Abstract
Among the many theories that have been advanced to explain the mechanism by which auditory verbal hallucinations (AVH) arise, 2 that have received a degree of empirical support are: the hypothesis that AVHs arise from misinterpreted inner speech and the proposal that they arise from aberrant activation of the primary auditory cortex. To test these hypotheses, we were fortunate to be able to study the interesting and rare case of a woman with schizophrenia who experienced continuous AVH which disappeared when she listened to loud external speech. Functional magnetic resonance imaging (fMRI) was used to measure the patient's brain activity in the temporal and inferior frontal regions during the AVHs and while the she was listening to external speech. The brain activity of a matched control subject was also recorded under the same experimental conditions. AVHs were associated with increased metabolic activity in the left primary auditory cortex and the right middle temporal gyrus. Our results suggest a possible interaction between these areas during AVHs and also that the hypotheses of defective internal monitoring and aberrant activation are not mutually exclusive. Potential limitations to the generalization of our results are discussed.
Medical subject headings: auditory cortex, brain mapping, hallucinations, magnetic resonance imaging, schizophrenia, speech perception
Abstract
Parmi les nombreuses théories qu'on a avancées pour expliquer le mécanisme déclencheur des hallucinations auditivo-verbales (HAV), voici les deux propositions qui ont reçu une certaine confirmation empirique : l'hypothèse selon laquelle les HAV émanent d'une mauvaise interprétation de la parole intérieure, et l'hypothèse selon laquelle elles émanent d'une anomalie de l'activation du cortex auditif primaire. Nous avons la chance d'avoir pu étudier le cas intéressant et rare d'une schizophrène qui entendait continuellement des HAV, sauf lorsqu'elle écoutait des paroles extérieures fortes, afin de vérifier ces hypothèses. L'imagerie par résonance magnétique fonctionnelle (IRMf) a servi à mesurer l'activité cérébrale de la patiente dans les régions temporale et frontale inférieure au cours des HAV et tandis qu'elle écoutait des paroles extérieures. L'activité du cerveau d'un sujet témoin jumelé a également été enregistrée dans ces conditions expérimentales. Les HAV étaient associées à une activité métabolique accrue du cortex auditif primaire gauche et de la circonvolution temporale moyenne droite. Nos résultats indiquent qu'au cours des HAV, il pourrait y avoir une interaction entre ces régions, et que les hypothèses traitant respectivement de l'anomalie du contrôle interne et de l'anomalie de l'activation ne sont pas inconciliables. Les limites possibles de la généralisation de nos résultats sont présentées.
Introduction
Auditory verbal hallucinations (AVHs) are one of the most characteristic symptoms of schizophrenia, but the mechanism by which they arise remains uncertain. Among the many theories that have been advanced,1 2 that have received a degree of empirical support are the hypothesis that AVHs arise from misinterpreted inner speech2,3 and the proposal that they arise from aberrant activation of the auditory cortex.4
The hypothesis that auditory hallucinations arise from misinterpreted inner speech is supported by the observation that AVHs are sometimes associated with perioral movements.5,6 In a single-case study, Dooley and George7 found that perioral activity seen during AVHs was related to inner speech. Inner speech generation activates Broca's area,8,9 a brain region known to be crucially involved in language production, and several neuroimaging studies have reported activation of Broca's area during auditory hallucinations.10,11 Furthermore, a corpus of data reported by McGuire et al12,13,14 regarding self-monitoring and its relation to AVHs also supports the “misrecognition of inner speech” hypothesis and suggests the left middle temporal gyrus (MTG) is involved. However, several neuroimaging studies have failed to observe activation of Broca's area during AVHs,3,15,16 although not all of these studies examined this region specifically. Although this might reflect a type II statistical error, it does cast some doubt on the inner speech hypothesis. In the same vein, results from a recent study conducted by Evans et al17 suggest inner speech and AVHs are not linked in a simple or direct way.
With regard to the hypothesis of aberrant activity in the auditory cortex, Dierks et al4 reported an activation of the primary auditory cortex (PAC) while patients were experiencing AVHs. They then posited that it might be this abnormal activity in the PAC that lends the quality of real sound to the experience and leads to the misinterpretation of its source as external.
