Abstract
Tyrosine O-sulfation is a post-translational modification mediated by one of two Golgi tyrosylprotein sulfotransferases (TPST-1 and -2) expressed in all mammalian cells. Tyrosine sulfation plays an important role in the function of some known TPST substrates by enhancing protein-protein interactions. To explore the role of these enzymes in vivo, and gain insight into other potential TPST substrates, TPST-2 deficient mice were generated by targeted disruption of the Tpst2 gene. Tpst2+/- mice appear normal and, when interbred, yield litters of normal size with a Mendelian distribution of the targeted mutation. Tpst2-/- mice have moderately delayed growth, but appear healthy and attain normal body weight by 10 weeks of age. In contrast to Tpst1-/- males that have normal fertility, Tpst2-/- males are infertile. Tpst2-/- sperm are normal in number, morphology, and motility in normal media and appear to capacitate and undergo acrosomal exocytosis normally. However, they are severely defective in their motility in viscous media and in their ability to fertilize zona pellucida (ZP)-intact eggs. Adhesion of Tpst2-/- sperm to the egg plasma membrane is reduced compared to wild type sperm, but sperm-egg fusion is similar or even increased. These data strongly suggest that tyrosine sulfation of unidentified substrate(s) play a crucial role in these processes and document for the first time the critical importance of post-translational tyrosine sulfation in male fertility.
Tyrosine O-sulfation is a widespread posttranslational modification that was first described about 50 years ago (1,2). Tyrosine-sulfated proteins and/or tyrosylprotein sulfotransferase (TPST4) activity have been described in many species throughout the plant and animal kingdoms, including Volvox carteri, one of the earliest multicellular organisms. At this time, 37 tyrosine-sulfated proteins have been identified in man, many of which play important roles in inflammation, hemostasis, immunity, and other processes (3-5). These include certain adhesion molecules, G-protein coupled receptors, coagulation factors, serpins, extracellular matrix proteins, hormones, and others. It has been demonstrated that some of these proteins require tyrosine sulfation for optimal function (6). Nevertheless, it is very likely that we are only beginning to appreciate the complexity of the TPST substrate repertoire.
In mouse and man tyrosine O-sulfation is mediated by one of two tyrosylprotein sulfotransferases, called TPST-1 and TPST-2, which are localized to the trans-Golgi network. (7-9). Mouse TPST-1 and-2 are 370 and 376 residue type II transmembrane proteins, respectively. Each has a short N-terminal cytoplasmic domain, followed by a single ≈ 17 residue transmembrane domain, a membrane proximal ≈ 40 residue stem region, and a luminal catalytic domain containing four conserved cysteine residues and two N-glycosylation sites. The amino acid sequence of human and mouse TPST-1 are ≈ 96% identical and human and mouse TPST-2 have a similar degree of identity. TPST-1 is ≈ 65-67% identical to TPST-2 in both man and mouse. Both isoenzymes are broadly expressed in human and murine tissues and cell lines and are co-expressed in most, if not all, cell types (6). However, the relative abundance of the two proteins in tissues and cells is uncertain. The human TPST1 and TPST2 genes are on 7q11.21 and 22q12.1, respectively. The mouse Tpst1 and Tpst2 genes are ≈ 18.5 Mbp apart on chromosome 5.
In vitro studies using synthetic peptide acceptors indicate that the two TPST isoenzymes differ in substrate preference. Peptides modeled on the N-terminus of P-selectin glycoprotein ligand-1 are sulfated by the two isoenzymes with equal efficiency. In contrast, peptides modeled on sulfation sites of human C4 α chain and heparin cofactor II are sulfated more efficiently by TPST-1, than by TPST-2 (8). Seibert et al studied sulfation of a peptide modeled on the N-terminal extracellular domain of human CCR5 that has 4 potential tyrosine sulfation sites (10). They showed that TPST-1 preferred Tyr14 over Tyr15 as the initial sulfation site, whereas TPST-2 preferred Tyr15 over Tyr14. Nevertheless, it is not known if TPST-1 and -2 have distinct macromolecular substrate specificities in vivo.
To gain an understanding of the biological role(s) of TPSTs, we generated TPST-deficient mice by targeted disruption of either the Tpst1 or Tpst2 gene. Previous studies of the Tpst1-/- mice (Tpst1tm1Klm, MGI:2183366) revealed unexpected and pleiotropic effects, including modest effects on body weight and fecundity (11). Tpst1+/-mice appear normal and, when interbred, yield litters of normal size with a Mendelian distribution of the targeted mutation. A histological survey of the Tpst1 knockout did not reveal any abnormalities. Northern analyses showed that disruption of the Tpst1 gene did not effect transcription of the Tpst2 gene. Tpst1-/- mice appear healthy but have ≈ 5% lower average body weight than wild type mice. Fertility of Tpst1-/- males and females per se was normal. However, Tpst1-/- females have smaller litters than wild type females due to fetal death between 8.5 and 15.5 days post coitum.
Here we describe the production and characterization of Tpst2 knockout mice. In this study, we find that, in contrast to Tpst1-/- males, Tpst2-/- males are infertile. We find that Tpst2-/- males are eugonadal and have normal spermatogenesis. Epididymal sperm from Tpst2-/- males are normal in number, morphology, and motility and appear to capacitate in vitro and undergo acrosome exocytosis in response to agonist normally. However, they are severely defective in motility in viscous media and in their ability to fertilize ZP-intact eggs. In addition, in vitro fertilization (IVF) experiments designed to assess the gamete membrane interactions revealed that sperm from Tpst2-/- males have reduced ability to adhere to the egg plasma membrane but are able to undergo membrane fusion with the egg.
EXPERIMENTAL PROCEDURES
Gene Targeting. Mouse genomic clones were identified and plaque purified from a 129S6/SvEv spleen Lambda FIX II® genomic library (Stratagene) by high stringency hybridization with a full-length mouse TPST-2 cDNA probe. The organization of the Tpst2 gene was characterized by a combination of Southern blotting, restriction mapping, and sequencing. Comparison of genomic and cDNA sequences showed that most of the TPST-2 open reading frame is encoded by a 912 bp exon. This exon encodes the N-terminal 279 amino acids of the protein, including the 5’ PSB and 3’ PB motifs that are involved in binding the 5’ and 3’ phosphates of 3’, 5’-ADP in the known sulfotransferase crystal structures (Fig 1A) (12). A targeting construct was designed to replace a 0.88 kb fragment spanning the start codon and the remainder of exon 3 by a 1.7 kb PGKneo cassette (Fig. 1B). A 4.5 kb EcoRI-ApaI genomic fragment was used as the left arm for homologous recombination and was cloned into the pPGKneo vector upstream of the PGKneo cassette. A 1.6 kb ApaI-BglII genomic fragment 3’ to exon 3 was cloned downstream of the PGKneo cassette as the right arm for homologous recombination. The construct was linearized with NotI and electroporated into AB2.2 embryonic stem cells (Lexicon Genetics, Inc.). After G418 selection, five positive clones were identified from 900 G418-resistant colonies by PCR screening. Clones were analyzed by Southern blotting to confirm the correct homologous recombination event, and random integration of extra copies of the targeting construct was excluded by hybridization with a neomycin probe (not shown). ES clones were injected into blastocysts, and the blastocysts implanted into pseudopregnant mice to generate chimeras. Male chimeras were mated with 129S6/SvEvTac females (Taconic Farms) to generate Tpst2+/- mice that were then interbred to generate Tpst2-/- mice. Genotyping was performed by PCR using the following primers; 5’-GAG GAC CTC ATT GGC AAG CCT-3’, and 5’-CAA CCT GGT GGA GTC GCT TCT CTC-3’ for the 520 bp fragment from the wild type allele, and 5’-CCT CGT GCT TTA CGG TAT CGC CGC-3’, and 5’-CAA CCT GGT GGA GTC GCT TCT CTC-3’ for the 822 bp fragment from the mutant allele. All experiments reported here were performed on mice in the 129S6/SvEvTac background. Animals were mated, housed, and fed as previously described (11) and all animal procedures were approved by the Institutional Animal Care and Use Committees.
Fig. 1.
