Abstract
Because of expanding markets for high-value niche crops, opportunities have increased for the production of medicinal herbs in the USA. An experiment was conducted in 2001 and 2002 near Gilbert, IA, to study crop performance, weed suppression, and environmental conditions associated with the use of several organic mulches in the production of two herbs, catnip (Nepeta cataria L.) and St. John’s wort (Hypericum perforatum L. ‘Helos’). Treatments were arranged in a completely randomized design and included a positive (hand-weeded) control, a negative (nonweeded) control, oat straw, a flax straw mat, and a nonwoven wool mat. Catnip plant height was significantly greater in the oat straw than the other treatments at 4 wk through 6 wk in 2001; at 4 to 8 wk in 2002, catnip plant height and width was significantly lower in the negative control compared with the other treatments. Catnip yield was significantly higher in the flax straw mat than all other treatments in 2001. In 2002, St. John’s wort yields were not statistically different in any treatments. All weed management treatments had significantly fewer weeds than the non-weeded rows in 2002. Total weed density comparisons in each crop from 2 yr showed fewer weeds present in the flax straw and wool mat treatments compared with positive control plots. There was no significant weed management treatment effect on the concentration of the target compounds, nepetalactone in catnip and pseudohypericin–hypericin in St. John’s wort, although there was a trend toward higher concentrations in the flax straw treatment.
THE RISE IN INPUT COSTS for commodity crops may provide incentives for farmers to consider alternative cropping systems. An excellent opportunity exists for high-value niche crops, such as medicinal herbs in the USA, where an estimated 7600 ha of culinary and medicinal herbs were produced in 2001, with 33.3% of these acres under organic production (USDA/ERS, 2002). Concomitant with this growth is considerable pressure for growers to upgrade agricultural methods and improve management techniques to increase herb crop yields and quality (Janick and Simon, 1993; Hay and Waterman, 1993; Omidbaigi and Hornok, 1992; Pank, 1992; Simon et al., 1984, 1987; Weiss, 1997). Market demand for catnip medicinal, feline product, and insecticidal uses has increased in recent years (Peterson and Coats, 2001), along with St. John’s wort for antiviral and antidepressive properties (Bourrel et al., 1993; Cohen et al., 1996; Diwu, 1995; Prince et al., 2000; Simon et al., 1984 Tyler, 1999). Nepetalactone in catnip and pseudohypericin–hypericin in St. John’s wort are key biochemical constituents used in determining herbal quality. Information on cultural methods related to plant growth, yield, and chemical composition of botanical crops is very limited (Li, 1998), with weed management a key concern for organic growers in the USA (Walz, 1999).
The demand for integrated weed management approaches has resulted from increasing energy, labor, and material costs associated with weed control practices in conventional cropping systems (Clements et al., 1995; Feldman et al., 2000). Degraded herbicide compounds have also been detected in surface and ground waters at amounts exceeding maximum contamination levels established by the USEPA (Goolsby et al., 1991; Kolpin et al., 1997), generating an interest in alternative strategies. Economically and environmentally sustainable weed control alternatives, such as nonsynthetic or “natural” mulches, can provide many benefits, including weed suppression and delayed weed seed emergence (Teasdale and Mohler, 1993); soil moisture conservation and improved water infiltration (Hoyt and Hargrove, 1986); enhanced soil stabilization, soil porosity, water holding capacity, microbial population activity, and cation exchange capacity (Abdul-Baki and Teasdale, 1993; Cavigelli et al., 1998; Feldman et al., 2000); and decreased plant disease (Gleason et al., 2001).
Synthetic mulches, constructed from petroleum-based materials, have been used extensively in agriculture, but problems with these materials include increased runoff volume compared with living mulches (Rice et al., 2001), disposal and landfill concerns, and their restriction in “certified organic” production as a long-term management strategy (USDA/AMS, 2002). Natural mulches include fibers or residues from plants or animals, such as straw mulch, flax straw mats, and nonwoven wool mats. Flax straw mats were shown to suppress all weed growth in herb plantings (R.C Smith, North Dakota State Univ. Dep. of Plant Sciences, Fargo, ND, 2001, personal communication), and wool mats increased crop yields, plant biomass, and weed suppression in strawberry (Fragaria × ananassa Duch.), tomato (Lycopersicon esculentum Mill.), and apple (Malus domestica Borkh.) production (Hoover et al., 2000; Poppe et al., 2001; Forcella et al., 2003).
Our study examines in detail the effects of three alternative weed management treatments (oat straw, wool mat, and flax straw mat) on crop growth, yield, and weed suppression in a perennial herb production system, compared with hand-weeded, positive and nonweeded, negative controls. Other environmental effects, including soil temperature and moisture, were evaluated under the mulched and nonmulched treatments. Additionally, we compared the effect of the weed management treatments on the concentration of two target herbal compounds in catnip and St. John’s wort. The objective of our study was to determine whether the use of a specific mulch treatment would enhance crop performance and biochemical constituents, while suppressing weed populations below economic threshold levels.
MATERIALS AND METHODS
Field Site
The field study was conducted in 2001 and 2002 on uniformly sloped Nicollet fine sandy loam soil at the Iowa State University Horticulture Research Station, in Gilbert, IA, (USDA hardiness zone 5a; 42°3′ N lat.). The experimental site was previously in an alfalfa-grass fallow for >5 yr and managed according to organic certification standards (IDALS, 1999). Field dimensions were 36 by 29 m. Forty plots containing a total of 2176 plants were arranged in a completely randomized design in weed management-herb species combinations. Five weed management treatments were imposed on two medicinal herb species, catnip and St. John’s wort (Helos). Treatments were replicated four times and consisted of the following: a nonwoven wool mat (0.6-cm thickness) (Hobbs Bonded Fibers, Waco, TX), a flax straw mat (1.2-cm thickness) (FlaxTech LLC, Rock Lake, ND), organic oat straw (from oat fields at the certified organic Iowa State University Neely-Kinyon Research Farm at Greenfield, IA), a hand-weeded (positive) control, and a nonweeded (negative) control. Each experimental unit consisted of three rows of plants, 0.9 m apart, with the two outer rows serving as buffers. There were 17 plants per row, spaced 46 cm apart.
