Abstract
The immunomodulating agent FTY720 is a substrate for the sphingosine kinase and the phosphorylated form is able to bind to sphingosine 1-phosphate (S1P) receptors. In this study, we show that exposure of renal mesangial cells to phospho-FTY720 leads to a rapid and transient activation of several protein kinase cascades, including the mitogen- and stress-activated protein kinases. The nonphosphorylated FTY720 also increased MAPK phosphorylation, but with a reduced potency and a more delayed time course. In addition, phospho-FTY720 and FTY720 are able to increase phosphorylation of Smad proteins which are classical members of the transforming growth factor-β (TGF-β) signalling device, thus suggesting a crosstalk between FTY720 and TGF-β signalling.
Pretreatment with the S1P3 receptor antagonist suramin inhibits FTY720 and phospho-FTY720-induced Smad phosphorylation, whereas pertussis toxin pretreatment, which blocks Gi/0 proteins, has no effect on Smad phosphorylation.
Since TGF-β is a potent profibrotic cytokine in mesangial cells and upregulates the connective tissue growth factor (CTGF) and collagen as important hallmarks in the fibrotic sequelae, we investigated whether FTY720 and phospho-FTY720 are able to mimic these effects of TGF-β. Indeed, FTY720 and phospho-FTY720 markedly upregulate CTGF and collagen type IV protein expressions. In addition, the tissue inhibitor of metalloproteinase-1 is transcriptionally activated by FTY720, whereas cytokine-induced matrix metalloproteinase-9 is down-regulated by FTY720.
Depletion of the TGF-β receptor type II by the siRNA transfection technique blocks not only Smad phosphorylation but also CTGF upregulation. Similarly, Smad-4 depletion by siRNA transfection also abrogates CTGF upregulation induced by FTY720 and phospho-FTY720.
In summary, our data show that FTY720 and phospho-FTY720 not only activate the Smad signalling cascade in mesangial cells, but also upregulate the expression of CTGF and collagen. These findings suggest that FTY720 may have additional effects besides the established immunomodulatory action and, importantly, a profibrotic activity has to be considered in future experimental approaches.
Keywords: FTY720, mesangial cell, Smad, TGF-β2, CTGF, collagen
Introduction
Renal mesangial cells are involved in the regulation of the glomerular filtration as well as in the preservation of the structural integrity of the glomerulus. In addition, mesangial cells play an important role in most pathological processes of the renal glomerulus (Pfeilschifter, 1989; 1994; Kashgarian & Sterzel, 1992). Several proinflammatory functions of mesangial cell have been established. The three most prominent include (i) increased extracellular matrix production, (ii) increased inflammatory mediator production, and (iii) increased mesangial cell proliferation, which are all hallmarks of many forms of glomerulonephritis, finally leading to glomerulosclerosis (Pfeilschifter, 1989; 1994). However, the detailed mechanisms underlying these cell responses are still not completely understood. A particularly important factor in the mechanism of matrix accumulation is the transforming growth factor-β (TGF-β), whose production is highly induced in many fibrotic diseases, including atherosclerosis and fibrosis of the kidney, liver and lung (Kitamura & Suto, 1997; Leask & Abraham, 2004; Agrotis et al., 2005).
The connective tissue growth factor (CTGF) is a newly identified growth factor which is also thought to play an important role in wound repair and fibrosis (Leask & Abraham, 2003). It belongs to the family of CCN (cysteine-rich 61 (Cyr61), CTGF and neuroblastoma overexpressed (Nov) immediate early genes) and is proposed to stimulate fibroblast proliferation, matrix production and granulation tissue formation (Perbal, 2004). Furthermore, it may promote cell adhesion and migration of many cell types, although the mechanisms remain unclear (Shimo et al., 1999; Crean et al., 2004). Similar to TGF-β, CTGF is also significantly upregulated in fibrotic diseases including glomerulosclerosis (Gupta et al., 2000). Thus, it is not surprising that TGF-β is so far the best described stimulator of CTGF expression in many cell types, although other factors such as angiotensin II, lysophosphatidic acid and sphingosine 1-phosphate (S1P) have also been described.
Recently, a novel immunomodulating agent has been introduced, FTY720, which is a derivative of myriocin (also known as ISP-1), a metabolite of the fungus Isaria sinclairii (Brinkmann et al., 2000; Kiuchi et al., 2000). The detailed mechanism of immunosuppressive action of FTY720 is still not completely understood, although a characteristic depletion of circulating lymphocytes in the peripheral blood is observed with an acceleration of lymphocyte homing into peripheral lymph nodes. Later, it was discovered that FTY720, which has a sphingosine-like chemical structure, can function as a substrate for the sphingosine kinases to become phosphorylated (Brinkmann et al., 2002; Mandala et al., 2002). This phosphorylation not only occurs in vitro but can also be detected in vivo in the blood of mice and rats after administration of FTY720 (Brinkmann et al., 2002; Mandala et al., 2002). The phosphorylated form of FTY720 exerted the same immunosuppressive effects as FTY720 itself, suggesting that FTY720 is only a prodrug and its application leads to intracellular phosphorylation of the substance to the active form. Interestingly, the phosphorylated form of FTY720 is able to bind to S1P receptors (also denoted as endothelial differentiation gene (Edg) receptors), thus rendering it a potentially useful agonist of the S1P receptors (Brinkmann et al., 2002; Mandala et al., 2002). Moreover, differential binding affinities have been shown for the S1P4/Edg-6 receptor, which exerts a 20-fold higher affinity for the phospho-FTY720 than for the natural ligand S1P (Mandala et al., 2002). In contrast, the S1P2 receptor only binds S1P but does not bind phospho-FTY720. Moreover, FTY720 has been suggested to be a competitive inhibitor of S1P-induced chemotaxis in T cells (Graeler & Goetzl, 2002). Opposite to this proposed antagonistic effect of FTY720 on S1P receptors, it was also reported that, in in vitro S1P receptor-binding studies, FTY720 shows a KD value of 300 nM for the S1P1 receptor (Mandala et al., 2002). This would rather imply an agonistic effect of FTY720 at higher concentrations.
The sphingolipid S1P has attracted a lot of interest during the previous years because of its capability to trigger many important cellular responses, including proliferation (Zhang et al., 1991; Olivera & Spiegel, 1993), differentiation (Vogler et al., 2003), cytoprotection (Manggau et al., 2001) and cell migration (Wang et al., 1999). These cell responses are mainly mediated by S1P binding extracellularly to specific cell surface receptors. However, despite the existence of these cell membrane receptors, S1P generated inside the cell can also trigger intracellular events independent of the Edg receptors (van Brocklyn et al., 1998; Meyer zu Heringdorf et al., 2003), although the identity of these putative intracellular targets of S1P still remains to be unvealed.
In this study, we show that FTY720 and its phosphorylated derivative phospho-FTY720 are able to crossactivate the TGF-β signalling pathway in renal mesangial cells and lead to increased phosphorylation of Smad-1, -2 and -3 proteins. Furthermore, FTY720 and phospho-FTY720 are equally potent in inducing CTGF and collagen type IV, which are hallmarks in the fibrotic sequelae, thus suggesting that the therapeutic use of FTY720 may not only cause immunomodulation but also eventually trigger additional unwanted cell and tissue responses.
