Abstract
Chromium speciation has attracted attention because of the different toxicity of Cr(III), which is considered relatively non-toxic, and Cr(VI), which can cross cell membranes mainly as a chromate anion and has been classified as a class I human carcinogen. The aims of the present study were to measure soluble Cr(VI) levels in environmental samples, to develop a simple method of quantifying Cr(VI) in exhaled breath condensate (EBC), and to follow the kinetics of EBC Cr(VI) in chrome plating workers.
Personal air samples were collected from 10 chrome platers; EBC was collected from the same workers immediately after the work shift on Tuesday and before the work shift on the following Wednesday. Environmental and EBC Cr(VI) levels were determined by means of colorimetry and electrothermal absorption atomic spectrometry, respectively.
The method of detecting Cr(VI) in environmental air was based on the extraction of the Cr(VI)-diphenylcarbazide (Cr(VI)–DPC) complex in 1-butanol, whereas EBC Cr(VI) was determined using a solvent extraction of Cr(VI) as an ion pair with tetrabutylammonium ion, and subsequent direct determination of the complex (Cr(VI)–DPC) in EBC.
Kinetic data showed that airborne Cr(VI) was reduced by 50% in airway lining fluid sampled at the end of exposure and that there was a further 50% reduction after about 15 h. The persistence of Cr(VI) in EBC supports the use of EBC in assessing target tissue levels of Cr(VI).
Keywords: Cr(VI), Speciation, Exhaled breath condensate, ETAAS, Chrome plating
1. Introduction
Chromium (Cr) is present in the environment in two main oxidation states: Cr(III) and Cr(VI). Whereas Cr(III) can be present in non-polluted environments, Cr(VI) is largely anthropogenic. Both forms are detectable in the workplace air of stainless steel production plants, chromium plating plants, chromium pigment factories, etc. [1]. Chromium speciation has attracted a great deal of attention because of the differential toxicity of its stable species: Cr(III) cannot usually cross cell membranes and its toxicity is considered to be relatively low [2,3], whereas Cr(VI) is transported through anion channels as chromate (CrO42−) [4], and its reduction by different intracellular components (such as glutathione, ascorbate, tocopherols and different enzyme cofactors) generates stable Cr(III) or unstable Cr(IV) and Cr(V) intermediates, all of which are capable of forming complexes with peptides, proteins and DNA, and generating oxidative stress in vitro and in vivo, though Cr(VI) oxidizing potential in humans is not known with certainty [5–8]. Cr(VI) has been recognised as a highly toxic elemental species on the basis of experimental and epidemiological evidence, and has been classified as a class I human carcinogen by the International Agency for Research on Cancer (IARC) [2].
In occupational settings, Cr(VI) exposure mainly occurs as a result of inhalation, and it is known that Cr(VI) compounds cause lung cancer [1]. The ex vivo monitoring of Cr species at the target organ level is important in occupational toxicology, because the measurement of total Cr in white and red blood cells [9] can only give information concerning systemic levels, which however reflect only exposure to soluble Cr(VI) and are influenced by absorption from all routes, including dermal exposure. Similar information can be obtained from Cr in urine, where Cr is completely reduced to the trivalent state [10]. However, the most toxic Cr species are represented by Cr(VI) compounds that are poorly absorbed and persist in the lung, where – when taken up by resident cells – can cause cancer.
We have recently demonstrated that total Cr is measurable in the exhaled breath condensate (EBC) of chrome plating workers, and that its EBC levels correlate with biomarkers of inflammation and oxidative stress, such as malondialdehyde (MDA) and hydrogen peroxide (H2O2), thus suggesting that exhaled total Cr may reflect the lung dose responsible for toxic effects on the airways [11]. There are no published data concerning the Cr(VI)/Cr(III) equilibrium in the airway lining fluid, nor any data is available concerning its reducing power, although different concentrations of individual reducing agents (glutathione, ascorbic acid) may affect the reduction of Cr(VI) to Cr(III), and thus the levels of Cr(VI) that reach pulmonary tissue [12]. The measurement of Cr(VI) in EBC, which is also a suitable fluid for assessing the lung doses and effects of cobalt and tungsten in exposed workers [13], may therefore be a useful means of following the reduction pathways of Cr at target organ level and studying the mechanisms of pulmonary Cr(VI) toxicity.
Published methods of measuring Cr(VI) in environmental and water samples include colorimetry after the complexation of chromate ions with 1,5-diphenylcarbazide (DPC) or other molecules, with and without previous extraction and pre-concentration steps [14–18], and chromatographic techniques for the separation of different oxidation forms [19–21] and stripping voltammetry [22]. Furthermore, the website of the National Institute for Occupational Safety and Health (NIOSH) shows various protocols for determining Cr(VI) in environmental air [23], and a comparison of the different analytical methods [24]. The state of the art concerning the measurement of airborne Cr(VI) compounds in workplace aerosols and related samples has recently been reviewed [25]. Cr(VI) has never been assayed in EBC after exposure to hexavalent Cr compounds.
