Abstract
In cardiac myocytes, sustained (3 min) intracellular acidosis activates the ERK1/2 (extracellular-signal-regulated kinase 1/2) pathway and, through this pathway, increases sarcolemmal NHE (Na+/H+ exchanger) activity [Haworth, McCann, Snabaitis, Roberts and Avkiran (2003) J. Biol. Chem. 278, 31676–31684]. In the present study, we aimed to determine the time-dependence, pH-dependence and upstream signalling mechanisms of acidosis-induced ERK1/2 activation in ARVM (adult rat ventricular myocytes). Cultured ARVM were subjected to intracellular acidosis for up to 20 min by exposure to NH4Cl, followed by washout with a bicarbonate-free Tyrode solution containing the NHE1 inhibitor cariporide. After the desired duration of intracellular acidosis, the phosphorylation status of ERK1/2 and its downstream effector p90RSK (90 kDa ribosomal S6 kinase) were determined by Western blotting. This revealed a time-dependent transient phosphorylation of both ERK1/2 and p90RSK by intracellular acidosis (intracellular pH ∼6.6), with maximum activation occurring at 3 min and a return to basal levels by 20 min. When the degree of intracellular acidosis was varied from ∼6.8 to ∼6.5, maximum ERK1/2 phosphorylation was observed at an intracellular pH of 6.64. Inhibition of MEK1/2 [MAPK (mitogen-activated protein kinase)/ERK kinase 1/2) by pre-treatment of ARVM with U0126 or adenoviral expression of dominant-negative D208A-MEK1 protein prevented the phosphorylation of ERK1/2 by sustained intracellular acidosis, as did inhibition of Raf-1 with GW 5074 or ZM 336372. Interference with Ras signalling by the adenoviral expression of dominant-negative N17-Ras protein or with FPT III (farnesyl protein transferase inhibitor III) also prevented acidosis-induced ERK1/2 phosphorylation, whereas inhibiting G-protein signalling [by adenoviral expression of RGS4 or Lsc, the RGS domain of p115 RhoGEF (guanine nucleotide-exchange factor)] or protein kinase C (with bisindolylmaleimide I) had no effect. Our data show that, in ARVM, sustained intracellular acidosis activates ERK1/2 through proximal activation of the classical Ras/Raf/MEK pathway.
Keywords: acidosis, cardiac myocyte, extracellular-signal-regulated kinase (ERK) pathway, G-protein-coupled receptor (GPCR), protein kinase C (PKC), Raf, Ras
Abbreviations: ARVM, adult rat ventricular myocytes; BIM I, bisindolylmaleimide I; EGFP, enhanced green fluorescent protein; ERK, extracellular-signal-regulated kinase; FPT III, farnesyl protein transferase inhibitor III; GEF, guanine nucleotide-exchange factor; GPCR, G-protein-coupled receptor; HRP, horseradish peroxidase; I.U., international units; MAPK, mitogen-activated protein kinase; MEK, MAPK/ERK kinase; mM199, modified M199; NHE, Na+/H+ exchanger; NRVM, neonatal rat ventricular myocytes; p90RSK, 90 kDa ribosomal S6 kinase; pHi, intracellular pH; PKC, protein kinase C; RGS, regulator of G-protein signalling; Lsc, RGS domain of p115 RhoGEF
INTRODUCTION
In cardiac myocytes, as in other cell types, numerous cellular processes are sensitive to changes in pHi (intracellular pH). In particular, almost every step in the excitation–contraction process in cardiac myocytes is inhibited by intracellular acidosis [1], emphasizing the importance of pHi-regulatory mechanisms that have evolved to prevent or correct this condition. A critical regulator of pHi in cardiac myocytes is the sarcolemmal NHE (Na+/H+ exchanger), which is encoded by the NHE1 isoform of the multigene NHE family [2] and responds rapidly to intracellular acidosis [3], through an allosteric mechanism [4,5], to extrude H+. We have shown previously, in both neonatal and adult cardiac myocytes, that when intracellular acidosis is maintained for 3 min by blocking acid-extruding mechanisms (‘sustained intracellular acidosis’) subsequent sarcolemmal NHE activity is increased significantly more than after transient intracellular acidosis [6]. In a detailed examination of potential signalling mechanisms underlying this response in neonatal cardiac myocytes, we also found that sustained intracellular acidosis activates the ERK1/2 (extracellular-signal-regulated kinase 1/2) pathway, and that this activation of ERK1/2 is required for the potentiation of sarcolemmal NHE activity [6]. Although this work revealed a novel kinase-mediated mechanism for the acidosis-induced stimulation of sarcolemmal NHE activity, the minimum requirements for ERK1/2 activation, in terms of the duration and severity of intracellular acidosis, and the upstream elements of the pertinent pHi-sensitive signalling pathway, have remained unknown.
In the present study, we have determined the time- and pHi-dependence of ERK1/2 activation by intracellular acidosis and investigated the mechanism by which ERK1/2 is activated by sustained intracellular acidosis in ARVM (adult rat ventricular myocytes).
EXPERIMENTAL
Materials and animals
Antibodies for phospho-ERK1/2 (pThr202/pTyr204), phospho-p90RSK (90 kDa ribosomal S6 kinase; pSer381) and MEK1 [MAPK (mitogen-activated protein kinase)/ERK kinase 1) were from Cell Signaling Technology. The antibody to ERK2 was from Santa Cruz Biotechnology. HRP (horseradish peroxidase)-conjugated secondary antibodies and enhanced chemiluminescence reagents were from Amersham Pharmacia Biotech. U0126, ZM 336372, FPT III (farnesyl protein transferase inhibitor III) and BIM I (bisindolylmaleimide I) were from Merck. GW 5074 was from Tocris Cookson. Phenylephrine, thrombin and PMA were from Sigma. M199, penicillin and streptomycin were from Invitrogen. Adenovirus encoding a kinase-inactive form of MEK1 (D208A-MEK1), the upstream activator of ERK1/2, was a gift from Dr J. Molkentin (Department of Pediatrics, Cincinnati Children's Hospital Medical Center, Cincinnati, U.S.A.). Adenoviruses encoding either RGS (regulator of G-protein signalling) 4 or Lsc [the RGS domain of p115 RhoGEF (guanine nucleotide-exchange factor)], were gifts from Dr T. Wieland (Institute for Pharmacology and Toxicology, University of Heidelberg, Heidelberg, Germany). Adenovirus encoding the dominant-negative mutant of H-Ras (N17-Ras) was a gift from Dr B. Kahn (Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, U.S.A.) via Professor C. Proud (Department of Biochemistry and Molecular Biology, University of British Columbia, Vancouver, BC, Canada). Adult male Wistar rats (250 g) were from B & K Universal. The investigation was performed in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986.
Short-term culture and adenoviral infection of ARVM
ARVM were isolated as described previously [7,8], and washed with M199 medium with added penicillin [100 I.U. (international units)/ml] and streptomycin (100 I.U./ml). The cell suspension was centrifuged at 100 g for 2 min to pellet the myocytes, which were then resuspended in mM199 (modified M199) medium [M199 medium supplemented with penicillin (100 I.U./ml), streptomycin (100 I.U./ml), L-carnitine (2 mM), creatine (5 mM) and taurine (5 mM)]. To each well of a laminated six-well culture plate, 2 ml of cell suspension was added and the plates were placed in a humidified 5% CO2 incubator at 37 °C. After 2 h of pre-plating, the medium was aspirated, leaving only adherent cells, and 2 ml of fresh pre-warmed mM199 medium was added. For studies involving the measurement of pHi, an identical protocol was followed, except that ARVM were plated on to laminated glass coverslips placed into each well of a 12-well culture plate.