To test these hypotheses, we used functional magnetic resonance imaging (fMRI) in a female patient with schizophrenia who experiences continuous AVHs. We hypothesized that inner speech might be misinterpreted as “alien” because it abnormally reaches the PAC during AVHs, and this access to the PAC may result from an abnormal functioning of the left MTG. In accordance with this hypothesis, we predicted a priori that AVHs would be associated with an increased metabolic activity in the left PAC and in Broca's area, but not in the left MTG.
Methods
Two right-handed female subjects, subject DT and a normal control subject, participated in this study. These women were matched for age (36 years of age) and level of education (high school).
Subject DT had been suffering from paranoid schizophrenia, according to the criteria of the DSM-IV,18 for 10 years and was receiving an antipsychotic treatment (intramuscular depot clopenthixol, 200 mg every 15 days) at the time of the study. When at home, she usually abolished her AVHs by turning up the volume of her radio or TV set. Taking advantage of this fact, we made sure, 3 weeks before the beginning of this study, that listening to prerecorded loud speech completely inhibited her AVHs. This being confirmed, subject DT was then trained to recognize and to report the onset and the end of her AVH. The content of the AVH mostly consisted of a single voice commenting on her behaviour and sometimes ordering her to accomplish some acts. At times, however, subject DT heard the voices of several people talking to each other.
Experimental design
A single scanning run was conducted. This run consisted of 5 epochs of 60 s (2 blocks of 30 s) each. In 1 of the 30-s blocks, the subjects listened to the noise generated by the fMRI scanner (experimental condition). Before scanning, we verified that this noise did not prevent subject DT from experiencing AVHs. In the other 30-s block, the subjects listened, binaurally via headphones, to recorded speech (control condition). To make sure that subject DT was actually hallucinating during each of the experimental blocks, she was asked to press a button once to confirm the onset of an episode of AVHs and twice to signal its end.
Image acquisition and scanning
Scanning was performed on a 1.5-T system (Magneton Vision, Siemens Electric, Erlangen, Germany). Twenty-six slices (5-mm thick) were acquired every 6 s in an inclined axial plane, aligned with the AC-PC (anterior commissure – posterior commissure) axis, and covering all of the brain. The T2*-weighted functional images were acquired using an echo-planar imaging (EPI) pulse sequence (repetition time [TR] = 6 s, echo time [TE] = 54 ms, flip angle = 90°, field of view [FOV] = 215 mm, matrix = 128 х 128). After functional scanning, structural data were obtained via T1-weighted 3-dimensional volume acquisition using a gradient echo-pulse sequence (TR = 9.7 ms, TE = 4 ms, flip angle = 12°, FOV = 250 mm, matrix = 256 х 256).
Image analysis
Data were analyzed with the Statistical Parametric Mapping software (SPM 99, Wellcome Department of Cognitive Neurology, London, UK). Images were realigned to correct for artifacts due to small head movements and normalized into an MRI stereotaxic (i.e., Talairach coordinates) space. They were then convolved in space with a 3-dimensional isotropic Gaussian kernel (8 mm FWHM [full-width half-maximum]) to improve the signal-to-noise ratio. For statistical analysis, the time series of the images were correlated with the delayed box-car function that approximates the activation patterns, and a linear model for autocorrelated observations was applied voxelwise. Regional specific effects were assessed with t-tests. For patient DT, because we had predicted activation in the auditory cortical areas of the temporal lobes and in Broca's area, a directed regional search was carried out using the Worsley's small-volume correction within 3 boxes (with each side at 60 mm, located at 58, –16, 8; –58, –16, 8 and 44, 12, 13). The threshold for significance within these areas was set at p < 0.05, corrected for multiple comparisons.
Results
In an interview immediately after the scanning session, subject DT confirmed that she experienced AVHs during all of the experimental condition blocks and that the AVHs ended when she listened to external speech (control condition).
fMRI results
In subject DT, when the brain activity measured during the control condition (i.e., listening to an external speech) was subtracted from that measured during the experimental condition (i.e., AVHs), significant blood oxygenation level dependent (BOLD) signal increases were detected in the left superior temporal gyrus (Brodmann's area [BA] 41; coordinates 52, –21, 6) (z = 4.96; p ≤ 0.042) (Fig. 1C) and in the right middle temporal gyrus (BA 21, coordinates –55, –25, –10) (z = 4.96; p ≤ 0.027) (Fig. 1E). No significant activation was seen in Broca's area.
