Abstract
The addition of Ca2+ ionophore A23187 or ATP to freshly isolated or cultured pig coronary artery endothelial cells (PCEC) potentiated the release of ascorbate (Asc). Cultured PCEC were used to characterize the Ca2+-mediated release. An increase in Ca2+-mediated Asc release was observed from PCEC preincubated with Asc, Asc-2-phosphate or dehydroascorbic acid (DHAA).
The effects of various ATP analogs and inhibition by suramin were consistent with the ATP-induced release being mediated by P2Y2-like receptors.
ATP-stimulated Asc release was Ca2+-mediated because (a) ATP analogs that increased Asc release also elevated cytosolic [Ca2+], (b) Ca2+ ionophore A23187 and cyclopiazonic acid stimulated the Asc release, (c) removing extracellular Ca2+ and chelating intracellular Ca2+inhibited the ATP-induced release, and (d) inositol-selective phospholipase C inhibitor U73122 also inhibited this release.
Accumulation of Asc by PCEC was examined at Asc concentrations of 10 μM (Na+-Asc symporter not saturated) and 5 mM (Na+-Asc symporter saturated). At 10 μM Asc, A23187 and ATP caused an inhibition of Asc accumulation but at 5 mM Asc, both the agents caused a stimulation. Substituting gluconate for chloride did not affect the basal Asc uptake but it abolished the effects of A23187.
PCEC but not pig coronary artery smooth muscle cells show a Ca2+- mediated Asc release pathway that may be activated by agents such as ATP.
Keywords: ATP, oxidative stress, vitamin C, lectin, magnetic beads, P2Y2 receptors, dehydroascorbic acid
Introduction
Vitamin C (ascorbate, Asc) is an essential cofactor in carnitine and collagen synthesis, and an antioxidant that helps to protect cells against oxidative stress (May et al., 1995; 2003; Jones et al., 2002). Asc is involved in recycling oxidized tocopherol (Vitamin E) which protects the plasma membrane against lipid peroxidation (Jones et al., 2002; May et al., 2003). In humans and some other mammals, Asc is an essential vitamin and dietary intake leads to 50–100 μM of Asc in plasma (Weber et al., 1996; Jones et al., 2002; Woollard et al., 2002). In patients with low plasma levels of Asc, an increase in Asc intake prevents vascular endothelial dysfunction during atherosclerosis (Lehr et al., 1995; Weber et al., 1996; Carr et al., 2000; Woollard et al., 2002).
The absorption of Asc in the small intestine and uptake into various cells, including smooth muscle and endothelium, occurs via the Na+-Asc symporters SVCT1 and SVCT2 (Daruwala et al., 1999; Tsukaguchi et al., 1999; Holmes et al., 2000; May et al., 2001). SVCT1 is considered to be a low-affinity high-velocity carrier that is expressed in epithelial cells. SVCT2 has slightly higher affinity and is expressed in lower abundance in most cells including endothelial cells (Best et al., 2005). Asc that gets oxidized to dehydroascorbic acid (DHAA) can also be absorbed by the tissues via glucose transporters and recycled. The recycling requires enzymes that utilize reductants such as glutathione and NADPH and are present in most cells including vascular smooth muscle and endothelium (May et al., 2001; Holmes et al., 2002).
Several stimuli trigger an increase in cytosolic Ca2+ concentration ([Ca2+]i) and thus lead to increased NO production and release thereby inducing vasodilation (Bodin et al., 1991; Buxton et al., 2001; Braet et al., 2003; Sprague et al., 2003; Yamamoto et al., 2003). In vitro, Asc has been shown to protect NO from forming peroxynitrite but the Asc concentrations used in these experiments were 100-times higher than those present in the plasma (Jackson et al., 1998). Whereas the pathways for Asc entry into pig coronary artery endothelial cells (PCEC) have been identified, there are no reports to show if PCEC could release Asc upon stimulation that leads to increase in [Ca2+]i. Here, we report that A23187 and ATP elicit release of Asc from PCEC.
Methods
Materials
Niflumic acid, 5-nitro-2-(3-phenylpropylamino)benzoic acid, indanyloxyacetic acid-94, A23187, DHAA, ATP and its analogs, 1-[6-[((17b)-3-Methoxyestra-1,3,5[10]-trien-17-yl) amino] hexyl]-1H-pyrrole-2,5-dione (U73122), 1-[6-[((17b)-3-Methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-2,5-pyrrolidinedione (U73343, inactive analog used as a control), saponin, and lectin from Griffonia simplicifolia were purchased from Sigma-Aldrich Canada (Oakville, Canada). Fluo 3/AM was purchased from Teflabs (Austin, U.S.A.) and BAPTA/AM from Molecular Probes (Eugene, U.S.A.). Dynabeads M450 Epoxy were purchased from Dynal Biotech. Inc. (Lake Success, U.S.A.). Tissue culture supplies were obtained from Invitrogen (Burlington, Canada) and serum was purchased from CanSera (Etobicoke, Canada).
Asc release from freshly isolated PCEC
The following protocol was developed and optimized in initial experiments to study Asc release from freshly isolated PCEC attached to lectin-coated magnetic beads (Dynabeads) (Hewett & Murray, 1993; Wang et al., 2002). The magnet Dynal MPC-S (Dynal Biotech. Inc., Lake Success, U.S.A.) was used to concentrate the magnetic beads and a Dynal Biotech Sample Mixer was used for stirring. Magnetic beads (1.2 × 108 beads in 300 μl) were quickly washed two times in 0.1 M sodium borate buffer (pH 9.5) and then suspended in 480 μl of borate buffer and 120 μl of 1 mg lectin ml−1 in phosphate buffer saline solution (10 mM Na-phosphate and 140 mM NaCl pH 7.4). The beads were stirred for 24 h at 4°C, washed for 10 min in the phosphate buffer saline solution containing 0.1% bovine serum albumin, once each for 30 min and 20 h at 4°C and then resuspended in 300 μl of the same solution but containing 0.02% Na-azide. The lectin coated beads were stored at 4°C and used within 1–2 weeks. Pig hearts were obtained from Maple Leaf Meats (Burlington, Canada) and immediately placed in chilled physiological saline solution containing (in mM): 138 NaCl, 2 CaCl2, 10 glucose, 10 HEPES, 5 KCl, 1 MgCl2 at pH 6.4. Left anterior coronary artery was removed from the hearts and placed immediately in Krebs' solution containing (in mM): 115 NaCl, 5 KCl, 22 NaHCO3, 1.1 MgCl2, 1.7 CaCl2, 1.1 KH2PO4, 0.03 EDTA and 7.7 glucose at 22–24°C. The arteries were dissected open, endothelial cells dislodged using a cotton swab and placed in chilled Na-HEPES buffer containing (in mM): 134 NaCl, 5.4 KCl, 10 glucose, 0.8 MgSO4, 20 HEPES and 1.8 CaCl2, pH 7.3. Typically, endothelium from 40 hearts was pooled. The cells were mixed with 200 μl of beads and 200 μM DHAA in a 50 ml Ehrlenmeyer flask and gently shaken at 37°C for 30 min. Another 200 μM DHAA was added and the suspension containing beads and the cells was divided into 24 aliquots which were stirred at 22–24°C for another 30–60 min. The beads were washed 2 × with 25 μl of the Na-HEPES buffer and then suspended in 25 μl of the buffer with or without specified additives. Each sample was incubated for 5 min in a shaking water bath at 37°C and then placed on the magnet. The buffer was removed and added to a chilled tube containing 2.7 μl of 8.5% metaphosphoric acid. The contents of the tubes were then suspended in 25 μl of 0.85% metaphosphoric acid. The samples were stirred for 60 min at 4°C and then placed on the magnet and the suspension was removed and centrifuged at 14,000 × g for 2 min. The supernatant was saved for Asc measurement using HPLC. The samples for HPLC were cleared by centrifugation through 0.45 μM filters and saved at −80°C until use. The pellets containing the cell lysates were suspended in 2.5 μl 1 M tris and 22.5 μl of 100 mM tris-EDTA and used for protein estimation. Cells isolated by this method were positive for von Willebrand factor.
