Abstract
The small protein Bv8, isolated from the amphibian skin, belongs to a novel family of secreted proteins linked to several biological effects. We describe the expression of Bv8/prokineticins and their receptors in mouse macrophages, and characterize their proinflammatory activities.
The rodent analogue of Bv8, prokineticin-2, is expressed by macrophages, as well as its G-protein-coupled receptor prokineticin receptor (PKR-1 and PKR-2). PKR-1 is expressed more abundantly.
Bv8 induces potent chemotaxis of macrophages at concentrations as low as 10−12 M.
It stimulates lipopolysaccharide-induced production of the proinflammatory cytokines IL-1 and IL-12, reducing that of the anti-inflammatory cytokine IL-10. The effects are observed starting at the very low concentration of 10−11 M.
Effects on chemotaxis and cytokine are not pertussis-toxin sensitive, but are completely prevented by addition of the phospholipase inhibitor U73122, suggesting a Gq protein is involved in the Bv8-induced effects.
Studies in PKR-1 knockout mice indicate that all the activities exerted by Bv8 on macrophages are mediated by the PKR-1 receptor.
In conclusion, Bv8 appears to be able to induce the macrophage to migrate and to acquire a proinflammatory phenotype.
Keywords: Bv8, chemotaxis, anti-inflammatory cytokines, macrophages, prokineticins, proinflammatory cytokines
Introduction
The small 77-amino-acid (aa) protein Bv8, isolated from the amphibian skin (Mollay et al., 1999) belongs to a novel family of secreted proteins whose homologues have been found in snakes (VPRA, MIT1) (Joubert & Strydom, 1980; Schweitz et al., 1999), rodents (mouse Bv8 or prokineticin (PK) 1 and 2) (Melchiorri et al., 2001) and humans (PK 1 or EG-VEGF and PK 2) (Masuda et al., 2002). This peptide family has structural shared motives, such as the 20-aa terminal sequence and a pattern of cysteine sequence that folds the molecules into a globular form.
The mRNA of murine Bv8-like protein, PK 1 and 2 (PK-1 and PK-2), has been detected in the brain, spinal cord, dorsal root ganglia, gastrointestinal tract, endocrine glands, spleen and circulating leukocytes of mice, rats and humans (Li et al., 2001; Melchiorri et al., 2001; Masuda et al., 2002; Le Couter et al., 2004). Two receptors for this family of secretory proteins have been identified in humans, rats and mice (Lin et al., 2002; Masuda et al., 2002). These receptors, PKR-1 and PKR-2, belong to the G-protein-coupled receptor and share approximately 85% aa identity. They are distributed in the brain and peripheral organs (Melchiorri et al., 2001; Negri et al., 2002), including the spleen and leukocytes (Le Couter et al., 2004).
The list of biological activities associated with Bv8/PK peptides is rapidly growing. They seem to influence complex behaviors such as feeding and drinking (Negri et al., 2004), and circadian rhythms (Cheng et al., 2002), and are involved in neuronal survival (Melchiorri et al., 2001), angiogenesis (Le Couter et al., 2001; Le Couter & Ferrara, 2003), and the reproductive cycle (Wechselberger et al., 1999). Bv8 and EG-VEGF are also related to regulation of hematopoiesis and hematopoietic cell mobilization (Le Couter et al., 2004). Moreover, an hyperalgesic activity of Bv8 has been clearly demonstrated. When injected intravenously or subcutaneously in rats, Bv8 induces intense systemic nociceptive sensitization to mechanical and thermal stimuli applied to the tail and paws (Mollay et al., 1999; Negri et al., 2002).
The cytokines interleukin (IL-)1β and tumor necrosis factor alpha (TNF-α) are closely involved in peripheral nerve and neural hyperexcitability, leading to the development of persistent hyperalgesia (Watkins & Maier, 1999; 2002). However, anti-inflammatory cytokines have been reported to limit the hyperalgesic responses induced by inflammatory stimuli and by IL-1β and TNF (Poole et al., 1995).
Considering that lymphoid organs, circulating leukocytes and hematopoietic cells all express high levels of Bv8-like protein (Le Couter et al., 2004), we assessed whether Bv8 could influence the physiology of the macrophage, the main cell involved in inflammatory responses. Macrophages play a central role in both innate and adaptive immunity (Schiffmann, 1982; Beutler, 2004). They are fundamental to the innate immune response and their ability to be chemotactically attracted to the site of initial microbial invasion or to an inflammatory focus is crucial for full activation of the immune/inflammatory response that follows (Beutler, 2004). They are the main producers of the proinflammatory cytokines IL-1β and TNF and the major anti-inflammatory cytokine IL-10 (Moore et al., 1993; Beutler, 2004; Hoebe et al., 2004). Macrophages also synthesize and release IL-12, the critical factor driving the development of T helper (Th)-1 cells (Moore et al., 1993; Trincheri, 1995; Mosman & Sad, 1996; Hoebe et al., 2004), linked to cellular immune responses and tissue injury.
Therefore, considering the importance of macrophages in all aspects of the inflammatory response, we evaluated the effect of Bv8 in vitro on several macrophage functions.
First of all we investigated whether murine peritoneal macrophages expressed PK receptors and PK 1/2. We then analyzed how Bv8 affected macrophage migration and the production of the pro-inflammatory cytokines IL-1β, TNF and IL-12 and the anti-inflammatory cytokine IL-10. In order to identify the type of receptor involved, we designed some experiments with macrophages from PKR-1 knockout (KO) mice, and tried to characterize the type of G-protein signaling in the effects observed.
Methods
Animals
Balb/C male mice, 18–20 g body weight (Charles River, Calco, Italy), were used. They were kept on a 12-h light–dark cycle with water and food ad libitum and were housed six to a cage. When indicated, PKR-1 KO mice and wild-type (WT) controls were used.
PKR-1-deficient mice were generated by Lexicon Genetics Incorporated (The Woodlands, TX, U.S.A.). A targeting vector was constructed in which exon 1 of the PKR-1 gene was replaced with a neomycin resistance gene derived from LacZ/Neo vector. Lex-1 embryonic stem (ES) cells were electroporated with the targeting vector before selection of the cells expressing the targeted allele for the generation of chimeric mice. PKR-1 deficient mice were generated by breeding chimeric mice with C57BL/6 mice.