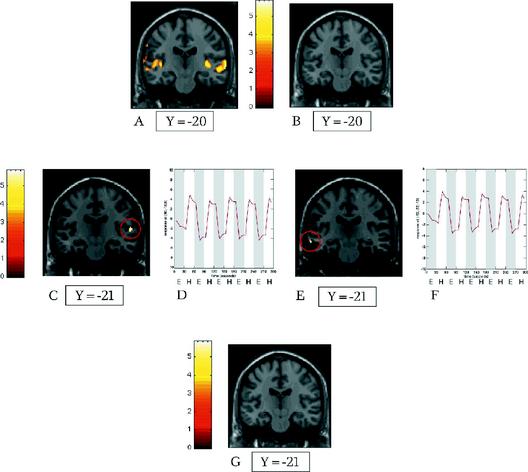
Fig 1A: In the normal control subject, coronal statistical map showing bilateral blood oxygenation level dependent (BOLD) signal increases in auditory cortex while subject was listening to external speech (subtraction of brain activity associated with listening to the noise produced by the functional magnetic resonance imaging [fMRI] machine from that associated with listening to an external speech and the noise of the fMRI machine).
B: Absence of activation in the auditory cortex and in the inferior frontal lobe when the brain activity associated with listening to external speech and the noise produced by fMRI machine was subtracted from that associated with listening to the fMRI machine alone.
C–F: In patient DT, the subtraction of brain activity associated with listening to external speech and fMRI machine noise from that associated with experiencing auditory verbal hallucinations (AVHs) and fMRI machine noise revealed a significant activation of the left superior temporal gyrus (primary auditory cortex, Brodmann's area 41) (C) and the right middle temporal gyrus (auditory association cortex, Brodmann's area 21) (E). The time courses of the BOLD signal increases for coronal statistical maps depicted in C and E are presented in D and F, respectively.
G: When the brain activity associated with experiencing an AVH and listening to the noise of the fMRI machine was subtracted from that associated with listening to external speech and the fMRI machine, there were BOLD signal increases seen in the areas of interest.
Interestingly, the time course analysis of these 2 clusters of voxels showed a striking correlation between the increase of the BOLD signal at the beginning of the experimental blocks and the return of the BOLD signal to baseline levels at the end of these blocks (Fig. 1D, Fig. 1F).
When the brain activity measured during the experimental condition was subtracted from that measured during the control condition, no BOLD signal increases were seen in the areas of interest (Fig. 1G).
In the control subject, when the brain activity measured during the experimental condition was subtracted from that measured during the control condition, significant activation was observed (Fig. 1A) in the right middle temporal gyrus (BA 21, coordinates –57, –34, –1) (z = 7.06); the right superior temporal gyrus (BA 22, coordinates –48, 6, –9) (z = 5.46); the left middle temporal gyrus (BA 21, coordinates 60, –30, –12) (z = 7.36); the left superior temporal gyrus (BA 38, coordinates 46, 14, –11) (z = 4.83); and the left inferior temporal gyrus (BA 20, coordinates 46, –14, –20) (z = 4.46) (all peaks at p ≤ 0.001).
In addition, when the brain activity measured during the control condition was subtracted from that measured during the experimental condition, no significant activation was seen either in the temporal lobes or in the left inferior frontal gyrus (Fig. 1B).
Discussion
The main findings of this study were that in the “absence of external speech,” the hallucinating subject showed elevated activation of the left STG and right MTG compared with the matched control subject and that “listening to external speech” was associated with bilateral activation of a range of temporal cortical areas in the control but not the hallucinating subject. There was no significant activation seen in the temporal cortical areas in patient DT while listening to external speech, however. This indicates that the areas activated by normal speech in healthy individuals are also activated by the experience of AVHs and suggests, as others have also reported,19,20 that experiencing AVHs and listening to external speech might be subserved by some common neurological substrates.