Cell cultures
PCEC were dislodged from the inner surface of coronary arteries using a sterile cotton swab and cultured as previously described (Grover & Samson, 1997). Briefly, the cells were plated in 6-well plates in Dulbecco's modified Eagle's medium supplemented with 0.5 mM 4-(2-hydroxyethyl-1-piperazine ethane sulfonate) (HEPES) pH 7.4, glutamine (2 mM), gentamicin (50 mg l−1), amphotericin B (0.125 mg l−1), and 10% fetal bovine serum. After being grown to confluence, the PCEC were removed from the plates by trypsinization (0.25% trypsin, 1 mM EDTA in Ca2+- and Mg2+-free Hank's balanced salt solution, Invitrogen) for 4 min at 37°C and replated. At the third passage a large stock of cells was frozen into aliquots. Confluent cell cultures from passage 4 were split one in six to 60 mm dishes and used on day 7 of growth. In Western blots, these cells reacted positively with anti-endothelial NO synthase and anti-von Willebrand factor but not with anti-smooth muscle α-actin (Grover & Samson, 1997). Pig coronary artery smooth muscle cells (PCSMC) were cultured and frozen after passage 2, thawed and cultured to be used at confluence after passage 4. PCSMC reacted positively with anti-smooth muscle α-actin and SERCA2b-selective antibody, IID8, but not with selective antibodies against SERCA2a, endothelial NO synthase or von Willebrand factor as described previously (Grover & Samson, 1997).
14C-Asc loading and release measurement
Cells were incubated for 18 h with 14C-Asc alone or with 14C-Asc plus 3H-deoxyglucose in the presence of 25 mM glucose. Preincubating the cells with 1 μM 3H-deoxyglucose did not affect cell growth, Asc accumulation or Asc release. The final Asc concentration was 200 μM including 20 μM of 14C-Asc (specific activity 13 mCi mmol−1, stored as an aqueous solution in 20 mM homocysteine at −80°C). Petridishes (60 mm) containing the cells were rinsed 2 × with a Na+-HEPES buffer prewarmed to 37°C (Holmes et al., 2000). This solution was 290–300 mOsm. Then the cells were placed in 1 ml of Asc-free Na+-HEPES buffer (with or without the specified additives) in a shaking water bath (30 r.p.m.) at 37°C. The cells were harvested in 1 ml water by scraping and used for protein estimation and scintillation counting. These procedures were modified as specified in the Results. A 60 mm dish of the cells contained 1.4±0.4 × 106 cells (0.20±0.07 mg protein). For monitoring 14C-Asc uptake over short times, the cells were washed with the Na+-HEPES buffer and placed in the same buffer with specified concentrations of 14C-Asc for 12 min at 37°C after which the plates were washed and the amount of radioactivity in the cells was determined.
Asc determination with HPLC in cultured cells
Cell scrapings or release solutions were acidified with metaphosphoric acid (final concentration 0.85%) and frozen immediately at −80°C and subsequently analyzed by HPLC with a Waters M460 amperometric detector as described previously (Holmes et al., 2000; 2002).
[Ca2+]i measurements
PCEC cultured on coverslips were rinsed with a solution containing (in mM): 115 NaCl, 5.8 KCl, 2 CaCl2, 0.6 MgCl2, 12 glucose, 25 HEPES-Na, pH 7.4 at 22–24°C. The cells, while still attached to the cover slips, were loaded with FLUO 3/AM and probenecid and then used for [Ca2+]i measurement as previously described (Grover & Samson, 1997).
Ethidium bromide staining
PCEC were cultured as described above but on a glass coverslip glued under a 5 mm hole in the Petridish, washed 2 × with the same buffer as used for the Asc efflux experiments and then placed in the same buffer containing ethidium bromide (20 μg ml−1) and specified additives. After 5 min at 37°C, the cells were washed 3 × in the buffer without the ethidium bromide or the additives and finally were viewed using an LSM510 confocal microscope.
Data analysis
Values presented are mean±s.e.m of the specified number of replicates. Student's t-test was used to test null hypotheses and P values <0.05 were considered to negate them. The results were also verified with a one-way ANOVA test. Each experiment was repeated two to four times with the specified number of replicates per experiment.
Results
Asc release from freshly isolated cells
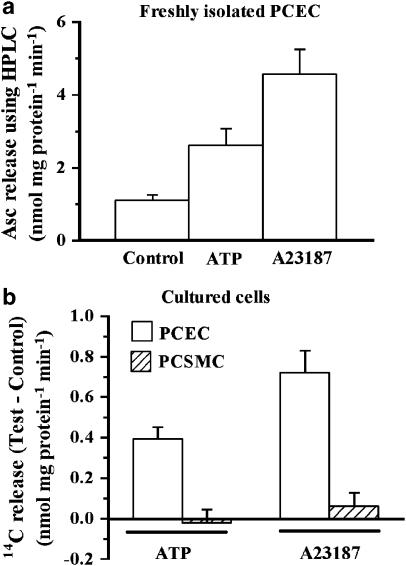
The total amount of PCEC cell protein obtained from 40 hearts was between 21 to 25 μg and we optimized the conditions for carrying out experiments using this small amount of material. Preincubation with DHAA, as described in the Methods, increased the authentic Asc content (determined using HPLC) of the cells from 13 nmol mg protein−1 to 134±10 nmol mg protein−1 (mean±s.e.m. of 100 measurements). This increase in Asc content permitted efflux studies using this small amount of material. Data pooled from experiments using PCEC on five different days showed that A23187 and ATP produced an increase in the release of authentic Asc (Figure 1a). The increase in Asc release obtained with A23187 was 314±62% over the release from control cells and it was significantly greater than that obtained with ATP (136±42%).
