Abstract
Cutaneous drug reactions (CDRs) associated with sulfonamides are believed to be mediated through the formation of reactive metabolites that result in cellular toxicity and protein haptenation. We evaluated the bioactivation and toxicity of sulfamethoxazole (SMX) and dapsone (DDS) in normal human dermal fibroblasts (NHDF). Incubation of cells with DDS or its metabolite (D-NOH) resulted in protein haptenation readily detected by confocal microscopy and ELISA. While the metabolite of SMX (S-NOH) haptenated intracellular proteins, adducts were not evident in incubations with SMX. Cells expressed abundant N-acetyltransferase-1 (NAT1) mRNA and activity, but little NAT2 mRNA or activity. Neither NAT1 nor NAT2 protein were detectable. Incubation of NHDF with S-NOH or D-NOH increased reactive oxygen species formation and reduced glutathione content. NHDF were less susceptible to the cytotoxic effect of S-NOH and D-NOH than are keratinocytes. Our studies provide the novel observation that NHDF are able to acetylate both arylamine compounds and bioactivate the sulfone, DDS, giving rise to haptenated proteins. The reactive metabolites of SMX and DDS also provoke oxidative stress in these cells in a time- and concentration-dependent fashion. Further work is needed to determine the role of the observed toxicity in mediating CDRs observed with these agents.
Keywords: sulfonamides, cutaneous drug reactions, fibroblasts, protein haptenation, toxicity, N-acetyltransferase
INTRODUCTION
Cutaneous drug reactions (CDRs) represent a significant cause of morbidity and mortality in the use of a wide array of medicinal agents (Svensson et al., 2001). While much remains to be learned about the mechanisms of the varied reactions that can occur in the skin after systemic drug administration, many of these reactions are consistent with delayed-type hypersensitivity – implicating the involvement of the immune system in their provocation (Cribb et al., 1996a; Svensson et al., 2001; Pichler et al., 2002; Pichler, 2003). The observation that patients with a history of such reactions may possess drug-reactive T-cells suggests that these responses are T-cell mediated (Mauri-Hellweg et al., 1995; Schnyder et al., 2000; Nassif et al., 2002). As drugs noted to provoke these reactions are small molecules, it has been proposed that bioactivation of drugs to reactive metabolites is an essential prerequisite to the initiation of CDRs (Park and Kitteringham, 1990; Park et al., 1998; Park et al., 2000; Svensson, 2003).
Sulfonamides are among the most widely studied compounds that are associated with delayed-type CDRs. Reactions to these drugs may range from a mild morbilliform reaction to more severe cutaneous involvement, such as Stevens Johnson Syndrome or toxic epidermal necrolysis (Cribb et al., 1996a; Svensson et al., 2001). Several of the sulfonamides associated with CDRs have been found to undergo bioactivation in the liver to reactive arylhydroxylamine metabolites (Cucinelli et al., 1972; Shear and Spielberg, 1985; Cribb and Spielberg, 1992; Cribb et al., 1995; Cribb et al., 1996b). Formation of these arylhydroxylamines may lead to covalent adduction with cellular proteins and cause cytotoxicity (Cribb et al., 1996b; Manchanda et al., 2002; Naisbitt et al., 2002). Such events are believed to be essential in the provocation of an immune response in selected individuals.
We have previously proposed that events occurring at the cutaneous level are critical in the initiation of CDRs to sulfonamides and related drugs (Reilly et al., 2000). In particular, the bioactivation of sulfonamides within keratinocytes to their respective arylhydroxylamine metabolites may result in protein haptenation. Indeed, we have found that incubation of normal adult human epidermal keratinocytes (NHEK) with the arylhydroxylamine metabolites of sulfamethoxazole (SMX) or dapsone (DDS) results in the formation of drug/metabolite-protein adducts (Reilly et al., 2000). In addition, we have recently demonstrated that incubation of these cells with the parent compounds also gives rise to protein haptenation (Roychowdhury et al., 2005); supporting a potential role for intracellular bioactivation.
As drugs and metabolites in the cutaneous environment will come into contact with a variety of cells, it is important to determine if other cell types are capable of bioactivating these drugs or forming metabolite-protein adducts when exposed to preformed metabolites. Since dermal fibroblasts have been shown to possess a number of drug metabolizing enzymes (Pascale et al., 1990; Eltom et al., 1998; Saeki et al., 2002), we hypothesized that fibroblasts are capable of metabolizing sulfonamides and giving rise to intracellular haptenated proteins when exposed to these drugs. In addition, since these cells appear to have a lower glutathione content (an important antioxidant that is capable of detoxifying reactive metabolites) than keratinocytes (Leccia et al., 1998), we postulated that fibroblasts are more susceptible to arylhydroxylamine-induced toxicity.
METHODS
Chemicals and reagents
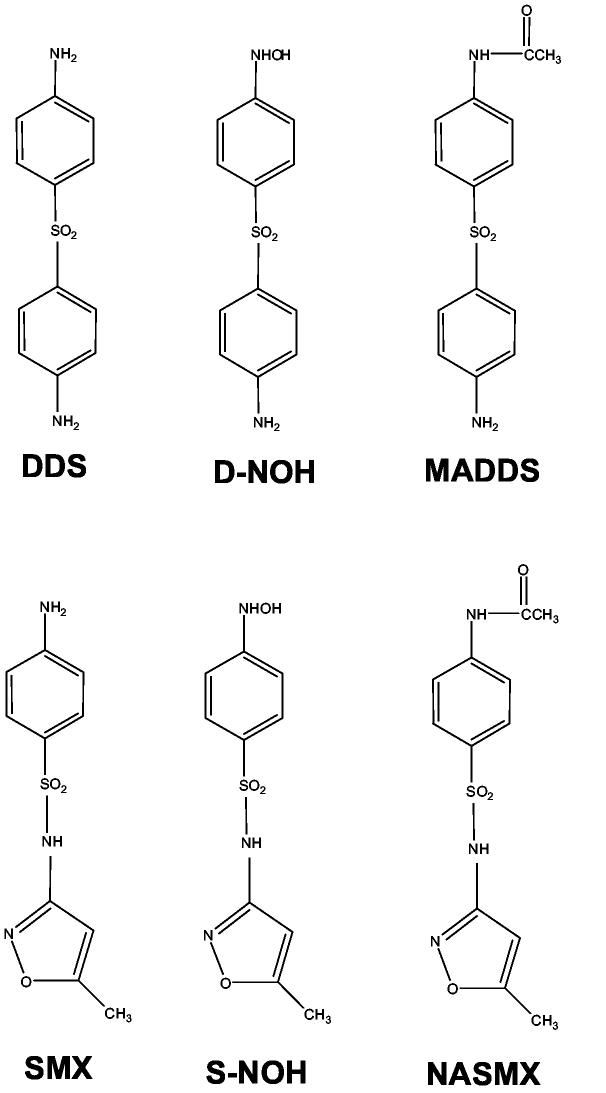
The compounds and metabolites used are illustrated in Scheme 1. The arylhydroxylamine metabolites of DDS and SMX were synthesized as described previously (Vyas et al., 2005). The purity of each metabolite was > 97% as determined by high performance liquid chromatography (HPLC). DDS and SMX were obtained from Sigma-Aldrich (St. Louis, MO) and found to be >99% pure. Rabbit antisera were raised against SMX- and DDS-keyhole limpet hemocyanine (KLH) conjugates and assayed for specificity as described previously (Reilly et al., 2000). Goat anti-rabbit antibody conjugated with alkaline phosphatase, goat-anti-rabbit IgG conjugated with Alexafluor 488, Yo-Yo-1 and monochlorobimane were purchased from Molecular Probes (Eugene, OR). Immunomount was obtained from Vector Laboratories (Burlingame, CA). Glutathione (GSH), bovine glutathione-S-transferase (GST) and rat tail collagen (type-1) were obtained from Sigma (St. Louis, MO). Microtiter ELISA plates (96 well) were obtained from Rainin Instruments (Woburn, MA), while Corning tissue cultured 6 well plates were obtained from Fisher Scientific (Chicago, IL). Bradford assay reagent kit was purchased from PIERCE (Rockford, IL). Human N-acetyltransferase-1 (NAT1) specific anti-sera was generated against a 12 amino acid peptide (CVPKHGDRFFTI) specific to the C-terminus of human NAT1 (Stanley et al., 1996). The human NAT2-specific anti-sera was generated against a 13 amino acid peptide (FLNSHLLPKKKHQ) specific to human NAT2. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Chicago, IL).
