Abstract
Mitochondrial dysfunction including decrease of mitochondrial membrane potential and reduced ATP production represents a common final pathway of many conditions associated with oxidative stress, for example, hypoxia, hypoglycemia, and aging.
Since the cognition-improving effects of the standard nootropic piracetam are usually more pronounced under such pathological conditions and young healthy animals usually benefit little by piracetam, the effect of piracetam on mitochondrial dysfunction following oxidative stress was investigated using PC12 cells and dissociated brain cells of animals treated with piracetam.
Piracetam treatment at concentrations between 100 and 1000 μM improved mitochondrial membrane potential and ATP production of PC12 cells following oxidative stress induced by sodium nitroprusside (SNP) and serum deprivation. Under conditions of mild serum deprivation, piracetam (500 μM) induced a nearly complete recovery of mitochondrial membrane potential and ATP levels. Piracetam also reduced caspase 9 activity after SNP treatment.
Piracetam treatment (100–500 mg kg−1 daily) of mice was also associated with improved mitochondrial function in dissociated brain cells. Significant improvement was mainly seen in aged animals and only less in young animals. Moreover, the same treatment reduced antioxidant enzyme activities (superoxide dismutase, glutathione peroxidase, and glutathione reductase) in aged mouse brain only, which are elevated as an adaptive response to the increased oxidative stress with aging.
In conclusion, therapeutically relevant in vitro and in vivo concentrations of piracetam are able to improve mitochondrial dysfunction associated with oxidative stress and/or aging. Mitochondrial stabilization and protection might be an important mechanism to explain many of piracetam's beneficial effects in elderly patients.
Keywords: Piracetam, mitochondrial function, oxidative stress, aging
Introduction
Piracetam, the prototype of the so-called ‘nootropic' drugs (Giurgea, 1982), is used in many countries to treat cognitive impairment in aging, brain injuries, as well as dementia (Croisile et al., 1993; Waegemans et al., 2002). Although its clinical efficacy is still a matter of dispute, a recent meta-analysis of all available (published and not published) clinical studies provided compelling evidence for the global efficacy of piracetam in a diverse group of older subjects with cognitive impairment (Waegemans et al., 2002).
Similar to the situation in man, piracetam has also been shown to improve cognitive function in animals, but its mode of action is not yet been finally known (Giurgea, 1982; Muller et al., 1999). Findings that piracetam's efficacy is usually associated with conditions of disturbed brain function like aging, that is, young healthy animals usually benefit little or nothing from piracetam treatment (Valzelli et al., 1980; Muller et al., 1997), has led to the speculation that piracetam's mechanism of action is associated with biochemical deficits of the aged brain. This assumption was later supported by observations that piracetam specifically enhances membrane fluidity in aged brain material, showing no effect at all in membranes from young brains (Muller et al., 1997). Since piracetam's effects at the membrane level were observed at concentrations also needed in pharmacological experiments to improve cognition (Muller et al., 1997), we proposed that by restoring age-related membrane alterations piracetam improves brain function and finally cognition (Scheuer et al., 1999). At the subcellular level, piracetam's effects on membrane fluidity could be demonstrated for synaptosomal plasma membranes of aged mouse brains and also for mitochondrial membranes (Muller et al., 1999).
Piracetam's improving effects on the fluidity of aged synaptosomal membranes could easily explain the beneficial effects of piracetam on age-related deficits of several mechanisms of signal transduction (receptor density and function, transmitter release) (Stoll et al., 1992; Muller et al., 1999), since these mechanisms are disturbed in the aging brain probably due to a decrease of membrane fluidity. Piracetam's recently reported effects on impaired glucose uptake might also be a consequence of its effects on membrane fluidity (Naftalin et al., 2004). On the other hand, evidences that piracetam's beneficial effects on the fluidity of aged mitochondrial membranes might contribute to its therapeutic efficacy are rather indirect and orginate from observations that piracetam might improve glucose uptake and utilization as well as ATP production (Domanska-Janik & Zaleska, 1977; Benzi et al., 1985; Heiss et al., 1988). Even if these effects led to the term ‘metabolic enhancer', sometimes used to characterise piracetam and related nootropics, the mechanism of this effect and its possible relationship to mitochondrial function remained obscure.
As already mentioned, piracetam's beneficial effects are usually associated with impaired brain function such as aging, hypoxia, glucose deprivation, injuries, or neurodegeneration (Giurgea, 1982; Muller et al., 1999). It is quite remarkable that all those situations, even if other deficits are also present, are associated with mitochondrial dysfunction. Accordingly, together with our initial observations of improved mitochondrial membrane properties by piracetam, we speculated that piracetam might enhance mitochondrial function or at least protect mitochondria in situations of enhanced damage. Using specific fluorescence dyes to monitor mitochondrial membrane potential but also using other means to characterize mitochondrial function, the present communication reports a series of in vitro and in vivo experiments strongly indicating improvements of mitochondrial function as a specific property of piracetam.
Methods
Chemicals
Rhodamine 123 (R123) and tetramethylrhodamineethylester (TMRE) were purchased from Invitrogen, Karlsruhe (Germany). ViaLight HT kit was purchased from Cambrex, Verviers (Brussels). Hydrogen peroxide (H2O2), sodium nitroprusside (SNP), rotenone, thenoyltrifluoroacetone (TTFA), antimycine, sodium azide (NaN3), and oligomycine were obtained from Sigma, Munich (Germany). Piracetam was a gift from UCB (Brussels).
Cell culture
PC12 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum and 5% heat-inactivated horse serum, 50 U ml−1 penicillin, 50 μg ml−1 streptomycin, and 400 μg ml−1 G418 at 37°C in a humidified incubator containing 5% CO2.
Animal brain tissue
Young (2–3 months) and old (22–24 months) female Naval Medical Research Institute (NMRI) mice used in this study were from Harlan-Winkelmann GmbH (Borchen, Germany). The latter were obtained at an age of 12 months and maintained at the Biocenter's animal care facility until use. All animals were housed in plastic cages with water and food ad libitum and were maintained on a 12-h light/dark cycle. Animals were handled according to the German guidelines for animal care.
Mice were killed by decapitation and brains were quickly dissected on ice (method modified after Stoll et al., 1992). After removing the cerebellum, the tissue was minced into 2 ml of medium I (NaCl 138, KCl 5.4, Na2HPO4 0.17, K2PO4 0.22, glucose 5.5 and sucrose 58.4 all in mmol l−1, pH 7.35) with a scalpel and further dissociated by trituration through a nylon mesh (pore diameter 1 mm) with a pasteur pipette. The resulting suspension was filtered by gravity through a fresh nylon mesh with a pore diameter of 102 μM and the dissociated cell aggregates were washed twice with medium II (NaCl 110, KCl 5.3, CaCl2·H2O 1.8, MgCl2·6 H2O 1, glucose 25, sucrose 70, and HEPES (4-2-hydroxyethyl)-1-piperazineethanesufonic acid) 20 all in mmol l−1, pH 7.4) by centrifugation (400 × g for 3 min at 4°C). In all, 100 μl of the suspension was used for protein determination. After centrifugation, cells were resuspended in 6 ml DMEM and distributed on a 48 well plate for the measurement of mitochondrial membrane potential. The data were expressed as fluoroscence unit per mg ml−1 protein.