Progeny were genotyped with PCR, which permitted the amplification of the WT PKR-1 gene (5′-GGTGACTATGACATGCCCCTGG-3′, 5′-CTCTCGGAAAGGGAGAGGCAAGG-3′) and the neomycin-resistant gene cassette, which was inserted to disrupt the PKR-1 coding region (5′-CAGCGCATCGCCTTCTATC-3′, 5′-CTCTCGGAAAGGGAGAGGCAAGG-3′). Genomic DNA was isolated from tail samples by proteinase K (Sigma, St Louis, MO, U.S.A.) digestion and ethanol precipitation, and 200 ng DNA was amplified (HotStarTaq DNA Polymerase, Qiagen, Milan, Italy) with the following cycle parameters: 95°C 3 min (one cycle); 95°C 1 min, 55°C 1 min, 72°C 1 min (30 cycles); 72°C 10 min (one cycle). Amplified products were resolved on 2% agarose gel. WT littermates were used as controls.
All the animal procedures were approved by the Institutional Review Board of the Department of Pharmacology of the University of Milan.
Drugs
Bv8 was extracted from the skin secretion of electrically-stimulated Bombina variegata and purified to 98% (HPLC) as previously described (Mollay et al., 1999). Formyl-met-leu-phe (fMLP), lipopolysaccharide (LPS), the Gi inhibitor pertussis toxin (PTX), U73122, inhibitor of all phospholipase C isoforms and its inactive analogue U73343 were purchased from Sigma. All substances were diluted to the concentrations required in endotoxin-free sterile medium.
Harvesting elicited peritoneal macrophages
Mice were inoculated intraperitoneally with 2 ml of 3% Brewer's thioglycollate medium (Difco, Detroit, MI, U.S.A.) for macrophage elicitation. Peritoneal exudate cells (PEC) were harvested in cold RPMI-1640 medium (Sigma) plus 10% fetal calf serum (FCS) (Gibco BRL, Life Technology, Italy) 3 days after elicitation (Limiroli et al., 2002, Major et al., 2002). PEC from 4–8 mice were pooled. Cell viability was checked by the Trypan blue exclusion test.
Harvesting resident peritoneal macrophages
Peritoneal cells from naive mice were harvested in cold RPMI-1640 plus 10% FCS (Limiroli et al., 2002). Cells from 20–40 mice were pooled in order to obtain enough cells for the experiments.
Gene expression analysis of PKR-1, PKR-2, PK-1 and PK-2 by RT–PCR
PEC were resuspended in RPMI plus 10% FCS at 4 × 106 ml−1 and 1 ml aliquots were dispensed into wells of a 24-well culture plate. Macrophages were isolated and purified by adherence to culture plates. After 2 h, nonadherent cells were removed with the medium and adherent cells were washed twice with warm RPMI plus 10% FCS (Limiroli et al., 2002). Adherent macrophages were used for RNA extraction.
Extracted RNA was purified using RNeasy columns (Qiagen, Milan, Italy) and checked for accidental degradation on agarose gel. Two micrograms of purified RNA were used for cDNA synthesis with reverse transcriptase (Promega, Milan, Italy) and the reaction product was diluted to 100 μl with deionized water; 2.5 μl of cDNA solution was used for PCR amplification (iCycler, Bio-Rad, Milan, Italy).
Beta-actin (housekeeping gene) gene expression was used as internal control. Specific sense and antisense primers were synthesized (Biogen, Rome, Italy), to PCR-amplify the mouse PKR-1, PKR-2, PK-1 and PK-2 cDNA, according to the following sequences:
PKR-1: 5′-ATTCTCGGACTTTCTTTGC-3′
5′-GCGGCTGTTTGACACTTC-3′ (379 bp);
PKR-2: 5′-GGCCATCGCTATTGACAGAT-3′
5′-GCGGTGAGGTAGTGCTTCTC-3′ (501 bp);
PK-1: 5′-AAGATCCCCTTCTTGAGGAAAC-3′
5′-CCAGAAAGGTTGCTTTAGGAAG-3′ (420 bp);
PK-2, 5′-AGCTGCCACCCCCTGACTCG-3′
5′-TTCCGGGCCAAGCAAATAAACC-3′(125 bp);
PCR products were separated by agarose gel electrophoresis, stained with ethidium bromide and the fluorescent bands were revealed with the Versa Doc 3000 imaging system (Bio-Rad, Milan, Italy), excised from the gel, eluted (CFXPCR Kit, Amersham Pharmacia, Italy) and sequenced (Primm s.r.l., Italy) to confirm the expected product.
Chemotaxis
Elicited or resident peritoneal macrophages were collected as described above and pooled. Cells and chemoattractant substances were suspended in RPMI-1640+BSA 1%.
Chemotaxis was measured using a Boyden modified 48-well microchemotaxis chamber, with the upper and the lower compartments separated by a polycarbonate filter (Biomap, Agrate Brianza, Italy), with a pore diameter of 5 μm. Cells (2 × 106 cells ml−1, 105 macrophages/well) were placed in the upper chamber, and samples of either medium (in order to assess spontaneous mobility), of the chemoattractant fMLP at the fixed concentration of 10−8 M, or Bv8 at concentrations from 10−8 to 10−14 M, were added to the lower chamber. The chambers were incubated for 100 min at 37°C in an atmosphere of 5% CO2, then the migrated cells adhering to the distal part of the filters were fixed and stained. These cells were quantitated by microscopically counting random fields by a scorer blind to the experimental conditions (Sacerdote et al., 2005).
To distinguish better between true chemotaxis, cell migration following a chemical gradient and chemokinesis, random activation of movement due to the presence of a chemical, a ‘checkerboard analysis' was performed, in which different concentrations of Bv8 were placed in the upper and lower compartments of the Boyden chamber.
In the experiments aimed at characterizing the type of G-protein involved, macrophages were preincubated at 37°C for 1 h with 1 μg ml−1 PTX, 10 μM of U73122 or the inactive molecule U73343, then tested for chemotaxis in the Boyden chamber with either Bv8 (10−11 M) or fMLP (10−8 M). As controls, the same macrophages were incubated for 1 h only with medium. To examine the chemotaxis of resident macrophages, Bv8 was used at the optimal concentration of 10−11 M.
In another series of experiments elicited peritoneal macrophages were isolated, as above, from PKR-1 KO mice and their WT controls. Cells were tested in the Boyden chamber for their ability to migrate in the presence of either Bv8 (10−11 M) or fMLP (10−8 M).
