Abstract
In this study, we aimed to characterize in vitro the effects of the benzofuran 5-HT4 receptor agonists prucalopride, R149402 and R199715 and the indolic agents tegaserod and 5-HT in the atria of young pigs (10–11 weeks) and newborn piglets.
In the paced left atrium of young pigs, only 5-HT results in positive inotropic responses when administered cumulatively (maximal effect relative to isoprenaline=53%, pEC50=6.8); however, all agonists showed lusitropic effects. Noncumulative administration results in greater positive inotropic responses for 5-HT and induces moderate positive inotropic responses for the other agonists; these responses fade.
Phosphodiesterase (PDE) enzyme inhibition with 3-isobutyl-1-methylxanthine (IBMX; 20 μM) enhances the responses to cumulatively administered 5-HT (maximal effect=89%, pEC50=7.7) and reveals clear positive inotropic effects for prucalopride, tegaserod, R149402 and R199715; fading is abolished. The maximal effect of the benzofurans is less pronounced than that of the indoles.
In the spontaneously beating right atrium of young pigs, all agonists show chronotropic activity when administered cumulatively in the absence of IBMX, without fade. Benzofurans behaved as partial agonists compared to 5-HT (maximal effect=54%, pEC50=6.5).
In newborns, the inotropic activity of the agonists in the IBMX-treated left atrium was less pronounced than in the young pig; the same applied for the chronotropic response in the right atrium, except for 5-HT.
In conclusion, the atrial responses to 5-HT4 receptor activation increase in the first months of life; the inotropic response is regulated by PDEs. Prucalopride, R149402 and R199715 are partial agonists compared to 5-HT.
Keywords: 5-HT4 receptor, phosphodiesterase, pig atrium, 3-isobutyl-1-methylxanthine, inotropic, chronotropic, lusitropic, prucalopride, tegaserod
Introduction
Effects of serotonin (5-HT) are attributed to the interaction with several subtypes of 5-HT receptors. Molecular cloning studies during the last decade have confirmed the existence of at least 14 genes encoding 5-HT receptors, which are grouped in seven subfamilies: 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-ht5, 5-HT6 and 5-HT7 (for reviews, see Saudou & Hen, 1994; Saxena, 1995). Except for 5-HT3 receptors, which belong to a family of ligand-gated ion channels, 5-HT receptors are G protein-coupled receptors. Together with 5-HT6 and 5-HT7 receptors, 5-HT4 receptors are positively linked to cAMP (Raymond et al., 2001). Depending on their localization, activation of 5-HT4 receptors results in various central and peripheral effects (reviewed by Eglen et al., 1995; Langlois & Fischmeister, 2003).
In human atria, 5-HT4 receptors are the only 5-HT receptor subtype present (Blondel et al., 1997). Until now, up to ten human splice variants of the 5-HT4 receptor have been described (Bockaert et al., 2004; Brattelid et al., 2004a). Six of them have been found in the human atrium: 5-HT4(a), 5-HT4(b), 5-HT4(c), 5-HT4(g), 5-HT4(i) and 5-HT4(n) (Blondel et al., 1998; Mialet et al., 2000; Bach et al., 2001; Medhurst et al., 2001; Vilaro et al., 2002; Brattelid et al., 2004a). Very recently, in contrast to earlier reports (Jahnel et al., 1992; Schoemaker et al., 1993), the presence of functional 5-HT4 receptors was also shown in human ventricles (Brattelid et al., 2004a). The activation of these cardiac 5-HT4 receptors results in phosphorylation of L-type Ca2+ channels through a cAMP-dependent increase in PKA activity. Consequently, the opening probability of the channels increases, resulting in a rise in the amplitude of the L-type Ca2+ current (Ouadid et al., 1992). These actions finally result in an increase in contractile force (Kaumann et al., 1991; Sanders & Kaumann, 1992). Besides these inotropic effects, the increase in PKA activity also hastens the onset of muscle relaxation (Kaumann et al., 1989; 1990). Moreover, cAMP, that is synthesized upon 5-HT4 receptor activation, can modulate hyperpolarization-activated cyclic nucleotide-gated (HCN) cation channels, and increase the inward pacemaker current If (Pino et al., 1998; Ulens & Tytgat, 2001). This mechanism can explain the positive chronotropic effects observed upon activation of sinoatrial 5-HT4 receptors. The increase in If currents and L-type Ca2+ currents could also trigger atrial arrhythmias (Kaumann, 1994; Kaumann & Sanders, 1994; Workman & Rankin, 1998; Rahme et al., 1999; Pau et al., 2003).
Human right atrial tissue, obtained during coronary artery bypass surgery, has been used as the human in vitro model for cardiac 5-HT4 receptor-function. To study 5-HT4 receptor-mediated responses in a laboratory animal, the options are very limited. Only pigs and monkeys have functional cardiac 5-HT4 receptors. In addition, 5-HT4 receptor mRNA was detected in rat atria and ventricles, but, until very recently, without any proven functional response (Gerald et al., 1995; Qvigstad et al., 2005). Indeed, Qvigstad et al. (2005) showed an upregulation of rat ventricular 5-HT4 receptor mRNA and the manifestation of an inotropic response through 5-HT4 receptors in failing hearts. Also in human hearts, an upregulation of 5-HT4 receptor mRNA associated with heart failure has been demonstrated (Brattelid et al., 2004b). The pig has served as a model for human atrial 5-HT4 receptors. Activation of porcine atrial 5-HT4 receptors results in positive inotropic and chronotropic responses (Kaumann, 1990; Schoemaker et al., 1992; Medhurst & Kaumann, 1993; Parker et al., 1995). Porcine sinoatrial receptors received most attention because of the lack of a proper human in vitro model to study chronotropy (Kaumann, 1990; Parker et al., 1995). Only a confined number of published experiments make mention of 5-HT4 receptors in the porcine left atrium, all using newborn piglets (Lorrain et al., 1992; Parker et al., 1995). As far as we know, the functional presence of 5-HT4 receptors in human left atrial tissue has only been reported in a single study (Sanders & Kaumann, 1992).
Using human and porcine ventricular tissue, Brattelid et al. (2004b) showed the inhibitory role of phosphodiesterases (PDEs) in the establishment of a functional 5-HT4 receptor-mediated response. Moreover, they showed an increase in the generation of the 5-HT4 receptor-mediated effect in porcine ventricle during the first months after birth.
In this study, we aimed to study in more detail the 5-HT4 receptor-mediated inotropic and lusitropic effects in porcine left atrium, and chronotropic effects in porcine right atrium. The influence of development on the atrial 5-HT4 receptor-mediated responses, as well as the role of PDEs in the fading of these responses, was evaluated. The effect of 5-HT was compared with that of four 5-HT4 receptor agonists: prucalopride and tegaserod have known gastroprokinetic effects and tegaserod is used in constipation-predominant irritable bowel syndrome. R149402 and R199715 are experimental 5-HT4 receptor agonists with a benzamide-derived benzofuran structure cf. prucalopride, developed by Johnson & Johnson Pharmaceutical Research & Development. In binding assays, R149402 (pKi against the 5-HT4 receptor antagonist GR113808=8.5) shows selectivity ratios of 160 and 400 between 5-HT4 receptors and 5-HT1B and 5-HT1D receptors, respectively, while it was at least 1000-fold more selective for 5-HT4 receptors compared to over 40 other receptors. R199715 (pKi against GR113808=9.1) shows an overall selectivity ratio of at least 1000 (Johnson & Johnson, unpublished data).
Methods
Tissue preparation
Female young pigs (10–11 weeks, 22–27 kg) and newborn female pigs (2–3 days, 1.6–2.8 kg), obtained from local farms, were deeply anaesthetized with an intravenous (50 mg kg−1) or intraperitoneal (100 mg kg−1) sodium pentobarbital (Kela NV, Hoogstraten, Belgium) injection, respectively. After exsanguination, the heart was dissected and placed in Krebs–Henseleit solution (composition in mM: glucose 11.1, CaCl2 2.51, NaHCO3 25, MgSO4 1.18, KH2PO4 1.18, KCl 4.69, CaNa2 EDTA 0.033 and NaCl 118). The right and left atrium were rapidly removed and thoroughly washed free of blood under continuous oxygenation. For chronotropic effect studies, the spontaneously beating right atrium was mounted in a 100 ml (20 ml for newborns) organ bath containing Krebs–Henseleit solution, continuously aerated with 95% O2 and 5% CO2. Guo et al. (1983) showed that pectinate muscles serve as a useful model of cardiac function, superior to atrial strips. Therefore, left atrial pectinate muscles with a thickness <1 mm and a length between 3 and 7 mm were carefully dissected away from the endocardial surface (9–12 per left atrium). The thickest end of the pectinate muscle was attached to a tissue holder and the tissue was lowered vertically into a 20 ml water-jacketed tissue bath. The tissue holders were equipped with two electrodes, designed for contact stimulation. Electrodes were oriented perpendicularly to the tissue, forcing the current through the axis of the tissue. Owing to the small dimensions of the pectinate muscles in newborn pigs, the left atria of these animals were cut into strips (<1 mm width; 4 per left atrium), defined by the orientation of the pectinate muscles, and mounted as described above. Preliminary experiments showed that a temperature of 37°C and a CaCl2 concentration of 2.51 mM in the bathing solution generated optimal conditions for all atrial experiments. Changes in isometric force of the tissues were recorded via Statham UC2 force transducers (Gould, Cleveland, U.S.A.) and DBA 18 digital bridge amplifiers (Anerma, Belgium) on a Powerlab data acquisition system and recorded using Chart v5.1.1 software. In experiments with newborn piglets, the amplifiers were from Janssen Pharmaceutica, Belgium and data were recorded directly on a chart recorder (Graphtec, Linearcorder WR 3701). Electrical field stimulation was performed with a constant voltage stimulator (Janssen Pharmaceutica, Belgium). All tissues were used on the day of preparation. The study was approved by the ethical committee from Johnson & Johnson Pharmaceutical Research & Development, a division of Janssen Pharmaceutica NV, Beerse, Belgium.
Experimental protocols
1. Left atrial pectinate muscles: Left atrial pectinate muscles were placed under a minimal resting load (5–10 mN) and were driven with square-wave pulses (0.5 Hz, 5 ms duration, 4 V, corresponding current 0.03–0.05 A). The preparation was allowed to equilibrate and stabilize for 90 min, with rinsing every 30 min. After this equilibration period, a length–tension curve was constructed by increasing the resting load stepwise (3–5 mN per step) to determine the length at which maximal paced contractions occurred (Lmax) and the tissues were then adjusted to 50% of Lmax. Strips showing no clear relation between length and developed tension were excluded. Before starting the experimental protocol, the solution described above was refreshed and supplemented with 0.2 μM propranolol and 6 μM cocaine, to avoid possible β-adrenoreceptor-mediated effects due to release of noradrenaline, and to reduce the uptake of 5-HT by the tissue, respectively (Medhurst & Kaumann, 1993). The voltage of the electrical stimulation was then decreased to just above threshold voltage.
Once a stable response was obtained, the nonselective PDE inhibitor 3-isobutyl-1-methylxanthine (IBMX) was added to one organ bath (20 μM). After 30 min, the IBMX-treated as well as a nontreated pectinate muscle were used to establish a cumulative concentration–response curve to an agonist with half log-units ascending concentration increments (depending on the agonist from 0.1 to 1 nM onwards). The contact time for each concentration was approximately 3 (5-HT), 7 (prucalopride and R149402) and 10 min (tegaserod and R199715). Seven consecutive concentrations of agonist were added as a single concentration to different parallel tissues and the response was followed for 30 min (5-HT, prucalopride and R149402) or up to 60 min (tegaserod and R199715), whereupon the 5-HT4 receptor antagonist GR113808 (0.1 μM) was added, except in experiments with 5-HT. This concentration of GR113808 is expected to induce a concentration ratio of at least 200 for 5-HT4 receptor-mediated effects, based on affinity data in the literature (Gale et al., 1994). After 30–60 min, experiments were terminated by the administration of a saturating concentration of isoprenaline (0.1 mM), still in the presence of previously administered agonist and antagonist. In separate tissues, the cumulative concentration–response curve to 5-HT, both in the presence and absence of IBMX, was also obtained after incubation with 0.3 μM GR113808 for 30 min. The influence of a 30 min incubation period with 0.3 μM GR113808 was also tested versus a single concentration of 5-HT (10 μM); the response of 5-HT was followed for 30 min.