Scheme 1.
Chemical structures for sulfamethoxazole (SMX), sulfamethoxazole hydroxylamine (S-NOH), N-acetylsulfamethoxazole (NASMX), dapsone (DDS), dapsone hydroxylamine (D-NOH), monoacetyldapsone (MADDS).
Cell culture
Cryopreserved 1st passage normal human dermal fibroblasts (NHDF), fibroblast growth media, basal media and trypsinizing reagents were obtained from CAMBREX (Walkersville, MD). Cells were cultured in 75 cm2 flasks using basal media (FBM) supplemented with growth factors, recombinant human fibroblast growth factor (rhFGF-B, 0.1%), insulin (0.1%), GA-1000 (0.1%) and fetal bovine serum (2%) at 37°C in a sterile atmosphere containing 5% CO2. This growth media was replaced every 2-3 days. When cell cultures reached near confluence (75-90%), cells were harvested using 0.025% Trypsin/0.01% EDTA in HEPES, followed by neutralization with double the volume of trypsin neutralizing solution. The cell suspension was then centrifuged at 220 × g for 5 min, followed by washing in basal media and re-suspension in growth media. These cells were either subjected to sub-culturing to further passages until 5th passage cells were obtained or cryopreserved for later experiments. All experiments were performed using 5th passage cells from the same individual.
Determination of covalent adducts via immunofluorescent confocal microscopy
Drug or metabolite-protein covalent adduct formation was assessed using confocal microscopy as described previously for keratinocytes (Roychowdhury et al., 2005), with minor modifications. Briefly, 1 × 105 cells were grown on collagen coated (0.1 mg/ml) coverslips and placed in Petri dishes containing 2 ml of complete growth medium (FGM-2). After 24 h, cultures were washed with basal media and drug (with or without ascorbic acid) or metabolite was added followed by incubation for an additional 24 h. Drugs and metabolites were dissolved in DMSO and then added to the incubation (final DMSO content 0.25% vol/vol). After 24 h cultures were washed with phosphate buffered saline (PBS, 0.05 M sodium phosphate, 0.15 M NaCl, pH 7.4) followed by fixation with 4% paraformaldehyde in PBS for 20 min. After fixation, cultures were prepared for and analyzed using confocal microscopy as previously described (Roychowdhury et al., 2005).
Determination of covalent adducts via ELISA
Formation of covalent adducts following SMX or DDS exposure, in the presence or absence of ascorbic acid (2 mM), was determined by cultivating 1×106 NHDF cells for 24 h in a 6 well plate in 5 ml of complete growth medium. After 24 h cells were washed with basal media and incubated with SMX or DDS (800 μM) in presence or absence of 2 mM ascorbic acid in basal media. After 24 h, media was removed and 1 ml lysis buffer (1:1 0.2% Triton-X 100 in PBS) was added to all wells. The cells were scraped and vortexed with intermittent cycles of freezing and thawing (3 times) to ensure complete lysis. The cell suspension was centrifuged at 220 × g for 5 min and the pellet containing cell debris discarded. The supernatant containing cellular soluble proteins was collected for protein assay and subsequent ELISA analysis of covalent adducts as described previously (Reilly et al., 2000), with minor modifications.
Cellular metabolite formation
Biotransformation of SMX and DDS to their arylhydroxylamine (S-NOH or D-NOH) or N-acetyl metabolites in NHDF were determined using methods similar to those described previously (Reilly et al., 2000), with minor modifications. N-hydroxy and N-acetyl metabolites of DDS and SMX were quantified based upon externally generated standard curves using HPLC methods described previously (Reilly et al., 2000).
Quantitative determination of NAT1 and NAT2 mRNA in NHDF
NAT1-specific mRNA levels were determined as previously described (Husain et al., 2004). NAT2-specific mRNA levels were determined in an identical manner except that the forward NAT2 5′-ttggaagcaagaggattgcat-3′ and reverse NAT2 5′-gatctggtgctcaagaatgtcagt-3′ primers spanned an 8.6 kb intron in NAT2 and the TaqMan probe was fam-tcctggttgctggcc. Absolute levels of NAT1 and NAT2 mRNA were determined by calibration to a standard curve prepared with plasmids containing amplified NAT1 and NAT2 mRNA standards quantified by UV absorbance at 260 nm. All samples and standards were normalized to beta-actin mRNA as described previously (Husain et al., 2004).
Western blot analysis for NAT
Attempts to measure NAT2 protein expression by Western blot analysis were conducted as previously described (Zang et al., 2004). The human NAT2-specific antisera was generated against a 13 amino acid peptide (FLNSHLLPKKKHQ) specific to human NAT2. This method has been successfully used to measure NAT2 protein expression in transfected COS-1 cells (Zang et al., 2004) and human hepatocytes (Doll and Hein, unpublished data). Attempts to measure NAT1 protein expression were conducted using the same protocol with human NAT1-specific antisera generously donated by Dr. Edith Sim (University of Oxford). This antisera was generated against a 12 amino acid peptide (CVPKHGDRFFTI) specific to the C-terminus of human NAT1 (Stanley et al., 1996). This antisera has been successfully used to measure human NAT1 expression in urinary bladder (Stanley et al., 1996), skeletal muscle (Rodrigues-Lima et al., 2003), and human hepatocytes (Doll and Hein, unpublished data). Positive controls for Western blots were yeast (Schizosaccromyces pombe) lysates that were transformed with recombinant human NAT1 or NAT2.
Assessment of NAT1 and NAT2 catalytic activity in cytosol from NHDF
P-Aminobenzoic acid (PABA) and sulfamethazine (SMZ) N-acetyltransferase assays were conducted as previously described (Leff et al., 1999). Briefly, cell cytosol, PABA or SMZ substrate (300 μM) and acetyl coenzyme A (1 mM) were incubated for 20 min at 37°C. Controls substituted water for acetyl coenzyme A. N-acetyl-PABA or N-acetyl-SMZ product was separated by HPLC and quantified by absorbance at 280 or 260 nm, respectively.
Cytotoxicity of arylhydroxylamines toward NHDF
The cytotoxicity of parent drug (DDS/SMX) and their metabolites (DNOH/SNOH) towards NHDF were determined as described previously (Reilly et al., 2000), with minor modifications. Briefly, 2×104 NHDF cells were plated on 96-well plates and incubated for 24 h in 200 μl of FGM-2 media at 37°C, 5% CO2. After 24 h, FGM-2 was replaced by FBM and different concentrations (0 - 5mM) of the metabolite or drug were added to respective wells. DMSO concentration was 1% in all incubations. Cells were then incubated for 3 h, after which the plate was washed once with FBM. Fresh FBM containing 4 μM of Yo-Yo-1 (a non-permeable DNA binding dye) was added to all wells and cytotoxicity determined as described previously (Reilly et al., 2000).