Measurement of mitochondrial membrane potential
PC12 cells were plated the day before at a density of 2 × 105 cells well−1 in a 24-well plate. The mitochondrial membrane potential was measured using the fluorescence dye R123 (Baracca et al., 2003). Transmembrane distribution of the dye depends on the mitochondrial membrane potential. The dye was added to the cell culture medium at a concentration of 0.4 μM for 15 min. The cells were washed twice with Hanks' balanced salt solution and the fluorescence was determined with a fluorescence reader (Victor® multilabel counter) at 490/535 nm.
The mitochondrial membrane potential of dissociated brain cells was also measured with R123 at a concentration of 0.4 μM for 15 min. For detailed information about the preparation, see under Animal brain tissue.
To test acute and fast changes in mitochondrial membrane potential, the fluorescence dye TMRE (Collins et al., 2002) was used at a concentration of 0.4 μM for 15 min. TMRE exhibits a characteristic increase in fluorescence at 490/590 nm after challenging mitochondria with membrane potential-decreasing drugs (Krohn et al., 1999). The mitochondrial membrane potential was recorded and then the complex inhibitors (rotenone 2 μM, TTFA 10 μM, antimycine 2 μM, oligomycine 10 μM, and NaN3 10 mM) were added.
Determination of ATP levels with a bioluminescence assay (ViaLight™ HT)
PC12 cells were plated the day before at a density of 2 × 104 cells well−1 in a white 96-well plate. The kit is based upon the bioluminescent measurement of ATP (Crouch et al., 1993). The bioluminescent method utilizes an enzyme, luciferase, which catalyzes the formation of light from ATP and luciferin. The emitted light is linearly related to the ATP concentration and is measured using a luminometer.
Piracetam treatment
PC12 cells were treated in three different ways. First, the protective effect of piracetam on recovery after oxidative stress was tested. Thus, PC12 cells were treated for 24 h with SNP (0.5 mM), piracetam was added 30 min after the onset of SNP exposure. Second, PC12 cells were pretreated for 30 min with SNP, then the medium was exchanged and piracetam was added for 23 h. Third, PC12 cells were pretreated for 6 h with piracetam, after that the mitochondrial membrane potential was recorded, and then the complex inhibitors were added.
Dissociated brain cells were treated for 6 h with SNP (2 mM), and piracetam was added 1 h after the onset of SNP exposure.
Treatment of animals
The treated animals received 100, 250, or 500 mg kg−1 piracetam in 0.9% NaCl solution p.o. once daily for 2 weeks. Control animals were treated with 0.9% NaCl alone. The tests were implemented 24 h after the last feeding. The dissociated brain cells of untreated and treated mice were incubated for 4 or 6 h with H2O2 (2 mM) or SNP (2 mM).
Measurement of caspase 9 activity
PC12 cells were plated the day before at a density of 5 × 106 cells per culture dish (10 cm diameter). After pretreatment for 22 h with piracetam, SNP was added for 2 h. The cells were harvested, and, after a centrifugation step, the culture medium was aspirated. The cell-containing pellet was washed with PBS and lysed in 100 μl of lysis buffer (10 mM HEPES, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM EDTA, 1 mM dithiothreitol, 1 μg ml−1 pepstatin A, 1 μg ml−1 leupeptin, 0.1% CHAPS, pH 7.4). Lysates were centrifuged at 20,000 × g for 10 min at 4°C, and the supernatant was used for caspase assay.
Caspase 9 activity was measured by cleavage of the colorimetric substrate Ac-LEHD-pNA for caspase 9. The production of pNA was monitored over 30 min in a photometer at 405 nm. The caspase activity is expressed as change in absorption units. One unit was defined as the amount of enzyme required to cleave 1 pmol of NA per min of incubation per 5 × 106 cells. For details, see Marques et al. (2003).
Cu/Zn-superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR) activity assays
For determination of antioxidative enzyme activities, we prepared frozen brain hemispheres. Tissue samples without cerebellum were washed in 1 ml EDTA and 1 ml Tris buffer (20 mM, pH 7.4) and homogenized with a Potter homogenizer in 20 mM Tris buffer. The resulting homogenates were centrifuged at 8500 × g for 10 min at 4°C. The clear supernatants were used to determine the activity of SOD, GPx and GR according to Leutner et al. (2001) and Schuessel et al. (2004).
SOD activity
The assay of Cu/Zn-SOD is based on the method of Nebot et al. (1993), utilizing the commercially available SOD Assay Kit from Merck Biosciences, Bad Soden (Germany). For elimination of interfering substances and Mn-SOD activity, Cu/Zn-SOD enzyme activity was assayed after the extraction procedure with chloroform and ethanol according to the supplier's manual. SOD activity was determined from the Vs/Vc ratio of the autoxidation rates of the chromophore BXT-01050 at 37°C measured in the presence (Vs) and absence (Vc) of sample.
One SOD activity unit is defined as the activity that doubles the autoxidation background (Vs/Vc=2).
GPx activity
The assay of GPx (cytosolic GPx) is based on the reaction described by Paglia & Valentine (1967) using the commercially available Cellular GPx Assay Kit from Merck Biosciences, Bad Soden (Germany). Tert-butylhydroperoxide was used as substrate.
One unit of GPx is defined as the activity that converts 1 mmol of reduced glutathione per litre per minute at 25°C.
GR activity
The GR-activity assay is based on the method of Mizuno & Ohta (1986). Enzymatic activity was assayed photometrically by measuring NADPH consumption during the enzymatic reaction: in the presence of GSSG and NADPH, GR reduces GSSG and oxidizes NADPH to yield NADP, resulting in a decrease of absorbance at 340 nm. We used the commercially available GR Assay Kit from Merck Biosciences, Bad Soden (Germany).
One unit of GR is defined as the activity that converts 1 mmol of NADPH per litre per minute at 25°C.
Statistics
Data are given as mean±s.e.m. n is the number of independent experiments usually performed in triplicates. In animal studies it is the number of animals. For statistical comparison, Student's t-test, one-way ANOVA followed by Tukeys post hoc test or repeated-measures ANOVA followed by Tukeys post hoc test were used. P-values less than 0.05 were considered statistically significant.
Results
Protection against NO-induced oxidative stress in PC12 cells
Oxidative stress accumulates with aging and plays an important role in the pathogenesis of neurodegenerative disorders including Alzheimer's disease (AD) (Butterfield et al., 1999; Christen, 2000; Wei & Lee, 2002; Sastre et al., 2003; Emerit et al., 2004). For our initial experiments using PC12 cells in tissue culture, we experimentally induced acute oxidative stress using different approaches to mirror acutely damages, which are usually seen in the aged brain only after years (Marques et al., 2003; Keil et al., 2004).