For prucalopride, an extra set of tissues was used to study the effects of single concentrations in the presence of IBMX (20 μM). For comparison, the cumulative concentration–response curve to prucalopride in the presence of IBMX was also studied again. In these animals, one pectinate muscle preparation was used to evaluate the effect of a single concentration of tegaserod (1 μM) in the presence of IBMX. GR113808 (0.1 μM) was added 30 or 60 min after the administration of the single concentration of prucalopride and tegaserod, respectively. In this part of the study, basal contraction force in the strips that were not treated with IBMX was 12.0±0.3 mN, while isoprenaline increased the twitch force to 22.3±0.5 mN (n=247). In the strips that were to receive IBMX, the contraction force before the administration of IBMX was 13.5±0.4 mN, IBMX increased the contraction force to 16.9±0.6 mN, while at the end of the experiment isoprenaline increased it to 27.4±0.7 mN (n=150).
2. Newborn piglet left atrial strips: Pacing frequency was here set at 1 Hz. Preliminary experiments showed little variation in Lmax between muscle strips, probably because of the rather fixed dimensions of the strips, which made us decide in favour of a fixed resting tension of 20 mN, corresponding to 50% of the preliminary Lmax. In all strips, a cumulative concentration–response curve with log unit increments in the presence of propranolol (0.2 μM), cocaine (6 μM) and IBMX (20 μM) was constructed. Experiments were terminated by the administration of a saturating concentration of isoprenaline (0.1 mM), still in the presence of previously administered agonist. Basal contraction force in the presence of IBMX was 12.8±0.6 mN and isoprenaline increased this contraction force to 24.5±1.0 mN (n=32). Since in these experiments IBMX was administered together with propranolol and cocaine, its effect per se could not be assessed. In preliminary experiments in the absence of IBMX, basal contraction force was 8.8±0.4 mN (n=91).
3. Right atrium: The spontaneously beating right atrium of the newborn and young female pigs was stretched to a resting tension just sufficient to accurately measure developing force. These conditions were chosen to avoid stretch-induced sinus acceleration. The solution was refreshed every 15 min, until excessive foam production came to an end, and complemented with 0.2 μM propranolol and 6 μM cocaine. In experiments with right atria from newborn piglets, cocaine was only present in the experiments with 5-HT. Approximately 55% of the atria of young pigs showed irregular and/or very small contractions because conduction block episodes occurred due to the relatively large dimensions of these atria (ischemia); these atria were not used. Once a stable inotropic and chronotropic response was obtained, a cumulative concentration–response curve was established for all the agonists under study (one curve per preparation). To end the protocol, a saturating concentration of isoprenaline (0.1 mM) was administered to the solution.
Data analysis
1. Inotropic and chronotropic effects: The average contraction or beating rate during 2 min before the addition of agonist was taken as the initial value. All responses were expressed relative to the increase above the initial value caused by 0.1 mM isoprenaline. Atrial preparations that showed an increase in contraction to isoprenaline smaller than 1 mN were excluded from the analysis. An increase in contraction force was quantified using the maximal response, while for a decreasing response the minimal contraction was used. To account for the individual bell-shaped concentration–effect curves in fitting procedures, the higher concentrations where the response declined under the maximal response were excluded. Rarely, IBMX-treated pectinate muscles showed agonist-induced arrhythmic contractions in the cumulative protocol; these results were excluded in the fitting procedures (1/6 for 5-HT and R199715). The collected data were iteratively fitted to the Hill equation, obtaining curve parameter estimates for mid-point location (EC50, estimated as −log(EC50)), upper asymptote of the observed maximal effect (α) and Hill slope (nH):
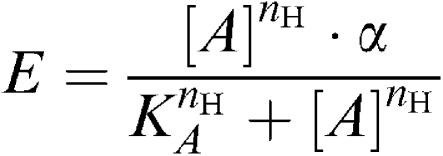 |
This was carried out using a nonlinear mixed effects model (PROC NLMIXED; SAS v9.1). These models include additional random effects (besides the fixed effects) to account for interindividual variability of the fitted curve-location parameters (see Vonesh & Chinchilli, 1997; Pinheiro & Bates, 2000; Verbeke & Molenberghs, 2005 for more information on mixed effects modelling).
Besides maximal twitch force, the change in contraction velocity was analysed in all experiments with 5-HT and prucalopride, and in experiments with cumulative administration of tegaserod, R149402 and R199715 in the absence of IBMX. The change in maximal contraction (+dF/dt) and maximal relaxation (−dF/dt) rate was obtained by taking the first derivative of isometric force recordings.
2. Lusitropic effects: For the analysis of the hastening of contraction and lusitropic effect in the left atrial pectinate muscles, the time from the beginning of contraction till the peak (time to peak force (tpf)) and the time from peak contraction till 50% relaxation (t50) were analysed with Peak Parameters v2.1.1, an extension of Chart v5.1.1 for Windows. The changes in tpf and t50 for each agonist were expressed in terms of percentage relative to the change caused by isoprenaline. Pectinate muscles in which isoprenaline did not reduce the tpf or t50 (compared to the basal value) were excluded from the analysis. The numbers used in the analysis are indicated between brackets. Concentration–effect curves for tpf and t50 were fitted to the Hill equation using GraphPad Prism v.4.02; no mixed-effects procedure was used.
Contraction and relaxation are two interconnected processes. A change in the contraction phase (+dF/dt) induces a coordinated change in the relaxation phase (−dF/dt). Therefore, −dF/dt cannot assess relaxation independently of the contraction phase. To follow the time course of the relaxant effect, we therefore also analysed a mechanical parameter that takes simultaneous changes in contraction and relaxation into account. The coefficient R2=(+dF/dt)/(−dF/dt) tests the coupling between contraction and relaxation. It has been shown to be a mechanical parameter that tests the lusitropic state in a manner that is less dependent on the inotropic changes. Indirectly it reflects myofilament calcium sensitivity (Mattiazzi et al., 1986; Hanouz et al., 2004).
Statistics
To check for differences between two administration methods, the fit of a three-parameter logistic model with common curve location parameters for the two administration methods is compared to the fit of a three-parameter logistic model with separate curve location parameters for each administration method. In the mixed-effects procedure in SAS, this is carried out using a likelihood ratio (LR) test. For the lusitropic data, the global fitting approach in GraphPad Prism v.4.02 was used; in this method the goodness of fit of both models are compared with an F-test (assessed by the sum of squares), adjusting for difference in the number of degrees of freedom. If the LR test or the F-test resulted in a P-value <0.05, the individual curve location parameters (α, pEC50, nH) were compared by a t-test and the corresponding P-values are shown. To test whether the 5-HT4 receptor agonists caused a reduction in tpf and t50 in the presence of IBMX, the raw data were analysed using one-way ANOVA for repeated measures, followed by a Dunett's multiple comparison test to compare the responses against the basal value. Comparisons between two data groups were carried out using t-tests. When more than two groups were to be compared, one-way ANOVA was performed with a Tukey–Kramer post test. Unless otherwise stated, data are presented as mean±s.e.m., based on results from 5–7, 5–8 and 6–10 different animals for left atrium, right atrium (10–11 weeks) and right atrium (newborn), respectively. Significance is associated with a P-value <0.05.
Drugs
The following drugs were used (abbreviations and respective suppliers in parentheses): 3-isobutyl-1-methyl-xanthine (IBMX; Fluka, Switzerland); propranolol HCl (Sigma, Belgium); [1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl-1-methyl-1H-indole-3-carboxylate (GR 113808; Tocris Cookson, U.K.); 5-hydroxytryptamine creatinine sulphate (5-HT; Acros chimica, Belgium); cocaine HCl, isoprenaline HCl, tegaserod, prucalopride HCl, 4-amino-5-chloro-2,2-dimethyl-2,3-dihydro-benzofuran-7-carboxylic acid [3-hydroxy-1-(3-methoxy-propyl)-piperidin-4ylmethyl]-amide (R149402 HCl) and 4-amino-5-chloro-2,3-dihydro-benzofuran-7-carboxylic acid [3-hydroxy-1-(3-methoxy-propyl)-piperidin-4ylmethyl]-amide (R199715 HCl; Johnson & Johnson Research and Development, Beerse, Belgium). All compounds were dissolved and diluted in distilled water, except for GR 113808 and tegaserod. GR 113808 was dissolved in dimethyl sulphoxide (DMSO) to obtain a stock solution of 10 mM. Dilutions were made with distilled water. A stock solution of tegaserod (1 mM) was made in distilled water containing 20% cyclodextrin and diluted with distilled water. For experiments with 5-HT in newborn piglets, 5-HT was dissolved in 0.2 mM ascorbic acid.
Results
Effects of 5-HT on the left atrium of young pigs
Inotropic effects
In left atrial pectinate muscles, 5-HT caused a concentration-dependent increase in contractile force when administered in a noncumulative manner (single concentrations to different tissues) as well as in a cumulative manner. The positive inotropic effect following the addition of a single concentration of 5-HT reached a maximum after 1–2 min (Figure 1a). After this, the contractile force quickly returned to the basal value in approximately 10–15 min, and often stabilized at a level below basal within 30 min. The decrease below basal level was significant for the higher concentrations of 5-HT (3 and 10 μM 5-HT; P<0.05). When the change in contractile force per time unit (+dF/dt) was examined, the same transient response was observed (Figure 3a). However, +dF/dt stabilized at a level that was not significantly different from basal within 30 min for all concentrations of 5-HT (results not shown). The cumulative concentration–response curve (Figure 1b) has a typical bell shape (Figure 2) as described earlier (Parker et al., 1995). When adding the concentrations in the decreasing part of this curve (from 3 μM 5-HT onwards), no or a very small transient inotropic response occurred and contraction force progressively declined. The concentration–effect curves for cumulative and noncumulative administration significantly (P<0.001) differed in their maximal effect (Figure 2, Table 1). When the cumulative concentration–effect curve was constructed in the presence of IBMX (Figure 1c), the maximal effect and the pEC50 were significantly increased compared to cumulative administration in the absence of IBMX (Figure 2, Table 1). When compared with the location parameters of the noncumulative concentration–response curve to 5-HT in the absence of IBMX, there is a significant increase in pEC50 and nH values (Figure 2, Table 1). When changes in contraction rate (+dF/dt) were expressed relative to isoprenaline, the location and shape of the concentration–effect curves were the same as described for the changes in force in the corresponding conditions (results not shown).
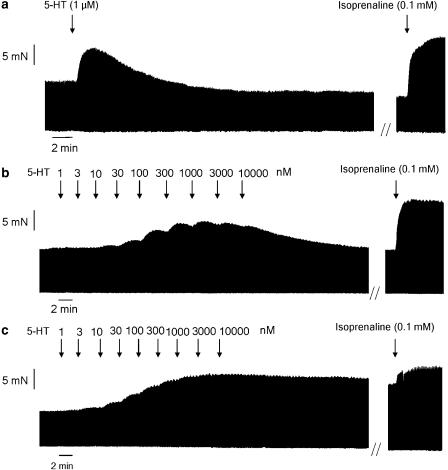
Figure 1.
Representative tracings showing the response to 5-HT in porcine left atrial pectinate muscles. The upper tracing (a) represents the effect caused by the administration of a single concentration of 5-HT (1 μM). The recording in (b) shows the response following the cumulative administration of 5-HT with half log unit concentration increments, indicated by the arrows. In (c), the response to a cumulative administration of 5-HT in the presence of the nonselective PDE inhibitor IBMX (20 μM) is shown. Experiments were terminated by the administration of a supramaximal concentration of isoprenaline (0.1 mM), still in the presence of previously administered substances.
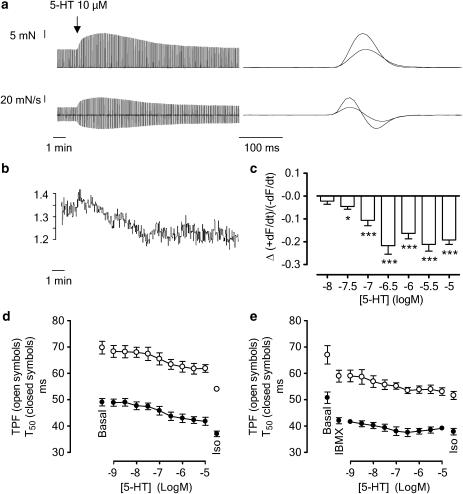
Figure 3.
(a) Positive inotropic and lusitropic effects to 5-HT (10 μM) on a left atrial pectinate muscle. Tracings show a recording of the contractile force (upper panel) and the corresponding first derivative (lower panel). The diagrams on the right show a superimposed fast-speed recording of a contraction before administration of 5-HT and at maximal inotropic response by 5-HT. Basal values for +dF/dt and −dF/dt were 312±12 and −210±8 mN s−1, respectively; 0.1 mM isoprenaline increased +dF/dt and −dF/dt to 737±34 and −557±25 mN s−1, respectively (n=48). (b) Tracing showing the time-related decrease of R2 (+dF/dt/−dF/dt) of the correlating tracings in (a). (c) Concentration-dependent decrease of R2, measured 30 min after the administration of a single concentration of 5-HT. On average, the basal value of R2 was 1.47±0.02 in these experiments (n=40). *P<0.05, ***P<0.001 versus basal values. (d, e) tpf and t50 by 5-HT in the presence of increasing cumulatively administered concentrations of 5-HT in the absence (d) and presence (e) of IBMX (20 μM). IBMX per se reduced tpf and t50. The vertically averaged experimental data points are connected by a line. The influence of the administration of isoprenaline (0.1 mM) at the end of the experiments is also shown. Mean±s.e.m. is given; error bars smaller than symbols are not shown.