Adenoviral infection of cultured myocytes was performed after the initial 2 h pre-plating step. The number of rod-shaped cells in a field of 1 mm2 (as defined by an eye-piece graticule) was counted in several wells and used to estimate the number of cells/well. Myocytes were exposed to adenovirus at an MOI (multiplicity of infection) of 100 plaque-forming units/cell for 1 h at 37 °C, before the medium containing residual virus was aspirated and replaced with fresh pre-warmed (37 °C) mM199 medium. ARVM were maintained in culture in a humidified tissue culture incubator (37 °C; 5% CO2) for 18–42 h before use in experiments.
Induction of intracellular acidosis
Intracellular acidosis was induced using the NH4Cl pulse method, as in our previous studies [6,9–11]. Cultured ARVM were initially bathed in bicarbonate-free Tyrode solution consisting of 137 mM NaCl, 5.4 mM KCl, 1.0 mM CaCl2, 0.5 mM MgCl2, 10 mM Hepes (pH 7.4) and 10 mM glucose for 90 min in room air at 37 °C. After this, intracellular acidosis was induced by exposure to bicarbonate-free Tyrode solution containing 5–30 mM NH4Cl for 3 min, followed by washout of NH4Cl with Tyrode solution containing 3 μM cariporide (a selective NHE1 inhibitor [12]) for the desired period. Under these conditions, all acid-extruding mechanisms are inactive, resulting in intracellular acidosis (the severity of which depends on the NH4Cl concentration; see below) which is maintained for several minutes [6].
Pharmacological protocols
All inhibitors, except FPT III, were added to cells 10 min prior to NH4Cl exposure and were present throughout the rest of the protocol. FPT III was added to cells immediately after plating. Since the inhibitors were dissolved in DMSO, the final concentration of this vehicle (0.1%) was included in the appropriate solutions in the untreated groups. Where used as positive controls, phenylephrine (100 μM), thrombin (100 units/ml) or PMA (30 nM) were added 10 min after inhibitor or vehicle for 5 min.
Western blotting
Cells were lysed in Laemmli buffer and protein samples were separated by SDS/PAGE. After transfer on to a PVDF membrane, Western analysis was performed using rabbit polyclonal antibodies which detect dual phosphorylated ERK1/2, phosphorylated p90RSK or MEK1, or mouse monoclonal antibodies which detect ERK2 or p90RSK. Where both phosphorylated and total protein were determined, duplicate blots were used. Bound antibody was detected by labelling with HRP-conjugated secondary antibody, followed by enhanced chemiluminescence. Phosphorylation status was quantified using a laser densitometer (Beckman GS-800).
Measurement of pHi
pHi was determined in single cells superfused with bicarbonate-free Tyrode solution by microepifluorescence, using the fluorescent pH indicator SNARF-1 (carboxyseminaphthorhodafluor-1), as we have described previously for ARVM [6,9].
Statistics
Data are expressed as means±S.E.M. Inter-group comparisons were by ANOVA, followed by the Bonferroni t test. P<0.05 was considered significant.
RESULTS
Time- and pHi-dependence of ERK1/2 activation
ERK1/2 phosphorylation and activity are increased by sustained intracellular acidosis in a time-dependent manner
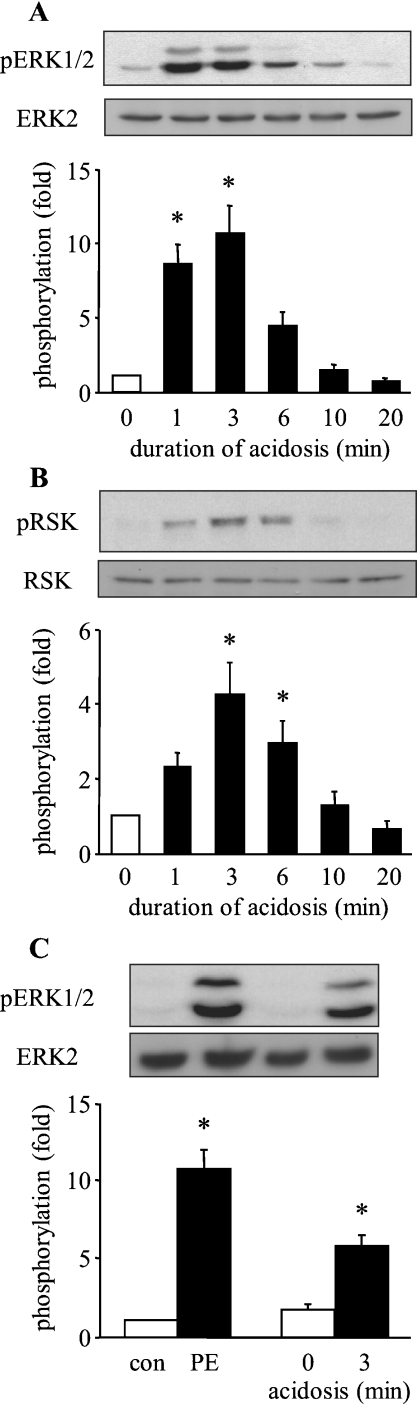
To determine the effect of the duration of intracellular acidosis on ERK1/2 activation, ARVM were exposed for 3 min to 20 mM NH4Cl, which was washed out in the presence of cariporide (3 μM), lowering pHi to ∼6.6 (see [6] and below). Samples were harvested at 0, 1, 3, 6, 10 and 20 min after NH4Cl washout and analysed for ERK1/2 and p90RSK phosphorylation by Western blotting. The upper panels of Figure 1(A) show representative Western blots of phosphorylated ERK1/2 and total ERK2, and the lower panel shows quantitative data from six similar experiments. ERK2 protein levels did not change over the duration of the experiment. Basal ERK1/2 phosphorylation was low in the absence of intracellular acidosis. Following NH4Cl washout, an increase in ERK1/2 phosphorylation was observed, which was significant at 1 min and reached a maximum at 3 min. ERK1/2 phosphorylation subsequently declined, reaching basal levels after 20 min of acidosis. To confirm that ERK1/2 phosphorylation correlated with ERK1/2 activity, we also analysed the samples for the phosphorylation of p90RSK, a downstream target of ERK1/2 [13]. p90RSK phosphorylation was also increased by intracellular acidosis, being detectable at 1 min and reaching a maximum at 3 min, before declining to near basal levels by 20 min (Figure 1B). p90RSK protein levels did not change over the duration of the experiment. Thus intracellular acidosis rapidly and transiently phosphorylates and activates ERK1/2 in ARVM.
Figure 1. Effect of the duration of intracellular acidosis on the activation of ERK1/2 in ARVM.
(A) Western blots of phosphorylated ERK1/2 (pERK1/2) and total ERK2 following intracellular acidosis for 0, 1, 3, 6, 10 and 20 min, and quantitative data from six separate experiments. Data are expressed as fold phosphorylation normalized to control (0 min; open bar). (B) Western blots of phosphorylated p90RSK (pRSK) and total p90RSK (RSK), and quantitative data from six separate experiments. (C) Western blots of phosphorylated ERK1/2 (pERK1/2) and total ERK2 in cells following exposure to vehicle (con) or 100 μM phenylephrine (PE), or to intracellular acidosis for 0 or 3 min, and quantitative data from six separate experiments. Data are expressed as fold phosphorylation normalized to vehicle control (con). *P<0.05 compared with control.