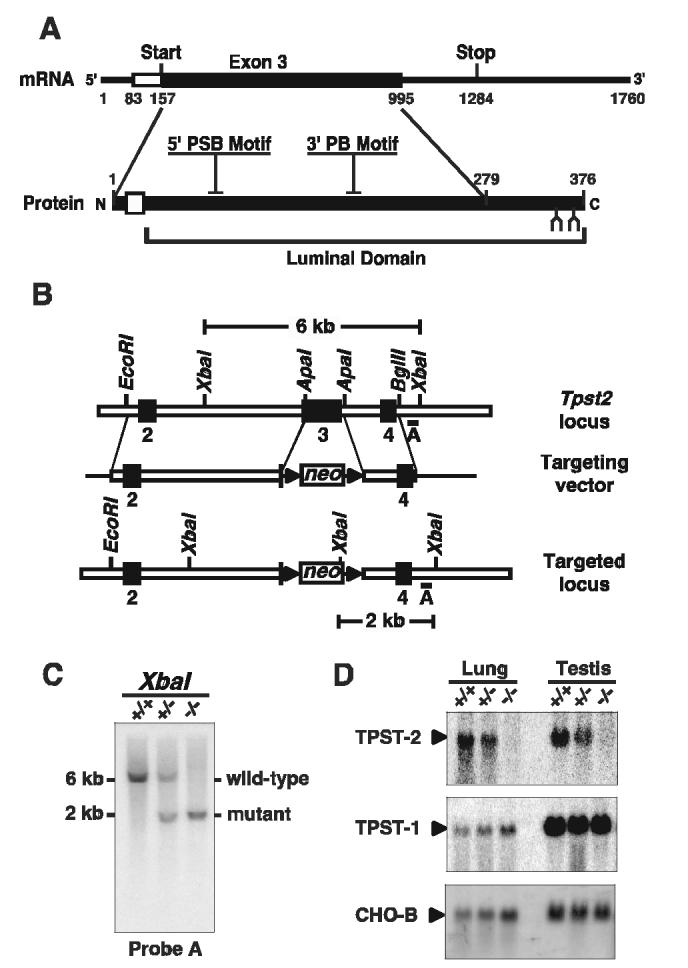
Targeted Disruption of the Tpst2 Gene. A. Diagram of the mouse TPST-2 mRNA and corresponding primary structural features of the TPST-2 protein. The untranslated and translated portions of exon 3 are indicated by open and solid rectangles, respectively. The transmembrane domain is indicated by an open rectangle, N-glycosylation sites are indicated (lancet), and the location of the 5’ PSB and 3’ PB motifs common to the sulfotransferase gene family are shown. B. Diagram of the wild type Tpst2 locus, targeting vector, and targeted locus. A 0.88 kb fragment spanning the start codon and the remainder of exon 3 was replaced by a 1.7 kb PGKneo cassette. Triangles indicate the location of loxP sites. C. Southern analysis of XbaI digested genomic DNA. Homologous recombination at the Tpst2 locus adds an XbaI site that results in a shorter XbaI genomic fragment (2 kb) than that from the wild type allele (6 kb) that is detected by hybridization with Probe A. D. Northern analysis of total RNA from tissues of Tpst2+/+, Tpst2+/-, Tpst2-/- mice using either a TPST-2 (top), TPST-1 (middle), or control CHO-B (bottom) cDNA probe.
Blot Analysis. Total RNA was isolated from tissues using Trizol Reagent (Gibco/BRL), separated on 1% denatured gels, and transferred to nitrocellulose membranes. For Northern blotting, a 489 bp fragment corresponding to nt 958-1446 of the full-length cDNA was used as a probe. For Southern blotting, tail DNA was isolated (Qiagene Genomic DNA kit), exhaustively digested with XbaI, separated on 1% agarose gels, and transferred to nitrocellulose membranes. A 469 bp TPST-2 DNA fragment downstream of the right arm was used as a probe. CHO-B (GeneBank Accession No. L22552), a partial cDNA probe for ribosomal protein S2 from Chinese hamster, was used as a control probe (13). Blots were processed and imaged as previously described (11).
Sulfotyrosine analysis -Tissues were rinsed with PBS and cultured in sulfate-free Joklik modified Eagles medium containing 2% dialyzed FBS and 0.15 mCi/ml carrier-free Na35SO4 at 37°C in 5% CO2/95% air. After 24 hrs, conditioned media was collected and the tissue explants were rinsed three times in PBS, solubilized in 2% SDS, 62.5 mM Tris-HCl, pH 6.8 and the extracts were boiled for 5 min. Conditioned media and the SDS extracts were clarified by centrifugation (16,000g, 10 min), proteins were precipitated with cold acetone, the precipitates washed three times with cold acetone, and air-dried. These samples were hydrolyzed (0.2 M Ba(OH)2 in degassed H2O) for 18 hr at 110°C and then neutralized by slow addition of H2SO4 using phenol red as an indicator, as previously described (14). Neutralized hydrolysates were centrifuged (16,000g, 10 min) and supernatants were lyophilized. Samples were dissolved in 20 μL of electrophoresis buffer (5% formic acid, 15.6% glacial acetic acid, pH 1.9), spiked with 2 μg of unlabeled sulfoamino acid standards, spotted on cellulose plates (EMD Chemicals), and subjected to thin layer electrophoresis (600 v, 10 psi, 2 hrs) using a Hunter TLE system (CBS Scientific, Model HTLE-7000). After electrophoresis, standards were visualized by spraying the plates with 0.25% ninhydrin in acetone followed by heating (100°C, 5 min). The plates were exposed to pre-flashed BioMax MS film (Kodak) and [35S]sulfotyrosine quantitated using Image J software from a standard curve of serial dilutions of [35S] standards spotted on the plates.
Serum Assays. Serum testosterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) levels assays were performed by Anilytics, Inc. (Gaithersburg, MD). LH and FSH levels were determined by radioimmunoassay. Testosterone levels were measured using the Coat-a-Count® total testosterone kit (Diagnostic Products Corporation).
Analysis of Natural Matings. Superovulated 4 week-old FVB/N female mice (Charles Rivers) were placed in cages pairwise with 10-12 week-old virgin wild type or Tpst2-/- males beginning at noon. The next morning females were checked for copulatory plugs. In one group of matings, plugs were removed from the females, washed in PBS, and the washings examined microscopically for the presence of sperm. The uteri of the plugged females were then excised en block and the proximal end of the uterus was cannulated and each horn of the uterus was separately inflated with ≈ 1 ml of PBS. The distal end of the uterine horn was transected to release the fluid and the washings from each horn were pooled and examined microscopically for the presence of sperm. In a separate group of matings, egg clutches were recovered from the ampullae of the oviducts into M2 media (Cell and Molecular Technologies) and incubated with 0.03% hyaluronidase to remove cumulus cells. A single observer blinded to the genotype of the animal, counted and scored each egg as having been fertilized as indicated by the presence of a male pronucleus.
Sperm Analysis. Male mice (10-12 week-old) were anesthetized and the cauda of the epididymis was removed and placed in a 35-mm Petri dish containing 0.5 ml of pre-warmed HTF media (100 mM NaCl, 4.7 mM KCl, 0.2 mM MgSO4, 0.37 mM KH2PO4, 2 mM CaCl2, 25 mM NaHCO3, 2.78 mM glucose, 0.33 mM sodium pyruvate, and 21.4 mM sodium lactate, pH 7.4), a media based on the composition of human tubal fluid (15). Sperm were released by slicing the epididymides several times with a 22-gauge needle. After a 5 min “swim-out” period, sperm-containing supernatant was collected, a sample was loaded on a hemacytometer, and the number of immotile sperm counted. After this count, 1/10th volume of Kimura stain was added to another sample of the supernatant and the total sperm count determined. The percentage of motile sperm was then calculated as ((total-immotile) ÷ total) x 100. A single observer that was blinded to the genotype of the animal performed all counts. An additional sample of the supernatant was fixed by addition of an equal part of 8% paraformaldehyde in PBS, centrifuged at 20 g for 1 min, and the pellet resuspended in mounting media and examined by phase contrast imaging.
For computer-assisted sperm analysis (CASA) and IVF assay with cumulus-intact eggs, the epididymides and vas deferentia from each male were dissected into 1 ml HTF media containing 0.075% penicillin G, 0.05% streptomycin sulfate, 0.001% phenol red, 0.4% BSA; the pH was adjusted by gassing with 5% CO2, 5% O2 and 90% N2 at 37°C. Sperm were released by slicing the epididymides several times with a 30-gauge needle and by “walking” a pair of 30-gauge needles along the length of the vas deferentia to extrude the contents. The tissues were then allowed to stand for a 10 min “swim-out” period at 37°C. Sperm samples were diluted 1:10 with a modification of Whittingham’s PB1 media (0.8% (w/v) NaCl, 0.02% KCl, 0.01% MgCl2, 0.01% CaCl2, 0.1% dextrose, 0.115% Na2HPO4, 0.0036% Na pyruvate, 0.0075% penicillin G, 0.005% streptomycin sulfate, 0.3 % BSA) and then loaded into cannulae and analyzed using an IVOS™ Sperm Analyzer (Hamilton Thorne Research). The following parameters were measured: mean straight line velocity (μm/sec, straight line distance divided by time), mean curvilinear velocity (μm/sec, total distance divided by time), linearity (distance in straight line divided by total distance), mean track width (μm, deviation of the head from the mean head trajectory), and beat cross frequency (Hz, the number of times the sperm head crosses the mean head trajectory per second).