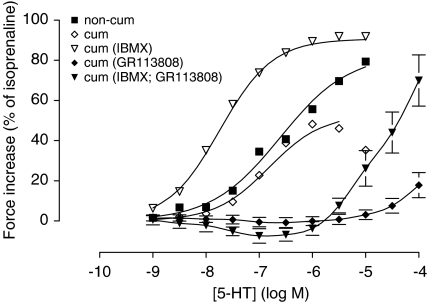
Figure 2.
Concentration-dependent increase in contractile force by 5-HT in porcine left atrium pectinate muscle preparations. 5-HT was administered in a noncumulative (non-cum), or cumulative way in the absence (cum) or presence of 20 μM IBMX (cum IBMX). The cumulative concentration–response curves were also obtained after 30 min pre-incubation with the 5-HT4 receptor antagonist GR113808 (0.3 μM). The vertically averaged experimental data points were expressed as mean percentage of the contraction caused by 0.1 mM isoprenaline; for the datapoints with GR113808, the s.e.m. is shown. The curves shown superimposed on the vertically averaged data points of (non-cum), (cum) and (cum IBMX) represent the population mean obtained from an iterative fitting procedure to the Hill equation in a nonlinear mixed effects model. To account for bell-shaped curves, the higher concentrations where the response declined under the maximal response were excluded in the fitting procedures. Therefore, the bell shape only appears in the averaged data points, and not in the superimposed curve (cum). The data points of the experiments with GR113808 were connected by a line.
Table 1.
Curve parameters for the concentration–effect curves of the inotropic and lusitropic effects of 5-HT in left atrial pectinate muscles of young pigs
| Administration method | pEC50 | α | nH |
|---|---|---|---|
| Inotropic effect | |||
| Noncumulative | 6.61±0.14 | 85.5±6.5*** | 0.66±0.05 |
| Cumulative | 6.82±0.13### | 52.7±5.4### | 0.86±0.09 |
| Cumulative (IBMX) | 7.71±0.10$$$ | 88.7±5.2 | 0.93±0.07$ |
| Reduction in time to peak force (tpf) | |||
| Noncumulative | 6.38±0.13* | 71.1±6.0* | ND |
| Cumulative | 6.99±0.21 | 50.7±5.4 | ND |
| Cumulative (IBMX) | ND | ND | ND |
| Reduction in time to reach 50% relaxation (t50) | |||
| Noncumulative | 6.40±0.22 | 67.3±8.6 | ND |
| Cumulative | 6.88±0.20 | 58.9±6.0 | ND |
| Cumulative (IBMX) | ND | ND | ND |
The parameters α, pEC50 and nH were obtained from the iterative fitting procedure to the Hill equation; a mixed-effects procedure was used for the inotropic data. nH values for tpf and t50 are not shown since no animal-specific correlation was used in the fitting procedures, which can cause an underestimation of the Hill slope. Experiments were performed in the presence of propranolol (0.2 μM) and cocaine (6 μM), and 5-HT was administered noncumulatively, cumulatively or cumulatively in the presence of 20 μM IBMX. Results were expressed as the percentage increase compared to the response by isoprenaline (0.1 mM). Since the reduction in tpf and t50 by isoprenaline in the presence of IBMX was very small, normalizing the data to isoprenaline was not meaningful and no fitting procedures were performed on these data. The values are expressed as mean±s.e.m.
P<0.05
P<0.001 noncumulative versus cumulative.
P<0.001 cumulative versus cumulative (IBMX).
P<0.05
P<0.001 cumulative (IBMX) versus noncumulative, ND: not determined.
Lusitropic effects
5-HT causes a reduction in tpf and t50 (as illustrated for a single concentration in Figure 3a and for cumulative administration in Figure 3d). The curve parameters for the concentration–effect curves upon noncumulative and cumulative administration are given in Table 1. From 0.03 μM onwards, noncumulative administration of 5-HT significantly reduced R2 (Figure 3b and c). This reduction of R2 was not transient and occurred more slowly than the inotropic response (R2 was not significantly decreased at the inotropic maximum of a given concentration; compare Figure 3b to a). The maximal reduction of R2 occurred at 0.3 μM 5-HT and was 14±1% (measured 30 min after agonist administration). In the cumulative assay, 5-HT appeared less efficacious and more potent in reducing the tpf but not the t50, compared to the noncumulative conditions. In the cumulative protocol, R2 was measured 30 min after the administration of the highest concentration, to be comparable with the noncumulative administration of 5-HT; 5-HT reduced R2 with 17±2%.
IBMX per se induced significant reductions of both the tpf and the t50 by 7.67±1.16 and 8.27±1.41 ms, respectively (n=6, P<0.01; Figure 3e). 5-HT caused an additional decrease in tpf and t50, which were not further reduced by isoprenaline. The tpf and t50 after the administration of isoprenaline in the presence of IBMX were on average only 6.20±1.51 and 1.33±1.95 ms lower than the values in the presence of IBMX per se (n=6). Therefore, normalizing the data to the isoprenaline response was not meaningful and no fitting procedures were performed on the lusitropic data in the presence of IBMX. IBMX reduced R2, and no additional decrease of R2 was observed with 5-HT.
Effects of prucalopride, tegaserod, R149402, R199715 on the left atrium of young pigs
Inotropic effects
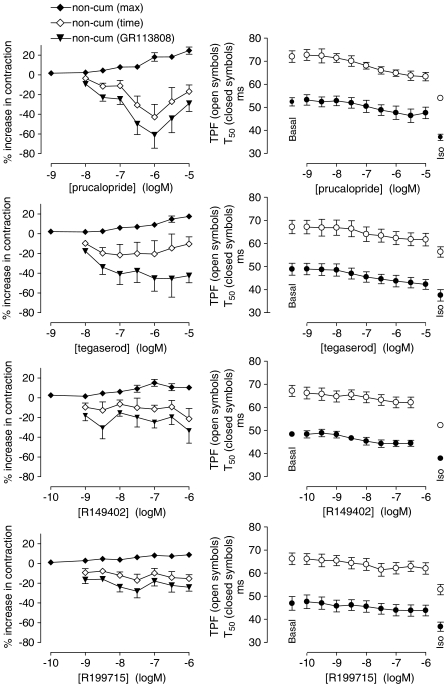
In left atrial pectinate muscles, all 5-HT4 receptor agonists tested caused positive inotropic effects when administered in a noncumulative manner (as demonstrated for prucalopride and tegaserod in Figure 4a and c, respectively). The inotropic response for a given concentration of agonist developed at a slower rate than for 5-HT, but reached a maximum within 7 min. The capricious nature of the individual curves made it impossible to construct meaningful fittings to the Hill equation for each animal. The vertically averaged and connected data points (Figure 5), however, show a clear concentration-dependent, significant increase in contraction force after stimulation with the 5-HT4 receptor agonists (P<0.001; ANOVA with post-test for linear trend on log-transformed data to obtain equal variances). The maximal developed increase in contraction force was 24.7±3.7% for prucalopride, 17.6±2.3% for tegaserod, 15.2±3.5% for R149402 and 8.8±2.3% for R199715. For prucalopride, the increase in contraction velocity (+dF/dt) was analysed, revealing a maximal increase of 20.5±2.9%. Stimulation with 3 and 10 μM tegaserod caused an increase in contraction force with kinetics comparable to the other concentrations (maximum reached within 7 min), but for these concentrations this ‘early' response was followed by a supplementary ‘late' increase of contraction after 15–30 min (Figure 4c). After reaching a maximum, the response to all agonists tended to fade with time. The effect of prucalopride and R149402 tended to stabilize within 30 min, while that of tegaserod and R199715 often needed up to 60 min to consolidate. This decreased response was significantly below the basal contraction force for some concentrations of prucalopride (0.03, 0.3 and 1 μM), tegaserod (0.01 and 0.03 μM), R149402 (0.001, 0.003, 0.1 and 1 μM) and R199715 (0.001, 0.003, 0.01, 0.03 and 1 μM) (Figure 5). The contraction rate of prucalopride also faded to a level that was not significantly below basal, except for 0.3 μM prucalopride.
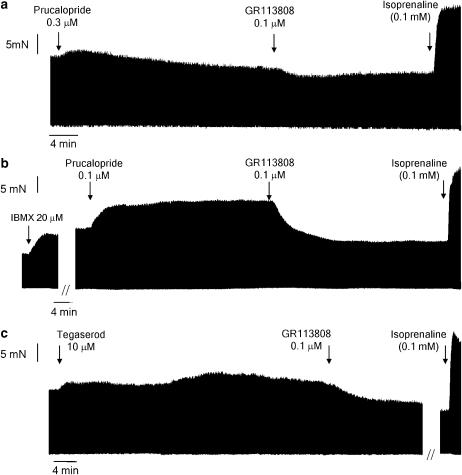
Figure 4.
Representative tracings showing the response to prucalopride (a, b) and tegaserod (c) in porcine left atrial pectinate muscles. The effect caused by the administration of a single concentration of prucalopride in the absence (a) or presence (b) of 20 μM IBMX is shown. In (c) the biphasic effect caused by a single concentration, in the absence of IBMX, of tegaserod is demonstrated. The effect of the administration of GR113808 (0.1 μM), still in the presence of the agonist, is shown in (a–c). Experiments were terminated by the administration of a supramaximal concentration of isoprenaline (0.1 mM), still in the presence of previously administered substances.
Figure 5.
Concentration-dependent effects of the 5-HT4 receptor agonists prucalopride, tegaserod, R149402 and R199715 on contractile force (left panels) and on tpf and t50 (right panels) in left atrial pectinate muscles. In the left panels, the maximal responses and the responses after 30 min (prucalopride, R149402) or up to 60 min (tegaserod, R199715) upon noncumulative administration of the agonist is shown (non-cum time). At 30 or 60 min, GR113808 (0.1 μM) was administered and the minimal contraction force in the presence of GR113808 is shown (non-cum GR113808). The vertically averaged experimental data points were expressed as mean percentage±s.e.m. of the contraction to isoprenaline (0.1 mM) and connected by a line. In the right panels, the responses to a cumulative administration of agonist are shown and averaged data points±s.e.m were connected by a line.
When the different concentrations were added cumulatively, no consistent increase in contraction was obtained. On average, with increasing concentrations of agonist, contractile force, but not +dF/dt (results not shown), declined. For tegaserod, R149402 and R199715, this reduction was representative for the response in at least five out of six animals. In only two out of six pectinate muscle preparations, the contraction force decreased after a cumulative treatment with prucalopride. The maximal decrease in contraction force was 7.9±8.2% for prucalopride, 22.2±7.6% for tegaserod, 10.5±4.5% for R149402 and 14.2±6.8% for R199715. When the response to the highest cumulatively administered concentration (10 μM) of tegaserod was followed for another 30 min, the same ‘late' response as in the noncumulative administration regime was observed.
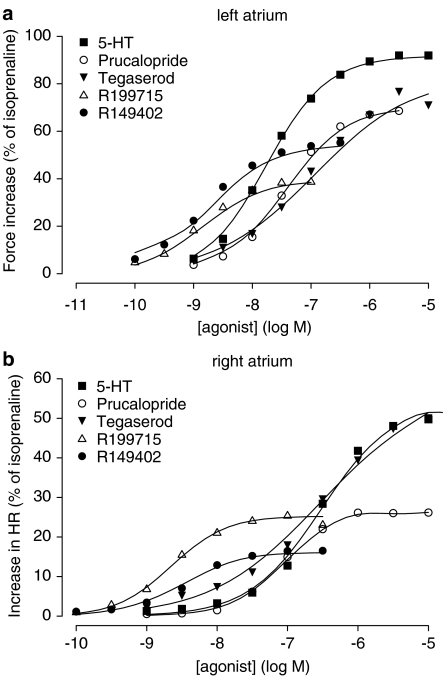
When IBMX was added to the organ bath, all 5-HT4 receptor agonists showed a clear concentration-dependent increase in contraction force (Figure 6a). The responses to tegaserod showed a higher variability compared to the other agonists, as reflected in the larger s.e.m. of the pEC50 when compared to the other compounds (Table 2). Only tegaserod shows an upper asymptote that did not differ from 5-HT, while only prucalopride was equipotent with the natural ligand for the 5-HT4 receptor (P<0.001 for all differences).
