Abstract
1,25-Dihydroxyvitamin D3 and several of its analogs, such as EB1089, induce growth arrest and apoptosis of breast cancer cells in culture. EB1089 has also been shown to limit growth of xenografts in nude mice and carcinogen-induced mammary tumors in rats. Coupled with the fact that the vitamin D receptor is highly expressed in a large proportion of breast tumors, these data suggest that it may be a broad spectrum therapeutic target. We utilized a transgenic model of hormone-induced mammary cancer, the LH-overexpressing mouse, to assess, for the first time, the efficacy of EB1089 in a spontaneous tumor model. Similar to human breast cancers, the pre-neoplastic mammary glands and mammary tumors in these mice express high levels of vitamin D receptor. Treatment with EB1089 decreased proliferation of mammary epithelial cells in pre-neoplastic glands by 35%. Moreover, half of hormone-induced mammary tumors treated with EB1089 demonstrated a decreased rate of growth, with a subset of these tumors even regressing, suggesting that 1,25-dihydroxyvitamin D3 analogs may be effective chemopreventive and chemotherapeutic agents for breast cancer.
Keywords: Breast cancer; 1,25-Dihydroxyvitamin D3; Vitamin D receptor; EB1089; Luteinizing hormone; Ovarian hyperstimulation; Transgenic mice
1. Introduction
Breast cancer is the most common cancer in women in the United States, who exhibit a 1 in 7 chance of being diagnosed with this disease over their lifetime [1]. Although currently available treatment paradigms, which include hormone modulators such as tamoxifen, are often effective, many cancers are refractory to therapy and others recur following initial responsiveness. The identification of novel therapeutic targets and subsequent development of effective modulators of these targets will assist in reducing the incidence and mortality of this disease.
The vitamin D endocrine system represents one emerging target of therapeutic interest in breast tissue. Vitamin D3 is acquired through the diet or produced in the skin upon exposure to ultraviolet light. 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3], the bioactive metabolite of vitamin D3, binds to the vitamin D receptor (VDR), a member of the nuclear receptor superfamily, and regulates transcription of select target genes (reviewed in [2]). Several lines of epidemiological evidence suggest that activation of VDR may impart protection against breast cancer progression. Risk of fatal breast cancer is inversely proportional to the intensity of local sunlight [3] and post-treatment disease prognosis is improved if chemotherapy is administered during seasons of high levels of vitamin D3 exposure [4]. Furthermore, high dietary intake of vitamin D has been correlated with decreased breast cancer risk in women [5,6]. Immunohistochemical analysis has revealed that the vitamin D receptor is elevated in human breast cancers compared to normal breast tissue [7]. VDR is present in more than 75% of breast tumors and is associated with a diverse set of molecular phenotypes, evoking the possibility that VDR may be a broad spectrum therapeutic target [8].
Studies of the endogenous role of vitamin D receptor in the mammary gland have been performed using genetically engineered mice. Mice deficient in VDR have revealed that it is able to limit hormone-driven mammary gland development. Compared to age-matched wild type littermates, peripubertal female VDR−/− mice exhibit enhanced ductual elongation and increased secondary branching [9], events ascribed to estrogen and progesterone activity, respectively [10]. Furthermore, mammary glands from mice lacking VDR display enhanced responsiveness to exogenous estrogen, progesterone, and lactogenic hormones both in vivo and ex vivo [9]. The importance of VDR in resistance to tumorigenesis has been verified by a recent report that loss of a single copy of VDR is sufficient to shorten tumor latency and increase tumor incidence in the MMTV-neu mouse model of mammary cancer [11].
In addition to mouse models, numerous studies have demonstrated the ability of 1,25-(OH)2D3 to inhibit growth of human breast cancer cells in culture [12–15]. This antiproliferative effect is due to G1 arrest and is correlated with a decrease in cyclin D1, increases in the cell cycle inhibitors p21 and p27 [15], and a decrease in Cdk2 activity [16]. In MCF-7 cells, 1,25-(OH)2D3 treatment is also associated with downregulation of estrogen receptor (ER) [17,18]; however, VDR agonists also deter growth of several estrogen receptor negative breast cancer cell lines, demonstrating the existence of estrogen receptor-independent mechanisms of action [12,19,20]. In addition to growth inhibition, cells exposed to 1,25-(OH)2D3 also display trademarks of apoptosis, such as cell shrinkage, chromatin condensation and DNA fragmentation [21].
Initially, the hypercalcemic effects of 1,25-(OH)2D3 limited in vivo scrutiny of the therapeutic potential of VDR activation, but the development and characterization of several 1,25-(OH)2D3 analogs that maintain the ability to inhibit mammary epithelial cell growth while exerting a diminished effect on calcium homeostasis has facilitated investigation in experimental animal models [22,23]. One such compound, EB1089, inhibits growth of MCF-7 cells with a potency at least one order of magnitude greater than that exhibited by the native hormone. Most importantly, this agent is significantly less hypercalcemic than 1,25-(OH)2D3 in mice [24]. Systemic administration of EB1089 prevented expansion of established MCF-7 xenografts in nude mice, even inducing regression of a subset of tumors [25]. Treatment with EB1089 also inhibited growth of established nitrosomethyl urea (NMU)-induced mammary tumors in rats [26]; however, to date, prevention and treatment efficacies of 1,25-(OH)2D3 analogs have not been assessed in a spontaneous model of mammary cancer.