Reactive Oxygen Species Generation
Oxidative stress induced by the generation of reactive oxygen species (ROS) when NHDF cells were exposed to the arylhydroxylamine metabolites was determined as described previously (Vyas et al., 2005). Briefly, 2 × 104 cells were cultured in a 96 well plate and incubated in 200 μl of FGM-2 media at 37°C, 5% CO2 for 24 h. After 24 h, FGM-2 was washed and replaced with 200 μl PBS. Various concentrations (0-1.5mM) of the drug/metabolite were added to the wells. ROS formation was measured by immediate addition of 20 μM of a fluorescent dye, 2′-7′-dichlorodihydrofluorescein diacetate (DCHF-DA). ROS generation was determined by fluorescence as described (Vyas et al., 2005).
Intracellular Depletion of Reduced Glutathione
Reduced Glutathione (GSH) depletion following drug and metabolite exposure was determined by cultivating NHDF cells (1 × 106 cells) in a 6 well plate in 2 ml of FGM-2 for 24 h. Cells were then washed with FBM and incubated with drug or metabolite (1.5 mM) in the basal media for 3 h. After 3 h incubation, media was removed and the cells were washed with ice cold Dulbeco's PBS, followed by addition of 1 ml Lysis Buffer (1:1 0.2% Triton-X 100 in Nanopure water: Dulbeco's PBS). Plates were kept on ice through out the extraction process. The cells were scraped and vortexed with intermittent cycles of freezing and thawing (3 times) to ensure complete lysis. The cell suspension was then centrifuged at 220×g for 5 min. The pellets containing the cell debris were discarded, while the supernatant containing cellular soluble proteins was collected for protein assay and subsequent GSH content assay. Protein content was determined using the Bradford Reagent Kit.
GSH Assay
Eighty μl of cell lysate was added to each well of a 96 well plate, followed by addition of 10 μl glutathione-S-transferase (10U/ml) and 10 μl monochlorobimane (0.5 mg/ml). The plate was shaken for 1 min and then incubated at 37°C in the dark for 30 min. The fluorescence was then measured in a microplate reader (Molecular Devices, Sunnyvale, CA) with an excitation and emission wavelength of 380 nm and 460 nm, respectively. Absolute levels of GSH were determined by calibration to an externally generated standard curve. All samples were normalized and reported as GSH content/g protein.
Statistical analysis
Data are presented as mean (SD). Statistical comparisons between groups were made using ANOVA and the Holm-Sidak method for multiple pairwise comparisons. A value of p<0.05 was considered to be significant.
RESULTS
Visualization and quantification of protein haptenation in NHDF exposed to drugs or metabolites
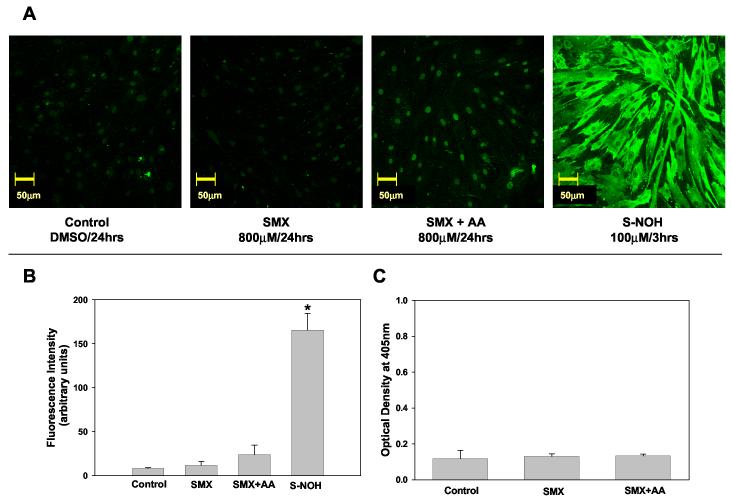
Confocal microscopy was used to visualize and semi-quantify covalent protein adducts formed in SMX and DDS treated NHDF. Cells treated with SMX alone did not give rise to detectable protein adduct formation (Fig. 1A & 1B). While previous studies in normal human epidermal keratinocytes (NHEK) demonstrated that ascorbic acid significantly increased the level of SMX-associated protein haptenation (Roychowdhury et al., 2005), incubation of NHDF with SMX and ascorbic acid produced only a marginal level of adduct (Fig 1A & B). Similar results were obtained when protein haptenation was assessed using an adduct-specific ELISA (Fig. 1C). In contrast, when NHDF were incubated with S-NOH, haptenation proteins were readily detected and appeared to be diffusely distributed throughout the cytosolic region of the cell (Fig. 1A and 1B). Use of pre-immune sera did not give rise to detectable fluorescence.
Figure 1.
Sulfamethoxazole (SMX) - protein adducts formation in NHDF.
A) Detection of SMX-protein adducts using confocal microscopy. NHDF cells (5x104 cells/mL) were incubated with SMX (800 μM, 24 h) in presence or absence of ascorbic acid (2 mM) or S-NOH (100 μM, 3 h). Controls were exposed to the vehicle alone (dimethyl sulfoxide). At the end of the incubation cells were fixed with paraformaldehyde, permeabilized, immunostained, and imaged on a confocal microscope using 20X objective. B) Confocal Images were analyzed as detailed in the Materials and Methods and fluorescence intensity from a minimum of 3 view fields of 4 different slides (incubations) of each treatment (with 10 cells per field) were averaged and expressed as mean (+SD) fluorescence intensity (arbitrary units). Hence, each bar represents mean (SD) of the mean of 4 incubations with 30 measurements of fluorescence intensity for each incubation. Results were analyzed using ANOVA with Holm-Sidak method for multiple pairwise comparisons.*p<0.05 compared to control/SMX/SMX+AA. C) Detection of SMX-protein adducts using ELISA technique. NHDF cells from the same individual as in A & B were seeded at a density of 2x105 cells/mL and incubated in the presence or absence of SMX 800 μM and/or ascorbic acid (AA) 2 mM. Data presented as optical density (OD) mean (+SD) of 4 separate incubations for each condition. Results were analyzed using ANOVA with Holm-Sidak method for multiple pairwise comparisons. There is no statistical difference (p<0.05) between the treatments.
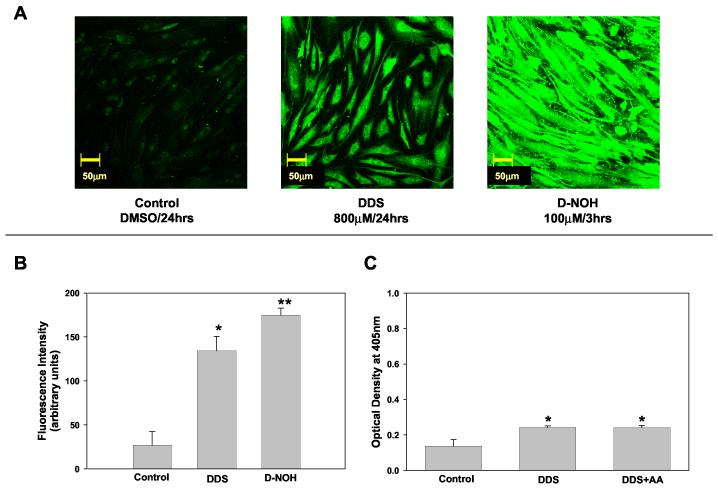
Incubation of NHDF with 800 μM of DDS for 24 h resulted in protein adducts that were readily detected via confocal microscopy and ELISA (Fig. 2). The level of adduct formed after exposure to 100 μM of D-NOH for 3 h was substantially greater than that seen after exposure to the parent compound (Fig. 2A & B). The amount of protein haptenation after exposure to DDS was not altered by the addition of ascorbic acid (Fig. 2C).