The aims of this study were to develop a simple liquid/liquid extraction and colorimetric method for monitoring soluble Cr(VI) in environmental samples without the need for more complex and expensive separation methods such as solid phase extraction (SPE), to measure EBC Cr(VI) levels in exposed workers, and to follow the kinetics of Cr(VI) exhalation using an even more precise and sensitive method based on ion pair extraction followed by electrothermal absorption atomic spectrometry (ETAAS).
2. Experimental
2.1. Subjects
The study involved 10 workers employed in a chrome plating plant who had normal spirometric indices and did not report any significant current or past respiratory disease; none of them had experienced any symptoms of acute respiratory illness in the 4 weeks preceding the study. Their demographic and clinical data are summarised in Table 1. Two EBC samples were collected: one immediately after the end of the work shift on Tuesday evening (t0) and the other before the beginning of the work shift on Wednesday morning (t1), about 15 h after the last Cr exposure.
Table 1.
Demographic and clinical data of the study subjects
| Chrome plating workers | |
|---|---|
| Number of subjects | 10 |
| Sex (M/F) | 10/0 |
| Age (years) | 39.3 ± 7.3 |
| Smokers/ex-smokers/non-smokers | 1/0/9 |
| FVC (% predicted) | 91.2 ± 8.3 |
| FEV1 (% predicted) | 92.5 ± 9.1 |
| FEV1/FVC (%) | 83.4 ± 9.2 |
The pulmonary parameters and age are expressed as mean values ± S.D. FVC: forced expiratory vital capacity; FEV1: forced expiratory volume in 1 s.
All of the enrolled subjects gave their written informed consent to the procedures, which were approved by our local Institutional Human Ethics Committee. The biological material was sampled as laid down in the Declaration of Helsinki [26].
2.2. EBC collection
EBC was collected using a TURBO-DECCS (Transportable Unit for Research on Biomarkers Obtained from Disposable Exhaled Condensate Collection Systems) (ItalChill, Parma, Italy) as previously described [11]. Briefly, the subjects were asked to breathe tidally through the mouthpiece for 15 min, while sitting comfortably in the workplace office where the level of total Cr was below 0.1 μg/m3. The collecting temperature was −5 °C. We also measured total Cr in EBC of controls [11], but Cr levels were too low to detect the fraction of Cr(VI).
2.3. Instrumentation
Sonication was carried out in a Transsonic 460 ultrasonic bath (Elma, Singen, Germany). The solid phase (SPE) was extracted using a VacMaster™-10 SPE Manifold (IST, Glamorgan, UK) connected to a vacuum pump by means of a pressure control valve. The PTFE valve liners came from IST (Glamorgan, UK), and the strong anion-exchange solid-phase extraction (SAE-SPE) cartridges were Supelclean™ LC-Florisil SPE Tubes (6 ml) from Supelco (Bellefonte, PA, USA). A DU 640 spectrophotometer (Beckman, Fullerton, CA, USA) was used for the colorimetric measurements, and a 220 Z atomic absorption spectrometer (VARIAN, Palo Alto, USA) with Zeeman effect background correction was used to monitor Cr in the biological samples.
2.4. Reagents
The standard certified Cr(VI) solution came from Fluka (Buchs, Germany), and the standard Cr(III) atomic absorption solution from Aldrich (Milwaukee, WI, USA) as tetrabutylammonium bromide 99% (TBAB), 4-methyl-2-pentanone and nitric acid 69.5%. The 1,5-diphenylcarbazide (DPC), acetonitrile, ammonium sulphate and ammonium hydroxide (all reagent grade) came from Sigma (St. Louis, MI, USA). Hydrochloric acid 37% (HCl), 1-butanol and absolute ethanol came from Carlo Erba (Milan, Italy).
2.5. Environmental measurements
2.5.1. Environmental sample collection and extraction procedure
Ambient monitoring was carried out by means of personal samplers. Briefly, airborne particulates were collected on PVC membrane filters (5.0 μm porosity, 25 mm diameter, Supelco, Bellofonte, PA, USA) at a constant flow of 3 l/min−1 for 90–150 min in the morning or afternoon of the Tuesday work shift. The reducing power of the filters was excluded by incubating some of them with known Cr(VI) concentrations for 24 h (data not shown). The 7703 NIOSH method [23] for the extraction of total Cr by sonication of the membranes (10 ml of extraction buffer – 0.05 M ammonium sulphate, 0.05 M ammonium hydroxide – was used for every membrane) was strictly followed. During the 24 h following the environmental air collection, Cr(VI) and total Cr were, respectively, measured by means of a colorimetric method and ETAAS with Zeeman effect background correction.