Figure 1.
A23187 and ATP-induced release of Asc from different cells. (a) Freshly isolated PCEC. Cells were isolated and incubated with DHAA as described in the Methods. For each tube, Asc released in 5 min and that remaining in the cells was determined by HPLC. Percent release was computed as 100 × Asc released/(Asc released+Asc remaining). The values are mean±s.e.m. from data pooled from experiments on five different days from a total of 32, 20 and 17 tubes for control, 100 μM ATP and 10 μM A23187, respectively. Both agents caused a significant (P<0.05) increase in the release. (b) Comparison of 14C release from cultured PCEC and PCSMC. PCEC and PCSMC were preincubated with 14C-Asc and used for release in paired experiments. Mean value of total Asc content was 12.9 nmol mg protein−1 in PCEC and 20.9 nmol mg protein−1 in PCSMC. Values are mean±s.e.m. of six replicates. Test–Control values differed significantly from zero (P<0.05) for PCEC but not for PCSMC from the no additive control. The paired experiment was replicated three times and the effect on the two cell types was also examined several times in non-paired experiments.
ATP and A23187 release 14C-Asc from PCEC but not PCSMC
Next, we tested if cells cultured from PCEC and PCSMC also showed similar increases in release. Characteristics of the PCEC and PCSMC have been reported earlier (Grover & Samson, 1997). In Western blots, the lysates from PCEC react positively to anti-von Willebrand factor and anti-endothelial NO synthase but negatively to anti-smooth muscle α-actin. Conversely, the lysates from PCSMC react positively to anti-smooth muscle α-actin but not to anti-von Willebrand factor or anti-endothelial NO synthase.
The cultured cells when tested using HPLC did not contain any Asc, unlike freshly isolated cells. Therefore, PCEC were first preincubated with 14C-Asc and then 14C release was examined. As cultured cells did not have the same limitation of availability of material as the fresh cells, they were preincubated with 14C-Asc to attain total cell levels of Asc (12.9 nmol mg protein−1) similar to the natural Asc content of the freshly isolated PCEC (13 nmol mg protein−1). Hence, the absolute values of release with the cultured PCEC were significantly smaller than those obtained in Figure 1a. However, A23187 and ATP increased 14C release (Figure 1b), with the increase being significantly greater with A23187 than with ATP. In contrast, neither agent increased 14C release from PCSMC. Thus, freshly isolated or cultured PCEC showed this release but PCSMC did not. As the amount of freshly isolated PCEC was so limited, cultured PCEC were used in subsequent experiments.
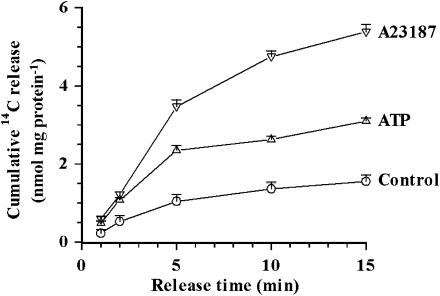
Time course of 14C-Asc release from cultured PCEC
Figure 2 shows the time course of cumulative release of 14C from PCEC preincubated with 14C-Asc. Both, A23187 and ATP, produced a significant increase in the 14C release that corresponded to 200–300 and 100–150% acceleration over the basal values, respectively. Rates of the ATP-induced 14C-Asc release were significantly greater for the first 5 min than for later periods. Therefore, release over 5 min was examined in all the subsequent experiments.
Figure 2.
Time course of cumulative release from PCEC preincubated with 14C-Asc for 18 h. Cumulative 14C-release after 1, 2, 5, 10 and 15 min calculated as nmol mg protein−1. The values are mean±s.e.m. of five replicates. At 2, 5, 10 and 15 min the release with 10 μM A23187 and 100 μM ATP differed significantly (P<0.05) from the control values without any additives.
ATP and A23187 do not alter permeability of PCEC
Cells were incubated with 14C-Asc and 3H-deoxyglucose. The latter is converted intracellularly to the anion 3H-deoxyglucose-phosphate which has a molecular weight similar to Asc. ATP and A23187 increased the efflux of 14C-Asc but not that of 3H-deoxyglucose (data not shown). We also estimated the amount of protein in the efflux solution to test the possibility that A23187 or ATP may damage or dislodge the cells during the experiment. However, neither agent significantly affected the protein in the efflux solution.
Staining of nuclei of PCEC with ethidium bromide was used as another measure of cell permeability. Ethidium bromide does not readily cross the cell membrane but can go through membranes of damaged cells, large channels created by P2X7 receptors (Ke et al., 2003) or large pores created by saponin. Nuclei were not stained for control cells or for those incubated with ATP or A23187, but nearly all of the nuclei were stained in the saponin permeabilized PCEC used as a positive control (data not shown). Thus, the 14C-Asc release induced by ATP and Ca2+ ionophore did not represent a general increase in membrane permeability.
HPLC and 14C-Asc measurements of release from PCEC
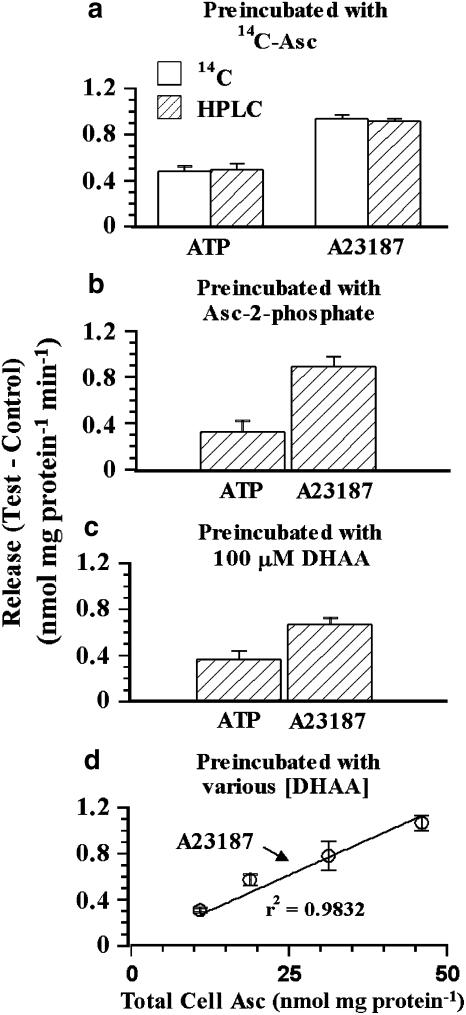
We preincubated PCEC with 14C-Asc and then examined the release in a 5 min period for authentic Asc by HPLC-based electrochemical assay and by 14C scintillation counting (Figure 3a). Analysis using HPLC and scintillation counting gave identical results (P>0.05) for the increase in the release with A23187 or ATP. Thus, the released material was Asc and not a degradation product (Figure 3a).
Figure 3.