Herb Culture
Catnip (common variety) and St. John’s wort Helos seeds (Johnny’s Selected Seeds, Albion, ME) were sown in plastic trays in a commercial germination mix (Beautiful Land Products, West Branch, IA) on 10 Mar. 2001. Soil was moistened and catnip seed trays were placed in the Iowa State Univ. Horticulture Dep. greenhouse and maintained at 18°C. The St. John’s wort trays were moistened and kept in the dark in 4°C refrigeration for 5 d, after which time they were moved to the greenhouse with natural light and ambient temperature (18°C). Every 4 wk the plants were fertilized at a rate of 4 mL L−1 with commercial fish emulsion containing nitrogen, phosphate, and potash (2-4-0.5) (Bonide Products, Inc., Yorkville, NY). Four-week-old plants were transplanted into 4- by 4-cm cells containing commercial organic potting mix (Beautiful Land Products, West Branch, IA), and 3 wk later into 6- by 6-cm Fertil Pots (Carlin Horticulture Supplies, Milwaukee, WI).
Catnip and St. John’s wort plants that were 7 to 9 cm in height were transplanted into the Gilbert field on 13 June 2001 with a one-row mechanical transplanter. All plots were manually mulched with their respective treatments on 14 June, and a knife was used to cut holes into the mats for placement of the herbs. Both flax straw and wool mats were tacked down with 15-cm landscape staples. The oat straw mulch was layered to a 10-cm depth. All three mulch treatments were centered over the planted rows to provide a 46-cm weed management zone, 23 cm on each side of the herb plants.
Each row of the positive control plots was hand-weeded within the same dimensions of the weed management zone (46-cm by 7.8-m row length) every 2 wk during the growing season. Mechanical cultivation between the mulched areas of the rows (46 cm between each mulch treatment) occurred 2 and 8 wk after transplanting in 2001, and mid-May in 2002. Irrigation from overhead risers was applied immediately after transplanting and as needed during the first two months of establishment. The flax straw mat and wool mat remained in place throughout the 2 yr of the experiment, while an additional amount of oat straw was added 10 May 2002 to maintain a 10-cm depth for the 2002 growing season.
Data Collection
All plant and soil data were collected from the 46-cm weed management zone of the middle row of each experimental unit. Four randomly selected plants in each experimental unit were assessed for plant height and width. Measurements were taken on 10 July, 24 July, 8 Aug., and 28 Aug. 2001 and 17 May and 14 June 2002. On 14 Sept. 2001 (in the 50% bloom stage) four catnip plants in each plot were manually harvested by cutting the upper 15 to 16 cm of each shoot on the plant, according to protocols established by the herb industry (FNP, 2000; R. Soberg, Organic Herb Producer Cooperative, Lakeville, MN, 2001, personal communication). The plants were placed in individual nylon mesh bags and fresh weights were immediately obtained. Plants in bags were dried at 30 to 32°C for 72 h (FNP, 2000), and dry weights were recorded. A second catnip harvest occurred on 28 June 2002 (in the 25% bloom stage) by cutting and drying eight plants per experimental unit in the aforementioned manner. The St. John’s wort plants were manually harvested 17 June 2002 by cutting the upper 15 cm of each budding and flowering shoot (collectively known as flowering tops) on the plant. The harvested plant material was processed by the same methods as the catnip but dried at 37 to 38°C for 5 d (Porter et al., 1998; Buter et al., 1998). The dried plant material was stored in the dark at room temperature (24°C) until subsequent chemical analysis.
Weeds were counted in 2001 at 3, 8, and 12 wk after the initiation of the experiment and again in 2002 at 6, 11, and 16 wk after the herbs were 9 cm in height. A 0.9-m2 quadrat was placed within the 46-cm weed management zone in three random locations along the row, and counted weeds were classified as either grass, broadleaf, or oats. In addition, at the end of each growing season, weed biomass was harvested from the 46-cm weed management zone across the entire row and a dry weight was determined for each experimental unit (Teasdale and Daughtry, 1993).
Tissue and roots samples from plants that displayed disease symptoms were submitted to the Iowa State Univ. Plant Disease Diagnostic Laboratory (Ames, IA) for analysis. On 7 June 2002, disease occurrence and severity were assessed for each plant in the middle row of each experimental unit. Numbers of diseased vs. healthy plants were recorded, along with the number of diseased vs. healthy shoots in each infected plant. On 17 June 2002 (the St. John’s wort harvest), the diseased shoots from each harvested plant were cut and bagged separately from healthy plant material.
To compare soil temperatures between the mulched and bare soil treatments, copper-constantan fixed thermocouple wires were placed 5 cm beneath the soil surface in each plot (except for the nonweeded, negative control treatments) (Teasdale and Daughtry, 1993) from 25 June to 23 Aug. 2001 and from 24 May to 26 Aug. 2002. Each wire was placed in the middle of two herb plants in the center of the weed management zone within each middle row. The temperature data were collected with a Campbell 21X Micrologger (Campbell Scientific, Inc., Logan, UT). Hourly average, daily average, and daily maximum/minimum temperatures were calculated.
Moisture readings of the top 5 cm of the soil surface were taken using a Theta Probe (meter type HH1, sensor type mL1; Delta-T Devices Ltd., Cambridge, UK). Readings were taken at both 24- and 48-h intervals after a moisture event three times each year. The probe was randomly placed near the center of the row in each treatment for each sampling. Three readings were taken during the first collection of the first year, and five readings were taken on all subsequent collections each year.
Catnip Chemical Analysis
A homogeneous 10-g sample of dried catnip leaves and flowers from each experimental unit was collected into separate 250-mL French square jars. For the second harvest of catnip, two 10-g homogeneous samples were collected and treated as replications throughout the extraction and chemical analysis. The plant material in each jar was completely submerged in acetone (approximately 210 mL) for the extraction and kept at room temperature (24°C) for 2 d. Following this period, the contents were mechanically shaken for 2 min and then gravity-filtered with Whatman #1 15-cm filter paper. The final volume was recorded and the filtrate was stored in the dark at 3°C until subsequent chemical analysis.
