Abstract
An increase in circulating levels of specific NEFAs (non-esterified fatty acids) has been implicated in the pathogenesis of insulin resistance and impaired glucose disposal in skeletal muscle. In particular, elevation of SFAs (saturated fatty acids), such as palmitate, has been correlated with reduced insulin sensitivity, whereas an increase in certain MUFAs and PUFAs (mono- and poly-unsaturated fatty acids respectively) has been suggested to improve glycaemic control, although the underlying mechanisms remain unclear. In the present study, we compare the effects of palmitoleate (a MUFA) and palmitate (a SFA) on insulin action and glucose utilization in rat L6 skeletal muscle cells. Basal glucose uptake was enhanced approx. 2-fold following treatment of cells with palmitoleate. The MUFA-induced increase in glucose transport led to an associated rise in glucose oxidation and glycogen synthesis, which could not be attributed to activation of signalling proteins normally modulated by stimuli such as insulin, nutrients or cell stress. Moreover, although the MUFA-induced increase in glucose uptake was slow in onset, it was not dependent upon protein synthesis, but did, nevertheless, involve an increase in the plasma membrane abundance of GLUT1 and GLUT4. In contrast, palmitate caused a substantial reduction in insulin signalling and insulin-stimulated glucose transport, but was unable to antagonize the increase in transport elicited by palmitoleate. Our findings indicate that SFAs and MUFAs exert distinct effects upon insulin signalling and glucose uptake in L6 muscle cells and suggest that a diet enriched with MUFAs may facilitate uptake and utilization of glucose in normal and insulin-resistant skeletal muscle.
Keywords: glucose transport, insulin, mono-unsaturated fatty acid, non-esterified fatty acid (NEFA), protein kinase B (PKB)/Akt, saturated fatty acid, skeletal muscle
Abbreviations: AMPK, AMP-activated protein kinase; FBS, foetal bovine serum; GS, glycogen synthase; GSK3, GS kinase 3; HBS, Hepes-buffered saline; HRP, horseradish peroxidase; MAPK, mitogen-activated protein kinase; Me-AIB, α-methylaminoisobutyrate; MEM, minimal essential medium; mTOR, mammalian target of rapamycin; MUFA, mono-unsaturated fatty acid; NEFA, non-esterified fatty acid; p70S6K, p70 S6 kinase; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; PKC, protein kinase C; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; SNAT2, System A transporter 2
INTRODUCTION
Upon binding to its receptor, insulin regulates numerous cellular responses including, for example, stimulation of glucose uptake, glycogen synthesis and lipogenesis in key target tissues, such as skeletal muscle and white fat, while simultaneously suppressing hepatic gluconeogenesis. However, impaired insulin responsiveness (i.e. insulin resistance) of the above-mentioned tissues leads to dysregulation in the hormonal control of these important physiological responses and contributes to the development of Type 2 diabetes mellitus. The mechanisms that contribute to the pathogenesis of insulin resistance are poorly understood, but NEFAs (non-esterified fatty acids) have been implicated strongly in its development in tissues such as skeletal muscle [1,2]. In particular, there is considerable evidence showing that increased circulating SFAs (saturated fatty acids) promote insulin resistance and reduce skeletal muscle glucose utilization [3,4].
One potential mechanism that may help explain lipid-induced insulin resistance is the reciprocal relationship that exists with respect to skeletal muscle metabolism of glucose and fatty acids, as proposed by Randle in the early 1960s [3]. The Randle hypothesis or the ‘glucose–fatty acid cycle’, as it is also known, proposes that increased availability of NEFAs to muscle would lead to elevated mitochondrial acetyl CoA/CoA and NADH/NAD ratios, which, in turn, would promote inhibition of pyruvate dehydrogenase, resulting in increased citrate levels and an attendant inhibition in phosphofructokinase activity. The effect of inhibiting this latter enzyme would be an overall reduction in glycolytic flux and accumulation of glucose 6-phosphate, which would inhibit hexokinase and thereby decrease glucose uptake. However, the finding that an increase in circulating fatty acids can impair glycogen synthesis in human muscle prior to any significant rise in intramuscular glucose 6-phosphate [4] implies the existence of additional regulatory mechanisms to the Randle cycle.
Indeed, recent work from our laboratory [5] and that of others has shown that palmitate, the most prevalent circulating SFA, can impair the insulin-dependent activation of PKB (protein kinase B; also known as Akt), which has been implicated strongly in the hormonal regulation of glucose transport and glycogen synthesis [6]. This inhibition appears to rely upon de novo synthesis of ceramide from palmitate, since inhibitors of serine palmitoyl-transferase, the enzyme that commits palmitoyl-CoA to ceramide synthesis, antagonize the inhibitory effects of palmitate on PKB [5]. Indeed, short-chain cell-permeant analogues of ceramide mimic the inhibitory effect of palmitate on PKB activation, and such studies have revealed that this inhibition, depending on the experimental system used, involves the ceramide-mediated activation of either a type 2A-like phosphatase, which dephosphorylates PKB on its regulatory phosphorylation sites, or of an atypical PKC (protein kinase C) isoform, which physically interacts with and negatively regulates PKB activation [7–12].
Intriguingly, although SFAs have been implicated in the development of insulin resistance, some MUFAs and PUFAs (mono- and poly-unsaturated fatty acids respectively) appear to either have no adverse effects or positively enhance insulin action [13,14]. Many studies carried out in whole animals have reported contradictory results with respect to the effects of MUFAs and PUFAs on insulin action. This inconsistency may, in part, be explained by the fact that dietary fat is often administered to animals as a mixture of several different fatty acids and there is good evidence that insulin sensitivity is influenced by the dietary fatty acid profile (for a review, see [15]). However, distinct effects of individual SFAs and unsaturated fatty acids have been documented in vitro on cell proliferation (for a review, see [16]), and perhaps the best characterized example seems to be the ability of MUFAs to protect against β-cell apoptosis induced by SFAs [17–19]. In addition, oleate has been shown to stimulate basal glucose uptake in rat adipocytes, probably by mediating changes in the intrinsic activity of glucose transporters [20]. A previous attempt to characterize the effects of fatty acids in murine C2C12 myotubes focused mainly on the effect of SFAs on glycogen synthesis [5a], as these cells do not serve as a particularly good model for investigating GLUT4-mediated glucose uptake. Recent work from our laboratory has characterized the inhibitory effects of palmitate on insulin-stimulated glucose uptake in rat L6 myotubes [5]. In the present study, we have investigated the effect of MUFAs (in particular the effect of palmitoleate) and PUFAs on insulin signalling and glucose metabolism. We show that, unlike SFAs, palmitoleate increases basal glucose uptake by inducing an increase in GLUT1 and GLUT4 abundance in the plasma membrane. Although the precise mechanism underlying this change in carrier abundance remains currently unknown, it appears that exposing muscle cells to palmitoleate results in enhanced glucose oxidation and glycogen synthesis and over-rides the inhibitory effect of palmitate on insulin-stimulated glucose uptake.