For analysis of sperm motility in viscous media the epididymides and vas deferentia were removed and placed in 250 μl of capacitation media consisting of M199 with 0.4% BSA, (EquitechBio, Inc.), equilibrated at 37°C and 5% CO2. Sperm were released by slicing the tissue with a 22-gauge needle and incubated for 10 min to allow the sperm to “swim out”. The swim-out sperm were layered under 750 μl M199-BSA in 12 x 75 mm culture tubes, and incubated for 45 min to allow motile sperm to “swim up”. The upper 500 μl swim-up fraction was collected, and incubated for an additional 60 min at 37°C, 5% CO2. For observation of sperm in normal viscosity media, a 40 μl drop of the swim-up sample in M199-BSA was placed in glass bottom culture dishes (MatTek Corp.) on a heatable mounting frame held at 37°C by a Tempcontrol 37-2 digital temperature controller (Carl Zeiss). For observation of sperm in viscous media, swim-up samples were injected under a drop of 2% long chain polyacrylamide (Scientific Polymer Products, Inc.) in M199-BSA equilibrated at 37°C using a 25-gauge needle. Video images were acquired at 30 frames per second using a Zeiss Axiovert 200 inverted microscope equipped with a CCD camera (Hammamatsu) and a VHS recorder. For normal viscosity media, a 1 min recording of a single field was acquired, whereas for viscous media, two 1 min recordings of separate fields were acquired and results from the two fields were averaged. Video images were analyzed off-line. The number of sperm that were inactive, active but with no forward velocity, or active with forward velocity were characterized as immotile, non-progressive, and progressive, respectively. Sperm velocities were measured by tracking individual cells frame-by-frame using NanoTrack Software (Isee Imaging Systems).
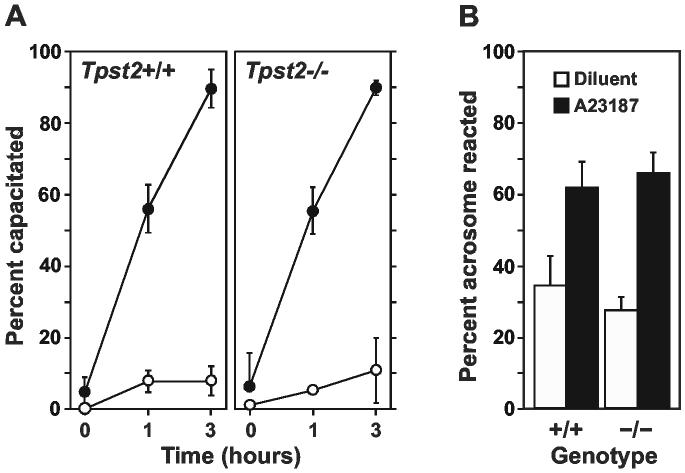
Capacitation. Extraction of cholesterol from sperm membranes during in vitro capacitation results in the redistribution of cholesterol-rich lipid rafts as assessed by immunofluorescence staining with cholera toxin ß-subunit (CTß) that binds ganglioside GM1 that is enriched in lipid rafts. CTß staining is restricted to the equatorial segments and head cap on uncapacitated sperm. However, after capacitation the toxin diffusely stains both the head and tail (16). To assess sperm capacitation, sperm were incubated in M199 with or without 0.4% BSA at 37°C. At various time points sperm samples were diluted with one part of 10 μg/ml FITC-CTß (Sigma) in PBS, 0.1% BSA for 30 min at room temperature (16). After washing, the stained sperm were fixed (4% paraformaldehyde, 10 min) and then mounted in Vectashield (Vector Labs). A total of two hundred sperm were scored as being capacitated (green staining of sperm head and tail) or uncapacitated (green staining of sperm head only) by an observer blinded to the genotype of the sample.
Acrosomal Exocytosis. Sperm were isolated as described above and were capacitated in M199-BSA for 2 hr at 37°C. Calcium ionophore A23187 (50 μM) or diluent was added and the sperm incubated for an additional hour. Samples were fixed (70% ethanol, 10 min), air-dried onto glass slides, and then stained with 10 μg/ml FITC-conjugated Arachis hypogaea lectin (Peanut agglutinin, Vector Labs) in PBS for 10 min at room temperature. After washing, the slides were mounted in Vectashield containing 0.5 μg/ml propidium iodide. The samples were examined using an Olympus IMT-2 fluorescence microscope equipped with a dual band pass filter. Two hundred sperm were scored as being acrosomeintact (red nucleus with green acrosomal cap) or acrosome-reacted (red nucleus without green cap) by an observer blinded to the genotype of the sample.
IVF of Cumulus-Intact Eggs. Metaphase II-arrested eggs were collected from 23 ± 2 day-old superovulated CB6F1/J mice at 13 hr after human chorionic gonadotropin (hCG) injection. Females were sacrificed and oviducts were dissected into HTF media. Egg clutches (eggs and surrounding cumulus cell mass) were released by tearing the ampullae of the oviducts. IVF was carried out as described by Sztein et al. (17) with minor modification. Eggs and 5 μl sperm were added to a 250 μl drop of HTF in a Petri dish under light mineral oil (Sigma) at 37°C under an atmosphere of 5% CO2, 5% O2 and 90% N2. After 4-5 hr, eggs were washed by transfer through drops of fresh HTF and allowed to incubate overnight at 37°C under the same atmosphere. The next morning an observer blinded to the genotype of the animal scored the number of 2 cell embryos.
IVF of ZP-intact and ZP-free Eggs. Egg collection was performed as previously described (18). Metaphase II-arrested eggs were collected from 6-8 week-old superovulated CF-1 (Harlan) mice at 13 hr after hCG injection. Cumulus cells were removed by brief incubation (<5 min) in Whitten’s-HEPES media containing 3% BSA (Albumax I, Gibco-BRL) and 0.02% Type IV-S hyaluronidase from bovine testis (Sigma). Eggs that were to be inseminated ZP-intact were cultured in Whitten’s media with 22 mM NaHCO3 (hereafter referred to as Whitten’s) supplemented with 10% FBS until the time of insemination to prevent premature ZP conversion (19). For eggs that were to be inseminated ZP-free, the ZP were removed after cumulus cell removal by a brief incubation (∼15 sec) in acidic culture media compatible buffer (10 mM HEPES, 1 mM NaH2PO4, 0.8 mM MgSO4, 5.4 mM KCl, 116.4 mM NaCl, pH 1.5). The eggs were then allowed to recover for 60 min in Whitten’s, 1.5% BSA. All egg cultures were performed in a humidified atmosphere of 5% CO2 in air.
Caudal epididymal sperm were collected from Tpst2-/- mice or wild type control mice. The sperm from one epididymis were collected in 125 μl Whitten’s, 1.5% BSA. After 10-15 minutes, tissue was removed from the droplet and the sperm were pipetted into the bottom of a tube containing 750 μl Whitten’s, 1.5% BSA. After 45 min, 225 μl from the top of the swim-up culture was removed and placed in a culture dish and covered with light mineral oil. The sperm were cultured for a total of 2.5-3 hr in Whitten’s, 1.5% BSA to allow the sperm to undergo capacitation and spontaneous acrosome exocytosis.
IVF of ZP-intact and ZP-free eggs was performed essentially as described previously (18,20) with the following additions. ZP-intact eggs were washed through three drops of Whitten’s, 1.5% BSA to wash away the FBS, then inseminated with 250,000 sperm/ml for 3 hr, with ∼25 eggs per 100 μl drop. For IVF of ZP-free eggs to assess sperm-egg membrane interactions, 10 eggs per 10 μl drop were inseminated for 1 hr with 25,000 sperm/ml or 100,000 sperm/ml, for sperm:egg ratios of 25:1 or 100:1 respectively. The eggs were washed through three drops of Whitten’s, 1.5% BSA using a thin bore pipette to detach any loosely attached sperm. Eggs were then fixed in 3.7% paraformaldehyde in PBS and stained with 1.5 μg/ml 4’, 6’-diamidino-2-phenylindole (DAPI) to assess the stage of maternal DNA and visualize decondensing sperm heads in the egg cytoplasm. For the assays using ZP-free eggs, data are expressed in terms of sperm bound (but not fused) per egg and sperm fused per egg. Statistical analysis of the data from these IVF experiments used nested analysis of variance (ANOVA) in order to assess differences among the means of the various parameters examined (R statistics software). This analysis assesses the differences between individual mice, as well as differences between the two genotypes.
RESULTS
Characterization of Tpst2-/- Mice. To disrupt the Tpst2 gene, a targeting vector was constructed in which most of exon 3 of the Tpst2 gene was replaced with a PGKneo cassette (Fig. 1B). Linearized targeting vector was electroporated into ES cells that were then subjected to positive selection. Homologous recombinants were detected using PCR and confirmed by Southern analysis of XbaI digested genomic DNA. ES cells were injected into blastocysts and the blastocysts implanted into pseudopregnant mice. Male chimeras were mated with 129S6/SvEvTac females and heterozygotes were crossed to generate Tpst2-/- mice. This targeted allele has been designated Tpst2tm1Klm (MGI:3512111).