Nepetalactone levels in the catnip plant material were determined by a Varian 3700 GC (Walnut Creek, CA), equipped with a flame ionization detector and employing a 15-m DB5 phase capillary column (Supelco, Bellefonte, PA). The injection volume of each sample was 1 μL. The temperature program started at 100°C, ramped at 15°C min−1 until 220°C, and was held there for 8 min. The extraction and analysis of the first harvest occurred on 18 Jan. and 28 Feb. 2002, respectively. The extraction and analysis of the second harvest occurred on 15 July and 17 Sept. 2002, respectively.
St. John’s Wort Chemical Analysis
The extraction of the St. John’s wort plants occurred on 25 to 26 Sept. 2002. The upper 5 cm of the dried flowering tops were ground and sifted through a US #40 mesh screen. Two homogeneous 10-g samples were collected to replicate each treatment. A representative 1-g subsample from each sample was placed in a 250-mL round bottom flask, and 20 mL of acetone and 2 mL of 0.1 M NaOH were added to each flask. These flasks were individually wrapped with flame retardant material to protect the St. John’s wort extract from light and refluxed for 30 min. The flask contents were gravity filtered by means of GF-A 70-mm glass fiber filter paper and rinsed twice with 5 mL of acetone. The filter paper and extractant were returned to the boiling flask, and the extraction, filtration, and rinses were repeated. Four milliliters of 0.1 M HCl was added to the filtrate in each flask, and a volume of 50 mL was obtained by adding acetone. The samples were then diluted four times before pseudohypericin and hypericin concentration determination. The final volume used for analysis was 2 mL. The absorption spectrum of each sample was determined with an Ultraviolet/Visible spectrometer Lambda 18 (Perkin Elmer, Norwalk, CT).
Beer-Lambert’s law [A = ecl, where A is the sample absorption, ec is the extinction coefficient, and l is the sample path-length] was used to determine the concentration of hypericin-like molecules, on the basis of an extinction coefficient of 46 000 at 596 nm. From this equation, we calculated the final concentrations of total hypericin and pseudohypericin in each sample by the following formula: Concentration (mmol mL−1) × Volume of sample (mL) = Molecular wt (mg mmol−1) = total hypericin + pseudohypericin (mg), on the basis of a molecular weight of 504 mg mmol−1.
Data Analysis
All data were analyzed by analysis of variance (SAS Inst., 2001). The biomass measurements were subjected to a repeated measures analysis. Within each year and species combinations, treatment means of herb yield, weed density, weed biomass, and soil temperature were statistically separated by Fisher’s PLSD at P ≤0.05. Within each year, species, and hour combinations, treatment means of soil moisture were statistically separated by Fisher’s PLSD at P ≤0.05.
RESULTS AND DISCUSSION
Plant Productivity
Catnip plant height was significantly greater in the oat straw treatment than the other treatments at 4 to 6 wk in the first growing season (Fig. 1). By 10 wk of growth, plant height of each herb crop was statistically equivalent among all weed management treatments. On average, catnip plants were 33% wider in the wool mat treatment than in the weeded, positive control, and St. John’s wort plants were 24% wider in the flax straw mat than the nonweeded, negative control, though no statistical differences in plant width among treatments were observed at 10 wk. At both 4 and 8 wk of the second growing season, however, catnip plant height and width was significantly lower in the nonweeded, negative control treatment than the other treatments (Fig. 2).
Fig. 1.
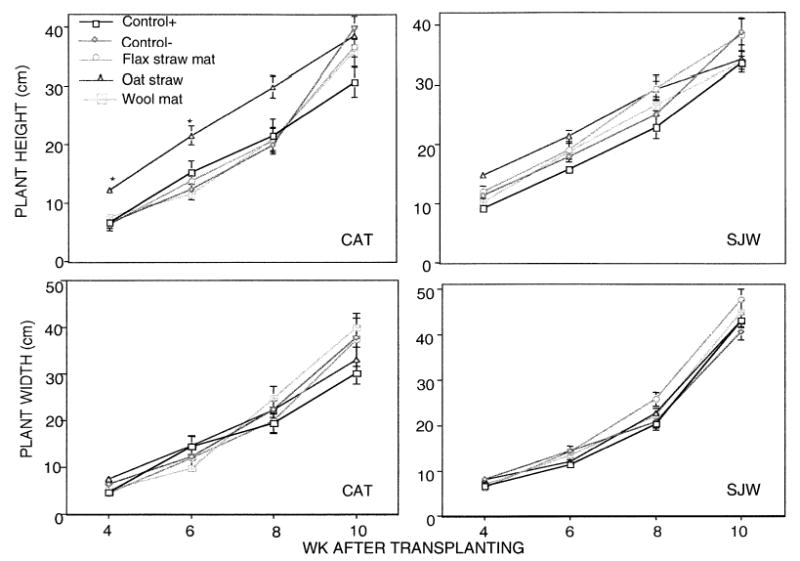
Catnip (CAT) and St. John’s wort (SJW) biomass measurements during first growing season, 2001. Significant differences between treatments within each sampling date are indicated using * to represent significance at the P = 0.05 level.
Fig. 2.

Catnip (CAT) and St. John’s wort (SJW) biomass during second growing season, 2002. Bars within the same sampling period and with the same letter above them are not significantly different at the P = 0.05 level.
This treatment effect was less apparent in the St. John’s wort plots. Catnip plant height was significantly greater in the weeded, positive control treatment than the oat straw and nonweeded treatments by 8 wk of growth. Plants were significantly wider in the weeded, positive control compared with the flax and oat straw mulches by this same time. St. John’s wort plant height was significantly greater in the flax straw mat and oat straw in 2002 than the wool mat treatment at 8 wk. St. John’s wort plant width was significantly greater in the flax straw mat than either non-mulched control treatments.
In summary, biomass comparisons between the treatments were variable, but general trends suggested that the oat straw initially increased plant growth more rapidly, and the mulches supported equivalent or greater aboveground biomass than the weeded, positive control treatment. No significant treatment × date interactions were shown in the repeated measures analysis.