EXPERIMENTAL
Materials
α-MEM (α-minimal essential medium), FBS (foetal bovine serum) and antibiotic/antimycotic solution were from Life Technologies. All other reagent-grade chemicals, including insulin, BSA, palmitate, palmitoleate, oleate, linoleate and linoleneate, were obtained from Sigma–Aldrich. Wortmannin, LY-294002, rapamycin, SB-203580, PD-98059 and Ro 31.8220 were purchased from Calbiochem-Novabiochem, and genistein and SB-415286 were from Tocris. Antibodies against PKB and GS (glycogen synthase), and phospho-PKB Ser473, phospho-p70S6K Thr389 (where p70S6K is p70 S6 kinase) and phospho-GSK3β Ser9 (where GSK3 is GS kinase 3) were from New England Biolabs. The anti-GLUT1 antibody was purchased from Chemicon International, the anti-GLUT4 monoclonal antibody (clone 1F8) was obtained from Genzyme Diagnostics, the polyclonal antibody against the SNAT2 (System A transporter 2) was generated in-house, as described previously [22], and a monoclonal antibody against the α1 subunit of the Na+/K+-ATPase was generously given by Dr K. Sweadner (Laboratory of Membrane Biology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, U.S.A.). An antibody against phospho-GS Ser641–645 [residues 635–650 of murine GS (RYPRPVpSVPPpSPSLSR)] was raised in-house by the MRC Protein Phosphorylation Unit in conjunction with the Division of Signal Transduction and Therapy at the University of Dundee. HRP (horseradish peroxidase)-conjugated anti-(rabbit IgG) and anti-(mouse IgG) were obtained from New England Biolabs. Complete protein phosphatase inhibitor tablets were purchased from Boehringer-Roche Diagnostics. D-[U-14C]Glucose and [1-14C]oleate were purchased from Amersham Biosciences, and [14C]Me-AIB (α-methylaminoisobutyrate) and 2-deoxy-D-[3H]glucose were from PerkinElmer Life Sciences.
Cell culture
Rat L6 muscle cells were cultured to myotubes as described previously [23] in α-MEM containing 2% (v/v) FBS and 1% (v/v) antibiotic/antimycotic solution (100 units/ml penicillin, 100 mg/ml streptomycin and 250 ng/ml amphotericin B) at 37 °C with 5% CO2.
Fatty acid treatment
L6 myotubes were maintained in medium containing 2% FBS (v/v), 1% (v/v) antibiotic/antimycotic and 2% BSA (w/v) for up to 24 h, and serum-deprived during the last 2 h prior to any treatment with insulin. L6 muscle cells were exposed to fatty acids that had been conjugated to BSA (fraction V) for the times and at the concentrations indicated in the Figure legends (controls were incubated with vehicle containing BSA but lacking the fatty acid) and incubated with insulin (100 nM) in the penultimate 15 min incubation period for immunoblotting analysis or 30 min for glucose uptake assays.
Cell lysis and cellular fractionation
L6 myotubes were incubated for the time and with the appropriate amount of effectors described in the Figure legends. Following appropriate treatment, cells were washed three times by aspiration with 0.9% (w/v) ice-cold saline and then lysed using lysis buffer [50 mM Tris/HCl (pH 7.4), 0.27 M sucrose, 1 mM sodium ortho-vanadate, 1 mM EDTA, 1 mM EGTA, 10 mM sodium β-glycero-phosphate, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 1% (w/v) Triton X-100, 0.1% (v/v) 2-mercaptoethanol, 0.1 mM microcystin-LR and protease inhibitors]. Whole-cell lysates were centrifuged (15000 g at 4 °C for 10 min) and stored at −20 °C. In some experiments, confluent L6 myotubes were subfractionated following pre-treatment with NEFAs and/or insulin. Subcellular membranes from L6 myotubes were isolated as described previously [22,24]. Following treatment, cells from five dishes (15 cm) were harvested, pooled and pelleted gently. The cell pellet was homogenized [250 mM sucrose, 20 mM Hepes, 5 mM NaN3, 2 mM EGTA (pH 7.4) plus one protease inhibitor tablet per 50 ml] and subjected to a series of differential centrifugation steps to isolate crude cell membranes which were subsequently fractionated on a discontinuous sucrose gradient (32, 40 and 50% sucrose by mass) at 210000 g for 2.5 h. Membranes from top of the 32% sucrose cushion (plasma membrane fraction) were recovered and used subsequently for immunoblotting. The protein content of membrane samples was determined using the Bradford assay [25].
SDS/PAGE and immunoblotting
Cell lysates (50 μg of protein) and plasma membrane fractions from L6 myotubes (20 μg of protein) were subjected to SDS/PAGE on 10% (w/v) acrylamide resolving gels and transferred on to Immobilon-P or Hybond-C membranes (Millipore), as described previously [23]. Membranes were probed with primary antibodies against the proteins of interest. Primary antibody detection was performed using either HRP-conjugated anti-(rabbit IgG) or anti-(mouse IgG) and visualized using enhanced chemiluminescence (Pierce-Perbio Biotechnology) on Kodak X-OMAT film (Eastman-Kodak).
Glucose and amino acid uptake
L6 myotubes were incubated with insulin and/or fatty acids in the absence or presence of various kinase inhibitors at the times and concentrations indicated in the Figure legends. The inhibitors were added 15 min prior to fatty acid treatment. Cells were washed three times with warm HBS [Hepes-buffered saline; 140 mM NaCl, 20 mM Hepes, 5 mM KCl, 2.5 mM MgSO4 and 1 mM CaCl2 (pH 7.4)]. Glucose uptake was assayed by incubation with 10 μM 2-deoxy-D-[3H]glucose (1 μCi/ml) for 10 min as described previously [23,26]. For amino acid transport activity, cells were incubated for 10 min with 10 μM [14C]Me-AIB (47.6 kBq/ml). Non-specific binding was determined by quantifying cell-associated radioactivity in the presence of either 10 μM cytochalasin B or a saturating dose of unlabelled Me-AIB. Medium was aspirated prior to washing adherent cells three times with 0.9% (w/v) ice-cold saline. Cells were subsequently lysed in 50 mM NaOH and the radioactivity was quantified using a Beckman LS 6000IC scintillation counter. Protein concentration in cell lysates was determined using the Bradford reagent, as described previously [25].
