Abstract
Acetylcholine is synthesized in the majority of non-neuronal cells, for example in human skin. In the present experiments, the in vivo release of acetylcholine was measured by dermal microdialysis.
Two microdialysis membranes were inserted intradermally at the medial shank of volunteers. Physiological saline containing 1 μM neostigmine was perfused at a constant rate of 4 μl min−1 and the effluent was collected in six subsequent 20 min periods. Acetylcholine was measured by high-pressure liquid chromatography (HPLC) combined with bioreactors and electrochemical detection.
Analysis of the effluent by HPLC showed an acetylcholine peak that disappeared, when the analytical column was packed with acetylcholine-specific esterase, confirming the presence of acetylcholine.
In the absence of neostigmine, 71±51 pmol acetylcholine (n=4) was found during a 120 min period. The amount increased to 183±43 pmol (n=34), when the perfusion medium contained 1 μM neostigmine.
Injection of 100 MU botulinum toxin subcutaneously blocked sweating completely, but the release of acetylcholine was not affected (botulinum toxin treated skin: 116±70 pmol acetylcholine/120 min; untreated skin: 50±20 pmol; n=4).
Quinine (1 mM), inhibitor of organic cation transporters, and carnitine (0.1 mM), substrate of the Na+-dependent carnitine transporter OCTN2, tended to reduce acetylcholine release (by 40%, not significant).
Our experiments demonstrate, for the first time, the in vivo release of non-neuronal acetylcholine in human skin. Organic cation transporters are not predominantly involved in the release of non-neuronal acetylcholine from the human skin.
Keywords: Non-neuronal acetylcholine, human skin, in vivo microdialysis, botulinum toxin, dermal microdialysis, organic cation transporter
Introduction
Acetycholine, regarded as an exemplary neurotransmitter, is also synthesized by the vast majority of human non-neuronal cells. For example, epithelial and endothelial cells contain acetylcholine, which acts as a local cell molecule to control basic cell functions such as proliferation, differentiation and cell–cell contact via paracrine and autocrine mechanisms (Grando, 1997; Wessler et al., 1998; 1999; Kawashima & Fujii, 2000; Grando et al., 2003). The release of non-neuronal acetylcholine has been shown in isolated tissue such as human placenta or in isolated tumor cells (Sastry & Sadavongvivad, 1978; Wessler et al., 2001; Song et al., 2003); however, the in vivo release of non-neuronal acetylcholine from human tissue has never been demonstrated.
It has been repeatedly shown that the human skin expresses considerable amounts of acetylcholine (Grando, 1997; Klapproth et al., 1997); about 200–500 pmol acetylcholine per gram wet weight have been found in 5 mm depth punch biopsy specimens (Wessler et al., 2003). The human skin is directly accessible to dermal microdialysis. Thus, the human skin may be a useful tissue to study the release of non-neuronal acetylcholine under in vivo conditions. The present experiments addressed two points: first, to test whether acetylcholine can be collected by dermal microdialysis from the human skin and second, to demonstrate the existence of non-neuronal acetylcholine, that is, to exclude a contribution of neuronal acetylcholine.
Methods
Experimental protocol
Study protocol and informed consent was approved by the concerned ethics committee. In total, 17 male and 17 female volunteers (mean age: 27±0.9 years) were informed verbally and by means of a study information letter. After signing the informed consent, the volunteers were placed horizontally in a resting position with one shank fixed comfortably in a vacuum cushion. After removing hairs and placing a cooling bag onto the skin for about 3 min, two microdialysis membranes were inserted intradermally at the left or right medial shank. Microdialysis membranes, 200 μm in width, had a cutoff of 3000 kDa. Both membranes were placed in parallel (10 mm apart) within the superficial 2 mm skin layer along a distance of 15 mm (Schmelz et al., 1997). Flow-constant perfusion (4 μl min−1) was started with physiological saline solution containing 1 μM neostigmine, unless otherwise stated. Additionally, two further microdialysis membranes could be placed at the same shank about 300 mm away. The eluate was collected at the end of the microdialysis membrane and pooled from both membranes. The eluate collection was performed in 20 min periods and six subsequent samples were assayed. The individual sample volume was determined and thereafter the samples were stored at −26°C until high-pressure liquid chromatography (HPLC) analysis.