To determine the extent of ERK1/2 activation induced by intracellular acidosis relative to that induced by a potent neurohormonal stimulus, we compared the effect of a 3 min exposure to 100 μM phenylephrine with that of 3 min of intracellular acidosis. As illustrated in Figure 1(C), intracellular acidosis promoted ERK1/2 phosphorylation to approx. 60% of that seen on exposure to phenylephrine. Thus intracellular acidosis activates ERK1/2 to an extent that is likely to have functional consequences.
ERK1/2 phosphorylation and activity are increased by sustained intracellular acidosis in a pHi-dependent manner
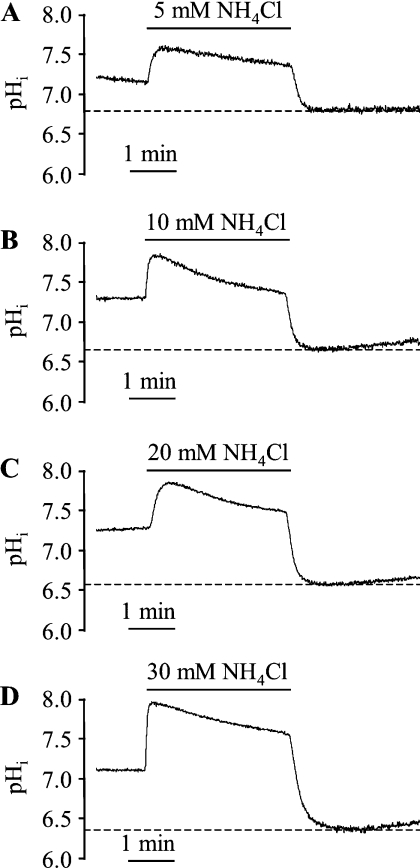
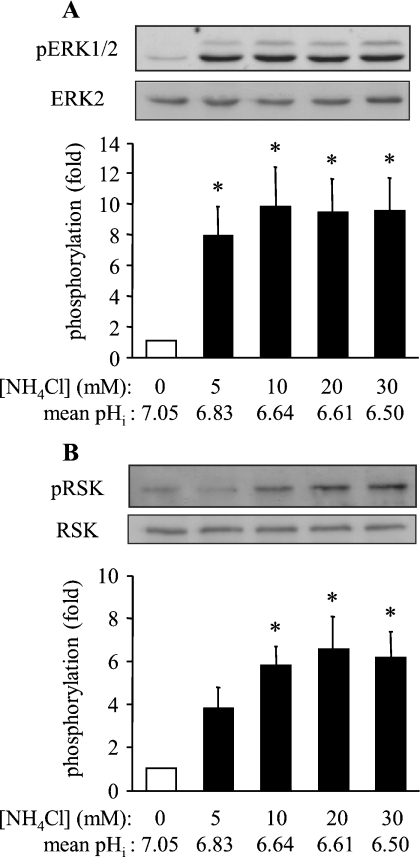
To determine the effect of the extent of intracellular acidosis on ERK1/2 activation, ARVM were exposed for 3 min to different concentrations (5, 10, 20 or 30 mM) of NH4Cl which were washed out in the presence of cariporide. Samples were harvested at 3 min after NH4Cl washout [the time-point at which maximum ERK1/2 phosphorylation was seen with 20 mM NH4Cl; see Figure 1(A)]. Initially, experiments were performed to determine the degree of acidosis achieved with each concentration of NH4Cl. Upon washout of NH4Cl with cariporide-containing Tyrode solution, pHi was clamped at various degrees of acidosis for the desired period of time (3 min), as illustrated in Figure 2. The mean acidified pHi values in cells exposed to 5, 10, 20 and 30 mM NH4Cl were 6.83±0.06, 6.64±0.03, 6.61±0.07 and 6.50±0.07 respectively. The phosphorylation of ERK1/2 and p90RSK were determined in parallel experiments (Figure 3). Basal ERK1/2 phosphorylation was low before the induction of intracellular acidosis, and had a robust increase even with 5 mM NH4Cl, corresponding to a pHi of 6.83 (Figure 3A). In addition, a significant increase in p90RSK phosphorylation was observed with 10 mM NH4Cl, corresponding to a pHi of 6.64 (Figure 3B). In the same samples, there was no significant increase in p38 MAPK phosphorylation in response to intracellular acidosis (results not shown), indicating the absence of a general activation of MAPK pathways. These data indicate that even a modest degree of intracellular acidosis (pHi 6.8) sustained for 3 min is sufficient to activate ERK1/2 in a selective manner.
Figure 2. Representative pHi recordings in ARVM exposed to (A) 5 mM, (B) 10 mM, (C) 20 mM and (D) 30 mM NH4Cl for 3 min prior to washout with bicarbonate-free Tyrode solution containing the NHE1 inhibitor cariporide.
Basal pHi was 7.14±0.04, 7.12±0.04, 7.18±0.03 and 7.17±0.05 in the groups subsequently exposed to 5, 10, 20 and 30 mM NH4Cl respectively (n=6 cells/group). Maximal acidification obtained following NH4Cl washout (dotted line) was 6.83±0.06, 6.64±0.03, 6.61±0.07 and 6.50±0.07 for 5, 10, 20 and 30 mM respectively.
Figure 3. Effect of the extent of sustained intracellular acidosis on the activation of ERK1/2 in ARVM.
(A) Western blots of phosphorylated ERK1/2 (pERK1/2) and total ERK2 after 3 min exposure to 0, 5, 10, 20 and 30 mM NH4Cl, followed by washout with bicarbonate-free Tyrode solution containing the NHE1 inhibitor cariporide for 3 min, and quantitative data from eight separate experiments. Data are expressed as fold phosphorylation normalized to control (0 min; open bar). Mean pHi data are values for maximal acidification, determined from the experiments presented in Figure 2. (B) Western blots of phosphorylated p90RSK (pRSK) and total p90RSK (RSK), and quantitative data from six separate experiments. Data are expressed as fold phosphorylation normalized to control (0 min; open bar). *P<0.05 compared with control.