In this regard, transgenic mice that overexpress luteinizing hormone (LH) provide a unique model of hormone-induced mammary cancer [27,28]. Elevated LH causes ovarian hyperstimulation and leads to increased levels of several mammogenic hormones, including estradiol, progesterone and prolactin. Exposure to this altered hormonal milieu results in mammary gland hyperplasia and the formation of spontaneous mammary tumors [28], making the LH-overexpressing mouse one of the few experimental models that reflects the critical contribution of reproductive hormones to human breast cancer. This contribution has been long acknowledged and is evidenced by numerous epidemiological studies that correlate the timing and occurrence of several hormonally regulated events, such as menarche, pregnancy and menopause, with risk of breast cancer diagnosis [29–33]. Furthermore, women treated with tamoxifen, a selective estrogen receptor modulator that antagonizes ER in the breast, display a significantly decreased risk of developing invasive breast cancer [34]. Hence, identifying approaches to combat the pathological effects of hormones on the mammary gland, both preventatively and therapeutically, should contribute to reductions in disease incidence and mortality and models such as the LH-overexpressing mouse should facilitate this process.
The studies presented herein provide evidence that treatment with a vitamin D3 analog, EB1089, has the ability to limit hormone-induced proliferation of endogenous mammary epithelial cells and reduce the growth rate of a subset of spontaneous mammary tumors in vivo.
2. Materials and methods
2.1. Animals
Mice were housed in microisolator plus units with a 12 h light/dark cycle and given food and water ad libitum. During treatment with EB1089 or vehicle, all mice were maintained on a 0.1% low-calcium diet (BioServe, Frenchtown, NJ) to avoid the potential development of hypercalcemia. All mouse studies were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University.
2.2. Gene expression profiling
Affymetrix U74Av2 gene chips were probed with biotinylated cRNA generated from mammary glands of wild type and transgenic mice at the specified ages. Double stranded cDNA was reverse transcribed from 10 μg of total RNA using the Superscript Double Stranded cDNA Synthesis kit (Invitrogen, Carlsbad, CA; cat# 11917-010) and an oligo-dT primer coupled to the T7 RNA polymerase promoter. Subsequently, biotinylated cRNA was synthesized using the Enzo BioArray High Yield RNA Transcript Labeling Kit (Affymetrix, Santa Clara, CA; cat# 900182). Data was analyzed using Affmetrix MicroArray Suite 5.0 (MAS 5.0). VDR was identified as part of a list of genes that were at least two-fold changed in at least one transgenic sample relative to the appropriate wild type sample.
2.3. Immunohistochemistry
Thoracic mammary glands were removed and fixed in 4% paraformaldehyde. Five micron sections were deparaffinized in xylene, rehydrated with graded concentrations of ethanol and rinsed in phosphate buffered saline (PBS). Unless otherwise noted, all incubations were performed at room temperature and washes done in PBS. The primary antibodies utilized were the 9A7 rat anti-chicken VDR antibody (Affinity Bioreagents, Golden, CO; cat# MA1-710) and mouse anti-BrdU (Becton Dickinson Immunocytometry Systems, San Jose, CA, cat# 347580). Vector ABC kits containing biotinylated anti-rat and anti-mouse secondary antibodies were used (Vector Laboratories, Burlingame, CA; cat# PK-4004, PK-4002) and diamino-benzidine (DAB) was employed for detection. Nuclei were counterstained with hematoxylin QS (Vector Laboratories; cat# H-3404).
For VDR, antigen retrieval was performed in 2N HCl for 30 min at 37 °C. Non-specific binding was blocked by pre-incubation in 1.5% normal rabbit serum in PBS for 30 min. The antibody for VDR was used at a concentration of 5 μg/ml with 1.5% normal goat serum in PBS and incubated overnight at 4 °C. After washing, biotinylated anti-rat secondary antibody (1:200) was incubated for 1 h at 37 °C. The percentage of VDR positive cells was quantitated from 10 to 20 individual sections per animal (n=5 WT, 6 LH) and data analyzed with a Student’s t-test.
Sections to be assayed for BrdU incorporation were first boiled for 10 min in 10 mM citric acid, pH 6.0 to expose antigenic sites. After 1 h incubation with the primary antibody (1:150) and subsequent washes, sections were exposed to secondary antibody (1:300) for 1 h. The percentage of BrdU positive nuclei was determined in 10–20 sections (1100–1200 total cells) from each animal and data was analyzed by a one-tailed Student’s t-test (n=4 WT, 4 LH). Epithelial cells comprising both ducts and terminal end buds were included in the analysis.