Transmission of the targeted mutation was confirmed by PCR and Southern blot analysis of genomic DNA (Fig. 1C). Functional inactivation of the Tpst2 gene was confirmed by Northern analysis of total RNA isolated from Tpst2+/+, Tpst2+/-, and Tpst2-/- organs (Fig. 1D, upper panel). This showed a ≈ 50% reduction of full-length Tpst2 mRNA (≈ 1.8 kb) in lung and testes of Tpst2+/- mice. Transcripts were not detected in Tpst2-/- mice. To determine if disruption of the Tpst2 gene affected expression of the Tpst1 gene, the blots were stripped and re-probed with a TPST-1 probe (Fig. 1D, middle panel) and TPST-1 transcripts were quantified and normalized to CHO-B transcript levels (Fig. 1D, bottom panel). In the lungs of Tpst2+/+, Tpst2+/-, and Tpst2-/- mice, the ratios of TPST-1 to CHO-B mRNA were 0.055, 0.056, 0.059, respectively. Likewise, in the testes of Tpst2+/+, Tpst2+/-, and Tpst2-/- mice, the ratios of TPST-1 to CHO-B mRNA were 0.41, 0.30, 0.45, respectively. Thus, disruption of the Tpst2 gene had no detectable effect on expression of the Tpst1 gene in lung or testes.
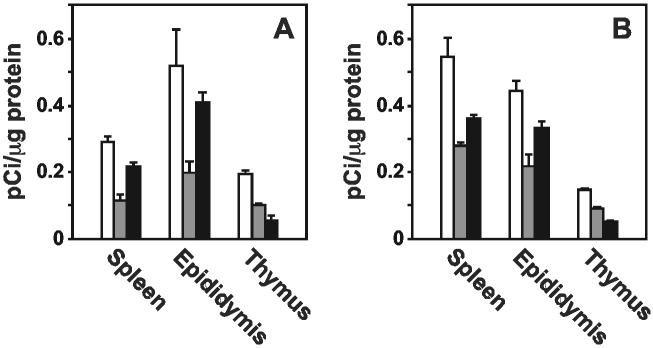
To determine the effect of disruption of the Tpst2 gene on protein-tyrosine sulfation, the amount of sulfotyrosine incorporated into proteins was determined as described in Experimental Procedures. We observed that disruption of either the Tpst1 or Tpst2 gene resulted in a decrease in tyrosine sulfation of secreted (Fig. 2A) and cellular proteins (Fig. 2B) in spleen, epididymus, and thymus compared to wild type tissues.
Fig 2.
Sulfotyrosine Analysis. Tissue explants were metabolically labeled with Na35SO4 and quantitative sulfotyrosine analysis was performed as described in Experimental Procedures. Sulfotyrosine analysis of secreted (A) and cellular proteins (B) from wild type (open bars), Tpst1-/-(gray bars), and Tpst2-/-(black bars) tissues are shown. Results are expressed as the mean ± SEM of three independent experiments.
Growth and Development. Tpst2+/- mice appeared normal. The reproductive performance of Tpst2+/- mating pairs was normal [6.24 ± 2.20 pups/litter (mean ± SD, n = 402)] when compared to that of wild type mating pairs [5.95 ± 2.12 pups/litter (mean ± SD, n = 254)]. The genotypes of offspring from Tpst2+/- x Tpst2+/- matings were consistent with Mendelian inheritance (26% wild type, 50% heterozygotes, 24% homozygotes, n = 2507) indicating that Tpst2-/- mice develop normally in utero. The sex ratio in each Tpst2 genotype was ≈ 50:50.
To assess the growth of Tpst2-/- mice, a cohort of offspring from Tpst2+/- mating pairs were weighed weekly from the age of 2 to 10 weeks (Fig. 3). At 2 weeks of age, the mean body weights of male and female Tpst2-/- mice were not different from wild type littermates. However, beginning at 3 weeks of age the body weights of male and female of Tpst2-/- mice lagged behind that of wild type littermates. The maximum difference in body weight occurred at 4-5 weeks of age at which time the body weight of both male and female homozygotes was on average ≈ 3 grams (≈ 20%) below that of wild type littermates. However, despite the growth delay, Tpst2-/- mice attained normal body weights at 10 weeks of age and Tpst2-/- mice appeared healthy out to 12 months of age. The body weights of Tpst2+/-mice were indistinguishable from wild type littermates at all ages (not shown).
Fig 3.
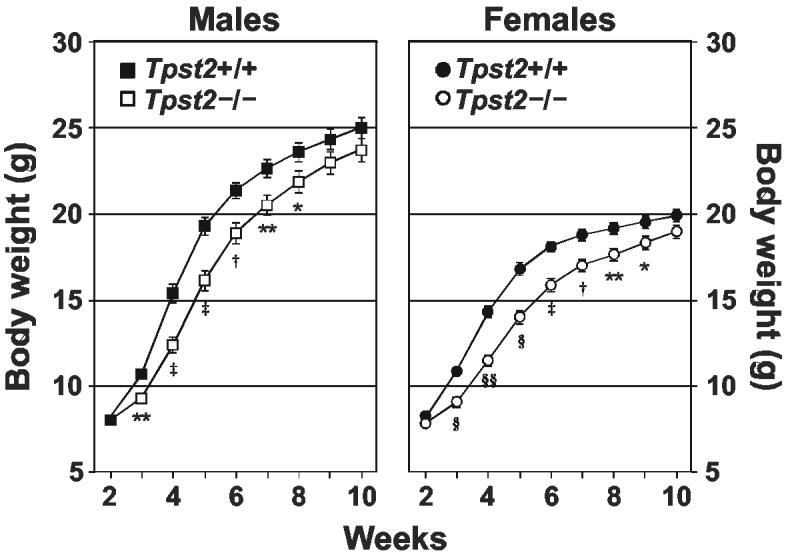
Growth Curves. Offspring from heterozygote mating pairs were weighed weekly starting at 3 weeks of age and pups were weaned at 3 weeks. Results are shown as the age vs. mean ± SE of body weight in grams. The number of mice in each group were; Tpst2+/+ male (n = 39), Tpst2-/- male (n = 26), Tpst2+/+ female (n = 41), Tpst2-/- female (n = 34). Statistical significance between groups at each age was determined using a one-tailed Student’s t-test with unequal sample variance. (*p <0.05, **p = < 0.01, † p = <10-3, ‡ p = <10-4, § p = <10-5, §§ p = <10-7).
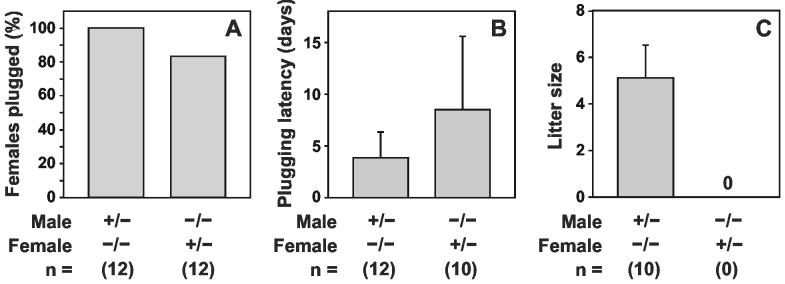
Reproductive Performance. After two months of continuous mating between Tpst2-/- males and females (n = 8 mating pairs) no litters were born. To examine the reproductive performance of Tpst2-/- mice in more detail, 10 week-old virgin female Tpst2+/- and Tpst2-/- mice were mated with either Tpst2+/- or Tpst2-/- males. Females were examined for copulatory plugs each morning to determine if and when copulation occurred and the size of the firstborn litter was recorded (Fig. 4). Copulatory plugs were detected in 12 of 12 Tpst2-/- females mated with Tpst2+/-males. The time between set-up of the matings and the detection of copulatory plugs was 3.8 ± 2.5 days (mean ± SD, n = 12) indicating that estrous cycle length in Tpst2-/- females is normal. Ten of 12 of the plugged females had litters (5.1 ± 1.4 pups/litter, mean ± SD, n = 10). Copulatory plugs were found in 10 of 12 of the Tpst2+/-females mated with Tpst2-/- males. There was a trend towards a longer latency period (8.5 ± 7.1 days, n = 10). However, this difference did not achieve statistical significance (p = 0.072, two-tailed Student’s t-test with unequal sample variance). Most strikingly, none of the plugged females had litters, and after a two-month period of continuous co-habitation, none of these 12 mating pairs had litters and none of the females were pregnant at necropsy at the end of that period. These data demonstrate that Tpst2-/- males are infertile, whereas the fertility of Tpst2-/- females is not compromised.
Fig 4.
Reproductive Performance. Ten week-old virgin Tpst2+/- or Tpst2-/- females were mated with either Tpst2+/- or Tpst2-/- males. Females were examined for copulatory plugs each morning to determine the percentage of females plugged (A), the plugging latency (B), and the size of any firstborn litter (C). Results are expressed as the mean ± SD. The sample size is indicated in parentheses. Neither the percentages of females plugged (p = 0.478, two-tailed Fisher’s exact test) nor the plugging latencies (p = 0.072, two-tailed Student’s t-test with unequal sample variance) were statistically different between groups.