Methods
Chemicals
FTY720, phospho-FTY720 and TGF-β2 were kindly donated by Novartis Pharma Ltd, Basel, Switzerland; phospho-specific antibodies against p42/p44-MAPK, JNK, p38-MAPK, phospho-Smad-1(Ser463/465) and phospho-Smad-2(Ser465/467) and total Smad-2 were from Cell signalling, Frankfurt am Main, Germany; the TGF-βRI kinase inhibitor and the total polyclonal anti-human Smad-1 antibody (directed against amino-acid residues 147–258 of human Smad-1) were from Merck Biosciences, Schwalbach, Germany; the total Smad-3 antibody (FL-425) and the collagen type IV (H-234) antibody were from Santa Cruz, Heidelberg, Germany; the neutralizing pan-specific TGF-β antibody and the TGF-β ELISA were from Biosource Deutschland GmbH, Solingen, Germany; [35S]methionine/cysteine pro-mixture (specific activity >1000 Ci mmol−1), [α-32P]dCTP (specific activity 3000 Ci mmol−1), protein A sepharose 4B, anti-rabbit and anti-mouse horseradish peroxidase-linked IgGs and Hyperfilm were purchased from Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany; all cell culture nutrients were from Gibco, Karlsruhe, Germany.
Cell culture
Rat renal mesangial cells were isolated from kidneys of male Sprague–Dawley rats (70–100 g body weight), and cultivated and characterized as described previously (Pfeilschifter et al., 1986). Passages 8–23 were used for the experiments in this study.
Cell stimulation and Western blot analysis
Confluent mesangial cells in 60-mm-diameter dishes were stimulated with the indicated substances in Dulbecco's modified Eagle medium (DMEM) containing 0.1 mg ml−1 of fatty-acid-free bovine serum albumin. Thereafter, the medium was withdrawn and the cells washed once with ice-cold phosphate-buffered saline solution. Cells were scraped into ice-cold lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X100, 2 mM EDTA, 2 mM EGTA, 40 mM β-glycerophosphate, 50 mM sodium fluoride, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 1 μM pepstatin A, 1 mM phenylmethyl sulphonyl fluoride) and homogenized by 10 passes through a 26-G needle fitted to a 1 ml syringe. Samples were centrifuged for 10 min at 14,000 × g and the supernatant was taken for protein determination. Cell extracts containing 50 μg of protein were prepared in SDS-sample buffer and subjected to SDS–PAGE. Proteins were transferred on to nitrocellulose and immunostaining was performed as described previously in detail (Huwiler et al., 2000). Antibodies were diluted in blocking buffer as indicated in the legends of the figures. Bands were detected by the enhanced chemiluminescence (ECL) method as recommended by the manufacturer.
Metabolic labelling of cells and immunoprecipitation
Confluent cells in 100-mm-diameter dishes were incubated in methionine-free DMEM in the absence or presence of the indicated stimuli for 24 h. For the last 4 h of incubation, [35S]methionine/cysteine mixture was added (140 μCi per plate). After labelling, cells were processed exactly as described previously (Franzen et al., 2001). For immunoprecipitation, an anti-collagen type IV antibody was used at a dilution of 1 : 200.
Northern blot analysis
Total cellular RNA was extracted from mesangial cells using Trizol reagent (Invitrogen, Karlsruhe, Germany), and RNA was hybridized following standard procedures using probes for rat tissue inhibitor of metalloproteinase-1 (TIMP-1), matrix metalloproteinase-9 (MMP-9) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as described previously (Eberhardt et al., 2000).
Cell transfections
Gene silencing was performed using sequence-specific siRNA reagents of: rat Smad-4 (AAUACACCGACAAGCAAUGACdTdT and GUCAUUGCUUGUCGGU-GUAUUdTdT); rat TGF-βR II (AAAGUCGGUUAACAGCGAUCUdTdT and AGAUCGCUGU-UAACCGACUUUdTdT). Mesangial cells were transfected at approximately 50% confluency with 200 nM of the 21-nucleotide duplexes using OligofectAMINE as recommended by the manufacturer (Dharmacon Research Inc., Boulder, CO, U.S.A.). After 48 h cells were stimulated as indicated in the figure legends. The silencing efficiency was detected by Western blot analyses using specific antibodies.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by a Bonferroni's post hoc test for multiple comparisons (GraphPad InStat version 3.00 for Windows NT, GraphPad Software, San Diego, CA, U.S.A.).
Results
FTY720 and phospho-FTY720 activate the different MAPK cascades
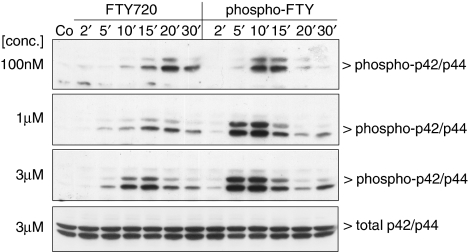
Stimulation of mesangial cells with a low concentration (100 nM) of phospho-FTY720 leads to an increased phosphorylation and hence activation of the classical p42/p44-MAPKs with a maximal effect at 10–15 min (Figure 1). The same concentration of the nonphosphorylated FTY720 causes a more delayed and less pronounced activation of p42/p44-MAPK with a maximum at 20–30 min (Figure 1). When increasing the concentration of phospho-FTY and FTY720, the maximal activation of the p42/p44 is shifted towards earlier time points. It is worth noting that FTY720 does not achieve the maximal level of MAPK phosphorylation triggered by phospho-FTY, thus suggesting that FTY720 is only a partial agonist.
Figure 1.
Effect of FTY720 and phospho-FTY720 on p42/p44-MAPK activation in renal mesangial cells. Quiescent mesangial cells were stimulated with either vehicle (Co, 10 min) or FTY720 and phospho-FTY720 at concentrations of 100 nM each (upper panel), 1 μM (middle panel) and 3 μM (lower panel) for the indicated time periods (in min). Thereafter, cells were harvested and Western blot analyses were performed using an anti-phospho-specific antibody against p42/p44-MAPK at a dilution of 1 : 1000. Bands were detected by the ECL method according to the manufacturer's recommendation. Data are representative of three independent experiments giving similar results.
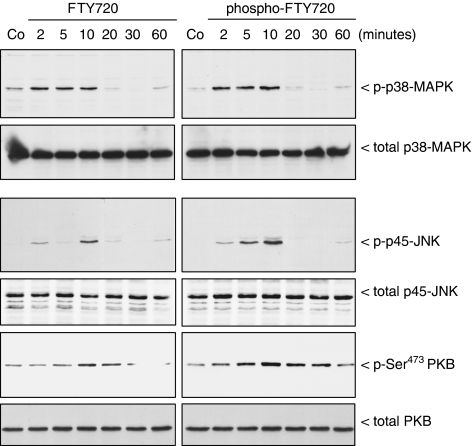
A similar differential effect of phospho-FTY and FTY720 is also seen for the activation of the stress-activated protein kinase p45 (SAPK)/JNK (Figure 2). In contrast, the stress-activated p38-MAPK and the protein kinase B/Akt (PKB) are activated by both substances, phospho-FTY and FTY720, over the same time period and to the same extent. During the whole stimulation period, the total amounts of the different protein kinases did not change.
Figure 2.