2.5.2. Cr(VI) determinations
The standards were freshly prepared from a certified Cr(VI) solution after dilution with water. Moreover, adequate amounts of standards were added directly to the membrane and extracted as the samples, with a recovery close to 100%. In the original NIOSH 7703 protocol, Cr(VI) was extracted from SPE cartridges with 9 ml of elution solution (0.5 M ammonium sulphate, 0.1 M ammonium hydroxide), but better Cr(VI) recovery was obtained by slightly changing the SPE procedure to include a cartridge washing step with methanol 3 ml, the cartridge loading of 3 ml of the aqueous extraction samples obtained from membranes after sonication, and the subsequent addition of 1 ml water to clean up the impurities. Finally, Cr(VI) was extracted from the cartridge with 9 ml of buffer elution 0.01 M ammonium sulphate and 0.01 M ammonium hydroxide and 100 μl of HCl 37% and 3 ml of DPC complexation solution (25 mM of DPC in acetonitrile) were added to the SPE recovery samples. The determinations of the Cr(VI)–DPC complex were made by measuring solution absorbance at 540 nm.
The same analysis was carried out in parallel by changing the Cr(VI) colorimetric detection protocol. We found experimentally that the Cr(VI)–DPC complex tended to concentrate in some organic solvents such as 1-butanol (see Section 3), and so 9 ml of the elution solution with Cr(VI) and 3 ml of DPC standard solution (2 mM) in 1-butanol were added together with 400 μl of HCl 37%. The samples were mixed thoroughly and, after five minutes, centrifuged (2000 × g, 5 min) to separate the phases. The determinations of the Cr(VI)–DPC complex were made on the organic phase by measuring absorbance at 545 nm as the use of 1-butanol as solvent shifted the absorbance peak the complex from 540 to 545 nm.
2.5.3. Cr(VI) determinations without SPE procedures
The great affinity between the Cr(VI)–DPC complex and 1-butanol induced us to develop a simpler method of detecting Cr(VI) in environmental samples that avoided the SPE procedure. Because of the relatively high concentrations of Cr(VI) in the membranes, 1 ml of the extracted Cr(VI) was mixed directly with 1 ml of butanolic DPC solution (2 mM) without any previous extraction. In the mixing phase, we added 100–150 μl of HCl 37% and directly measured the absorbance of the organic phase at 545 nm after centrifugation at 2000 × g for 2 min.
2.6. Cr(VI) and total Cr determinations in EBC
The protocol for measuring Cr(VI) in EBC was derived from published solvent extraction procedures for water samples [27]. Briefly, 1 ml of TBAB in 4-methyl-2-pentanone, 50 mM solution, and 50 μl nitric acid 69.5% were added to 1 ml of EBC, and the biphasic system was thoroughly mixed for 10 min. After centrifugation at 2000 × g for 5 min, 800 μl of the organic phase containing the ion couple between the chromate and tetrabutylammonium ion were mixed with 50 μl of a concentrated aqueous solution of DPC (20 mM) in 60% ethanol and 7% nitric acid, and 1 ml of pure water. After mixing for 2–3 min, the two phases were separated by centrifuging the solution at 3000 × g for 1 min. Because of the low concentration of Cr(VI) in EBC, the Cr(VI) complexed with DPC in the aqueous phase was measured by means of ETAAS, which is about 2.5 times more sensitive than the colorimetric method used for the environmental analysis.
Total Cr in EBC was measured by means of ETAAS as previously reported [11].
2.7. Statistical analysis
The Bland–Altman method was used to compare the different methods of Cr(VI) detection [28]. Because of the relatively small number of studied subjects, parametric tests were used on logarithms to reduce the effect of outliers (dependent Student’s t-test or ANOVA for repeated measures followed by Tukey’s post hoc test) with a significant p value of 0.05. The data are expressed as geometric means (geometric S.D.), and also ranges.
3. Results
3.1. Detection of Cr(VI) in environmental samples
In order to test the liquid/liquid Cr(VI)/DPC extraction efficiency of 1-butanol and compare it with that of the colorimetric method suggested by the NIOSH 7703 protocol, in which DPC is dissolved in acetonitrile and directly added to the Cr(VI) solution [23], two different calibration lines were used in the range of 0–200 μg l−1 of Cr(VI) in elution buffer (Fig. 1). Within this range, both methods showed a linear response (R = 0.999), but the accumulation of Cr–DPC in 1-butanol enormously enhanced the signal: the slope of the regression lines passed from 0.60 ± 0.01 for the standard method to 1.83 ± 0.01 for butanol liquid/liquid extraction, and the limit of detection (LOD) calculated as 3 S.D. of the blank variability changed from about 1.5 to 0.5 μg l−1. The use of 1-butanol as solvent shifted the wavelength of maximum absorbance to 545 nm without changing the intensity of the peak (data not shown).