2.4. EB1089 treatment of mice during development
EB1089 was obtained from Leo Pharmaceutical Products (Ballerup, Denmark). LH-overexpressing female mice heterozygous for the VDR null allele were generated in a mixed genetic background of CF1, FVB and C57BL/6J. Female mice were injected subcutaneously with 50 μl of sesame oil or with 50 μl of freshly prepared EB1089 (0.027 μg per animal) diluted in sesame oil once per day from 3 to 5 weeks of age. Mice were weighed daily to monitor for any global toxicity. Weight gains of mice in vehicle and EB1089 treated groups were indistinguishable. Twenty four hours following the last injection, the mice were injected intraperitoneally with 0.1 mg of 5-bromo-2-deoxyuridine (BrdU; Sigma, cat# B5002) per gram body weight. Two hours later, the mice were asphyxiated with carbon dioxide and their mammary glands processed for immunohistochemistry.
2.5. EB1089 treatment of tumor bearing mice
Transgenic mice were palpated weekly. Magnetic resonance imaging (MRI) was performed upon detection of a mammary tumor and again 2 weeks later in order to determine a basal growth rate for each tumor. Treatment was then initiated. Mice were given intraperitoneal injections of vehicle or 0.05 μg EB1089 in PBS every 48 h. After 14 days of treatment, tumors were reevaluated using MRI. Percent change in growth rate (GR) was calculated as follows: % change GR=((GRpost−GRb)/GRb)×100. GRb (basal growth rate)=change in tumor volume over 14 days prior to treatment initiation. GRpost (post-treatment growth rate)=change in tumor volume from Days 0 to 14 of treatment. Hence, a percent change in growth rate between − 100 and 0 reflects a decreased tumor growth rate, while a percent change in growth rate below − 100 indicates tumor regression.
2.6. Magnetic resonance imaging
Mice were anesthetized under 2.0% isoflurane gas in oxygen for 5 min and transferred to a clinical 1.5T MRI scanner (Siemens Sonata). A nosecone was used to deliver 1–2% isoflurane in oxygen to the animals during the MRI scans. The sedated animals were placed into a cylindrical, phased-array mouse coil (ID=33 mm) developed in-house to maximize the signal-to-noise ratio for the high resolution murine images. To visualize potentially small mammary tumors, the mice were scanned with a high resolution (270 μm×270 μm), T1-weighted spin echo acquisition (TR/TE=700/13 ms, slice thickness=1 mm, number of averages=6). The slices were acquired with zero gap in between to ensure an accurate tumor volume measurement. The T1-weighted acquisition was selected to provide good contrast between the normally bright, lipid-rich mammary glands and the darker tumors. The area of the tumors was measured in each image (slice) by manually tracing along the edge of the tumor. The total tumor volume was calculated by multiplying the slice-by-slice tumor areas by the slice thickness (1 mm) and summing the individual slice volumes.
3. Results
3.1. Mammary glands of LH-overexpressing mice demonstrate elevated expression of vitamin D receptor
In order to identify the molecular profile associated with the development of hormone-induced mammary gland hyperplasia and tumorigenesis, Affymetrix gene expression profiling was performed on the mammary glands of LH-overexpressing mice and wild type littermates at 5, 8, 12, and 19 weeks of age, as well as on spontaneous mammary tumors and age-matched controls. Included among the genes that are differentially expressed between wild type and transgenic mammary glands is the vitamin D receptor (Fig. 1A). While the signal associated with the VDR transcript is below the limit of detection in most of the wild type samples, mammary glands from 8, 12, and 19-week-old LH-overexpressing mice display robust expression, with relative signal intensities between 6- and 17-fold higher than those of age-matched controls. Increased expression of VDR is maintained in tumors, with tumors having 2-fold more VDR mRNA compared to age-matched wild type mice.
Fig. 1.
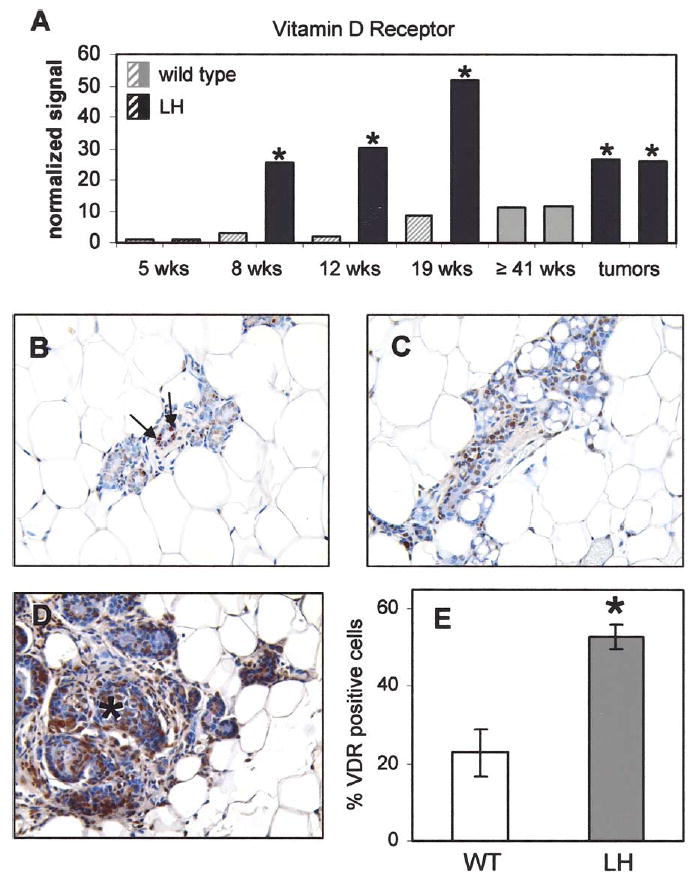
Mammary glands of LH-overexpressing mice demonstrate elevated expression of vitamin D receptor. (A) Affymetrix gene expression analysis reveals increased VDR mRNA in mammary glands of LH-overexpressing compared to age-matched wild type mice at 8, 12, and 19 weeks of age as well as in tumors. Data was analyzed using Affymetrix MAS 5.0 (gene detection: hatched bars=absent, solid bars=present; signal intensities normalized to 5-wk wild type; asterisk indicates sample significantly increased compared to age-matched wild type control). Immunohistochemistry for VDR was performed on mammary glands of adult wild type (B), and LH-overexpressing mice (C), as well as mammary tumors (marked by asterisk) from LH-overexpressing mice (D). (E) The percentage of epithelial cells expressing VDR is increased 2.3-fold in the mammary glands of adult LH-overexpressing mice relative to age-matched wild type mice (*P<0.01). Examples of positively staining nuclei are denoted by arrows in (B). Images are magnified 200×.