Figure 6.
Influence of cumulatively administered increasing concentrations of 5-HT and the 5-HT4 receptor agonists prucalopride, tegaserod, R149402 and R199715 on the contraction force in IBMX-treated left atrial pectinate muscles (a) and the beating rate of right atrium (b) of young pigs. The experiments in (b) were carried out in the absence of IBMX. The vertically averaged experimental data points were expressed as mean percentage of the contraction caused by 0.1 mM isoprenaline. The curves shown superimposed on the vertically averaged data points represent the population mean obtained from an iterative fitting procedure to the Hill equation in a nonlinear mixed-effect model.
Table 2.
Curve parameters for the concentration–effect curves of the inotropic effect in left atrial pectinate muscles and the chronotropic effect in right atria of young pigs
| Agonist | pEC50 | α | nH |
|---|---|---|---|
| Inotropic effect | |||
| 5-HT | 7.71±0.10 | 88.7±5.2 | 0.93±0.07 |
| Prucalopride | 7.70±0.09 | 64.3±6.7*** | 0.86±0.05 |
| Tegaserod | 6.85±0.17*** | 93.5±6.8 | 0.58±0.02 |
| R149402 | 8.97±0.08*** | 51.6±5.5*** | 0.86±0.05 |
| R199715 | 8.94±0.09*** | 32.8±6.7*** | 0.91±0.06 |
| Chronotropic effect | |||
| 5-HT (0/6) | 6.53±0.14 | 54.4±4.8 | 0.87±0.05 |
| Prucalopride (2/7) | 7.01±0.14* | 27.6±4.6*** | 1.16±0.11 |
| Tegaserod (3/6) | 6.58±0.17 | 57.6±5.7 | 0.62±0.04 |
| R149402 (1/8) | 8.53±0.15*** | 16.3±4.2*** | 1.30±0.16 |
| R199715 (1/5) | 8.70±0.17*** | 25.6±5.4*** | 1.23±0.15 |
All experiments were carried out in the presence of cocaine (6 μM) and propranolol (0.2 μM), while only in experiments with left atria IBMX (20 μM) also was added; agonists were added cumulatively. The parameters were obtained using a mixed-effects fitting procedure with the Hill equation. The values are expressed as mean±s.e.m. Numbers between brackets for the chronotropic effects represent the number of animals showing agonist-induced arrhythmic contractions.
P<0.05
P<0.001 versus corresponding values for 5-HT.
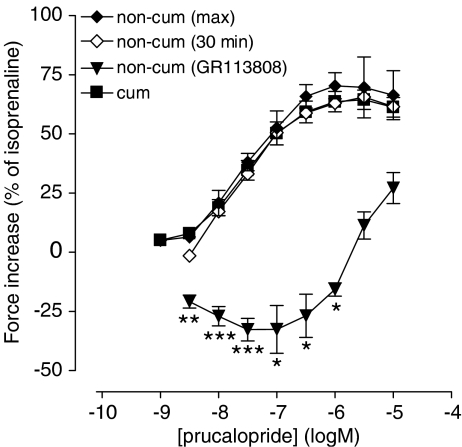
To investigate the role of PDEs in the observed difference in the inotropic response between cumulative and noncumulative administrations of agonists, we constructed concentration–effect curves to prucalopride, added noncumulatively as well as cumulatively, both in the presence of IBMX, in left atrial pectinate muscle preparations from the same animals. In these studies, the addition of a single concentration of agonist caused a persistent (Figure 4b), concentration-dependent increase in contraction (Figure 7). This contraction was stable for 30 min; the same was observed for +dF/dt. An LR test was used to compare the goodness of fit and no evidence was found to reject the hypothesis that the curve parameters of the noncumulative concentration–effect curve of prucalopride were equal to those of the cumulative concentration–response curve in the presence of IBMX (α, EC50, nH; P=0.78). The administration of a single concentration of tegaserod (1 μM) to an IBMX-treated pectinate muscle also caused a perseverant inotropic response of 51.5±8.6% (n=4) that remained stable during 60 min. This was not significantly different from the response at the same concentration in the cumulative protocol in the presence of IBMX (66.7±12.8%).
Figure 7.
Concentration-dependent increase in contractile force by noncumulative and cumulative administration of prucalopride to left atrial pectinate muscles in the presence of IBMX. The maximal response by a given concentration of prucalopride is shown (non-cum max), as well as the response after 30 min (non-cum 30 min). The 5-HT4 receptor antagonist GR113808 (0.1 μM), administered 30 min after prucalopride, reduced the contractile force to the value shown (non-cum GR113808). The vertically averaged experimental data points were expressed as mean percentage±s.e.m. of the contraction to isoprenaline (0.1 mM) and connected by a line. *P<0.05, **P<0.01, ***P<0.001: responses significantly below basal value.
Lusitropic effects
The noncumulative administration of the 5-HT4-receptor agonists caused a reduction of the tpf and the t50 (Table 3). In comparison to 5-HT, prucalopride was more potent and less efficacious than 5-HT in reducing the tpf; it was also less efficacious than 5-HT in reducing the t50, but the potency difference was not significant (Table 3). The influence of tegaserod on tpf and t50 was not different from that of 5-HT, except for a smaller efficacy in reducing the tpf. This efficacy (48.8%) was still twice as high as that of prucalopride (P<0.01). Both R149402 and R199715 were significantly more potent and less efficacious than 5-HT in reducing tpf and t50. From 0.01 μM onwards, noncumulative administration of prucalopride significantly reduced R2 by maximally 12±3% (measured 30 min after agonist administration; no significant change in R2 was observed at the time of maximal inotropic response for a given concentration).
Table 3.
Effect of 5-HT4 receptor agonists on time to peak force (tpf) and time to 50% relaxation (t50) in left atrial pectinate muscles of young pigs
| tpf | t50 | ||||
|---|---|---|---|---|---|
| Compound | Administration method | PEC50 | α | pEC50 | α |
| 5-HT | non | 6.38±0.13 | 71.1±6.0 | 6.40±0.22 | 67.3±8.6 |
| cum | 6.99±0.21# | 50.7±5.4# | 6.88±0.20 | 58.9±6.0 | |
| Prucalopride | non | 7.23±0.17*** | 24.6±2.4*** | 6.96±0.29 | 35.8±5.5** |
| cum | 6.78±0.22 | 51.6±6.1### | 6.81±0.23 | 39.3±5.5* | |
| Tegaserod | non | 6.58±0.25 | 48.8±7.0* | 6.98±0.17 | 45.8±4.8 |
| cum | 6.99±0.211 | 50.8±5.71 | 6.95±0.222 | 58.1±6.42 | |
| R149402 | non | 8.15±0.242*** | 16.6±2.12*** | 8.07±0.17*** | 23.0±2.6*** |
| cum | 8.14±0.25*** | 34.5±4.4*,### | 8.26±0.18*** | 37.8±4.1**,## | |
| R199715 | non | 8.61±0.42*** | 11.1±2.6*** | 7.97±0.34*** | 25.7±4.7*** |
| cum | 8.44±0.341*** | 30.4±5.01**,### | 8.27±0.48** | 35.0±7.8* | |
Agonists were added in a cumulative (cum) and noncumulative (non) way. nH values are not shown since no animal-specific correlation was used in the fitting procedures, which can cause an underestimation of the Hill slope. Experiments were carried out in the presence of cocaine (6 μM) and propranolol (0.2 μM). Values are expressed as mean±s.e.m.
P<0.05
P<0.01
P<0.001 versus corresponding values for 5-HT.
P<0.05
P<0.01
P<0.001 cumulative versus noncumulative.
One strip excluded from calculations because isoprenaline had no effect above basal, or
because a second contraction occurred in the relaxation phase.
Although not having a positive inotropic effect when added cumulatively, the 5-HT4 receptor agonists did reduce the tpf and t50 when administered in a cumulative fashion (Figure 5, Table 3). For tegaserod the curve-location parameters of the two effects were not significantly different from the parameters obtained with 5-HT. Prucalopride was less efficacious in reducing the t50 compared to 5-HT. R149402 and R199715, on the other hand, were also less efficacious but more potent in reducing tpf and t50 compared to 5-HT. In comparison to the noncumulative protocol, prucalopride, R149402 and R199715 caused a significantly higher reduction in the tpf, without a change in the pEC50. Except for R149402, which is significantly more efficacious (P<0.01) when administered cumulatively, no significant differences in the curve parameters for the t50 between the two administration protocols were found. In case of tegaserod, the administration method did not show a significant influence on the curve parameters. The response to isoprenaline was consistent in all experiments with noncumulative and cumulative administration of the agonists. At 30 min after a cumulative administration of prucalopride, tegaserod, R149402 and R199715, R2 was reduced by maximally 5±2, 14±1, 6±2 and 4±3 %, respectively.
IBMX reduced the tpf and t50 by 9.94±0.94 and 8.46±1.18 ms, respectively (pooled data for experiments with all agonists, n=26). The four 5-HT4 receptor agonists caused a small additional decrease in the tpf and t50. But since the decrease in tpf and t50 caused by isoprenaline in the presence of IBMX was very small (on average 4.28±0.99 and 2.18±0.76 ms, respectively, n=26), normalizing the data to the isoprenaline response was not meaningful and no fitting procedures were performed on the lusitropic data in the presence of IBMX. IBMX reduced R2 by 11±1% (n=48). Prucalopride caused no additional changes of R2 in the presence of IBMX.
Antagonism of the inotropic effects by GR113808 in the left atrium of young pigs
Preincubation of left atrial pectinate muscles with 0.3 μM GR113808 was able to block the responses caused by a cumulative administration of 5-HT in the absence of IBMX (Figure 2). GR113808 shifted the cumulative concentration–effect curve in the presence of IBMX to the right by approximately a factor 1000, which corresponds to the shift that can be expected from its KB values in the literature (Gale et al., 1994). After pre-incubation with the antagonist, most of the response following the administration of a single concentration of 5-HT (10 μM) was prevented (results not shown). In these conditions, 5-HT caused an increase in contraction force of 6.8±3.0% that diminished to −7.1±3.5% within 30 min. This corresponds to the response in the absence of GR113808 of a concentration of 5-HT that is approximately 1000-fold lower (10 nM). These observations are in line with the 5-HT4 receptor being the only 5-HT receptor subtype involved in the observed responses in our preparations.
In single concentration experiments, adding 0.1 μM GR113808 after the agonist caused an additional decrease in contraction force, even when the response to the agonist had stabilized below basal at the moment of administration of GR113808 (Figures 4 and 5). This effect was most prominent for prucalopride and tegaserod, and was also observed for 5-HT (preliminary experiments). For prucalopride and particularly for tegaserod, the effect of GR113808 increased when the preceding concentration of agonist increased. At a concentration of 0.3 μM prucalopride and 10 μM tegaserod, GR113808 resulted in maximal decreases in contraction force of 19.1±4.5 and 32.1±4.7% respectively (compared to the situation before the addition of antagonist and normalized for the response of isoprenaline above basal). GR113808 had the same effect on the contraction velocity of prucalopride, with a maximal decrease of +dF/dt of 13.2±2.9%. The stable decrease of R2, on the other hand, was not reversed by GR113808. In the experiments where IBMX-treated pectinate muscles received a single concentration of prucalopride, which induced a nearly stable increase of the EFS-induced contraction amplitude, the administration of 0.1 μM GR113808 reduced the contraction amplitude (as well as the change in +dF/dt, results not shown) to a level (Figure 4b) that was significantly below basal for 3 nM to 1 μM prucalopride (Figure 7). In the presence of IBMX, 0.1 μM GR113808 also reverted the increased contraction by a single concentration of tegaserod (1 μM) to a level below basal (−19.4±1.6%, n=4).
Spontaneously beating right atrium
The spontaneously beating right atria of the young pigs (10–11 weeks) showed a basal beating rate of 65.9±2.6 beats min−1 (b.p.m.) (averaged data for all experiments, n=33). Preliminary data showed no fading of the response in beating rate upon a single concentration of 5-HT, while the inotropic effect declined. Chronotropic responses to a cumulative administration of all agonists under study were determined (Figure 6, Table 2). Except for experiments with 5-HT, arrhythmic contractions occurred in experiments with all agonists in at least one animal. These arrhythmic contractions were probably caused by the induction of conduction block episodes, rather than by the induction of ectopic loci. The number of right atria in which at least one agonist concentration was followed by arrhythmia was: prucalopride, n=2/7; tegaserod, n=3/6, R149402, n=1/8; R199715, n=1/5. All data were included in the mixed-effects model, including those curves in which arrhythmic contractions occurred. Tegaserod was not different from 5-HT with respect to the maximal chronotropic effect or the pEC50. All the other agonists were more potent and less efficacious in increasing the heart rate than 5-HT. We also fitted the model to the data set in which we excluded, for each animal, all concentrations greater than or equal to the lowest concentration that evoked an arrhythmic response. The curve parameters obtained as such were not different from the parameters that were obtained when all data were included (results not shown). The response to isoprenaline was not different between the experiments with the different agonists. The beating rate after the administration of isoprenaline was 144.5±2.5 b.p.m. (averaged data for all experiments, n=33).