Experiments with botulinum toxin and measurement of sweat gland activity
Botulinum toxin was injected subcutaneously into the lateral aspect of the shank using a 26 gauge needle (Braune et al., 2001; Birklein et al., 2003). A fixed square injection scheme with four injections (2 × 2 cm) was used. These injections were performed 2 weeks before acetylcholine measurements, since suppression of sweating is maximum after 2 weeks (Krämer et al., 2003; Schlereth et al., 2005). Directly before the experiment, sweating was visualized by iodine starch staining (Minor, 1927) to detect the anhidrotic skin area, in which the microdialysis membranes were placed. Sweating was elicited in a thermoregulatory manner by drinking about 1 l hot tea and placing the volunteers onto an electric blanket.
Measurement of acetylcholine
Acetylcholine was measured by cationic exchange HPLC combined with bioreactors and electrochemical detection as described in detail previously (Klapproth et al., 1997). A micropore HPLC system was used (BAS, Lafayette, U.S.A.). The specificity of the acetylcholine peak detected in the incubation medium was checked by using an analytical column packed with 40 U acetylcholinesterase. This application specifically eliminates the acetylcholine peak, but the choline peak remains (Klapproth et al., 1997). Detection limit was 10 fmol acetylcholine per injection (20 μl).
Calculations and statistics
Results were expressed as mean value±s.e.m. of n experiments. The volume of each sample could slightly vary, because there could be some leakage at the outlet end of the membranes, where the perfusate was collected with capillary tubes. Therefore, the acetylcholine content of each individual sample was normalized to a 200 μl volume. Statistical analysis of the results was performed by Student's paired t-test or ANOVA using Statistica 6.0.
Drugs and special chemicals
The following compounds were purchased from Sigma Chemie, Munich, Germany: acetylcholine chloride, choline chloride. Quinine corresponding to the European Pharmacopoeia was a generous gift from Merck (Darmstadt, Germany), neostigmine (human use) was taken from CuraMED Pharma (Karlsruhe, Germany) and Levocarnitine (human use) from Medice (Iserlohn, Germany). Botulinum toxin for human use (Dysport®) was purchased from Ipsen Pharma (Ettlingen, Germany).
Results
In vivo release of acetylcholine by dermal microdialysis
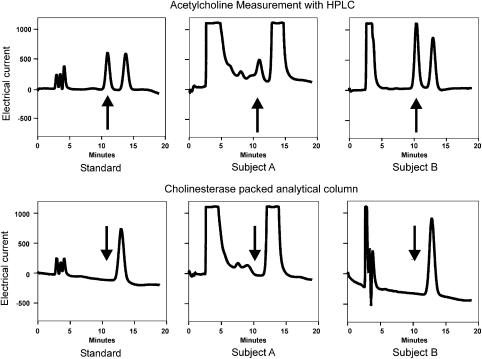
Figure 1 shows exemplary chromatograms of samples obtained from two different volunteers. In both samples, a peak was detected with a retention time corresponding to the respective acetylcholine peak of the standard. The retention time of the standard acetylcholine peak was 11.0 min and in a comparable experiment carried out 4 months later, 10.5 min. Similar peaks were obtained with the microdialysis samples, which gives first evidence that acetylcholine is released into the dermal dialysate. To prove the identity of the respective peak, samples were checked by an acetylcholinesterase packed analytical column. In this case, acetylcholine is already hydrolyzed when passing the analytical column resulting in the elimination of the acetylcholine peak and a corresponding increase of the choline peak (see standard in Figure 1). Likewise, the respective peak disappeared in the microdialysis samples obtained from two volunteers confirming the presence of acetylcholine. It should be noted that both volunteers released quite different amounts of acetylcholine, volunteer A about 14 pmol in 20 min and volunteer B about 340 pmol, although the experimental protocol and daytime period were identical.
Figure 1.
HPLC analysis of samples obtained by human dermal microdialysis. HPLC samples of two different subjects. Left column: standard, Middle column: subject A, right column: subject B. In the upper row standard HPLC: two peaks are detectable, the first one (arrow) represents acetylcholine, the second choline. Samples collected from subjects contained acetylcholine, but in a quite different amount: sample B contained so much acetylcholine that it had to be diluted by a factor of 10. To prove the authenticity of the ACh peak, the samples were tested with a acetylcholine-specific cholinesterase-packed analytical column (lower row). Under this condition the ACh peak was eliminated indicating that the samples really contained acetylcholine.