Figure 2.
Dapsone (DDS) - protein adducts formation in NHDF.
A) Detection of DDS protein adducts using confocal microscopy. NHDF cells (5x104cells/mL) were incubated with DDS (800 μM, 24 h) or D-NOH (100 μM, 3 h). Controls were exposed to the vehicle alone (dimethyl sulfoxide). At the end of the incubation cells were fixed with paraformaldehyde, permeabilized, immunostained, and imaged on a confocal microscope using 20X objective. B) Confocal Images were analyzed as detailed in the Materials and Methods and fluorescence intensity from a minimum of 3 view fields of 4 different slides (incubation) of each treatment (with 10 cells per field) were averaged and expressed as mean (+SD) fluorescence intensity (arbitrary units). Hence, each bar represents mean (SD) of the mean of 4 incubations with 30 measurements of fluorescence intensity for each incubation. Results were analyzed using ANOVA with Holm-Sidak method for multiple pairwise comparisons. *p< 0.05 compared to control, **p< 0.05 compared to control/DDS. C) Detection of DDS protein adducts formation using ELISA technique. NHDF cells were seeded at a density of 2x105cells/mL and incubated in the presence or absence of DDS 800 μM and/or Ascorbic Acid (AA) 2 mM. Data presented as optical density (OD) mean (+SD) of 4 separate incubations for each condition. Results were analyzed using ANOVA with Holm-Sidak method for multiple pairwise comparisons. *p<0.05 compared to control.
Cellular metabolism of SMX and DDS
The protein haptenation of NHDF exposed to DDS suggested these cells are able to bioactivate this compound, which was investigated further using high performance liquid chromatography (HPLC). When NHDF were incubated with 800 μM SMX for 24 h, the level of S-NOH was below the limit of detection. Similar incubations of NHDF with DDS resulted in detectable D-NOH peaks that were below the level of quantification. Incubation of NHDF with SMX resulted in a concentration of 4.49 nmoles/ml of the acetyl metabolite. While the monoacetyl metabolite of dapsone was detected in incubations of NHDF with DDS, it was below the level of quantification.
NAT1 and NAT2 activity, message and protein content in NHDF
As cellular metabolism yielded detectable N-acetylated metabolites, we sought to determine which N-acetyltransferase (NAT) enzymes were present in these cells. Using isolated cell cytosol, the PABA NAT activity was 1.42 nmol/min/mg, while the SMZ NAT activity was undetectable (limit of detection is 0.15 nmol/min/mg). The concentration of NAT1 and NAT2 mRNA in NHDF cells was 8.76±0.62 zeptomoles/μg of mRNA and 0.00650±0.00044 zeptomoles/μg of mRNA, respectively. Neither NAT1 nor NAT2 protein in NHDF cell lysates was detectable by Western blot. The positive control proteins (recombinant NAT1 and NAT2 expressed in yeast lysates) were readily detected.
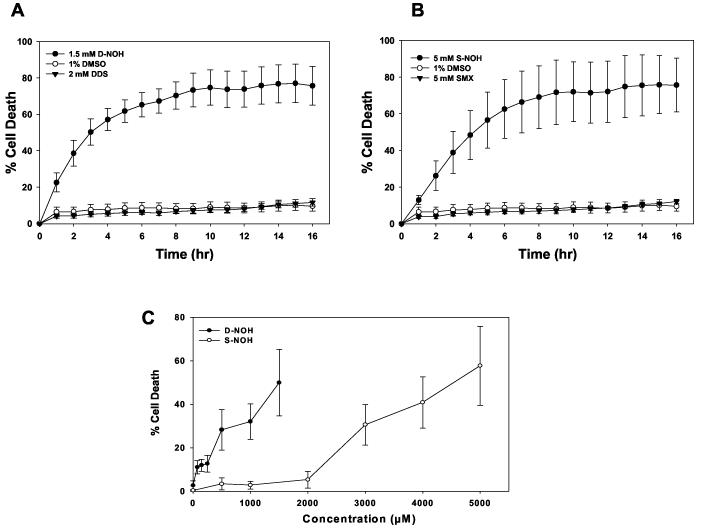
Cytotoxicity of arylhydroxylamine metabolites toward NHDF
These arylhydroxylamine metabolites have been shown to be cytotoxic to a variety of cell types, including peripheral blood mononuclear cells (Rieder et al., 1988) and NHEK (Reilly et al., 2000). Incubation of NHDF cells with SMX or DDS did not alter the level of cell death compared to that seen with vehicle alone (Fig. 3A & B). In contrast, both arylhydroxylamine metabolites resulted in a concentration- and time-dependent cytotoxicity in NHDF (Fig 3). Maximum cell death occurred at about 10 h after exposure to either metabolite. There was a significant difference in the level of cell death induced by D-NOH compared to S-NOH at equimolar concentrations (Fig. 3C). The solubility of the metabolites prevented a determination of the maximal cytotoxicity. However, use of the data from Fig. 3 from 20 – 60% cell death resulted in an estimate for the LC50 (concentration producing death in 50% of cells) for D-NOH of ∼1.5 mM and ∼4.5 mM for S-NOH.
Figure 3.
Time- and concentration- dependent cytotoxicity caused by DDS/SMX or their hydroxylamine metabolite in NHDF. NHDF cells were seeded at a density of 105 cells/mL on 96 well plates and incubated with A) DDS (2 mM), D-NOH (1.5 mM). B) SMX (5 mM), S-NOH (5 mM) or C) S-NOH (0 to 5 mM) or D-NOH (0 to 1.5 mM) in the basal media (FBM) for 3 h. After 3 h incubation, the plate was washed with FBM followed by the addition of 4 μM of YO-YO-1, a DNA binding dye. After 3 h incubation with the respective metabolites, the plate was washed with basal media (FBM) followed by the addition of 4 μM of YO-YO-1, a DNA binding dye. Fluorescence was measured for 16 h (A & B) or at 16 h (C) at 37° C using a fluorescent microtiter plate reader followed by the addition of Triton-X (0.1%) for 3 h. The % cytotoxicity was determined as described in the Materials and Methods section. Data presented as the mean (+SD) fluorescence of 5 separate incubations for each condition.
ROS generation in NHDF cells
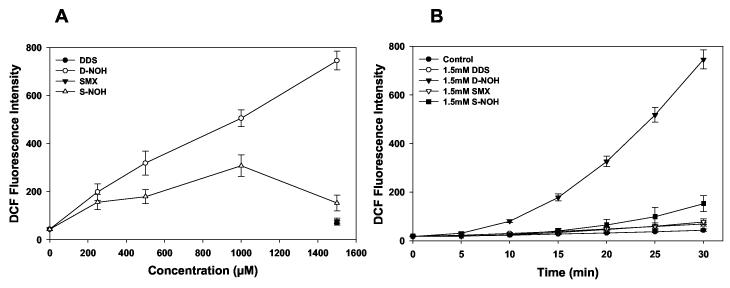
Generation of ROS in cells exposed to drugs/metabolites may induce cell death or provoke the release of molecules that may stimulate an inflammatory response. As previous studies have demonstrated that these metabolites increase ROS generation in NHEK (Vyas et al., 2005), we assessed ROS generation in NHDF incubated with S-NOH and D-NOH. Both S-NOH and D-NOH induced a time- and concentration-dependent increase in the amount of ROS generated in NHDF, whereas ROS levels in cells exposed to DDS or SMX did not differ from those exposed to vehicle alone (Fig. 4). As observed with cytotoxicity, the ROS generated with D-NOH exposure was much higher than that observed with S-NOH (Fig. 4). Incubation of NHDF with 1.5 mM of either metabolite or parent compound revealed a rate of ROS formation (measured as change in DCF fluorescence (RFU) per min) of 24.3 RFU/min with D-NOH, while that for S-NOH was 4.28 RFU/min.