Nitric oxide (NO) and its derivative peroxynitrite radical induce oxidative stress. NO inhibits cytochrome c oxidase activity (Brunori et al., 1999; Sarti et al., 2000; Cooper, 2002). Inhibition of mitochondrial respiratory chain by NO may cause apoptosis (Brown & Borutaite, 2002; Vieira & Kroemer, 2003). In PC12 cells, SNP, a NO donor, led to a reduction of mitochondrial membrane potential and ATP levels (Figure 1).
Figure 1.
(a) Treatment with piracetam after NO (SNP) insult improves the reduction of mitochondrial membrane potential and enhances ATP levels. PC12 cells were incubated for 24 h with 0.5 mM SNP, piracetam was added 30 min after insult. (b) Improvement of recovery of mitochondrial membrane potential and ATP levels by piracetam following NO exposure. PC12 cells were incubated for 30 min with 0.5 mM SNP; after washing, piracetam was added for 23 h. Data are expressed as means±s.e.m. (n=6–12. **P<0.01, *P<0.05 vs SNP control, one-way ANOVA followed by Tukey's post hoc test.
Under basal conditions without additional damage, piracetam did not affect mitochondrial membrane potential and ATP levels in PC12 cells even after 24 h incubation and at rather high concentrations (data not shown). However, piracetam was able to reduce mitochondrial membrane potential changes and to enhance ATP levels, when added 30 min after the onset of SNP exposure (Figure 1a). Additionally, piracetam improved the recovery of mitochondrial membrane potential and ATP levels after exchanging the medium following SNP exposure (Figure 1b).
Protection against mitochondrial damage due to reduction of neurotrophic support in PC12 cells
Cells require serum to maintain growth in vitro. Serum provides growth and survival factors and its removal causes cellular stress. Serum deprivation leads to a rise of ROS and a decrease of ATP levels in neurons (Atabay et al., 1996). Additionally, serum and glucose deprivation in PC12 cells cause peroxidation of their cell membrane lipids and decrease intracellular SOD activity (Ochiai et al., 2004). We have previously shown that serum deprivation enhances apoptosis in PC12 cells (Leutz et al., 2002). Here, we could demonstrate that serum deprivation leads to a decrease of mitochondrial membrane potential (Figure 2a). Interestingly, piracetam was able to protect mitochondria against cellular stress following serum deprivation. The decrease of mitochondrial membrane potential after serum deprivation was significantly reduced (Figure 2a).
Figure 2.
(a) Treatment with piracetam improves the reduction of mitochondrial membrane potential induced by 24 h serum deprivation. Piracetam was added 30 min after insult. Data are expressed as means±s.e.m. (n=6). **P<0.01 vs serum deprivation control, one-way ANOVA followed by Tukey's post hoc test. (b) Treatment with piracetam ameliorates the decrease of mitochondrial membrane potential induced by 24 h stepwise serum deprivation. Piracetam was added 30 min after insult. Data are expressed as means±s.e.m. (n=10–15). ***P<0.001 vs % control, Student's paired t-test. (c) Treatment with piracetam ameliorates the reduction of ATP levels induced by 24 h stepwise serum deprivation. Piracetam was added 30 min after insult. Data are expressed as means±s.e.m. (n=10–15). ***P<0.001 vs % control, Student's paired t-test.
Under conditions of mild serum deprivation, when serum concentrations not lower than 2% were used, piracetam (500 μM) induced a nearly complete recovery of mitochondrial membrane potential (Figure 2b). ATP levels are more sensitive to serum deprivation (Figure 2c). Reduced ATP levels were already seen at 10% serum. Under this condition, piracetam completely restored ATP levels, while at lower serum concentrations only a partial restoration was seen.
Piracetam protects PC12 cells against damage induced by inhibitors of the respiratory chain
Biochemical analysis of CNS tissue from patients with AD has yielded evidence for abnormalities of components of the electron transport chain (Parker Jr et al., 1994b). In many AD patients, cytochrome c oxidase activity is impaired in the CNS (Kish et al., 1992) and even in other tissues, including platelets (Parker Jr et al., 1994a; Bosetti et al., 2002; Cardoso et al., 2004).
Thus, we investigated the possible efficacy of piracetam to protect individual complexes of the mitochondrial respiratory chain after treatment with different respiratory chain complex inhibitors. Complexes I, II, and III were protected at concentrations as low as 500 μM piracetam (Figure 3, Table 1). A significant protection of complexes IV and V was observed at a concentration of 1000 μM piracetam (Table 1).
Figure 3.
Pretreatment with piracetam reduces antimycine-induced changes in mitochondrial membrane potential evoked by inhibition of complex III. PC12 cells were pretreated for 6 h with piracetam. Mitochondrial membrane potential was recorded with the dye TMRE and antimycine was added after 100 s. Data are expressed as means±s.e.m. (n=4).
Table 1.
Protective efficacy of piracetam on the individual complexes of the mitochondrial respiratory chain
| Protection by piracetam (mmol l−1) | |||||
|---|---|---|---|---|---|
| Complex | Damage | 0.50 | 1.00 | 10.00 | |
| Complex | I | Rotenone | + | + | + |
| Complex | II | Thenoyltrifluoroacetone | + | + | + |
| Complex | III | Antimycine | + | + | ++ |
| Complex | IV | Natriumazide | + | ++ | |
| Complex | V | Oligomycine | + | ++ | |
Pretreatment with piracetam diminished mitochondrial membrane potential changes evoked by different complex inhibitors. Unpaired student's t-test, ++P<0.01, +P<0.05 vs control cells (n=4–10).
Other antidementiva and antipsychotic drugs had no effect on mitochondrial membrane potential and ATP levels
We investigated the putative protective effects of other antidementiva and psychotropic drugs like memantine, galantamine, haloperidol, imipramine, fluoxetine, or trolox on mitochondrial function by measuring mitochondrial membrane potential and ATP levels under similar conditions. PC12 cells were incubated with SNP for 24 h, 30 min after insult the drugs were added. Interestingly, none of the investigated drugs was able to diminish the decrease of mitochondrial membrane potential and the reduction of ATP levels (Figure 4a and b). Thus, the protective effect of piracetam under similar conditions shows a substantial specificity.
Figure 4.
(a) No protective effect of other drugs on mitochondrial membrane potential. The concentration of all drugs was 10 μM. (b) No protective effect of other drugs on ATP levels.