To determine whether the observed expression change also occurs at the protein level, we assessed VDR expression immunohistochemically. As expected, nuclear VDR staining is present in a relatively small subset of mammary epithelial cells in adult virgin wild type mice [9] (Fig. 1B); however, the hyperplastic mammary glands of adult LH-over-expressing mice display a 2.3-fold higher proportion of VDR-positive epithelial cells (Fig. 1C and E; P<0.01). Furthermore, VDR is detected in nearly every cell of the hormone-induced mammary tumors (Fig. 1D).
3.2. EB1089 decreases mammary epithelial cell proliferation in LH-overexpressing mice
The presence of high levels of vitamin D receptor in the mammary glands of LH-overexpressing mice led us to speculate that the growth inhibitory potential of this receptor could be exploited to limit the pathological effects of the altered hormonal milieu. In order to determine whether activation of VDR has the ability to inhibit the proliferative effects of the elevated mammogenic hormones in these mice, we implemented a 2-week-treatment of peripubertal transgenic mice with EB1089, a 1,25-(OH)2D3 analog with less in vivo hypercalcemic activity than the native hormone. BrdU incorporation was used to assess epithelial cell proliferation. Exposure to EB1089 reduced the number of proliferating mammary epithelial cells by 35% compared to vehicle treated controls (Fig. 2; P<0.05).
Fig. 2.
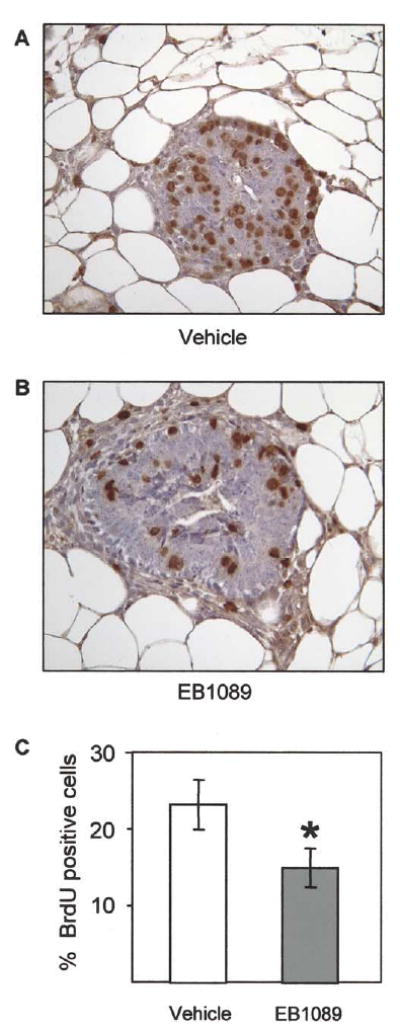
EB1089 decreases mammary epithelial cell proliferation in LH-overexpressing mice. EB1089 or vehicle was injected subcutaneously into LH-expressing female mice for 2 weeks after weaning. At 5 weeks of age, the mammary glands from vehicle (A) and EB1089 (B) treated animals were assessed for proliferation using BrdU labeling analysis (400× magnification). (C) EB1089 treated animals exhibited 35% fewer BrdU positive mammary epithelial cells than vehicle treated controls (*P<0.05).