Figure 4.
Reactive oxygen species (ROS) generation in the presence/absence of various drugs and their metabolites in NHDF. NHDF were seeded at a density of 105 cells/mL on a 96 well plate. A) Concentration dependent response. Cells were exposed to increasing concentrations of the hydroxylamine metabolites (S-NOH or DNOH) or parent compounds (DDS-1.5 mM and SMX-1.5 mM) in phosphate buffer. 2',7'-dichlorodihydrofluoroscein-diacetate (DCHF-DA) (20 μM) a fluorescent dye was added immediately. Relative fluorescence intensity at 30 min time point is presented as the mean (SD) of 8 replicate incubations. B) Time dependent response. Cells were exposed to 1.5 mM of parent compound or the metabolite in phosphate buffer. 2',7'-dichlorodihydrofluoroscein-diacetate (20 μM) was added immediately. Relative fluorescence intensity was measured every 5 min for 30 min at an excitation/emission of 485/530. Data shown as mean (+SD) 8 separate incubations.
GSH depletion in NHDF cells following drug/metabolite exposure
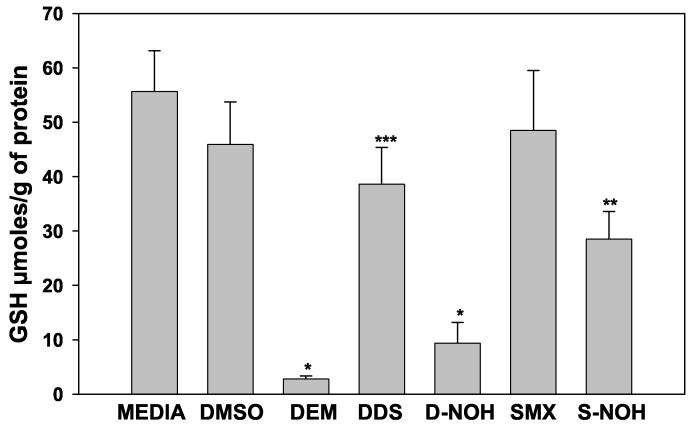
As GSH is a critical component in maintaining the redox balance in cells, we evaluated the effect of these arylamines and arylhydroxylamines on GSH content in NHDF. As expected, incubation of NHDF with DEM resulted in a substantial reduction in GSH content (Fig. 5). Cells exposed to DDS, S-NOH or D-NOH exhibited depletion in GSH content of 16%, 38% and 80%, respectively, as compared to the control (DMSO). Interestingly, DDS exposed cells exhibited a significantly lower level of GSH than that seen in control (media or vehicle) or SMX-exposed cells (Fig 5). This reduction may reflect the utilization of GSH in the detoxification of the D-NOH formed upon exposure to the parent compound. In contrast, the lack of effect of SMX on GSH content is consistent with the lack of observable formation of S-NOH or protein haptenation (Fig. 5).
Figure 5.
Glutathione depletion in the presence/absence of parent drugs and their metabolites in NHDF cells: NHDF cells were seeded at a density of 5×105 cells/mL on a 6 well plate and incubated for 3 h with 1.5 mM of parent drug or metabolite. Media with NHDF cells alone were used to measure the basal GSH content, DMSO (0.25%) was used as the control, and DEM 0.4 mM was used as a positive control for GSH reduction. After 3 h incubation, the cells were washed with ice cold PBS and treated with 1 ml ice cold lysis buffer. Cells were lysed, extracted and analyzed for GSH content as described in Materials and Methods. GSH contents were determined using a standard curve generated from known concentrations and expressed as μmoles/g of protein. Data presented as mean (+SD) of four separate incubations for each condition. Results were analyzed using ANOVA with Holm-Sidak method for multiple pairwise comparisons. *p<0.05 compared to media, DMSO, DDS, SMX and SNOH; **p<0.05 compared to media, DMSO, DDS, and SMX; ***p<0.05 compared to media, DMSO, and SMX.
DISCUSSION
Despite being among the most common adverse drug reactions, the mechanism of CDRs remains to be elucidated. As small molecular weight compounds are generally unable to provoke an immune response, it is believed that protein haptenation is an essential step in the provocation of such reactions (Park et al., 1998; Svensson et al., 2001). Since most drug molecules are not highly reactive, bioactivation to reactive species appears to be an essential step in the initiation of immune reactions to these drugs. For sulfonamides in particular, it has been proposed that the formation of arylhydroxylamine metabolites is a determining factor in the CDRs associated with these drugs (Reilly and Ju, 2002; Svensson, 2003).
Early work regarding the role of reactive metabolites and immune-mediated drug reactions focused on the formation of such metabolites in the liver and circulating cells (Park et al., 1987; Uetrecht, 1989; Park et al., 1992; Uetrecht, 1992). Recently, however, we suggested that bioactivation and protein haptenation occurring in the cutaneous environment may play a key role in the precipitation of immune reactions to drugs in the skin (Reilly et al., 2000). Indeed, we have demonstrated that exposure of normal human epidermal keratinocytes (NHEK) to SMX, DDS, or their arylhydroxylamine metabolites results in intracellular protein haptenation (Reilly et al., 2000; Roychowdhury et al., 2005). As the predominant cell type in the epidermal region, and one which plays an important role in the regulation of cutaneous immune responses, the ability of keratinocytes to metabolize these drugs to reactive metabolites has potential implications for the mechanism of CDRs. In addition to keratinocytes, other cell types in the skin contain enzymes known to metabolize drugs. Fibroblasts are the most abundant cell type in the dermal region and have been reported to contain cytochromes P450 and other drug metabolizing enzymes (Pascale et al., 1990; Eltom et al., 1998; Saeki et al., 2002). Importantly, drugs distributing to the skin from the systemic circulation would encounter these cells prior to distribution to the epidermal region and interaction with keratinocytes. Thus, we determined the bioactivation and protein haptenation of arylamine xenobiotics in fibroblasts as an initial step in probing the role of fibroblast in these immune mediated CDRs.
While intracellular protein haptenation was readily demonstrated in NHDF exposed to DDS, similar protein adducts were not detected when cells were exposed to SMX (Fig. 1 & 2) by either method (confocal microscopy or adduct-specific ELISA). Though previous studies have demonstrated that ascorbic acid increases the intracellular protein haptenation in NHEK exposed to SMX (Roychowdhury et al., 2005), haptenated proteins were not detected under these conditions in NHDF. The concentration of SMX selected (800 μM) is in the range of expected peak concentrations of the drug in plasma at doses used in the treatment of Pneumocystis carinii pneumonia, while the S-NOH concentration (100 μM) is at the lower end of estimated peak concentration for this metabolite (Manchanda et al., 2002). In addition, these concentrations have been found to produce readily detected haptenated proteins in NHEK (Roychowdhury et al., 2005). For comparison purposes, identical concentrations of SMX/DDS and S-NOH/D-NOH were used in these studies. Our studies in NHEK have suggested that the level of adduct formed is much higher after DDS than SMX exposure, a result consistent with the present observations in NHDF. These results, combined with the inability to detect the arylhydroxylamine metabolite after SMX and the below quantifiable levels of D-NOH after DDS exposure in NHDF, suggests that the ability of these cells to bioactivate the parent compounds is substantially less than that seen in keratinocytes. Hence, it would appear unlikely that bioactivation of these drugs by NHDF would play a significant role in the CDRs associated with their use.