Effect of FTY720 and phospho-FTY720 on SAPK/JNK, p38-MAPK and PKB activation in renal mesangial cells. Quiescent mesangial cells were stimulated with either vehicle (Co, 10 min) or FTY720 and phospho-FTY720 at concentrations of 3 μM for the indicated time periods (in min). Thereafter, cells were harvested and Western blot analyses were performed using an anti-phospho-specific antibody against the p45-SAPK/JNK, p38-MAPK and Ser473-PKB at dilutions of 1 : 1000 each. Bands were detected by the ECL method according to the manufacturer's recommendation.
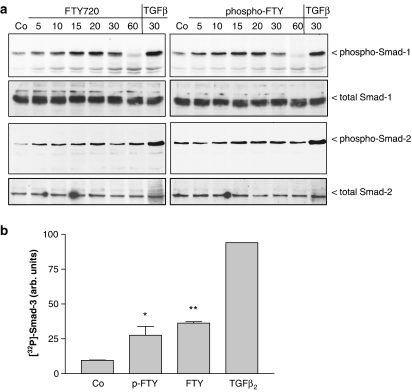
Since we recently identified the Smad signalling cascade as an additional S1P-triggered pathway, we further investigated whether FTY720 and phospho-FTY720 can phosphorylate and activate the Smad proteins. As seen in Figure 3a, both substances induce increased phosphorylation of Smad-1 at Ser463 and Ser465, and of Smad-2 at Ser465 and Ser467, although the effect is more pronounced for Smad-1, which is considered to be mainly activated by bone morphogenic proteins (BMPs), but also by TGF-β (Goumans et al., 2002; Shi & Massagué, 2003). This indicates that FTY720 derivatives are able to crossactivate the TGF-β signalling device, reminiscent of the S1P effect on Smad activation (Xin et al., 2004a). Since no phospho-specific antibodies against Smad-3 are available, in vivo 32P-labelled cells were stimulated and subjected to immunoprecipitation with an anti-Smad-3 antibody. Figure 3b shows that 10 min of FTY720 and phospho-FTY720 stimulation significantly enhanced phosphorylation of Smad-3, although TGF-β2 stimulation leads to the most potent phosphorylation of Smad-3.
Figure 3.
Effect of FTY720 and phospho-FTY720 on Smad-1, -2 and -3 phosphorylations in mesangial cells. (a) Quiescent mesangial cells were stimulated with either vehicle for 10 min (Co), FTY720 (3 μM) and phospho-FTY720 (3 μM) for the indicated time periods (in minutes). Thereafter, cells were harvested and Western blot analyses were performed using specific anti-phospho-Smad-1, total Smad-1, anti-phospho-Smad-2 or total Smad-2 antibodies at a dilution of 1 : 1000 each. Bands were detected by the ECL method according to the manufacturer's recommendation. Data are representative of two independent experiments giving similar results. (b) In vivo-32Pi-labelled cells were stimulated for 10 min with either vehicle (Co), 1 μM of FTY, 1 μM of phospho-FTY or 20 ng ml−1 TGF-β2. Thereafter, Smad-3 was immunoprecipitated from cell lysates as described in Methods. Immunoprecipitates were separated on SDS–PAGE and bands corresponding to Smad-3 were quantitated on an Imaging System. Results are expressed as % of control values and are means±s.d. (n=3).
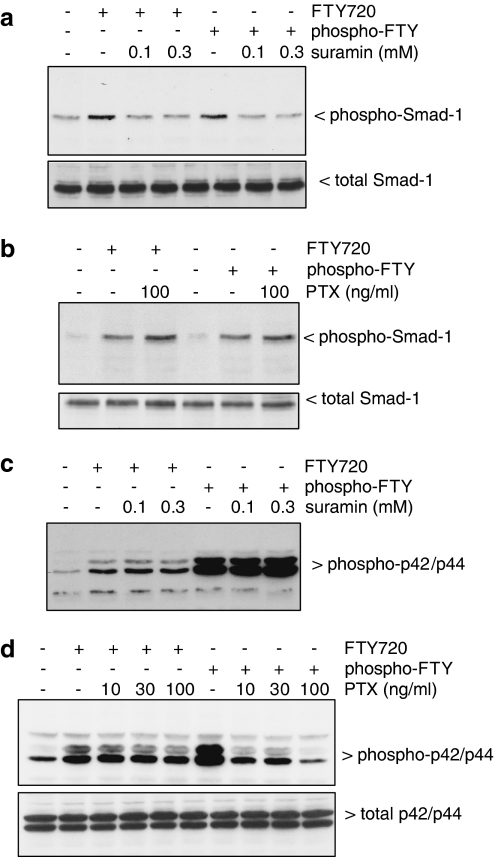
In a further attempt to characterize the S1P receptor subtype involved in the crossactivation of the Smad signalling cascade, suramin was tested, which is a selective S1P3 antagonist when compared to the other S1P receptor subtypes (Ancellin & Hla, 1999). Suramin pretreatment completely blocks phospho-FTY- and FTY720-stimulated Smad-1 phosphorylation (Figure 4a). By contrast, pertussis toxin, an inhibitor of Gi/o proteins (Hewlett et al., 1983), has no inhibitory effect (Figure 4b). These data suggest that both FTY derivatives may act via the S1P3 receptor and independent of Gi/o proteins to activate the Smad cascade. In contrast, the p42/p44-MAPK phosphorylation stimulated by the FTY derivatives is not reduced by suramin pretreatment (Figure 4c) but is markedly blocked by pertussis toxin pretreatment (Figure 4d), further proposing that different S1P receptor subtypes are involved in MAPK activation and Smad activation.
Figure 4.
Effect of suramin and pertussis toxin on FTY- and phospho-FTY-stimulated Smad and p42/p44-MAPK phosphorylation in mesangial cells. Cells were pretreated with either suramin (in mM) for 20 min (a and c) or with pertussis toxin (in ng ml−1) for 16 h (b and d) prior to stimulation with either vehicle (Co), FTY720 (FTY; 3 μM) or phospho-FTY720 (p-FTY; 3 μM) for 20 min. Thereafter, cell lysates were subjected to Western blot analysis using antibodies against phospho-Smad-1 (a and b, upper panels), total Smad-1 (a and b, lower panels), phospho-p42/p44 (c and d, upper panels) or total p42/p44-MAPKs (d, lower panel) at dilutions of 1 : 1000 each. Data are representative of two independent experiments giving similar results.
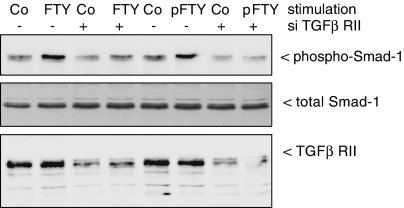
To see whether the TGF-β receptor (TGF-βR) is required for FTY720-induced Smad activation, the TGF-βR type II was depleted by using the siRNA technique. As shown in Figure 5, lower panel, TGF-βR type II-siRNA transfected cells show a drastic, although not complete, reduction of the TGF-βR type II protein compared to the control-transfected cells. Under these TGF-βR type II-depleted conditions, both the phospho-FTY and the FTY720-stimulated Smad phosphorylation is abolished suggesting that the TGF-βR is essential for the activation of the Smad cascade by both FTY derivatives.
Figure 5.