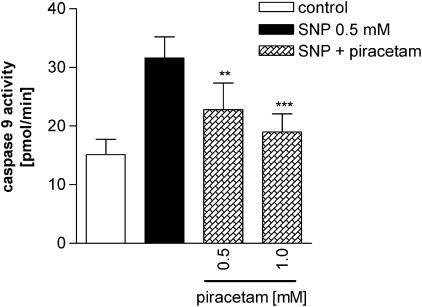
Piracetam reduces caspase 9 activity after SNP exposure
Caspase 9 represents the last specific step of the intrinsic pathway of apoptosis, which is triggered from mitochondria following oxidative stress (Kroemer & Reed, 2000). The activation of caspase 9 leads to activation of the executor caspase 3, which is common also for the extrinsic apoptotic pathway, and finally leads to apoptotic cell death. Caspase 9 is activated in PC12 cells after SNP exposure with a maximum after 2 h of SNP treatment (data not shown). Pretreatment of PC12 cells with piracetam for 22 h, following SNP for 2 h, reduced the caspase 9 activity significantly (Figure 5).
Figure 5.
Treatment with piracetam reduces caspase 9 activity after SNP exposure. PC12 cells were pretreated for 22 h with piracetam, then SNP 0.5 mM was added for 2 h. Data are expressed as means±s.e.m. (n=5). ***P<0.001, **P<0.01 vs SNP control, Repeated-measures ANOVA followed by Tukey's post hoc test.
Protection against NO-induced oxidative stress in dissociated brain cells from young and aged mice in vitro
Consistent with our findings in PC12 cells, piracetam is not protective by itself in dissociated brain cells in vitro, since the mitochondrial membrane potential of dissociated brain cells did not differ between in vitro untreated control cells and cells treated with piracetam (data not shown). However, piracetam was protective against SNP-induced mitochondrial dysfunction in cells of aged (Figure 6b) but not of young mice (Figure 6a). This effect was already seen at the rather low piracetam concentration of 500 μM.
Figure 6.
(a) Treatment with piracetam after NO (SNP) insult improves the reduction of mitochondrial membrane potential. Membrane potential was measured after 1 h preincubation with 0.5 mM SNP and 6 h incubation with piracetam in dissociated brain cells of young mice. (b) Dissociated brain cells of aged mice. Data are expressed as means±s.e.m. (n=4–7). *P<0.05 vs SNP control, one-way ANOVA followed by Tukey's post hoc test. (c) Treatment with piracetam stabilizes basal levels of mitochondrial membrane potential in aged mice. The treated animals received 500 mg piracetam kg−1 in 0.9% NaCl solution p.o. once daily for 2 weeks. Control animals were treated with 0.9% NaCl solution alone. Data are expressed as means±s.e.m. (n=6–8). *P<0.05 vs control, Student's unpaired t-test. (d) Piracetam treatment protects against NO induced mitochondrial damage. Young (2–3 months) and aged mice (22 months) were treated with 500 mg piracetam kg−1 in 0.9% NaCl solution p.o. once daily for 2 weeks. Control animals were treated with 0.9% NaCl solution alone. Membrane potential of dissociated brain cells was measured after 4 h incubation with 2 mM SNP. Data are expressed as means±s.e.m. (n=6–8). *P<0.05 vs SNP control, Student's unpaired t-test.
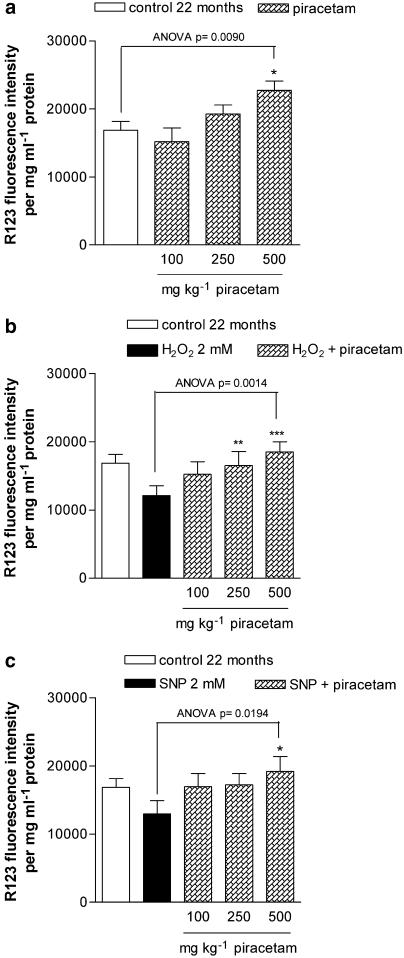
Piracetam normalizes the basal level of mitochondrial membrane potential in vivo
Brain cells of aged mice show a decreased mitochondrial membrane potential compared to young mice (Figure 6c). Contrary to our in vitro data, piracetam treatment of young and aged mice for 14 days normalized mitochondrial membrane potential in aged mice without showing an effect in young animals (Figure 6c).
Protection against H2O2- and NO-induced oxidative stress in dissociated brain cells from young and aged mice treated in vivo
When piracetam was given orally for 2 weeks, decrease of mitochondrial membrane potential by H2O2 was significantly diminished in young and old treated mice (data not shown). Piracetam was protective against NO-induced mitochondrial dysfunction in aged mice only (Figure 6d). The protection in aged mice was always more pronounced than in young animals. Therefore, the dose dependence of piracetam's protective effects was investigated in aged mice only.
Consistent with our first study, piracetam normalized basal levels of mitochondrial membrane potential and protected against H2O2- and SNP-induced mitochondrial dysfunction, with first effects already seen at concentrations of 250 mg kg−1 (Figure 7a–c).
Figure 7.
(a) Treatment with piracetam stabilizes concentration dependently the basal levels of mitochondrial membrane potential in aged mice. (b) Treatment with piracetam protects against H2O2-induced mitochondrial damage in a concentration dependent manner. (c) Treatment with piracetam protects against SNP induced mitochondrial damage concentration dependently. The treated animals received 100, 250 and 500 mg kg−1 piracetam in 0.9% NaCl solution p.o. once daily for 2 weeks. Control animals were treated with 0.9% NaCl solution alone. Membrane potential of dissociated brain cells was measured after 4 h incubation with H2O2 or SNP. Data are expressed as means±s.e.m. (n=8). ***P<0.001, *P<0.01, *P<0.05 vs control, repeated-measures ANOVA followed by Tukey's multiple comparion test.