The absence of external speech was correlated with significant activity in the left PAC and the right MTG in the hallucinating patient, but with no activity of the temporal lobes in the control subject. Given that the patient reported hearing voices during the absence of external speech, this finding indicates even greater activity in the left PAC and the right MTG during hallucinations than during external speech. This activity of the left PAC might be due to greater attention being paid to the strange content of AVHs than to external speech. However, it does not explain the asymmetry of the 2 hemispheres. The increased activity in the right MTG is consistent with the report of McGuire et al12 who found greater involvement of the right temporal cortex during self-monitoring. The right MTG is probably less dysfunctional than the left one in our patient, leading to a better monitoring of the source of the stimuli. In concordance with the finding of Dierks et al4 regarding the PAC and that of McGuire et al,12 our finding that the MTG and the PAC are not activated in the same hemisphere supports our hypothesized interaction between the MTG and the PAC. Indeed, we postulated that a defective MTG (in patients who are hallucinating) might permit access of internal speech to the PAC. This, in turn, might lend the quality of a real voice to the inner speech. However, the lack of activation of Broca's area in the patient during the absence of external speech is not consistent with our hypothesis that AVHs arise through misidentified internal speech. Although we can't reject the possibility of a type II statistical error, our failure to find activation of Broca's area is consistent with the observations of Silbersweig et al15 and Woodruff et al.16
Potential limitations to the generalization of our results must be considered. First, our study is a single case study. Given the rarity of this particular problem and the difficulty finding patients who will agree to participate in research, it is reasonable to adopt this approach. Indeed, patient DT was fascinating for a number of reasons: she was continuously hallucinating, her hallucinations were inhibited by loud external speech and she was able to recognize the onset and the end of her AVH. As emphasized by Ramachadran,21 valuable lessons about the dysfunctional brain can be learned, not only “from statistical analysis involving large numbers of patients,” but also from the study of “those who believe that doing the right kind of experiments on the right patients, even a single case patient, can yield much more information.”
In the study of the relation between hallucinations and cerebral activity, 3 main research designs have been employed: (i) assessing correlations between symptoms and regional cerebral blood flow (rCBF) across patients; (ii) comparing rCBF in the presence and absence of symptoms within patients; (iii) determining correlations between symptoms and rCBF within patients.22 Further investigation of the relation between activity in the MTG and the PAC during auditory hallucinations using 1 of these designs is warranted.
Our findings are the product of a series of contrasts after subtracting a control condition (scanner noise and external speech) from an experimental condition (scanner noise) and vice versa. In our analysis, we assumed that any cortical activity due to the scanner noise, external speech and AVHs could be independently partitioned off in both subjects. Interpretation of our results is therefore dependent on the following assumptions:
· that the cortical activity associated with AVHs is present during the experimental condition and “inhibited” during the control condition and
· that the presence of AVHs or external speech (or both), in addition to scanner noise, does not result in an interaction that is not accounted for. There is compelling evidence that the introduction of some form of auditory stimuli to background MR scanner noise results in a complex interaction in the temporal cortex.23,24,25 However, there is also evidence that the distribution of activation within individual subjects is relatively constant across several iterations of the scanner noise stimulus.25
Although violation of these assumptions does not invalidate our results, our interpretation of the data is dependent on them. For example, we cannot be 100% sure that the cortical activity related to AVHs simply dissipates when external speech is played. In addition, as with all fMRI studies of auditory processing, there is the potential problem of reduced signal in the auditory cortex due to the background scanner noise.26 There is also the possibility that we may have failed to find more extensive activation of the left temporal cortex, as noted by Shergill et al,27 because of the background scanner noise.
To date, our results suggest that the hypotheses of defective internal monitoring and of aberrant activation of PAC are not mutually exclusive. Rather, they suggest that defective internal monitoring in the left hemisphere is associated with aberrant activation of PAC. To the best of our knowledge, this is the first time that neural correlates of AVHs have been investigated in this manner. This is due to the unusual clinical characteristics of DT, who kindly agreed to participate in this study.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Emmanuel Stip, Fernand-Seguin Research Centre, 7331, rue Hochelaga, Montréal QC H1N 3V2; fax 514 251-2617; emmanuel.stip@umontreal.ca
Submitted Nov. 12, 2001 Accepted Jan. 18, 2002
References
- 1.Ait Bentaleb L, Stip E, Beauregard M. Psychopathologie et bases neurobiologiques des hallucinations auditives dans la schizophrénie. Sante Ment Que 2000;25:241-57. [PubMed]
- 2.Frith CD, Done DJ. Towards a neuropsychology of schizophrenia. Brit J Psychiat 1988;153:437-43. [DOI] [PubMed]
- 3.David AS. The neuropsychology of auditory-verbal hallucinations. In: David A, Cutting J, editors. The neuropsychology of schizophrenia. New York: Psychology Press; 1994. p. 269-312.