Yields
Catnip yield was significantly greater in the flax straw mat than all other treatments in the first growing season (Table 1), yielding three times more dry matter than the nonweeded control treatment (920 kg ha−1 vs. 278 kg ha−1). The weeded, positive control treatment yield was equivalent to catnip yields in the oat straw and wool mat treatments. In 2002, the highest catnip yield was from the weeded control treatment (4138 kg ha−1), though this yield did not differ statistically from the flax or wool mat treatments (3647 kg ha−1 and 3578 kg ha−1, respectively). The weeded, positive control treatment mean yields were 116% and 62% greater than the non- weeded, negative control treatment in 2001 and 2002, respectively. Overall, the average yield in the second growing season was approximately six times greater than the yield in the first year (3415 kg ha−1 vs. 595 kg ha−1). When grown as a perennial crop, catnip yields are normally expected to be significantly greater in the second season. All 2002 yields compared well above the typical second season commercial yield of 1680 kg ha−1 (FNP, 2000).
Table 1.
Yields (dry matter) from catnip (CAT) and St. John’s wort (SJW) harvests, 2001 and 2002.
| CAT
|
SJW
|
||
|---|---|---|---|
| Treatment | 2001 | 2002 | 2002 |
| ———kg ha−1——— | |||
| Control + | 600b† | 4138c | 1903 |
| Control − | 278a | 2558a | 1650 |
| Flax straw mat | 920c | 3647bc | 2236 |
| Oat straw | 632b | 3154ab | 2167 |
| Wool mat | 546ab | 3578bc | 2299 |
| LSD (0.05) | 282 | 728 | NS |
Within a year, means followed by the same letter are not significantly different at P = 0.05.
There was no significant treatment effect in St. John’s wort yields, which ranged from 1650 kg ha−1 in the negative control plots to 2299 kg ha−1 in the wool mat treatment. The mean yield of the positive control treatment was 15% higher than the negative control. In a similar study, fruit yield, dry matter, and plant height of tomatoes, catnip, and St. John’s wort grown in wool mat mulch were also equivalent to hand-weeded plots (Poppe et al., 2001).
Weed Management
Weed densities in both catnip and St. John’s wort were significantly greater in the nonweeded control and oat straw mulch treatments compared with the other treatments in the first growing season (Table 2). The least amount of total weeds in 2001 in either herb crop were observed in the flax straw and wool mat treatments, though these mulches were not statistically different than the weeded control treatment. In the second year, all weed management treatments had significantly fewer weeds than the nonweeded rows, with the least number of total weeds found in the oat straw mulch in St. John’s wort (6 weeds m−2) and in the flax straw mat in catnip (7 weeds m−2). Total weed density comparisons in each crop from two growing seasons showed fewer weeds in the flax straw mat and wool mat treatments compared with the weeded control plots, even through a consecutive season of mulch use. This decrease in weed density may be positively correlated with the increased crop performance in the second growing season in these mulch treatments. Some nutrients may also have been released into the soil from the mulch fibers as they slowly decomposed.
Table 2.
Weed density and biomass response to different weed management treatments in St. John’s wort (SJW) and catnip (CAT), 2001 and 2002.
| Total density
|
Grass density
|
Broadleaf density
|
Total dry wt
|
|||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 2001 | 2002 | 2001 | 2002 | 2001 | 2002 | 2001 | 2002 |
| ———m−2——— | ———g m−2——— | |||||||
| SJW | ||||||||
| Control+ | 8b† | 26b | 6b | 19b | 1.7 | 7a | 11b | 30b |
| Control− | 19b | 232a | 18a | 225a | 1.8 | 7a | 6218a | 4194a |
| Flax straw mat | 2b | 22b | 2bc | 22b | 0 | 0b | 117b | 394b |
| Oat straw | 59a | 6b | 1c | 1b | 0.3 | 1b | 235b | 95b |
| Wool mat | 2b | 12b | 2bc | 11b | 0.5 | 1b | 618b | 376b |
| LSD (0.05) | 27 | 57 | 4.6 | 56 | NS | 4.9 | 1593 | 498 |
| CAT | ||||||||
| Control+ | 9bc | 25b | 6b | 22b | 2 | 3 | 42b | 28b |
| Control− | 31b | 376a | 30a | 372a | 1 | 5 | 7922a | 2618a |
| Flax straw mat | 2c | 7b | 1b | 4b | 1 | 3 | 70b | 84b |
| Oat straw | 71a | 18b | 2b | 2b | 0 | 4 | 998b | 142b |
| Wool mat | 4c | 16b | 4b | 15b | 0 | 1 | 256b | 211b |
| LSD (0.05) | 23 | 85 | 5.7 | 84 | NS | NS | 1026 | 572 |
Within a column, means of each species followed by the same letter are not significantly different at P = 0.05.
Volunteer oat (Avena sativa L.), germinated in the oat straw, were included in total weed density and dry weight analyses, but were not counted as a grass species in the analysis of grass vs. broadleaf weedy species (Table 2). Though these oat were the main contributing factor in the high weed density in the oat straw mulch in 2001, their effect on end-of-season weed biomass was negligible compared with the biomass of other weeds in the nonweeded control treatment. There was a significant treatment effect on total weed dry weight due to weed management treatments in both years: 2001 and 2002 end-of-season weed biomass in both herb species averaged 95% less in each respective year in the mulched treatments than in the nonweeded control treatment. Giant foxtail (Setaria faberi Herrm.) produced the greatest biomass and was the most dominant weed species each year. Other major weedy grasses included yellow foxtail (S. glauca L.), green foxtail (S. viridis L.), large crabgrass (Digitaria sanguinalis L. Scop.), witchgrass (Panicum capillare L.), and barnyardgrass (Echinochloa crusgalli L.). Broadleaf species were predominately Virginia pepperweed (Lepidium virginicum L.), common purslane (Portulaca oleracea L.), smooth groundcherry (Physalis subglabrata Mack. and Bush.), and horsenettle (Solanum carolinense L.).