Fig. 1.
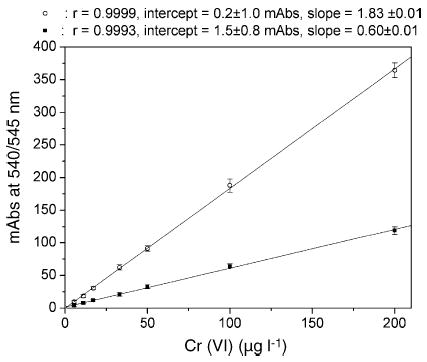
Calibration lines of Cr(VI) in the range 0–200 μg l−1 in elution buffer with the extraction of Cr(VI)–DPC in 1-butanol measuring absorbance at 545 nm (○), and following the NIOSH 7703 protocol measuring absorbance at 540 nm (▪). The figure also shows Pearson’s r-value, the intercept and the slope of the lines with their S.D.s.
Fig. 2A shows the experimental detection of Cr(VI) in environmental samples (20 experimental points in the range of 0.1–7.0 μg/membrane) with and without SPE (see Section 2 for further details). The slope of the straight line in the regression fit was 1.01 ± 0.03, and the correlation coefficient 0.995 (Fig. 2A), whereas the Bland–Altman graph (Fig. 2B) showed only one point between 2 and 3 S.D. (as expected with 20 experimental points), and the mean deviation between the two methods was near to 0 (0.03 μg/membrane).
Fig. 2.
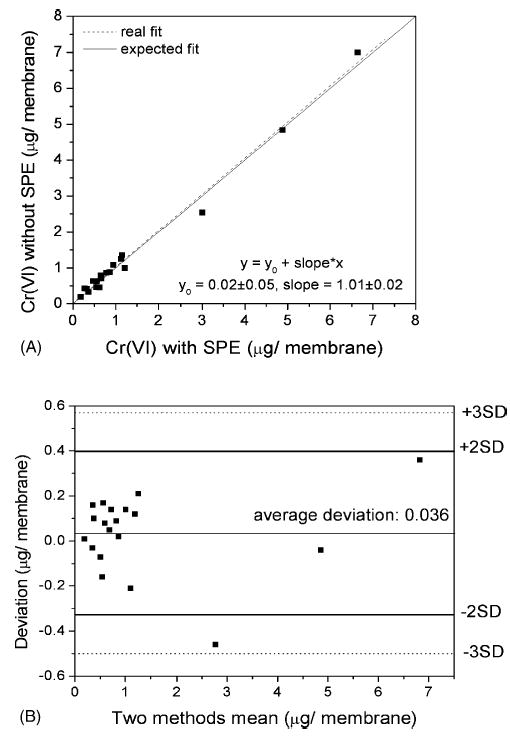
(A) Correlation between the Cr(VI) concentrations determined with and without SPE in the environmental air samples, also showing the function of the fit with the values of the intercept with y-axis (y0) and slope. The expected fit is y = x. (B) Bland–Altman graph in which the “two methods mean” is the average of the values measured with and without SPE, and “deviation” the difference between these values. The figure also shows the lines representing ± 2 S.D. (—) and 3 S.D. (…).
In relation to the selected workers, the morning exposure to total Cr [3.7 (2.8) μg m−3, range 0.8–14.1 μg m−3] was not statistically different from the afternoon exposure [4.6 (2.7) μg m−3, range 1.4–23.4 μg m−3]. This was confirmed by the measurements of Cr(VI), which passed from 2.5 (2.8) (range 0.5–10.3) μg m−3 in the morning to 3.3 (3.2) (range 1–17.6) μg m−3 in the afternoon. In terms of % Cr(VI) content, 67.8% (1.1) (range 56.7–76.6%) of the total Cr in the morning, and 70.1% (1.3) (range 43.9–100%) in the afternoon, was in the form of soluble Cr(VI).
On the basis of these results, the weighted average exposure of the workers during the work shift was 4.3 (2.6) (range 1.2–18.6) μg m−3 of total Cr, of which 2.9 (2.6) (range 0.8–13.8) μg m−3 was soluble Cr(VI). Soluble Cr(VI) accounted for 67.5% (1.1) (range 49.9–78.3%) of the total.