Effect of siRNA of the TGF-βR type II on FTY- and phospho-FTY-stimulated Smad phosphorylation in mesangial cells. Cells were transfected with either vehicle (−) or siRNA of TGF-βR type II (siRNA-TGF-βRII; +) as described in Methods prior to stimulation with either vehicle (Co), FTY720 (FTY; 3 μM) or phospho-FTY720 (p-FTY; 3 μM) for 20 min. Thereafter, cell lysates were subjected to Western blot analysis using antibodies against phospho-Smad-1 (upper panel), total Smad-1 (middle panel) or TGF-βRII (lower panel) at dilutions of 1 : 1000 (phospho-Smad-1, total Smad-1) and 1 : 2000 (TGF-βRII). Data are representative of four independent experiments giving similar results.
FTY720 and phospho-FTY720 mimic the TGF-β-induced profibrotic cell response in mesangial cells
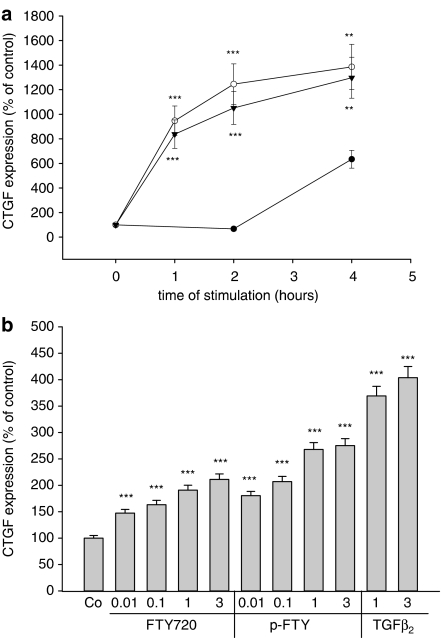
In the next step, we investigated whether FTY720 and phospho-FTY are able to mimic TGF-β-mediated cell responses. It is well established that TGF-β exerts a profibrotic activity in the kidney (Kitamura & Suto, 1997; Chen et al., 2003; Schnaper et al., 2003). One hallmark in this fibrotic sequelae is the potent upregulation of CTGF by TGF-β (Gupta et al., 2000; Riser et al., 2000). As seen in Figure 6a, stimulation of cells with phospho-FTY or FTY720 leads to an equally potent upregulation of CTGF protein expression, which is detectable already after 1 h and increases upto 4 h. The induction of CTGF measured after 4 h of stimulation occurs in a concentration-dependent manner, with maximal effects seen with 3 μM of FTY720 and phospho-FTY (Figure 6b).
Figure 6.
Effect of FTY720, phospho-FTY720 and TGF-β2 on CTGF expression in mesangial cells. (a) Mesangial cells were stimulated for the indicated time periods (in h) with either vehicle (•), FTY720 (3 μM, ○) or phospho-FTY (3 μM, ▾). (b) Cells were stimulated for 4 h with the indicated concentrations of FTY720 (in μM), phospho-FTY (p-FTY; in μM) or TGF-β2 (in ng ml−1). Thereafter, cell lysates were subjected to Western blot analysis using antibodies against CTGF at a dilution of 1 : 1000. Bands corresponding to CTGF were densitometrically evaluated. Results are means±s.d. (n=4), *P<0.05, **P<0.01, ***P<0.001 considered statistically significant when compared to vehicle-stimulated control values.
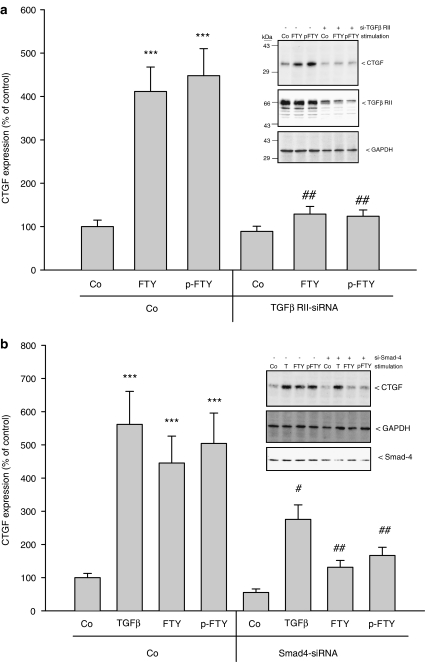
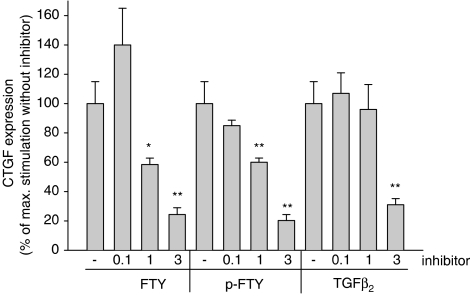
In order to elucidate the mechanism by which phospho-FTY and FTY720 induce CTGF protein expression, the TGF-βR type II, which has turned out to be essential for Smad activation (see Figure 5), was depleted by siRNA transfection. Under these conditions, the upregulation of CTGF by FTY720 and phospho-FTY is abolished (Figure 7a). Furthermore, Smad-4 depletion by siRNA transfection is able to at least partially block the upregulation of CTGF induced by FTY720, phospho-FTY720 and TGF-β2 (Figure 7b). The partial effect may be due to the only partial reduction of Smad-4 protein expression by siRNA transfection. Additionally, the recently developed inhibitor of the TGF-βR type I (Sawyer et al., 2003) also dose-dependently reduced FTY720- and phospho-FTY-induced CTGF expression (Figure 8).
Figure 7.
Effect of siRNA of the TGF-βR type II and Smad-4 on FTY- and phospho-FTY-stimulated CTGF expression in mesangial cells. (a) Cells were transfected with either vehicle (Co) or siRNA of TGF-βR type II (siRNA-TGF-βRII; +) as described in Methods prior to stimulation with either vehicle (Co), FTY720 (FTY; 3 μM) or phospho-FTY720 (p-FTY; 3 μM) for 4 h. Thereafter, cell lysates were subjected to Western blot analysis using antibodies against CTGF (a and b, insets: upper panels), GAPDH (a, inset: lower panel and b, inset: middle panel), TGF-βRII (a, inset: middle panel) or Smad-4 (b, inset: lower panel) at dilutions of 1 : 1000 (CTGF, GAPDH, Smad-4) and 1 : 2000 (TGF-βRII). Bands corresponding to CTGF were densitometrically evaluated. Results are means±s.d. (n=4), ***P<0.001, considered statistically significant when compared to vehicle-stimulated control values; #P<0.05, ##P<0.01, considered statistically significant when compared to the respective empty-transfected values.
Figure 8.
Effect of a TGF-βR type I kinase inhibitor on FTY720-, phospho-FTY- and TGF-β-stimulated CTGF expression in mesangial cells. Cells were pretreated for 30 min with either vehicle (−) or the indicated concentrations (in μM) of the TGF-βRI inhibitor prior to stimulation with either FTY720 (FTY; 3 μM), phospho-FTY720 (p-FTY; 3 μM) or TGF-β2 (20 ng ml−1) for 4 h. Thereafter, cell lysates were subjected to Western blot analysis using antibodies against CTGF or GAPDH at dilutions of 1 : 1000. Bands corresponding to CTGF were densitometrically evaluated. Results are means±s.d. (n=3), *P<0.05, **P<0.01, considered statistically significant when compared to the stimulated values in the absence of the inhibitor.