- 4.Dierks T, Linden DEJ, Jandl M, Formisano E, Goebel R, Lanfermann H, et al. Activation of Heschl's gyrus during auditory hallucinations. Neuron 1999;22:615-21. [DOI] [PubMed]
- 5.Gould LN. Auditory hallucinations and subvocal speech. J Nerv Ment Dis 1949;109:418-27. [DOI] [PubMed]
- 6.Green P, Preston M. Reinforcement of vocal correlates of auditory hallucinations by auditory feedback: A case study. Br J Psychiatry 1981;139:204-8. [DOI] [PubMed]
- 7.Dooley C, George RE. A single case study illustrating the reduction in subvocalisation and electromyography. Behav Psychother 1988;16:231-40.
- 8.Hinke RM, Hu X, Stillman AE, Kim SG, Merkle H, Salmi R, et al. Functional magnetic resonance imaging of Broca's area during internal speech. Neuroreport 1993;4:675-8. [DOI] [PubMed]
- 9.Shergill SS, Bullmore E, Simmons A, Murray R, McGuire P. Functional anatomy of auditory verbal imagery in schizophrenic patients with auditory hallucinations. Am J Psychiatry 2000;157:1691-3. [DOI] [PubMed]
- 10.Cleghorn JM, Garnett ES, Nahmias C, Brown GM, Kaplan RD, Szechtman H, et al. Regional brain metabolism during auditory hallucinations in chronic schizophrenia. Br J Psychiatry 1990;157:562-70. [DOI] [PubMed]
- 11.McGuire PK, Shah GMS, Murray RM. Increased blood flow in Broca's area during auditory hallucinations in schizophrenia. Lancet 1993;342:703-6. [DOI] [PubMed]
- 12.McGuire PK, Silbersweig DA, Frith CD. Functional neuroanatomy of self monitoring. Brain 1996;119:907-17. [DOI] [PubMed]
- 13.David AS. Auditory hallucinations: phenomenology, neuropsychology and neuroimaging update. Acta Psychiatr Scand Suppl 1999;395;95-104. [DOI] [PubMed]
- 14.McGuire PK, Silbersweig DA, Wright I, Murray RM, David AS, Frackowiak RS, et al. Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. Lancet 1995;346(8975):596-600. [DOI] [PubMed]
- 15.Silbersweig DA, Stern E, Frith CD, Cahill C, Holmes A, Grootoonk S, et al. A functional neuroanatomy of auditory hallucinations in schizophrenia. Nature 1995;378:176-9. [DOI] [PubMed]
- 16.Woodruff PWR, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SC, et al. Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry 1997;154:1676-82. [DOI] [PubMed]
- 17.Evans CL, McGuire PK, David AS. Is auditory imagery defective in patients with auditory hallucinations? Psychol Med 2000;30:137-48. [DOI] [PubMed]
- 18.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington DC: American Psychiatric Association; 1994
- 19.Barta PE, Pearlson GD, Brill LB 2nd, Royall R, McGilchrist IK, Pulver AE, et al. Planum temporale asymmetry reversal in schizophrenia: replication and relationship to gray matter abnormalities. Am J Psychiatry 1997;154:661-7. [DOI] [PubMed]
- 20.Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990;117:1457-62. [DOI] [PubMed]
- 21.Ramachadran VS, Blakeslee S. Phantoms in the brain. New York: William Morrow & Co.; 1998.
- 22.Liddle P. Functional brain imaging of schizophrenia. In: Reveley MA, Deakin JFW, editors. The Psychopharmacology of schizophrenia. London: Arnold; 2000. p. 109-30.
- 23.Bandettini PA, Jesmanowicz A, Van Kylen J, Birn RM, Hyde JS. Functional MRI of brain activation induced by scanner acoustic noise. Magn Reson Med 1998;39:410-6. [DOI] [PubMed]
- 24.Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, et al. “Sparse” temporal sampling in auditory fMRI. Hum Brain Mapp 1999; 7:213-23. [DOI] [PMC free article] [PubMed]
- 25.Ulmer JL, Biswal BB, Yetkin FZ, Mark LP, Mathews VP, Prost RW, et al. Cortical activation response to acoustic echo planar scanner noise. J Comput Assist Tomogr 1998;22:111-9. [DOI] [PubMed]
- 26.Shah NJ, Jancke L, Grosse-Ruyken ML, Muller-Gartner HW. Influence of acoustic masking noise in fMRI of the auditory cortex during phonetic discrimination.J Magn Reson Imaging 1999;9:19-25. [DOI] [PubMed]
- 27.Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 2000;57:1033-8. [DOI] [PubMed]