Newborn pig
To investigate the influence of development on atrial 5-HT4 receptors, the inotropic and chronotropic responses to 5-HT and the 5-HT4-receptor agonists were determined on IBMX-treated left atrial muscle strips and the spontaneously beating right atrium of newborn pigs (Table 4, Figure 8).
Table 4.
Curve parameters for concentration–effect curves of the left atrial inotropic and the right atrial chronotropic effect in newborn piglets
| Agonist | pEC50 | α | nH |
|---|---|---|---|
| Inotropic effect | |||
| 5-HT (n=7) | 6.54±0.11 | 69.8±6.1 | 0.91±0.07 |
| Prucalopride (n=6/7) | 6.79±0.38 | 30.4±8.0 | 0.91±0.14 |
| Tegaserod (n=5/7) | 7.18±0.20 | 32.7±6.6 | 0.54±0.07 |
| R149402 (n=6/7) | 4.83±0.37 | 56.3±12.2 | 0.37±0.04 |
| R199715 (n=5/6) | 6.19±0.40 | 28.3±6.8 | 0.41±0.07 |
| Chronotropic effect | |||
| 5-HT (n=6) | 6.85±0.12 | 64.3±3.3 | 1.23±0.12 |
| Prucalopride (n=7) | 7.06±0.14 | 14.4±3.5 | 0.85±0.16 |
| Tegaserod (n=10) | 6.49±0.12 | 21.8±2.8 | 0.85±0.09 |
| R149402 (n=4/7) | 8.00±0.14 | 0.8±0.7 | 3.16±1.70 |
| R199715 (n=8/10) | 8.83±0.06 | 10.0±3.3 | 10.58±5.27 |
Inotropic experiments in left atrium were carried out in the presence of cocaine (6 μM), propranolol (0.2 μM) and IBMX (20 μM). In experiments with the spontaneously beating right atrium, propranolol was added to the organ bath in all experiments, while cocaine was only present in experiments with 5-HT. The parameters were obtained using a mixed-effects fitting procedure with the Hill equation. The values are expressed as mean±s.e.m. Only responsive atria were used to obtain curve parameters; the numbers used for fitting versus total number studied are shown within brackets. The inotropic and chronotropic results for R149402 and R199715 are shown in italic; for the inotropic results, the very low Hill slope is indicative for low, inconsistent responses and misleading fits; for the chronotropic results, the high Hill slope results from the fact that the first chronotropically active agonist concentration resulted in a nearly maximal response because of the low response in some animals.
Figure 8.
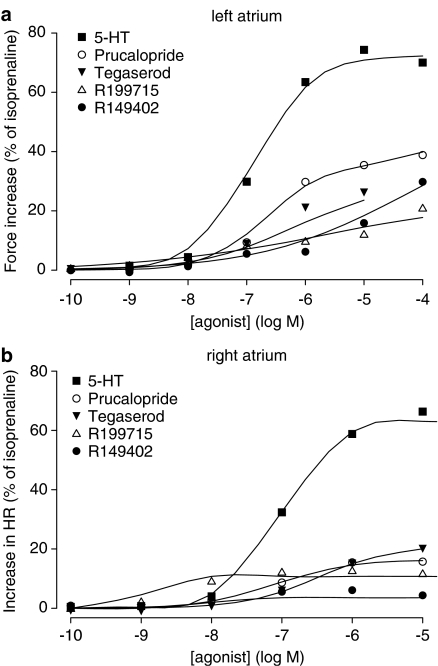
Influence of cumulatively administered increasing concentrations of 5-HT and the 5-HT4 receptor agonists prucalopride, tegaserod, R149402 and R199715 on contraction force in IBMX-treated left atrial muscle strips (a) and on beating rate in spontaneously beating right atria (b) of newborn pigs. The experiments in (b) were performed in the absence of IBMX. The data points represent the vertically averaged responses, expressed as percentage increase of force (a) or rate (b) relative to the increase in force (a) or rate (b) caused by 0.1 mM isoprenaline. The superimposed lines are the population averaged curves obtained through a nonlinear mixed-effects modelling approach using the Hill equation.
Only 5-HT showed clear sigmoid concentration–effect curves in the left atrium. Prucalopride was less consistent in its response, but still produced a curve shape that allowed meaningful fitting in 6/7 animals. The other agonists produced variable responses, capricious in nature, and often not reaching a clear maximum before 0.1 mM. Still, the use of a mixed-effects procedure resulted in reasonable fittings on the responding animals for prucalopride and tegaserod (Table 4, Figure 8a). However, the very low Hill slopes and fairly low potencies of R149402 and R199715 are probably the result of the low and inconsistent response. For the chronotropic effect in the right atrium, 5-HT, prucalopride and tegaserod induced clear sigmoid concentration–effect curves. The response to R199715 was variable and the maximal response was reached in a very narrow concentration range, as reflected in the high Hill slope. For R149402 three animals out of seven and for R199715 two animals out of 10 showed no response. Only the responding animals were used to determine the curve parameters (Table 4). The basal beating rate of the atria from newborns was 98.8±1.9 b.p.m. and the addition of isoprenaline resulted in a beating rate of 178.6±3.1 b.p.m. (averaged data for all agonists, n=40). The response to isoprenaline was not different between agonists.
Discussion
In this study, we aimed to characterize in the pig the inotropic and lusitropic effects of 5-HT, and the 5-HT4 receptor agonists prucalopride, tegaserod, R149402 and R199715 in the left atria, and their chronotropic effects in spontaneously beating right atria; the role of PDE enzymes in the regulation of the inotropic response was studied by using the PDE enzyme inhibitor IBMX. To verify whether the responses are developmentally related, we also studied the left atrial myocardial and sinoatrial 5-HT4 receptors of newborn piglets.
Inotropic effects in the porcine left atrium
Involvement of the 5-HT4 receptor: 5-HT, as well as the 5-HT4 receptor agonists prucalopride, tegaserod, R149402 and R199715, evoked positive inotropic effects in the porcine left atrium. 5-HT4 receptors have been shown to be the only functional receptor for 5-HT in porcine right and left atrium (Lorrain et al., 1992; Medhurst & Kaumann, 1993; Parker et al., 1995). The 5-HT4 receptor antagonist GR113808 was able to prevent and/or reverse the agonist-induced inotropic responses, supporting the idea that the effects are 5-HT4 receptor-mediated. The increased contraction force upon 5-HT4 receptor activation is probably due to an increase in L-type Ca2+ current, an effect that has been described in human atrial myocytes for 5-HT (Ouadid et al., 1992; Pau et al., 2003), and very recently also for prucalopride (Pau et al., 2005). A mechanism involving the phosphorylation of these Ca2+ channels by cAMP-dependent protein kinase was proposed, similar to the well-documented cardiac effects of β-adrenergic receptor activation by agonists (Ouadid et al., 1992). This is in accordance with the 5-HT4 receptor-induced stimulation of adenylyl cyclase and PKA in human atrial strips (Kaumann et al., 1990). The increased L-type Ca2+ current and the subsequent Ca2+-induced release of Ca2+ from the sarcoplasmatic reticulum, through the activation of ryanodine channels, is thus a plausible explanation of the observed positive inotropic action of the 5-HT4 receptor agonists.
Fading of 5-HT4 receptor-induced inotropic response: The positive inotropic response observed after the administration of a single concentration of agonist was transient. This time dependency was most clear for 5-HT, where the response returned to basal values within 15 min. For the higher concentrations, the response stabilized below the basal contraction level. The same time dependency was observed for the increase in contraction rate (+dF/dt). This excludes the possibility that a hastened relaxation causes the fading of the contraction force. For experiments with 5-HT, the contraction rate stabilized at a level that was not significantly different from basal for all concentrations of 5-HT tested. The other agonists showed slower kinetics in the rising as well as in the descending phase of the inotropic response, that could also stabilize below basal. Furthermore, there is a clear influence of the administration method on the concentration–response relationship. In a cumulative administration protocol, 5-HT appeared less efficacious compared to a noncumulative administration, while for the other agonists no positive inotropic response was observed in the cumulative protocol. Moreover, the cumulative concentration–response curve of 5-HT shows a typical bell shape. These observations suggest the involvement of a mechanism inducing fading of the inotropic response. In the cumulative protocol, the administration of the highest 5-HT concentration was not able to overcome the progressive fading of the response. Therefore, we can exclude the possibility that degradation of the ligand contributes to the fading response.
As in this study, in the human paced left atrium, the cumulative administration of 5-HT generates a bell-shaped concentration–response curve (Sanders & Kaumann, 1992). On the other hand, the response to a single concentration of 5-HT did not fade in the paced human right atrium, while the response to 5-CT and renzapride faded after a prolonged exposure to the agonists (Kaumann et al., 1991). It is noteworthy that the response following a cumulative administration of renzapride appeared bell shaped, while the response to 5-HT was not. This indicates that the mechanisms regulating the generated response can be different between different species, between both atria or even between different agonists.
Different mechanisms can be involved in the observed fading of the inotropic response. Some splice variants of the 5-HT4 receptor have been shown to couple to Gi proteins in transfected cells as well as in rat cardiac myocytes (Pindon et al., 2002; Castro et al., 2005). We consider it unlikely that the fading response is caused by a time-dependent switch in G protein selectivity from Gs to Gi, which has been described for β2-adrenergic receptors (Heubach et al., 2004; Martin et al., 2004) since GR113808 would also block this Gi-mediated effect and would thus not decrease the contraction force once it has stabilized.
Receptor desensitization
5-HT4 receptors have been shown to desensitize upon activation by their ligands (Ansanay et al., 1992; Ronde et al., 1995; Mialet et al., 2003). The 5-HT4(a) receptor undergoes rapid (t(1/2)∼2 min) concentration-dependent phosphorylation after stimulation with 5-HT (Ponimaskin et al., 2005), which is the initial trigger for desensitization leading to uncoupling of the receptor from the G proteins (Ferguson, 2001). Both in the presence and absence of IBMX a maximal inotropic response for 5-HT was reached within 1–2 min after the administration of a single concentration of 5-HT, which is in accordance with the phosphorylation time profile described for the 5-HT4(a) receptor. The occurrence of desensitization tallies with the difference in maximal effect of 5-HT between noncumulative and cumulative concentration–response curves (and the absence of positive inotropic effects upon cumulative administration of prucalopride, tegaserod, R149402 and R199715) since in a cumulative protocol, previously administered agonist concentrations, causing (partial) receptor desensitization, will interfere with the equilibrium between the additional agonist concentrations and the remaining receptors. When the inotropic response to a 5-HT4 receptor agonist stabilized below basal levels, GR113808 was still able to induce a further small reduction of the contraction force. The contraction velocity, stabilized near basal, was also further reduced to a level below basal by GR113808. Similar to what has been observed in the human ventricle after stimulation of IBMX-treated ventricular trabeculae with 5-HT (Brattelid et al., 2004b), GR113808 also reversed the stable increased contraction force upon stimulation with prucalopride or tegaserod in the presence of IBMX. Although 5-HT4 receptors have been shown to possess a large, splice variant-dependent, constitutive activity (Claeysen et al., 1999), it seems unlikely that GR113808 acted as an inverse agonist at a constitutively activated 5-HT4 receptor, as GR113808 did not show any effect on the basal contraction force, both in the absence or presence of IBMX (data not shown). It can thus be deduced that the 5-HT4 receptors are at least still partially active and thus certainly not completely desensitized, as they remain accessible for GR113808 when the response has stabilized.
Role of PDE enzymes
PDE enzymes, which catalyse the breakdown of cAMP, obviously play a role in the observed fade. Indeed, the inhibition of these enzymes with IBMX results in a marked increased responsiveness to single concentrations of prucalopride and tegaserod with no fading for 30 or 60 min, respectively (contraction force as well as +dF/dt). Furthermore, IBMX greatly potentiates the 5-HT-induced contractions, and unravels the contractions upon the cumulative administration of prucalopride, tegaserod, R149402 and R199715. This is in line with a recent study, in which the inhibition of PDE led to the discovery of functional porcine and human ventricular 5-HT4 receptors (Brattelid et al., 2004b), hereby contradicting previous reports, where no 5-HT4 receptor-mediated effects in human and porcine ventricles could be shown, probably because no PDE inhibitor was used (Jahnel et al., 1992; Schoemaker et al., 1992; 1993). Furthermore, in the presence of IBMX, the concentration–effect relation for prucalopride was the same for cumulative and noncumulative administrations. This suggests that PDEs are the major cause of the observed contrast between the effect of the agonists with both administration methods in the absence of IBMX. This does not exclude the possibility of the involvement of concentration-dependent (partial) rapid desensitization since this might be masked by IBMX in the cumulative protocol.