To see whether the FTY720-exerted effect on Smad activation involves TGF-β release and autocrine action, two different approaches were used. For the first, cells were preincubated with a neutralizing anti-TGF-β antibody before stimulation with FTY720 or phospho-FTY. Under this experimental setting, no alteration of FTY720-induced Smad phosphorylation is seen (data not shown). Secondly, the effect of FTY720 on the release of active TGF-β was measured using an ELISA. However, no increase of TGF-β release was observed either under short-term or long-term stimulations (data not shown). These data suggest that FTY720 does not exert its action via TGF-β release.
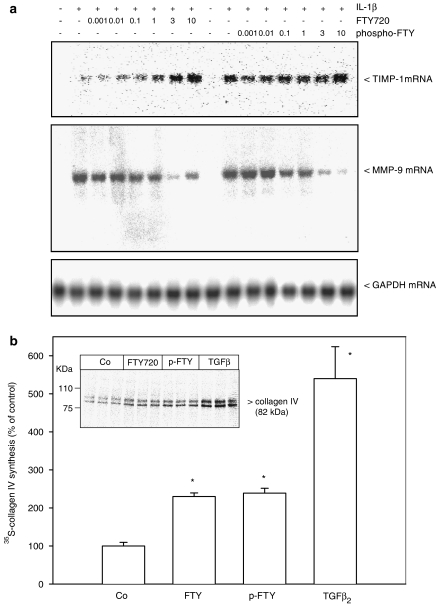
Finally, we investigated whether other potential profibrotic events are also activated by FTY720. In this context, it is well reported that the TIMP-1 is upregulated by TGF-β due to a Smad-dependent promoter activation (Hall et al., 2003). To see whether FTY720 mimics this effect of TGF-β on TIMP-1 gene transcription, Northern blot analyses were performed. As seen in Figure 9a (upper panel), FTY720 and also phospho-FTY dose-dependently upregulate TIMP-1 mRNA expression. In parallel, the IL-1β-induced MMP-9 mRNA expression known to be downregulated by TGF-β (Ogawa et al., 2004) is also reduced by FTY720 and phopsho-FTY (Figure 9a, middle panel). As an internal control, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression is detected, which is not altered upon cell stimulation (Figure 9a, lower panel). As a final readout of MMP-9 expression and action, the accumulation of collagen type IV was investigated. Figure 9b shows that the de-novo synthesis of collagen type IV by mesangial cells is significantly enhanced by FTY720, phospho-FTY and most potently by TGF-β.
Figure 9.
Effect of FTY720 and phospho-FTY on TIMP-1 and MMP-9 mRNA expression and on collagen type IV de-novo synthesis in mesangial cells. (a) Confluent mesangial cells were stimulated for 24 h with either vehicle (Co, -), or IL-1β (1 nM) in the presence of the indicated concentrations of FTY720 and phospho-FTY (in μM). Thereafter, RNA was extracted and subjected to a Northern blot analysis using a 32P-labelled probe of TIMP-1 (upper panel), MMP-9 (middle panel) and GAPDH (lower panel). (b) Cells were stimulated for 24 h with either vehicle (Co), FTY720 (3 μM), phospho-FTY (3 μM) or TGF-β2 (20 ng ml−1) in methionine-free DMEM. For the last 4 h of stimulation [35S]methionine/cysteine mixture was added. Cell lysates were prepared and equal amounts of radioactivity were subjected to immunoprecipitation using an antibody against collagen type IV at a dilution of 1 : 200. Immunoprecipitates were separated by SDS–PAGE and radioactive collagen (at 82 kDa) was analysed on an Imaging System. Data in (a) are representative of three independent experiments giving similar results. Data in (b) are means±s.d. (n=3), *P<0.05 considered statistically significant when compared to vehicle-stimulated control values using a two-tailed t-test.
Discussion
In this study, we show for the first time that the novel immunomodulatory drug FTY720 and its phosphorylated derivative phospho-FTY are both able to crossactivate the TGF-β/Smad signalling cascade and may thereby mimic TGF-β-triggered cell responses.
TGF-β is one member of the TGF-β superfamily, which consists of nearly 30 proteins (Shah et al., 2001). It is active as a dimer and possesses multifunctional properties enabling it to regulate diverse cellular activities, including differentiation, extracellular matrix production and cell proliferation (Kitamura & Suto, 1997; Shah et al., 2001; Zhu & Burgess, 2001; Leask & Abraham, 2004; Agrotis et al., 2005). Interestingly, TGF-β can induce a variety of rather opposing biological effects. Under acute conditions, it acts as an efficient anti-inflammatory agent to reduce proinflammatory mediator expression, whereas under chronic conditions, it rather displays a profibrotic potential in the kidney, resulting in glomerulosclerosis and interstitial fibrosis (Kitamura & Suto, 1997; Chen et al., 2003; Schnaper et al., 2003).
Furthermore, TGF-β is a very potent immunosuppressive agent and it has been proposed to promote the migration and homing of T cells (Luethviksson & Gunnlaugsdottir, 2003; Wahl et al., 2004). Reminiscent of this TGF-β-mediated homing of T-cells, another recently developed potent immunosuppressive agent, FTY720, has been reported to stimulate T-cell homing and thereby exert its immunosuppressive effect (Brinkmann et al., 2000). From these data, it is tempting to speculate that an overlapping signalling mechanism exists between TGF-β and FTY720, although no evidence has yet been presented.
Indeed, our study clearly shows that TGF-β and FTY720 share the same signalling device, that is, the Smad signalling cascade. Noteworthy, an overlapping signalling has also been suggested by us and others between TGF-β and S1P. We reported that S1P mimics the anti-inflammatory effect of TGF-β on mesangial cells, measured as reduction of iNOS expression and NO release, secretory phospholipase A2 and MMP-9 expression and secretion induced by cytokines (Xin et al., 2004a). Furthermore, Sauer et al. (2004) showed that S1P is able to activate the Smad-3 cascade in keratinocytes (Sauer et al., 2004), and also in murine Langerhans cells a similar S1P-activated Smad signalling cascade was reported (Radeke et al., 2005). In the latter study, a role of S1P as endogenous immunosuppressive mediator affecting migration and development of tolerogenic dendritic cells was proposed, which most probably involves S1P1 or S1P3 receptors (Radeke et al., 2005).
Interestingly, Smad activation in mesangial cells occurs with the same potency and the same time course for FTY720 and phospho-FTY, suggesting that there is no interconversion necessary and both substances are active per se and most probably act via at the same S1P receptor subtype. Several other reports have shown that FTY720 has agonistic effects on S1P receptors, which was proposed to occur via phosphorylation to phospho-FTY (Brinkmann et al., 2002; Mandala et al., 2002). In vitro radioligand competition-binding assays of FTY720 with [33P]S1P showed an IC50 value of 300 nM for the S1P1 receptor, but hardly any effect (IC50>10 μM) at the other S1P receptor subtypes. Despite this competitive effect, FTY720 by itself did neither stimulate Ca2+ mobilization nor did it desensitize S1P-triggered receptor activation in CHO cells overexpressing the S1P1 receptor (Mandala et al., 2002). Additionally, in vivo, FTY720 is inducing T-cell homing just as S1P. Mechanistically, it was suggested that FTY720 induces depletion of the S1P1 receptor (Matloubian et al., 2004). This was supported by the finding that S1P1 receptor knockout mice showed the same phenotype as FTY720-treated mice, that is, retention and accumulation of activated T cells in lymphoid organs (Matloubian et al., 2004).