In contrast to the low level of oxidative metabolism, the N-acetylated metabolite of SMX was readily detected when NHDF were incubated with this compound. The amount of monoacetyldapsone formed was, however, below the limit of quantification. While there are two known human N-acetyltransferase enzymes (NAT1 and NAT2), it appears that only NAT1 exhibits a wide tissue distribution (Hein et al., 2000; Windmill et al., 2000; Upton et al., 2001). Studies indicate that SMX is metabolized by NAT1 (Cribb et al., 1993), while DDS is primarily metabolized by NAT2 (Gelber et al., 1971; Palamanda et al., 1995). Our finding that NAT1 mRNA is highly expressed in NHDF, while very low levels of NAT2 mRNA were observed, suggests that expression of NAT1 in these cells is substantially higher than NAT2. Our finding that the in vitro acetylation of PABA (an NAT 1 substrate) was readily detected, while the level of SMZ (an NAT2 substrate) acetylation was much less, supports the conclusion that expression of NAT1 predominates in NHDF. Theses observations are consistent with that of previous investigations which have shown the presence of NAT1 activity, but not NAT2, in human skin and keratinocytes(Kawakubo et al., 1990; Kawakubo et al., 2000; Reilly et al., 2000). The failure to detect either NAT protein via Western blot may reflect the combined impact of a low level of expression and the well known instability of the enzymes.
As expected, protein haptenation was readily detected in NHDF exposed to either S-NOH or D-NOH (Fig. 1 & 2). This observation indicates that the arylhydroxylamine metabolites readily enter these cells and presumably autooxidize to their respective arylnitroso derivative prior to covalent adduction with proteins. The ability to detect the arylhydroxylamine metabolites of SMX (Cribb and Spielberg, 1992; van der Ven et al., 1994; van der Ven et al., 1995) and dapsone (Gordon et al., 1979; May et al., 1990; Mitra et al., 1995) in the urine of patients receiving these drugs suggests that the stability of these metabolites in blood is sufficient for distribution throughout the body after formation in the liver. Hence, dermal fibroblasts may be exposed to these arylhydroxylamine metabolites in vivo after formation in the liver and distribution to the skin. Further studies are needed to determine if protein haptenation occurs in vivo after such exposure and whether this plays a role in mediating the associated CDRs.
As necrotic cell death may release a variety of ‘danger’ signals that serve as potent immune stimulants, the cytotoxic effects of reactive metabolites may play an important role in immune-mediated drug reactions (Matzinger, 1994; Uetrecht, 1999; Gruchalla, 2001). Since glutathione content has been reported to be lower in fibroblasts compared to keratinocytes (Leccia et al., 1998), we postulated that NHDF may also be more susceptible to arylhydroxylamine-induced cytotoxicity. Our studies reveal that the LC50 for D-NOH in NHDF was 1.5 mM, while the LC50 in NHEK has been found to be ∼300 μM (Reilly et al., 2000). While there are no published reports regarding the concentrations of metabolites in skin after administration of SMX or DDS, the concentrations necessary to induce cytotoxicity in NHDF are unlikely to be achieved in vivo. Hence, it is improbable that metabolite-induced cytotoxicity in dermal fibroblasts plays a role in CDRs associated with these drugs.
While induction of cell death can certainly lead to the release of important immune signals, less overt toxicity may also give rise to biochemical changes that result in the release of danger signals that may results in immune activation. As it has been demonstrated that contact allergens may results in oxidative stress in keratinocytes (Picardo et al., 1992), we sought to determine if such stress is produced in NHDF exposed to arylhydroxylamine metabolites. Previous studies in our laboratory have demonstrated that both S-NOH and D-NOH provoke the generation of ROS in NHEK (Vyas et al., 2005). In the studies reported herein, we observed similar increases in ROS in NHDF exposed to these arylhydroxylamines (Fig. 4). Further evidence for the induction of oxidative stress is provided by the ability of these metabolites to reduce GSH content in NHDF (Fig. 5). Whether or not this magnitude of oxidative stress is sufficient to provoke the release of important danger signals is currently under investigation.
While the hapten hypothesis has been widely accepted as providing an underlying basis for immune-mediated drug reactions, recent work has challenged the notion that the immune system does not recognize small molecules apart from protein haptenation (Pichler, 2002; Pichler, 2003). The ability of reversible binding of SMX and antigen presenting cells to activate drug-specific T-cells from patients with a history of hypersensitivity to the drug in an MHC-dependent fashion suggests that elicitation of an immune response in sensitized individuals may not require bioactivation of the drug to reactive species (Zanni et al., 1998). It has also been suggested that non-reactive drug may stimulate the production of drug-specific T-cells in non-sensitized individuals (Engler et al., 2004). Though such observations suggest that bioactivation may not be essential for provocation of an immune response to drugs in the skin, they do not refute the experimental evidence that indicates that such bioactivation can give rise to immune responses. Hence, the demonstration that protein haptenation may be observed in NHDF exposed to drugs or reactive metabolites suggest these cells may play a role in immune-mediated CDRs. Further studies are needed to elucidate that role more specifically. In particular, it will be important to determine if these cells are targeted by infiltrating T-cells and whether or not they are able to transfer drug-protein adducts to dermal dendritic cells.
ACKNOWLEDGEMENTS
We wish to thank the staff of the Central Microscopy Research Facility at The University of Iowa, which is supported by the Office of the Vice President for Research, for their technical assistance. This work was supported in part by NIH Grants AI41395 and GM63821 to CKS and CA34627 to DWH.
Abbreviations
- AA
ascorbic acid
- CDRs
cutaneous drug reactions
- DDS
dapsone
- D-NOH
dapsone hydroxylamine
- ELISA
enzyme-linked immunosorbant assay
- GSH
glutathione
- HPLC
high performance liquid chromatography
- NAT
N-acetyltransferase
- NHEK
normal human epidermal keratinocytes
- NHDF
normal human dermal fibroblasts
- PABA
p-aminobenzoic acid
- PBS
phosphate buffered saline
- ROS
reactive oxygen species
- SMX
sulfamethoxazole
- SMZ
sulfamethazine
- S-NOH
sulfamethoxazole hydroxylamine
Footnotes
This work was presented in part at the joint 13th North American ISSX and 20th JSSX Meeting in Maui, Hawaii on October 27, 2005.
CONFLICT OF INTEREST
There are no conflicts of interest for any of the authors of this manuscript.