For the β2-adrenoreceptor a mechanism has been demonstrated by which β-arrestins, which are also involved in 5-HT4 receptor desensitization (Ponimaskin et al., 2005), contribute to the degradation of cAMP by recruiting cAMP-specific PDEs to ligand-activated receptors (Perry et al., 2002). Both β2-adrenergic and 5-HT4 receptors are expressed on the same cardiac cells and can even form heterodimers (Ouadid et al., 1992; Berthouze et al., 2005). Therefore, in analogy with β-adrenoreceptors, β-arrestins could also be involved in the recruitment of PDEs upon 5-HT4 receptor activation, and the observed fading in our experiments could thus well be caused by an integrated cascade of events that involve the coordinated activation of receptor-desensitizing mechanisms and the quenching of the receptor-induced activity.
Furthermore, PKA anchor proteins (AKAPs) serve to sequester PKA to distinct subcellular compartments for local activation, and the AKAP–PKA complex scaffolds and activates PDEs, which maintain compartmentation by limiting the diffusion of the second messenger (Dodge et al., 2001; Baillie & Houslay, 2005). This mechanism generates local PDE activity and therefore controls the spatial gradient of cyclic nucleotides (Zaccolo & Pozzan, 2002; Carlisle Michel et al., 2004; Rochais et al., 2004) and provides a molecular framework for a negative feedback mechanism by which cAMP may regulate its own levels (Rochais et al., 2004). It is possible that, also for the 5-HT4 receptor, PDEs play a role in the targeting of the response, or, as Brattelid et al. (2004b) suggested, that they have a protective role against 5-HT-induced cardiostimulation. The cardiac compartmentation has been shown to be greatly reduced by IBMX (Jurevicius & Fischmeister, 1996). The increased inotropic effect of the 5-HT4 receptor agonists in the presence of IBMX could therefore be a direct consequence of the inhibition of PDE action and/or, since compartmentation is lost, result from the more diffuse activation of cAMP substrates.
The reduction of the contraction force below basal after stimulation with an agonist is in line with the loss of the positive inotropic response by PDE action. The observation that the contraction force stabilized below basal, while the +dF/dt stabilized at the basal level, can be explained by the decrease of R2 at this moment. This indicates that there is still a lusitropic effect present, which is thus less sensitive to the negative influence of the PDEs than the positive inotropic response. This prolonged relaxant effect might be related to the lusitropic role of phospholamban and troponin I (see below), as they have slow dephosphorylation kinetics (Garvey et al., 1988).
The same argumentation could explain the observations with GR113808. Just as PDEs, GR113808 preferentially blocks the positive inotropic response over the lusitropic one, since R2 remains decreased. This is in line with the study of Brattelid et al. (2004b), who found that the positive inotropic effects of 5-HT in human ventricle were completely reversed by GR113808, whereas the lusitropic effects were only partially reversed. However, in the presence of IBMX, R2 was unaffected by the stimulation with prucalopride, yet the maximal contraction force and velocity (independent of lusitropic responses) was also reduced below its basal level by GR113808 (which cannot be accounted for by PDEs in this condition). Therefore, some negative inotropic mechanism other than PDE activation seems to be involved here.
Comparison of 5-HT4 receptor agonists: In the presence of IBMX, prucalopride, R149402 and R199715 acted as partial agonists in the paced left atrium, while no significant difference in efficacy was found between tegaserod and 5-HT. These results confirm previous findings with piglet sinoatrial and human right atrial tissue, in which substituted benzamides behaved as partial agonists (Kaumann et al., 1991; Villalon et al., 1991; Medhurst & Kaumann, 1993). Langlois & Fischmeister (2003) already stated that the stimulation of 5-HT4 receptors in piglet isolated right atrium is a useful method for evaluating the pharmacological profile of 5-HT4 receptor ligands. Prucalopride also acted as a partial agonist on the L-type Ca2+ current through 5-HT4 receptors in human atrial cells (Pau et al., 2005). This study together with our current findings show the partial agonistic character of prucalopride in a cardiac assay. It is interesting that R149402 and R199715, which are both structurally related to prucalopride, are more potent but less efficacious than their parent molecule. Tegaserod results in slow inotropic responses, and, for the higher concentrations, displays an additional inotropic response following a lag phase. In the literature, tegaserod is described as a high-affinity, potent 5-HT4 receptor agonist. Compared to these reports, the potency by which tegaserod increased left atrial contraction force in the present study was considerably lower (Beattie et al., 2004). But, since in our experiments, the curve to tegaserod has a low Hill slope, these findings might be related to a very difficult penetration of the chemical into the tissue. Although tegaserod has been shown to interact with 5-HT1 and 5-HT2 receptors (Briejer et al., 2001; Beattie et al., 2004), we consider it doubtful that these interactions occur in porcine atrium as 5-HT4 receptors are the only ones described in pig atria. However, the possibility that tegaserod interacts with non-5-HT4 receptors cannot be excluded. Interestingly, a low Hill slope is also observed for the noncumulative concentration–response curve to 5-HT, which is structurally related to tegaserod as they both are indolic compounds. Another alternative explanation could be that these indoles stabilize a specific receptor state that activates multiple G proteins, a mechanism that is called agonist-directed trafficking (Kenakin & Morgan, 1989; Kenakin, 1995).
Influence of development: In newborn piglets, the 5-HT4 receptor agonists were less potent and/or less efficacious in inducing left atrial inotropic responses than in young pigs; results with R149402 and R199715 could even not be adequately fitted. A similar age dependency was recently shown for 5-HT4 receptor-associated inotropic responses induced by 5-HT in porcine ventricular trabeculae carnae. 5-HT was 15-fold more potent and twice more efficacious in the trabeculae of 3 months old pigs than of newborn piglets. Furthermore, the increase of PKA activity caused by 10 μM 5-HT was twice as big in young pigs than in newborns (Brattelid et al., 2004b). In our left atrial experiments, a similar potency shift, but a smaller efficacy difference for 5-HT, is found. The developmental changes that cause the development-related increase in response to 5-HT4 receptor activation are not yet understood. One possibility is that the 5-HT4 receptor expression increases during development. The expression level of 5-HT4 receptors in the atria of piglets is indeed very low, being less than 1% of that of β1-adrenoreceptors (Kaumann et al., 1995). In comparison, in human (adult) atria the expression of 5-HT4 receptors equals 10 and 20% of that of β1- and β2-adrenoreceptors, respectively (Kaumann et al., 1996). In accordance with this higher receptor density, the 5-HT-induced inotropic responses on human atria are bigger than on piglet atria (Kaumann & Sanders, 1998).
Another possibility is that the 5-HT4 receptors become more efficiently coupled to their effectors during development. It has indeed been shown that chronic treatment with β-blockers causes an increased response to 5-HT at 5-HT4 receptors (Sanders et al., 1995; Pau et al., 2003), while it is documented that treatment with β-blockers does not change 5-HT4 receptor mRNA (Grammer et al., 2001). The increased sensitivity could be induced by an increased expression of G-protein. An age dependency of the expression, as well as the receptor-mediated activation of G-proteins, has been shown in human atrium (Kilts et al., 2003).
Lusitropic effects in the porcine left atrium
It has previously been shown that 5-HT changes the time to reach peak force in human left and right atria and ventricles (Kaumann et al., 1991; Sanders & Kaumann, 1992; Brattelid et al., 2004b). This correlates with the following sequence of events, proposed by Kaumann et al. (1990): Activated PKA catalyses the phosphorylation of the inhibitory regulatory protein phospholamban. This results in liberation of Ca2+ ATPase activity, resulting in an accelerated Ca2+ re-uptake in the sarcoplasmatic reticulum. PKA also phosphorylates troponin I, which causes a reduction in the affinity of Ca2+ for troponin C. This mechanism has been observed with catecholamines through β1- and β2-adrenoreceptors (Kaumann et al., 1999). We observed a hastening of the onset of relaxation (tpf) as well as a shortening of the relaxation time (t50) for 5-HT and all 5-HT4 receptor agonists. Interestingly, prucalopride, tegaserod, R149402 and R199715 induced lusitropic effects when administered cumulatively, while they had no clear positive inotropic effect. This can be explained by the preferential inhibition of the inotropic response by PDEs as discussed above. The potencies observed for both lusitropic effects of all agonists are comparable with each other. As observed for the inotropic response in the presence of IBMX, R149402 and R199715 were more potent than the other agonists.
An interesting observation is that prucalopride, R149402 and R199715, which are benzofuran compounds, are half as efficacious in reducing the tpf when administered noncumulatively than when administered cumulatively, while this is not the case for 5-HT and tegaserod, which possess an indolic structure. This phenomenon is not consistently observed for the reduction in t50. As a consequence, these benzamide-derived compounds induce a smaller reduction in the tpf than 5-HT in a noncumulative administration regime. A structural differentiation between 5-HT4 receptor agonists has been made previously by Pindon et al. (2002). They showed a cAMP-independent Ca2+ influx through 5-HT4(a), but not 5-HT4(b), receptor activation that was 2–3 times higher for benzamide-like structures than for indolamines in HEK 293 cells. Supposed this mechanism also occurs in the porcine atrial system, the additional inward Ca2+ transient would promote systolic contraction but hamper diastolic relaxation. Indeed, during diastole, the activation of 5-HT4 receptors results in a decreased Ca2+ concentration at the contractile proteins through a PKA-dependent phosphorylation of phospholamban and the linked increase in Ca2+ ATPase activity. The activation of the receptor with benzofurans, however, would then at the same time induce an inward Ca2+ flux and thus counteracting relaxation. The more efficacious relaxation with the benzofurans in the cumulative protocol could then be explained by the time-related inactivation of this additional Ca2+ effect or by 5-HT4 receptor desensitization. The latter should then reduce the Ca2+ influx, while phospholamban and/or troponin I remain phosphorylated for a longer time. The slow dephosphorylation kinetics for these proteins have indeed been shown, in agreement with our hypothesis (Garvey et al., 1988). The lower lusitropic efficacy of 5-HT in the cumulative protocol, compared to the noncumulative protocol, supports the idea of 5-HT4 receptor desensitization. The different coupling of benzamide-derived compounds fits into the concept of agonist-directed trafficking mentioned before (Kenakin & Morgan, 1989).
Chronotropic effects in the porcine right atrium
Porcine sinoatrial 5-HT4 receptors gained a lot of attention because of the lack of a human model to study 5-HT4-receptor-mediated tachycardia (Kaumann, 1990; Medhurst & Kaumann, 1993; Kaumann, 1994). 5-HT and all 5-HT4 receptor agonists tested caused positive chronotropic effects in the isolated right atrium of young pigs. An interesting difference with the left atrial inotropic response is that all agonists induced their effect when added cumulatively in the absence of IBMX. It is possible that the receptor is more efficiently coupled to the transducing mechanism in the sinus node than in the myocardium, and/or that PDEs are less involved in the 5-HT4-mediated response in the sinus node. Receptor-induced cAMP binds indeed directly to HCN channels to provoke the chronotropic effect, thus without the involvement of PKA, and steps further downstream in the cascade (Ulens & Tytgat, 2001), which might contribute to more efficient coupling. Furthermore, preliminary data also showed no fading of the response upon the administration of a single concentration of 5-HT, corresponding to a less pronounced influence of PDEs on the sinus rhythm. This may be explained by a different transduction mechanism in the compartments responsible for the frequency coupling, in the sinus node. It is possible that the influence of PDE in these compartments is limited. An interesting thought would be that the 5-HT4 receptor splice variants responsible for frequency coupling are different from those responsible for contraction coupling. If these splice variants show a different affinity for arrestin, AKAP or other members of the cascade complex, this could result in a lack of PDE recruiting. For example, it has been shown that the Na+/H+ exchanger regulatory factor (NHERF) interacts with AKAP. For β2-adrenoreceptors, this interaction has been shown to be functionally involved in the tight regulation of the signalling molecules into microdomains (Xiang & Kobilka, 2003). Furthermore, NHERF interacts with PDZ motifs on the 5-HT4(a) receptor, but not on the 5-HT4(e) receptor or with the 5-HT4(b) receptor (Joubert et al., 2004). Thus, there may exist different compartments for the different 5-HT4 receptor splice variants.
