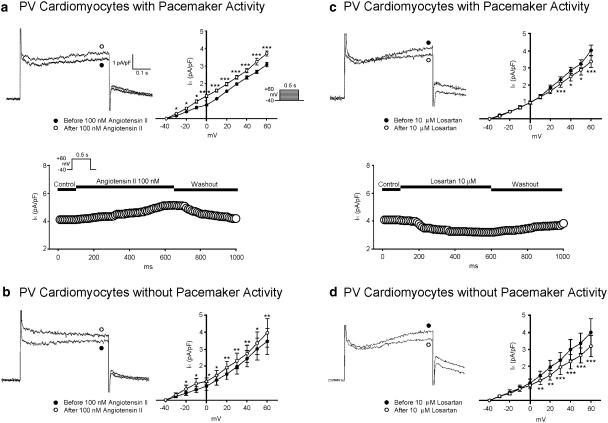
Ito was decreased after the administration of angiotensin II (100 nM) (Figures 5a and b) and losartan (10 μM) (Figures 5c and d) in the PV cardiomyocytes with and without pacemaker activity. IK was increased with the administration of angiotensin II (100 nM) (Figures 6a and b) and decreased with losartan (10 μM) (Figures 6c and d) in the PV cardiomyocytes with and without pacemaker activity.
Figure 5.
Effect of angiotensin II and losartan on Ito in PV cardiomyocytes. (a and b) Current traces, time course, and the I–V relationship (lower panels) of Ito before and after angiotensin II administration in PV cardiomyocytes with (n=7) and without (n=8) pacemaker activity. (c and d) Traces, time course, and the I–V relationship before and after the administration of losartan in PV cardiomyocytes with (n=8) and without (n=13) pacemaker activity. *P<0.05, **P<0.01, ***P<0.001 versus before drugs. The insets in the current traces show the various clamp protocols.
Figure 6.
Effect of angiotensin II and losartan on IK in the PV cardiomyocytes. (a and b) Superimposed current traces (depolarization from –40 to +60 mV), time course, and the I–V relationship (lower panels) of IK before and after the administration of angiotensin II in PV cardiomyocytes with (n=10) and without (n=7) pacemaker activity. (c and d) Traces, time course, and the I–V relationship before and after the administration of losartan in PV cardiomyocytes with (n=9) and without (n=7) pacemaker activity. The insets in the current traces show the various clamp protocols. *P<0.05, **P<0.01, ***P<0.001 versus before drugs.
IK1
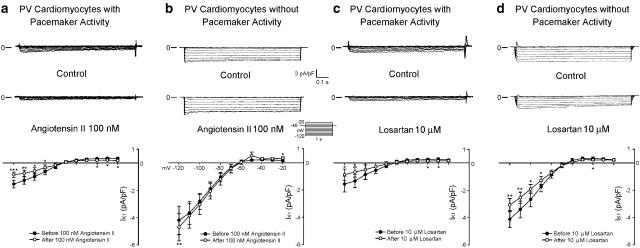
Angiotensin II decreased the IK1 in the PV cardiomyocytes with pacemaker activity (Figure 7a), but slightly increased the IK1 at the test potentials of −120 and −20 mV in the PV cardiomyocytes without pacemaker activity (Figure 7b). Losartan decreased the IK1 in both the PV cardiomyocytes with and without pacemaker activity (Figures 7c and d).
Figure 7.
Effect of angiotensin II and losartan on IK1 in PV cardiomyocytes. (a and b) Current traces and the I–V relationship (lower panels) of IK1 before and after the administration of angiotensin II in PV cardiomyocytes with (n=7) and without (n=6) pacemaker activity. (c and d) Traces and the I–V relationship before and after losartan in PV cardiomyocytes with (n=6) and without (n=6) pacemaker activity. The insets in the current traces show the various clamp protocols. *P<0.05, **P<0.01, ***P<0.001 versus before drugs.