Effects of ATP and A23187 on Asc release by HPLC-based electrochemical assay. (a) PCEC were preincubated with 14C-Asc and the cells were rinsed with the release solution used in Figure 2. The induced release (Test (ATP, A23187)–Control) was determined for 5 min using scintillation counting (14C) or HPLC (Asc). Values are mean±s.e.m. of five replicates. The Test–Control value for ATP or A23187 did not depend (P>0.05) on the method of measurement (HPLC or 14C). (b) Asc release induced by 10 μM A23187 or 1 mM ATP using HPLC from PCEC preincubated for 18 h with 400 μM Asc 2-phosphate. (c) Asc release from PCEC preincubated with 100 μM DHAA for 30 min. Values are mean±s.e.m. of 12 replicates. A23187 and ATP increased the Asc release significantly when the cells were preincubated with Asc 2-phosphate or DHAA (P<0.05). (d) Relationship between total Asc content of cells and A23187-induced Asc release. Different levels of total cell Asc were obtained as in (c) but by incubating the cells in the presence of various concentrations of DHAA. The amount released mg protein−1 min−1 correlated linearly (r2=0.9832, P<0.05) with the total loading (nmol mg protein−1).
Cells can take up Asc-2-phosphate and convert it into Asc. ATP and A23187 stimulated Asc release from PCEC which had been preincubated with Asc for 18 h using 400 μM Asc-2-phosphate (Figure 3b). Cells can also take up DHAA via a glucose transporter and reduce it to Asc (Holmes et al., 2002; May et al., 2003). ATP and A23187 stimulated Asc release from PCEC which had been preincubated with DHAA (Figure 3c). Thus, ATP and A23187 stimulated authentic Asc release in PCEC preincubated with Asc, Asc-2-phosphate or DHAA.
Next, we preincubated the cells with different concentrations of DHAA to examine the relationship between total cell Asc content on the A23187-induced Asc release. The absolute amount of A23187 stimulated release was proportional to the total Asc content of the cells (Figure 3d), that is, Asc released when determined as percent of uptake was independent of the level of loading. In another set of experiments, PCEC were incubated for a short time (1 h instead of 18 h) and at a lower concentration of 14C-Asc (50 μM instead of 200) to decrease the 14C-Asc accumulation. Values for the Asc released upon stimulation with ATP and A23187 as percent of total cell Asc content were independent of the total cell content (data not shown). These experiments, using different agents and conditions to alter the Asc content of the cultured PCEC, argue against the A23187-induced release being from only certain subcellular 14C-Asc pools.
Nature of receptors involved in ATP-induced 14C-Asc release from cultured PCEC
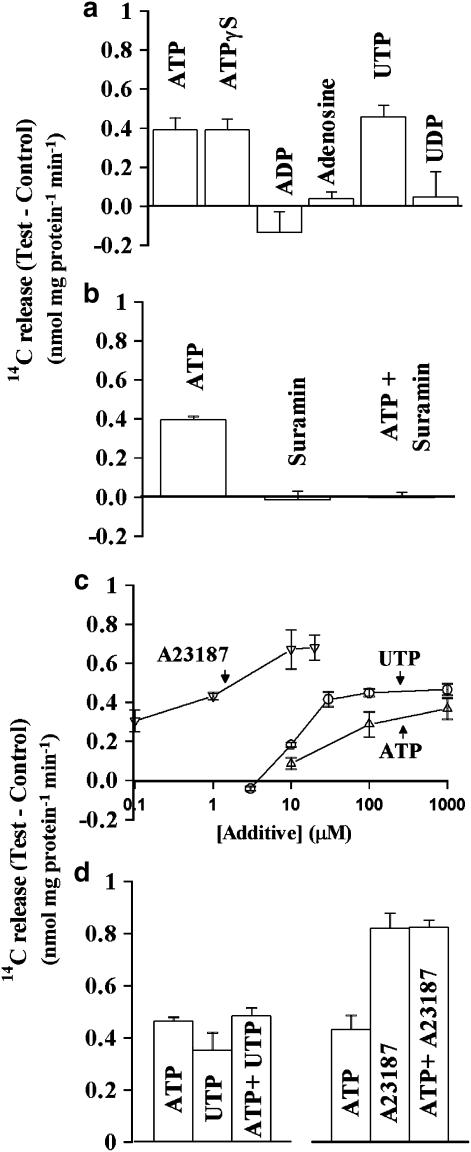
We determined the effects of adding several ATP metabolites and analogs on the 14C-Asc release from PCEC (Figure 4a). ATP, ATP-γ-S and UTP increased the release but adenosine, ADP and UDP (100 μM for all agents) did not. The ATP-induced release was inhibited by suramin (Figure 4b). Figure 4c shows that the Asc release depended on the concentrations of A23187, ATP and UTP. A23187 at 10 μM was saturating because the release obtained using 20 μM A23187 was not any greater. Release with UTP occurred at slightly lower concentrations than with ATP (Figure 4c) but both agents caused a similar maximum release. Adding UTP with a saturating concentration of ATP did not further increase the 14C-Asc release (Figure 4d) suggesting that the two agents acted via a common pathway. Based on the actions of various analogs and on the inhibition by suramin, ATP appeared to act through P2Y2-like nucleotide receptors (Von & Wetter, 2000). ATP did not increase the release produced with 10 μM A23187 (Figure 4d) suggesting that the ATP-induced release occurred via a Ca2+-dependent pathway which was saturated in the presence of 10 μM A23187. CPA-induced Asc release (0.76±0.12 nmol mg protein−1 min−1) was comparable to that with A23187 (0.89±0.06 nmol mg protein−1 min−1) thus confirming the role of Ca2+.
Figure 4.
Effects of ATP analogs and A23187 on 14C-Asc release from PCEC. (a) Effects of ATP analogs. PCEC were preincubated with 14C-Asc and used for 14C release over 5 min. The data are from several individual experiments. In each experiment, the effect of an agent was compared in five plates with 5–7 plates of the control. The experiments have been replicated more than 30 times with ATP and 2–3 times with each of the other agents. Additional release was observed with 100 μM for ATP, ATP-γ-S, UTP, and 10 μM for A23187 (P<0.05) but not with 100 μM ADP and UDP or 1 mM adenosine (P>0.05). (b) Effect of 300 μM suramin on the increase in 14C-Asc release produced by 100 μM ATP. Values are mean±s.e.m. of five replicates. Suramin inhibited the release induced by ATP (P<0.05). (c) Effects of different concentrations of A23187, ATP and UTP. The data are mean±s.e.m. from 4–5 replicates. (d) Effects of 1 mM ATP alone or with 100 μM UTP or 10 μM A23187. The data are mean±s.e.m. from 4 to 5 replicates. Inclusion of UTP with ATP did not produce any additional release (P>0.05). The release obtained with ATP plus A23187 did not differ significantly from that obtained with A23187 alone (P>0.05).