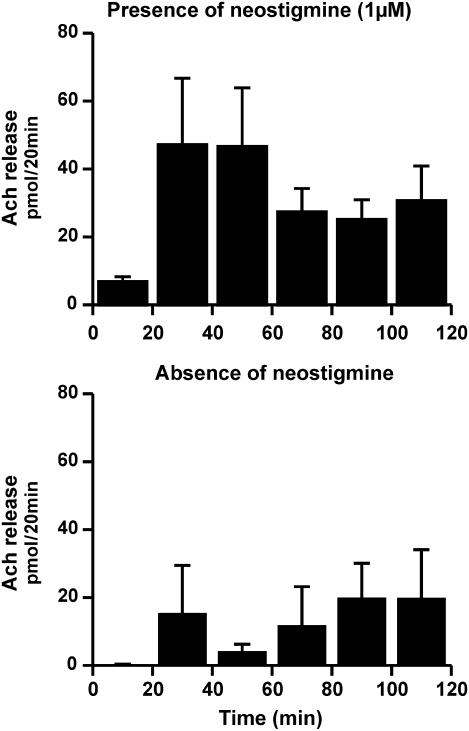
Figure 2 shows the spontaneous release of acetylcholine within the collecting period of 120 min in the presence and absence of neostigmine. Obviously, the amount of collected acetylcholine increased by a factor of 4 when cholinesterase was inhibited. During the 120 min collecting period, 71±51 pmol acetylcholine were found in the absence (n=4) and 183±43 pmol in the presence of 1 μM neostigmine (n=34). The neostigmine-induced increase of the acetylcholine peak can be regarded as additional evidence for the presence of acetylcholine.
Figure 2.
In vivo acetylcholine release from human skin assayed by dermal microdialysis in the presence and absence of neostigmine. Dermal microdialysis from 34 volunteers in the presence of 1 μM neostigmine (top row) and four volunteers in the absence of neostimgine (bottom row). Given are the means±s.e.m. In the presence of neostigmine, much more acetylcholine could be found in the samples.
The release pattern was comparable in the absence and presence of neostigmine, that is acetylcholine release increased from the first to the second sample and thereafter showed some maintenance. In the presence of neostigmine, the increase from the first to the second sample can be explained by the time required to block cholinesterases. In addition, an equilibrium process within the diffusion radius of the dialysis membrane may occur, which additionally explains the increase between the first and second sample observed in the experiments with or without neostigmine. It should be noted that the absolute release of acetylcholine varied considerably within the 120 min period and also between the individual volunteers (see Figures 1 and 2, which shows the means±s.e.m. of 34 individuals). So far, the reason for this variation remains unclear. The experimental procedure as well as the daytime, when the experiments were carried out, were more or less identical. Despite this variation, the release of acetylcholine appear to differ between male and female volunteers, 253±34 pmol (120 min; n=17) acetylcholine were found in female volunteers and only 114±34 in male volunteers (P<0.05).
Effect of botulinum toxin on the activity of sweat glands and the release of acetylcholine
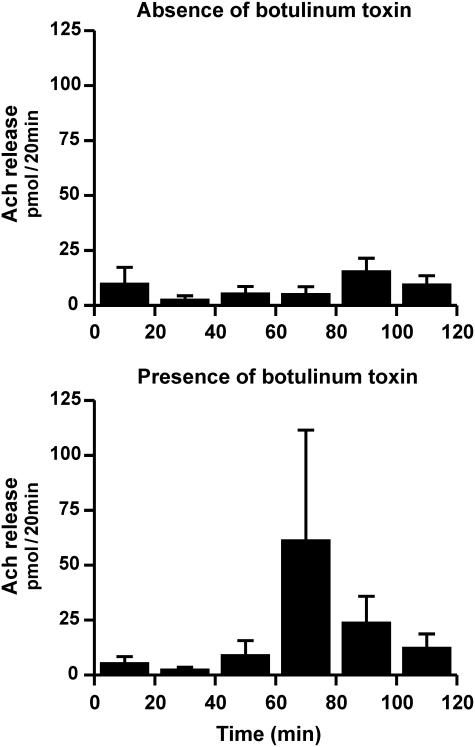
100 MU botulinum toxin were applied subcutaneously within a 2 cm2 area localized at the upper (two volunteers) or lower (two volunteers) part of the medial shank. The respective untreated area was used as control. The treated area was labeled, and sweating activity and the release of acetylcholine were measured 2 weeks later. The microdialysis membranes were placed within the treated and an untreated reference area, in intimate contact to the area where sweating was measured. In the untreated area sweating could be easly demonstrated by thermoregulatory stimulation (not shown), but in the botulinum toxin treated area, the activity of the sweat glands was completey blocked (not shown). However, in contrast to the effect of botulinum toxin on sweating, the release of acetylcholine was not affected, particularly not reduced (see Figure 3). During the 120 min collecting period, 116±70 pmol ACh were found at the botulinum toxin treated area compared to 50±20 pmol at the untreated area (P=0.28).