Endocrine Function and Histology of the Male Genital Tract. To assess endocrine function, serum testosterone, LH, and FSH levels were determined in 10-12 week-old virgin males. Serum testosterone, LH, and FSH levels in Tpst2-/- males were not statistically different from those in wild type controls (Table I). Histological examination showed that the testes appear normal (Fig. 5A and 5B), as do the epididymides that are filled with spermatozoa (Fig. 5C &5D), indicating that spermatogenesis is normal in Tpst2-/- males. The vas deferentia and seminal vesicles, as well as a variety of other tissues including, heart, lung, liver, spleen, thymus, lymph node, and kidney were also normal (not shown).
Table I.
Endocrine evaluation. Serum testosterone, LH, and FSH levels were assayed in 10-12 week-old Tpst2+/+ and Tpst2-/- males (10 animal per group). Values are expressed as the mean ± SD. Statistica significance between groups was determined by using two-tailed Student's t-test with unequal sample variance.
| Assay | Tpst2+/+ | Tpst2-/- | p-value |
|---|---|---|---|
| Testosterone (ng/ml) | 4.15 ± 4.15 | 3.74 ± 3.26 | 0.807 |
| LH (ng/ml) | 0.11 ± 0.13 | 0.13 ± 0.11 | 0.709 |
| FSH (ng/ml) | 11.29 ± 5.01 | 16.31 ± 6.21 | 0.063 |
Fig 5.
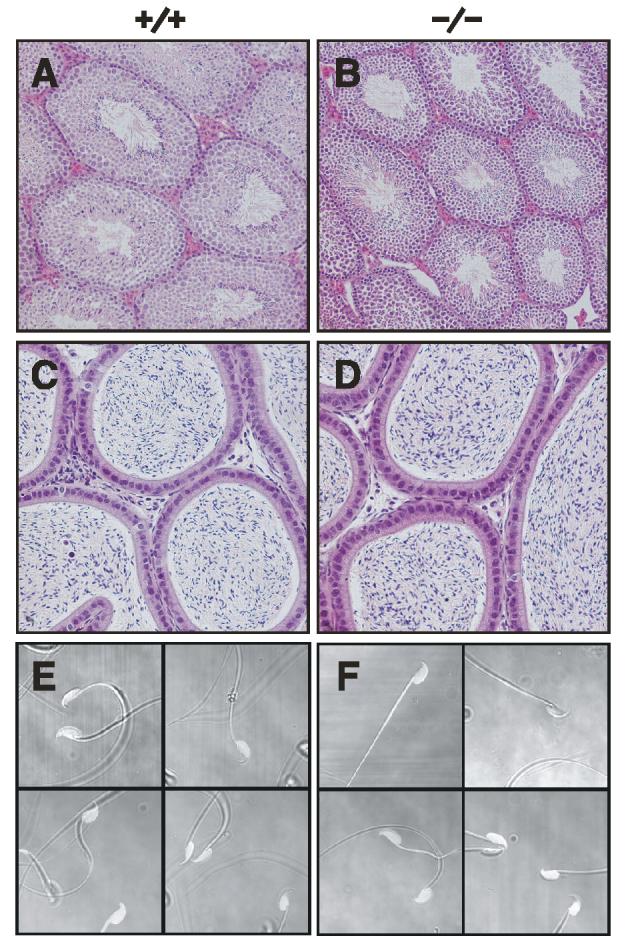
Tissue Histology and Sperm Cytology. Hematoxylin-eosin stained section of testes (A, B) and epididymides (C, D), and representative phase contrast images of caudal epididymal sperm from four different Tpst2+/+ and Tpst2-/- mice (E and F) are shown.
Matings with Superovulated Females. As noted above, copulatory plugs were found in 10 of 12 Tpst2+/-females mated with Tpst2-/- males, but none of the females had litters. To determine if the poor reproductive performance of Tpst2-/- males might be due to a failure to ejaculate sperm during mating, wild type and Tpst2-/- males were mated with superovulated FVB/N females and females examined for the presence of copulatory plugs the next morning. Plugs were removed from the females, washed in PBS, and the washings examined microscopically for the presence of sperm. Copulatory plugs were detected in 13 of 16 females mated with wild type males and in 15 of 24 females mated with Tpst2-/- males. Sperm was detected in all the plugs from both wild type and Tpst2-/- males. Thus, Tpst2-/- males can successfully inseminate females. We also sought to assess if Tpst2-/- sperm could ascend the female genital tract. To do so, the uteri of the plugged females were excised and the uterine horns flushed with PBS as described in Experimental Procedures. We observed that sperm was present in all 13 of the females plugged by wild type males, whereas sperm were present in only 8 of the 15 female plugged by Tpst2-/- males.
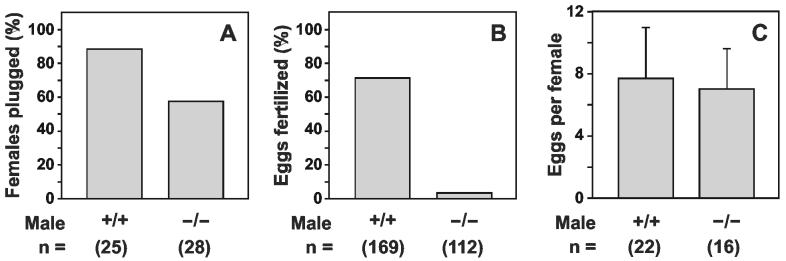
In a separate study, wild type and Tpst2-/- males (n = 10 males per group) were mated with one superovulated FVB/N female once per week for three weeks. The morning after each mating, females were examined for copulatory plugs and then euthanized. Eggs were collected from the ampullae of the oviducts and then counted and scored for fertilization as described in Experimental Procedures. In these experiments, copulatory plugs were observed in 22 of 25 females mated with wild type males, and in 16 of 28 females mated with Tpst2-/- males (Fig. 6A). All 10 wild type males and 9 of 10 Tpst2-/- males plugged at least one of the three females presented to it in the course of the study. We observed that 120 of 169 (71.0%) eggs harvested from females plugged by wild type males (n = 22 females) were fertilized. In contrast, only 4 of 112 (3.6%) eggs harvested from females plugged by Tpst2-/- males (n = 16 females) were fertilized (Fig. 6B). These four fertilized eggs were accounted for by one mating with a single Tpst2-/- male. This male plugged all three females presented to it over the course of the experiment and the genotype of this male was re-confirmed by PCR analysis of a second tissue sample. The number of eggs harvested from each group of females was not statistically different (Fig. 6C, p = 0.773, one-tailed Student’s t-test with unequal sample variance). These data show that the ability of Tpst2-/- males to fertilize eggs in vivo was severely compromised. In addition, the observation that Tpst2-/- sperm can fertilize eggs in vivo, albeit very inefficiently, indicates that Tpst2-/- sperm can reach the site of fertilization in vivo. We could not quantitatively assess the efficiency of sperm transport based on these data. However, the lower frequency with which sperm can be recovered from the uterus of females mated with Tpst2-/- males indicates that transport of Tpst2-/- sperm up the female genital tract is inefficient in comparison with wild type sperm.
Fig 6.
Analysis of Natural Matings. Each of ten virgin 10-12 week-old Tpst2+/+ and Tpst2-/- males was mated overnight with a superovulated FVB/N female. One week, and then again two weeks later, each male was mated overnight with a different superovulated female, for a total of two or three matings for each male. The morning after each mating, females were examined for copulatory plugs (A) and then euthanized. Egg clutches were collected from the ampullae of the oviducts, all the eggs collected were scored for fertilization (B), and the total number of eggs harvested from each female determined (C) as described in Experimental Procedures. Results are expressed as the mean ± SD. The sample size is indicated in parentheses. The difference in the percentage of eggs fertilized between groups was highlysignificant (p <10-32, two-tailed Fisher’s exact test). The number of eggs harvested per female was not statistically different (p = 0.773, two-tailed Student’s t-test with unequal sample variance).
In all our analyses of matings with superovulated FVB/N females described above, copulatory plugs were observed in 35 of 41 (85%) females mated with wild type males, and 31 of 52 (60%) females mated with Tpst2-/- males, a difference that is statistically significant (p = 0.011, two-tailed Fisher’s exact test).
Sperm Counts, Morphology, and Motility. Caudal epididymal sperm counts and the percentage of motile sperm in wild type (n = 10) and Tpst2-/- mice (n = 11) were indistinguishable. Sperm counts were 1.48 ± 0.63 x 107 (mean ± SD) in wild type and 1.49 ± 0.83 x 107 in Tpst2-/- mice, whereas, the percentage of motile sperm were 63.8 ± 12.7% in wild type and 55.6 ± 18.5% and Tpst2-/- mice, (p = 0.984 and p = 0.255, two-tailed Student’s t-test, respectively). In addition, Tpst2-/- sperm were cytologically normal (Fig. 5E and 5F). Furthermore, we observed no gross, reproducible changes in relative abundance of proteins in extracts of caudal epididymal sperm from Tpst2-/- compared to wild type sperm as assessed by SDS-PAGE (n = 3, data not shown).