Results are reported either as chemotactic index, that is, cells that migrated in the presence of the chemoattractant/cell that migrated with medium only, or as the number of macrophages migrated/microscopic field.
All experiments were repeated 2–4 times.
Cytokine production
Macrophages were collected as above and pooled. Cells were resuspended in RPMI plus 10% FCS at either 1 × 106 cells ml−1 (elicited cells) or 2 × 106 ml−1 (resident macrophages), and 1-ml portions were dispensed into a 24-well culture plate. Macrophages were isolated and purified by adherence to culture plates. After 2 h, nonadherent cells were removed with the medium and adherent cells were washed twice with warm RPMI plus 10% FCS (Limiroli et al., 2002; Major et al., 2002; Cailhier et al., 2005).
Differential staining with Diff-Quick (Dade, Biomap, Milan, Italy) was used to establish the percentage of macrophages in the adherent cells and to rule out the presence of different cell types. The proportion of macrophages in the adherent cells was always ⩾98%.
Elicited macrophages were primed with 1 μg ml−1 of LPS (Sigma) for TNF, IL-1 and IL-10 production or with 1 μg ml−1 LPS and 50 U ml−1 interferon (IFN)-γ (Pharmingen, San Diego, CA, U.S.A.) for IL-12 stimulation. The stimuli were added to the macrophage cultures in a final volume of 1 ml/well of RPMI plus 10% FCS, 1% glutamine (Sigma), 2% penicillin/streptomycin solution (Sigma), 0.1% 2-mercaptoethanol (Sigma) (complete medium).
Nonelicited macrophages were stimulated with 1 μg ml−1 of LPS for TNF, IL-1β and IL-10 production or with 10 μg ml−1 of LPS and 100 U ml−1 of IFN-γ for IL-12 (Limiroli et al., 2002). Bv8 was added to macrophage cultures at the same time as LPS at concentrations ranging from 10−7 to 10−13 M.
The plates were incubated at 37°C and 5% CO2, and supernatants were collected after 24 h culture and stored frozen at −80°C for cytokine analysis (Limiroli et al., 2002). In the experiments with the inhibitors, macrophages were separated by adherence as described, and preincubated for 1 h in the presence of PTX (1 μg ml−1), U73122 or U73343 (10 μM). At the end of the preincubation the stimuli for the production of cytokines (1 μg ml−1 LPS for TNF, IL-1β and IL-10 production, and 1 μg ml−1 LPS plus 50 U ml−1 IFN-γ for IL-12 stimulation) were added together with Bv8 at the concentration of 10−9 M. Supernatants for cytokine evaluation were collected 24 h later (Sacerdote et al., 2000).
When macrophages from PKR-1 KO and WT mice were used, the cells were isolated as described and Bv8 was added at the concentration of 10−9 M.
Cytokine ELISA
IL-12 p70 protein levels were determined by an enzyme-linked immunosorbent assay (ELISA) protocol standardized by Pharmingen (San Diego, CA, U.S.A.). The anti-IL-12 capture monoclonal antibody (mAb) (4 μg ml−1) was absorbed on a polystyrene 96-well plate and the IL-12 in the sample was bound to the antibody-coated wells. The biotinylated anti-IL-12 detecting mAb (0.25 μg ml−1) was added to bind the IL-12 captured by the first antibody. After washing, avidin–peroxidase (Sigma) was added to the wells to detect the biotinylated detecting antibody and finally 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (Sigma) substrate was added. A colored product formed in proportion to the amount of IL-12 in the sample and was measured at optical density 405 nm. The amount of cytokine in each supernatant was extrapolated from the standard curve. The standards were recombinant cytokine curves generated in doubling dilutions from 30 to 4000 pg ml−1.
IL-10 production was measured with the same ELISA protocol except for the use of anti-IL-10 capture mAb at 2 μg ml−1, biotinylated anti-IL-10 detecting mAb at 0.5 μg ml−1 and a standard curve from 15 to 2000 pg ml−1 (all mAbs and recombinant cytokines were from Pharmingen).
TNF-α concentrations in culture media were measured with an OptEIA set for mouse TNF, with standard curves ranging from 15 to 1000 pg ml−1 (BD Biosciences, Milan, Italy). Streptavidin–peroxidase and tetramethylbenzidine were used for color development. The color reaction was stopped with 2 N H2SO4 and read at an optical density of 450 nm.
For IL-1β measurements, a CytoSet Elisa kit for mouse IL-1β was used (Biosource, Prodotti Gianni, Milan, Italy). The concentrations of the capture and of the secondary biotinylated antibodies were 1.25 and 0.125 μg ml−1, respectively. Standard curves generated from recombinant protein ranged from 15 to 1000 pg ml−1. Color was developed as for TNF.
Statistical analysis
Results are expressed as the mean±s.d. Data were analyzed by one-way analysis of variance followed by Bonferroni's t-test for multiple comparisons. Significance was accepted at P<0.05.
Results
Expression of PK receptors and of PKs in murine macrophages
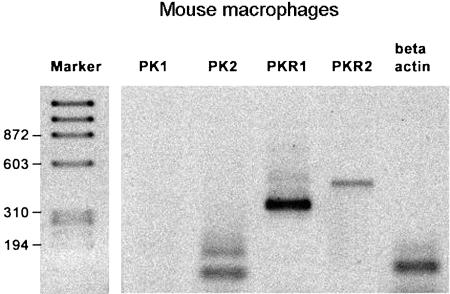
Both PKR isoforms were expressed in thioglycollate-elicited mouse macrophages, but PKR-1 was more abundant than PKR-2 (Figure 1). Moreover, these cells only expressed PK-2. PK-2 mRNA was always present as a tissue-specific double-splice variant: the short form encodes for a protein similar to amphibian Bv8 but the long form encodes for an additional 21 aa domain, inserted in the center of the polypeptide chain, already described only in mouse and human testis (Wechselberger et al., 1999).
Figure 1.
RT–PCR amplification of mRNAs for prokineticin receptors (PKR) PKR-1 and PKR-2 and of prokineticin-1 (PK-1) and -2 (PK-2) in elicited purified peritoneal macrophages. The left lane indicates the molecular marker.