Ca2+ dependency of the 14C-Asc release
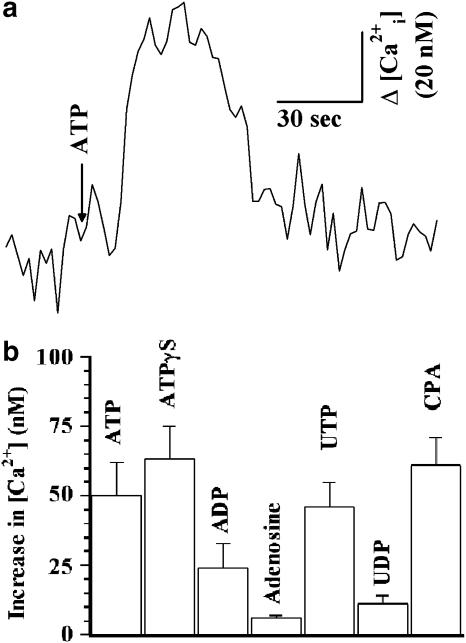
Because A23187 and the sarco/endoplasmic reticulum pump inhibitor CPA stimulated the 14C-Asc release from PCEC, we examined the effects of various agents on [Ca2+]i. Figure 5a is a typical tracing showing the effect of 100 μM ATP on [Ca2+]i in PCEC. ATP at concentrations of 10, 100 and 1000 μM increased [Ca2+]i by 5±1, 50±12 and 65±15 nM, respectively. The increase in [Ca2+]i was observed with 100 μM ATP, ATPγS and UTP, and 10 μM CPA (Figure 5b). ADP and UDP (both 100 μM) produced smaller increases in [Ca2+]i and adenosine did not produce a significant increase. The enhancement in 14C-Asc release obtained with different agents and at different concentrations of ATP correlated positively (r2=0.6530, P<0.05) with the increase in [Ca2+]i.
Figure 5.
(a) Tracing showing the effect of 100 μM ATP on [Ca2+]i in PCEC. [Ca2+]i were determined as described in the Methods. A change in [Ca2+]i is shown as Δ[Ca2+]i in nM. (b) Effects of different agents on [Ca2+]i in PCEC. In 5–10 tracings such as in (a), an increase in [Ca2+]i was observed with 100 μM ATP, ATP-γ-S or UTP, 10 μM A23187 or CPA (P<0.05), a much smaller increase was observed with 100 μM ADP and UDP but none with 1 mM adenosine.
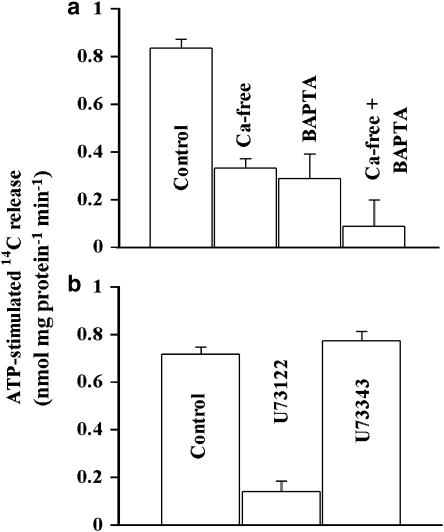
The ATP-induced 14C-Asc release was lower in nominally Ca2+-free solution (0 mM added CaCl2) than in the solution used in most experiments (1.5 mM CaCl2, Figure 6a). The ATP-induced release was also significantly lower from cells loaded with BAPTA (intracellular Ca2+-chelating agent) than from the control PCEC (Figure 6a). The ATP-dependent release was abolished when cells were loaded with BAPTA and placed in nominally Ca2+-free solution (Figure 6a). The inositol phosphate (IP3)-specific phospholipase C inhibitor U73122 (Agarwal et al., 1993) decreased the ATP-induced 14C-Asc release. The effect of U73122 was selective in that the structurally related inactive analog U73343 had no effect (Figure 6b) suggesting that the release of IP3 is a step in the ATP-induced 14C-Asc release. The L-type voltage gated Ca2+-channel blocker nitrendipine (10 μM) did not alter the 14C-Asc release (data not shown).
Figure 6.
Effects of Ca2+ manipulations on Asc release. (a) Effects of removing extracellular Ca2+ and chelating intracellular Ca2+ on ATP-stimulated 14C-Asc release. Note that the basal 14C-Asc release was examined under each condition to determine the ATP-stimulated release. The data are mean±s.e.m. from 4 to 5 replicates. Placing the cells in nominally Ca2+-free solution or loading them with BAPTA significantly lowered (P<0.05) the ATP-induced 14C-Asc release. The two treatments together lowered the ATP-induced release even further (P<0.05). The experiments were replicated three times. (b) Effect of IP3-specific phospholipase inhibitor on 14C-Asc release. Appropriate vehicle controls were used in the experiments. U73122 significantly decreased the ATP-stimulated 14C-Asc release (P<0.05) but U73343 had no effect (P>0.05).
As an increase in [Ca2+]i would also activate endothelial NO synthase, we also tested if the NO synthase inhibitor L-nitroarginyl methyl ester (L-NAME) would affect the A23187-stimulated Asc release. However, 100 μM L-NAME which is sufficient to block the endothelium-dependent relaxation produced by A23187 (Garcia-Villalon et al., 1993), did not affect the stimulated Asc release (Table 1).
Table 1.
Effect of L-NAME on Asc efflux
| Additive | 14C-Asc release (nmol mg protein−1 min−1) |
|---|---|
| None | 1.12±0.06 |
| A23187 (10 μM) | 2.07±0.15 |
| L-NAME (100 μM) | 1.33±0.05 |
| A23187 (10 μM)+L-NAME (100 μM) | 1.95±0.08 |
Note: The above values are mean±s.e.m. of 12 replicates. A23187 increased the Asc release (P<0.05). L-NAME did not affect basal or the A23187-stimulated 14C-Asc release (P>0.05).
Effects of anion channel inhibitors on 14C-Asc release
The anion channel blockers (Landry et al., 1987; Nilius & Droogmans, 2003) 300 μM niflumic acid, 5-nitro-2-(3-phenylpropylamino) benzoic acid, indanyloxyacetic acid-94, and 1 mM sulfinpyrazone inhibited the ATP-induced 14C-Asc release from PCEC by 87±17, 95±9, 97±6, and 97±12%, respectively. PCEC also contain a Na+-Asc symporter with a Km value of 27±3 μM for Asc (Best et al., 2005). Similarly, the anion channel blockers 300 μM niflumic acid, 5-nitro-2-(3-phenylpropylamino)benzoic acid, indanyloxyacetic acid-94, and 1 mM sulfinpyrazone also inhibited the Asc accumulation through the Na+-Asc symporter by 85±4, 70±6, 88±5, and 92±1%, respectively.