CASA was performed to examine Tpst2-/- sperm for more subtle motility abnormalities. We observed that mean straight line velocity, mean curvilinear velocity, mean track width, and beat cross frequency were not significantly different between wild type and Tpst2-/- sperm (Table II). However, the movement of Tpst2-/- sperm was slightly more linear than wild type sperm. Thus, sperm number, morphology, and sperm motility parameters were normal in Tpst2-/- mice.
Table II.
Computer-assisted sperm analysis. CASA was performed on caudal epididymal sperm from 10-12 week-old Tpst2+/+ (n = 5) and Tpst2-/- (n = 4) males as described in Experimental Procedures. Results are expressed as mean ± SD. Statistical differences between means weighted for sample size was determined by a two-tailed Student's t-test. The number of sperm analyzed for each animal ranged from 87 to 438 for Tpst2+/+ and from 98 to 862 for Tpst2-/- mice.
| Parameter | Tpst2+/+ | Tpst2-/- | p-value |
|---|---|---|---|
| Straight line velocity (μm/sec) | 40.0 ± 2.6 | 39.5 ± 8.5 | 0.381 |
| Curvilinear velocity (μm/sec) | 192.2 ± 12.3 | 164.7 ± 27.0 | 0.105 |
| Track width (μm) | 12.8 ± 1.2 | 11.7 ± 1.3 | 0.629 |
| Linearity | 0.228 ± 0.026 | 0.253 ± 0.054 | 0.047 |
| Beat cross frequency (Hz) | 34.5 ± 4.4 | 31.7 ± 6.5 | 0.410 |
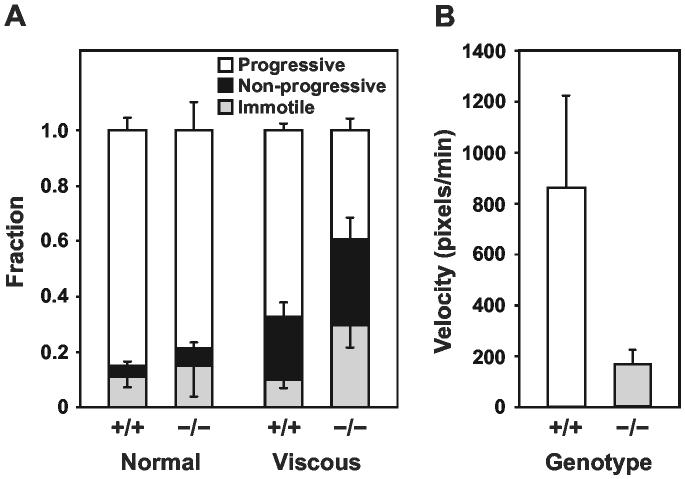
We also examined the quality of sperm motility in viscous media in an attempt to mimic the viscoelastic mucus that sperm encounter in the female genital tract. We observed no difference between wild type and Tpst2-/- mice in the fraction of sperm that were immotile or that displayed progressive or non-progressive motility in normal viscosity media (ϰ22 = 4.56, p = 0.102)(Fig. 7A). However, in viscous media a significantly lower fraction of Tpst2-/- sperm displayed progressive motility (0.39 vs. 0.67) while a comparably higher fraction was immotile (0.30 vs. 0.10) when compared to wild type sperm (ϰ22 = 46.37, p = 8.51 x 10-11). In addition, the straight line velocity of progressively motile Tpst2-/- sperm is substantially lower in comparison to wild type sperm (Fig. 7B)
Fig 7.
Sperm Motility in Viscous Media. Swim-up fractions of sperm from wild type and Tpst2-/- males were prepared and capacitated as described in Experimental Procedures. Sperm were observed in normal viscosity media (M199-BSA) or in viscous media (2% long chain polyacrylamide in M199-BSA). A. Video images were analyzed off-line to determine the number of sperm that were inactive (immotile), active with no forward velocity (non-progressive), or active with forward velocity (progressive). Results are expressed as mean ± SD of the fraction of immotile, non-progressive, and progressive sperm from three different animals. The mean number of spermatozoa analyzed (mean ± SD, n = 3) were; Tpst2+/+ (n = 146 ± 33) and Tpst2-/-(n = 91 ± 30) in normal media, and Tpst2+/+ (n = 83 ± 13) and Tpst2-/-(n = 84 ± 6) in viscous media. B. Sperm velocities were measured by tracking individual cells frame-by-frame using NanoTrack Software (Isee Imaging Systems). Results are expressed as the mean ± SD of straight line velocities of progressively motile sperm in viscous media. The combined number of spermatozoa analyzed from the three different animals were; Tpst2+/+ (n = 62) and Tpst2-/-(n = 61). The difference in sperm straight line velocities was highly significant (p <10-23, two-tailed Student’s t-test with unequal sample variance).
Capacitation and Acrosomal Exocytosis. The abilities of Tpst2-/- sperm to capacitate and undergo acrosomal exocytosis were determined as described in Experimental Procedures. We found that Tpst2-/- sperm undergo time-dependent capacitation normally (Fig. 8A). In addition, we found no abnormality in either spontaneous or A23178-induced acrosomal exocytosis in Tpst2-/- sperm (Fig. 8B).
Fig 8.
Sperm Capacitation and Acrosomal Exocytosis. A. The ability of sperm to capacitate in vitro as a function of time under capacitating (M199, 0.4% BSA, closed circles) or non-capacitating (M199 alone, open circles) conditions was assessed by fluorescence microscopy of sperm stained with FITC-CTß as described in Experimental Procedures. Results are expressed as mean ± SD of three independent experiments. B. The ability of sperm to undergo acrosomal exocytosis in response to 50 μM calcium ionophone A23187 (solid bars) or diluent (open bars) was assessed by fluorescence staining FITC-Peanut agglutinin as described in Experimental Procedures. Results are expressed as mean ± SD of four independent experiments.
In Vitro Fertilization Assays. To assess if the infertility of Tpst2-/- mice was a consequence of the inability of Tpst2-/- sperm to fertilize eggs, three types of IVF assays were performed. Cumulus-intact egg clutches collected from superovulated wild type females were inseminated in duplicate IVF drops with epididymal sperm from Tpst2-/- or wild type male (n = 4) as described in Experimental Procedures. We observed 305 of 318 eggs inseminated with wild type sperm were fertilized as assessed by progression to the 2-cell stage (95.6 ± 4.3%, mean ± SD of weighted means of duplicate assays, n = 4). In contrast, when inseminated with Tpst2-/- sperm, only 112 of 348 eggs were fertilized (35.0 ± 5.3%). This difference in fertilization rates is highly statistically significant (p = 10-6, one-tailed Student’s t-test). Similar results were obtained in IVF assays with cumulus-free, ZP-intact eggs, in which eggs were examined 3 hr post-insemination for the presence of sperm DNA in the egg cytoplasm (not shown). These results demonstrate that the ability of Tpst2-/- sperm to fertilize cumulus-intact eggs is severely compromised and that Tpst2-/- sperm can penetrate the cumulus mass.
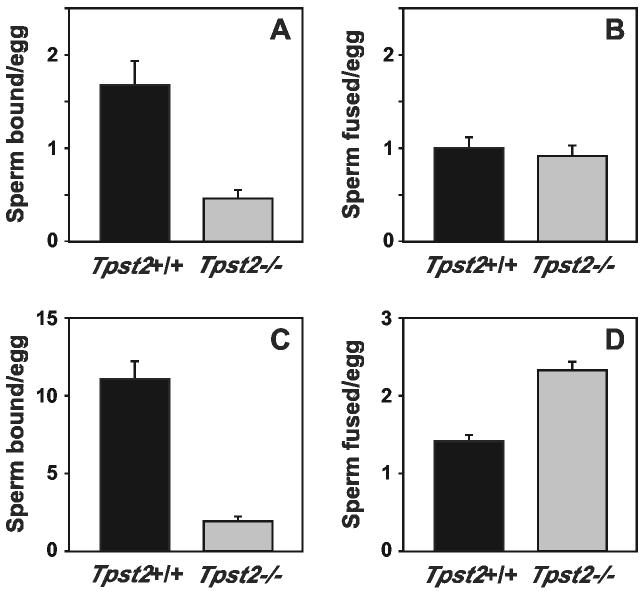
Reduced fertilization of cumulus-intact and ZP-intact eggs could be attributable to a failure of sperm to bind to and penetrate the ZP and/or a failure of sperm to bind to and/or fuse with the egg plasma membrane after successful ZP penetration. Therefore, IVF assays using ZP-free eggs were performed to assess the ability of Tpst2-/- sperm to interact with the egg plasma membrane. These assays used sperm:egg ratios of 100:1 and 25:1, with the latter being more capable of detecting subtle defects than the higher sperm:egg ratios (Fig. 9). Taking into account some mouse-to-mouse variability in our statistical analysis, there is some evidence that the Tpst2-/- sperm do not bind as well to the egg membrane compared to wild type sperm (p = 0.09 for assays at 100:1 sperm:egg ratio, p = 0.13 for assays at 25:1 sperm:egg ratio). There was weak evidence that sperm from the Tpst2-/- mice fuse with eggs at rates greater than sperm from wild type mice at a sperm:egg ratio of 100:1 (p = 0.15), but no evidence for this in assays at a sperm:egg ratio of 25:1 (p = 0.85).