Upstream regulators of ERK1/2 activation
Role of MEK1/2
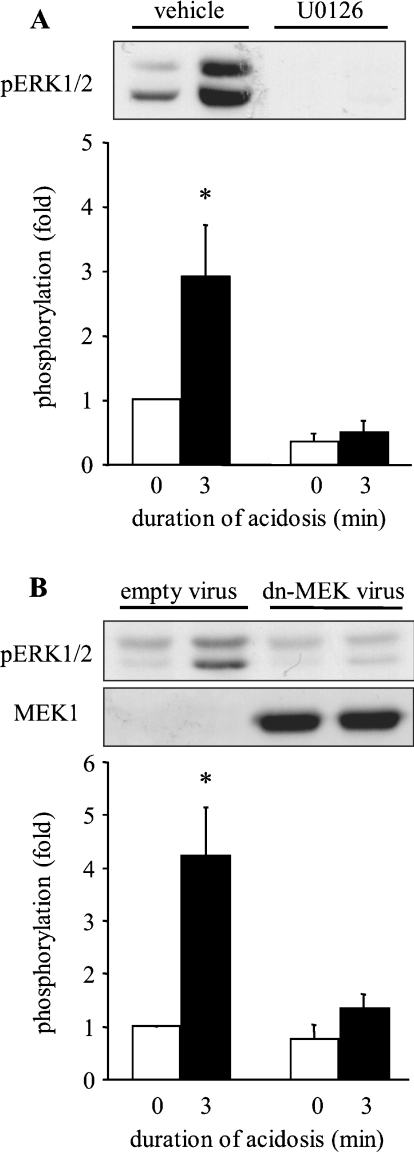
Our previous work in NRVM (neonatal rat ventricular myocytes) indicated that, in those cells, MEK1/2 activity was required for the acidosis-induced activation of ERK1/2 [6]. To determine the role of MEK1/2 in ARVM, MEK1/2 activity was inhibited either by using the selective inhibitor U0126 or by adenoviral expression of a kinase-inactive dominant-negative MEK1 [14]. ARVM were exposed to U0126 for 10 min prior to the induction and maintenance of intracellular acidosis, followed by Western blot analysis. The upper panel of Figure 4(A) shows a representative Western blot of phosphorylated ERK1/2, and the lower panel shows quantitative data from four similar experiments. Vehicle alone did not prevent the phosphorylation of ERK1/2 by sustained acidosis, whereas the presence of U0126 completely blocked ERK1/2 phosphorylation. To confirm that the effect of U0126 was indeed due to the inhibition of MEK1/2 activity, ARVM were infected with adenovirus encoding the dominant-negative D208A-MEK1 18 h prior to the induction of intracellular acidosis. Western blotting analysis with an antibody that recognizes MEK1 protein showed overexpression of MEK1 in the appropriate groups (Figure 4B). Infection of ARVM with control (empty) adenovirus did not affect the phosphorylation of ERK1/2 by intracellular acidosis, whereas the expression of D208A-MEK1 completely prevented the phosphorylation of ERK1/2 (Figure 4B). These data indicate that MEK1/2 is activated by sustained intracellular acidosis and mediates ERK1/2 phosphorylation.
Figure 4. Effect of MEK1/2 inhibition on the activation of ERK1/2 by sustained intracellular acidosis in ARVM.
(A) Western blot of phosphorylated ERK1/2 (pERK1/2) in control cells or following sustained intracellular acidosis in the absence (vehicle) or presence of 3 μM U0126, and quantitative data from four separate experiments. Data are expressed as fold phosphorylation normalized to vehicle control. (B) Western blots of phosphorylated ERK1/2 (pERK1/2) and MEK1 in control cells or following sustained intracellular acidosis 18 h after infection with control adenovirus (empty virus) or D208A-MEK1 (dn-MEK virus), and quantitative data from six separate experiments. Data are expressed as fold phosphorylation normalized to control. *P<0.05 compared with control.
Role of Raf
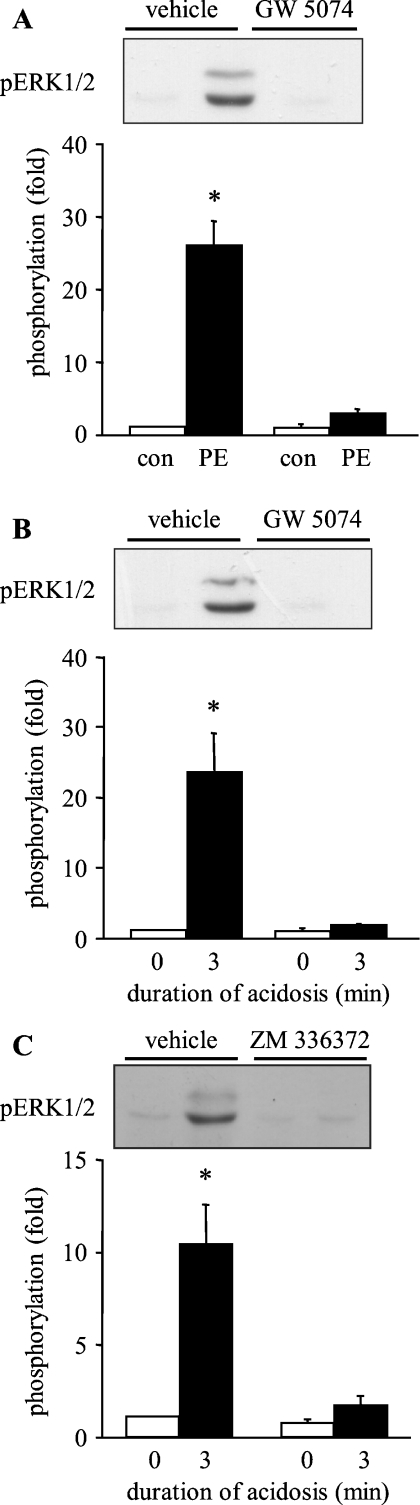
To establish the role of Raf in upstream activation of the MEK/ERK axis in response to sustained intracellular acidosis, the selective Raf-1 inhibitors GW 5074 and ZM 336372 were used [15,16]. Initially, experiments were performed to confirm the effectiveness of 3 μM GW 5074 (selected on the basis of preliminary concentration–response experiments; results not shown) by determining its effects on ERK1/2 phosphorylation by the α1-adrenergic receptor agonist phenylephrine. Vehicle alone did not prevent the phosphorylation of ERK1/2 by phenylephrine, whereas the presence of GW 5074 completely blocked ERK1/2 phosphorylation (Figure 5A). Subsequently, ARVM were exposed to GW 5074 prior to sustained intracellular acidosis. Once again, vehicle alone did not prevent the phosphorylation of ERK1/2 by intracellular acidosis, whereas the presence of GW 5074 completely blocked ERK1/2 phosphorylation (Figure 5B). Alternatively, ARVM were exposed to the chemically distinct Raf-1 inhibitor ZM 336372 (50 μM) prior to sustained intracellular acidosis. Vehicle alone did not prevent the phosphorylation of ERK1/2 by intracellular acidosis, whereas the presence of ZM 336372 largely prevented ERK1/2 phosphorylation (Figure 5C). These data indicate that Raf-1 is activated by sustained intracellular acidosis and mediates the downstream activation of MEK1/2 and ERK1/2.
Figure 5. Effect of Raf inhibition on the activation of ERK1/2 in ARVM.
(A) Western blot of phosphorylated ERK1/2 (pERK1/2) in control cells or following 5 min exposure to 100 μM phenylephrine in the absence (vehicle) or presence of 3 μM GW 5074, and quantitative data from four separate experiments. Data are expressed as fold phosphorylation normalized to vehicle control. (B) Western blot of phosphorylated ERK1/2 (pERK1/2) in control cells or following sustained intracellular acidosis in the absence (vehicle) or presence of 3 μM GW 5074, and quantitative data from four separate experiments. Data are expressed as fold phosphorylation normalized to vehicle control. (C) Western blot of phosphorylated ERK1/2 (pERK1/2) in control cells or following sustained intracellular acidosis in the absence (vehicle) or presence of 50 μM ZM 336372, and quantitative data from four separate experiments. Data are expressed as fold phosphorylation normalized to vehicle control. *P<0.05 compared with control.