In this particular field, fewer broadleaf weeds were found than grass species in both years of the experiment. Broadleaf weed densities were not significantly different among treatments in either year in catnip or in St. John’s wort in the first growing season. By 2002, however, broadleaf weed densities were significantly lower in all mulched treatments in St. John’s wort than in either control treatment. Broadleaf density averages were similar among herb species, though the number of grass weeds found in catnip was higher than St. John’s wort each growing season.
Disease Management
In April 2002, Alfalfa mosaic virus was detected in several catnip plants, and all plants displaying infected symptoms (approximately 20 plants) were removed from the experiment. Two other catnip diseases, detected one month later, were bacterial leaf spot [caused by Pseudomonas syringae pv. tabaci (Wolf & Foster) Young et al.] and Fusarium wilt (caused by Fusarium oxysporum Schlechtend.:Fr.), though no control measures were warranted in this field. The former disease may be important to producers who market catnip leaves for teas or culinary purposes, while Fusarium affects whole plants and can substantially reduce crop yields. Despite variability in disease development, there were no significant treatment effects on numbers of diseased catnip plants and disease severity (Table 3).
Table 3.
Disease occurrence and severity in catnip (CAT) and St. John’s wort (SJW) in weed management treatments during the second growing season, 7 June 2002.
| Diseased plants
|
Disease severity
|
|||
|---|---|---|---|---|
| Treatment | CAT | SJW | CAT | SJW |
| ———%——— | ||||
| Control+ | 41 | 45 | 28 | 32ab† |
| Control− | 31 | 36 | 21 | 26ab |
| Flax straw mat | 29 | 10 | 25 | 29ab |
| Oat straw | 17 | 12 | 25 | 11a |
| Wool mat | 59 | 39 | 31 | 46b |
| LSD (0.05) | NS | NS | NS | 35 |
Within a column, means followed by the same letter are not significantly different at P = 0.05.
In May 2002, symptoms of St. John’s wort anthracnose, caused by Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. in Penz. f. sp. hypericum, were observed on several St. John’s wort plants. One commercial grower in the Midwestern U.S. reported total crop destruction due to this disease in 2001 (B. Tornow, Commercial Producer, Tornow Herb Farms, Wausau, WI, 2001, personal communication). A study in eastern Canada attributed a mortality rate of 36 to 96% to this virulent fungus in noncultivated habitats of St. John’s wort (Morrison et al., 1998). In at least one instance, anthracnose was reported to have spread more quickly in St. John’s wort plants growing in plastic mulch (Schooley, 2001). Therefore, disease development was closely monitored in the mulched and non-mulched treatments. Before harvest, disease severity was significantly lower in the oat straw than the wool mat treatment (Table 3). Straw mulches have been reported to slow down the spread of disease in cultivation, such as Colletotrichum acutatum J.H. Simmonds in strawberry production (Gleason et al., 2001), though the cause of inhibition is not clearly understood.
Environmental Effects
Soil moisture levels among mulch treatments were variable across seasons and herb crops. Soil moisture levels in the top 5 cm of the soil surface were significantly higher under the flax straw mat compared with the wool mat both 24 and 48 h in catnip rows in 2001 (Table 4). The oat straw mulch retained significantly higher soil moisture values after 48 h in St. John’s wort than either the flax straw or wool mats in 2001. Among mulch treatments, there were no significant differences in any soil moisture comparisons of either herb crop in 2002.
Table 4.
Soil moisture content 0 to 5 cm below the soil surface, separated by growing season and interval after a moisture event in St. John’s wort (SJW) and catnip (CAT).
| 2001
|
2002
|
|||
|---|---|---|---|---|
| Treatment | 24 h | 48 h | 24 h | 48 h |
| ———%——— | ||||
| SJW | ||||
| Control+ | 25.3b† | 22.3c | 30.3 | 23.7b |
| Control− | 23.6b | 20.6c | 29.3 | 22.6b |
| Flax straw mat | 28.9a | 26.7b | 29.8 | 27.1a |
| Oat straw | 30.0a | 29.5a | 30.3 | 26.3a |
| Wool mat | 27.9a | 25.6b | 30.6 | 27.4a |
| LSD (0.05) | 2.3 | 1.9 | NS | 1.6 |
| CAT | ||||
| Control+ | 26.5c | 23.9c | 30.9 | 25.4b |
| Control− | 25.4c | 22.9c | 30.4 | 23.7c |
| Flax straw mat | 30.4a | 28.4a | 31.0 | 27.9a |
| Oat straw | 29.5ab | 27.8ab | 29.8 | 27.3a |
| Wool mat | 27.7bc | 26.1b | 31.1 | 27.1a |
| LSD (0.05) | 2.4 | 2.1 | NS | 1.5 |
Within a column, means of each species followed by the same letter are not significantly different at P =0.05.
Comparing between herb species, average soil moisture levels of all treatments were 1% lower in St. John’s wort than in catnip. This difference may be attributed to root architecture and water requirements of each species. Though higher soil moisture levels were retained under all mulches compared with nonmulched treatments 48 h after precipitation events, disease manifestation was not significantly greater in the mulched treatments. There was a trend toward greater soil moisture loss in the nonweeded versus weeded control treatments in all factor comparisons, though levels were not statistically different. This difference may be due to greater competition for resources among higher densities and greater biomass of weeds.
Daily average and daily maximum soil temperatures were significantly reduced under the oat straw and flax straw mat treatments compared with the hand-weeded treatment in both species each year (Table 5). The wool mat also significantly reduced daily average and daily maximum soil temperatures compared with the positive control treatment in the catnip plots in 2001 and 2002. Overall, soil temperatures beneath the mulches increased in the following order: oat straw < flax straw mat < wool mat. The warmer daily average and daily maximum soil temperatures under the wool mat may be related to the dark mottled black/gray color of the fabric, which may help retain more heat. During the 2001 growing season, significantly warmer daily minimum soil temperatures were detected beneath the flax straw mat than the other mulch treatments in St. John’s wort plots. However, no statistical differences were found in daily minimum soil temperatures in any other treatment comparisons between growing seasons or crops. It cannot be ascertained from this data if either the wool mat or the flax straw mat is a better insulator.