The benzamide-derived compounds prucalopride, R149402 and R199715 behaved as partial agonists, while tegaserod reached a maximal effect that was not different from 5-HT. The occurrence of arrhythmia was also most prominent in experiments with tegaserod. As for the inotropic response, the very low Hill slope associated with tegaserod suggests a very difficult penetration of the chemical into the tissue or an additional transducing mechanism associated with a specific receptor state.
5-HT and the 5-HT4 receptor agonists also increased the right atrial frequency in newborn piglets; the potency was similar as in young pigs. Results for 5-HT were in good agreement with the literature on sinoatrial 5-HT4 receptors in newborn piglets (Kaumann, 1990; Medhurst & Kaumann, 1993). Prucalopride, tegaserod, R149402 and R199715, but not 5-HT, are less efficacious in newborns. The decrease in efficacy was also observed for the inotropic responses in left atrium. It is likely that a general developmental change in receptor density and/or coupling efficiency is responsible for a bigger 5-HT4 receptor-mediated left atrial inotropic and right atrial chronotropic response. It is thus surprising that 5-HT, as well as isoprenaline, induced the same absolute increase in heart rate in newborns as in young pigs, irrespective of the higher basal beating rate in the former.
General conclusion
In conclusion, we demonstrate the inotropic, lusitropic and chronotropic effects of 5-HT and the 5-HT4 receptor agonists prucalopride, tegaserod, R149402 and R199715 in young pigs. PDEs are involved in the regulation of the left atrial inotropic responses, contributing to the fading of the response. If PDEs are also involved in the regulation of the chronotropic response, this influence is certainly less pronounced as all agonists produced nonfading chronotropic responses in the absence of PDE inhibition. In accordance with what was found on porcine ventricles (Brattelid et al., 2004b), experiments with left and right atria of newborn pigs clearly demonstrate an increase in 5-HT4 receptor-mediated effects with age.
Acknowledgments
We thank Luc Hoskens for his excellent technical assistance. The study was financially supported by Interuniversity Attraction Poles Programme P5/20, Belgian Science Policy.
Abbreviations
- AKAP
A kinase anchor protein
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- IBMX
3-isobutyl-1-methylxanthine
- LR
likelihood ratio
- NHERF
Na+/H+ exchanger regulatory factor
- PDE
phosphodiesterase
- t50
time to reach 50% relaxation
- tpf
time to peak force
References
- ANSANAY H., SEBBEN M., BOCKAERT J., DUMUIS A. Characterization of homologous 5-hydroxytryptamine4 receptor desensitization in colliculi neurons. Mol. Pharmacol. 1992;42:808–816. [PubMed] [Google Scholar]
- BACH T., SYVERSVEEN T., KVINGEDAL A.M., KROBERT K.A., BRATTELID T., KAUMANN A.J., LEVY F.O. 5HT4(a) and 5-HT4(b) receptors have nearly identical pharmacology and are both expressed in human atrium and ventricle. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:146–160. doi: 10.1007/s002100000299. [DOI] [PubMed] [Google Scholar]
- BAILLIE G.S., HOUSLAY M.D. Arrestin times for compartmentalised cAMP signalling and phosphodiesterase-4 enzymes. Curr. Opin. Cell. Biol. 2005;17:129–134. doi: 10.1016/j.ceb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- BEATTIE D.T., SMITH J.A., MARQUESS D., VICKERY R.G., ARMSTRONG S.R., PULIDO-RIOS T., MCCULLOUGH J.L., SANDLUND C., RICHARDSON C., MAI N., HUMPHREY P.P. The 5-HT4 receptor agonist, tegaserod, is a potent 5-HT2B receptor antagonist in vitro and in vivo. Br. J. Pharmacol. 2004;143:549–560. doi: 10.1038/sj.bjp.0705929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTHOUZE M., AYOUB M., RUSSO O., RIVAIL L., SICSIC S., FISCHMEISTER R., BERQUE-BESTEL I., JOCKERS R., LEZOUALC'H F. Constitutive dimerization of human serotonin 5-HT4 receptors in living cells. FEBS Lett. 2005;579:2973–2980. doi: 10.1016/j.febslet.2005.04.040. [DOI] [PubMed] [Google Scholar]
- BLONDEL O., GASTINEAU M., DAHMOUNE Y., LANGLOIS M., FISCHMEISTER R. Cloning, expression, and pharmacology of four human 5-hydroxytryptamine4 receptor isoforms produced by alternative splicing in the carboxyl terminus. J. Neurochem. 1998;70:2252–2261. doi: 10.1046/j.1471-4159.1998.70062252.x. [DOI] [PubMed] [Google Scholar]
- BLONDEL O., VANDECASTEELE G., GASTINEAU M., LECLERC S., DAHMOUNE Y., LANGLOIS M., FISCHMEISTER R. Molecular and functional characterization of a 5-HT4 receptor cloned from human atrium. FEBS Lett. 1997;412:465–474. doi: 10.1016/s0014-5793(97)00820-x. [DOI] [PubMed] [Google Scholar]
- BOCKAERT J., CLAEYSEN S., COMPAN V., DUMUIS A. 5-HT4 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004;3:39–51. doi: 10.2174/1568007043482615. [DOI] [PubMed] [Google Scholar]
- BRATTELID T., KVINGEDAL A.M., KROBERT K.A., ANDRESSEN K.W., BACH T., HYSTAD M.E., KAUMANN A.J., LEVY F.O. Cloning, pharmacological characterisation and tissue distribution of a novel 5-HT4 receptor splice variant, 5-HT4(i) Naunyn-Schmiedeberg's Arch. Pharmacol. 2004a;369:616–628. doi: 10.1007/s00210-004-0919-4. [DOI] [PubMed] [Google Scholar]
- BRATTELID T., QVIGSTAD E., LYNHAM J.A., MOLENAAR P., AASS H., GEIRAN O., SKOMEDAL T., OSNES J.B., LEVY F.O., KAUMANN A.J. Functional serotonin 5-HT4 receptors in porcine and human ventricular myocardium with increased 5-HT4 mRNA in heart failure. Naunyn-Schmiedeberg's Arch. Pharmacol. 2004b;370:157–166. doi: 10.1007/s00210-004-0963-0. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., BOSMANS J.P., VAN DAELE P., JURZAK M., HEYLEN L., LEYSEN J.E., PRINS N.H., SCHUURKES J.A. The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound. Eur. J. Pharmacol. 2001;423:71–83. doi: 10.1016/s0014-2999(01)01087-1. [DOI] [PubMed] [Google Scholar]
- CARLISLE MICHEL J.J., DODGE K.L., WONG W., MAYER N.C., LANGEBERG L.K., SCOTT J.D. PKA-phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signalling complex. Biochem J. 2004;381:587–592. doi: 10.1042/BJ20040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTRO L., MIALET-PEREZ J., GUILLEMEAU A., STILLITANO F., ZOLK O., ESCHENHAGEN T., LEZOUALCh F., BOCHET P., FISCHMEISTER R. Differential functional effects of two 5-HT4 receptor isoforms in adult cardiomyocytes. J. Mol. Cell. Cardiol. 2005;39:335–344. doi: 10.1016/j.yjmcc.2005.04.009. [DOI] [PubMed] [Google Scholar]
- CLAEYSEN S., SEBBEN M., BECAMEL C., BOCKAERT J., DUMUIS A. Novel brain-specific 5-HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol. Pharmacol. 1999;55:910–920. [PubMed] [Google Scholar]
- DODGE K.L., KHOUANGSATHIENE S., KAPILOFF M.S., MOUTON R., HILL E.V., HOUSLAY M.D., LANGEBERG L.K., SCOTT J.D. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., WONG E.H., DUMUIS A., BOCKAERT J. Central 5-HT4 receptors. Trends Pharmacol. Sci. 1995;16:391–398. doi: 10.1016/s0165-6147(00)89081-1. [DOI] [PubMed] [Google Scholar]
- FERGUSON S.S. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W., OXFORD A.W., BUNCE K.T., HUMPHREY P.P. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARVEY J.L., KRANIAS E.G., SOLARO R.J. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochem. J. 1988;249:709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERALD C., ADHAM N., KAO H.T., OLSEN M.A., LAZ T.M., SCHECHTER L.E., BARD J.A., VAYSSE P.J., HARTIG P.R., BRANCHEK T.A. The 5-HT4 receptor: molecular cloning and pharmacological characterization of two splice variants. EMBO J. 1995;14:2806–2815. doi: 10.1002/j.1460-2075.1995.tb07280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAMMER J.B., ZENG X., BOSCH R.F., KUHLKAMP V. Atrial L-type Ca2+-channel, β-adrenorecptor, and 5-hydroxytryptamine type 4 receptor mRNAs in human atrial fibrillation. Basic Res. Cardiol. 2001;96:82–90. doi: 10.1007/s003950170081. [DOI] [PubMed] [Google Scholar]
- GUO Z.G., LEVI R., AARONSON L.M., GAY W.A. The isolated human pectinate muscle: a reliable preparation of human cardiac tissue. J. Pharmacol Methods. 1983;9:127–135. doi: 10.1016/0160-5402(83)90004-9. [DOI] [PubMed] [Google Scholar]
- HANOUZ J.L., PERSEHAYE E., ZHU L., LAMMENS S., LEPAGE O., MASSETTI M., BABATASI G., KHAYAT A., BRICARD H., GERARD J.L. The inotropic and lusitropic effects of ketamine in isolated human atrial myocardium: the effect of adrenoceptor blockade. Anesth. Analg. 2004;99:1689–1695. doi: 10.1213/01.ANE.0000136466.85913.3C. [DOI] [PubMed] [Google Scholar]
- HEUBACH J.F., RAVENS U., KAUMANN A.J. Epinephrine activates both Gs and Gi pathways, but norepinephrine activates only the Gs pathway through human β2-adrenoceptors overexpressed in mouse heart. Mol. Pharmacol. 2004;65:1313–1322. doi: 10.1124/mol.65.5.1313. [DOI] [PubMed] [Google Scholar]
- JAHNEL U., RUPP J., ERTL R., NAWRATH H. Positive inotropic response to 5-HT in human atrial but not in ventricular heart muscle. Naunyn-Schmiedebergs Arch. Pharmacol. 1992;346:482–485. doi: 10.1007/BF00169000. [DOI] [PubMed] [Google Scholar]
- JOUBERT L., HANSON B., BARTHET G., SEBBEN M., CLAEYSEN S., HONG W., MARIN P., DUMUIS A., BOCKAERT J. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4(a) receptor splice variant: roles in receptor targeting. J. Cell Sci. 2004;117:5367–5379. doi: 10.1242/jcs.01379. [DOI] [PubMed] [Google Scholar]
- JUREVICIUS J., FISCHMEISTER R. cAMP compartmentation is responsible for a local activation of cardiac Ca2+ channels by β-adrenergic agonists. Proc. Natl. Acad. Sci. U.S.A. 1996;93:295–299. doi: 10.1073/pnas.93.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A., BARTEL S., MOLENAAR P., SANDERS L., BURRELL K., VETTER D., HEMPEL P., KARCZEWSKI P., KRAUSE E.G. Activation of β2-adrenergic receptors hastens relaxation and mediates phosphorylation of phospholamban, troponin I, and C-protein in ventricular myocardium from patients with terminal heart failure. Circulation. 1999;99:65–72. doi: 10.1161/01.cir.99.1.65. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Piglet sinoatrial 5-HT receptors resemble human atrial 5-HT4-like receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:619–622. doi: 10.1007/BF00169055. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Do human atrial 5-HT4 receptors mediate arrhythmias. Trends Pharmacol. Sci. 1994;15:451–455. doi: 10.1016/0165-6147(94)90058-2. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., LYNHAM J.A., BROWN A.M. Labelling with [125I]-SB 207710 of a small 5-HT4 receptor population in piglet right atrium: functional relevance. Br. J. Pharmacol. 1995;115:933–936. doi: 10.1111/j.1476-5381.1995.tb15900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., LYNHAM J.A., BROWN A.M. Comparison of the densities of 5-HT4 receptors, β1- and β2-adrenoceptors in human atrium: functional implications. Naunyn-Schmiedebergs Arch. Pharmacol. 1996;353:592–595. doi: 10.1007/BF00169181. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., MURRAY K.J., BROWN A.M., SANDERS L., BROWN M.J.A receptor for 5-HT in human atrium Br. J. Pharmacol. 198998664P [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., SANDERS L. 5-Hydroxytryptamine causes rate-dependent arrhythmias through 5-HT4 receptors in human atrium: facilitation by chronic β-adrenoceptor blockade. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;349:331–337. doi: 10.1007/BF00170877. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., SANDERS L.5-Hydroxytryptamine and human heart function: the role of 5-HT4 receptors 5-HT4 Receptors in the Brain and Periphery 1998Georgetown, Tex: RG Landes; 127–148.ed. Eglen, R.M. pp [Google Scholar]
