Abstract
Recent studies in human and animal models of narcolepsy have suggested that obesity in narcolepsy may be due to deficiency of hypocretin signaling, and is also under the influence of environmental factors and the genetic background. In the current study, using two hypocretin/orexin deficient narcoleptic mouse models (i.e. prepro-orexin knockout (KO) and orexin/ataxin-3 transgenic (TG) mice) with cross-sectional assessments, we have further analyzed factors affecting obesity. We found that both KO and TG narcoleptic mice with mixed genetic backgrounds (N4-5, 93.75-96.88 % genetic composition of C57BL/6) tended to be heavier than wild type (WT) mice of 100 to 200 days old. The body weight of heterozygous mice was intermediate between those of KO and WT mice. Obesity was more prominent in females in both KO and TG narcoleptic mice and was associated with higher serum leptin levels, suggesting a partial leptin resistance. Obesity is less prominent in the congenic TG narcoleptic mice, but is still evident in females. Our results confirmed that hypocretin/orexin ligand deficiency is one of the critical factors for the obese tendency in narcolepsy. However, multiple factors are also likely to affect this phenotype, and a gender specific alteration of leptin-hypocretin signaling may be involved.
Keywords: narcolepsy, obesity, hypocretin, orexin, leptin, gender
1. Introduction
Increasing evidence has suggested that narcolepsy is not a simple sleep disorder, but a condition with abnormalities in energy homeostasis, autonomic and neuroendocrine functions, and loss of hypocretin/orexin signaling underlies its pathophysiology [20, 21, 28]. Obesity has been reported in narcolepsy for several decades [4, 5, 13, 27], but this phenotype has not been given much attention. This is partially due to the facts that obesity in narcolepsy can be secondary to reduced daytime activities, and that obesity is only observed in a subset of patients, suggesting that this phenotype may not be the cardinal symptom of narcolepsy. Experiments using animal models led to the discovery of major pathophysiology of human narcolepsy, and narcolepsy has been identified to associate with deficiencies in hypocretin/orexin neurotransmission [2, 11, 17]. Hypocretins/orexins are neuropeptides involved in various hypothalamic functions, including appetite regulation, energy homeostasis, neuroendocrine function and sleep/wake control [1, 2, 6, 17, 24, 25]. Several experimental evidences have suggested that obesity in narcolepsy may also be due to deficiencies in hypocretin signaling. Theses findings include (1) hypocretin-deficient narcoleptic patients tend to be more obese compared to narcoleptic patients with normal CSF hypocretin levels [21] and non-hypocretin-deficient hypersomnias [23]; (2) hypocretin-deficient narcoleptic mice (i.e. orexin/ataxin-3 transgenic (TG) mice; hypocretin cell ablated mice) eat less, but are obese compared to their wild type (WT) littermates, suggesting a contribution of reduced metabolism to obesity in hypocretin-deficient narcoleptic mice [11, 12]. Detailed analysis in two mouse narcoleptic models (ligand knockout (KO) and cell ablation TG mice) suggested that obesity is under the influence of hypocretin deficiency as well as genetic background of the animals: (1) cell-ablated mice are more obese than ligand KO mice suggesting that the loss of substances colocalized in the hypocretin neurons, such as dynorphins, may also contribute to obesity; (2) Obesity of TG mice was found to be less prominent in pure C57BL/6 line than C57BL/6-DBA hybrid line. Other factors, such as gender, age, environment, and diet are also likely to significantly contribute to obesity in narcolepsy [11, 12]. Involvement of multiple factors may explain why only a subset of human narcoleptics are obese.
Hypocretin/orexin, when injected intracerebroventricularly, increases food intake, energy expenditure, and locomotor activity [1, 7, 10]. Thus, hypocretin deficiency decreases appetite and energy expenditure, but the latter may have larger impact, resulting in a positive weight gain. Hypocretin neuronal activity is under the influence of peripheral metabolic clues: leptin inhibits activities of hypocretin neurons, while low circulating glucose and ghrelin (an appetite-stimulatory hormone mostly produced by the stomach) stimulate hypocretin neurons [29]. Leptin is a peptide hormone produced by the ob gene in adipocytes in proportion to visceral fat mass; decreased leptin is a starvation signal for the hypothalamus [8, 9, 30]. Two independent human studies also demonstrated that peripheral leptin signaling is reduced in hypocretin-deficient narcolepsy [16, 26], and this may contribute to obesity in narcolepsy.
In order to further dissect factors affecting obesity in hypocretin-deficient narcolepsy, we carried out cross-sectional assessments of the body weight of two narcoleptic mouse models, namely preproorexin KO and orexin/ataxin-3 TG mice, and their respective WT mice housed in the same environment. Serum leptin levels were also assessed in a selected population of TG and WT mice.