3.2. Detection of Cr(VI) in EBC
Various experiments were performed to validate the procedure for EBC Cr(VI) determinations described in Section 2.
The signals of standard Cr(VI) alone and complexed with DPC in aqueous solution measured by means of ETAAS were similar (differences <5%, data not shown).
About 90% of the Cr(VI) initially present in the solution was extracted as an ion pair with TBAB in water; the extraction efficiency was slightly lower in EBC (about 85%); the calibration curves in Fig. 3 shows that the extraction in both water and EBC was independent of the Cr(VI) concentration. On the contrary, <2% of Cr(III) was extracted in the organic phase with TBAB, and its contribution to the final Cr(VI) signal was negligible up to 50 μg l−1 (data not shown).
The contribution of the contamination of DPC, nitric acid, TBAB and 4-methyl-2-pentanone to the blank signal was low, but not negligible (data not shown).
To test the effects of other anions on the complexation procedures (i.e. competition effects of different ions forming the ion pair) and the ETAAS Cr(VI) signal, Cr(VI) was determined in two different drinking waters in which the concentrations of anions such as chloride, carbonate, nitrate and sulfate can be easily measured. The spectrum of a standard Cr(VI) concentration (5 μg l−1) was unaffected by the matrix (data not shown), and the simultaneous presence in solution of 5 μg l−1 Cr(VI) and 500 μg l−1 Fe2+ (which tends to reduce Cr(VI) at acidic pH) reduced the ETAAS Cr(VI) absorption peak by only about 10%.
The calibration curve of Cr(VI) in a pool of different EBCs (total Cr = 0.1 μg l−1) showed an approximately 10% decrease in the Cr signal within the range 0–5 μg l−1 (Fig. 3). Moreover, as in the case of water, the presence of up to 50 μg l−1 of Cr(III) in EBC did not interfere with the Cr(VI) signal.
Considering 3 S.D. of the blank, the LOD of EBC Cr(VI) was about 0.2 μg l−1.
The coefficient of variation (CV) of the measurements of standard samples (0.8, 2 and 5 μg l−1) in water and a pool of EBCs was <10% (2 and 5 μg l−1) and <30% (0.8 μg l−1) for all of the intra- and inter-day determinations.
Fig. 3.
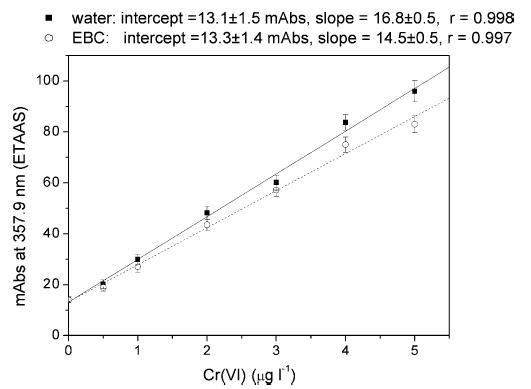
Calibration curve of Cr(VI) in the range 0–5 μg l−1 after extraction with TBAB and complexation with DPC in water, and in a pool of EBC; the slopes of the curves and Pearson’s r-value are also shown. The intercept represents the value of the blank in the absence of standard Cr(VI).
Fig. 4A shows total Cr in EBC, with the corresponding Cr(VI) concentrations. At t0, total Cr was 2.6 (1.5) (range 0.8–6.1) μg l−1, significantly higher (p < 0.05) than at t1 [1.5 (2.0), range 0.4–4.1 μg l−1], and Cr(VI) passed from 0.9 (3.4) (range 0.1–2.9) μg l−1 at t0 with only one of the 10 EBC samples below the LOD, to 0.2 (3.6) (range 0.1–2.1) μg l−1 at t1 (p < 0.01), with six of the 10 EBC samples below the LOD. Where Cr(VI) was less than 0.2 μg l−1, half of the LOD (0.1 μg l−1) was assigned. The Cr(VI)/total Cr ratio (Fig. 4B) showed that EBC Cr(VI) levels were significantly lower at t1 [16.5% (2.5), range 3.0–51.2% of total Cr] than at t0 [33.5% (2.0), range 9.7–79.0%; p < 0.05] and in the environmental air (p < 0.01); the difference between t0 and environmental air was also significant (p < 0.05).
Fig. 4.