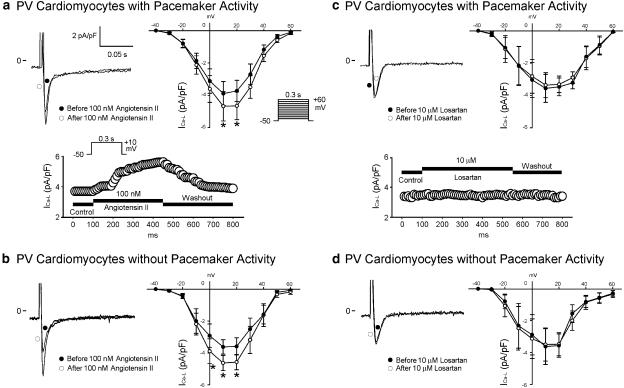
If
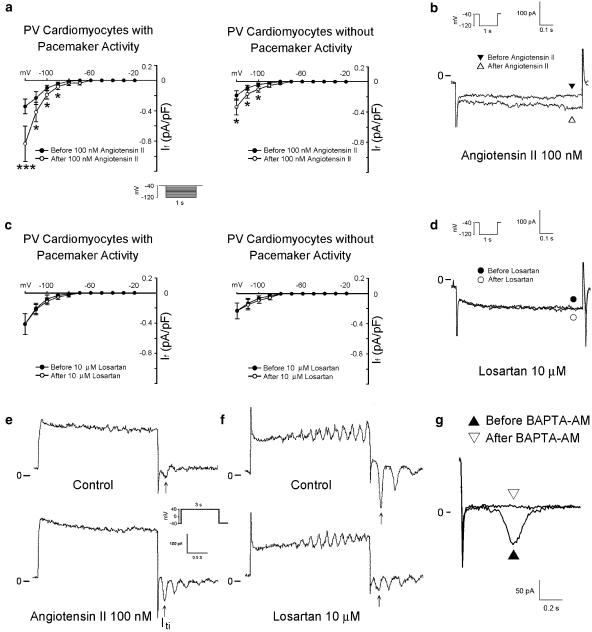
Angiotensin II (100 nM) increased If in the PV cardiomyocytes with and without pacemaker activity (Figure 8a). Figure 8b shows the tracings of If in the PV cardiomyocytes before and after the administration of angiotensin II (100 nM). Moreover, the increase in If due to angiotensin II (100 nM) was significantly larger in the PV cardiomyocytes with pacemaker activity than in those without pacemaker activity (at −120 mV, 0.49±0.15 versus 0.15±0.05 pA pF−1, P<0.05). The administration of losartan (10 μM) did not change If in the PV cardiomyocytes (Figure 8c). Figure 8d shows the tracings of If in the PV cardiomyocytes before and after the administration of losartan (10 μM).
Figure 8.
Effect of angiotensin II and losartan on If and Iti in PV cardiomyocytes. (a) The I–V relationship of If before and after the administration of angiotensin II in PV cardiomyocytes with (n=14) and without (n=17) pacemaker activity. (b) Superimposed current traces before and after the administration of angiotensin II. (c) The I–V relationship of If before and after the administration of losartan in PV cardiomyocytes with (n=8) and without (n=10) pacemaker activity. (d) There was a similar amplitude of If before and after the administration of losartan. (e and f) Angiotensin II increased Iti and losartan decreased Iti. The insets in the current traces show the clamp protocols. *P<0.05 versus before drugs. (g) The administration of BAPTA-AM (50 μM) reduces the inward currents in the PV cardiomyocytes confirms that this inward current on repolarization represents Iti.
Iti
Angiotensin II (100 nM) increased Iti in the PV cardiomyocytes with (from 0.43±0.10 to 0.68±0.08 pA pF−1, n=11, P<0.05) and without (from 0.31±0.09 to 0.45±0.12 pA pF−1, n=10, P<0.05) pacemaker activity. Figure 8e shows an example from a single PV cardiomyocyte with pacemaker activity before and after the administration of angiotensin II (100 nM). In contrast, losartan (10 μM) decreased the Iti in the PV cardiomyocytes with (from 0.57±0.18 to 0.25±0.09 pA pF−1, P<0.05, n=8) and without (from 0.43±0.11 to 0.18±0.07 pA pF−1, P<0.05, n=6) pacemaker activity. Figure 8f shows an example from a single PV cardiomyocyte with pacemaker activity before and in the presence of losartan (10 μM).