3.3. EB1089 displays anti-tumorigenic activity in a subset of hormone-induced mammary tumors
Evidence for EB1089 efficacy in the hyperproliferative endogenous mammary gland prompted us to investigate the influence of this compound on established hormone-induced mammary tumors of LH-overexpressing mice. Mice were palpated weekly for the presence of tumors. Upon tumor detection, mice were subjected to magnetic resonance imaging (MRI); the resulting images were used to determine tumor volume (see Section 2). As inter-mouse variability in tumor growth rate is observed in this model, each tumor was used as its own control. The change in tumor volume over the 2 weeks preceding initiation of treatment established the basal growth rate of each tumor. Subsequent to the second measurement of the tumor, treatment with vehicle or EB1089 was begun. After 2 weeks of treatment, a third MR image was generated and changes in tumor volume were calculated. The tumor growth rate actually increased in all but one of the vehicle treated animals (Fig. 3A). On the other hand, half of the mice that were treated with EB1089 displayed a decreased rate of tumor growth. Although variability in tumor response precluded statistical significance, it is remarkable that EB1089 actually caused regression of two tumors to less than half of their pre-treatment volume. Representative MR images of a mammary tumor that regressed in response to EB1089 are shown in Fig. 3B (pre-treatment) and C (post-treatment).
Fig. 3.
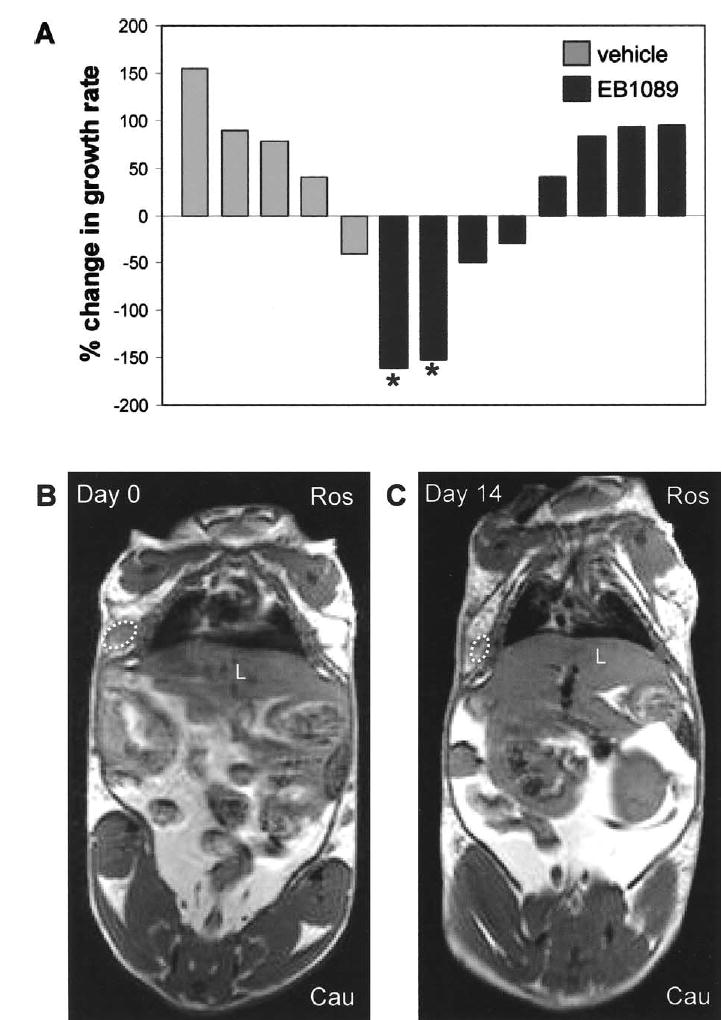
EB1089 displays anti-tumorigenic activity in a subset of hormone-induced mammary tumors. Tumor bearing animals were treated with vehicle or EB1089 and tumor mass was evaluated using magnetic resonance imaging (MRI). (A) Percent change in growth rate of tumors upon treatment with vehicle or EB1089 (see Section 2). Treatment with EB1089 for 14 days resulted in decreased rate of tumor growth in half of the mice; two of these tumors regressed more than 50% (indicated by asterisks). MR images of a tumor-bearing mouse on Day 0 (B) and Day 14 (C) of EB1089 treatment (tumor is outlined by a dotted line; Ros (rostral) and Cau (caudal) indicate the orientation of the mouse, L=liver).
4. Discussion
Extensive epidemiological and in vitro evidence supports a role for vitamin D signaling in limiting growth of mammary epithelial cells. Although estrogen receptor is not required for VDR-mediated growth inhibition of breast cancer cell lines [12,19, 20], mammary glands from mice deficient in VDR demonstrate an enhanced proliferative response to estrogen combined with other mammogenic hormones [9]. These data suggest that at least one facet of vitamin D anti-proliferative action involves opposition to proliferative hormonal stimuli. With this in mind, we attempted to harness the growth inhibitory qualities of VDR activation in order to hinder hormone-induced mammary gland pathology within a transgenic mouse model.
Importantly, vitamin D receptor is strongly expressed in the mammary glands and tumors of LH-overexpressing mice. Although expression of this protein is well documented in human breast cancers [35,36,7], to date, interrogation of mouse models of this disease has been limited to the MMTV-neu mouse [11]. The LH-overexpressing mouse represents a unique model of mammary tumorigenesis with a molecular profile and morphology distinct from that of MMTV-neu (data not shown), hence providing a valuable outlet for probing the potential of VDR activation in tumors not associated with amplification of HER2/neu.