Effects of ATP and A23187 on Asc influx
Using HPLC, Asc could not be detected in cultured PCEC unless they were previously incubated with Asc. Next, we studied the acute 14C-Asc accumulation in these cells to test the hypothesis that the ATP- or A23187-stimulated Asc release occurred by diffusion and not by Na+-Asc symporter. The rationale was that in a solution containing 5 mM Asc, the inward uptake via the Na+-ascorbate symporter (Km for Asc=27+3 μM) (Best et al., 2005) would be fully saturated. Hence, an increase in Asc accumulation with ATP or A23187 would signify either an increase in the maximum velocity of the Na+-ascorbate symporter or Asc entry by diffusion. At 10 μM extracellular Asc, the Na+-ascorbate symporter would not be saturated and hence uptake via the symporter would dominate. If ATP and A23187 were to increase maximum velocity of the symporter, they would also increase Asc accumulation at 10 μM Asc. Accumulation via the symporter has been shown to produce very high concentrations of cytosolic Asc. Therefore, if ATP and A23187 were to stimulate Asc by diffusion, they would decrease the net Asc accumulation at 10 μM Asc and increase it at 5 mM Asc. Consistent with the diffusion hypothesis, ATP and A23187 decreased Asc accumulation at 10 μM Asc (Figure 7a) but they increased it at 5 mM Asc (Figure 7b). The increased Asc accumulation was monitored with 14C-Asc and also verified using HPLC. As in the efflux experiments, the effects of ATP on Asc accumulation were inhibited by suramin (data not shown).
Figure 7.
Effects of ATP and A23187 on 14C-Asc accumulation by PCEC from solutions containing 10 μM or 5 mM Asc. PCEC were not previously incubated with Asc. [Ascout] indicates the concentration of 14C-Asc in the uptake solution. The data are mean±s.e.m. of five replicates in a and 10 replicates in b, c and d. (a) Effects of 1 mM ATP and 10 μM A23187 on accumulation from a solution containing 10 μM Asc. The data are mean±s.e.m. of 10 replicates. A23187 and ATP inhibited the accumulation (P<0.05). (b) Effects of 1 mM ATP and 10 μM A23187 on accumulation from a solution containing 5 mM Asc. A23187 and ATP activated the accumulation (P<0.05). (c) Effect of anion substitution and A23187 on Asc accumulation from a solution containing 10 μM Asc. Substituting gluconate for chloride overcame the inhibition of accumulation produced with A23187 (P<0.05). (d) Effect of anion substitution and A23187 on Asc accumulation from a solution containing 5 mM Asc. Substituting gluconate for chloride overcame the activation of accumulation produced with A23187 (P<0.05).
Substituting gluconate for chloride in the bathing solution abolished both the effects of A23187, that is, the decrease in Asc accumulation at 10 μM Asc and the increase at 5 mM Asc (Figure 7c and d). These data suggest the Ca2+-dependent increase in diffusion of Asc is mediated by carriers or channels.
Discussion
Stimulation of PCEC with ATP and Ca2+ ionophore resulted in release of authentic Asc, as identified by an HPLC-based electrochemical assay. It differs from the loss of Asc owing to oxidation that occurs when the cells are exposed to hydrogen peroxide or superoxide (Holmes et al., 2000). In the latter instance, there is a loss of Asc from the cells but no increase in Asc in the medium. The accelerated Asc release was not due to a general increase in membrane permeability. 14C-Asc was dissolved in homocysteine and this resulted routinely in 30 μM homocysteine in the medium. Homocysteine in the medium may alter cellular metabolism (Raposo et al., 2004). Asc was introduced into cells using ascorbate-2-phosphate or DHAA but no homocysteine and, under these conditions, ATP and A23187 accelerated the Asc release, thereby ruling out homocysteine as the cause of ATP- or A23187-stimulated Asc release. Furthermore, to vary the 14C-Asc concentrations, 20–60 μM homocysteine was used in initital experiments and the accelerated release was observed. We also conducted an experiment where 1 mM dithiothreitol was included during the preincubation with 14C-Asc. The accelerated release was still observed (data not shown). It is noted that oxidizing and reducing agents such as dithiothreitol and hydrogen peroxide cannot be used in the efflux solution because they modify the nature of the released substance.
The increase in release caused by several agents correlated with the increase in [Ca2+]i they produced. However, the increase in [Ca2+]i appeared to precede the Asc release. Reasons for this delay are not known at present. In the presence of A23187, ATP did not produce an additional release. Thus, the actions of the various agents used was consistent with a Ca2+-mediated release of Asc. This was also confirmed in the experiments using nominally Ca2+-free solutions and chelation of [Ca2+]i with BAPTA. The results with U73122 suggest the involvement of the IP3 selective phospholipase C (Agarwal et al., 1993) in the ATP-stimulated Asc release. In astrocytes, glutamate activates Asc efflux but it has not been established if the mechanism requires an increase in [Ca2+]i (Siushansian et al., 1996; Wilson et al., 2000). Asc is also released from heptatocytes and hepatic endothelium but the mechanism underlying the release is not known (Upston et al., 1999). Also, the pathways reported here for PCEC differ from those in PGF2α luteal cells where Ca2+-ionophore does not potentiate Asc release (Pepperell et al., 2003).
As the increase in Asc release by several agents correlated with the increase in [Ca2+]i, which may also activate the endothelial NO synthase, one needs to consider if the two are related. It is also interesting to note that smooth muscle that does not express the endothelial NO synthase did not show any increase in Asc release with ATP or A23187. ATP, CPA and A23187 can cause endothelium-dependent relaxation and a large component of it is blocked with the NO synthase inhibitor L-NAME (White & Angus, 1987; Grover & Samson, 1997; Yamamoto et al., 2003). However, L-NAME did not affect the stimulation of Asc release by A23187 indicating that there is no direct link between stimulation of the two pathways. Asc also plays a key antioxidant role in endothelial cells as shown with menadione (May et al., 2003). Menadione causes an increase in oxidative stress and increases L-arginine uptake by endothelial cells, but it inhibits endothelial NO synthase. The inhibition of NO synthase is prevented by acute loading with ascorbate. Oxidative stress caused by relatively high concentrations of NO is also prevented by ascorbate (May & Qu, 2004). Thus, there is no proven link between stimulation of NO and Asc release. However, one cannot rule out the possibility that the co-release of Asc may protect either NO or the endothelial cell surface from damage owing to oxidative stress. In patients with low plasma levels of Asc, an increased Asc intake prevents vascular endothelial dysfunction during atherosclerosis (Lehr et al., 1995; Weber et al., 1996; Carr et al., 2000; Woollard et al., 2002). Organic nitrates have been used for the treatment of ischemic heart disease (Csont & Ferdinandy, 2005). ATP causes increased NO release from endothelium (Yamamoto et al., 2003). Organic nitrates and the released NO act to protect by causing vasodilation. However, NO can also react with superoxide to produce the highly deleterious peroxynitrite during ischemic reperfusion and preconditioning (Ferdinandy & Schulz, 2003). We conjecture that a co-release of the antioxidant Asc could protect the released NO by preventing peroxynitrite formation. If so, the release of Asc from endothelium may have implications in ischemic heart disease and preconditioning.