Roles of potential upstream activators of Raf
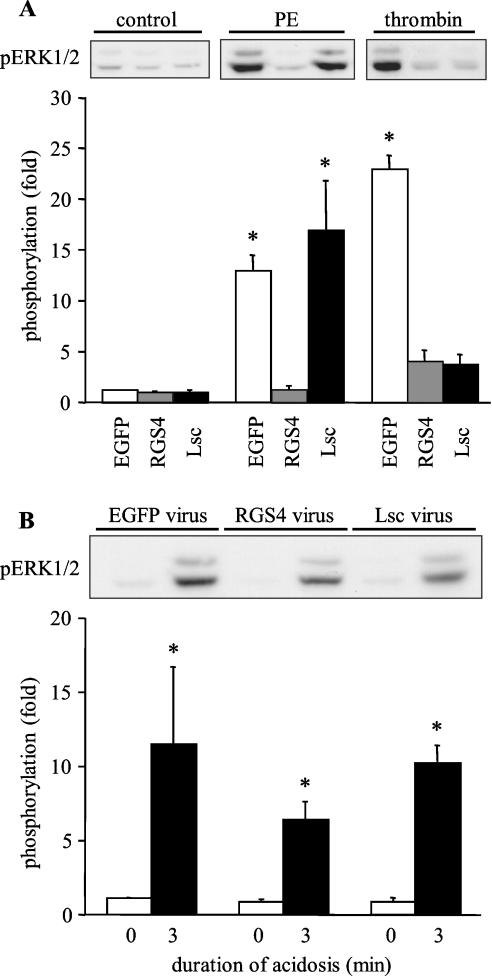
GPCRs (G-protein-coupled receptors), such as the α1-adrenergic receptor, induce ERK1/2 activation in cardiac myocytes via the activation of Raf [17]. Furthermore, acid-sensitive GPCRs have been identified in several cell types, including cardiac myocytes [18]. Although these receptors are thought to be sensitive to extracellular rather than intracellular acidosis, the method that has been used previously [19–21] to stimulate acid-sensitive receptors experimentally (incubation in acid medium) also promotes intracellular acidosis. To determine whether there is a role for GPCR signalling in the acidosis-induced activation of ERK1/2, we interfered with G-protein signalling by expressing the following GTPase-activating proteins of the RGS family: RGS4 (which inhibits signalling through Gαq and Gαi) or Lsc (which inhibits signalling through Gα12/13). In initial experiments, we determined the effects of adenoviral expression of these proteins on ERK1/2 phosphorylation by the α1-adrenergic receptor agonist phenylephrine and the protease-activated receptor agonist thrombin. The pertinent data, illustrated in Figure 6(A), were consistent with our previous study [7], confirming the effectiveness of the RGS protein expression protocol. Having confirmed the efficacy of the adenoviral constructs under the pertinent experimental conditions, experiments were performed to determine whether interfering with G-protein signalling affected the activation of ERK1/2 by sustained intracellular acidosis. Since the RGS4 and Lsc adenoviruses co-express EGFP (enhanced green fluorescent protein) under the control of a separate cytomegalovirus promoter [7], control cells were infected with adenovirus expressing only EGFP; in these cells, robust phosphorylation of ERK1/2 by sustained (3 min) intracellular acidosis was observed (Figure 6B). Comparable acidosis-induced ERK1/2 phosphorylation was also seen in cells expressing Lsc (Figure 6B). In cells expressing RGS4, there was a partial attenuation of the acidosis-induced response, but significant phosphorylation of ERK1/2 was retained (Figure 6B). These data indicate that, in ARVM, acid-sensitive GPCRs are not the principal initiators of ERK1/2 activation by sustained intracellular acidosis.
Figure 6. Effect of the inhibition of GPCR signalling on the activation of ERK1/2 in ARVM.
(A) Western blots of phosphorylated ERK1/2 (pERK1/2) in control cells or following 5 min exposure to 100 μM phenylephrine (PE) or 100 units/ml thrombin 18 h after infection with control adenovirus (EGFP), RGS4 or Lsc, and quantitative data from three separate experiments. Data are expressed as fold phosphorylation normalized to EGFP control. (B) Western blot of phosphorylated ERK1/2 (pERK1/2) in control cells or following sustained intracellular acidosis 18 h after infection with control adenovirus (EGFP), RGS4 or Lsc, and quantitative data from four or five separate experiments. Data are expressed as fold phosphorylation normalized to EGFP control. *P<0.05 compared control.
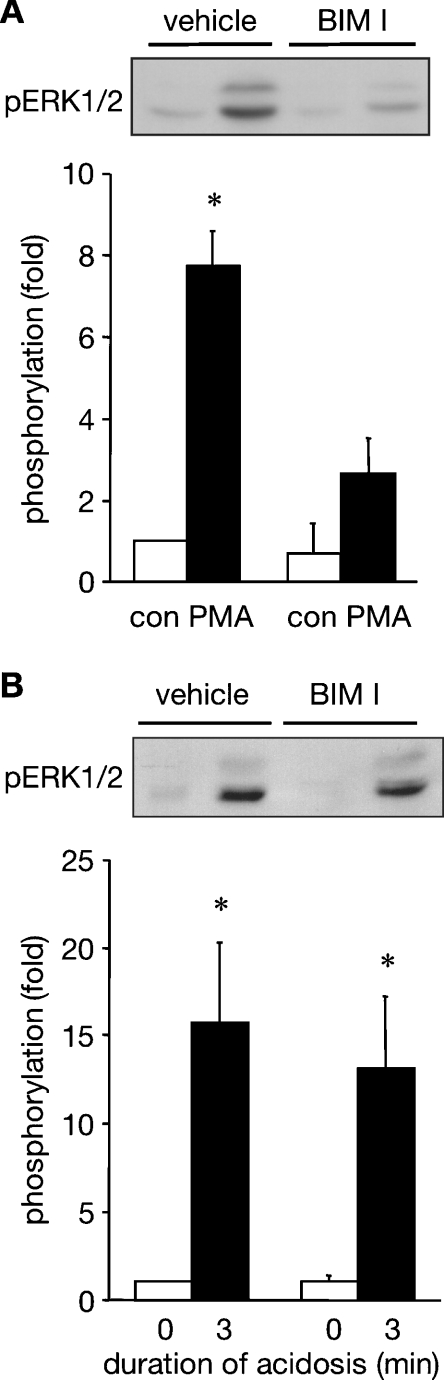
PKC (protein kinase C) is able to activate the ERK pathway in cardiac myocytes through the activation of Ras [22] or Raf [23]. Interestingly, although PKC is commonly activated by GPCRs in cardiac myocytes, PKC translocation can be induced by intracellular acidosis in brain cells [24]. We have therefore explored whether there is a role for PKC in the acidosis-induced activation of ERK1/2. ARVM were treated with 1 μM BIM I, which we have shown previously to effectively and selectively inhibit PKC in this cell type [8], prior to their exposure to PMA. PMA-induced ERK1/2 phosphorylation was attenuated by pre-treatment with BIM I, as expected (Figure 7A). Subsequently, ARVM were treated with BIM I prior to their exposure to sustained (3 min) intracellular acidosis. Comparably robust acidosis-induced phosphorylation of ERK1/2 was seen in both vehicle and BIM I-treated cells (Figure 7B). These data indicate that PKC is not involved in ERK1/2 activation by sustained intracellular acidosis.