From our data, we cannot exclude that the effect seen with FTY720 in mesangial cells is due to rapid phosphorylation to phospho-FTY by a sphingosine kinase. However, since the kinetics of Smad phosphorylation is very rapid (within minutes) and does not differ among the two substances, it is questionable whether phosphorylation of FTY720 is necessary to elicit this rapid response. Moreover, we previously showed that FTY720 pretreatment did not reduce S1P-stimulated MAPK activation in mesangial cells, suggesting that FTY720 is also no direct antagonist at one of the S1P receptors (Xin et al., 2004b). Rather it is more likely that FTY720 can directly activate one of the other receptors or intracellular targets, which still need to be identified. In this context, it has recently been reported that FTY720 can directly activate a protein phosphatase of the type 2A, which results in a rapid dephosphorylation and thus inactivation of PKB/Akt and increased apoptosis of human Jurkat T cells (Matsuoka et al., 2003).
These indirect agonistic effects of FTY720 on S1P receptor signalling contrast to a recent study showing that FTY720 in its nonphosphorylated form has an inhibitory effect on S1P receptors by inducing their internalization in T cells, and subsequently also blocks S1P-induced T-cell migration (Graeler & Goetzl, 2004). However, the target of FTY720 mediating the internalization of the S1P receptors was not further identified.
In addition, our data show that the FTY720-induced effect on Smad activation occurs independent of a Gi-protein, since pertussis toxin was ineffective in blocking the effect (Figure 4b), but was suramin sensitive (Figure 4a). In contrast, FTY720-triggered MAPK activation was abolished by pertussis toxin (Figure 4d) and insensitive to suramin (Figure 4c), thus suggesting that two different S1P receptors may be involved in MAPK and Smad activation. This is reminiscent of our previous data showing that S1P-stimulated Smad activation is also Gi-protein insensitive but suramin sensitive, whereas S1P-stimulated MAPK activation is pertussis toxin sensitive and suramin insensitive (Xin et al., 2004a). Since suramin has been shown to selectively antagonize the S1P3 receptor among the S1P receptors, it is tempting to speculate that the S1P3 receptor could be involved in the Smad activation by FTY720. In this context, it is worth noting that one of the in vivo observed side effects of FTY720, that is, induction of a transient bradykardia, was appointed to the S1P3 receptor (Sanna et al., 2004). On the other side, the immunosuppressive effect of FTY720, a process thought to mainly involve changes in T-lymphocyte signalling, was reported to be pertussis toxin-dependent (Henning et al., 2001). Clearly, mesangial cells and T-lymphocytes are equipped with different S1P receptor subtypes that also couple to different signalling devices and thereby guarantee unique cell type-specific responses.
Interestingly, CTGF is not only a key factor in the fibrotic sequelae but has also been implicated in tumor development and progression (Jiang et al., 2004). When comparing the levels of CTGF with cancer prognosis and metastatic potential, it appears that CTGF is significantly downregulated in breast tumors of patients with poor prognosis as compared to patients with good prognosis (Jiang et al., 2004). In addition, it was shown that patients with colorectal tumors that displayed high CTGF levels had a higher overall survival rate (Lin et al., 2005). Thus, it seems that an upregulation of CTGF may be beneficial in tumor therapy.
In this context, it is worth noting that few reports have pointed towards an antitumor and antimetastatic activity of FTY720. Thus, Azuma et al. (2002) showed a proapoptotic effect of FTY720 on cancer cells in vitro. Also in vivo, in a mouse breast cancer model, FTY720 at a concentration of 5 mg kg−1 day−1 or higher led to a drastic reduction of tumor volume (Azuma et al., 2002). Whether this observed antitumor effect of FTY720 mechanistically involves the upregulation of CTGF is an attractive hypothesis that certainly deserves further investigation.
FTY720 is currently used in clinical trials for prevention of kidney graft rejection after transplantation. However, not much is known about potential adverse effects. The only side effect exerted of FTY720 described so far is a transient bradycardia, which is most probably due to a conversion of FTY720 to phospho-FTY720, and binding to the S1P3 receptor on atrial sinus node myocytes, which in turn leads to a negative chronotropic response (Guo et al., 1999; Sanna et al., 2004). Regarding kidney function, no acute alteration has so far been detected upon FTY720 long-term treatment of rats and mice at doses of 1 mg kg−1 day−1 (Tawadrous et al., 2002). Also, prolonged administration of FTY720 does not cause renal toxicity in mice (Suleiman et al., 2005). Interestingly however, it was shown that rats treated with doses of 5 mg kg−1 day−1 of FTY720 for 3 weeks showed a significant decrease in their body weight. Furthermore, a significant reduction of sodium excretion was observed at high repeated doses of FTY720 (Tawadrous et al., 2002). Nevertheless, histological examination of kidneys revealed no signs of tubular changes, infiltrates or fibrosis. However, since time periods longer than 3 weeks were not investigated in that study, a chronic profibrotic effect of FTY720, as may be concluded from our data, cannot be excluded.
Additionally, in a rat model of anti-Thy1-induced chronic progressive glomerulosclerosis, it was proposed that FTY720 has beneficial effects and is able to reduce the progression of the disease towards chronic tubulointerstitial fibrosis and renal insufficiency by its ability to deplete lymphocytes and to stop the inflammatory reaction (Peters et al., 2002). This obvious dual role of FTY720, that is, anti-inflammatory and profibrotic, is very reminiscent of the spectrum of actions of TGF-β, which has been extensively studied (Kitamura & Suto, 1997; Chen et al., 2003; Schnaper et al., 2003; Leask & Abraham, 2004; Xin et al., 2004a; Agrotis et al., 2005).
In summary, we have shown that FTY720 and phospho-FTY720 crossactivate the TGF-β signalling cascade in mesangial cells and lead to activation of the Smad proteins with subsequent gene transcription, as exemplified for the profibrotic factor CTGF. These findings suggest that FTY720 has additional effects besides the immunosuppressive one and a profibrotic activity has to be considered in future experimental approaches.
Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (HU 842/2-3, PF361/2-1).