Fig 9.
Fertilization of ZP-free eggs. ZP-free eggs were prepared from CF-1 mice and inseminated with sperm from either Tpst2+/+ or Tpst2-/- mice for 60 min, then washed, fixed, and assessed for sperm-egg binding (A and C) or sperm-egg fusion (B and D). ZP-free eggs were inseminated with sperm at a sperm:egg ratio of 25:1 (A and B) or at a sperm:egg ratio of 100:1 (C and D). Results are expressed as mean ± SE from analyses of sperm from three wild type and four Tpst2-/- mice.
DISCUSSION
To assess the role of tyrosine sulfation in vivo, Tpst1 (11) and Tpst2 knockout mice have been successfully generated. Our analyses show that there are features that are common to both knockouts. Disruption of one allele at either the Tpst1 or the Tpst2 loci has no discernible effect on growth, longevity, or reproductive performance. In both strains, when heterozygotes are interbred the targeted mutation is inherited at the expected Mendelian frequency, indicating that neither isoenzyme is required for normal embryonic development. Furthermore, female fertility per se is not impaired in either knockout. However, other features of the two knockouts lines are clearly distinct. The most striking phenotypic features that are not shared between Tpst1 and Tpst2 knockouts are post-natal growth patterns and the fertility of the male homozygotes.
Body weights of Tpst2-/- mice and wild type littermates are the same at 2 weeks of age. Thereafter, growth of Tpst2-/- mice lags significantly behind wild type littermates. The maximum difference in body weight occurs at 4-5 weeks of age when the body weight of male and female homozygotes is ≈ 20% below that of wild type littermates. However, the homozygotes subsequently attain normal body weights at 10 weeks of age. The body weights of male and female Tpst1-/- mice are indistinguishable from wild type littermates up to the time of weaning at 3 weeks of age. However, after weaning the growth of Tpst1-/- mice modestly lags behind that of wild type mice. On average, Tpst1-/- mice have ≈ 5% lower body weight up through 10 weeks of age (11). As is the case for Tpst1 null mice, the physiological basis for the moderate growth lag in Tpst2 null mice remains unknown. Among the tyrosine-sulfated proteins described to date (6), only cholecystokinin and gastrin are known to play a direct role in feeding behavior and gastrointestinal function and require tyrosine sulfation for optimal function (21). Both the cholecystokinin and gastrin genes have been disrupted in mice, as have been their cognate G-protein-coupled receptors, the CCK-A and CCK-B/gastrin receptor, respectively (22-25). However, disruption of these genes has no discernible effect on post-natal growth.
The most interesting difference between the Tpst1 and Tpst2 knockouts relates to male fertility. Tpst2-/- males have normal testosterone and gonadotropin levels, normal spermatogenesis, sperm counts, sperm morphology and normal sperm motility in non-viscous media. In addition, Tpst2-/- males ejaculate sperm during mating and the sperm appear to capacitate and undergo agonist-induced acrosomal exocytosis normally. However, Tpst2-/- males are effectively infertile.
We identified three prominent, and perhaps causally related, abnormalities that explain the infertility of Tpst2-/- males. First, there is an impaired ability of sperm to ascend the female genital tract after coitus. Second, there is impaired sperm motility in viscous media, but not in media with normal viscosity. Third, there is severe impairment in the ability of Tpst2-/- sperm to fertilize ZP-intact eggs in vitro, as well as, a reduced ability of sperm to bind to the plasma membrane of ZP-free eggs.
After coitus, sperm must penetrate a barrier of viscous cervical mucus that effectively filters out sperm with sub-optimal motility such that only a small fraction of ejaculated sperm enter the uterus. After passage through the uterus, sperm interact with the oviductal epithelium and are effectively stored and maintained there in a fertile state, the so-called sperm reservoir. Here, sperm become capacitated and, when ovulation occurs, they become hyperactivated, enabling them to proceed to the ampullae. The vigorous hyperactivated motility is thought to facilitate movement in this viscous environment, as well as facilitate penetration of the cumulus mass and ZP surrounding the egg (26).
Our data show conclusively that the ability of Tpst2-/- sperm to navigate in viscous media is severely impaired and thus provides a coherent explanation of the abnormalities we have observed in Tpst2-/- sperm function. This analysis was performed in an attempt to assess if Tpst2-/- sperm displayed defective hyperactivation. However, under the conditions of our analyses, we did not observe the circular or erratic patterns of sperm movement that has been classically described for hyperactivation (27,28). The reasons for this are uncertain. Although, the classic pattern of hyperactivated motility has been observed in mice, to our knowledge it has not been reported in the 129SvEv strain.
We also observed that copulatory plugs were found significantly less frequently in superovulated females mated with Tpst2-/- compared to wild type males. Although the reason(s) for this is unclear, these data suggest the possibility that Tpst2-/- males may have a modest defect in sexual function and/or mating behavior.
Fertilization per se is a multi-step process, which involves penetration of the cumulus cell layer surrounding the ovulated egg by the sperm, sperm-ZP adhesion, triggering of the acrosome reaction, penetration of the ZP by acrosome-reacted sperm, sperm-egg adhesion, fusion of the sperm and egg plasma membrane, and subsequent egg activation (29,30). We show that fertilization of the ZP-intact egg by Tpst2-/- sperm is severely impaired. Our data also indicate that this impairment is not due to the inability of the sperm to capacitate, penetrate the cumulus mass, or undergo acrosomal exocytosis. The sperm appear to have a reduced ability to bind to the egg plasma membrane, and our data on motility in viscous media further indicate that defective fertilization of ZP-intact eggs by Tpst2-/- sperm is due to a reduced ability to penetrate the ZP.
In man, 37 tyrosine-sulfated proteins have been identified to date, some of which have been shown to require tyrosine sulfation for optimal function, including certain adhesion molecules, G-protein coupled receptors, coagulation factors, serpins, and hormones. The LH and FSH receptors are two proteins of key importance in reproductive biology. The human LH, FSH, and TSH receptors have been shown to be sulfated at a membrane proximal site in their respective N-terminal extracellular domains that are highly conserved in many species including the mouse (31). Sulfation of these receptors has been shown to be required for optimal affinity of their cognate ligand in vitro. However, our observations that serum LH, FSH, and testosterone levels are normal in Tpst2-/- males, excludes defective sulfation of these receptors as an explanation for infertility of Tpst2-/- males. Our observations coupled with the fact that Tpst1-/- mice are fertile (11) and have normal LH, FSH, and testosterone levels (data not shown) suggests that these receptors are good acceptor substrates for either TPST isoenzyme. Alternatively, it is possible that sulfation of these receptors has marginal impact on ligand binding in vivo or that the mouse homologs are not sulfated
Tpst2 is one of a number of examples of mouse genes whose ablation results in a subfertile or infertile male phenotype despite normal spermatogenesis and an otherwise normal appearance. Some of these targeted genes encode proteins that transit the secretory pathway during biosynthesis, and thus could potentially undergo tyrosine sulfation, including testes-specific angiotensin converting enzyme (Ace), Catsper1 (32), Catsper2 (33), fertilin ß (Adam2) (34), cyritestin (Adam3) (35), and Izumo (36). However, none of these proteins have been shown to be tyrosine-sulfated. Nevertheless, the phenotypes of these knockout mice are distinct from those we observe in Tpst2-/- males, and thus are less likely to be causally related to the infertility of Tpst2-/- males. For example, Catsper1-/- sperm motility is abnormal in standard CASA assays (32) Adam2-/-(37) and Adam3-/-(38) sperm are defective in adhesion to the ZP and egg plasma membrane as well as transit from the uterus to the oviduct, and Izumo-/- sperm are incompetent in sperm-egg fusion (36), whereas these functions are preserved in Tpst2-/- sperm.
Our observation that Tpst2-/- males, but not Tpst1-/- males, are infertile, strongly indicates that one or more proteins required for normal male reproductive function must undergo tyrosine O-sulfation to function normally and that this protein(s) can be sulfated in vivo in the absence of TPST-1, but not in the absence of TPST-2. The identity of this protein(s) remains to be determined. It is also possible that the infertility of Tpst2-/- males may be due to unexpected changes in the expression of certain sperm proteins, such as has been described in the fertilin ß and cyritestin knockouts (38). Our analysis of sperm proteins argues against this possibility, but does not rule it out. Nevertheless, a full understanding of the infertility phenotype must await the identification of tyrosine-sulfated proteins expressed in the male reproductive tract and a more detailed knowledge of the relative abundance and the cellular expression patterns of TPST-1 and TPST-2. At this point, it is not feasible to address the later two questions at the protein level because isoenyzmespecific antibodies and isoenzyme-specific substrates are not yet available. However, microrarray data in the public domain does shed some light on the cellular expression patterns of the TPST transcripts in the male reproductive tract. For example, transcripts for both TPST isoenzymes have been detected in all segments of the epididymus in C57BL/6 mice (39). In the same report TPST-1, but not TPST-2 transcripts, were detected in type A and B spermatogonia, pachytene spermatocytes, and round spermatids from CD-1 mice and in Sertoli cells isolated from BL/6-129 mice (40). However, in another microarray analysis TPST-2 transcripts were detected in both Sertoli cells and laminin-binding germ cells in the rat (GEO DataSet Accession numbers GDS698, GDS174).