[1-14C]Oleate uptake
L6 myotubes cultured in six-well plates were supplemented with medium containing 0.1 μCi/ml [1-14C]oleate for the times indicated in the Figure legends. At specified time points, the incubation medium was carefully removed and set aside. Muscle cells were then washed three times by aspiration with PBS prior to being solubilized in 50 mM NaOH. Radioactivity in the retained medium and solubulized cell lysate was then determined by liquid-scintillation counting.
Glycogen synthesis and glucose oxidation assay
Following treatment of muscle cells with insulin or palmitoleate, as indicated in the Figure legends, muscle cells were incubated with HBS containing 5 mM glucose and 2 μCi/ml D-[U-14C]glucose for 1 h at 37 °C. Following incubation, the culture medium was carefully removed and set aside in a culture flask for analysis of liberated [14C]CO2, whereas the cells were used for determination of the incorporation of 14C into cellular glycogen. Briefly, a glass fibre filter (Whatman) was dipped in 1 M KOH and suspended over the medium in the culture flask. The medium was then acidified by the addition of 6% (v/v) perchloric acid, and the flasks were placed at 37 °C with mild agitation for 2 h. The amount of [14C]CO2 released from the medium and captured on the filters was then measured by liquid-scintillation counting. For analysis of glycogen synthesis, cells were lysed in 10 M KOH and boiled for 30 min. To assist precipitation, 5 mg/ml glycogen was added together with 66% ethanol and lysates kept overnight at 4 °C. Following this period, samples were centrifuged, the supernatant was removed by aspiration and ethanol (66%) was added again. Samples were kept at −20 °C for 4 h prior to being centrifuged at 13000 g for 20 min. The glycogen-containing pellet was dissolved in water and the radioactivity associated with the pellet was measured by scintillation counting.
Statistical analysis
Data analysis was performed using GraphPad Prism software and considered statistically significant at values of P<0.05. Bands from immunoblots were quantified using ImageJ software.
RESULTS
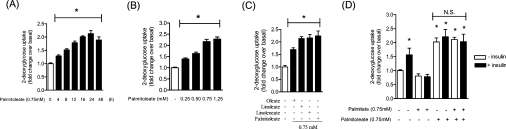
In an attempt to assess the effects of MUFAs on basal glucose uptake, we initially performed time course and dose–response studies. Figure 1(A) shows that glucose uptake was progressively stimulated with increasing exposure time to 0.75 mM palmitoleate (C16:1). Maximal stimulation was achieved by 16 h, as there were no significant increase in uptake beyond this incubation period. The increase in glucose uptake induced by the MUFA was also dose-dependent, being maximally stimulated at palmitoleate concentrations of 0.75 mM (Figure 1B). Given that in previous studies we have shown that 0.75 mM palmitate (C16:0) for 16 h induces a maximal suppression in the insulin-dependent regulation of key signalling intermediates, such as PKB and GSK3, and that of glucose transport [5], subsequent incubation of muscle cells with fatty acids were conducted for 16 h using a concentration of 0.75 mM. The increase in glucose uptake was not restricted to palmitoleate, and was also observed in response to a 16 h incubation of L6 myotubes with equivalent concentrations of oleate (C18:1), linoleate (C18:2) and α-linoleneate (C18:3) (Figure 1C). As shown in Figure 1(D), insulin increased glucose uptake by approx. 50% and, in line with our previous study [5], this hormonal stimulation was abolished when cells had been pre-incubated for 16 h with 0.75 mM palmitate. In contrast, irrespective of whether insulin was present or not, palmitate was unable to suppress the increase in glucose uptake elicited by palmitoleate (Figure 1D). It is noteworthy that maximal concentrations of both insulin and palmitoleate did not seem to exert any additive effect, suggesting that both stimuli either activate the same post-receptor signalling molecules or that signals initiated by each stimulus may ultimately converge upon a common end-point that promotes an increase in hexose uptake (e.g. recruitment and/or activation of glucose transporters).
Figure 1. Effect of MUFAs/PUFAs on basal and insulin-stimulated glucose uptake in L6 myotubes.
Uptake of 2-deoxyglucose was assayed in L6 myotubes following (A) incubation with 0.75 mM palmitoleate for the times indicated, (B) incubation with palmitoleate for 16 h at the concentrations indicated, (C) incubation with 0.75 mM of oleate, palmitoleate, linoleate or linoleneate for 16 h, and (D) incubation with 0.75 mM palmitate and/or 0.75 mM palmitoleate for 16 h, followed by incubation in the absence or presence of insulin (100 nM) during the last 30 min of incubation with the fatty acids. Controls were treated with vehicle alone. Values are expressed as a fold change relative to the untreated control (values are means±S.E.M. of three separate experiments each performed in triplicate). *P<0.05 compared with the control (vehicle only); N.S., non-significant change.
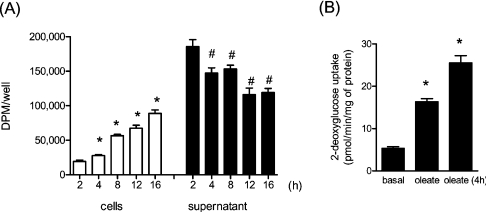
Since half-maximal stimulation of glucose uptake with MUFAs was observed between 4 and 8 h of incubation with the fatty acid, it was important to establish whether there was significant fatty acid uptake into the cells during this period. As shown in Figure 2(A), within 8 h of incubation with 0.75 mM [1-14C]oleate, muscle cells had accumulated a significant proportion of the labelled MUFA presented to them, which was associated with a corresponding loss in fatty-acid-associated radioactivity from the culture medium. After 16 h, approx. 60% of the initial oleate presented remained in the culture medium (Figure 2A). Intriguingly, in parallel experiments, the impact of replacing the fatty-acid-containing medium every 4 h with fresh medium supplemented with 0.75 mM oleate on glucose uptake was tested. This experimental manipulation led to a greater increase in glucose uptake (approx. 50%) than that observed when the MUFA was not replenished in the incubation medium (Figure 2B). This finding implies that maintaining the fatty acid concentration in the medium by regular renewal of the culture medium is likely to enhance the intracellular accumulation of the fatty acid and, thereby, increase its effect on glucose uptake in this cell system.