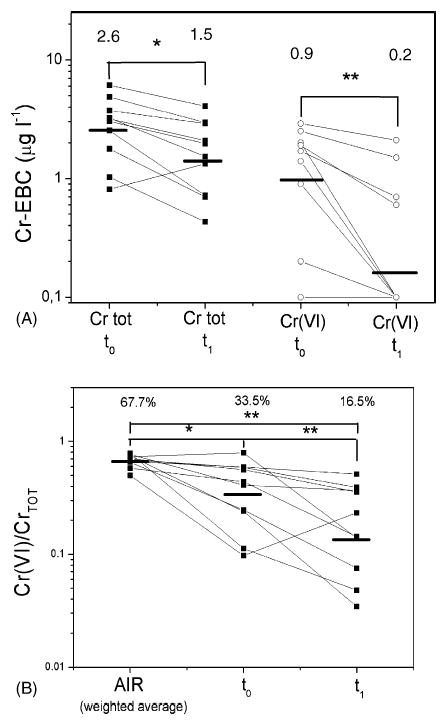
(A) Cr and Cr(VI) levels at the end of the Tuesday work shift (t0) and at the beginning of the Wednesday work shift (t1), and their geometric means (top of the graph). (B) The fraction of Cr(VI) in the environmental samples (AIR) at t0 and t1, and their geometric means (top of the graph). *p < 0.05; **p < 0.05 with a paired Student’s t-test (A) and repeated measures ANOVA followed by Tukey’s post hoc tests (B).
4. Discussion
Our study shows that it is possible to determine Cr(VI) in EBC and that the fractional contribution of Cr(VI) to total Cr decreased over time from the last exposure, thus ruling out its meaning as a simple marker of environmental contamination. This time-dependent decrease can in fact only be explained as an interaction between inhaled Cr(VI) and the pulmonary lining fluid, with a consequent reduction to Cr(III). The persistence of Cr(VI) in EBC reinforces the idea that the lower airways are the main target of Cr(VI) toxicity [10].
Cr(VI) can be detected in environmental samples using different approaches: soluble or total Cr(VI) can be distinguished by changing the extraction solution from the membrane buffer [29], and more sophisticated separation can be obtained by means of SPE or chromatography techniques [23]. However, as these are time-consuming and relatively expensive methods, we propose a very simple and rapid method of detecting soluble Cr(VI) based on the direct extraction of the Cr(VI)–DPC complex in 1-butanol and by-passing such extraction procedures as SPE. On the other hand, interferences may be problematic in workplaces where potentially interfering metals, like Fe in different oxidation states, could be present in much greater concentrations. Sample preparation procedures may be necessary in such cases. Our method has the advantage that Cr(VI) can be concentrated in the organic phase simply by changing the volume of the DPC/butanol solution: using a 3:1 ratio of sample:DPC/butanol solution (9:3 ml), tripled the increase in signal intensity and reduced the LOD by a factor of 3 (Fig. 1). This means that about 75% of Cr(VI) complexed with DPC was concentrated in the organic phase. Cr(VI) extraction of some pieces of membrane with the buffer suggested by Hazelwood et al. [29] in order to monitor the soluble and insoluble Cr(VI) compounds did not significantly change the results, the concentration of insoluble Cr(VI) in this study being considered negligible.
Fig. 2A and B also shows negligible interferences from other cationic transition elements in environmental samples. The results with and without the SPE step recommended in the literature to clean the samples from other cationic transition elements or impurities were perfectly consistent in the investigated range of concentrations, and this was confirmed by in vitro experiments in which 500 μg l−1 of Cr(VI) were incubated with equal absolute quantities of cationic Fe, Pb, Ni, Cd, Cu and Co (data not shown). Another advantage of our method is that the SPE cartridges could be saturated by high concentrations of Cr(VI), whereas a direct colorimetric measure is linear in the range of the linearity of the spectrophotometer (Abs about 1.5). Further work is needed to compare our method with the ISO 16740 speciation method recently published [30].
In determining EBC Cr(VI), we adopted a different and more sensitive method that is useful for small concentrations and small volume samples (the LOD passes from 0.5 to 0.2 μg l−1). Tetraalkylammonium (tetrapropyl or tetrabutylammonium) bromide tends to form ion pairs with metal cyanides, alkyl sulfonate, sulfur oxide, inorganic anions and oxyanions [31–34] and, in particular, tetrabutylammonium ions form stable ion pairs with chromate at acidic pH in aqueous solutions [17,27]. This ion pair can be selectively extracted in organic solvents as 4-methyl-2-pentanone or chloroform, or in aqueous two-phase systems [17,27]. We adapted the method of Noroozifar and Khorasani-Motlagh [27] to the small volumes of collected EBC (never more than 2 ml over 15 min), and the use of ETAAS also allowed good sensitivity in the generally diluted EBC samples. The percent recovery of Cr(VI) as an ion pair in 4-methyl-2-pentanone was in line with that observed in previous studies [17,27] despite the small volumes, and did not depend on the Cr(VI) concentration in the considered range (Fig. 3).