In summary, our results demonstrate conclusively that TPST-1 and TPST-2 have distinct biological roles in vivo and document for the first time the critical importance of proteintyrosine O-sulfation in male fertility. Finally, the high degree of homology between mouse and human TPST-2 (95% identical) and the conservation of biochemical mechanisms involved in mammalian fertilization suggests that proteintyrosine sulfation may be relevant to male infertility in humans.
Acknowledgments
Acknowledgments-We thank Dr. Randy Thresher (University of North Carolina Animal Models Core Facility) for blastocyst injections, Dr. Florea Lupu for assistance with sperm imaging, Drs. Swapan Nath (Oklahoma Medical Research Foundation) and Karl Broman (Johns Hopkins Bloomberg School of Public Health) for help with statistical analyses, and Joni Hodgson, Martin Lansdale, Kristin Edwards, KimberlyEwing, and Jane Farley for technical assistance. We also thank Dr. Susan Suarez (Cornell University) for advice and helpful suggestions.
Footnotes
This work was supported in part by National Institutes of Health (NIH) Grants HL74015 (to K.L.M.), RR01262 and RR09781 (to C.P.L.), and HD037696 and HD045671 (to J.P.E.).
- TPST
- tyrosylprotein sulfotransferase
- ZP
- zona pellucida
- LH
- luteinizing hormone
- FSH
- follicle-stimulating hormone
- HTF
- human tubal fluid
- CTß
- cholera toxin ß-subunit
- IVF
- in vitro fertilization
- CASA
- computer-assisted sperm analysis
REFERENCES
- 1.Bettelheim FR. J Am Chem Soc. 1954;76:2838–2839. [Google Scholar]
- 2.Huttner WB. Nature (London) 1982;299:273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- 3.Moore KL. J Biol Chem. 2003;278:24243–24246. doi: 10.1074/jbc.R300008200. [DOI] [PubMed] [Google Scholar]
- 4.Önnerfjord P, Heathfield TF, Heinegård D. J Biol Chem. 2004;279:26–33. doi: 10.1074/jbc.M308689200. [DOI] [PubMed] [Google Scholar]
- 5.Choe H, Moore MJ, Owens CM, Wright PL, Vasilieva N, Li W, Singh AP, Shakri R, Chitnis CE, Farzan M. Mol Microbiol. 2005;55:1413–1422. doi: 10.1111/j.1365-2958.2004.04478.x. [DOI] [PubMed] [Google Scholar]
- 6.Moore KL. J Biol Chem. 2003;278:24243–24246. doi: 10.1074/jbc.R300008200. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang YB, Lane WS, Moore KL. Proc Natl Acad Sci USA. 1998;95:2896–2901. doi: 10.1073/pnas.95.6.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouyang YB, Moore KL. J Biol Chem. 1998;273:24770–24774. doi: 10.1074/jbc.273.38.24770. [DOI] [PubMed] [Google Scholar]
- 9.Beisswanger R, Corbeil D, Vannier C, Thiele C, Dohrmann U, Kellner R, Ashman K, Niehrs C, Huttner WB. Proc Natl Acad Sci USA. 1998;95:11134–11139. doi: 10.1073/pnas.95.19.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seibert C, Cadene M, Sanfiz A, Chalt BT, Sakmar TP. Proc Natl Acad Sci USA. 2002;99:11031–11036. doi: 10.1073/pnas.172380899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang YB, Crawley JTB, Aston CE, Moore KL. J Biol Chem. 2002;277:23781–23787. doi: 10.1074/jbc.M202420200. [DOI] [PubMed] [Google Scholar]
- 12.Kakuta Y, Pedersen LG, Pedersen LC, Negishi M. Trends Biochem Sci. 1998;23:129–130. doi: 10.1016/s0968-0004(98)01182-7. [DOI] [PubMed] [Google Scholar]
- 13.Harpold MM, Evans RM, Salditt-Georgieff M, Darnell JE. Cell. 1979;17:1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- 14.Huttner WB. Meth Enzymol. 1984;107:200–223. doi: 10.1016/0076-6879(84)07013-0. [DOI] [PubMed] [Google Scholar]
- 15.Quinn P, Kerin JF, Warnes GM. Fertil Steril. 1985;44:493–498. doi: 10.1016/s0015-0282(16)48918-1. [DOI] [PubMed] [Google Scholar]
- 16.Roberts KP, Wamstad JA, Ensrud KM, Hamilton DW. Biol Reprod. 2003;69:572–581. doi: 10.1095/biolreprod.102.013771. [DOI] [PubMed] [Google Scholar]
- 17.Sztein JM, Farley JS, Mobraaten LE. Biol Reprod. 2000;63:1774–1780. doi: 10.1095/biolreprod63.6.1774. [DOI] [PubMed] [Google Scholar]
- 18.McAvey BA, Wortzman GB, Williams CJ, Evans JP. Biol Reprod. 2002;67:1342–1352. doi: 10.1095/biolreprod67.4.1342. [DOI] [PubMed] [Google Scholar]
- 19.Kalab P, Kopf GS, Schultz RM. Biol Reprod. 1991;45:783–787. doi: 10.1095/biolreprod45.5.783. [DOI] [PubMed] [Google Scholar]
- 20.Evans JP, Schultz RM, Kopf GS. J Cell Sci. 1995;108:3267–3278. doi: 10.1242/jcs.108.10.3267. [DOI] [PubMed] [Google Scholar]
- 21.Rehfeld JF. Physiol Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 22.Lacourse KA, Swanberg LJ, Gillespie PJ, Rehfeld JF, Saunders TL, Samuelson LC. Am J Physiol. 1999;276:G1302–G1309. doi: 10.1152/ajpgi.1999.276.5.G1302. [DOI] [PubMed] [Google Scholar]
- 23.Friss-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Am J Physiol. 1998;274:G561–G568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 24.Nagata A, Mitsuhiro I, Iwata N, Kuno J, Takano H, Minowa O, Chihara K, Matsui T, Noda T. Proc Natl Acad Sci USA. 1996;93:11825–11830. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanerek R, Beinborn M. J Clin Invest. 1999;103:383–391. doi: 10.1172/JCI4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suarez SS, Pacey AA. Hum Reprod Update. 2005. [DOI] [PubMed] [Google Scholar]
- 27.Suarez SS, Ho HC. Cell Mol Biol (Noisy-le-grand) 2003;49:351–356. [PubMed] [Google Scholar]
- 28.Suarez SS, Ho HC. Reprod Domest Anim. 2003;38:119–124. doi: 10.1046/j.1439-0531.2003.00397.x. [DOI] [PubMed] [Google Scholar]
- 29.Evans JP, Florman HM. Nat Cell Biol. 2002;(4 Suppl):s57–63. doi: 10.1038/ncb-nm-fertilityS57. [DOI] [PubMed] [Google Scholar]
- 30.Primakoff P, Myles DG. Science. 2002;296:2183–2185. doi: 10.1126/science.1072029. [DOI] [PubMed] [Google Scholar]
- 31.Costagliola S, Panneels V, Bonomi M, Koch J, Many MC, Smits G, Vassart G. EMBO J. 2002;21:504–513. doi: 10.1093/emboj/21.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers DL. Proc Natl Acad Sci USA. 2003;100:14869–14874. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho C, O’Dell Bunch D, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG. Science. 1998;281:1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- 35.Shamsadin R, Adham IM, Nayeria K, Heinlein UAO, Oberwinkler H, Engel W. Biol Reprod. 1999;61:1445–1451. doi: 10.1095/biolreprod61.6.1445. [DOI] [PubMed] [Google Scholar]
- 36.Inoue N, Ikawa M, Isotani A, Okabe M. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 37.Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG. Science. 1998;281:1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura H, Cho C, Branciforte DR, Myles DG, Primakoff P. Dev Biol. 2001;233:204–213. doi: 10.1006/dbio.2001.0166. [DOI] [PubMed] [Google Scholar]
- 39.Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, Kopf GS, Turner TT. Biol Reprod. 2005;73:404–413. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- 40.Shima JE, McLean DJ, McCarrey JR, Griswold MD. Biol Reprod. 2004;71:319–330. doi: 10.1095/biolreprod.103.026880. [DOI] [PubMed] [Google Scholar]