Figure 2. Uptake of oleate in L6 myotubes.
(A) L6 myotubes were treated with 0.75 mM oleate containing 0.1 μCi/ml [1-14C]oleate for the indicated times. Radioactivity in medium (supernatant) and lysates (cells) was measured as described in the Experimental section. Values are means±S.E.M. from six experimental determinations. *P<0.05 compared with the 2 h group (cells); #P<0.05 compared with the 2 h group (supernatant). (B) Cells were treated either with 0.75 mM oleate for 16 h or the medium was refreshed with 0.75 mM oleate every 4 h over a period of 16 h. Cells were then assayed for glucose uptake. *P<0.05 compared with basal.
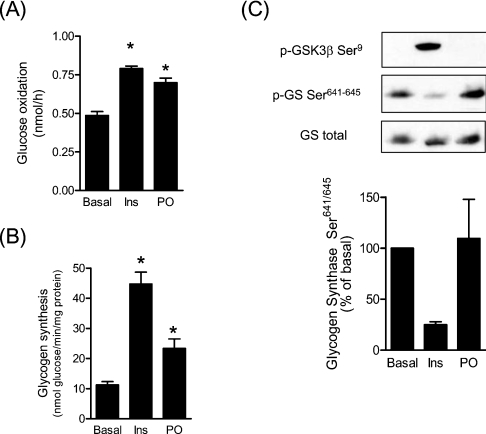
Given the increase in glucose uptake elicited by palmitoleate, we next examined the fate of glucose taken up by muscle cells by monitoring glucose oxidation and glycogen synthesis. Palmitoleate treatment of muscle cells increased glucose oxidation approx. 50% and was comparable with that seen in response to insulin, which was used as a positive control (Figure 3A). The MUFA also stimulated incorporation of glucose into glycogen approx. 2-fold, but the increase was significantly lower than that induced by insulin (approx. 4-fold; Figure 3B). The ability of insulin to stimulate glycogen synthesis is associated with a significant phosphorylation (inactivation) of GSK3 and an associated dephosphorylation of GS on Ser641 and Ser645, two of the residues phosphorylated by GSK3 that play a critical role in regulating its activity. However, unlike insulin, palmitoleate did not promote GSK3 phosphorylation nor did it induce dephosphorylation of these GS residues (Figure 3C).
Figure 3. Effects of palmitoleate on glucose oxidation, glycogen synthesis and phosphorylation of GS in L6 myotubes.
L6 myotubes were pre-incubated in the absence or presence of 0.75 mM palmitoleate (PO) and then the medium was replaced with HBS buffer containing 5 mM glucose (2 μCi/ml [U-14C]glucose) in the absence or presence of insulin (Ins). Subsequently, the culture medium was processed for glucose oxidation (A) and cells were lysed for determination of glycogen synthesis (B), as described in the Experimental section. In (C), L6 cells were treated either with 100 nM insulin for 15 min or with 0.75 mM palmitoleate for 16 h, then lysed and immunoblotted with phospho-specific antibodies against GSK3β Ser9, GS Ser641–645 or total GS. Immunoblots are representative of three separate experiments. The lower panel shows the quantification of the immunoblots from three separate experiments (values are means±S.E.M.) *P<0.05 compared with the control (vehicle only).
To assess the possibility that MUFAs may either activate molecules implicated in insulin signalling or antagonize the suppressive effect of SFAs on molecules mediating the insulin signal to processes regulating fuel use, we immunoblotted lysates from muscle cells following incubation with maximally effective concentrations of insulin, palmitate and/or palmitoleate with phospho-specific antibodies to PKB, GSK3 or p70S6K. Figure 4 shows that an acute (15 min) insulin incubation induced a robust phosphorylation of all three kinases (Figure 4, lane 2), but that cell treatment with palmitate or palmitoleate alone for 16 h (Figure 4, lanes 3 and 5 respectively) had no effect on the phosphorylation of these kinases. However, when cells were incubated with palmitate for 16 h prior to an acute insulin challenge, the insulin-dependent phosphorylation of PKB (Ser473), GSK3β (Ser9) and p70S6K (Thr389) was reduced substantially (Figure 4, lane 4). In contrast, the ability of insulin to induce phosphorylation of all three proteins was unaffected following incubation of myotubes with 0.75 mM palmitoleate (Figure 4, lane 6) and, as such, this MUFA was unable to antagonize the inhibitory effects of palmitate when the two fatty acids were presented to muscle cells simultaneously (Figure 4, lane 8). Another kinase implicated in the regulation of glucose uptake in response to stress stimuli (e.g. hypoxia and exercise) is AMPK (AMP-activated protein kinase) [27]. However, analysis of AMPK phosphorylation on Thr172, a residue considered to reflect the activation status of the kinase, revealed that there was no significant induction in phosphorylation of this site in response to cell treatment with palmitoleate compared with the activation induced by energy depletion using high concentrations of 2-deoxyglucose (results not shown).
Figure 4. Effects of palmitate and palmitoleate on the phosphorylation/activation status of PKB, GSK3 and p70S6K in L6 myotubes.
L6 myotubes were pre-incubated with 0.75 mM palmitoleate and/or palmitate for 16 h prior to incubation with either 100 nM insulin for 15 min. Whole-cell lysates were prepared and immunoblotted with phospho-specific antibodies against PKB Ser473 (p-PKB Ser473), GSK3β Ser9 (p-GSK3β Ser9) and p70S6K Thr389 (p-p70S6K Thr389), and an antibody against total PKB. Immunoblots from three separate experiments were quantified and are shown in the right-hand panels (values are means±S.E.M.).