Table 5.
Mean soil temperatures 5 cm below the soil surface of several weed management treatments in St. John’s wort (SJW) and catnip (CAT), taken by fixed thermocouples in 2001 and 2002.
| Daily average
|
Daily maximum
|
Daily minimum
|
||||
|---|---|---|---|---|---|---|
| Treatment | 2001 | 2002 | 2001 | 2002 | 2001 | 2002 |
| ———°C——— | ||||||
| SJW | ||||||
| Control+ | 26.6a† | 24.0a | 34.8a | 29.1a | 20.7c | 20.5a |
| Flax straw mat | 24.9bc | 23.2b | 28.3c | 27.7b | 21.7b | 20.1a |
| Oat straw | 24.3c | 22.4c | 28.3c | 25.1c | 21.3a | 20.3a |
| Wool mat | 25.1b | 24.4a | 30.0b | 29.5a | 21.3a | 20.7a |
| CAT | ||||||
| Control+ | 25.9a | 23.6a | 32.4a | 29.3a | 21.0a | 19.8a |
| Flax straw mat | 25.2b | 23.0b | 29.4b | 26.9b | 21.6a | 20.1a |
| Oat straw | 23.5c | 22.6b | 26.5c | 25.5c | 21.2a | 20.3a |
| Wool mat | 25.2b | 23.0b | 30.1b | 26.9b | 21.3a | 20.1a |
Within a column, means of each species followed by the same letter are not significantly different at P =0.05.
Comparing diurnal fluctuation patterns underneath the mulched and bare soils on 8 Aug. 2001, the soil temperature was 5 to 7°C lower in the mulch treatments during the warmest hour of the day (Fig. 3). The smallest soil temperature amplitude was found underneath the oat straw mulch, whereas the largest amplitude was recorded under the weeded control treatment. A phase shift was also apparent for the soil temperatures under the flax straw and wool mats, with soil temperatures reaching maximum levels an hour later in the day than the nonmulched and oat straw treatments. Because of lower daily average and daily maximum temperatures in the soil beneath them, these mulches may reduce the germination of weed seeds that have a diurnal temperature fluctuation requirement. This requirement was demonstrated by Taylorson (1987), who showed that some weed seeds will not germinate without a 10°C diurnal change. These findings offer one explanation for the lower weed densities reported in the mulch treatments.
Fig. 3.

Soil temperature diurnal fluctuation 5 cm below the soil surface in each weed management treatment of catnip and St. John’s wort plants combined, 8 August 2001. Thermocouples were placed midrow and centered between plants.
Herbal Quality Analysis
There was no significant treatment effect on the concentrations of the target compounds, nepetalactone (NEP), pseudohypericin (PHYP) or hypericin (HYP) (Table 6). The greatest quantity of nepetalactone was detected in the herbs harvested in the second growing season (2002). The trend that year showed higher concentrations of each target compound in the flax straw mat treatment (NEP = 11.8, PHYP = 0.83 and HYP = 0.21 mg g−1) compared with the average concentration over all other treatments (NEP = 9.4 mg g−1, PHYP = 0.63 mg g−1, HYP = 0.52 mg g−1).
Table 6.
Concentrations of selected secondary metabolites from dried plant material of catnip (nepetalactone) and St. John’s wort (hypericin and pseudohypericin) harvests, 2001 and 2002.
| Nepetalactone
|
Hypericin
|
Pseudohypericin
|
||
|---|---|---|---|---|
| Treatment | 2001 | 2002 | 2002 | 2002 |
| ———mg g−1——— | ||||
| Control+ | 2.3 | 9.1 | 0.16 | 0.64 |
| Control− | 2.5 | 9.6 | 0.14 | 0.55 |
| Flax straw mat | 2.5 | 11.8 | 0.21 | 0.83 |
| Oat straw | 2.4 | 9.2 | 0.16 | 0.66 |
| Wool mat | 2.4 | 9.8 | 0.17 | 0.67 |
| LSD | NS | NS | NS | NS |
Within years and individual compound, means were not significantly different at P = 0.05.
Nepetalactone levels in the catnip were approximately four times higher in 2002 than in 2001. This result may be correlated with the later chemical extraction of the dried catnip leaves and flowers after the first growing season compared with the second season (4 mo as opposed to 1 mo, respectively). Moreover, the catnip plants were harvested at a late developmental stage in 2001 (seed formation), rather than at the early to midbloom stage (20%) established in herb industry protocols (FNP, 2000). Highest lactone and essential oil yield and quality have been reported in catnip harvested at full bloom (Bourrel et al., 1993; Chalchat and Lamy, 1997; Ibrahim and El Din, 1999). Essential oils are comprised of volatile, aromatic compounds, and will be less concentrated over time in plant matter. Because of reported variation in nepetalactone concentration in essential oil analysis of catnip (Ibrahim and El Din, 1999; Malizia et al., 1996; Pappas, 2001), oil content in each treatment could not be accurately calculated on the basis of the detected nepetalactone concentrations only in this experiment. Quality assurance specifications from a Midwest herb cooperative include a minimum 3 mg g−1 (oil content in leaves and flowers, but no standards for nepetalactone have been developed; FNP, 2000).
The hypericin and pseudohypericin concentrations in St. John’s wort plants from each weed management treatment fell within reported ranges of hypericin from whole plants with flowers reported by Benigni et al. (1971) and by Briskin et al. (2000); Jensen et al. (1995 and 2000); and Kirakosyan et al. (2000). Plants grown in the flax mat treatments tended to exhibit greater levels of hypericin and pseudohypericin (Table 6), approximating the minimum hypericin concentration of 0.25 mg g−1 required for herbal extracts for a Midwest herb cooperative (Letchworth, B. Personal communication. Commodity Manager. Frontier Natural Products, Norway, IA, 2002). This effect was correlated with the trend toward more luxuriant growth in the St. John’s wort plants grown with flax mats.