- KAUMANN A.J., SANDERS L., BROWN A.M., MURRAY K.J., BROWN M.J. A 5-hydroxytryptamine receptor in human atrium. Br. J. Pharmacol. 1990;100:879–885. doi: 10.1111/j.1476-5381.1990.tb14108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., SANDERS L., BROWN A.M., MURRAY K.J., BROWN M.J. A 5-HT4-like receptor in human right atrium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:150–159. doi: 10.1007/BF00167212. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Agonist-receptor efficacy. I: Mechanisms of efficacy and receptor promiscuity. Trends Pharmacol. Sci. 1995;16:188–192. doi: 10.1016/s0165-6147(00)89020-3. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P., MORGAN P.H. Theoretical effects of single and multiple transducer receptor coupling proteins on estimates of the relative potency of agonists. Mol. Pharmacol. 1989;35:214–222. [PubMed] [Google Scholar]
- KILTS J.D., AKAZAWA T., EL MOALEM H.E., MATHEW J.P., NEWMAN M.F., KWATRA M.M. Age increases expression and receptor-mediated activation of Gαi in human atria. J. Cardiovasc. Pharmacol. 2003;42:662–670. doi: 10.1097/00005344-200311000-00013. [DOI] [PubMed] [Google Scholar]
- LANGLOIS M., FISCHMEISTER R. 5-HT4 receptor ligands: applications and new prospects. J. Med. Chem. 2003;46:319–344. doi: 10.1021/jm020099f. [DOI] [PubMed] [Google Scholar]
- LORRAIN J., GROSSET A., O'CONNOR S.E. 5-HT4 receptors, present in piglet atria and sensitive to SDZ 205-557, are absent in papillary muscle. Eur. J. Pharmacol. 1992;229:105–108. doi: 10.1016/0014-2999(92)90293-d. [DOI] [PubMed] [Google Scholar]
- MARTIN N.P., WHALEN E.J., ZAMAH M.A., PIERCE K.L., LEFKOWITZ R.J. PKA-mediated phosphorylation of the β1-adrenergic receptor promotes Gs/Gi switching. Cell. Signal. 2004;16:1397–1403. doi: 10.1016/j.cellsig.2004.05.002. [DOI] [PubMed] [Google Scholar]
- MATTIAZZI A., GARAY A., CINGOLANI H.E. Critical evaluation of isometric indexes of relaxation in rat and cat papillary muscles and toad ventricular strips. J. Mol. Cell. Cardiol. 1986;18:749–758. doi: 10.1016/s0022-2828(86)80946-4. [DOI] [PubMed] [Google Scholar]
- MEDHURST A.D., KAUMANN A.J. Characterization of the 5-HT4 receptor mediating tachycardia in piglet isolated right atrium. Br. J. Pharmacol. 1993;110:1023–1030. doi: 10.1111/j.1476-5381.1993.tb13916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEDHURST A.D., LEZOUALC'H F., FISCHMEISTER R., MIDDLEMISS D.N., SANGER G.J. Quantitative mRNA analysis of five C-terminal splice variants of the human 5-HT4 receptor in the central nervous system by TaqMan real time RT–PCR. Brain Res. Mol. Brain Res. 2001;90:125–134. doi: 10.1016/s0169-328x(01)00095-x. [DOI] [PubMed] [Google Scholar]
- MIALET J., BERQUE-BESTEL I., EFTEKHARI P., GASTINEAU M., GINER M., DAHMOUNE Y., DONZEAU-GOUGE P., HOEBEKE J., LANGLOIS M., SICSIC S., FISCHMEISTER R., LEZOUALC'H F. Isolation of the serotoninergic 5-HT4(e) receptor from human heart and comparative analysis of its pharmacological profile in C6-glial and CHO cell lines. Br. J. Pharmacol. 2000;129:771–781. doi: 10.1038/sj.bjp.0703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIALET J., FISCHMEISTER R., LEZOUALC'H F. Characterization of human 5-HT4(d) receptor desensitization in CHO cells. Br. J Pharmacol. 2003;138:445–452. doi: 10.1038/sj.bjp.0705061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUADID H., SEGUIN J., DUMUIS A., BOCKAERT J., NARGEOT J. Serotonin increases calcium current in human atrial myocytes via the newly described 5-hydroxytryptamine4 receptors. Mol. Pharmacol. 1992;41:346–351. [PubMed] [Google Scholar]
- PARKER S.G., TAYLOR E.M., HAMBURGER S.A., VIMAL M., KAUMANN A.J. Blockade of human and porcine myocardial 5-HT4 receptors by SB 203186. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;353:28–35. doi: 10.1007/BF00168912. [DOI] [PubMed] [Google Scholar]
- PAU D., WORKMAN A.J., KANE K.A., RANKIN A.C. Electrophysiological effects of 5-hydroxytryptamine on isolated human atrial myocytes, and the influence of chronic β-adrenoceptor blockade. Br. J. Pharmacol. 2003;140:1434–1441. doi: 10.1038/sj.bjp.0705553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAU D., WORKMAN A., KANE K., RANKIN A. Electrophysiological effects of prucalopride, a novel enterokinetic agent, on isolated atrial myocytes from patients treated with β-adrenoceptor antagonists. J. Pharmacol. Exp. Ther. 2005;313:146–153. doi: 10.1124/jpet.104.076869. [DOI] [PubMed] [Google Scholar]
- PERRY S.J., BAILLIE G.S., KOHOUT T.A., MCPHEE I., MAGIERA M.M., ANG K.L., MILLER W.E., MCLEAN A.J., CONTI M., HOUSLAY M.D., LEFKOWITZ R.J. Targeting of cyclic AMP degradation to β2-adrenergic receptors by β-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- PINDON A., VAN HECKE G., VAN GOMPEL P., LESAGE A.S., LEYSEN J.E., JURZAK M. Differences in signal transduction of two 5-HT4 receptor splice variants: compound specificity and dual coupling with Gαs- and Gαi/o-proteins. Mol. Pharmacol. 2002;61:85–96. doi: 10.1124/mol.61.1.85. [DOI] [PubMed] [Google Scholar]
- PINHEIRO J.C., BATES D.M. Mixed Effects Models in S and S-plus 2000New York: Springer-Verlag; ed. Chambers, J., Eddy, W., Hardle, W., Sheather, S. & Tierney, L [Google Scholar]
- PINO R., CERBAI E., CALAMAI G., ALAJMO F., BORGIOLI A., BRACONI L., CASSAI M., MONTESI G.F., MUGELLI A. Effect of 5-HT4 receptor stimulation on the pacemaker currentIf in human isolated atrial myocytes. Cardiovasc. Res. 1998;40:516–522. doi: 10.1016/s0008-6363(98)00198-9. [DOI] [PubMed] [Google Scholar]
- PONIMASKIN E., DUMUIS A., GAVEN F., BARTHET G., HEINE M., GLEBOV K., RICHTER D.W., OPPERMANN M. Palmitoylation of the 5-hydroxytryptamine4(a) receptor regulates receptor phosphorylation, desensitization and β-Arrestin mediated endocytosis. Mol. Pharmacol. 2005;67:1434–1443. doi: 10.1124/mol.104.008748. [DOI] [PubMed] [Google Scholar]
- QVIGSTAD E., BRATTELID T., SJAASTAD I., ANDRESSEN K.W., KROBERT K.A., BIRKELAND J.A., SEJERSTED O.M., KAUMANN A.J., SKOMEDAL T., OSNES J.B., LEVY F.O. Appearance of a ventricular 5-HT4 receptor-mediated inotropic response to serotonin in heart failure. Cardiovasc. Res. 2005;65:869–878. doi: 10.1016/j.cardiores.2004.11.017. [DOI] [PubMed] [Google Scholar]
- RAHME M.M., COTTER B., LEISTAD E., WADHWA M.K., MOHABIR R., FORD A.P., EGLEN R.M., FELD G.K. Electrophysiological and antiarrhythmic effects of the atrial selective 5-HT4 receptor antagonist RS-100302 in experimental atrial flutter and fibrillation. Circulation. 1999;100:2010–2017. doi: 10.1161/01.cir.100.19.2010. [DOI] [PubMed] [Google Scholar]
- RAYMOND J.R., MUKHIN Y.V., GELASCO A., TURNER J., COLLINSWORTH G., GETTYS T.W., GREWAL J.S., GARNOVSKAYA M.N. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol. Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- ROCHAIS F., VANDECASTEELE G., LEFEBVRE F., LUGNIER C., LUM H., MAZET J.L., COOPER D.M., FISCHMEISTER R. Negative feedback exerted by cAMP-dependent protein kinase and cAMP phosphodiesterase on subsarcolemmal cAMP signals in intact cardiac myocytes: an in vivo study using adenovirus-mediated expression of CNG channels. J. Biol. Chem. 2004;279:52095–52105. doi: 10.1074/jbc.M405697200. [DOI] [PubMed] [Google Scholar]
- RONDE P., ANSANAY H., DUMUIS A., MILLER R., BOCKAERT J. Homologous desensitization of 5-hydroxytryptamine4 receptors in rat esophagus: functional and second messenger studies. J. Pharmacol. Exp. Ther. 1995;272:977–983. [PubMed] [Google Scholar]
- SANDERS L., KAUMANN A.J. A 5-HT4-like receptor in human left atrium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;345:382–386. doi: 10.1007/BF00176614. [DOI] [PubMed] [Google Scholar]
- SANDERS L., LYNHAM J.A., BOND B., DEL MONTE F., HARDING S.E., KAUMANN A.J. Sensitization of human atrial 5-HT4 receptors by chronic β-blocker treatment. Circulation. 1995;92:2526–2539. doi: 10.1161/01.cir.92.9.2526. [DOI] [PubMed] [Google Scholar]
- SAUDOU F., HEN R. 5-Hydroxytryptamine receptor subtypes: molecular and functional diversity. Adv. Pharmacol. 1994;30:327–380. doi: 10.1016/s1054-3589(08)60178-7. [DOI] [PubMed] [Google Scholar]
- SAXENA P.R. Serotonin receptors: subtypes, functional responses and therapeutic relevance. Pharmacol. Ther. 1995;66:339–368. doi: 10.1016/0163-7258(94)00005-n. [DOI] [PubMed] [Google Scholar]
- SCHOEMAKER R.G., DU X.Y., BAX W.A., BOS E., SAXENA P.R. 5-Hydroxytryptamine stimulates human isolated atrium but not ventricle. Eur. J. Pharmacol. 1993;230:103–105. doi: 10.1016/0014-2999(93)90417-g. [DOI] [PubMed] [Google Scholar]
- SCHOEMAKER R.G., DU X.Y., BAX W.A., SAXENA P.R. 5-Hydroxytryptamine increases contractile force in porcine right atrium but not in left ventricle. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;346:486–489. doi: 10.1007/BF00169001. [DOI] [PubMed] [Google Scholar]
- ULENS C., TYTGAT J. Gi- and Gs-coupled receptors up-regulate the cAMP cascade to modulate HCN2, but not HCN1 pacemaker channels. PflugersArch. 2001;442:928–942. doi: 10.1007/s004240100617. [DOI] [PubMed] [Google Scholar]
- VERBEKE G., MOLENBERGHS G. Linear Mixed Models in Practice. A SAS-Oriented Approach. Berlin: Springer-Verlag; 2005. [Google Scholar]
- VILARO M.T., DOMENECH T., PALACIOS J.M., MENGOD G. Cloning and characterization of a novel human 5-HT4 receptor variant that lacks the alternatively spliced carboxy terminal exon. RT–PCR distribution in human brain and periphery of multiple 5-HT4 receptor variants. Neuropharmacology. 2002;42:60–73. doi: 10.1016/s0028-3908(01)00154-x. [DOI] [PubMed] [Google Scholar]
- VILLALON C.M., DEN BOER M.O., HEILIGERS J.P., SAXENA P.R. Further characterization, by use of tryptamine and benzamide derivatives, of the putative 5-HT4 receptor mediating tachycardia in the pig. Br. J. Pharmacol. 1991;102:107–112. doi: 10.1111/j.1476-5381.1991.tb12140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VONESH E.F., CHINCHILLI V.M. Linear and Nonlinear Models for the Analysis of Repeated Measurements. New York: Marcel-Dekker; 1997. [Google Scholar]
- WORKMAN A.J., RANKIN A.C. Serotonin, If and human atrial arrhythmia. Cardiovasc. Res. 1998;40:436–437. doi: 10.1016/s0008-6363(98)00258-2. [DOI] [PubMed] [Google Scholar]
- XIANG Y., KOBILKA B. The PDZ-binding motif of the β2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10776–10781. doi: 10.1073/pnas.1831718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZACCOLO M., POZZAN T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]