There is evidence in the literature that certain fatty acids can induce activation of PI3K (phosphoinositide 3-kinase) [28], MAPK (mitogen-activated protein kinase) signalling [29] or modify, via palmitoylation, members of the Src family of tyrosine kinases [30]. To assess whether the stimulatory effect of palmitoleate on glucose uptake involves activation of specific signalling pathways, we investigated the effect of various inhibitors known to target the activation of selected protein kinase cascades. As it was not desirable to have these inhibitors present in the culture medium for 16 h, their ability to suppress the stimulatory effect of palmitoleate was monitored over 4 h, a period over which glucose uptake was stimulated significantly by the MUFA (Figure 1A). It is important to stress that the efficacy of each of the inhibitors used was confirmed in parallel control experiments (results not shown). None of the inhibitors tested, with the exception of rapamycin, was able to suppress the increase in glucose uptake elicited by palmitoleate (Figure 5A). Rapamycin induced a modest, but significant, reduction in the stimulation of glucose uptake by palmitoleate (the net increase in hexose uptake falling from 67% to 40% in the presence of the inhibitor). Although this latter finding implies that mTOR (mammalian target of rapamycin) may feature in the stimulatory action of palmitoleate, it is inconsistent with the lack of any stimulation of p70S6K (a downstream mTOR target) by the MUFA (Figure 4, lane 5). PKC isoforms have also been implicated in the action of certain NEFAs and thus, in separate experiments, we tested whether Ro 31.8220, a bisindolemaleimide that inhibits conventional and novel PKCs in the submicromolar range [31,32] and atypical PKCs in the micromolar range [32], antagonized the stimulation of hexose uptake elicited by palmitoleate. Although Ro 31.8220 induced a dose-dependent inhibition in basal glucose transport, it was unable to prevent the net increase in hexose uptake upon cell incubation with palmitoleate (Figure 5B).
Figure 5. Effect of protein kinase and protein synthesis inhibitors on palmitoleate-induced glucose uptake in L6 myotubes.
L6 myotubes were pre-incubated in the absence or presence of (A) 100 nM wortmannin (Wort), 10 μM LY-294002 (LY), 100 nM rapamycin (Rap), 50 μM SB-415286 (SB4), 10 μM SB-203580 (SB2), 10 μM PD-98059 (PD), 5 μM cycloheximide (ChX) or 100 μM genistein (Gen), or (B) with either 0.1 or 1 μM Ro 31.8220, for 15 min prior to incubation with (+) or without (−) 0.75 mM palmitoleate (PO) for a further 4 h prior to assaying 2-deoxyglucose uptake. Values are means±S.E.M. of at least three separate experiments, each performed in triplicate. *Significant (P<0.05) increase compared with the appropriate untreated control; #significant inhibition (P<0.05) compared with palmitoleate treatment alone.
The finding that cycloheximide failed to prevent the increase in glucose uptake elicited by palmitoleate suggested that de novo protein synthesis was not required to support the stimulatory effect of MUFAs. By extension, this implies that the fatty acid either alters the activity of resident transporters in the plasma membrane or affects their abundance. To test this possibility, we assayed glucose transport over a range of extracellular glucose concentrations following cell treatment with or without palmitoleate. Figure 6 shows that glucose uptake displayed saturable Michaelis–Menten-type kinetics, and that following a 16 h incubation of muscle cells with 0.75 mM palmitoleate the capacity to transport glucose was significantly greater than that seen in non-treated myotubes. The data shown in Figure 6 were subsequently subjected to non-linear regression curve-fitting analysis using GraphPad Prism software to determine the Vmax and Km values. This analysis revealed that palmitoleate induced a significant (2.4-fold) increase in maximal transport from 924±153 to 2193±142 pmol/min per mg of protein, whereas the concentration at which transport was half-maximal (Km) was not altered significantly from 470±90 μM.
Figure 6. Effects of palmitoleate upon the kinetics of 2-deoxyglucose uptake in L6 myotubes.
2-Deoxyglucose transport was assayed over a range of extracellular glucose concentrations following cell treatment with (●) or without (○) palmitoleate (0.75 mM for 16 h). The data were subsequently subjected to non-linear regression curve-fitting analysis using GraphPad Prism software to determine the Vmax and Km. Palmitoleate induced a significant (2.4-fold) increase in Vmax from 924±153 to 2193±142 pmol/min per mg of protein, whereas the concentration at which transport was half-maximal (Km) was not altered significantly from 470±90 μM (values are means±S.E.M. from three separate experiments conducted in triplicate at each hexose concentration). *P<0.05 compared with the appropriate untreated control for each hexose concentration.
To assess whether palmitoleate may have modified the cellular abundance or distribution of GLUT1 and GLUT4, we immunoblotted total cell membranes, as well as isolated plasma membranes, from L6 cells following treatment with the MUFA. Consistent with the cycloheximide data shown in Figure 5(A), we could not detect any increase in the total amounts of either GLUT1 or GLUT4 (Figure 7A). However, analysis of plasma membranes isolated by subcellular fractionation revealed that the abundance of both transporters was noticeably elevated following palmitoleate treatment (approx. 3.5-fold for GLUT1 and 1.7-fold for GLUT4; Figure 7B). In line with the well-documented insulin-dependent translocation of both of these transporters in this particular muscle cell line [23,33,34], acute treatment of L6 myotubes with insulin induced a comparable increase in the cellsurface abundance of both transporters (Figure 7). In separate experiments, exploring the effects of palmitate on palmitoleate-induced transporter recruitment, we could not detect any inhibitory effect of the SFA (results not shown), consistent with the uptake results shown in Figure 1(D). It is also noteworthy that the ability of palmitoleate to induce an increase in cell-surface GLUT1/GLUT4 content with an associated enhancement in glucose uptake does not form part of a generalized cell response to increase nutrient uptake. As with GLUT1 and GLUT4, SNAT2 is also recruited to the plasma membrane in response to insulin [24], but, unlike the glucose transporters, it does not undergo an increase in cell-surface abundance in response to palmitoleate (Figures 8A and 8B). This finding is fully consistent with the lack of any stimulation in functional System A transport over the 16 h incubation period with the MUFA (Figure 8C). The abundance of the α1 subunit of Na+/K+-ATPase was used as a gel-loading control to ensure that changes in immunoreactive content of the three carrier proteins were not due to aberrant loading of protein on SDS/acrylamide gels.
Figure 7. Effect of palmitoleate on GLUT1 and GLUT4 plasma membrane abundance.