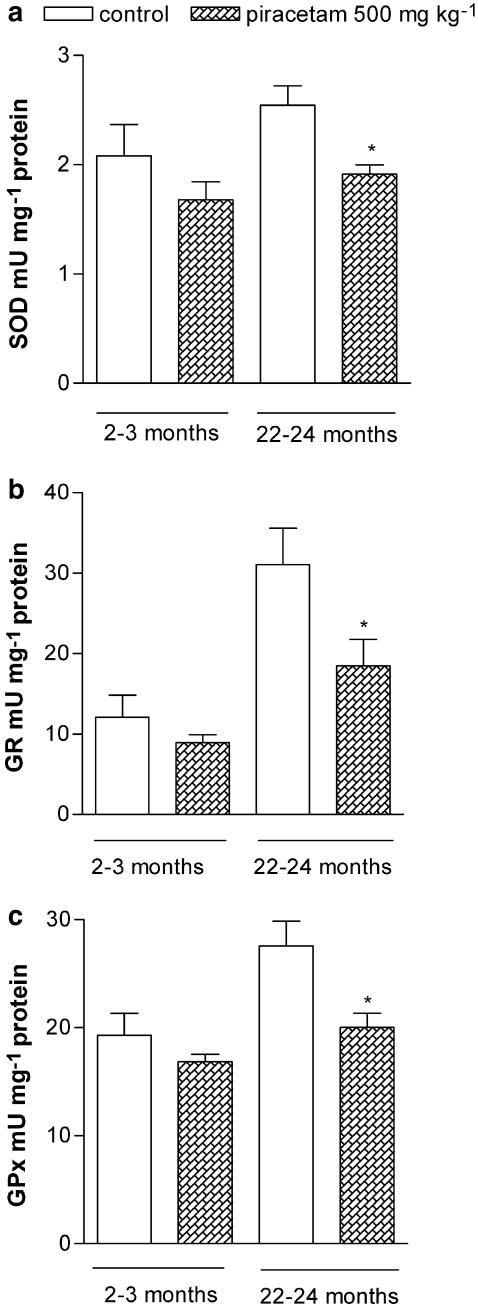
Effects of aging and treatment with piracetam on antioxidative enzyme activities in the mouse brain
Antioxidative enzymes are the primary defense mechanism to protect biological macromolecules from oxidative damage, and are upregulated in aged mouse brain as an adaptive response to oxidative stress (Leutner et al., 2001; Schuessel et al., 2005). Therefore, we investigated the effect of piracetam treatment on the activities of SOD, GPx, and GR in young mice (2–3 months old) and old mice (22–24 months old).
We confirmed a significant increase in GPx and GR activity in aged mice compared to young mice (Figure 8b and c). The activity of SOD had also a tendency to increase with age (Figure 8a). Piracetam treatment decreased the activities of all three enzymes in aged mice nearly to the level of young animals (Figure 8a–c). In young mice, a only small and not significant decrease of antioxidative enzymes could be observed (Figure 8).
Figure 8.
Effects of aging and treatment with piracetam on SOD (a), GPx (b) and GR activity (c) in young and aged mice. The treated animals received 500 mg kg−1 piracetam in 0.9% NaCl solution p.o. once daily for 2 weeks. Control animals were treated with 0.9% NaCl solution alone. Activities of antioxidant enzymes are increased in aged mice. Treatment with piracetam reduces the enzyme activities significantly in old mice. Data are expressed as means±s.e.m. (n=7). *P<0.05 vs control, Student's unpaired t-test.
Discussion
The data presented clearly show that piracetam protects mitochondria against different conditions associated with oxidative stress including aging. Piracetam's protecting effects on mitochondrial damage induced in vitro are small, but reproducible and highly significant. This is not surprising, since the conditions used to induce oxidative stress in vitro are not physiological and rather massive, in contrast to the small and slowly occurring changes induced by aging, which however were sometimes completely reversed by piracetam treatment. This was also the case for the adaptive elevation of antioxidant enzyme activities. When mild conditions were uesd in vitro (e.g. partial serum deprivation), a complete protection of mitochondria was seen by piracetam treatment in PC12 cells. Moreover, piracetam was highly effective in vivo in pathophysiologically relevant situations of brain dysfunction.
Piracetam does not possess radical scavenging properties (Bentue-Ferrer et al., 1989; Horvath et al., 2002). This observation was recently confirmed by us since piracetam up to 5 mM had no activity at all in two typical in vitro antioxidant assays (guajacol and oxyhemoglobin assay) (unpublished observations). Moreover, the classical radical scavenger trolox was not able to protect mitochondria under similar conditions (Figure 4). Thus, it seems quite likely that piracetam acts directly at the mitochondrial level, presumably by improving mitochondrial membrane properties (Muller et al., 1999). This is also supported by our observation that the protective effects were also seen in the recovery phase, when the oxidative stressor was already removed. Moreover, initial experiments from our lab also indicate similar effects of piracetam on isolated mouse brain mitochondria of animals treated with piracetam (unpublished observations). The concentrations of piracetam effective in vitro (100–1000 μM) and the doses used in the in vivo experiments (100–500 mg kg−1) are quite well within the plasma concentrations seen in patients treated with the standard dose of about 5 g daily, which range between 200 and 2000 μM (Saletu et al., 1986; 1995). Thus, it is quite likely that similar effects are also taking place in the brain of piracetam treated patients (Heiss et al., 1988; Eckert et al., 1999).
At such therapeutically relevant concentrations, piracetam seems to interact rather unspecifically with the polar head groups of the lipid bilayer of cellular membranes (Peuvot et al., 1995; Muller et al., 1997). Its structural analogue aniracetam binds to specific recognition sites associated with AMPA-sensitive glutamate receptors (Fallarino et al., 1995; O'Neill et al., 2004). However, piracetam does not interact with these sites up to 2 mM (Fallarino et al., 1995).
While the interaction of piracetam with membranes shows little further changes of membrane properties under normal conditions, it significantly enhances reduced membrane fluidity, for example, in the aging or even Alzheimer brain (Muller et al., 1997; Eckert et al., 1999). All the conditions associated with positive effects of piracetam on mitochondrial function in the present report may also be associated with decreased membrane fluidity due to enhanced lipid peroxidation and other damages. Thus, it seems quite plausible that piracetam improves mitochondrial function by enhancing membrane fluidity. However, direct proof for this mechanism still needs to be given. Piracetam also improved mitochondrial membrane potential after stimulation with several inhibitors of the mitochondrial respiratory chain, where complexes I, II, and III were specifically sensitive. Since the complexes are located in the inner mitochondrial membrane, this structure might benefit specifically by piracetam.
Impaired mitochondrial function and decreased activity of the respiratory chain will lead to reduced ATP levels, decreased glucose uptake, and finally to neuronal dysfunction, situations which all can be improved by piracetam (Benzi et al., 1985; Saletu et al., 1986; 1995; Heiss et al., 1988; Szelies et al., 2001). Impaired mitochondrial function will finally also lead to activation of caspases and apoptotic cell death. Observations with piracetam of Gabryel et al. (2002), about improved MTT reduction and enhanced ATP levels in astrozytes after hypoxia and reoxygenation, and by Pelsman et al. (2003), about reduced apoptosis after H2O2 treatment of human cortical neurons, together with our observation of reduced caspase 9 activity following oxidative stress, fit perfectly into the findings of a direct mitochondrial stabilization by piracetam. This might lead to improved respiratory chain function and ATP production on the one hand and reduced apoptosis signalling on the other hand.