In order to consider potential mechanisms of the stimulated Asc release, we first present the kinetic parameters for the Na+-Asc symporter of PCEC: maximum velocity of 0.3–0.6 nmol mg protein−1 min−1, Km for Na+ is 73±14 mM, Hill coefficient for Na+ is 2 (Best et al., 2005). The rates of Asc release with A23187 (0.6–0.8 nmol mg protein−1 min−1) exceeded this maximum velocity of the symporter. Even the rate of ATP-induced release (0.2–0.4 nmol min−1 mg protein−1) would require an unattainable intracellular Na+ concentration of 65–93 mM. Thus, it is not kinetically feasible for the Ca2+-mediated release to occur via the Na+-Asc symporter. Further, the experiments on Asc uptake at low and high extracellular [Asc] provide evidence for a pathway involving diffusion. It is possible that the Asc diffusion occurred through Ca2+-dependent anion channels. Most, but not all, anion channels are inhibited by sulfinpyrazone and other sulfonate anion transport inhibitors such as 5-nitro-2-(3-phenylpropylamino) benzoic acid, indanyloxyacetic acid-94, and niflumic acid (White & Angus, 1987; Grover & Samson, 1997; Nilius & Droogmans, 2003; Yamamoto et al., 2003). The Asc release was inhibited by these agents but this does not provide conclusive evidence as these agents also inhibited the Na+-Asc symporter. Inhibition of the effects of A23187 in experiments where gluconate was substituted for chloride, favors the anion channel hypothesis because gluconate permeates slowly through Ca2+-dependent anion channels (Kim et al., 2003; Nilius & Droogmans, 2003) and it inhibited the stimulated Asc release.
Acknowledgments
We thank Melanie E. Holmes, Ewa Jaworski and James Mwanjewe for help with some of the experiments, and Dr S.J. Dixon (University of Western Ontario) for helpful suggestions. This work was funded by a grant-in-aid (Grant No. T-5299), a Career Investigator Award to AKG, and summer studentships to KAD from the Heart & Stroke Foundation of Ontario and to KKM from the Hypertension Society of Canada and a grant-in-aid (A2200) from NSERC to JXW.
Abbreviations
- Asc
ascorbic acid
- BAPTA/AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- [Ca2+]i
cytosolic [Ca2+]
- CPA
cyclopiazonic acid
- EC
endothelial cells
- EDTA
(ethylenedinitrilo)-tetraacetic acid
- EGTA
ethyleneglycol bis(b-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- Fluo 3/AM
4-(6-acetoxymethoxy-2,7-dichloro-3-oxo-9-xanthenyl)-4′-methyl-2,2'-(ethylenedioxy)dianiline-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl) ester
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- L-NAME
L-nitroarginyl methyl ester
- PCEC
endothelial cells cultured from pig coronary artery
- PCSMC
smooth muscle cells cultured from pig coronary artery
- ROS
reactive oxygen species
References
- AGARWAL M.L., LARKIN H.E., ZAIDI S.I., MUKHTAR H., OLEINICKN L. Phospholipase activation triggers apoptosis in photosensitized mouse lymphoma cells. Cancer Res. 1993;53:5897–5902. [PubMed] [Google Scholar]
- BEST K.A., HOLMES M.E., SAMSON S.E., MWANJEWE J., WILSON J.X., DIXON S.J., GROVER A.K. Ascorbate uptake in pig coronary artery endothelial cells. Mol. Cell Biochem. 2005;271:43–49. doi: 10.1007/s11010-005-3442-0. [DOI] [PubMed] [Google Scholar]
- BODIN P., BAILEY D., BURNSTOCK G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br. J. Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAET K., ASPESLAGH S., VANDAMME W., WILLECKE K., MARTIN P.E., EVANS W.H., LEYBAERT L. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J. Cell Physiol. 2003;197:205–213. doi: 10.1002/jcp.10365. [DOI] [PubMed] [Google Scholar]
- BUXTON I.L., KAISER R.A., OXHORN B.C., CHEEK D.J. Evidence supporting the Nucleotide Axis Hypothesis: ATP release and metabolism by coronary endothelium. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1657–H1666. doi: 10.1152/ajpheart.2001.281.4.H1657. [DOI] [PubMed] [Google Scholar]
- CARR A.C., ZHU B.Z., FREI B. Potential antiatherogenic mechanisms of ascorbate (vitamin C) and alpha-tocopherol (vitamin E) Circ. Res. 2000;87:349–354. doi: 10.1161/01.res.87.5.349. [DOI] [PubMed] [Google Scholar]
- CSONT T., FERDINANDY P. Cardioprotective effects of glyceryl trinitrate: beyond vascular nitrate tolerance. Pharmacol. Ther. 2005;105:57–68. doi: 10.1016/j.pharmthera.2004.10.001. [DOI] [PubMed] [Google Scholar]
- DARUWALA R., SONG J., KOH W.S., RUMSEY S.C., LEVINE M. Cloning and functional characterization of the human sodium-dependent vitamin C transporters hSVCT1 and hSVCT2. FEBS Lett. 1999;460:480–484. doi: 10.1016/s0014-5793(99)01393-9. [DOI] [PubMed] [Google Scholar]
- FERDINANDY P., SCHULZ R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-VILLALON A.L., FERNANDEZ N., GARCIA J.L., MONGE L., GOMEZ B., DIEGUEZ G. Reactivity of the dog cavernous carotid artery. The role of the arterial and venous endothelium. Pflugers Arch. 1993;425:256–262. doi: 10.1007/BF00374175. [DOI] [PubMed] [Google Scholar]
- GROVER A.K., SAMSON S.E. Peroxide resistance of ER Ca2+ pump in endothelium: implications to coronary artery function. Am. J. Physiol. 1997;273:C1250–C1258. doi: 10.1152/ajpcell.1997.273.4.C1250. [DOI] [PubMed] [Google Scholar]
- HEWETT P.W., MURRAY J.C. Human lung microvessel endothelial cells: isolation, culture, and characterization. Microvasc. Res. 1993;46:89–102. doi: 10.1006/mvre.1993.1037. [DOI] [PubMed] [Google Scholar]
- HOLMES M.E., MWANJEWE J., SAMSON S.E., HAIST J.V., WILSON J.X., DIXON S.J., KARMAZYN M., GROVER A.K. Dehydroascorbic acid uptake by coronary artery smooth muscle: effect of intracellular acidification. Biochem. J. 2002;362:507–512. doi: 10.1042/0264-6021:3620507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES M.E., SAMSON S.E., WILSON J.X., DIXON S.J., GROVER A.K. Ascorbate transport in pig coronary artery smooth muscle: Na(+) removal and oxidative stress increase loss of accumulated cellular ascorbate. J. Vasc. Res. 2000;37:390–398. doi: 10.1159/000025755. [DOI] [PubMed] [Google Scholar]