Chemotaxis in Balb/c mice
To test whether Bv8 induced macrophage migration, the protein was added in the lower chemotaxis chamber in a wide range of concentrations and elicited macrophages were seeded in the upper chamber. Bv8 was a potent and efficient stimulator of macrophage chemotaxis (Figure 2), with efficacy comparable to that of the chemotactic peptide fMLP, reaching a maximum chemotaxis index of 3 at the concentration of 10−12 M. Its potency in inducing chemotaxis is clear from the significant effect already present at the very low concentration of 10−14 M. The bell-shaped response curve is typical of most chemotactic substances, and is probably due to rupture of the gradient, that happens with the highest concentrations.
Figure 2.
Macrophage chemotactic activity of Bv8, added in the lower Boyden chamber at the concentrations indicated. Thioglycollate-elicited macrophages were added in the upper chamber. The chemotactic activity of the standard chemoattractant peptide fMLP at the concentration of 10−8 M is also indicated. Results are reported as chemotactic index, that is, the number of cells migrated with Bv8/number of cells migrated in the presence of medium only. Means±s.d. of four experiments. *P<0.05 vs background migration (chemotactic index=1).
To better distinguish between true chemotaxis, cell migration following a chemical gradient and chemokinesis, random activation of movement due to the presence of a chemical, a checkerboard analysis was performed, placing different concentrations of Bv8 in both the upper and lower chambers. Table 1 shows cell migration primarily in response to a positive chemical gradient, indicating the true chemotactic activity of the protein.
Table 1.
Checkerboard analysis of Bv8-induced chemotaxis
| Lower chamber | Upper chamber | ||
|---|---|---|---|
| Bv8 (−log M) | 0 | 11 | 10 |
| 0 | 28.5±3.2 | 22.5±3.4 | 26.3±3.9 |
| 11 | 90.0±13.0* | 26.0±4.6 | 30.0±1.5 |
| 10 | 88.0±6.0* | 30.0±2.3 | 28.1±3.6 |
Chemotactic responsiveness (migration towards a chemical gradient) of thioglycollate-elicited macrophages to Bv8, placed in both the upper and lower compartments of a Boyden chamber, as indicated. Results are cells/fields±s.d. from triplicate determinations.
P<0.01 vs medium only (without Bv8 in either chamber).
To identify the type of G-protein coupled to the chemotactic effect of Bv8, we used the inhibitor of the Gi protein PTX , the phospholipase inhibitor U73122 and its inactive control reagent U73343. Elicited macrophages were preincubated for 1 h together with 1 μg ml−1 PTX, 10 μM U73122 or 10 μM U73343, and checked for their ability to migrate in the presence of an optimal active concentration (10−11 M) of Bv8 or fMLP (10−8 M).
As reported in the upper panel of Figure 3, PTX pretreatment did not affect the migration induced by Bv8 but, as expected, the chemotactic activity of fMLP was completely abolished in PTX-treated macrophages. In contrast (lower panel of Figure 3), after pretreatment with the phospholipase C inhibitor U73122, fMLP and Bv8-stimulated chemotaxis was significantly reduced, while U73343, its structurally close analogue that lacks the phospholipase-C inhibiting activity, had no such effect.
Figure 3.
Effect of pretreatment with pertussis toxin, PTX, (upper panel) or the phospholipase inhibitors U73122 and U73343 (lower panel) on Bv8 and fMLP-induced chemotaxis. Elicited macrophages were preincubated with 1 μg ml−1 PTX, 10 μM U73122 or U73343 or medium only for 1 h. The viability of macrophages was checked and they were added in the chemotaxis chamber in the presence of medium only or with optimal concentrations of fMLP (10−8 M) or Bv8 (10−11 M). The chambers were then incubated for 100 min as indicated in Methods. The preincubation with PTX and U73122 prevented fMLP-chemotaxis, while only U73122 blocked Bv8's effect. Results are the number of cells migrated/microscopic field. *P<0.01 vs medium, that is, spontaneous migration.
In order to rule out a toxic effect of the inhibitors on macrophages, at the end of pretreatment, before incubating the cells in the Boyden chamber, we checked the viability by the Trypan blue exclusion test. The percentage of viable cells was not affected by the inhibitors (data not shown).
Although thioglycollate-elicited macrophages are commonly utilized for macrophage studies (Limiroli et al., 2002; Major et al., 2002; Cook et al., 2003; Sacerdote et al., 2005), the possibility exists that thioglycollate-mediated stimulation might interfere with the results, affecting the stage of macrophage activation. To rule this out, we examined whether Bv8 also stimulated chemotaxis of resident, not elicited macrophages. As reported in Table 2, Bv8 (10−11 M) induced significant migration of resident macrophages. This effect was not blocked by PTX pretreatment, while the phospholipase C inhibitor U73122, but not U73343, prevented it.
Table 2.
Resident peritoneal macrophage chemotaxis induced by fMLP and Bv8
| Chemotactic stimuli | Pretreatment (1 h) | |||
|---|---|---|---|---|
| Medium | +PTX | +U73122 | +U73343 | |
| Medium | 15.2±8.1 | 17.0±9.5 | 12.2±8.1 | 16.4±6.3 |
| fMLP 10−8 M | 39.3±6.3* | 21.1±6.7 | 15.0±4.2 | 44.0±8.0* |
| Bv8 10−11 M | 43.1±6.2* | 45.0±5.8* | 16.1±9.4 | 41.2±9.6* |
Effect of pretreatment with PTX, the phospholipase inhibitor U73122 and its inactive control U73343 on Bv8 and fMLP-induced chemotaxis of resident macrophages. Cells were preincubated with 1 μg ml−1 PTX, 10 μM U73122, 10 μM U73343 or medium only for 1 h before testing in the chemotaxis chamber with medium only, fMLP or Bv8. Results are cells/field±s.d. from triplicate determinations.
P<0.01 vs medium/medium (spontaneous migration).
Chemotaxis in PKR-1 KO and WT control mice
Mice knocked-out for PKR-1 have recently been generated. As this receptor is the most widely expressed on macrophages, we tested whether macrophages from these animals were still able to respond chemotactically to Bv8. Because the previous chemotaxis experiments were performed in Balb/C mice, and the PKR-1 KO animals had been generated on a C5/7BL6 background, we used WT littermates as controls. In these experiments, we used only thioglycollate-elicited macrophages. The results are described in Figures 4. Macrophages from WT animals showed chemotactic activity towards both fMLP and Bv8 similar to that with cells from the BalbC/J mice (see Figures 1 and 2). Macrophages from the KO animals also gave a significant chemotactic response when stimulated with fMLP. In contrast, Bv8 completely lost its ability to induce chemotaxis with the macrophages from the PKR-1 KO animals.