Moreover, the experiments using drinking water and Fe2+, together with the results of interference studies published in the literature [27], make the developed method ideal for diluted samples such as EBC, although the slight decrease in the extraction efficiency of Cr(VI) as an ion pair and then as a complex with DPC (about 10% overall) suggested adding the standard directly to the pools of EBCs and treating them as samples. This can be explained as a probable interference of traces of EBC anions (salts and proteins) present upon the extraction of Cr(VI). The slight effect of the different components on the signal of the blank suggested using highly pure reagents to minimise these effects, but the blank absorbance was in any case acceptable: about 14 mAbs against the 6 mAbs of pure water.
The optimisation of the methods of measuring soluble Cr(VI) in environmental samples and EBC allowed us to study the kinetics of Cr(VI) in the airways. First of all, total Cr in EBC decreased 15 h after the last exposure, a finding that is in line with previous results observed before and after a weekend, and confirms that Cr can pass through airway lining fluid [11]: the kinetics of pulmonary lung desorption is relatively slow [35], and Cr accumulation has recently been observed in the bronchi and lungs of chromate workers [36]. Most importantly, the EBC Cr(VI) data confirmed that Cr(VI) was reduced to Cr(III) by airway lining fluid, but not completely so (Fig. 4A and B), even in workers exposed to smaller environmental Cr(VI) concentrations than the limits suggested by ACGIH [37], and those exposed to lower Cr concentrations than those reported in our previous study [11]. About 33.5% of Cr was still in the hexavalent form immediately after chromate exposure (about 67% of the environmental samples were hexavalent): 9/10 workers had clearly detectable EBC Cr(VI) levels, and 4/10 had detectable levels about 15 h after the last exposure. The persistence of Cr(VI) in the airways can justify the previously reported increase in biomarkers of inflammation in EBC [11], because unreduced Cr(VI) can be absorbed at pulmonary level and reveal its toxicity in pulmonary cells. We are currently attempting to relate the reduction kinetics of EBC Cr(VI) to individual susceptibility and EBC biomarkers of inflammation in a larger number of subjects.
5. Conclusions
In conclusion, we have developed a method for measuring EBC Cr(VI) based on ion pair extraction followed by ETAAS determination. The results of this study highlight the potential of EBC as a medium for assessing lung dose and effects after exposure to inhaled pneumotoxic substances (particularly transition elements) with different oxidation states. The integrated use of EBC and classic biological matrices such as urine and blood, which reflect systemic exposure, may therefore allow the fundamental completion of the biological monitoring of pneumotoxic compounds.
Acknowledgments
This study was supported by the National Heart, Blood and Lung Institute (NHLBI), Bethesda, MD, USA (grant R01 HL72323), and the Italian Ministry of Education, University and Research (PRIN 200306145).
References
- 1.ATSDR, Toxicological profile for Chromium (final report), NTIS Accession No. PB2000-108022. Agency for Toxic Substances and Disease Registry, Atlanta, GA, 2000, 461pp.
- 2.International Agency for Research on Cancer, Chromium, nickel and welding, IARC Monographs on the Evaluation of Carcinogenic Risk to Humans, vol. 49, IARC Scientific Pubblications, IARC, Lyon, 1999.
- 3.De Flora S, Bagnasco M, Serra D, Zanacchi P. Mutat Res. 1990;238:99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- 4.De Flora S, Wetterhahn KE. Life Chem Rep. 1989;7:169–244. [Google Scholar]
- 5.Bagchi D, Stohs SJ, Downs BW, Bagchi M, Preuss HG. Toxicology. 2002;180:5–22. doi: 10.1016/s0300-483x(02)00378-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhitkovich A. Chem Res Toxicol. 2005;18:3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
- 7.Wise JP, Sr, Wise SS, Little JE. Mut Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 8.Levina A, Lay PA. Coord Chem Rev. 2005;249:281–298. [Google Scholar]