- JACKSON T.S., XU A., VITA J.A., KEANEY J.F., JR Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998;83:916–922. doi: 10.1161/01.res.83.9.916. [DOI] [PubMed] [Google Scholar]
- JONES W., LI X., QU Z.C., PERRIOTT L., WHITESELL R.R., MAY J.M. Uptake, recycling, and antioxidant actions of alpha-lipoic acid in endothelial cells. Free Radic. Biol. Med. 2002;33:83–93. doi: 10.1016/s0891-5849(02)00862-6. [DOI] [PubMed] [Google Scholar]
- KE H.Z., QI H., WEIDEMA A.F., ZHANG Q., PANUPINTHU N., CRAWFORD D.T., GRASSER W.A., PARALKAR V.M., LI M., AUDOLY L.P., GABEL C.A., JEE W.S., DIXON S.J., SIMS S.M., THOMPSON D.D. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 2003;17:1356–1367. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- KIM S.J., SHIN S.Y., LEE J.E., KIM J.H., UHM D.Y. Ca2+-activated Cl- channel currents in rat ventral prostate epithelial cells. Prostate. 2003;55:118–127. doi: 10.1002/pros.10214. [DOI] [PubMed] [Google Scholar]
- LANDRY D.W., REITMAN M., CRAGOE E.J., JR, AL AWQATI Q. Epithelial chloride channel. Development of inhibitory ligands. J. Gen. Physiol. 1987;90:779–798. doi: 10.1085/jgp.90.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHR H.A., FREI B., OLOFSSON A.M., CAREW T.E., ARFORS K.E. Protection from oxidized LDL-induced leukocyte adhesion to microvascular and macrovascular endothelium in vivo by vitamin C but not by vitamin E. Circulation. 1995;91:1525–1532. doi: 10.1161/01.cir.91.5.1525. [DOI] [PubMed] [Google Scholar]
- MAY J.M., QU Z., LI X. Requirement for GSH in recycling of ascorbic acid in endothelial cells. Biochem. Pharmacol. 2001;62:873–881. doi: 10.1016/s0006-2952(01)00736-5. [DOI] [PubMed] [Google Scholar]
- MAY J.M., QU Z.C. Nitric oxide-induced oxidant stress in endothelial cells: amelioration by ascorbic acid. Arch. Biochem. Biophys. 2004;429:106–113. doi: 10.1016/j.abb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- MAY J.M., QU Z.C., LI X. Ascorbic acid blunts oxidant stress due to menadione in endothelial cells. Arch. Biochem. Biophys. 2003;411:136–144. doi: 10.1016/s0003-9861(02)00715-4. [DOI] [PubMed] [Google Scholar]
- MAY J.M., QU Z.C., WHITESELL R.R. Ascorbic acid recycling enhances the antioxidant reserve of human erythrocytes. Biochemistry. 1995;34:12721–12728. doi: 10.1021/bi00039a031. [DOI] [PubMed] [Google Scholar]
- NILIUS B., DROOGMANS G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- PEPPERELL J.R., PORTERFIELD D.M., KEEFE D.L., BEHRMAN H.R., SMITH P.J. Control of ascorbic acid efflux in rat luteal cells: role of intracellular calcium and oxygen radicals. Am. J. Physiol Cell Physiol. 2003;285:C642–C651. doi: 10.1152/ajpcell.00587.2002. [DOI] [PubMed] [Google Scholar]
- RAPOSO B., RODRIGUEZ C., MARTINEZ-GONZALEZ J., BADIMON L. High levels of homocysteine inhibit lysyl oxidase (LOX) and downregulate LOX expression in vascular endothelial cells. Atherosclerosis. 2004;177:1–8. doi: 10.1016/j.atherosclerosis.2004.06.015. [DOI] [PubMed] [Google Scholar]
- SIUSHANSIAN R., DIXON S.J., WILSON J.X. Osmotic swelling stimulates ascorbate efflux from cerebral astrocytes. J. Neurochem. 1996;66:1227–1233. doi: 10.1046/j.1471-4159.1996.66031227.x. [DOI] [PubMed] [Google Scholar]
- SPRAGUE R.S., OLEARCZYK J.J., SPENCE D.M., STEPHENSON A.H., SPRUNG R.W., LONIGRO A.J. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am. J. Physiol Heart Circ. Physiol. 2003;285:H693–H700. doi: 10.1152/ajpheart.01026.2002. [DOI] [PubMed] [Google Scholar]
- TSUKAGUCHI H., TOKUI T., MACKENZIE B., BERGER U.V., CHEN X.Z., WANG Y., BRUBAKER R.F., HEDIGER M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- UPSTON J.M., KARJALAINEN A., BYGRAVE F.L., STOCKER R. Efflux of hepatic ascorbate: a potential contributor to the maintenance of plasma vitamin C. Biochem. J. 1999;342 (Part 1):49–56. [PMC free article] [PubMed] [Google Scholar]
- VON K., WETTER A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- WANG D., LEHMAN R.E., DONNER D.B., MATLI M.R., WARREN R.S., WELTON M.L. Expression and endocytosis of VEGF and its receptors in human colonic vascular endothelial cells. Am. J. Physiol Gastrointest. Liver Physiol. 2002;282:G1088–G1096. doi: 10.1152/ajpgi.00250.2001. [DOI] [PubMed] [Google Scholar]
- WEBER C., ERL W., WEBER K., WEBER P.C. Increased adhesiveness of isolated monocytes to endothelium is prevented by vitamin C intake in smokers. Circulation. 1996;93:1488–1492. doi: 10.1161/01.cir.93.8.1488. [DOI] [PubMed] [Google Scholar]
- WHITE T.D., ANGUS J.A. Relaxant effects of ATP and adenosine on canine large and small coronary arteries in vitro. Eur. J.Pharmacol. 1987;143:119–126. doi: 10.1016/0014-2999(87)90741-2. [DOI] [PubMed] [Google Scholar]
- WILSON J.X., PETERS C.E., SITAR S.M., DAOUST P., GELB A.W. Glutamate stimulates ascorbate transport by astrocytes. Brain Res. 2000;858:61–66. doi: 10.1016/s0006-8993(99)02433-6. [DOI] [PubMed] [Google Scholar]
- WOOLLARD K.J., LORYMAN C.J., MEREDITH E., BEVAN R., SHAW J.A., LUNEC J., GRIFFITHS H.R. Effects of oral vitamin C on monocyte: endothelial cell adhesion in healthy subjects. Biochem. Biophys. Res. Commun. 2002;294:1161–1168. doi: 10.1016/S0006-291X(02)00603-4. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO K., SOKABE T., OHURA N., NAKATSUKA H., KAMIYA A., ANDO J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am. J. Physiol Heart Circ. Physiol. 2003;285:H793–H803. doi: 10.1152/ajpheart.01155.2002. [DOI] [PubMed] [Google Scholar]