Figure 4.
Migration of macrophages from wild-type (WT) or PKR-1 knockout (KO) mice. Thioglycollate-elicited macrophages were incubated in the Boyden chamber with medium only, fMLP (10−8 M) or Bv8 (10−11 M). Results are the number of cells migrated/microscopic field. Macrophages from WT mice were chemotactically attracted by both fMLP and Bv8 (*P<0.01 vs number of cells migrated in the present of medium only) while the macrophages from PKR-1 KO mice responded chemotactically to fMLP but not to BV8 (*P<0.01 vs number of cells migrated in the presence of medium only). Means±s.d. of two experiments.
Effect of Bv8 on cytokines in Balb/C mice
Bv8 was added in vitro for 24 h to thioglycollate-elicited macrophage cultures with or without LPS for the stimulation of IL-1β, TNF and IL-10 production, and LPS + IFN-γ for IL-12. As reported in panel (a) of Figure 5, Bv8 never affected spontaneous production of IL-1β. However, the protein significantly boosted the production of this cytokine in LPS-stimulated macrophages. The increase was evident at 10−12, 10−11, 10−10, 10−9 and 10−8 M, but lower and higher concentrations were not effective. Panel (b) of the Figure 5 shows the levels of TNF. When cells were cultured without LPS, this cytokine was not detectable. LPS induced TNF production but Bv8 never had any such effect at the concentrations tested. In contrast, as shown in the panel (c) of Figure 5, Bv8 added in vitro to macrophages significantly reduced LPS-induced production of the antiinflammatory cytokine IL-10. This effect was significant at the concentrations of 10−11, 10−10, 10−9 and 10–8 M, while lower and higher concentrations had no noticeable activity.
Figure 5.
Effect of the addition of Bv8 on IL-1β (a), TNF-α (b), IL-10 (c) and IL-12 (d) production by macrophages. Thioglycollate-elicited macrophages (1 × 106 ml−1), purified by adherence, were used. Bv8 was added alone (effect on spontaneous production) or together with stimuli to induce cytokine production. IL-1β, TNF-α and IL-10 were stimulated with 1 μg ml−1 of LPS. The production of IL-12 was stimulated with 1 μg ml−1 of LPS+ 50 U ml−1 INF-γ. After 24 h incubation, culture media were collected and cytokines measured by specific ELISA. TNF and IL-12 levels were undetectable in unstimulated cells. Means±s.d. of four experiments. *P<0.01 vs 0 Bv8 (i.e. macrophage cultures without Bv8 addition).
Unstimulated cells, with or without Bv8, did not produce measurable amounts of IL-12. LPS+IFN-γ stimulated significant production of IL-12 and the addition of Bv8, at the concentrations of 10−11 and 10−9 M, significantly enhanced this output (Figure 5, panel d).
In order to rule out that thioglycollate elicitation could interfere with the results, we evaluated the ability of Bv8 to alter stimulated cytokine production also by resident, not elicited macrophages. Cells from 20/40 mice were pooled for each experiment.
As shown in Table 3, the levels of IL-1β, TNF and IL-10 produced by resident macrophages after in vitro stimulation with LPS (1 μg ml−1) were comparable in thioglycollate-elicited and resident macrophages, while the LPS+IFN-γ-stimulated production of IL-12 was low in resident macrophages. However, BV8 at the concentration of 10−11 and 10−9 M affected the cytokine levels similarly to thioglycollate-elicited macrophages: IL-1β and IL-12 production was increased, IL-10 production decreased and TNF was not affected.
Table 3.
Effect of Bv8 on stimulated cytokine production by resident peritoneal macrophages
| IL-1 (pg ml−1) | TNF (pg ml−1) | IL-10 (pg ml−1) | IL-12 (pg ml−1) | |
|---|---|---|---|---|
| Stimuli alone | 376±58 | 65.7±9.5 | 552.6±80 | 32±2 |
| Bv8 10−11 M | 564±34* | 72.9±6.7 | 234.7±42* | 65±11* |
| Bv8 10−9 M | 669±74* | 71.3±5.8 | 324.4±25* | 83±15* |
Resident peritoneal macrophages were purified by adherence. The production of IL-1, TNF and IL-10 was stimulated with 1 μg ml−1 LPS, and IL-12 production was stimulated with 10 μg ml−1 of LPS and 100 U ml−1 IFN-γ. Bv8 was added together with the stimuli. After 24-h incubation, culture media were collected and cytokines measured by specific ELISA.
Mean±s.d. from triplicate determinations.
P<0.01 vs stimuli alone.
Considering that Bv8 has similar effects on elicited and resident macrophages, subsequent experiments were performed only with elicited macrophages, as this enabled us to use far fewer animals.
PTX and the phospholipase inhibitor U73122 were used to investigate the type of G-protein involved in Bv8-induced cytokine modulation. Purified thioglicollate-elicited macrophages were preincubated for 1 h in the presence of 1 μg ml−1 PTX or 10 μM U73122 and U73343 before the addition of LPS and 10−9 M Bv8. As reported in Figure 6, PTX did not affect the cytokines' responses to Bv8. With PTX treatment Bv8 significantly increased the concentrations of IL-1β (upper panel) and IL-12 (lower panel), while IL-10 (middle panel) remained low. The figure also shows that pretreatment with U73122, but not with its inactive analogue U73343, completely prevented Bv8's effects on the three cytokines.
Figure 6.
Effect of pretreatment with PTX, the phospholipase inhibitor U73122 and its inactive control U73343 on Bv8's effects on IL-1β (upper panel), IL-10 (middle panel) and IL-12 (lower panel) production by macrophages. Thioglycollate-elicited macrophages (1 × 106 ml−1) were purified by adherence. Cells were preincubated with 1 μg ml−1 PTX, 10 μM U73122 or U73343 or medium only for 1 h. At the end of the preincubation, IL-1β, TNF-α and IL-10 were stimulated with 1 μg ml−1 of LPS. The production of IL-12 was stimulated with 1 μg ml−1 of LPS+ 50 U ml−1 INF-γ. Bv8 at the concentration of 10−9 M was added together with the stimuli. After 24 h incubation, culture media were collected and cytokines measured by specific ELISA. *P<0.01 vs blank columns, representing LPS alone (IL-1β, and IL-10) or LPS+ 50 U ml−1 INF-γ (IL-12).