REFERENCES
- Cribb AE, Lee BL, Trepanier LA, Spielberg SP. Adverse reactions to sulphonamide and sulphonamide-trimethoprim antimicrobials: clinical syndromes and pathogenesis. Adverse Drug React. Toxicol. Rev. 1996a;15:9–50. [PubMed] [Google Scholar]
- Cribb AE, Nakamura H, Grant DM, Miller MA, Spielberg SP. Role of polymorphic and monomorphic human arylamine N-acetyltransferases in determining sulfamethoxazole metabolism. Biochem. Pharmacol. 1993;45:1277–1282. doi: 10.1016/0006-2952(93)90280-a. [DOI] [PubMed] [Google Scholar]
- Cribb AE, Nuss CE, Alberts DW, Lamphere DB, Grant DM, Grossman SJ, Spielberg SP. Covalent binding of sulfamethoxazole reactive metabolites to human and rat liver subcellular fractions assessed by immunochemical detection. Chem. Res. Toxicol. 1996b;9:500–507. doi: 10.1021/tx950167j. [DOI] [PubMed] [Google Scholar]
- Cribb AE, Spielberg SP. Sulfamethoxazole is metabolized to the hydroxylamine in humans. Clin. Pharmacol. Ther. 1992;51:522–526. doi: 10.1038/clpt.1992.57. [DOI] [PubMed] [Google Scholar]
- Cribb AE, Spielberg SP, Griffin GP. N4-hydroxylation of sulfamethoxazole by cytochrome P450 of the cytochrome P4502C subfamily and reduction of sulfamethoxazole hydroxylamine in human and rat hepatic microsomes. Drug Metab. Dispos. 1995;23:406–414. [PubMed] [Google Scholar]
- Cucinelli SA, Israili ZH, Dayton PG. Microsomal N-oxidation of dapsone as a cause of methemoglobin formation in human red cells. Am. J. Trop. Med. Hyg. 1972;21:322–331. doi: 10.4269/ajtmh.1972.21.322. [DOI] [PubMed] [Google Scholar]
- Eltom SE, Larsen MC, Jefcoate CR. Expression of CYP1B1 but not CYP1A1 by primary cultured human mammary stromal fibroblasts constitutively and in response to dioxin exposure: Role of the Ah receptor. Carcinogenesis. 1998;19:1437–1444. doi: 10.1093/carcin/19.8.1437. [DOI] [PubMed] [Google Scholar]
- Engler OB, Strasser I, Naisbitt DJ, Cerny A, Pichler WJ. A chemically inert drug can stimulate T cells in vitro by their T cell receptor in non-sensitised individuals. Toxicology. 2004;197:47–56. doi: 10.1016/j.tox.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Gelber R, Peters J, Gordon G, Glazko A, Levy L. The polymorphic acetylation of dapsone in man. Clin. Pharmacol. Ther. 1971;12:225–238. doi: 10.1002/cpt1971122part1225. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Murray JF, Peters JH, Gelber RH, Jacobson RR. Studies on the urinary metabolites of dapsone in man. Int. J. Lepr. 1979;47:681–682. [Google Scholar]
- Gruchalla R. Drug metabolism, danger signals, and drug-induced hypersensitivity. J. Allergy Clin. Immunol. 2001;108:475–488. doi: 10.1067/mai.2001.118509. [DOI] [PubMed] [Google Scholar]
- Hein D, Doll M, Fretland A, Leff M, Webb S, Xiao G, Devanaboyina U-S, Nangju N, Feng Y. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol. Biomarkers Prev. 2000;9:29–42. [PubMed] [Google Scholar]
- Husain A, Barker DF, States JC, Doll MA, Hein DW. Identification of the major promoter and non-coding exons of the human arylamine N-acetyltransferase 1 gene (NAT1) Pharmacogenetics. 2004;14:397–406. doi: 10.1097/01.fpc.0000114755.08559.6e. [DOI] [PubMed] [Google Scholar]
- Kawakubo Y, Merk HF, Al Masaqudi T, Sieben S, Blomeke B. Nacetylation of paraphenylenediamine in human skin and keratinocytes. J. Pharmacol. Exp. Ther. 2000;292:150–155. [PubMed] [Google Scholar]
- Kawakubo Y, Yamazoe Y, Kato R, Nishikawa T. High capacity of human skin for N-acetylation of arylamines. Skin Pharmacol. 1990;3:180–185. doi: 10.1159/000210868. [DOI] [PubMed] [Google Scholar]
- Leccia M-T, Richard M-J, Joanny-Crisci F, Beani J-C. UV-A1 cytotoxicity and antioxidant defence in keratinocytes and fibroblasts. Eur. J. Dermatol. 1998;8:478–482. [PubMed] [Google Scholar]
- Leff MA, Epstein PN, Doll MA, Fretland AJ, Devanaboyina US, Rustan TD, Hein DW. Prostate-specific human N-acetyltransferase 2 (NAT2) exprssion in the mouse. J. Pharmacol. Exp. Ther. 1999;290:182–187. [PubMed] [Google Scholar]
- Manchanda T, Hess D, Dale L, Ferguson SG, Rieder MJ. Haptenation of sulfonamide reactive metabolites to cellular proteins. Mol. Pharmacol. 2002;62:1011–1026. doi: 10.1124/mol.62.5.1011. [DOI] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- Mauri-Hellweg D, Bettens F, Mauri D, Brander C, Hunziker T, Pichler WJ. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J. Immunol. 1995;155:462–472. [PubMed] [Google Scholar]
- May DG, Porter JA, Uetrecht JP, Wilkinson GR, Branch RA. The contribution of N-hydroxylation and acetylation to dapsone pharmacokinetics in normal subjects. Clin. Pharmacol. Ther. 1990;48:619–627. doi: 10.1038/clpt.1990.204. [DOI] [PubMed] [Google Scholar]
- Mitra AK, Thummel KE, Kalhorn TF, Kharasch ED, Unadkat JD, Slattery JT. Metabolism of dapsone to its hydroxylamine by CYP2E1 in vitro and in vivo. Clin. Pharmacol. Ther. 1995;58:556–566. doi: 10.1016/0009-9236(95)90176-0. [DOI] [PubMed] [Google Scholar]
- Naisbitt DJ, Farrell J, Gordon SF, Maggs JL, Burkhart C, Pichler WJ, Pirmohamed M, Park BK. Covalent binding of the nitroso metabolite of sulfamethoxazole leads to toxicity and major histocompatibility complex-restricted antigen presentation. Mol. Pharmacol. 2002;62:628–637. doi: 10.1124/mol.62.3.628. [DOI] [PubMed] [Google Scholar]
- Nassif A, Bensussan A, Dorothee G, Mami-Chouaib F, Bachot N, Bagot M, Boumsell L, Roujeau JC. Drug specific cytotoxic T-cells in the skin of lesions of a patient with toxic epidermal necrolysis. Journal of Investigative Dermatology. 2002;118:728–733. doi: 10.1046/j.1523-1747.2002.01622.x. [DOI] [PubMed] [Google Scholar]
- Palamanda JR, Hickman D, Ward A, Sim E, Romkes-Sparks M, Unadkat JD. Dapsone acetylation by human liver arylamine N-acetyltransferases and interaction with antiopportunistic infection drugs. Drug Metab. Dispos. 1995;23:473–477. [PubMed] [Google Scholar]
- Park BK, Coleman JW, Kitteringham NR. Drug disposition and drug hypersensitivity. Biochem. Pharmacol. 1987;36:581–590. [PubMed] [Google Scholar]
- Park BK, Kitteringham NR. Drug-protein conjugation and its immunological consequences. Drug Metab. Rev. 1990;22:87–144. doi: 10.3109/03602539008991445. [DOI] [PubMed] [Google Scholar]
- Park BK, Kitteringham NR, Powell H, Pirmohamed M. Advances in molecular toxicology - towards understanding idiosyncratic drug toxicity. Toxicology. 2000;153:39–60. doi: 10.1016/s0300-483x(00)00303-6. [DOI] [PubMed] [Google Scholar]
- Park BK, Pirmohamed M, Kitteringham NR. Idiosyncratic drug reactions: a mechanistic evaluation of risk factors. Br. J. Clin. Pharmacol. 1992;34:377–395. doi: 10.1111/j.1365-2125.1992.tb05647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BK, Pirmohamed M, Kitteringham NR. Role of drug disposition in drug hypersensitivity: A chemical, molecular, and clinical perspective. Chem. Res. Toxicol. 1998;11:969–988. doi: 10.1021/tx980058f. [DOI] [PubMed] [Google Scholar]