2. Method
2.1 Animals and body weight measurement
The breeding pairs of N3 generations of preproorexin KO (N3: 87.5% C57BL/6 and 6.3% 129SvEv) and orexin/ataxin 3 TG (N3: 87.5 % C57BL/6 and 6.3% DBA1) mice arrived at Stanford University in April 2002 from University of Tsukuba, Ibaraki, Japan, and breeding was conducted at the Stanford Center for Narcolepsy. Since majority of our breeding crosses represented homozygous (H) with heterozygous (Hz), Hz with Hz, or Hz with WT for KO (90 %) and hemizygous (Hz) x WT for TG (100%) lines, the sample population in this study was mostly from littermate animals. All mice were provided with regular rodent oval pellets (Prolab RMH 3000, PMI Nutrition International) and water ad libitum, and maintained under a 12h:12h light-dark cycle. All animals were under controlled conditions of temperature and humidity. In August 2003, the weights of N4-5 mice in the colony were recorded (Numbers of animals: 207 H KO, 212 Hz KO, 193 WT for KO group; 335 TG, and 284 WT for TG group), and weight, age and genotype of the mice were analyzed.
In addition to these data collections, we further backcrossed the mice and obtained a congenic strain of TG mouse (N9: 99.8 % C57BL/6 and 0.2 % DBA1). Weight, age and genotype of the congenic TG mice (n = 290) and the respective WT mice (n = 402) were analyzed in Summer 2005.
2.2. Leptin measurement
In a selected population of TG (n=53, 162.8 ± 30 [SD] days of age) and respective WT (n=39, 159.6 ± 31.2 [SD] days of age) mice from N4-5 generation, we collected their blood to examine serum leptin levels. All blood samples were collected by cardiac puncture under isoflurane anesthesia (1-3 %) between 9 -11a.m. followed by euthanasia. The blood samples were kept in room temperature for 30 min, and then centrifuged (3000 rpm, 5 min) to obtain sera. The serum samples were stored at-80 °C until radioimmunoassay (RIA). Serum leptin levels were measured in duplicate using a 125I-RIA (Meddiagnost, Tubingen, Germany).
2.3. Statistical analysis
Data are shown as means ± SEM. Comparisons between groups were performed using the Mann-Whitney U test, Kruskal-Wallis analysis of variance (ANOVA), or Friedman’s test depending on data type and form. Correlations between serum leptin levels and body weight were analyzed by Pearson’s correlation. Comparison of the relationship of serum leptin levels and body weight between TG and WT was analyzed by one-way analysis of covariance (ANCOVA). Statistical significance was set at p < 0.05 (two-tailed).
All experiments were carried out in accordance with the guidelines described in The National Institutes of Health Guide for the Care and Use of Laboratory Animals.
3. Results
3.1. Obesity in N4, N5 narcoleptic mice
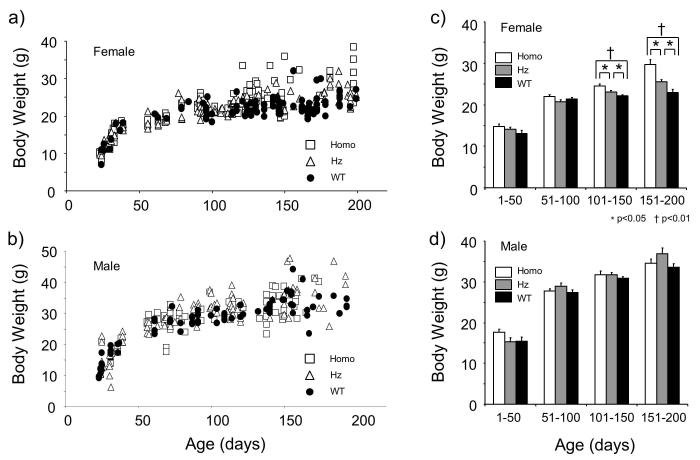
The body weight of KO mice became heavier than that of WT mice after 100 days of age (Fig. 1a, b). This tendency was noticeable in female mice, but less clear in the male mice. In order to examine the influence of age on body weight in detail and to apply statistical comparison, we analyzed the weight data of both male and female mice with 50-day age bins up to 200 days old (Fig. 1c, d). We found that female KO mice were significantly heavier than WT mice at 101 to 150 days and 151 to 200 days (p<0.01, Kruskal-Wallis test followed by Sheffe’s post hoc test). (Fig. 1c). Interestingly, Hz animals exhibited intermediate body weight in these age groups; the mean body weight of Hz mice were significantly heavier than that of WT mice, and significantly lighter than that of KO mice (p<0.05, Kruskal-Wallis test followed by Sheffe’s post hoc test) (Fig. 1c). In contrast, no statistically significant difference was observed in male mice of any age groups (p>0.05. Kruskal-Wallis test) (Fig. 1d).