Effect of Bv8 on cytokines in PKR-1 KO and WT control mice
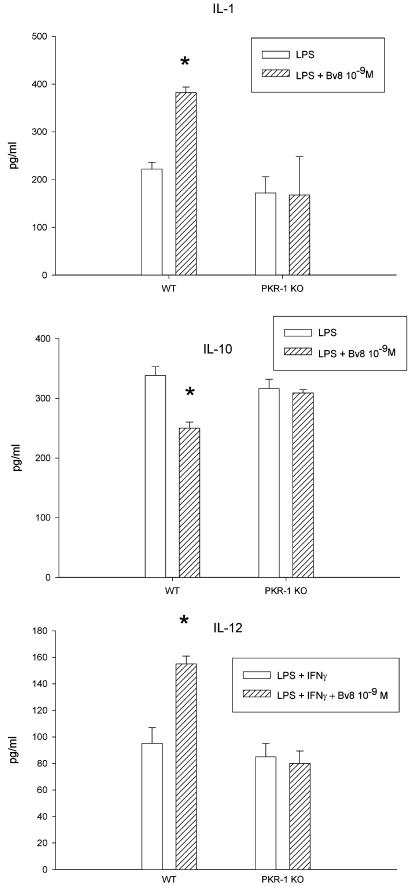
Figure 7 shows the effect of Bv8 on cytokine production in PKR-1 KO mice and their WT controls. In these experiments, we used only thioglycollate-elicited macrophages. Like in Balb/C mice, Bv8 (10−9 M) increased IL-1β and IL-12 but lowered IL-10 concentrations in WT controls. These effects were completely abolished in the PKR-1 KO mice, demonstrating its dependency on this receptor.
Figure 7.
Effect of Bv8 (10−9 M) on IL-1β (upper panel), IL-10 (middle panel) and IL-12 (lower panel) production by macrophages from wild type and PKR-1 KO mice. Thioglycollate-elicited macrophages (1 × 106 ml−1) were purified by adherence and were stimulated with 1 μg ml−1 of LPS for IL-1β and IL-10, and 1 μg ml−1 of LPS+50 U ml−1 INF-γ for IL-12. After 24 h incubation, culture media were collected and cytokines measured by specific ELISA. Bv8 influenced stimulated cytokine production by macrophages from WT animals but not from PKR-1 KO mice. Means±s.d. of two experiments. *P<0.01 vs blank columns, representing LPS alone (IL-1β, and IL-10) or LPS+50 U ml−1 INF-γ (IL-12).
Discussion
The macrophage is a fundamental effector cell of innate immune responses, but it also constitutes the interface between innate and adaptive immunity thanks to its ability to produce important regulatory cytokines such as IL-12 and IL-10 (Beutler, 2004; Hoebe et al., 2004). The present study found that Bv8 strongly influences all aspects of macrophage physiology, driving a proinflammatory macrophage phenotype.
The recruitment of macrophages and monocytes to sites of inflammation, injury and infection is a crucial step in inflammation and antimicrobial immune responses. The ability to migrate towards chemoattractants is a first, important event in macrophage physiology (Schiffmann, 1982; Beutler, 2004; Mantovani et al., 2004). Bv8 promotes substantial macrophage migration at very low concentrations indeed – as low as 10−12 M – with an effect similar to that of the classical chemoattractant fMLP.
High levels of Bv8 expression have been detected in infiltrating neutrophils at sites of inflammation (Le Couter et al., 2004). Neutrophils are the first cells to be recruited at sites of inflammation and injury, followed by macrophages. It is therefore possible that Bv8's potent chemotactic activity plays an important role in directing macrophage migration. In the same study, Le Couter et al. (2004) also showed that Bv8 can induce migration of human monocytes, indicating that the chemotactic properties are common to myeloid cells of different species.
However, besides attracting macrophages, Bv8 further stimulates them. The addition of the protein boosts the production of the proinflammatory cytokines IL-1β and IL-12 induced by LPS. Like chemotaxis, cytokine production is increased by Bv8 at low doses.
The observation that TNF production was not affected by Bv8 is unexpected, but it is known that in the proinflammatory cytokine cascade TNF is frequently the first to be synthesized and secreted, followed by IL-1β (Cavaillon et al., 2003; Cunha et al., 2005). It is therefore possible that Bv8 acts on specific mechanisms for IL-1β, downstream of TNF regulation, and not on common pathways (Beutler, 2004). Mirroring the Bv8-induced increase of IL-1β and IL-12, there was also a significant decrease in the anti-inflammatory cytokine IL-10. Bv8 therefore strongly skews the pro inflammatory macrophage profile by raising IL-1β and lowering IL-10.
IL-12, produced by antigen-presenting cells, links the innate and cell-mediated components of the immune response by inducing CD4+ T-cell differentiation to Th1-like cells, while IL-10 underlies the development of a Th2 profile (Mosman & Sad, 1996). Hypothetically, therefore, Bv8's effects on the macrophage might have an indirect impact on the Th1/Th2 balance, influencing the development of immune responses. This interesting aspect is being evaluated (Sacerdote et al., in preparation).
Bv8 and its mammalian analogues are ligands for two G-protein-coupled receptors, PKR-1 and PKR-2, which show distinct expression patterns in different organs. Although the PCR analysis did showed that macrophages express both PKR-1 and PKR-2 transcript, PKR-1 is expressed more abundantly. This observation is in agreement with other reports that PKR-2 is the form expressed mostly in the central nervous system (Melchiorri et al., 2001; Negri et al., 2002), while PKR-1 is largely present on immune tissue (Melchiorri et al., 2001; Le Couter et al., 2004).
In this study, we used, for the first time, recently generated PKR-1-deficient mice, clearly demonstrating that all the activities exerted by Bv8 on macrophages are completely mediated by the PKR-1 receptor. Bv8-induced chemotaxis and cytokine modulation were in fact completely abolished in these PKR-1 KO animals. We also characterized the early signal mechanisms activated immediately after Bv8 binds to its receptor. Pretreatment with PTX toxin did not affect either Bv8-induced chemotaxis or cytokine modulation. These results seem to rule out the coupling of macrophage PKR-1 receptors to a Gαi/0 protein. The complete blockade of Bv8 activity on macrophage with the phospholipase inhibitor U73122 suggests there is a Gq-coupled PKR-1 acting through the IP-3/phospholipase-C signaling pathways. The phospholipase-C isoenzymes hydrolyze PI 4,5-bisphosphate (PIP2) to produce DAG and inositol 1,4,5 triphosphate (IP3), a calcium mobilizer, second messenger. Mammalian phospholipase-C comprises four subtypes, β, δ, γ and ɛ. Classically, phospholipase C-β is considered to be regulated by G protein-coupled receptors through a Gαq protein (Rhee, 2001).