The VDR promoter is activated in response to estradiol [37] and VDR mRNA levels increase in human breast cancer cell lines that have been exposed to progestins [38], suggesting that the altered hormonal milieu of the LH-overexpressing mice may contribute to the observed changes in vitamin D receptor expression. However, while pre-neoplastic mammary epithelial cells in these mice express steroid hormone receptors and exhibit ovarian dependency, tumors largely lack expression of both progesterone and estrogen receptors ([28] and Milliken and Keri, unpublished observations), revealing that VDR overexpression is not dependent on ER or PR. The p38 and JNK MAPK pathways have also been shown to activate VDR [39], indicating that a broad array of signaling pathways may impinge on the regulation of this protein.
The study reported herein shows, for the first time, that treatment with a VDR agonist can have a significant impact on the proliferative index of the intact mammary gland in the presence of elevated mammogenic hormones. The anti-proliferative effect of vitamin D and several related analogs has been extensively observed in the controlled environment of cultured human breast cancer cells [12–15] and has also been documented in xenograft models [25]; however, verification of in vivo efficacy in targeting an endogenous tissue should stimulate enthusiasm for the potential of VDR agonists to act as chemopreventive agents. The value of chemoprevention is illustrated by the Breast Cancer Prevention Trial, in which tamoxifen significantly decreased the incidence of both invasive and non-invasive breast cancers in high risk patients [40]. While promising, it is important to note that tamoxifen did not alter the incidence of estrogen receptor negative breast cancers. The identification of chemopreventive agents that impact a broad spectrum of cancer phenotypes is, as yet, an unresolved challenge. In this regard, the vitamin D receptor provides a promising target and 1,25-(OH)2D3 analogs such as EB1089 may facilitate this effort.
Data regarding the chemotherapeutic potential of 1,25-(OH)2D3 analogs in breast cancer is limited. A Phase I clinical trial reports low toxicity, but no tumor response to EB1089 in patients with advanced disease [41]; however, studies done with earlier stage patients have not yet been published. In animal models, a report that EB1089 can reduce the volume of carcinogen-induced mammary tumors in rats is significant and intriguing [26], but it is important to determine the effectiveness of such compounds in a diverse assemblage of models, including those that approximate a more natural disease progression. The LH-overexpressing model of hormone-induced mammary cancer reflects the important role that hormones play in breast cancer, thus providing an informative outlet for discovery of chemopreventive and chemotherapeutic agents. In the current study, half of tumors exposed to EB1089 decreased their growth rate, suggesting that activation of VDR signaling can counteract the intrinsic proliferative drive of at least a subset of tumors. The reason for variability in tumor response is unknown, although it is not a function of tumor size or basal growth rate (data not shown). Furthermore, VDR expression is not a distinguishing factor, as the protein was detected by immunohistochemistry in both responding and non-responding tumors (data not shown). Morphological and molecular variability among mammary tumors of the LH-overexpressing mouse has been observed and may be partially due to the presence of genomic instability early in tumorigenic progression (Milliken et al., in preparation). The occurrence of both EB1089 responsive and non-responsive tumors in LH-overexpressing mice may make it a useful model for identification of indicators that predict the outcome of treatment with VDR agonists.
One possibility is that these mammary tumors possess differing capacities for transcriptional modulation of vitamin D receptor target genes in response to agonist. Hampered response may be due to limiting levels of cooperative signaling components or the presence of an inhibitory factor. In either case, basal expression of VDR target genes may gauge pathway functionality. In this regard, variability in mRNA levels of the VDR target genes osteopontin and 25-hydroxyvitamin D-24 hydroxylase has been observed in mammary tumors of LH-overexpressing mice despite equivalent levels of VDR (data not shown). To determine whether relatively low levels of these genes predict refractoriness to EB1089, correlation of pre-treatment mRNA expression, obtained via biopsy, with treatment response should be performed in future trials.
The efficacy of EB1089 in this study, in combination with previously published evidence, suggests that vitamin D receptor agonists warrant attention as chemopreventive and chemotherapeutic agents. While VDR activation has demonstrated a beneficial effect on its own, it is likely that the observed anti-tumorigenic consequences would be magnified if it were coupled with additional approaches. This scheme has been validated in mice harboring MCF-7 xenografts; EB1089 treatment in combination with tamoxifen [42], paclitaxel [43], or ionizing radiation [44] was more effective than the individual components at inhibiting growth or inducing tumor regression. It should be of value to extend this combinatorial paradigm to transgenic mouse models as well as other in vivo models of cancer to assist determination of optimal treatment paradigms utilizing vitamin D3 analogs prior to introduction into clinical therapeutic programs.
Acknowledgments
The Gene Expression Array Core Facility of the Comprehensive Cancer Center at Case Western Reserve University/University Hospitals provided valuable services for the generation of the microarray data. Lise Binderup at Leo Pharmaceutical Products provided EB1089 and helpful advice. We also thank Kristen Lozada and Amelia Sutton for assistance with animal husbandry. This work was funded by DOD grant DAMD 17-01-1-0195 (RAK), NIH RO1 CA-90398 APRC supplement (RAK, JLD), NIH RO1 PK53980 (PNM) and NIH training grants CA59366 and GM08056 (ELM).