(A) L6 myotubes were incubated with either 0.75 mM palmitoleate for 16 h or 100 nM insulin for 30 min (basal, control cells were treated with vehicle alone). Following this incubation period, cells were harvested and total cell or plasma membrane fractions were isolated as described in the Experimental section. Membrane fractions were loaded on to SDS/acrylamide gels and immunoblotted with antibodies against GLUT1, GLUT4 and Na+/K+-ATPase. Immunoblots are representative of three separate experiments. (B) GLUT1 and GLUT4 immunoreactive bands from plasma membrane fractions were quantified, and the abundance expressed relative to α1 subunit of Na+/K+-ATPase, a plasma membrane protein not affected by fatty acid treatment. Values (expressed as a ratio) represent means±S.E.M. of three separate experiments. *P<0.05 compared with the untreated control (basal). PO, palmitoleate; Ins, insulin.
Figure 8. Effect of palmitoleate on SNAT2 and Me-AIB uptake.
(A) L6 myotubes were incubated with either 0.75 mM palmitoleate for 16 h or with 100 nM insulin for 30 min (basal, control cells were treated with vehicle alone). Following this incubation period, cells were harvested and total cell or plasma membrane fractions were isolated as described in the Experimental section. Membrane fractions were loaded on to SDS/acrylamide gels and immunoblotted with antibodies against SNAT2 and Na+/K+-ATPase. (B) SNAT2 immunoreactive bands from plasma membrane fractions were quantified and the abundance expressed relative to α1 subunit of Na+/K+-ATPase, a plasma membrane protein not affected by fatty acid treatment. Values (expressed as a ratio) are means±S.E.M. from two experiments. *P<0.05 compared with untreated control (basal). PO, palmitoleate; Ins, insulin. (C) L6 myotubes were incubated with 0.75 mM palmitoleate for various times and then assayed for Me-AIB uptake as described in the Experimental section. Values are means±S.E.M. of three separate experiments, each performed in triplicate. N.S., non-significant change.
DISCUSSION
Accumulation of intramuscular triacylglycerol (triglyceride) and increased synthesis of fatty-acid-derived metabolites, such as ceramide, are features commonly associated with an elevation in circulating NEFAs and are thought to play an important role in the pathogenesis of insulin resistance in skeletal muscle [5,12,21,35]. The composition of accumulated lipid in skeletal muscle has been reported as being highly reflective of dietary fat [36], and there is general acceptance that intake and deposition of saturated fat, in particular, reduces the sensitivity of skeletal muscle to insulin [15]. Indeed, studies in rodents and human subjects have revealed that changing from a diet rich in SFAs to one with a high proportion of MUFAs improves glucose tolerance and insulin sensitivity [37,38]. Although the molecular basis of this beneficial shift in glucose utilization and insulin sensitivity remains poorly understood, it is possible that it may, in part, arise via effects of unsaturated fatty acids on skeletal muscle, a primary target for insulin action and a major site of whole-body glucose disposal [39]. To assess whether this may be the case, the present study compared the effects of different NEFAs on insulin signalling and glucose transport in cultured L6 skeletal muscle cells. We show that, in line with a previous study [5], incubation of muscle cells with palmitate (C16:0) induced a state of insulin resistance, as judged by the reduction in PKB-directed insulin signalling and the associated loss in insulin-stimulated glucose transport. In contrast, incubation of muscle cells with MUFAs [palmitoleate (C16:1) and oleate (C18:1)] or PUFAs [linoleate (C18:2) and linoleneate (C18:3)] led to a significant increase in basal glucose uptake. Some of the glucose taken up under these circumstances is oxidized, but a significant proportion is also channelled into synthesis of glycogen. Interestingly, the increase in glycogen synthesis induced by the MUFA is not dependent on dephosphorylation of GS on GSK3 target residues, which represent the principal sites of phosphate loss in response to insulin and which underpin the hormonal activation of GS [40]. Although we cannot exclude the possibility that activation of GS might be mediated by dephosphorylation of other sites, a more likely mechanism by which the enzyme is stimulated is via an increase in cytosolic glucose 6-phosphate [41]. The concentration of this allosteric activator of GS will increase as a direct consequence of the greater influx of glucose into cells exposed to the MUFA. The finding that insulin induces a much greater activation of glycogen synthesis than palmitoleate is completely in keeping with the fact that the hormone not only elevates glucose 6-phosphate via stimulation of glucose uptake, but will also simultaneously promote the dephosphorylation and thereby greater activation of GS.
We hypothesized that the increase in glucose uptake elicited by palmitoleate may involve activation of signalling molecules regulating glucose uptake in response to stimuli such as insulin and various stress-inducing agents [6,27]. This possibility is supported by the observation that oleate, but not palmitate, activates PI3K in human breast cancer cells and that this underlies the increase in cell proliferation induced by this MUFA [28]. However, our findings indicate that it is highly unlikely that palmitoleate stimulates PI3K in L6 muscle cells given that the fatty acid does not activate PKB [a molecule whose activation is dependent on PtdIns(3,4,5)P3, a PI3K reaction product] and its stimulatory effect upon glucose uptake was insensitive to PI3K inhibitors (wortmannin and LY-294002). Moreover, palmitate, which ablates insulin-stimulated glucose uptake and causes a significant reduction in the hormonal activation of PKB via a ceramide- and PKCζ-dependent mechanism [5], was unable to antagonize the MUFA-induced stimulation in glucose uptake, most likely because palmitoleate acts at a point downstream of PKB. We currently do not know the precise signalling proteins involved in the MUFA response, but our results do not support the involvement of p38 MAPK, mTOR or AMPK, which can be activated by changes in nutrient availability (including that of certain NEFAs) [27,42,43], nor, based on using a variety of kinase inhibitors, do they support the participation of tyrosine kinases, classical MAPKs or members of the PKC family.