CONCLUSIONS
The oat straw, flax straw mat, and wool mat mulches evaluated in this study effectively suppressed weed populations below economic threshold levels. Crop growth and yield in the flax straw and wool mat mulches were significantly greater or comparable to yield from hand-weeded plots over two growing seasons. Similar yield responses and weed suppression were noted in wool-mulched plots compared with hand-weeded plots in strawberry field production (Forcella et al., 2003). Temperature modification, as a result of mulching, was associated with optimum herb plant productivity, in contrast to reducing crop seedling growth from cool, moist conditions in the spring (Bristow, 1988). Lower diurnal soil temperature fluctuations (Teasdale and Mohler, 1993) contributed to reduced weed establishment in mulch treatments. Though initial purchasing and implementation costs may be higher than on-farm sources of grain straw, flax and wool mulches offer farmers an environmentally sustainable alternative to hand-weeding, synthetic mulches, and herbicides. Enhanced crop performance, effective weed suppression, and enhanced bioactive constituents in the case of hypericin in St. John’s wort extracts support the use of natural mulches as weed management alternatives to manual labor and herbicides.
References
- Abdul-Baki AA, Teasdale JR. A no-tillage tomato production system using hairy vetch and subterranean clover mulches. HortScience. 1993;28:106–108. [Google Scholar]
- Benigni R, Capra C, Cattorin PE. Inverni & Della Beffa; Milano, Italy: 1971. Hypericum. Plante medicinali: Chimica, farmacologia e terapia. [Google Scholar]
- Bourrel C, Perineau C, Michel G, Bessiere JM. Catnip (Nepeta cataria L.) essential oil: Analysis of chemical constituents, bacteriostatic and fungistatic properties. J Essent Oil Res. 1993;5:159–167. [Google Scholar]
- Briskin DP, Leroy A, Gawienowski M. Influence of nitrogen on the production of hypericins by St. John’s wort. Plant Physiol Biochem. 2000;38(5):413–420. [Google Scholar]
- Bristow KL. The role of mulch and its architecture in modifying soil temperature. Aust J Soil Res. 1988;26:269–280. [Google Scholar]
- Buter B, Orlacchio C, Soldati A, Berger K. Significance of genetic and environmental aspects in the field cultivation of Hypericum perforatum. Planta Med. 1998;64:431–437. doi: 10.1055/s-2006-957475. [DOI] [PubMed] [Google Scholar]
- Cavigelli MA, Deming SR, Probyn LK, Harwood RR, editors. Michigan State Univ. Extension Bulletin E-2646; East Lansing, MI: 1998. Michigan Field Crop Ecology: Managing biological processes for productivity and environmental quality. [Google Scholar]
- Chalchat JC, Lamy J. Chemical composition of the essential oil isolated from wild catnip Nepeta cataria L. cv. Citriodora from the Drôme region of France. J Essent Oil Res. 1997;9:527–532. [Google Scholar]
- Clements DR, Weise SF, Brown R, Stonehouse DP, Hume DJ, Swanton CJ. Energy analysis of tillage and herbicide inputs in alternative weed management systems. Agric Ecosyst Environ. 1995;52:119–128. [Google Scholar]
- Cohen PA, Hudson JB, Towers GHN. Antiviral activities of anthraquinones, bianthrones, and hypericin derivatives from lichens. Experientia. 1996;52:180–183. doi: 10.1007/BF01923366. [DOI] [PubMed] [Google Scholar]
- Diwu Z. Novel therapeutic and diagnostic applications of hypocrellins and hypericins. Photochem Photobiol. 1995;61:529–539. doi: 10.1111/j.1751-1097.1995.tb09903.x. [DOI] [PubMed] [Google Scholar]
- Feldman RS, Holmes CE, Blomgren TA. Use of fabric and compost mulches for vegetable production in a low tillage, permanent bed system: Effects on crop yield and labor. Am J Altern Agric. 2000;15(4):146–153. [Google Scholar]
- Forcella F, Poppe SR, Hansen NC, Head WA, Hoover E, Propsom F, McKensie J. Biological mulches for managing weeds in transplanted strawberry (Fragaria × ananassa) Weed Technol. 2003;17:782–787. [Google Scholar]
- Frontier Natural Products (FNP) FNP; Norway, IA: 2000. Catnip grower’s crop monograph. [Google Scholar]
- Gleason M, Wegulo S, Nonnecke G. Iowa State Univ.; Ames, IA: 2001. Efficacy of straw mulch for suppression of anthracnose on day-neutral strawberries. ISU Ext. FG-601:48. [Google Scholar]
- Goolsby DA, Thurman EM, Koplin DW. Geographic and temporal distribution of herbicides in surface waters of the upper Midwestern United States, 1989–90. In: Mallard GE, Aronson DA, editors. U.S. Geological Survey Toxic Substances Hydrology Program. Proc. Technical Meeting; Monterey, CA: 1991. pp. 183–188. 11–15 Mar. 1991. U.S. Geological Survey Water-Resources Investigations Report 91–4034. [Google Scholar]
- Hay R, Waterman P, editors. Longman, Essex; England: 1993. Volatile oil crops: Their biology, biochemistry and production. [Google Scholar]
- Hoover E, Propsom F, Poppe S, Forcella F, Hansen N, Head B, Jacobson B. Greenbook 2000. Energy and Sustainable Agriculture Program. Minnesota Dep. of Agriculture; St. Paul, MN: 2000. Bio-based weed control in strawberries using sheep wool mulch, canola mulch, and canola green manure. [Google Scholar]
- Hoyt GD, Hargrove WL. Legume cover crops for improving crop and soil management in the southern United States. Hort-Science. 1986;23(3):397–402. [Google Scholar]
- Ibrahim ME, El Din AAE. Cultivation of Nepeta cataria L. in Egypt: Its growth, yield, and essential oil content as influenced by some agronomic practices. Egypt J Hortic. 1999;26(3):281–302. [Google Scholar]