References
- 1.Ries LAG, Eisner MP, Kosary CL, et al., editors. National Cancer Institute; Bethesda, MD: 2004. SEER Cancer Statistics Review, 1975–2001. http://seer.cancer.gov/csr/1975-2001. [Google Scholar]
- 2.Sutton AL, MacDonald PN. Vitamin D: more than a ‘bone-a-fide’ hormone. Mol Endocrinol. 2003;17:777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 3.Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614–622. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 4.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon-and prostate cancer (Norway) Cancer Causes Control. 2004;15:149–158. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 5.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971–1975 to 1992, National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- 6.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich M, Axt-Fliedner R, Villena-Heinsen C, Tilgen W, Schmidt W, Reichrath J. Analysis of vitamin D-receptor (VDR) and retinoid X-receptor alpha in breast cancer. Histochem J. 2002;34:35–40. doi: 10.1023/a:1021343825552. [DOI] [PubMed] [Google Scholar]
- 8.Berger U, Wilson P, McClelland RA, Colston K, Haussler MR, Pike JW, Coombes RC. Immunocytochemical detection of 1,25-dihydroxyvitamin D3 receptor in breast cancer. Cancer Res. 1987;47:6793–6799. [PubMed] [Google Scholar]
- 9.Zinser G, Packman K, Welsh J. Vitamin D(3) receptor ablation alters mammary gland morphogenesis. Development. 2002;129:3067–3076. doi: 10.1242/dev.129.13.3067. [DOI] [PubMed] [Google Scholar]
- 10.Hennighausen L, Robinson GW. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12:449–455. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- 11.Zinser GM, Welsh J. Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis. 2004 doi: 10.1093/carcin/bgh271. [DOI] [PubMed] [Google Scholar]
- 12.Chouvet C, Vicard E, Devonec M, Saez S. 1,25-Dihydroxyvitamin D3 inhibitory effect on the growth of two human breast cancer cell lines (MCF-7, BT-20) J Steroid Biochem. 1986;24:373–376. doi: 10.1016/0022-4731(86)90085-3. [DOI] [PubMed] [Google Scholar]
- 13.Eisman JA, Sutherland RL, McMenemy ML, Fragonas JC, Musgrove EA, Pang GY. Effects of 1,25-dihydroxyvitamin D3 on cell-cycle kinetics of T 47D human breast cancer cells. J Cell Physiol. 1989;138:611–616. doi: 10.1002/jcp.1041380323. [DOI] [PubMed] [Google Scholar]
- 14.Eisman JA, Koga M, Sutherland RL, Barkla DH, Tutton PJ. 1,25-Dihydroxyvitamin D3 and the regulation of human cancer cell replication. Proc Soc Exp Biol Med. 1989;191:221–226. doi: 10.3181/00379727-191-42912. [DOI] [PubMed] [Google Scholar]
- 15.Verlinden L, Verstuyf A, Convents R, Marcelis S, Van Camp M, Bouillon R. Action of 1,25(OH)2D3 on the cell cycle genes, cyclin D1, p21 and p27 in MCF-7 cells. Mol Cell Endocrinol. 1998;142:57–65. doi: 10.1016/s0303-7207(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 16.Wu G, Fan RS, Li W, Ko TC, Brattain MG. Modulation of cell cycle control by vitamin D3 and its analogue, EB1089, in human breast cancer cells. Oncogene. 1997;15:1555–1563. doi: 10.1038/sj.onc.1201329. [DOI] [PubMed] [Google Scholar]
- 17.Swami S, Krishnan AV, Feldman D. 1alpha,25-Dihydrox-yvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000;6:3371–3379. [PubMed] [Google Scholar]
- 18.Stoica A, Saceda M, Fakhro A, Solomon HB, Fenster BD, Martin MB. Regulation of estrogen receptor-alpha gene expression by 1, 25-dihydroxyvitamin D in MCF-7 cells. J Cell Biochem. 1999;75:640–651. [PubMed] [Google Scholar]
- 19.Flanagan L, Packman K, Juba B, O’Neill S, Tenniswood M, Welsh J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J Steroid Biochem Mol Biol. 2003;84:181–192. doi: 10.1016/s0960-0760(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 20.Love-Schimenti CD, Gibson DF, Ratnam AV, Bikle DD. Antiestrogen potentiation of antiproliferative effects of vitamin D3 analogues in breast cancer cells. Cancer Res. 1996;56:2789–2794. [PubMed] [Google Scholar]
- 21.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367–376. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 22.Maenpaa PH, Vaisanen S, Jaaskelainen T, Ryhanen S, Rouvinen J, Duchier C, Mahonen A. Vitamin D(3) analogs (MC 1288, KH 1060, EB 1089, GS 1558, and CB 1093): studies on their mechanism of action. Steroids. 2001;66:223–225. doi: 10.1016/s0039-128x(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 23.Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 24.Colston KW, Mackay AG, James SY, Binderup L, Chander S, Coombes RC. EB1089: a new vitamin D analogue that inhibits the growth of breast cancer cells in vivo and in vitro. Biochem Pharmacol. 1992;44:2273–2280. doi: 10.1016/0006-2952(92)90669-a. [DOI] [PubMed] [Google Scholar]