Figure 7. Effect of PKC inhibition on the activation of ERK1/2 in ARVM.
(A) Western blot of phosphorylated ERK1/2 (pERK1/2) in control cells or following 5 min exposure to 30 nM PMA in the absence (vehicle) or presence of 1 μM BIM I, and quantitative data from four separate experiments. Data are expressed as fold phosphorylation normalized to vehicle control. (B) Western blot of phosphorylated ERK1/2 (pERK1/2) in control cells or following sustained intracellular acidosis in the absence (vehicle) or presence of 1 μM BIM I, and quantitative data from six separate experiments. Data are expressed as fold phosphorylation normalized to vehicle control. *P<0.05 compared with control.
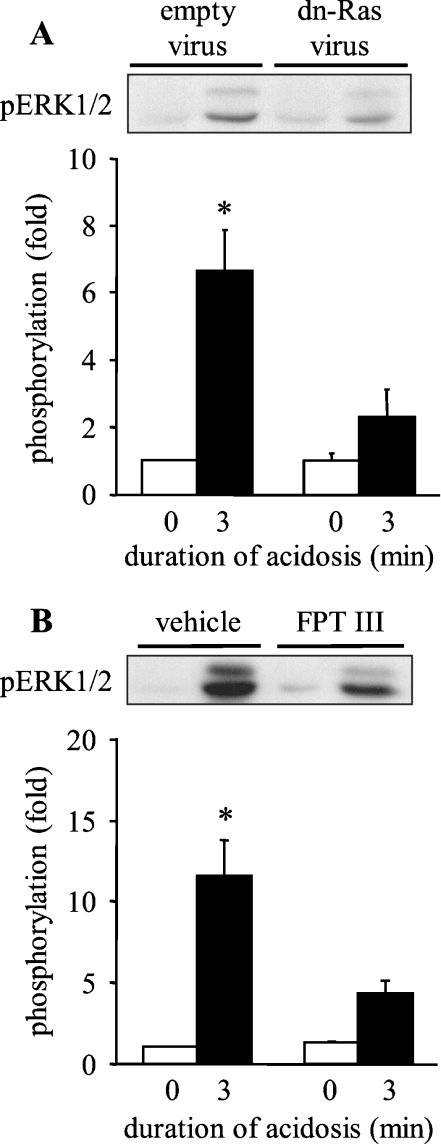
The classical activator of Raf in response to many stimuli is the small G-protein Ras [25]. To determine whether Ras triggers the downstream activation of the Raf/MEK/ERK pathway in response to sustained intracellular acidosis, ARVM were infected with adenovirus encoding dominant-negative N17-Ras 42 h prior to the induction of intracellular acidosis. Infection of ARVM with empty adenovirus again did not affect acidosis-induced phosphorylation of ERK1/2, whereas adenoviral expression of N17-Ras completely prevented such phosphorylation (Figure 8A). As an alternative approach to inhibiting Ras activity, ARVM were exposed to FPT III for 18 h prior to the induction of intracellular acidosis. Pre-treatment with vehicle did not affect acidosis-induced phosphorylation of ERK1/2, whereas pre-treatment with FPT III significantly reduced such phosphorylation (Figure 8B). These data indicate that Ras is activated by sustained intracellular acidosis and triggers the downstream activation of the Raf/MEK/ERK pathway.
Figure 8. Effect of Ras inhibition on the activation of ERK1/2 by sustained intracellular acidosis in ARVM.
(A) Western blot of phosphorylated ERK1/2 (pERK1/2) in control cells or following sustained intracellular acidosis 42 h after infection with control adenovirus (empty virus) or N17-Ras (dn-Ras virus), and quantitative data from six separate experiments. Data are expressed as fold phosphorylation normalized to empty virus control. (B) Western blot of phosphorylated ERK1/2 (pERK1/2) in control cells or following sustained intracellular acidosis in the absence (vehicle) or presence of 50 μM FPT III, and quantitative data from six separate experiments. Data are expressed as fold phosphorylation normalized to vehicle control. *P<0.05 compared with control.
DISCUSSION
Intracellular acidosis adversely affects most of the steps in cardiac myocyte excitation–contraction coupling, including the action potential, the regulation of intracellular [Ca2+] and the response of the myofilaments to Ca2+, resulting in reduced force production [1]. The molecular mechanisms by which intracellular acidosis affects these processes are still not well understood. Although some effects may be caused by conformational changes of key proteins by direct proton binding, resulting in altered protein function, it seems likely that at least some of these changes are brought about by alterations in pHi-sensitive kinase signalling. For example, there is good evidence that intracellular acidosis indirectly promotes the activation of CaMKII (Ca2+/calmodulin-dependent protein kinase II) by raising intracellular [Ca2+], leading to the phosphorylation of phospholamban and a subsequent increase in sarcoplasmic reticulum Ca2+-ATPase activity [26,27], which partially offsets the inhibitory effects of acidosis on other Ca2+-transporting proteins in the myocyte. Acidosis also leads to increased phosphorylation of cardiac troponin I, although it is not clear which kinase is involved [26]. In addition, the same group found an acidosis-dependent inhibition of the activity of the protein phosphatase PP1 [26]. Thus it is apparent that intracellular acidosis can have a profound effect on cardiac function by altering the phosphorylation status of several proteins.
The major finding of the present study is that sustained intracellular acidosis activates the classical Ras/Raf/MEK/ERK pathway in adult ventricular myocytes, as revealed by using a combination of pharmacological and genetic methods to interfere with components of the pathway. Our method of inducing intracellular acidosis, a brief exposure of cells to NH4Cl, does not significantly alter extracellular pH, making interpretation of the results simpler than when intracellular pH is reduced by lowering extracellular pH. Different approaches to inducing intracellular acidosis could have important consequences on the physiological responses of the cell. Zheng et al. [28] have shown recently that acidifying adult ventricular myocytes by exposing cells to low pH media activates p38 MAPK, whereas selectively acidifying the myocyte cytoplasm does not activate p38 MAPK in our hands. It is possible that GPCRs that are sensitive to extracellular acidosis can lead to the activation of p38 MAPK, whereas selective intracellular acidosis bypasses this activation. If this is indeed the case, the lack of any p38 MAPK activation in our hands would support the idea that GPCRs of the Ogr1 family are sensitive to extracellular, but not intracellular, acidosis.
Exposure of cells to NH4Cl does not in itself promote the activation of ERK1/2, as little ERK1/2 phosphorylation was observed after incubating cells in 20 mM NH4Cl for 3 min. Thus it appears that intracellular acidosis is the initiating factor behind ERK1/2 activation. The activation of ERK1/2 by sustained intracellular acidosis follows a transient pattern, consistent with a rapid but short-lived activating signal, followed by activation of the MAPK phosphatase to dephosphorylate ERK1/2. A relatively modest change in intracellular pH (7.05 to 6.83) is sufficient to promote the maximal activation of ERK1/2 that is achievable by intracellular acidosis (within the pHi range studied), indicating that the acid sensor is very sensitive to changes in pHi.