NCX currents
Angiotensin II increased the outward and inward nickel-sensitive NCX currents in the PV cardiomyocytes with and without pacemaker activity (Figures 9a and b). In contrast, losartan (10 μM) decreased the nickel-sensitive NCX currents in the PV cardiomyocytes with and without pacemaker activity (Figures 9c and d). As a small remnant current was present after the administration of nickel (10 mM), this study also investigated the effects of angiotensin II (100 nM) and losartan (10 μM) on the nickel-insensitive currents. In the presence of nickel (10 mM), there were no significant changes in the nickel-insensitive currents with the administration of angiotensin II (100 nM) (at +100 mV, 1.75±0.5 versus 1.81±0.43 pA pF−1, n=6) or losartan (10 μM) (at +100 mV, 1.97±0.23 versus 1.95±0.22 pA pF−1, n=6), which indicates that the currents affected by angiotensin II and losartan are the NCX currents in the PV cardiomyocytes.
Figure 9.
Effect of angiotensin II and losartan on the NCX currents in the PV cardiomyocytes. (a and b) Current traces and the I–V relationship of nickel-sensitive NCX currents before and after the administration of angiotensin II in PV cardiomyocytes with (n=6) and without (n=7) pacemaker activity. (c and d) Traces and the I–V relationship of nickel-sensitive NCX currents before and after the administration of losartan in PV cardiomyocytes with (n=6) and without (n=8) pacemaker activity. The insets in the current traces show the various clamp protocols. *P<0.05, **P<0.01, ***P<0.001 versus before drugs.
Interaction of losartan and angiotensin II on the arrhythmogenic activity of PV cardiomyocytes
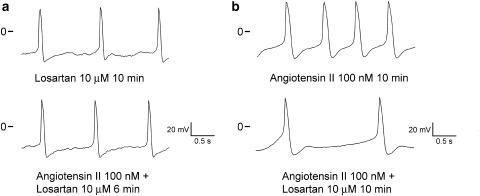
This study also examined whether angiotensin II had an effect on the arrhythmogenic activity of PV cardiomyocytes pretreated with losartan. As the examples show in Figure 10a, the administration of 100 nM angiotensin II in the presence of 10 μM losartan only slightly increased the automatic rhythm in the PV cardiomyocytes (from 0.7±0.2 to 0.9±0.3 Hz, n=5, P>0.05). Obviously, the concentration of 100 nM angiotensin II is not sufficient to overcome the block of 10 μM losartan. In addition, 10 μM losartan also inhibited the spontaneous activity of the PV cardiomyocytes (from 2.2±0.4 to 0.7±0.2 Hz, n=5, P<0.05) in the presence of 100 nM angiotensin II (Figure 10b).
Figure 10.
Interactions of losartan and angiotensin II on the PV electrical activity. (a) Angiotensin II (100 nM) only slightly increased the spontaneous activity in the presence of 10 μM losartan. (b) In the presence of angiotensin II (100 nM), 10 μM losartan inhibited the spontaneous activity of the PV cardiomyocytes.
Discussion
Effects of angiotensin II on the electrical activity of PVs
Previous studies have indicated that angiotensin II has positive inotropic and proarrhythmic effects (Allen et al., 1988; Chen et al., 1991; Gondo et al., 2001). In this study, we demonstrated that angiotensin II increased the PV spontaneous activity and triggered activity of delayed after-depolarizations, which confirmed that angiotensin II plays an important role in cardiac arrhythmias. As PVs play a pivotal role in the genesis of atrial fibrillation, these findings also indicated that angiotensin II may induce atrial fibrillation through increasing the PV arrhythmogenic activity.
AIIRBs have been shown to prevent the occurrence of atrial fibrillation; however, knowledge about the mechanisms is limited. Decreasing atrial stretch, preventing atrial fibrosis, or modifying the sympathetic tone has been proposed as the mechanisms of the AIIRB-related prevention of atrial fibrillation (Madrid et al., 2002). However, it is not clear whether AIIRBs may alter the PV electrical activity, thus reducing the occurrence of atrial fibrillation. In this study, we demonstrated that AIIRB can inhibit the PV spontaneous activity. As these effects were found in the absence of angiotensin II, these findings indicated that AIIRB had a direct antiarrhythmic effect through the reduction of the PV arrhythmogenic activity. In addition, treatment with losartan could prevent or attenuate the proarrhythmic effects of angiotensin II in the PV cardiomyocytes, which suggests that losartan also works as an antagonist of angiotensin II to prevent atrial fibrillation. Both these effects may reduce the occurrence of atrial fibrillation. It has been shown that the maximal plasma concentration of losartan is near 1 μM (Sasaki et al., 1996). Therefore, it is possible that the effects of losartan on the PVs are relevant to the clinical setting in vivo.