Figure 3.
Effect of botulinum toxin on the in vivo release of acetylcholine assayed by dermal microdialysis. Volunteers (n=4) were pretreated with 100 MU botulinum toxin either at an upper or lower area of the medial shank. After 14 days, dermal microdialysis was performed at the pretreated area or a respective untreated area 30 min apart. Shown are the results (means±s.e.m.) in the absence (control) and the presence of botulinum toxin. Botulinum toxin could not reduce the release of acetylcholine.
Effect of quinine alone and in combination with carnitine on the release of non-neuronal acetylcholine from the human skin
Two membranes were placed at the upper part of the shank and two membranes 300 mm distal. Quinine (1 mM) was added to the medium from the start of perfusion either at the upper or distal membranes that changed at random. The quinine-free medium represented the individual control. In experiments with 14 different volunteers, 155±73 pmol acetylcholine were released in 120 min in the absence of quinine and 105±43 pmol acetylcholine in the presence of quinine (P=0.55). In separate experiments, carnitine (0.1 mM), a substrate of the carnitine transporter (also known as OCTN2), was added in addition to quinine 1 mM. In experiments with 11 different volunteers, 207±50 pmol acetylcholine were released (120 min) under control conditions and 123±32 pmol in the presence of quinine together with carnitine (P<0.05). The percentage of female and male volunteers was comparable in these experiments.
Discussion
Acetylcholine, one of the most exemplary neurotransmitters, is widely expressed in non-neuronal cells in humans and is involved in the regulation of basic cell functions (Grando, 1997; Wessler et al., 1998; 1999; Kawashima & Fujii, 2000). To discriminate from the neurotransmitter function, the terms non-neuronal cholinergic system and non-neuronal acetylcholine have been introduced (Klapproth et al., 1997; Wessler et al., 1998; 1999). However, the release of non-neuronal acetylcholine from human tissue has, so far, not been demonstrated under in vivo conditions. Therefore, the present experiments with dermal microdialysis have been carried out to investigate the in vivo release of non-neuronal acetylcholine. It has been demonstrated that the epidermis as well as the underlaying dermal tissue including fat tissue express choline acetyltransferase activity; additionally, acetylcholine was found in isolated homogenized skin specimens (Grando et al., 1993; Grando, 1997; Klapproth et al., 1997; Wessler et al., 2003).
Importantly, the human skin is an easily accessible target for microdialysis and could be used for the investigation of the in vivo release of non-neuronal acetylcholine. The following results demonstrate that acetylcholine was present in the microdialysis eluate: (1) The retention times using HPLC with bioreactors, the latter increasing the specificity of the detection method, were more or less identical between authentic standard and the microdialysis sample. (2) The amount of the assayed compound increased significantly, when the cholinesterase in the skin was blocked by neostigmine. (3) The respective peak disappeared, when the analytical column was packed with the acetylcholine specific cholinesterase.