We have shown previously that sustained intracellular acidosis activated ERK1/2 in NRVM, and that such activation was required for the observed stimulation of NHE activity [6]. Activation of ERK1/2 by intracellular acidosis has also been observed in renal proximal tubule cells [29]. However, there seems to be a cell-type-dependent requirement for ERK1/2 activation for NHE stimulation. Thus acidosis-induced stimulation of NHE1 in neonatal myocytes [6] and NHE3 in proximal tubule cells [29] have been found to be dependent on ERK1/2 activity. In contrast, acidosis-induced stimulation of NHE1 activity in colonocytes was insensitive to ERK1/2 inhibition [30]. At present, however, it is not known if sustained intracellular acidosis activates ERK1/2 or other kinases in colonocytes. In NRVM, we found that a number of NHE1 kinases were activated [6], and it is possible that a different kinase is responsible for the stimulation of NHE1 activity in colonocytes.
It is clear from the data in the present study that the entire Ras/Raf/MEK/ERK pathway is activated by intracellular acidosis, indicating that the initiating acid sensor is found at the level of Ras or above. The method of activation of Ras is not clear from our experiments. Ras cycles between an inactive GDP-bound form and an active GTP-bound form, with the active form inducing the recruitment and activation of Raf at the membrane. The exchange of GDP for GTP is normally mediated by a family of regulatory proteins called GEFs. A recent study by Heo et al. [31] showed that acidosis can have a direct effect on Ras in vitro, promoting guanine nucleotide exchange in the absence of GEFs. Interestingly, acidosis appeared to cause a conformational change in Ras, which resembled that seen when the GEF Sos bound to Ras, suggesting a mechanistic basis for the activation of Ras by acidosis [31]. Although it is tempting to suggest that, in ARVM, sustained intracellular acidosis may activate Ras directly by promoting guanine nucleotide exchange, it is difficult to reconcile such a mechanism with the potent inhibitory effect of the dominant-negative N17-Ras that we observed. N17-Ras preferentially binds GDP over GTP [32], which allows N17-Ras to prevent endogenous Ras activation by sequestering GEFs [33]. If intracellular acidosis acts directly on Ras to promote a conformation which mimics GEF-bound Ras, and thereby induces GEF-independent Ras activation, then this process should not be inhibited by the sequestration of GEFs by N17-Ras. It would appear therefore that, in ARVM, acidosis-induced activation of Ras is GEF-mediated, rendering it sensitive to inhibition by N17-Ras. The precise molecular mechanism through which Ras, and therefore the downstream Raf/MEK/ERK cascade, is activated by sustained intracellular acidosis remains to be determined. Our experiments appear to rule out roles for Src family kinases, PKC and Gαq/i/Gα12/13 in the acidosis-induced activation of Ras. Gαs is also present in ARVM, although there is no evidence that it mediates the activation of ERK. We have considered whether Gβγ subunits could be involved, since there is evidence that these subunits contribute to the GPCR-mediated activation of Ras [34]. However, heterologous RGS4 and Lsc expression should promote the re-association of the Gβγ subunits with the targeted Gα subunits, thereby inhibiting downstream signalling through both the α and the βγ subunits. Our results therefore suggest that neither the α nor the βγ subunits are involved in acidosis-induced ERK activation. However, from our experiments, we cannot rule out a contribution from a non-G-protein-mediated mechanism for ERK activation by GPCR.
The inhibitory effects of GW 5074 and ZM 336372, which have been described as selective Raf-1 inhibitors [15,16], suggest that Raf-1 is involved in acidosis-induced ERK activation. However, our data do not rule out potential contributions from A-Raf and/or B-Raf, which are also expressed in cardiac myocytes [35,36].
In conclusion, we have determined that intracellular acidosis induces a rapid and transient activation of ERK1/2 in ARVM. This activation is mediated through the classical Ras/Raf/MEK/ERK pathway, with Ras acting as the upstream trigger.
Acknowledgments
This work was supported by a British Heart Foundation Intermediate Research Fellowship (FS/02/001/13240). S.D. was funded by a Medical Research Council Co-operative Group Core Grant (G0001112). We would like to thank Dr Barbara Kahn for providing us with the N17-Ras adenoviral construct, Dr Thomas Wieland for the RGS4 and Lsc adenoviral constructs, and Dr Jeffery Molkentin for the D208A-MEK1 adenoviral construct.
References
- 1.Orchard C. H., Kentish J. C. Effects of changes of pH on the contractile function of cardiac muscle. Am. J. Physiol. 1990;258:C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- 2.Fliegel L., Dyck J. R. B., Wang H., Fong C., Haworth R. S. Cloning and analysis of the human myocardial Na+/H+ exchanger. Mol. Cell. Biochem. 1993;125:137–143. doi: 10.1007/BF00936442. [DOI] [PubMed] [Google Scholar]
- 3.Leem C. H., Lagadic-Gossman D., Vaughan-Jones R. D. Characterisation of intracellular pH regulation in the guinea-pig ventricular myocyte. J. Physiol. 1999;517:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakabayashi S., Bertrand B., Shigekawa M., Fafournoux P., Pouysségur J. Growth factor activation and “H+-sensing” of the Na+/H+ exchanger isoform 1 (NHE-1) J. Biol. Chem. 1994;269:5583–5588. [PubMed] [Google Scholar]
- 5.Wakabayashi S., Ikeda T., Iwamoto T., Pouysségur J., Shigekawa M. Calmodulin-binding autoinhibitory domain controls ‘pH-sensing’ in the Na+/H+ exchanger NHE1 through sequence-specific interaction. Biochemistry. 1997;36:12854–12861. doi: 10.1021/bi9715472. [DOI] [PubMed] [Google Scholar]
- 6.Haworth R. S., McCann C., Snabaitis A. K., Roberts N., Avkiran M. Stimulation of the plasma membrane Na+/H+ exchanger NHE1 by sustained intracellular acidosis. J. Biol. Chem. 2003;278:31676–31684. doi: 10.1074/jbc.M304400200. [DOI] [PubMed] [Google Scholar]
- 7.Snabaitis A. K., Muntendorf A., Wieland T., Avkiran M. Regulation of the extracellular signal-regulated kinase pathway in adult myocardium: differential roles of Gq/11. Gi and G12/13 proteins in signalling by α1-adrenergic, endothelin-1 and thrombin-sensitive protease-activated receptors. Cell. Signalling. 2005;17:655–664. doi: 10.1016/j.cellsig.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Roberts N. A., Haworth R. S., Avkiran M. Effects of bisindolylmaleimide PKC inhibitors on p90RSK activity in vitro and in adult ventricular myocytes. Br. J. Pharmacol. 2005;145:477–489. doi: 10.1038/sj.bjp.0706210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasutake M., Haworth R. S., King A., Avkiran M. Thrombin activates the sarcolemmal Na+/H+ exchanger: evidence for a receptor-mediated mechanism involving protein kinase C. Circ. Res. 1996;79:705–715. doi: 10.1161/01.res.79.4.705. [DOI] [PubMed] [Google Scholar]