Effects of angiotensin II on membrane currents of PV cardiomyocytes
This study showed that angiotensin II increased ICa-L, IK, and inhibited Ito in PV cardiomyocytes. These results were similar to the known effects of angiotensin II on ventricular cardiomyocytes (Daleau & Turgeon, 1994; Boston et al., 1998). The increase in ICa-L with the administration of angiotensin II not only prolongs the AP duration but may also contribute to the enhanced contractile force of the PV specimen. Additionally, this study showed that AIIRB decreased Ito, and IK. These effects may contribute to the prolongation of the AP duration by AIIRB in PV cardiomyocytes.
In this study, angiotensin II was found to inhibit IK1 in the PV cardiomyocytes with pacemaker activity, which may facilitate the arrhythmogenic activity of the PV cells. However, angiotensin II only had a little effect on IK1 in the PV cardiomyocytes without pacemaker activity. Although the underlying mechanisms were not clear, the different pharmacological responses between PV pacemaker and nonpacemaker cells indicate the varying electrophysiological characteristics of PV cardiomyocytes. If is known to contribute to the automaticity of cardiomyocytes. However, the effects of angiotensin II and AIIRB on If are not clear. We found that angiotensin II increased If in PV cardiomyocytes, especially in those with pacemaker activity. Therefore, the angiotensin II-induced increase of If may enhance the automaticity of PV cardiomyocytes. However, the insignificant effects of AIIRB on If suggest that the direct antiarrhythmic effect of AIIRB in PV cardiomyocytes is not related to the function of If.
Iti has been shown to play an important role in the PV arrhythmogenic activity (Chen et al., 2001, 2002). Previous studies have shown that angiotensin II and AIIRB can increase and suppress Iti, respectively (Enous & Opie, 1994; Louch et al., 2000). In this study, we also demonstrated that Iti of PV cardiomyocytes was increased by the administration of angiotensin II and decreased by that of AIIRB. These findings may account for the antiarrhythmogenic effects of losartan and proarrhythmogenic effects of angiotensin II in PVs. Similar to the report in a previous study (Aiello et al., 2002), angiotensin II was found to increase the NCX currents in PV cardiomyocytes. In addition, we demonstrated that NCX currents were inhibited by AIIRB. NCX currents have been suggested to play a role in the genesis of Iti and also to contribute to the automaticity in cardiomyocytes. These results suggested that angiotensin II and AIIRB may alter the NCX currents and then change the Iti and arrhythmogenic activity of PV cardiomyocytes. It is possible that the decrease of NCX currents might be responsible for its direct antiarrhythmic effects of losartan and contribute to the low recurrence rate of atrial fibrillation in the patients receiving AIIRB. A previous study showed that the inhibition of the NCX currents may reduce the occurrence of atrial fibrillation through the prevention of atrial remodeling (Miyata et al., 2002). The decrease in the PV arrhythmogenic activity may be another important mechanism underlying the inhibition of NCX currents that result in a reduction in the atrial fibrillation. Moreover, changes in the NCX currents may contribute to the effects of angiotensin II and AIIRBs on the contractile force of the PV tissue specimens.
Conclusions
These studies showed that angiotensin II and AIIRB modulate the PV electrical activity, which may play a role in the pathophysiology of atrial fibrillation.
Acknowledgments
The present work was supported by the NSC 92-2314-B010-052, NSC 92-2314-B075-117, NSC92-2314-B038-054, VGH92-238, VGH92-243, VGH91-260, VGH91-44, VTY 92-P5-34, VTY92-P5-29, VTY91-P5-44, and RFCM 91-02019 grants.