Figure 1.
Body weight and age relationship of preprohypocretin/preproorexin knockout (KO) mice. Individual data for females (a) and males (b) are shown. After 100 to 200 days of age, a cluster of homozygotes (Homo) were heavier than other groups in females. This was not obviously observed in males. Body weight data were divided into 4 age groups (1-50, 51-100, 101-150, and 151-200 days of age) and shown as mean ± SEM (c, d). Homo mice were significantly heavier than wild-type mice (WT) in 101-150 and 151-200 age groups in females (c). Heterozygote (Hz) female mice were significantly heavier than WT and lighter than Homo in these age groups. This phenomenon was not observed in male mice (d).
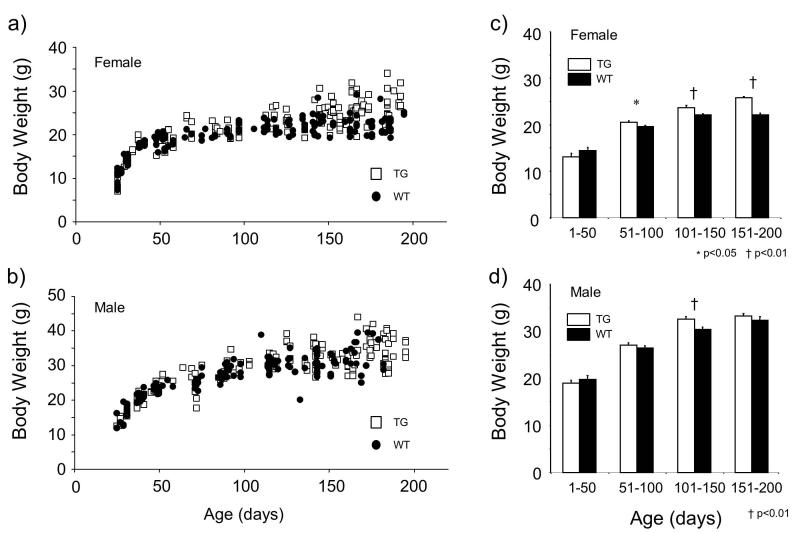
We found similar tendencies in body weight changes in TG mice. Female TG mice became heavier than female WT mice after 100 days of age, but this tendency was not clear in male mice (Fig. 2a, b). The mean body weight of female TG mice was significantly heavier than that of WT mice at 51 to 100 (P<0.05), 101 to 150 (P<0.01), and 151 to 200 (P<0.01) days of age (Mann-Whitney test) (Fig. 2c). The body weight of male TG mice was heavier than that of WT mice at 51 to 200 days old, but the difference reached significance level only at 101 to 150 days of age (Fig. 2d).
Fugure 2.
Body weight and age relationship of orexin/ataxin-3 transgenic (TG) mice. Individual data for females (a) and males (b) are shown. After 100 to 200 days of age, a cluster of TG mice were heavier than wild type in females. This was not obviously observed in males. Body weight data were divided into 4 age groups (1-50, 51-100, 101-150, and 151-200 days of age) and shown as mean ± SEM. TG were significantly heavier than wild-type mice (WT) in 51-100 (p < 0.05), 101-150 (p < 0.01) and 151-200 (p < 0.01) age groups in females (c). This phenomenon was only observed in the 101-150 age group (p < 0.01) in male mice (d).
3.2. Obesity in the congenic narcoleptic mice
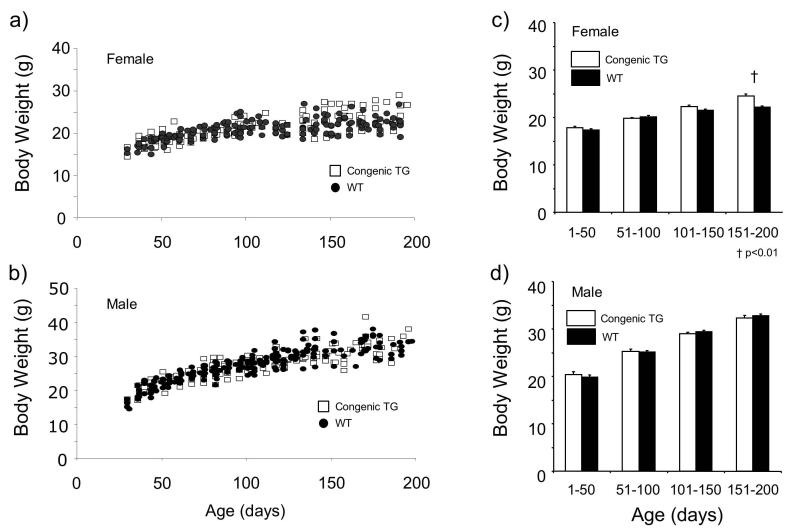
The body weight distribution across age of the congenic TG animals showed a similar pattern to that of animals in N4-5 generation (Fig. 3a, b). However, the tendency of body weight of female TG mice being heavier than that of WT mice was not as apparent; the significant difference between TG and WT was only observed in females of 151 to 200 days of age (P<0.01, Mann-Whitney test) (Fig. 3c, d).