- Iowa Dep. of Agriculture and Land Stewardship (IDALS) Iowa Organic Certification and Organic Standards; Des Moines, IA: 1999. [Google Scholar]
- Janick J, Simon JE, editors. John Wiley and Sons; New York: 1993. Progress in new crops. [Google Scholar]
- Jensen AG, Cornett C, Gudiksen L, Hansen SH. Characterization of extracts of Hypericum perforatum L. using an online HPLC system with UV/visible and fluorescence detection prior to and after photochemical conversion of the effluent. Phytochem Anal. 2000;11:387–394. [Google Scholar]
- Jensen KIN, Gaul SO, Specht EG, Doohan DJ. Hypericin content of Nova Scotia biotypes of Hypericum perforatum L. Can J Plant Sci. 1995;75:923–926. [Google Scholar]
- Kirakosyan A, Hayashi H, Inoue K, Charchoglyan A, Vardapetyan H. Stimulation of the production of hypericins by mannan in Hypericum perforatum shoot cultures. Phytochemistry. 2000;53:345–348. doi: 10.1016/s0031-9422(99)00496-3. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Kalkhoff SJ, Goolsby DA, Sneck-Fahrer DA, Thurman EM. Occurrence of selected herbicides and herbicide degradation products in Iowa’s ground water, 1995. Ground Water. 1997;35(4):679–688. [Google Scholar]
- Li T. Echinacea: Cultivation and medicinal value. HortTechnology. 1998;8:122–129. [Google Scholar]
- Malizia RA, Molli JS, Cardell DA, Retamar JA. Volatile constituents of the essential oil of Nepeta cataria L. grown in Cordoba Province (Argentina) J Essent Oil Res. 1996;8:565–567. [Google Scholar]
- Morrison KD, Reekie EG, Jensen KIN. Biocontrol of common St. Johnswort (Hypericum perforatum) with Chrysolina hyperici and a host-specific Colletotrichum gloeosporioides. Weed Technol. 1998;12:426–435. [Google Scholar]
- Omidbaigi R, Hornok L. Effect of N-fertilization on the production of fennel (Foeniculum vulgare Mill.) Acta Hortic. 1992;306:249–252. [Google Scholar]
- Pank F. The influence of chemical weed control on quality characters of medicinal and aromatic plants. Acta Hortic. 1992;306:145–154. [Google Scholar]
- Pappas RS. Essential Oil Univ.; New Albany, IN: 2001. Certificate of analysis—Pure catnip essential oil. [Google Scholar]
- Peterson C, Coats J. Insect repellents—Past, present, and future. Pesticide Outlook. 2001;12(4):154–158. [Google Scholar]
- Poppe S, Becker R, Hansen N, Solemsaas M, Fritz V, Forcella F, Wagner S, Head B, Padula B, Nennich T, Stordahl J. Greenbook 2003. Sustaining People, Land and Communities. Energy and Sustainable Agriculture Program. Minnesota Dep. of Agriculture; St. Paul, MN: 2001. Wool mulching systems for specialty crops. [Google Scholar]
- Porter B, McVicar R, Bader L. St. John’s wort in Saskatchewan. Saskatchewan Agriculture, Food, and Rural Revitalization. [verified 28 January 2004.];1998 http://www.agr.gov.sk.ca/docs/crops/special_crops/production_information/johnswort02.asp.
- Prince AM, Pascual D, Meruelo D, Liebes L, Mazur Y, Dubovi E, Mandel M, Lavie G. Strategies for evaluation of enveloped virus inactivation in red cell concentrates using hypericin. Photochem Photobiol. 2000;71(2):188–195. doi: 10.1562/0031-8655(2000)071<0188:sfeoev>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rice PJ, McConnell LL, Heighton LP, Sadeghi AJ, Isensee AR, Teasdale JR, Abdul-Baki AA, Harman-Fetcho JA, Hapeman CJ. Runoff loss of pesticides and soil: A comparison between vegetative mulch and plastic mulch in vegetable production systems. J Environ Qual. 2001;30:1808–1821. doi: 10.2134/jeq2001.3051808x. [DOI] [PubMed] [Google Scholar]
- SAS Institute. Statistical Analysis Service Institute Inc.; Cary, NC: 2001. SAS user’s guide: Statistics. Version 8.2 ed. [Google Scholar]
- Schooley J. St. John’s Wort and Chrysolina Beetles. Ontario Ministry of Agriculture, Food, and Rural Affairs. [verified 28 January 2004. >];2001 http://www.gov.on.ca/OMAFRA/english/crops/facts/info_sjwbeetles.htm.
- Simon JE, Chadwick AF, Craker LE. Archon Books; Hamden, CT: 1984. Herbs, an indexed bibliography, 1971–1980. The scientific literature on selected herbs, and aromatic and medicinal plants of the Temperate Zone. [Google Scholar]
- Simon JE, Mathe A, Craker LE, editors. Sixth International Symposium on Medicinal and Aromatic Plants. Technical Communication of the International Society for Horticultural Science. Acta Hortic. 1987;208:1–279. [Google Scholar]
- Taylorson RB. Environmental and chemical manipulation of weed seed dormancy. Rev Weed Sci. 1987;3:135–154. [Google Scholar]
- Teasdale JR, Daughtry CST. Weed suppression by live and desiccated hairy vetch (Vicia villosa) Weed Sci. 1993;41:207–212. [Google Scholar]
- Teasdale JR, Mohler CL. Light transmittance, soil temperature, and soil moisture under residue of hairy vetch and rye. Agron J. 1993;85:673–680. [Google Scholar]
- Tyler VE. Herbs affecting the central nervous system. p. 442–449. Perspectives on new crops and new uses. In: Janick J, editor. ASHS Press; Alexandria, VA: 1999. [Google Scholar]
- USDA Agriculture Marketing Service. (USDA/AMS) National Organic Program. Final rule: 7 CFR Part 205. [verified 28 January 2004.];2002 http://www.ams.usda.gov/nop.
- USDA Economic Research Service. (USDA/ERS) Organic production overview. [verified 28 January 2004.];2002 http://www.ers.usda.gov/Data/organic.
- Walz E. Organic Farming Research Foundation; Santa Cruz, CA: 1999. Final results of the third biennial national organic farming survey. [Google Scholar]
- Weiss EA. CAB International; New York: 1997. Essential oil crops. [Google Scholar]