Abbreviations
- AIIRB
angiotensin II receptor blocker
- AP
action potential
- APD90
AP duration at repolarization of 90% of the AP amplitude
- ICa-L
L-type calcium current
- If
pacemaker current
- IK
delayed rectifier potassium current
- IK1
inward rectifier potassium current
- Iti
transient inward current
- Ito
transient outward potassium current
- NCX
Na+–Ca2+ exchanger
- PV
pulmonary vein
References
- AIELLO E., VILLA-ABRILLE M.C., CINGOLANI H.E. Autocrine stimulation of cardiac Na+–Ca2+ exchanger currents by endogenous endothelin released by angiotensin II. Circ. Res. 2002;90:374–376. doi: 10.1161/hh0402.105373. [DOI] [PubMed] [Google Scholar]
- ALLEN I.S., COHEN N.M., DHALLAN R.S., GAA S.T., LEDERER W.J., ROGERS T.B. Angiotensin II increases spontaneous contractile frequency and stimulates calcium currents in cultured neonatal rat heart myocytes: insights into underlying biochemical mechanisms. Circ. Res. 1988;62:524–534. doi: 10.1161/01.res.62.3.524. [DOI] [PubMed] [Google Scholar]
- BLOM N.A., GITTENBERGER-DE GROOT A.C., DERUITER M.C., POELMANN R.E., MENTINK M.M., OTTENKAMP J. Development of the cardiac conduction tissue in human embryos using HNK-1 antigen expression: possible relevance for understanding of abnormal atrial automaticity. Circulation. 1999;99:800–806. doi: 10.1161/01.cir.99.6.800. [DOI] [PubMed] [Google Scholar]
- BOLDT A., WETZEL U., WEIGL J., GARBADE J., LAUSCHKE J., HINDRICKS G., KOTTKAMP H., GUMMERT J.F., DHEIN S. Expression of angiotensin II receptors in human left and right atrial tissue in atrial fibrillation with and without underlying mitral valve disease. J. Am. Coll. Cardiol. 2003;42:1785–1792. doi: 10.1016/j.jacc.2003.07.014. [DOI] [PubMed] [Google Scholar]
- BOSTON D.R., KOYAMA T., RODRIGUEZ-LARRAIN J., ZOU A., SU Z., BARRY W.H. Effects of angiotensin II on intracellular calcium and contracture in metabolically inhibited cardiomyocytes. J. Pharmacol. Exp. Ther. 1998;285:716–723. [PubMed] [Google Scholar]
- CHEN S.A., CHANG M.S., CHIANG B.N., CHENG K.K., LIN C.I. Electromechanical effects of angiotensin in human atrial tissues. J. Mol. Cell Cardiol. 1991;23:483–493. doi: 10.1016/0022-2828(91)90172-i. [DOI] [PubMed] [Google Scholar]
- CHEN S.A., HSIEH M.H., TAI C.T., TSAI C.F., PRAKASH V.S., YU W.C., HSU T.L., DING Y.A., CHANG M.S. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–1886. doi: 10.1161/01.cir.100.18.1879. [DOI] [PubMed] [Google Scholar]
- CHEN Y.C., CHEN S.A., CHEN Y.J., CHANG M.S., CHAN P., LIN C.I. Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J. Am. Coll. Cardiol. 2002;39:366–372. doi: 10.1016/s0735-1097(01)01731-4. [DOI] [PubMed] [Google Scholar]
- CHEN Y.J., CHEN S.A., CHANG M.S., LIN C.I. Arrhythmogenic activity of cardiac muscle in pulmonary vein of the dog: implication for the genesis of atrial fibrillation. Cardiovasc. Res. 2000;48:265–273. [Google Scholar]
- CHEN Y.J., CHEN S.A., CHEN Y.C., YEH H.I., CHAN P., CHANG M.S., LIN C.I. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: implication in initiation of atrial fibrillation. Circulation. 2001;104:2849–2854. doi: 10.1161/hc4801.099736. [DOI] [PubMed] [Google Scholar]
- DALEAU P., TURGEON J. Angiotensin II modulates the delayed rectifier potassium current of guinea pig ventricular myocytes. Pflugers Arch. 1994;427:553–555. doi: 10.1007/BF00374275. [DOI] [PubMed] [Google Scholar]
- ENOUS R., OPIE L.H. Effect of the angiotensin-converting enzyme inhibitor, perindoprilat, and of angiotensin-II on the transient inward current of rabbit ventricular myocytes. Cardiovasc. Drugs Ther. 1994;8:647–651. doi: 10.1007/BF00877418. [DOI] [PubMed] [Google Scholar]
- FEINBERG W.M., BLACKSHEAR J.L., LAUPACIS A., KRONMAL R., HART R.G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch. Intern. Med. 1995;155:469–473. [PubMed] [Google Scholar]