It also appears that different events in macrophage activation, such as chemotaxis and cytokine production, are governed by the same intracellular signaling, quite likely activated by Bv8 binding to PKR-1. The involvement of a G protein α subunit of the q type has been reported for other Bv8 effects too, such as the increase of [Ca2+] concentrations and the p42/p44 MAPK phosphorylation in CHO cells expressing PKRs and in the dorsal root ganglia cells, whereas PTX did not affect these phenomena (Negri et al., 2002). In contrast to our results, Le Couter et al. (2004) found that PTX blocked Bv8-induced migration of human monocytes. However, some explanations can be offered. First of all, the type of receptor involved in Bv8-induced human monocyte migration was not determined, and PKR-1 or PKR-2 could have different coupling systems. In addition, although monocytes and macrophages derive from a common myeloid lineage, tissue macrophages have different characteristics from monocytes.
It is also increasingly emerging that different macrophage subtypes might have different ‘inflammatory' functions (Cook et al., 2003). In our study, however, we found similar effects on both chemotaxis and cytokine production when Bv8 was administered to thioglycollate-elicited inflammatory macrophages, or to resident peritoneal macrophages from naive animals, the sentinel cells that respond to injury and orchestrate the innate immune responses (Cailhier et al., 2005). Bv8's ability to induce an inflammatory macrophage phenotype seems therefore general in macrophages at different stages and states of maturation or activation. In a previous study, we found a similar response as regards the effect on cytokines, using either resident or elicited macrophages (Limiroli et al., 2002).
Besides PK-receptors, macrophages also express high levels of PK-2 mRNA, which was always present as a tissue-specific double-splice variant: the short form encodes for a protein similar to amphibian Bv8 while the long form encodes for an additional 21 aa domain, inserted in the center of the polypeptide chain, already described only in mouse and human testis (Wechselberger et al., 1999). The insert was sequenced and was rich in basic residues, arginine and lysine, containing several potential cleavage sites for prohormone convertases. Nothing is known about the function of the longer PK-2 variant. Bullock et al. (2004) produced a recombinant protein of this splice variant that is about 200 times less potent than PK-2, in an aequorin-based assay for [Ca2+] mobilization in PKR-transfected CHO cells.
We have found a similar PK-2 long variant in rat macrophages and granulocytes, in human circulating granulocytes and in the promyelocytic cell line HL-60 (Negri et al., in preparation). Interestingly, coexpression of the ligand and receptors of this family of proteins has been observed in most immune cell types (Le Couter et al., 2004).
Recently, evidence has emerged that cytokines link the immune and the nervous systems and may be involved in the generation of pain and hyperalgesia. IL-1β has been associated with hyperalgesic states, acting directly on sensory neurons to increase their susceptibility to noxious stimuli (Safie Garabedian et al., 1995; Sommer & Kress, 2004). In contrast, IL-10 lowers nociceptive thresholds in several pain models (Poole et al., 1995). In view of Bv8's potent hyperalgesic activity (Negri et al., 2002), it is conceivable that this might be related to its effects on cytokines.
In conclusion, both resident and inflammatory macrophages seem to be targets for the Bv8/PK protein family. As these cells have functional PKR-1 receptors and produce Bv8, this molecule might possibly have some paracrine/autocrine role in regulating macrophage function.
Acknowledgments
This work was supported by grants from the Italian Ministry of University and Scientific Research (Cofin 2002/2004).
Abbreviations
- EG-VEGF
endocrine gland-derived vascular endothelial growth factor
- fMLP
formyl-met-leu-phe
- IL
interleukin
- LPS
lipopolysaccharide
- MIT1
mamba intestinal toxin
- PK
prokineticin
- PKR
prokineticin receptor
- PTX
pertussis toxin
- TNF
tumor necrosis factor
- U73122
(1-[6-[[(17b)-3-methoxyestra-1-3-5(10)-trien-17-yl]amino]hexyl])-1H pyrrole-2.5-dione
References
- BEUTLER B. Innate Immunity, an overview. Mol. Immunol. 2004;40:845–849. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- BULLOCK C.M., LI J.D., ZHOU Q.Y. Structural determinants required for the bioactivities of prokineticins and identification of prokineticin receptor antagonists. Mol. Pharmacol. 2004;65:582–588. doi: 10.1124/mol.65.3.582. [DOI] [PubMed] [Google Scholar]
- CAILHIER J.F., PARTOLINA M., VUTHOORI S., WU S., KO K., WATSON S., SAVILL J., HUGHES J., LANG R.A. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J. Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- CAVAILLON J.M., ADIB-CONQUY M., FITTINE C., ADRIE C., PAYEN D. Cytokine cascade in sepsis. Scand. J. Infect. Dis. 2003;35:535–544. doi: 10.1080/00365540310015935. [DOI] [PubMed] [Google Scholar]
- CHENG M.J., BULLOCK C.M., LI C., LEE A.G., BERMAK J.C., BELLUZZI J., WEAVER D.R., LESLIE F.M., ZHOU Q.Y. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- COOK A.D., BRAINE E.L., HAMILTON J.A. The phenotype of inflammatory macrophages is stimulus-dependent: implications for the nature of the inflammatory response. J. Immunol. 2003;171:4816–4823. doi: 10.4049/jimmunol.171.9.4816. [DOI] [PubMed] [Google Scholar]
- CUNHA T.M., VERRI W.A., SILVA J.S., POOLE S., CUNHA F.Q., FERREIRA S.H. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOEBE K., JANSSEN E., BEUTLER B. The interface between innate and adaptive immunity. Nat. Immunol. 2004;5:917–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- JOUBERT F.J., STRYDOM D.J. Snake venom. The amino acid sequence of protein A from Dendroaspis polylepsis (black mamba) venom. Hoppe Seylers Z. Physiol. Chem. 1980;361:1787–1794. doi: 10.1515/bchm2.1980.361.2.1787. [DOI] [PubMed] [Google Scholar]