In addition to the non-neuronal cholinergic system (Grando et al., 1993; Klapproth et al., 1997) the skin also contains elements of the neuronal cholinergic system. For example, eccrine sweat glands receive cholinergic nerve fibres, and afferent neurons may also contain cholinergic fibres (Landis, 1999). The microdialysis membrane was placed within the superficial 2 mm skin surface, that is, within the diffusion radius of eccrine sweat glands. Therefore, the assayed acetylcholine may consist of non-neuronal and neuronal origin. To exclude the neuronal origin, botulinum toxin was used, which is known to block the exocytotic acetylcholine release (Gundersen, 1980). In the present experiments, the release of acetylcholine from botulinum toxin-treated skin was even somewhat higher compared to the untreated skin of the same individual and did not differ from the neostigmine control. These results exclude a substantial contamination by neuronal acetylcholine and demonstrate, for the first time, the in vivo release of non-neuronal acetylcholine in humans.
Obviously, the release of non-neuronal acetylcholine showed considerable variation, although the experimental conditions did not vary. Sometimes a ‘spikelike' activity (time scale 20 min) was observed, that is, a considerable increase of the acetylcholine content occurred between two subsequent 20 min periods (e.g. see Figure 3). Such a pattern was observed also in the presence of botulinum toxin, that is, when neuronal cholinergic activity was blocked. Additionally, a sex-dependent difference appears to exist in the release of non-neuronal ACh, as the amount of ACh measured in female volunteers exceeded the amount of ACh measured in male volunteers.
In experiments with isolated human placenta, it has been demonstrated that the release of non-neuronal acetylcholine is mediated by organic cation transporters (OCT), subtype 1 and 3. Quinine (0.1 mM), an inhibitor of many transporters including OCTs, caused a rapid and substantial inhibition of the acetylcholine release in the human placenta (Wessler et al., 2001). In contrast to the human placenta, quinine did not cause a significant inhibition of acetylcholine release from the human skin, that is, transporters other than OCTs might be involved. When carnitine, a substrate of OCTN2, which is expressed in the human skin (Ohashi et al., 1999; Tein, 2003) and which may interfere via substrate competition with the release of non-neuronal acetylcholine after being taken up, was additionally present, the release of acetylcholine was reduced by about 40% (P<0.05). Thus, OCTs appear to contribute to the release of non-neuronal acetylcholine in the human skin, but do not play a dominant role in contrast to the human placenta.
In conclusion, the present experiments demonstrate for the first time the in vivo release of non-neuronal actylcholine from the human skin by dermal microdialysis. In contrast to the human placenta, the release of non-neuronal acetylcholine from the human skin is not dominantly mediated by OCTs, that is, the release mechanisms appear to differ in a tissue-specific manner.
Acknowledgments
The present work contains parts of the MD thesis of K. an Haack and S. Schiffmann. The work was supported by the Deutsche Forschungsgemeinschaft DFG Ki 210/9-3 and by DFG Bi 579-1/1-2.
Abbreviations
- HPLC
high-pressure liquid chromatography
- OCT
organic cation transporter
References
- BIRKLEIN F., EISENBARTH G., ERBGUTH F., WINTERHOLLER M. Botulinum toxin type B blocks sudomotor function effectively: a 6 month follow up. J. Invest. Dermatol. 2003;121:1312–1316. doi: 10.1046/j.1523-1747.2003.12620.x. [DOI] [PubMed] [Google Scholar]
- BRAUNE C., ERBGUTH F., BIRKLEIN F. Dose thresholds and duration of the local anhidrotic effect of botulinum toxin injections: measured by sudometry. Br. J. Dermatol. 2001;144:111–117. doi: 10.1046/j.1365-2133.2001.03961.x. [DOI] [PubMed] [Google Scholar]