Abbreviations
- DMEM
Dulbecco's modified Eagle medium
- ECL
enhanced chemiluminescence
- FTY720
2-amino-2-[2-(4-octyl-phenyl)ethyl]-1,3-propanediol hydrochloride
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IL-1β
interleukin-1β
- iNOS
inducible nitric oxide synthase
- MAPK
mitogen-activated protein kinase
- MMP-9
matrix metalloproteinase-9
- NO
nitric oxide
- PKB
protein kinase B
- SAPK/JNK
stress-activated protein kinase/N-terminal c-Jun kinase
- S1P
sphingosine 1-phosphate
- TGF-β
transforming growth factor-β
- TIMP-1
tissue inhibitor of metalloproteinase-1
References
- AGROTIS A., KALININA N., BOBIK A. Transforming growth factor-β, cell signaling and cardiovascular disorders. Curr. Vasc. Pharmacol. 2005;3:55–61. doi: 10.2174/1570161052773951. [DOI] [PubMed] [Google Scholar]
- ANCELLIN N., HLA T. Differential pharmacological properties and signal transduction of the sphingosine-1-phosphate receptors EDG-1, EDG-3, and EDG-5. J. Biol. Chem. 1999;274:18997–19002. doi: 10.1074/jbc.274.27.18997. [DOI] [PubMed] [Google Scholar]
- AZUMA H., TAKAHARA S., ICHIMARU N., WANG J.D., ITOH Y., OTSUKI Y., MORIMOTO J., FUKUI R., HOSHIGA M., ISHIHARA T., NONOMURA N., SUZUKI S., OKUYAMA A., KATSUOKA Y. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62:1410–1419. [PubMed] [Google Scholar]
- BRINKMANN V., DAVIES M.D., HEISE C.E., ALBERT R., COTTENS S., HOF R., BRUNS C., PRIESCHL E., BAUMRUKER T., HIESTAND P., FOSTER C.A., ZOLLINGER M., LYNCH K.R. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- BRINKMANN V., PINSCHEWER D., CHIBA K., FENG L. FTY720: a novel transplantation drug that modulates lymphocyte traffic rather than activation. Trends Pharmacol. Sci. 2000;21:49–52. doi: 10.1016/s0165-6147(99)01419-4. [DOI] [PubMed] [Google Scholar]
- CHEN S., JIM B., ZIYADEH F.N. Diabetic nephropathy and transforming growth factor-β: transforming our view of glomerulosclerosis and fibrosis build-up. Semin. Nephrol. 2003;23:532–543. doi: 10.1053/s0270-9295(03)00132-3. [DOI] [PubMed] [Google Scholar]
- CREAN J.K., FURLONG F., FINLAY D., MITCHELL D., MURPHY M., CONWAY B., BRADY H.R., GODSON C., MARTIN F. Connective tissue growth factor [CTGF]/CCN2 stimulates mesangial cell migration through integrated dissolution of focal adhesion complexes and activation of cell polarization. FASEB J. 2004;18:1541–1543. doi: 10.1096/fj.04-1546fje. [DOI] [PubMed] [Google Scholar]
- EBERHARDT W., BEEG T., BECK KF WALPEN S., GAUER S., BÖHLES H., PFEILSCHIFTER J. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 2000;57:59–69. doi: 10.1046/j.1523-1755.2000.00808.x. [DOI] [PubMed] [Google Scholar]
- FRANZEN R., PAUTZ A., BRÄUTIGAM L., GEISSLINGER G., PFEILSCHIFTER J., HUWILER A. Interleukin-1β induces chronic activation and de novo synthesis of neutral ceramidase in renal mesangial cells. J. Biol. Chem. 2001;276:35382–35389. doi: 10.1074/jbc.M102153200. [DOI] [PubMed] [Google Scholar]
- GOUMANS M.U., VALDIMARSDOTTIR G., ITOH S., ROSENDAHL A., SIDERAS P., TEN DIJKE P. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAELER M., GOETZL E.J. Activation-regulated expression and chemotactic function of sphingosine-1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- GRAELER M.H., GOETZL E.J. The immunosuppressant FTY720 down-regulates sphingosine-1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- GUO J., MACDONELL K.L., GILES W.R. Effects of sphingosine-1-phosphate on pacemaker activity in rabbit sino-atrial node cells. Pflügers Arch. 1999;438:642–648. doi: 10.1007/s004249900067. [DOI] [PubMed] [Google Scholar]
- GUPTA S., CLARKSON M.R., DUGGAN J., BRADY H.R. Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 2000;58:1389–1399. doi: 10.1046/j.1523-1755.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- HALL M.C., YOUNG D.A., WATERS J.G., ROWAN A.D., CHANTRY A., EDWARDS D.R., CLARK I.M. The comparative role of activator protein 1 and Smad factors in the regulation of TIMP-1 and MMP-1 gene expression by transforming growth factor-β1. J. Biol. Chem. 2003;278:10304–10313. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]
- HENNING G., OHL L., JUNT T., REITERER P., BRINKMANN V., NAKANO H., HOHENBERGER W., LIPP M., FOSTER R. CC chemokine receptor 7-dependent and -independent pathways for lymphocyte homing: modulation by FTY720. J. Exp. Med. 2001;194:1875–1881. doi: 10.1084/jem.194.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEWLETT E.L., SAUER K.T., MYERS G.A., COWELL J.L., GUERRANT R.L. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 1983;40:1198–1203. doi: 10.1128/iai.40.3.1198-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUWILER A., WARTMANN M., VAN DEN BOSCH H., PFEILSCHIFTER J. Extracellular nucleotides activate the p38-stress-activated protein kinase cascade in glomerular mesangial cells. Br. J. Pharmacol. 2000;129:612–618. doi: 10.1038/sj.bjp.0703077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG W.G., WATKINS G., FODSTAD O., DOUGLAS-JONES A., MOKBEL K., MANSEL R.E. Differential expression of the CCN family members Cyr61, CTGF and Nov in human breast cancer. Endocr. Rel. Cancer. 2004;11:781–791. doi: 10.1677/erc.1.00825. [DOI] [PubMed] [Google Scholar]
- KASHGARIAN M., STERZEL R.B. The pathobiology of the mesangium. Kidney Int. 1992;41:524–529. doi: 10.1038/ki.1992.74. [DOI] [PubMed] [Google Scholar]
- KITAMURA M., SUTO T.S. TGF-β and glomerulonephritis: anti-inflammmatory versus prosclerotic actions. Nephrol. Dial. Transplant. 1997;12:669–679. doi: 10.1093/ndt/12.4.669. [DOI] [PubMed] [Google Scholar]
- KIUCHI M., ADACHI K., KOHARA T., MINOGUCHI M., HANANO T., AOKI Y., MISHINA T., ARITA M., NAKAO N., OHTSUKI M., HOSHINO Y., TESHIMA K., CHIBA K., SASAKI S., FUJITA T. Synthesis and immunosuppressive activity of 2-substituted 2-aminopropane-1,3-diols and 2-aminoethanols. J. Med. Chem. 2000;43:2946–2961. doi: 10.1021/jm000173z. [DOI] [PubMed] [Google Scholar]
- LEASK A., ABRAHAM D.J. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem. Cell. Biol. 2003;81:355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- LEASK A., ABRAHAM D.J. TGF-β signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- LIN B.R., CHANG C.C., CHE S.T., CHEN S.T., CHEN R.J., YANG C.Y., JENG Y.M., LIANG J.T., LEE P.H., CHANG K.J., CHAU Y.P., KUO M.L. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology. 2005;128:9–23. doi: 10.1053/j.gastro.2004.10.007. [DOI] [PubMed] [Google Scholar]
- LUETHVIKSSON B.R., GUNNLAUGSDOTTIR R. Transforming growth factor-β as a regulator of site-specific T-cell inflammatory response. Scand. J. Immunol. 2003;58:129–138. doi: 10.1046/j.1365-3083.2003.01297.x. [DOI] [PubMed] [Google Scholar]
- MANDALA S., HAJDU R., BERGSTROM J., QUACKENBUSH E., XIE J., MILLIGAN J., THORNTON R., SHEI G.J., CARD D., KEOHANE C., ROSENBACH M., HALE J., LYNCH C.L., RUPPRECHT K., PARSONS W., ROSEN H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- MANGGAU M., KIM D.S., RUWISCH L., VOGLER R., KORTING H.C., SCHÄFER-KORTING M., KLEUSER B. 1α,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J. Invest. Dermatol. 2001;117:1241–1249. doi: 10.1046/j.0022-202x.2001.01496.x. [DOI] [PubMed] [Google Scholar]