- LE COUTER J., FERRARA N. EG-VEGF and Bv-8: a novel family of tissue-selective mediators of angiogenesis, endothelial phenotype and function. Trends Vasc. Med. 2003;13:276–282. doi: 10.1016/s1050-1738(03)00110-5. [DOI] [PubMed] [Google Scholar]
- LE COUTER J., ZLOT C., TEJADA M., PEALE F., FERRARA N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16813–16818. doi: 10.1073/pnas.0407697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE COUTER J., KOWALSKI J., FOSTER J., HASS P., ZHANG Z., DILLARD-TELM L., FRANTZ G., RANGELL L., DEGUZMAN L., KELLER G.A., PEALE F., GURNEY A., HILLAN K.J., FERRARA N. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:876–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- LI M., BULLOCK C.M., KNAUER D.J., EHLERT F.J., ZHOU Q.Y. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol. Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- LIMIROLI E., GASPANI L., PANERAI A.E., SACERDOTE P. Differential tolerance development in the modulation of macrophage cytokine production in mice. J. Leukoc. Biol. 2002;72:43–48. [PubMed] [Google Scholar]
- LIN D.C.H., BULLOCK C.M., EHLERT F.J., CHEN J.L., THIAN H., ZHOU Q.Y. Identification and molecular characterization of two closely related G-protein coupled receptors activated by prokineticins/EG-VEGF. J. Biol. Chem. 2002;277:19276–19280. doi: 10.1074/jbc.M202139200. [DOI] [PubMed] [Google Scholar]
- MAJOR J., FLETCHER J.E., HAMILTON T.A. IL-4 pretreatment selectively enhances cytokine and chemokine production in lipopolysaccaride-stimulated peritoneal macrophages. J. Immunol. 2002;168:2456–2463. doi: 10.4049/jimmunol.168.5.2456. [DOI] [PubMed] [Google Scholar]
- MANTOVANI A., SICA A., SOZZANI S., ALLAVENA P., VECCHI A., LOCATI M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;125:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- MASUDA Y., TAKATSU Y., TERAO Y., KUMANO S., ISHIBASHI Y., SUENAGA M., ABE M., FUKUSUMI S., WATANABE T., SHINTANI Y., YAMADA T., HINUMA S., INATOMI N., OHTAKI T., ONDA H., FUJINO M. Isolation and identification of EG-VEGF/prokineticins as cognate ligands for two orphan G-protein-coupled receptors. Biochem. Biophys. Res. Commun. 2002;293:396–402. doi: 10.1016/S0006-291X(02)00239-5. [DOI] [PubMed] [Google Scholar]
- MELCHIORRI D., BRUNO V., BESONG G., NGOMBA R.T., CUOMO L., DEBLASI A., COPANI A., MOSCHELLA C., STORTO M., NICOLETTI F., LEPPERDINGER G., PASSARELLI F. The mammalian homologue of the novel peptide Bv8 is expressed in the central nervous system and supports neuronal survival by activating the MAP kinase/PI-3-kinase pathways. Eur. J. Neurosci. 2001;13:1694–1702. doi: 10.1046/j.1460-9568.2001.01549.x. [DOI] [PubMed] [Google Scholar]
- MOLLAY C., WECHSELBERGER C., MIGNOGNA G., NEGRI L., MELCHIORRI P., BARRA D., KREIL G. Bv8, a small protein from frog skin and its homolog from snake venom induce hyperalgesia in rats. Eur. J. Pharmacol. 1999;374:189–196. doi: 10.1016/s0014-2999(99)00229-0. [DOI] [PubMed] [Google Scholar]
- MOORE K.W., O GARRA A., DE WAAL MALEFYT R., VIEIRA P., MOSMAN T.R. Interleukin-10. Annu. Rev. Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- MOSMAN T.R., SAD S. The expanding universe of T-cell subsets; Th1,Th2 and more. Immunol. Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- NEGRI L., LATTANZI R., GIANNINI E., DE FELICE M., COLUCCI A., MELCHIORRI P. Bv8, the amphibian homologue of the mammalian prokineticins, modulates ingestive behavior in rats. Br. J. Pharmacol. 2004;142:181–191. doi: 10.1038/sj.bjp.0705686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEGRI L., LATTANZI R., GIANNINI E., METERE A., COLUCCI M.A., BARRA D., KREIL G., MELCHIORRI P. Nociceptive sensitization by the secretory protein Bv8. Br. J. Pharmacol. 2002;137:1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POOLE S., CUNHAM F.Q., SELKIRK S., LORENZETTI B.B. Cytokine-mediated inflammatory hyperalgesia limited by IL-10. Br. J. Pharmacol. 1995;115:684–688. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHEE S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACERDOTE P., MANFREDI B., GASPANI L., PANERAI A.E. The opioid antagonist naloxone induces a shift from type 1 cytokine pattern to type 2 cytokine pattern in balb/cJ mice. Blood. 2000;95:2031–2036. [PubMed] [Google Scholar]
- SACERDOTE P., MARTUCCI C., VACCANI A., BARISELLI F., PANERAI A.E., COLOMBO A., PAROLARO D., MASSI P. The non-psychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J. Neuroimmunol. 2005;159:97–105. doi: 10.1016/j.jneuroim.2004.10.003. [DOI] [PubMed] [Google Scholar]
- SAFIE GARABEDIAN B., POOLE S., ALLCHORNE A., WINTER J., WOOLF C.J. Contribution of IL-1-beta to inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIFFMANN E. Leukocyte chemotaxis. Annu. Rev. Physiol. 1982;44:553–573. doi: 10.1146/annurev.ph.44.030182.003005. [DOI] [PubMed] [Google Scholar]
- SCHWEITZ H., BIDARD J.N., LAZDUNSKI M. Purification and pharmacological characterization of peptide toxins from the black mamba (Dendroaspis polylepsis) venom. Toxicon. 1999;28:847–856. doi: 10.1016/s0041-0101(09)80007-x. [DOI] [PubMed] [Google Scholar]
- SOMMER C., KRESS M. Recent findings on how pro-inflammatory cytokines cause pain: peripheral mechanism in inflammatory and neuropathic hyperalgesia. Neurosci. Let. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- TRINCHERI G. Interleukin-12: a pro-inflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- WATKINS L.R., MAIER S.F. Implications of immune-to-brain communication for sickness and pain. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS L.R, MAIER S.F. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- WECHSELBERGER C., PUGLISI R., ENGEL E., LEPPERDINGER G., BOITANI C., KREIL G. The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett. 1999;462:177–181. doi: 10.1016/s0014-5793(99)01473-8. [DOI] [PubMed] [Google Scholar]