- 10.Haworth R. S., Yasutake M., Brooks G., Avkiran M. Cardiac Na+/H+ exchanger during postnatal development in the rat: changes in mRNA expression and sarcolemmal activity. J. Mol. Cell. Cardiol. 1997;29:321–332. doi: 10.1006/jmcc.1996.0277. [DOI] [PubMed] [Google Scholar]
- 11.Gunasegaram S., Haworth R. S., Hearse D. J., Avkiran M. Regulation of sarcolemmal Na+/H+ exchanger activity by angiotensin II in adult rat ventricular myocytes. Circ. Res. 1999;85:919–930. doi: 10.1161/01.res.85.10.919. [DOI] [PubMed] [Google Scholar]
- 12.Hoshini K., Avkiran M. Effects of moderate hypothermia on sarcolemmal Na+/H+ exchanger activity and its inhibition by cariporide in cardiac ventricular myocytes. Br. J. Pharmacol. 2001;134:1587–1595. doi: 10.1038/sj.bjp.0704405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334:715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- 14.Braz J. C., Bueno O. F., de Windt L. J., Molkentin J. D. PKCα regulates the hypertrophic growth of cardiomyocytes through extracellular signal-regulated kinase 1/2 (ERK1/2) J. Cell Biol. 2002;156:905–916. doi: 10.1083/jcb.200108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lackey K., Cory M., Davis R., Frye S. V., Harris P. A., Hunter R. N., Jung D. K., McDonald O. B., McNutt R. W., Peel M. R., et al. The discovery of potent cRaf1 kinase inhibitors. Bioorg. Med. Chem. Lett. 2000;10:223–226. doi: 10.1016/s0960-894x(99)00668-x. [DOI] [PubMed] [Google Scholar]
- 16.Hall-Jackson C. A., Eyers P. A., Cohen P., Goedert M., Boyle F. T., Hewitt N., Plant H., Hedge P. Paradoxical activation of Raf by a novel Raf inhibitor. Chem. Biol. 1999;6:559–568. doi: 10.1016/s1074-5521(99)80088-x. [DOI] [PubMed] [Google Scholar]
- 17.Thorburn J., McMahon M., Thorburn A. Raf-1 kinase activity is necessary and sufficient for gene expression changes but not sufficient for cellular morphology changes associated with cardiac myocyte hypertrophy. J. Biol. Chem. 1994;269:30580–30586. [PubMed] [Google Scholar]
- 18.Xu Y., Casey G. Identification of human OGR1, a novel G protein-coupled receptor that maps to chromosome 14. Genomics. 1996;35:397–402. doi: 10.1006/geno.1996.0377. [DOI] [PubMed] [Google Scholar]
- 19.Ishii S., Kihara Y., Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J. Biol. Chem. 2005;280:9083–9087. doi: 10.1074/jbc.M407832200. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig M. G., Vanek M., Guerini D., Gasser J. A., Jones C. E., Junker U., Hofstetter H., Wolf R. M., Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 21.Wang J. Q., Kon J., Mogi C., Tobo M., Damirin A., Sato K., Komachi M., Malchinkhuu E., Murata N., Kimura T., et al. TDAG8 is a proton-sensing and psychosine-sensitive G-protein-coupled receptor. J. Biol. Chem. 2004;279:45626–45633. doi: 10.1074/jbc.M406966200. [DOI] [PubMed] [Google Scholar]
- 22.Chiloeches A., Paterson H. F., Marais R., Clerk A., Marshall C. J., Sugden P. H. Regulation of Ras.GTP loading and Ras-Raf association in neonatal rat ventricular myocytes by G protein-coupled receptor agonists and phorbol ester. J. Biol. Chem. 1999;274:19762–19770. doi: 10.1074/jbc.274.28.19762. [DOI] [PubMed] [Google Scholar]
- 23.Zou Y., Komuro I., Yamazaki T., Aikawa R., Kudoh S., Shiojima I., Hiroi Y., Mizuno T., Yazaki Y. Protein kinase C, but not tyrosine kinases or ras, plays a critical role in angiotensin II-induced activation of raf-1 kinase and extracellular signal-regulated protein kinases in cardiac myocytes. J. Biol. Chem. 1996;271:33592–33597. doi: 10.1074/jbc.271.52.33592. [DOI] [PubMed] [Google Scholar]
- 24.Katsura K., Kurihara J., Siesjo B. K., Wieloch T. Acidosis enhances translocation of protein kinase C but not Ca2+/calmodulin-dependent protein kinase II to cell membranes during complete cerebral ischemia. Brain Res. 1999;849:119–127. doi: 10.1016/s0006-8993(99)02072-7. [DOI] [PubMed] [Google Scholar]
- 25.Marais R., Light Y., Paterson H. F., Marshall C. J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mundina-Weilenmann C., Vittone L., Cingolani H. E., Orchard C. H. Effects of acidosis on phosphorylation of phospholamban and troponin I in rat cardiac muscle. Am. J. Physiol. 1996;270:C107–C114. doi: 10.1152/ajpcell.1996.270.1.C107. [DOI] [PubMed] [Google Scholar]
- 27.Hulme J. T., Colyer J., Orchard C. H. Acidosis alters the phosphorylation of Ser16 and Thr17 of phospholamban in rat cardiac muscle. Pflugers Arch. 1997;434:475–483. doi: 10.1007/s004240050423. [DOI] [PubMed] [Google Scholar]
- 28.Zheng M., Reynolds C., Jo S. H., Wersto R., Han Q., Xiao R. P. Intracellular acidosis-activated p38 MAPK signaling and its essential role in cardiomyocyte hypoxic injury. FASEB J. 2005;19:109–111. doi: 10.1096/fj.04-2607fje. [DOI] [PubMed] [Google Scholar]
- 29.Tsuganezawa H., Sato S., Yamaji Y., Preisig P. A., Moe O. W., Alpern R. J. Role of c-src and ERK in acid-induced activation of NHE3. Kidney Int. 2002;62:41–50. doi: 10.1046/j.1523-1755.2002.00418.x. [DOI] [PubMed] [Google Scholar]
- 30.Azriel-Tamir H., Sharir H., Schwartz B., Hershfinkel M. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J. Biol. Chem. 2004;279:51804–51816. doi: 10.1074/jbc.M406581200. [DOI] [PubMed] [Google Scholar]
- 31.Heo J., Gao G., Campbell S. L. pH-dependent perturbation of ras-guanine nucleotide interactions and ras guanine nucleotide exchange. Biochemistry. 2004;43:10102–10111. doi: 10.1021/bi035704a. [DOI] [PubMed] [Google Scholar]
- 32.Feig L. A., Cooper G. M. Inhibition of NIH 3T3 cell proliferation by a mutant ras protein with preferential affinity for GDP. Mol. Biol. Cell. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweighoffer F., Cai H., Chevalier-Multon M. C., Fath I., Cooper G. M., Tocque B. The Saccharomyces cerevisiae SDC25 C-domain gene product overcomes the dominant inhibitory activity of Ha-Ras Asn-17. Mol. Biol. Cell. 1993;13:39–43. doi: 10.1128/mcb.13.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crespo P., Xu N., Simonds W. F., Gutkind J. S. Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 35.Bogoyevitch M. A., Marshall C. J., Sugden P. H. Hypertrophic agonists stimulate the activities of the protein kinases c-Raf and A-Raf in cultured ventricular myocytes. J. Biol. Chem. 1995;270:26303–26310. doi: 10.1074/jbc.270.44.26303. [DOI] [PubMed] [Google Scholar]
- 36.Silberbach M., Gorenc T., Hershberger R. E., Stork P. J. S., Steyger P. S., Roberts C. T. Extracellular signal-regulated protein kinase activation is required for the anti-hypertrophic effect of atrial natriuretic factor in neonatal rat ventricular myocytes. J. Biol. Chem. 1999;274:24858–24864. doi: 10.1074/jbc.274.35.24858. [DOI] [PubMed] [Google Scholar]