Piracetam also markedly protects against mitochondrial dysfunction induced by serum deprivation and even seems to enhance or normalize mitochondrial function in situations of mildly reduced trophic support.
Serum deprivation leads to a decrease of cellular ATP/ADP ratio (Gottlieb et al., 2002) and to an energy deficiency. Piracetam is known to enhance cerebral glucose metabolism (Heiss et al., 1988). Moreover, Piracetam antagonizes inhibition of human erythrocyte D-glucose transport by barbiturates, diazepam, melatonin, and galanin (Naftalin et al., 2004). Thus, enhanced glucose transport in the cells might contribute to the stabilization of mitochondrial function during serum deprivation. In addition, serum deprivation induces opening of the permeability transition pore (Furuno et al., 2001). Due to the membrane-stabilizing properties, piracetam might inhibit the opening of the permeability transition pore and so protect mitochondria under serum-deprivated conditions. Furthermore, piracetam has been shown to be specifically active in preventing hypoxia-induced sensitization of animals for convulsive drugs (Rauca et al., 2000). Interestingly, this kindling-like phenomenon has been associated with reduced levels of trophic factors. The assumption that piracetam also compensates for cellular (mitochondrial) dysfunction associated with reduced neurotrophic support and might even improve neurotrophic mechanisms could be an explanation that piracetam might enhance the restitution and reorganization of neuronal circuits after periods of brain damage in experimental animals or patients following stroke (Coq & Xerri, 1999; Szelies et al., 2001).
In summary, protection against mitochondrial dysfunction, improved ATP production, and prevention of apoptotic signals may be important features of piracetam's beneficial effects in aging and neurodegeneration. Since mitochondrial dysfunction and reduced energy metabolism seem to be very early events during the course of AD (Mielke et al., 1994; Santens et al., 2001; Eckert et al., 2003; Keil et al., 2004), the present findings can easily explain piracetam's beneficial effects on glucose utilization in AD patients (Domanska-Janik & Zaleska, 1977; Benzi et al., 1985; Heiss et al., 1988) together with its effects on glucose uptake.
Acknowledgments
This study was supported by UCB, Belgium.
Abbreviations
- AD
Alzheimer's disease
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- ANOVA
analysis of variance
- CNS
central nervous system
- GPx
glutathione peroxidase
- GR
glutathione reductase
- H2O2
hydrogen peroxide
- NaN3
sodium azide
- PC12 cells
rat pheochromocytoma cells
- R123
rhodamine 123
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- TMRE
tetramethylrhodamineethylester
- TTFA
thenoyltrifluoroacetone
References
- ATABAY C., CAGNOLI C.M., KHARLAMOV E., IKONOMOVIC M.D., MANEV H. Removal of serum from primary cultures of cerebellar granule neurons induces oxidative stress and DNA fragmentation: protection with antioxidants and glutamate receptor antagonists. J. Neurosci. Res. 1996;43:465–475. doi: 10.1002/(SICI)1097-4547(19960215)43:4<465::AID-JNR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- BARACCA A., SGARBI G., SOLAINI G., LENAZ G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F(0) during ATP synthesis. Biochim. Biophys. Acta. 2003;1606:137–146. doi: 10.1016/s0005-2728(03)00110-5. [DOI] [PubMed] [Google Scholar]
- BENTUE-FERRER D., PHILOUZE V., PAPE D., REYMANN J.M., ALLAIN H., VAN DEN D.J. Comparative evaluation of scavenger properties of exifone, piracetam and vinburnine. Fundam. Clin. Pharmacol. 1989;3:323–328. doi: 10.1111/j.1472-8206.1989.tb00673.x. [DOI] [PubMed] [Google Scholar]
- BENZI G., PASTORIS O., VILLA R.F., GIUFFRIDA A.M. Influence of aging and exogenous substances on cerebral energy metabolism in posthypoglycemic recovery. Biochem. Pharmacol. 1985;34:1477–1483. doi: 10.1016/0006-2952(85)90687-2. [DOI] [PubMed] [Google Scholar]
- BOSETTI F., BRIZZI F., BAROGI S., MANCUSO M., SICILIANO G., TENDI E.A., MURRI L., RAPOPORT S.I., SOLAINI G. Cytochrome c oxidase and mitochondrial F(1)F(0)-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol. Aging. 2002;23:371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- BROWN G.C., BORUTAITE V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic. Biol. Med. 2002;33:1440–1450. doi: 10.1016/s0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- BRUNORI M., GIUFFRE A., SARTI P., STUBAUER G., WILSON M.T. Nitric oxide and cellular respiration. Cell Mol. Life Sci. 1999;56:549–557. doi: 10.1007/s000180050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTERFIELD D.A., HOWARD B., YATIN S., KOPPAL T., DRAKE J., HENSLEY K., AKSENOV M., AKSENOVA M., SUBRAMANIAM R., VARADARAJAN S., HARRIS-WHITE M.E., PEDIGO N.W., JR, CARNEY J.M. Elevated oxidative stress in models of normal brain aging and Alzheimer's disease. Life Sci. 1999;65:1883–1892. doi: 10.1016/s0024-3205(99)00442-7. [DOI] [PubMed] [Google Scholar]
- CARDOSO S.M., REGO A.C., PENACHO N., OLIVEIRA C.R. Apoptotic cell death induced by hydrogen peroxide in NT2 parental and mitochondrial DNA depleted cells. Neurochem. Int. 2004;45:693–698. doi: 10.1016/j.neuint.2004.03.003. [DOI] [PubMed] [Google Scholar]
- CHRISTEN Y. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- COLLINS T.J., BERRIDGE M.J., LIPP P., BOOTMAN M.D. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 2002;21:1616–1627. doi: 10.1093/emboj/21.7.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER C.E. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector. Trends Biochem. Sci. 2002;27:33–39. doi: 10.1016/s0968-0004(01)02035-7. [DOI] [PubMed] [Google Scholar]