Unlike insulin, which induces a rapid (within minutes) stimulation of glucose uptake, the increase observed in response to palmitoleate was slow and protracted, suggesting that the effects of this MUFA may depend upon increased synthesis of glucose transporters. However, this possibility is negated by the lack of any sensitivity to cycloheximide and the observation that there were no detectable changes in cellular GLUT1 or GLUT4 content following 16 h of incubation with the MUFA. The increase in maximal glucose transport activity (approx. 2.4-fold) elicited by palmitoleate does rely upon a rise in the plasma membrane abundance of both GLUT1 and GLUT4. This suggests that palmitoleate must either induce recruitment of these transporters from a subcellular compartment in a manner similar to that seen in response to insulin and/or promote retention of surface carriers by reducing their endocytic recycling. An elevation in plasma membrane GLUT1 and GLUT4 in response to unsaturated fatty acids is not unprecedented and has been reported previously in 3T3-L1 adipocytes following exposure to arachidonic acid [44]. Although the mechanism by which this PUFA increases cell-surface GLUTs in fat cells remains unknown, the associated increase in basal glucose uptake was partially sensitive to inhibitors of lipoxygenase and, in part, reliant upon PPARγ (peroxisome-proliferator-activated receptor γ) [44]. It is plausible that prolonged exposure of muscle cells to NEFAs may lead to changes in the phospholipid composition of the plasma membrane, influencing membrane fluidity with potential knock-on consequences for the activity of solute transporters resident in the plasma membrane. However, neither the work of Nugent et al. [44] nor pilot studies conducted in our laboratory (results not shown) were able to detect any significant changes in membrane fluidity. Moreover, it is noteworthy, that unlike GLUT1 and GLUT4, SNAT2, which is recruited to the plasma membrane in response to insulin [24] and whose insulin-stimulated activity is suppressed by palmitate [45], does not undergo changes in cell-surface abundance or activity following cell treatment with palmitoleate. These latter findings lend credence to the idea that the increase in glucose transport elicited by palmitoleate is unlikely to be part of a generalized response to the MUFA arising from non-specific effects on membrane fluidity.
Taken together, our results suggest that prolonged exposure of L6 skeletal muscle cells to increased concentrations of palmitoleate stimulates basal glucose uptake by promoting an increase in plasma membrane GLUT1 and GLUT4 abundance. Although the signalling processes that underlie this effect remain poorly understood, our studies exclude a number of potential candidates that have been implicated in the regulation of glucose transport by insulin, nutrients and stress-inducing agents. Moreover, although evidence exists in the literature showing that SFAs and MUFAs exert differential effects upon cell proliferation and apoptosis [18,28,42], our present study shows, for the first time, that SFAs and unsaturated fatty acids exert distinct effects upon glucose uptake in skeletal muscle cells. Taking into account that the average dietary intake of MUFAs (of which approx. 90% is oleate) accounts for approx. 14% of total energy intake, an amount that is comparable with the intake of SFA (PUFAs contribute less, approx. 7% of energy intake), our findings suggest that a change in dietary fatty acid composition to one weighted in favour of MUFAs and PUFAs may enhance glucose uptake and disposal in both normal and insulin-resistant skeletal muscle. This proposition is consistent with studies showing that diets rich in MUFAs protect against the risk of cardiovascular disease and improve glucose homoeostasis in Type 2 diabetes [46,47].
Acknowledgments
We thank the protein production and antibody purification teams (Division of Signal Transduction Therapy, University of Dundee, Dundee, U.K.) co-ordinated by Hilary McLauchlan and James Hastie. M.W. is supported by an Industrial BBSRC (Biotechnology and Biological Sciences Research Council) CASE (Co-operative Awards in Science and Engineering) award. This work was supported by an integrated project (contract LSHM-CT-20004-005272) from European Commission, Medical Research Council and Diabetes UK.
References
- 1.Wang L., Folsom A. R., Zheng Z. J., Pankow J. S., Eckfeldt J. H. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am. J Clin. Nutr. 2003;78:91–98. doi: 10.1093/ajcn/78.1.91. [DOI] [PubMed] [Google Scholar]
- 2.McGarry J. D. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Randle P. J. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Roden M., Price T. B., Perseghin G., Petersen K. F., Rothman D. L., Cline G. W., Shulman G. I. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell D. J., Turban S., Gray A., Hajduch E., Hundal H. S. Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem. J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Chavez J. A., Summers S. A. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Hajduch E., Litherland G. J., Hundal H. S. Protein kinase B: a key regulator of glucose transport? FEBS Lett. 2001;492:199–203. doi: 10.1016/s0014-5793(01)02242-6. [DOI] [PubMed] [Google Scholar]
- 7.Salinas M., Lopez-Valdaliso R., Martin D., Alvarez A., Cuadrado A. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol. Cell. Neurosci. 2000;15:156–169. doi: 10.1006/mcne.1999.0813. [DOI] [PubMed] [Google Scholar]
- 8.Teruel T., Hernandez R., Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-α in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 9.Stratford S., Hoehn K. L., Liu F., Summers S. A. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 10.Doornbos R. P., Theelen M., van der Hoeven P. C., van Blitterswijk W. J., Verkleij A. J., van Bergen en Henegouwen P. M. Protein kinase Cζ is a negative regulator of protein kinase B activity. J. Biol. Chem. 1999;274:8589–8596. doi: 10.1074/jbc.274.13.8589. [DOI] [PubMed] [Google Scholar]
- 11.Bourbon N. A., Sandirasegarane L., Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Cζ: implications for growth arrest. J. Biol. Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 12.Powell D. J., Hajduch E., Kular G., Hundal H. S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol. Cell. Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storlien L. H., Kraegen E. W., Chisholm D. J., Ford G. L., Bruce D. G., Pascoe W. S. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–888. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- 14.Storlien L. H., Jenkins A. B., Chisholm D. J., Pascoe W. S., Khouri S., Kraegen E. W. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40:280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 15.Manco M., Calvani M., Mingrone G. Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes. Metab. 2004;6:402–413. doi: 10.1111/j.1462-8902.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- 16.Hardy R. W., Wickramasinghe N. S., Ke S. C., Wells A. Fatty acids and breast cancer cell proliferation. Adv. Exp. Med. Biol. 1997;422:57–69. doi: 10.1007/978-1-4757-2670-1_5. [DOI] [PubMed] [Google Scholar]
- 17.Maedler K., Spinas G. A., Dyntar D., Moritz W., Kaiser N., Donath M. Y. Distinct effects of saturated and monounsaturated fatty acids on β-cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 18.Maedler K., Oberholzer J., Bucher P., Spinas G. A., Donath M. Y. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic β-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 19.Welters H. J., Tadayyon M., Scarpello J. H., Smith S. A., Morgan N. G. Mono-unsaturated fatty acids protect against β-cell apoptosis induced by saturated fatty acids, serum withdrawal or cytokine exposure. FEBS Lett. 2004;560:103–108. doi: 10.1016/S0014-5793(04)00079-1. [DOI] [PubMed] [Google Scholar]
- 20.Murer E., Boden G., Gyda M., Deluca F. Effects of oleate and insulin on glucose uptake, oxidation, and glucose transporter proteins in rat adipocytes. Diabetes. 1992;41:1063–1068. doi: 10.2337/diab.41.9.1063. [DOI] [PubMed] [Google Scholar]
- 21. Reference deleted.