- GOETTE A., ARNDT M., ROCKEN C. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation. 2000a;101:2678–2681. doi: 10.1161/01.cir.101.23.2678. [DOI] [PubMed] [Google Scholar]
- GOETTE A., STAACK T., ROCKEN C., ARNDT M., GELLER J.C., HUTH C., ANSORGE S., KLEIN H.U., LENDECKEL U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J. Am. Coll. Cardiol. 2000b;35:1669–1677. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- GONDO N., KUMAGAI K., NAKASHIMA H., SAKU K. Angiotensin II provokes cesium-induced ventricular tachyarrhythmias. Cardiovasc. Res. 2001;49:381–390. doi: 10.1016/s0008-6363(00)00249-2. [DOI] [PubMed] [Google Scholar]
- HAISSAGUERRE M., JAIS P., SHAH D.C., TAKAHASHI A., HOCINI M., QUINIOU G., GARRIGUE S., LE MOUROUX A., LE METAYER P., CLEMENTY J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- HOCINI M., HO S.Y., KAWARA T., LINNENBANK A.C., POTSE M., SHAH D., JAIS P., JANSE M.J., HAISSAGUERRE M., DE BAKKER J.M. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation. 2002;105:2442–2448. doi: 10.1161/01.cir.0000016062.80020.11. [DOI] [PubMed] [Google Scholar]
- LOUCH W.E., FERRIER G.R., HOWLETT S.E. Losartan improves recover of contraction and inhibits transient inward current in a cellular model of cardiac ischemia and reperfusion. J. Pharmacol. Exper. Ther. 2000;295:697–704. [PubMed] [Google Scholar]
- MADRID A.H., BUENO M.G., REBOLLO J.M., MARIN I., PENA G., BERNAL E., RODRIGUEZ A., CANO L., CANO J.M., CABEZA P., MORO C. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002;106:331–336. doi: 10.1161/01.cir.0000022665.18619.83. [DOI] [PubMed] [Google Scholar]
- MIYATA A., ZIPES D.P., HALL S., RUBART M. KB-R7943 prevents acute, atrial fibrillation-induced shortening of atrial refractoriness in anesthetized dogs. Circulation. 2002;106:1410–1419. doi: 10.1161/01.cir.0000028587.85711.f6. [DOI] [PubMed] [Google Scholar]
- NAKASHIMA H., KUMAGAI K., URATA H., GONDO N., IDEISHI M., ARAKAWA K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. 2000;101:2612–2617. doi: 10.1161/01.cir.101.22.2612. [DOI] [PubMed] [Google Scholar]
- PAPPONE C., ROSANIO S., ORETO G., TOCCHI M., GUGLIOTTA F., VICEDOMINI G., SALVATI A., DICANDIA C., MAZZONE P., SANTINELLI V., GULLETTA S., CHIERCHIA S. Circumferential radiofrequency ablation of pulmonaryvein ostia: a new anatomic approach for curing atrial fibrillation. Circulation. 2000;102:2619–2628. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- PEREZ-LUGONES A., MCMAHON J.T., RATLIFF N.B., SALIBA W.I., SCHWEIKERT R.A., MARROUCHE N.F., SAAD E.B., NAVIA J.L., MCCARTHY P.M., TCHOU P., GILLINOV A.M., NATALE A. Evidence of specialized conduction cells in human pulmonary veins of patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2003;14:803–809. doi: 10.1046/j.1540-8167.2003.03075.x. [DOI] [PubMed] [Google Scholar]
- SASAKI M., FUJIMURA A., HARADA K., SUNAGA K., EBIHARA A. Clinical pharmacology of multiple-dose losartan, an angiotensin II receptor antagonist, in patients with essential hypertension. J. Clin. Pharmacol. 1996;36:403–408. doi: 10.1002/j.1552-4604.1996.tb05026.x. [DOI] [PubMed] [Google Scholar]
- SUN Y., RAMIRES F.J.A., WEBER K.T. Fibrosis of atria and great vessels in response to angiotensin II or aldosterone infusion. Cardiovasc. Res. 1997;35:138–147. doi: 10.1016/s0008-6363(97)00097-7. [DOI] [PubMed] [Google Scholar]
- YU H., GAO J., WANG H., WYMORE R., STEINBERG S., MCKINNON D., ROSEN M.R., COHEN I.S. Effects of the rennin–angiotensin system on the currents I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ. Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- ZHOU S., CHANG C.M., WU T.J., MIYAUCHI Y., OKUYAMA Y., PARK A.M., HAMABE A., OMICHI C., HAYASHI H., BRODSKY L.A., MANDEL W.J., TING C.T., FISHBEIN M.C., KARAGUEUZIAN H.S., CHEN P.S. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1244–H1252. doi: 10.1152/ajpheart.01109.2001. [DOI] [PubMed] [Google Scholar]