- COQ J.O., XERRI C. Acute reorganization of the forepaw representation in the rat SI cortex after focal cortical injury: neuroprotective effects of piracetam treatment. Eur. J. Neurosci. 1999;11:2597–2608. doi: 10.1046/j.1460-9568.1999.00673.x. [DOI] [PubMed] [Google Scholar]
- CROISILE B., TRILLET M., FONDARAI J., LAURENT B., MAUGUIERE F., BILLARDON M. Long-term and high-dose piracetam treatment of Alzheimer's disease. Neurology. 1993;43:301–305. doi: 10.1212/wnl.43.2.301. [DOI] [PubMed] [Google Scholar]
- CROUCH S.P., KOZLOWSKI R., SLATER K.J., FLETCHER J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- DOMANSKA-JANIK K., ZALESKA M. The action of piracetam on 14C-glucose metabolism in normal and posthypoxic rat cerebral cortex slices. Pol. J. Pharmacol. Pharm. 1977;29:111–116. [PubMed] [Google Scholar]
- ECKERT A., KEIL U., MARQUES C.A., BONERT A., FREY C., SCHUSSEL K., MULLER W.E. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer's disease. Biochem. Pharmacol. 2003;66:1627–1634. doi: 10.1016/s0006-2952(03)00534-3. [DOI] [PubMed] [Google Scholar]
- ECKERT G.P., CAIRNS N.J., MULLER W.E. Piracetam reverses hippocampal membrane alterations in Alzheimer's disease. J. Neural Transm. 1999;106:757–761. doi: 10.1007/s007020050196. [DOI] [PubMed] [Google Scholar]
- EMERIT J., EDEAS M., BRICAIRE F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- FALLARINO F., GENAZZANI A.A., SILLA S., L'EPISCOPO M.R., CAMICI O., CORAZZI L., NICOLETTI F., FIORETTI M.C. [3H] Aniracetam binds to specific recognition sites in brain membranes. J. Neurochem. 1995;65:912–918. doi: 10.1046/j.1471-4159.1995.65020912.x. [DOI] [PubMed] [Google Scholar]
- FURUNO T., KANNO T., ARITA K., ASAMI M., UTSUMI T., DOI Y., INOUE M., UTSUMI K. Roles of long chain fatty acids and carnitine in mitochondrial membrane permeability transition. Biochem. Pharmacol. 2001;62:1037–1046. doi: 10.1016/s0006-2952(01)00745-6. [DOI] [PubMed] [Google Scholar]
- GABRYEL B., ADAMEK M., PUDELKO A., MALECKI A., TRZECIAK H.I. Piracetam and vinpocetine exert cytoprotective activity and prevent apoptosis of astrocytes in vitro in hypoxia and reoxygenation. Neurotoxicology. 2002;23:19–31. doi: 10.1016/s0161-813x(02)00004-9. [DOI] [PubMed] [Google Scholar]
- GIURGEA C.E. The nootropic concept and its prospective implications. Drug Dev. Res. 1982;2:441–446. [Google Scholar]
- GOTTLIEB E., ARMOUR S.M., THOMPSON C.B. Mitochondrial respiratory control is lost during growth factor deprivation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12801–12806. doi: 10.1073/pnas.202477599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEISS W.D., HEBOLD I., KLINKHAMMER P., ZIFFLING P., SZELIES B., PAWLIK G., HERHOLZ K. Effect of piracetam on cerebral glucose metabolism in Alzheimer's disease as measured by positron emission tomography. J. Cereb. Blood Flow Metab. 1988;8:613–617. doi: 10.1038/jcbfm.1988.104. [DOI] [PubMed] [Google Scholar]
- HORVATH B., MARTON Z., HALMOSI R., ALEXY T., SZAPARY L., VEKASI J., BIRO Z., HABON T., KESMARKY G., TOTH K. In vitro antioxidant properties of pentoxifylline, piracetam, and vinpocetine. Clin. Neuropharmacol. 2002;25:37–42. doi: 10.1097/00002826-200201000-00007. [DOI] [PubMed] [Google Scholar]
- KEIL U., BONERT A., MARQUES C.A., SCHERPING I., WEYERMANN J., STROSZNAJDER J.B., MULLER-SPAHN F., HAASS C., CZECH C., PRADIER L., MULLER W.E., ECKERT A. Amyloid beta-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J. Biol. Chem. 2004;279:50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- KISH S.J., BERGERON C., RAJPUT A., DOZIC S., MASTROGIACOMO F., CHANG L.J., WILSON J.M., DISTEFANO L.M., NOBREGA J.N. Brain cytochrome oxidase in Alzheimer's disease. J. Neurochem. 1992;59:776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- KROEMER G., REED J.C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- KROHN A.J., WAHLBRINK T., PREHN J.H. Mitochondrial depolarization is not required for neuronal apoptosis. J. Neurosci. 1999;19:7394–7404. doi: 10.1523/JNEUROSCI.19-17-07394.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUTNER S., ECKERT A., MULLER W.E. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J. Neural Transm. 2001;108:955–967. doi: 10.1007/s007020170015. [DOI] [PubMed] [Google Scholar]
- LEUTZ S., STEINER B., MARQUES C.A., HAASS C., MULLER W.E., ECKERT A. Reduction of trophic support enhances apoptosis in PC12 cells expressing Alzheimer's APP mutation and sensitizes cells to staurosporine-induced cell death. J. Mol. Neurosci. 2002;18:189–201. doi: 10.1385/JMN:18:3:189. [DOI] [PubMed] [Google Scholar]
- MARQUES C.A., KEIL U., BONERT A., STEINER B., HAASS C., MULLER W.E., ECKERT A. Neurotoxic mechanisms caused by the Alzheimer's disease-linked Swedish amyloid precursor protein mutation: oxidative stress, caspases, and the JNK pathway. J. Biol. Chem. 2003;278:28294–28302. doi: 10.1074/jbc.M212265200. [DOI] [PubMed] [Google Scholar]
- MIELKE R., PIETRZYK U., JACOBS A., FINK G.R., ICHIMIYA A., KESSLER J., HERHOLZ K., HEISS W.D. HMPAO SPET and FDG PET in Alzheimer's disease and vascular dementia: comparison of perfusion and metabolic pattern. Eur. J. Nucl. Med. 1994;21:1052–1060. doi: 10.1007/BF00181059. [DOI] [PubMed] [Google Scholar]
- MIZUNO Y., OHTA K. Regional distributions of thiobarbituric acid-reactive products, activities of enzymes regulating the metabolism of oxygen free radicals, and some of the related enzymes in adult and aged rat brains. J. Neurochem. 1986;46:1344–1352. doi: 10.1111/j.1471-4159.1986.tb01745.x. [DOI] [PubMed] [Google Scholar]
- MULLER W.E., ECKERT G.P., ECKERT A. Piracetam: novelty in a unique mode of action. Pharmacopsychiatry. 1999;32 (Suppl 1):2–9. doi: 10.1055/s-2007-979230. [DOI] [PubMed] [Google Scholar]
- MULLER W.E., KOCH S., SCHEUER K., ROSTOCK A., BARTSCH R. Effects of piracetam on membrane fluidity in the aged mouse, rat, and human brain. Biochem. Pharmacol. 1997;53:135–140. doi: 10.1016/s0006-2952(96)00463-7. [DOI] [PubMed] [Google Scholar]