- 22.Hyde R., Christie G. R., Litherland G. J., Hajduch E., Taylor P. M., Hundal H. S. Subcellular localization and adaptive up-regulation of the System A (SAT2) amino acid transporter in skeletal-muscle cells and adipocytes. Biochem. J. 2001;355:563–568. doi: 10.1042/bj3550563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajduch E., Alessi D. R., Hemmings B. A., Hundal H. S. Constitutive activation of protein kinase Bα (PKBα) by membrane targeting promotes glucose and System A amino acid transport, protein synthesis and GSK3 inactivation in L6 muscle cells. Diabetes. 1998;47:1006–1013. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- 24.Hyde R., Peyrollier K., Hundal H. S. Insulin promotes the cell surface recruitment of the SAT2/ATA2 System A amino acid transporter from an endosomal compartment in skeletal muscle cells. J. Biol. Chem. 2002;277:13628–13634. doi: 10.1074/jbc.M108609200. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 26.Blair A. S., Hajduch E., Litherland G. J., Hundal H. S. Regulation of glucose transport and glycogen synthesis in L6 muscle cells during oxidative stress: evidence for cross-talk between the insulin and SAPK2/p38 MAP kinase signalling pathways. J. Biol. Chem. 1999;274:36293–36299. doi: 10.1074/jbc.274.51.36293. [DOI] [PubMed] [Google Scholar]
- 27.Kahn B. B., Alquier T., Carling D., Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Hardy S., Langelier Y., Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000;60:6353–6358. [PubMed] [Google Scholar]
- 29.Brown J. M., Boysen M. S., Chung S., Fabiyi O., Morrison R. F., Mandrup S., McIntosh M. K. Conjugated linoleic acid induces human adipocyte delipidation: autocrine/paracrine regulation of MEK/ERK signaling by adipocytokines. J. Biol. Chem. 2004;279:26735–26747. doi: 10.1074/jbc.M401766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang X., Lu Y., Wilkes M., Neubert T. A., Resh M. D. The N-terminal SH4 region of the Src family kinase Fyn is modified by methylation and heterogeneous fatty acylation: role in membrane targeting, cell adhesion, and spreading. J Biol. Chem. 2004;279:8133–8139. doi: 10.1074/jbc.M311180200. [DOI] [PubMed] [Google Scholar]
- 31.Alessi D. R. The protein kinase C inhibitors RO318220 and GF109203X are equally potent inhibitors of MAPKAP kinase-1β (Rsk-2) and p70 S6 kinase. FEBS Lett. 1997;402:121–123. doi: 10.1016/s0014-5793(96)01510-4. [DOI] [PubMed] [Google Scholar]
- 32.Standaert M. L., Galloway L., Karnam P., Bandyopadhyay G., Moscat J., Farese R. V. Protein kinase c-ζ as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes – potential role in glucose transport. J. Biol. Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 33.Bilan P. J., Mitsumoto Y., Ramlal T., Klip A. Acute and long term effects of insulin-like growth factor-I on glucose transporters in muscle cells: translocation and biosynthesis. FEBS Lett. 1992;298:285–290. doi: 10.1016/0014-5793(92)80078-u. [DOI] [PubMed] [Google Scholar]
- 34.Mitsumoto Y., Klip A. Developmental regulation of subcellular distribution and glycosylation of GLUT1 and GLUT4 glucose transporters during myogenesis of L6 muscle cells. J. Biol. Chem. 1992;267:4947–4962. [PubMed] [Google Scholar]
- 35.Summers S. A., Garza L. A., Zhou H., Birnbaum M. J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson A., Nalsen C., Tengblad S., Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am. J. Clin. Nutr. 2002;76:1222–1229. doi: 10.1093/ajcn/76.6.1222. [DOI] [PubMed] [Google Scholar]
- 37.Rocca A. S., LaGreca J., Kalitsky J., Brubaker P. L. Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology. 2001;142:1148–1155. doi: 10.1210/endo.142.3.8034. [DOI] [PubMed] [Google Scholar]
- 38.Vessby B., Unsitupa M., Hermansen K., Riccardi G., Rivellese A. A., Tapsell L. C., Nalsen C., Berglund L., Louheranta A., Rasmussen B. M., et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia. 2001;44:312–319. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- 39.DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose: results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 40.Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle: effects of insulin on the state of phosphorylation of the 7 phosphoserine residues in vivo. Eur. J. Biochem. 1983;130:227–243. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- 41.Villar-Palasi C., Guinovart J. J. The role of glucose 6-phosphate in the control of glycogen synthase. FASEB J. 1997;11:544–558. [PubMed] [Google Scholar]
- 42.Miller T. A., LeBrasseur N. K., Cote G. M., Trucillo M. P., Pimentel D. R., Ido Y., Ruderman N. B., Sawyer D. B. Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem. Biophys. Res. Commun. 2005;336:309–315. doi: 10.1016/j.bbrc.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 43.Hornberger T. A., Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J. Cell. Biochem. 2005;97:1207–1216. doi: 10.1002/jcb.20671. [DOI] [PubMed] [Google Scholar]
- 44.Nugent C., Prins J. B., Whitehead J. P., Wentworth J. M., Chatterjee V. K., O'Rahilly S. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane. Evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2001;276:9149–9157. doi: 10.1074/jbc.M009817200. [DOI] [PubMed] [Google Scholar]
- 45.Hyde R., Hajduch E., Powell D. J., Taylor P. M., Hundal H. S. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 2005;19:461–463. doi: 10.1096/fj.04-2284fje. [DOI] [PubMed] [Google Scholar]
- 46.Kris-Etherton P. M. AHA science advisory: monounsaturated fatty acids and risk of cardiovascular disease. J. Nutr. 1999;129:2280–2284. doi: 10.1093/jn/129.12.2280. [DOI] [PubMed] [Google Scholar]
- 47.Ros E. Dietary cis-monounsaturated fatty acids and metabolic control in type 2 diabetes. Am. J. Clin. Nutr. 2003;78:617S–625S. doi: 10.1093/ajcn/78.3.617S. [DOI] [PubMed] [Google Scholar]