- 9.Coogan TP, Squibb KS, Motz J, Kinney PL, Costa M. Toxicol Appl Pharmacol. 1991;108:157–166. doi: 10.1016/0041-008x(91)90279-n. [DOI] [PubMed] [Google Scholar]
- 10.Paustenbach DJ, Panko JM, Fredrick MM, Finley BL, Proctor DM. Reg Toxicol Pharmacol. 1997;26:S23–S34. doi: 10.1006/rtph.1997.1135. [DOI] [PubMed] [Google Scholar]
- 11.A. Caglieri, M. Goldoni, O. Acampa, R. Andreoli, M.V. Vettori, M. Corradi, P. Apostoli, A. Mutti, Environ. Health Perspect., in press, doi:10.1289/ehp.8506. [DOI] [PMC free article] [PubMed]
- 12.De Flora S. Carcinogenesis. 2000;21:533–541. doi: 10.1093/carcin/21.4.533. [DOI] [PubMed] [Google Scholar]
- 13.Goldoni M, Catalani S, De Palma G, Manini P, Acampa O, Corradi M, Bergonzi R, Apostoli P, Mutti A. Environ Health Perspect. 2004;112:1293–1298. doi: 10.1289/ehp.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Ashley K, Marlow D. Anal Chem. 1999;71:1027–1032. doi: 10.1021/ac980501r. [DOI] [PubMed] [Google Scholar]
- 15.Adria-Cerezo DM, Llobat-Estelles M, Maurì-Aucejo AR. Talanta. 2000;51:531–536. [PubMed] [Google Scholar]
- 16.Balogh IS, Maga IM, Hargitai-Toth A, Andruch V. Talanta. 2000;53:543–549. doi: 10.1016/s0039-9140(00)00525-7. [DOI] [PubMed] [Google Scholar]
- 17.Akama Y, Sali A. Talanta. 2002;57:681–686. doi: 10.1016/s0039-9140(02)00076-0. [DOI] [PubMed] [Google Scholar]
- 18.Chwastowska J, Skwara W, Sterlinska E, Pszonicki L. Talanta. 2005;66:1345–1349. doi: 10.1016/j.talanta.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 19.Borai EH, El-Sofany EA, Abdel-Halim AS. Trends Anal Chem. 2002;21:741–745. [Google Scholar]
- 20.Wang JS, Chiu KH. Anal Sci. 2004;20:841–846. doi: 10.2116/analsci.20.841. [DOI] [PubMed] [Google Scholar]
- 21.Hossain MA, Kumita M, Michigami Y, Islam TSA, Mori S. J Chrom Sci. 2005;43:98–103. doi: 10.1093/chromsci/43.2.98. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez O, Arcos J. Anal Chim Acta. 2002;470:241–252. [Google Scholar]
- 23.Available at the site http://www.cdc.gov/niosh/topics/hexchrom/
- 24.Boiano JM, Wallace ME, Sieber WK, Groff JH, Wang J, Ashley K. J Environ Monit. 2000;2:329–333. doi: 10.1039/b002456m. [DOI] [PubMed] [Google Scholar]
- 25.Ashley K, Howe AM, Demange M, Nygren O. J Environ Monit. 2003;5:707–716. doi: 10.1039/b306105c. [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association, World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Ferney-Voltaire, France: The World Medical Association, 2002. Available: http://www.wma.net/e/policy/pdf/17c.pdf [DOI] [PubMed]
- 27.Noroozifar M, Khorasani-Motlagh M. Anal Sci. 2003;19:705–708. doi: 10.2116/analsci.19.705. [DOI] [PubMed] [Google Scholar]
- 28.Bland JM, Altman DG. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 29.Hazelwood KJ, Drake PL, Ashley K, Marcy D. J Occup Environ Hyg. 2004;1:613–619. doi: 10.1080/15459620490493810. [DOI] [PubMed] [Google Scholar]
- 30.ISO, 16740 method: workplace air – Determination of hexavalent chromium in airborne particulate matter – method by ion chromatography and spectrophotometric measurement using diphenyl carbazide. International Organization for Standardization, Geneva, Switzerland, 2005.
- 31.Maeck WJ, Booman GL, Kussy ME, Rein JE. Anal Chem. 1961;33:1775–1780. [Google Scholar]
- 32.Kusakabe S, Arai M. Bull Chem Soc Jpn. 1996;69:581–588. [Google Scholar]
- 33.Abramov AA, Dzhigirkhanov MS, Iofa BZ, Volkova SV. Radiochemistry. 2002;44:270–273. [Google Scholar]
- 34.Akama Y, Ito M, Tanaka S. Talanta. 2000;53:645–650. doi: 10.1016/s0039-9140(00)00555-5. [DOI] [PubMed] [Google Scholar]
- 35.O’Flaherty EJ. Toxicol Appl Pharmacol. 1996;138:54–64. doi: 10.1006/taap.1996.0097. [DOI] [PubMed] [Google Scholar]
- 36.Kondo K, Takahashi Y, Ishikawa S, Uchihara H, Hirose Y, Yoshizawa K, Tsuyuguchi M, Takizawa H, Miyoshi T, Sakiyama S, Monden Y. Cancer. 2003;98:2420–2429. doi: 10.1002/cncr.11818. [DOI] [PubMed] [Google Scholar]
- 37.ACGIH, Chromium. Documentation of the threshold limit values and biological exposure indices. American Conference of Governmental Industrial Hygienists. Cincinnati, OH, 1999.