- NAFTALIN R.J., CUNNINGHAM P., AFZAL-AHMED I. Piracetam and TRH analogues antagonise inhibition by barbiturates, diazepam, melatonin and galanin of human erythrocyte D-glucose transport. Br. J. Pharmacol. 2004;142:594–608. doi: 10.1038/sj.bjp.0705798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEBOT C., MOUTET M., HUET P., XU J.Z., YADAN J.C., CHAUDIERE J. Spectrophotometric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol. Anal. Biochem. 1993;214:442–451. doi: 10.1006/abio.1993.1521. [DOI] [PubMed] [Google Scholar]
- OCHIAI T., OHNO S., SOEDA S., TANAKA H., SHOYAMA Y., SHIMENO H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of alpha-tocopherol. Neurosci. Lett. 2004;362:61–64. doi: 10.1016/j.neulet.2004.02.067. [DOI] [PubMed] [Google Scholar]
- O'NEILL M.J., BLEAKMAN D., ZIMMERMANN D.M., NISENBAUM E.S. AMPA receptor potentiators for the treatment of CNS disorders. Curr. Drug Targets. 2004;3:181–194. doi: 10.2174/1568007043337508. [DOI] [PubMed] [Google Scholar]
- PAGLIA D.E., VALENTINE W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- PARKER W.D., JR, MAHR N.J., FILLEY C.M., PARKS J.K., HUGHES D., YOUNG D.A., CULLUM C.M. Reduced platelet cytochrome c oxidase activity in Alzheimer's disease. Neurology. 1994a;44:1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- PARKER W.D., JR, PARKS J., FILLEY C.M., KLEINSCHMIDT-DEMASTERS B.K. Electron transport chain defects in Alzheimer's disease brain. Neurology. 1994b;44:1090–1096. doi: 10.1212/wnl.44.6.1090. [DOI] [PubMed] [Google Scholar]
- PELSMAN A., HOYO-VADILLO C., GUDASHEVA T.A., SEREDENIN S.B., OSTROVSKAYA R.U., BUSCIGLIO J. GVS-111 prevents oxidative damage and apoptosis in normal and Down's syndrome human cortical neurons. Int. J. Dev. Neurosci. 2003;21:117–124. doi: 10.1016/s0736-5748(03)00031-5. [DOI] [PubMed] [Google Scholar]
- PEUVOT J., SCHANCK A., DELEERS M., BRASSEUR R. Piracetam-induced changes to membrane physical properties. A combined approach by 31P nuclear magnetic resonance and conformational analysis. Biochem. Pharmacol. 1995;50:1129–1134. doi: 10.1016/0006-2952(95)00225-o. [DOI] [PubMed] [Google Scholar]
- RAUCA C., JANTZE H., KRUG M. Does fucose or piracetam modify the effect of hypoxia preconditioning against pentylenetetrazol-induced seizures. Brain Res. 2000;880:187–190. doi: 10.1016/s0006-8993(00)02743-8. [DOI] [PubMed] [Google Scholar]
- SALETU B., GRUNBERGER J., CEPKO H. Pharmacokinetic and -dynamic studies in elderlies with a potential antihypoxidotic/nootropic drug tenilsetam utilizing pharmaco-eeg and psychometry. Drug Dev. Res. 1986;9:95–113. [Google Scholar]
- SALETU B., HITZENBERGER G., GRUNBERGER J., ANDERER P., ZYHLARZ G., LINZMAYER L., RAMEIS H. Double-blind, placebo-controlled, pharmacokinetic and -dynamic studies with 2 new formulations of piracetam (infusion and sirup) under hypoxia in man. Int J. Clin. Pharmacol. Ther. 1995;33:249–262. [PubMed] [Google Scholar]
- SANTENS P., DE BLEECKER J., GOETHALS P., STRIJCKMANS K., LEMAHIEU I., SLEGERS G., DIERCKX R., DE REUCK J. Differential regional cerebral uptake of (18)F-fluoro-2-deoxy-D-glucose in Alzheimer's disease and frontotemporal dementia at initial diagnosis. Eur. Neurol. 2001;45:19–27. doi: 10.1159/000052084. [DOI] [PubMed] [Google Scholar]
- SARTI P., GIUFFRE A., FORTE E., MASTRONICOLA D., BARONE M.C., BRUNORI M. Nitric oxide and cytochrome c oxidase: mechanisms of inhibition and NO degradation. Biochem. Biophys. Res. Commun. 2000;274:183–187. doi: 10.1006/bbrc.2000.3117. [DOI] [PubMed] [Google Scholar]
- SASTRE J., PALLARDO F.V., VINA J. The role of mitochondrial oxidative stress in aging. Free Radic. Biol. Med. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- SCHEUER K., ROSTOCK A., BARTSCH R., MULLER W.E. Piracetam improves cognitive performance by restoring neurochemical deficits of the aged rat brain. Pharmacopsychiatry. 1999;32 (Suppl 1):10–16. doi: 10.1055/s-2007-979231. [DOI] [PubMed] [Google Scholar]
- SCHUESSEL K., LEUTNER S., CAIRNS N.J., MULLER W.E., ECKERT A. Impact of gender on upregulation of antioxidant defence mechanisms in Alzheimer's disease brain. J. Neural Transm. 2004;111:1167–1182. doi: 10.1007/s00702-004-0156-5. [DOI] [PubMed] [Google Scholar]
- SCHUESSEL K., SCHAFER S., BAYER T.A., CZECH C., PRADIER L., MULLER-SPAHN F., MULLER W.E., ECKERT A. Impaired Cu/Zn-SOD activity contributes to increased oxidative damage in APP transgenic mice. Neurobiol. Dis. 2005;18:89–99. doi: 10.1016/j.nbd.2004.09.003. [DOI] [PubMed] [Google Scholar]
- STOLL L., SCHUBERT T., MULLER W.E. Age-related deficits of central muscarinic cholinergic receptor function in the mouse: partial restoration by chronic piracetam treatment. Neurobiol. Aging. 1992;13:39–44. doi: 10.1016/0197-4580(92)90006-j. [DOI] [PubMed] [Google Scholar]
- SZELIES B., MIELKE R., KESSLER J., HEISS W.D. Restitution of alpha-topography by piracetam in post-stroke aphasia. Int. J. Clin. Pharmacol. Ther. 2001;39:152–157. doi: 10.5414/cpp39152. [DOI] [PubMed] [Google Scholar]
- VALZELLI L., BERNASCONI S., SALA A. Piracetam activity may differ according to the age of the recipient mouse. Int. Pharmacopsychiatry. 1980;15:150–156. doi: 10.1159/000468431. [DOI] [PubMed] [Google Scholar]
- VIEIRA H., KROEMER G. Mitochondria as targets of apoptosis regulation by nitric oxide. IUBMB Life. 2003;55:613–616. doi: 10.1080/15216540310001639652. [DOI] [PubMed] [Google Scholar]
- WAEGEMANS T., WILSHER C.R., DANNIAU A., FERRIS S.H., KURZ A., WINBLAD B. Clinical efficacy of piracetam in cognitive impairment: a meta-analysis. Dement. Geriatr. Cogn. Disord. 2002;13:217–224. doi: 10.1159/000057700. [DOI] [PubMed] [Google Scholar]
- WEI Y.H., LEE H.C. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp. Biol. Med. (Maywood) 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]