- GRANDO S.A. Biological functions of keratinocyte cholinergic receptors. J. Investig. Dermatol. Symp. Proc. 1997;2:41–48. doi: 10.1038/jidsymp.1997.10. [DOI] [PubMed] [Google Scholar]
- GRANDO S.A., KAWASHIMA K., WESSLER I. Introduction: the non-neuronal cholinergic system in humans. Life Sci. 2003;72:2009–2012. doi: 10.1016/s0024-3205(03)00063-8. [DOI] [PubMed] [Google Scholar]
- GRANDO S.A., KIST D.A., QI M., DAHL M.V. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J. Invest. Dermatol. 1993;101:32–36. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- GUNDERSEN C.B. The effects of botulinum toxin on the synthesis, storage and release of acetylcholine. Prog. Neurobiol. 1980;14:99–119. doi: 10.1016/0301-0082(80)90019-2. [DOI] [PubMed] [Google Scholar]
- KAWASHIMA K., FUJII T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. [DOI] [PubMed] [Google Scholar]
- KLAPPROTH H., REINHEIMER T., METZEN J., MUNCH M., BITTINGER F., KIRKPATRICK C.J., HOHLE K.D., SCHEMANN M., RACKE K., WESSLER I. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn. Schmiedebergs Arch. Pharmacol. 1997;355:515–523. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- KRÄMER H., ANGERER C., ERBGUTH F., SCHMELZ M., BIRKLEIN F. Botulinum Toxin A reduces neurogenic flare but has almost no effect on pain and hyperalgesia in human skin. J. Neurol. 2003;250:188–193. doi: 10.1007/s00415-003-0971-x. [DOI] [PubMed] [Google Scholar]
- LANDIS S.C. Development of muscarinic receptors and regulation of secretory responsiveness in rodent sweat glands. Life Sci. 1999;64:381–385. doi: 10.1016/s0024-3205(98)00578-5. [DOI] [PubMed] [Google Scholar]
- MINOR V. Ein neues Verfahren zu der klinischen Untersuchung der Schweißabsonderung. Z. Neurologie. 1927;101:302–308. [Google Scholar]
- OHASHI O., TAMAI I., YABUUCHI H., NEZU J.I., OKU A., SAI Y., SHIMANE M., TSUJI A. Na-dependent carnitine transport by organic cation transporter (OCTN2): Its pharmacological and toxicological relevance. J. Pharmacol. Exp. Ther. 1999;292:778–784. [PubMed] [Google Scholar]
- SASTRY B.V., SADAVONGVIVAD C. Cholinergic systems in non-nervous tissues. Pharmacol. Rev. 1978;30:65–132. [PubMed] [Google Scholar]
- SCHLERETH T., MOUKA I., EISENBARTH G., WINTERHOLLER M., BIRKLEIN F. Botulinum toxin A (Botox®) and sweating – dose efficacy and comparison to other BoNT preparations. Auton. Neurosci. 2005;117:120–126. doi: 10.1016/j.autneu.2004.11.005. [DOI] [PubMed] [Google Scholar]
- SCHMELZ M., LUZ O., AVERBECK B., BICKEL A. Plasma extravasation and neuropeptide release in human skin as measured by intradermal microdialysis. Neurosci. Lett. 1997;230:117–120. doi: 10.1016/s0304-3940(97)00494-1. [DOI] [PubMed] [Google Scholar]
- SONG P., SEKHON H.S., JIA A., KELLER J.A., BLUSZTAJIN J.K., MARK J.K., SPINDEL E.R. Acetylcholine is syntesized by and acts as an autocrine growth factor for small cell lung carcinoma. Cancer Res. 2003;63:214–221. [PubMed] [Google Scholar]
- TEIN I. Carnitine transport: pathophysiology and metabolism of known molecular defects. J. Inherit. Metab. Dis. 2003;26:147–169. doi: 10.1023/a:1024481016187. [DOI] [PubMed] [Google Scholar]
- WESSLER I., KIRKPATRICK C.J., RACKE K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol. Ther. 1998;77:59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- WESSLER I., KIRKPATRICK C.J., RACKE K. The cholinergic ‘pitfall': acetylcholine, a universal cell molecule in biological systems, including humans. Clin. Exp. Pharmacol. Physiol. 1999;26:198–205. doi: 10.1046/j.1440-1681.1999.03016.x. [DOI] [PubMed] [Google Scholar]
- WESSLER I., REINHEIMER T., KILBINGER H., BITTINGER F., KIRKPATRICK C.J., SALOGA J., KNOP J. Increased acetylcholine levels in skin biopsies of patients with atopic dermatitis. Life Sci. 2003;72:2169–2172. doi: 10.1016/s0024-3205(03)00079-1. [DOI] [PubMed] [Google Scholar]
- WESSLER I., ROTH E., DEUTSCH C., BROCKERHOFF P., BITTINGER F., KIRKPATRICK C.J., KILBINGER H. Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. Br. J. Pharmacol. 2001;134:951–956. doi: 10.1038/sj.bjp.0704335. [DOI] [PMC free article] [PubMed] [Google Scholar]