- 25.VanWeelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology. 1998;139:2102–2110. doi: 10.1210/endo.139.4.5892. [DOI] [PubMed] [Google Scholar]
- 26.Colston KW, Pirianov G, Bramm E, Hamberg KJ, Binderup L. Effects of Seocalcitol (EB1089) on nitrosomethyl urea-induced rat mammary tumors. Breast Cancer Res Treat. 2003;80:303–311. doi: 10.1023/A:1024962316691. [DOI] [PubMed] [Google Scholar]
- 27.Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci USA. 1995;92:1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milliken EL, Ameduri RK, Landis MD, Behrooz A, Abdul-Karim FW, Keri RA. Ovarian hyperstimulation by LH leads to mammary gland hyperplasia and cancer predisposition in transgenic mice. Endocrinology. 2002;143:3671–3680. doi: 10.1210/en.2002-220228. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh CC, Trichopoulos D, Katsouyanni K, Yuasa S. Age at menarche, age at menopause, height and obesity as risk factors for breast cancer: associations and interactions in an international case-control study. Int J Cancer. 1990;46:796–800. doi: 10.1002/ijc.2910460508. [DOI] [PubMed] [Google Scholar]
- 30.Irwin KL, Lee NC, Peterson HB, Rubin GL, Wingo PA, Mandel MG. Hysterectomy, tubal sterilization, and the risk of breast cancer. Am J Epidemiol. 1988;127:1192–1201. doi: 10.1093/oxfordjournals.aje.a114912. [DOI] [PubMed] [Google Scholar]
- 31.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331:5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 32.MacMahon B, Purde M, Cramer D, Hint E. Association of breast cancer risk with age at first and subsequent births: a study in the population of the Estonian Republic. J Natl Cancer Inst. 1982;69:1035–1038. [PubMed] [Google Scholar]
- 33.Rao DN, Ganesh B, Desai PB. Role of reproductive factors in breast cancer in a low-risk area: a case-control study. Br J Cancer. 1994;70:129–132. doi: 10.1038/bjc.1994.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 35.Friedrich M, Rafi L, Tilgen W, Schmidt W, Reichrath J. Expression of 1,25-dihydroxy vitamin D3 receptor in breast carcinoma. J Histochem Cytochem. 1998;46:1335–1337. doi: 10.1177/002215549804601114. [DOI] [PubMed] [Google Scholar]
- 36.Friedrich M, Villena-Heinsen C, Tilgen W, Schmidt W, Reichrat J, Axt-Fliedner R. Vitamin D receptor (VDR) expression is not a prognostic factor in breast cancer. Anticancer Res. 2002;22:1919–1924. [PubMed] [Google Scholar]
- 37.Byrne IM, Flanagan L, Tenniswood MP, Welsh J. Identification of a hormone-responsive promoter immediately upstream of exon 1c in the human vitamin D receptor gene. Endocrinology. 2000;141:2829–2836. doi: 10.1210/endo.141.8.7618. [DOI] [PubMed] [Google Scholar]
- 38.Vienonen A, Miettinen S, Manninen T, Altucci L, Wilhelm E, Ylikomi T. Regulation of nuclear receptor and cofactor expression in breast cancer cell lines. Eur J Endocrinol. 2003;148:469–479. doi: 10.1530/eje.0.1480469. [DOI] [PubMed] [Google Scholar]
- 39.Qi X, Pramanik R, Wang J, Schultz RM, Maitra RK, Han J, et al. The p38 and JNK pathways cooperate to trans-activate vitamin D receptor via c-Jun/AP-1 and sensitize human breast cancer cells to vitamin D(3)-induced growth inhibition. J Biol Chem. 2002;277:25884–25892. doi: 10.1074/jbc.M203039200. [DOI] [PubMed] [Google Scholar]
- 40.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 41.Gulliford T, English J, Colston KW, Menday P, Moller S, Coombes RC. A phase I study of the vitamin D analogue EB 1089 in patients with advanced breast and colorectal cancer. Br J Cancer. 1998;78:6–13. doi: 10.1038/bjc.1998.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vink-van Wijngaarden T, Pols HA, Buurman CJ, van den Bemd GJ, Dorssers LC, Birkenhager JC, et al. Inhibition of breast cancer cell growth by combined treatment with vitamin D3 analogues and tamoxifen. Cancer Res. 1994;54:5711–5717. [PubMed] [Google Scholar]
- 43.Koshizuka K, Koike M, Asou H, Cho SK, Stephen T, Rude RK, et al. Combined effect of vitamin D3 analogs and paclitaxel on the growth of MCF-7 breast cancer cells in vivo. Breast Cancer Res Treat. 1999;53:113–120. doi: 10.1023/a:1006123819675. [DOI] [PubMed] [Google Scholar]
- 44.Sundaram S, Sea A, Feldman S, Strawbridge R, Hoopes PJ, Demidenko E, et al. The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin, Cancer Res. 2003;9:2350–2356. [PubMed] [Google Scholar]