- Pascale R, Ruggiu ME, Simile MM, Daino L, Vannini G, Seddaiu MA, Satta G, Feo F. Dependence of benzo(a)pyrene metabolism on NADPH pool in normal and glucose-6-phosphate dehydrogenase deficeint human fibroblasts. Res. Commun. Chem. Pathol. Pharmacol. 1990;69:361–364. [PubMed] [Google Scholar]
- Picardo M, Zompetta C, Marchese C, al e. Paraphenylenediamine, a contact allergen, induces oxidative stress and ICAM-1 expression in human keratinocytes. Br. J. Dermatol. 1992;126:450–455. doi: 10.1111/j.1365-2133.1992.tb11817.x. [DOI] [PubMed] [Google Scholar]
- Pichler WJ. Pharmacological interaction of drugs with anitgen-specific immune receptors: The p-i concept. Curr. Opin. Allergy Immunol. 2002;2:301–305. doi: 10.1097/00130832-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Pichler WJ. Delayed drug hypersensitivity reactions. Ann. Intern. Med. 2003;139:683–693. doi: 10.7326/0003-4819-139-8-200310210-00012. [DOI] [PubMed] [Google Scholar]
- Pichler WJ, Yawalkar N, Britschgi M, Depta JPH, Strasser I, Schmid S, Kuechler P, Naisbitt D. Cellular and molecular pathophysiology of cutaneous drug reactions. Am. J. Clin. Dermatol. 2002;3:229–238. doi: 10.2165/00128071-200203040-00001. [DOI] [PubMed] [Google Scholar]
- Reilly TP, Ju C. Mechanistic perspectives on sulfonamide-induced cutaneous drug reactions. Curr. Opin. Allergy Clin. Immunol. 2002;2:307–315. doi: 10.1097/00130832-200208000-00004. [DOI] [PubMed] [Google Scholar]
- Reilly TP, Lash LH, Doll MA, Hein DW, Woster PM, Svensson CK. A role for bioactivation and covalent binding within epidermal keratinocytes in sulfonamide-induced cutaneous drug reactions. Journal of Investigative Dermatology. 2000;114:1164–1173. doi: 10.1046/j.1523-1747.2000.00985.x. [DOI] [PubMed] [Google Scholar]
- Rieder MJ, Uetrecht J, Shear NH, Spielberg SP. Synthesis and in vitro toxicity of hydroxylamine metabolites of sulfonamides. J. Pharmacol. Exp. Ther. 1988;244:724–728. [PubMed] [Google Scholar]
- Rodrigues-Lima F, Cooper RN, Goudeau B, Atmane N, Chamagne A-M, Butler-Browne G, Sim E, Vicart P, Dupret J-M. Skeletal muscles express the xenobiotic-metabolizing enzyme arylamine N-acetyltransferase. J. Histochem. Cytochem. 2003;51:789–796. doi: 10.1177/002215540305100610. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, Vyas PM, Reilly TP, Gaspari AA, Svensson CK. Characterization of the formation and localization of sulfamethoxazole- and dapsone-associated drug-protein adducts in human epidermal keratinocytes. J. Pharmacol. Exp. Ther. 2005;314:43–52. doi: 10.1124/jpet.105.086009. [DOI] [PubMed] [Google Scholar]
- Saeki M, Saito Y, Nagano M, Teshima R, Ozawa S, Sawada J-I. mRNA expression of multiple cytochrome P450 isozymes in four types of cultured skin cells. Int. Arch. Allergy Immunol. 2002;127:333–336. doi: 10.1159/000057751. [DOI] [PubMed] [Google Scholar]
- Schnyder B, Burkhart C, Schnyder-Frutig K, von Greyerz S, Naisbitt DJ, Pirmohamed M, Park BK, Pichler WJ. Recognition of sulfamethoxazole and its reactive metabolites by drug-specific CD4+ T cells from allergic individuals. J. Immunol. 2000;164:6647–6654. doi: 10.4049/jimmunol.164.12.6647. [DOI] [PubMed] [Google Scholar]
- Shear NH, Spielberg SP. In vitro evaluation of a toxic metabolite of sulfadiazine. Can. J. Physiol. Pharmacol. 1985;63:1370–1372. doi: 10.1139/y85-225. [DOI] [PubMed] [Google Scholar]
- Stanley LA, Coroneos E, Cuff R, Hickman D, Ward A, Sim E. Immunochemmical detection of arylamine N-acetyltransferase in normal and neoplastic bladder. J. Histochem. Cytochem. 1996;44:1059–1067. doi: 10.1177/44.9.8773572. [DOI] [PubMed] [Google Scholar]
- Svensson CK. Do arylhydroxylamine metabolites mediate the idiosyncratic reactions associated with sulfonamides and sulfones? Chem. Res. Toxicol. 2003;16:1034–1043. doi: 10.1021/tx034098h. [DOI] [PubMed] [Google Scholar]
- Svensson CK, Cowen EW, Gaspari AA. Cutaneous drug reactions. Pharmacol. Rev. 2001;53:357–379. [PubMed] [Google Scholar]
- Uetrecht JP. Idiosyncratic drug reactions: possible role of reactive metabolites generated by leukocytes. Pharm. Res. 1989;6:265–273. doi: 10.1023/a:1015934104984. [DOI] [PubMed] [Google Scholar]
- Uetrecht JP. The role of leukocyte-generated reactive metabolites in the pathogenesis of idiosyncratic drug reactions. Drug Metab. Rev. 1992;24:299–366. doi: 10.3109/03602539208996297. [DOI] [PubMed] [Google Scholar]
- Uetrecht JP. New concepts in immunology relevant to idiosyncratic drug reactions: The “Danger Hypothesis” and innate immune system. Chem. Res. Toxicol. 1999;12:387–395. doi: 10.1021/tx980249i. [DOI] [PubMed] [Google Scholar]
- Upton A, Johnson N, Sandy J, Sim E. Arylamine N-acetyltransferases - of mice, men and microorganisms. Trends Pharmacol. Sci. 2001;22:140–146. doi: 10.1016/s0165-6147(00)01639-4. [DOI] [PubMed] [Google Scholar]
- van der Ven AJ, Mantel MA, Vree TB, Koopmans PP, van der Meer JW. Formation and elimination of sulphamethoxazole hydroxylamine after oral administration of sulphamethoxazole. Br. J. Clin. Pharmacol. 1994;38:147–150. doi: 10.1111/j.1365-2125.1994.tb04339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven AJ, Vree TB, van Ewijk-Beneken Kolmer EW, Koopmans PP, van der Meer JW. Urinary recovery and kinetics of sulphamethoxazole and its metabolites in HIV-seropositive patients and healthy volunteers after a single oral dose of sulphamethoxazole. Br. J. Clin. Pharmacol. 1995;39:621–625. doi: 10.1111/j.1365-2125.1995.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas PM, Roychowdhury S, Woster PM, Svensson CK. Reactive oxygen species generation and its role in the differential cytotoxicity of the arylhydroxylamine metabolites of sulfamethoxazole and dapsone in normal human epidermal keratinocytes. Biochem. Pharmacol. 2005;70:275–286. doi: 10.1016/j.bcp.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Windmill K, Gaedigk A, Hall P, Samaratunga H, Grant D, McManus M. Localization of N-acetyltransferases NAT1 and NAT2 in human tissues. Toxicol. Sci. 2000;54:19–29. doi: 10.1093/toxsci/54.1.19. [DOI] [PubMed] [Google Scholar]
- Zang Y, Zhao S, Doll MA, States JC, Hein DW. The T341C (Ile114Thr) polymorphism of N-acetyltransferase 2 yields slow acetylator phenotype by enhanced protein degradation. Pharmacogenetics. 2004;114:717–723. doi: 10.1097/00008571-200411000-00002. [DOI] [PubMed] [Google Scholar]
- Zanni M, von Greyerz S, Schnyder B, Brander K, Frutig K, Hari Y, Valitutti S, Pichler W. HLA-restricted, processing- and metabolism-independent pathway of drug recognition by human αβ T lymphocytes. J. Clin. Invest. 1998;102:1591–1598. doi: 10.1172/JCI3544. [DOI] [PMC free article] [PubMed] [Google Scholar]