- MATLOUBIAN M., LO C.G., CINAMON G., LESNESKI M.J., XU Y., BRINKMANN V., ALLENDE M.L., PROIA R.L., CYSTER J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- MATSUOKA Y., NAGAHARA Y., IKEKITA M., SHINOMIYA T. A novel immunosuppressive agent FTY720 induced Akt dephosphorylation in leukemia cells. Br. J. Pharmacol. 2003;138:1303–1312. doi: 10.1038/sj.bjp.0705182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., LILIOM K., SCHAEFER M., DANNEBERG K., JAGGAR J.H., TIGYI G., JAKOBS K.H. Photolysis of intracellular caged sphingosine-1-phosphate causes Ca2+ mobilization independently of G-protein-coupled receptors. FEBS Lett. 2003;554:443–449. doi: 10.1016/s0014-5793(03)01219-5. [DOI] [PubMed] [Google Scholar]
- OGAWA K., CHEN F., KUANG C., CHEN Y. Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-beta is mediated by a nuclear factor-κB site. Biochem J. 2004;381:413–422. doi: 10.1042/BJ20040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLIVERA A., SPIEGEL S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- PERBAL B. CCN proteins: multifunctional signaling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- PETERS H., MARTINI S., WANG Y., SHIMIZU F., KAWACHI H., KRÄMER S., NEUMAYER H.-H. Selective lymphocyte inhibition by FTY720 slows the progressive course of chronic anti-thy 1 glomerulosclerosis. Kidney Int. 2002;66:1434–1443. doi: 10.1111/j.1523-1755.2004.00906.x. [DOI] [PubMed] [Google Scholar]
- PFEILSCHIFTER J. Cross-talk between transmembrane signalling systems: a prerequisite for the delicate regulation of glomerular haemodynamics by mesangial cells. Eur. J. Clin. Invest. 1989;19:347–361. doi: 10.1111/j.1365-2362.1989.tb00241.x. [DOI] [PubMed] [Google Scholar]
- PFEILSCHIFTER J. Mesangial cells orchestrate inflammation in the renal glomerulus. News Physiol. Sci. 1994;9:271–276. [Google Scholar]
- PFEILSCHIFTER J., KURTZ A., BAUER C. The role of phospholipase C and protein kinase C in vasoconstrictor-induced prostaglandin synthesis in cultured rat renal mesangial cells. Biochem. J. 1986;234:125–130. doi: 10.1042/bj2340125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADEKE H.H., VON WENCKSTERN H., STOIDTNER K., SAUER B., HAMMER S., KLEUSER B. Overlapping signaling pathways of sphingosine-1-phosphate and TGF-β in the murine Langerhans cell line XS52. J. Immunol. 2005;174:2778–2786. doi: 10.4049/jimmunol.174.5.2778. [DOI] [PubMed] [Google Scholar]
- RISER B.L., DENICHILO M., CORTES P., BAKER C., GRONDIN J.M., YEE J., NARINS R.G. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J. Am. Soc. Nephrol. 2000;11:25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- SANNA M.G., LIAO J., JO E., ALFONSO C., AHN M.Y., PETERSON M.S., WEBB B., LEFEBVRE S., CHUN J., GRAY N., ROSEN H. Sphingosine-1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- SAUER B., VOGLER R., VON WENCKSTERN H., FUJII M., ANZANO M.B., GLICK A.B., SCHÄFER-KORTING M., ROBERTS A.B., KLEUSER B. Involvement of Smad signalling in sphingosine-1-phosphate-mediated biological responses of keratinocytes. J. Biol. Chem. 2004;279:38471–38479. doi: 10.1074/jbc.M313557200. [DOI] [PubMed] [Google Scholar]
- SAWYER J.S., ANDERSON B.D., BEIGHT D.W., CAMPBELL R.M., JONES M.L., HERRON D.K., LAMPE J.W., MCCOWAN J.R., MCMILLEN W.T., MORT N., PARSONS S., SMITH E.C., VIETH M., WEIR L.C., YAN L., ZHANG F., YINGLING J.M. Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J. Med. Chem. 2003;46:3953–3956. doi: 10.1021/jm0205705. [DOI] [PubMed] [Google Scholar]
- SCHNAPER H.W., HAYASHIDA T., HUBCHAK S.C., PONCELET A.C. TGF-β signal transduction and mesangial cell fibrinogenesis. Am. J. Physiol. Renal Physiol. 2003;284:F243–F252. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- SHAH P.K., BUSLJE C.M., SOWDHAMINI R. Structural determinants of binding and specificity in transforming growth factor–receptor interacations. Proteins. 2001;454:408–420. doi: 10.1002/prot.10010. [DOI] [PubMed] [Google Scholar]
- SHI Y., MASSAGUÉ J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- SHIMO T., NAKANISHI T., NISHIDA T., ASANO M., KANYAMA M., KUBOKI T., TAMATANI T., TEZUKA K., TAKEMURA M., MATSUMURA T., TAKIGAWA M. Connective tissue growth factor induces proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J. Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- SULEIMAN M., CURY P.M., PESTANA J.O., BURDMANN E.A., BUENO V. FTY720 prevents renal T-cell infiltration after ischemia/reperfusion injury. Transplant. Proc. 2005;37:373–374. doi: 10.1016/j.transproceed.2004.12.280. [DOI] [PubMed] [Google Scholar]
- TAWADROUS M.N., MABUCHI A., ZIMMERMANN A., WHEATLEY A.M. Effects of immunosuppressant FTY720 on renal and hepatic hemodynamics in the rat. Transplantation. 2002;74:602–610. doi: 10.1097/00007890-200209150-00004. [DOI] [PubMed] [Google Scholar]
- VAN BROCKLYN J.R., LEE M.J., MENZELEEV R., OLIVERA A., EDSALL L., CUVILLIER O., THOMAS D.M., COOPMAN P.J., THANGADA S., LIU C.H., HLA T., SPIEGEL S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J. Cell. Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGLER R., SAUER B., KIM D.S., SCHÄFER-KORTING M., KLEUSER B. Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J. Invest. Dermatol. 2003;120:693–700. doi: 10.1046/j.1523-1747.2003.12096.x. [DOI] [PubMed] [Google Scholar]
- WAHL S.M., SWISHER J., MCCARTNEY-FRANCIS N., CHEN W. TGF-β: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J. Leukoc. Biol. 2004;76:15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- WANG F., VAN BROCKLYN J.R., HOBSON J.P., MOVAFAGH S., ZUKOWSKA-GROJEC Z., MILSTIEN S., SPIEGEL S. Sphingosine-1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J. Biol. Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- XIN C., REN S., KLEUSER B., SHABAHANG S., EBERHARDT W., RADEKE H., SCHÄFER-KORTING M., PFEILSCHIFTER J., HUWILER A. Sphingosine-1-phosphate cross-activates the Smad signaling cascade and mimics TGFβ-induced cell responses. J. Biol. Chem. 2004a;279:35255–35280. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- XIN C., REN S., PFEILSCHIFTER J., HUWILER A. Heterologous desensitization of the sphingosine-1-phosphate receptors by purinoceptor activation in renal mesangial cells. Br. J. Pharmacol. 2004b;14:581–589. doi: 10.1038/sj.bjp.0705980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H., DESAI N.N., OLIVERA A., SEKI T., BROOKER G., SPIEGEL S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell. Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU H.J., BURGESS A.W. Regulation of transforming growth factor-β signalling. Mol. Cell. Biol. Res. Commun. 2001;4:321–330. doi: 10.1006/mcbr.2001.0301. [DOI] [PubMed] [Google Scholar]