Figure 3.
Body weight and age relationship of congenic (N7 and N8 generations) orexin/ataxin-3 transgenic (TG) mice. Individual data for females (a) and males (b) are shown. After 150 to 200 days of age, a cluster of TG female mice seemed to be heavier than wild type mice. This was not observed in male mice. Body weight data were divided into 4 age groups (1-50, 51-100, 101-150, and 151-200 days of age) and shown as mean ± SEM. Congenic TG mice were significantly heavier than wild-type mice (WT) in the 151-200 (p < 0.01) age group in females (a). This phenomenon was not observed in male mice (b).
3.3. Serum leptin level
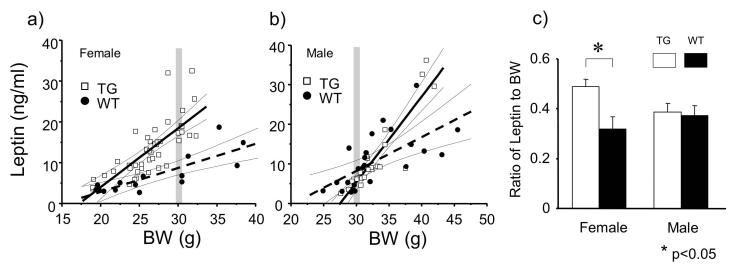
Since gender and body weight (fat weight) significantly affects serum leptin levels [8, 22], the leptin results are displayed in scattergram with the x-axis as body weight. Serum leptin levels and body weight showed clear linear correlations in female TG (Y= 1.66 X -29.87, r = 0.80, p < 0.0001), female WT (Y= 0.59 X -8.86, r = 0.82, p = 0.0002), male TG (Y= 2.17 X -59.56, r = 0.91, p < 0.0001), and male WT (Y= 0.85 X -17.27, r = 0.65, p = 0.0006) (Fig. 4a, b). Similar to other species, leptin levels in female mice were higher than those in male mice (Fig. 4a, b). We found significant difference between female TG and WT mice in relationship of body weight and serum leptin level (P < 0.01, ANCOVA), but not in male mice (p = 0.63, ANCOVA). The ratio of leptin to body weight of TG females was significantly larger than that of WT females (P < 0.01, Mann-Whitney test), but no significant difference was found in male mice (p = 0.9, Mann-Whitney test) (Fig 4c).
Figure 4.
Correlation between serum leptin level and body weight in female (a) and male (b) orexin/ataxin-3 transgenic (TG) mice. The leptin levels in females are higher than those in males with similar body weight (gray bars). Solid lines represent the regression lines for TG and broken lines represent the regression lines for wild type (WT). The thin lines indicate the 95% confidential lines for each data set. There was a significant difference between TG and WT in relationship of body weight and serum leptin level in females (p < 0.01, ANCOVA) (a), but not in males (b). The ratios of leptin to body weight (BW) of female TG mice (0.49 ± 0.03) were significantly larger than that of female WT mice (0.32 ± 0.04) (p < 0.05, t-test), but no difference was observed between male TG (0.39 ± 0.03) and male WT (0.37 ± 0.04) (c).
4. Discussion
We observed that both hypocretin/orexin-deficient KO and TG (ligand deficit and hypocretin/orexin cell death, respectively) narcoleptic mice with mixed genetic background are obese compared to their WT littermates housed in the same environment. The obesity is gender specific and prominent in female narcoleptic mice in both models. Preprohypocretin/preproorexin Hz KO animals were reported to produce 75 to 83 % of hypocretin peptides of WT mice [2] and these Hz mice exhibited intermediate mean body weight between homozygous KO and WT mice. These results strongly suggest that hypocretin/orexin deficiency is one of the most important factors for the obese tendency in narcoleptic mice. It is likely, however, that other factors also affect this phenotype. It has been previously reported [11, 12] that obesity is age-dependent (i.e. late onset) and less clear in the congenic lines of C57BL/6 strain background. In this regard, our results of cross-sectional evaluation are very consistent with those of time series evaluation by Hara et al [11, 12], demonstrating that maturation and genetic background significantly affect body weight gain.
In addition, we found that gender also significantly affects obesity in these mice. The studies by Hara et al included only male mice, and the gender effect has not been noted previously. The gender effect was observed in both KO and TG mouse models with mixed genetic background. Furthermore, a significant increase in body weight in females was still observed in the congenic TG line (151-200 days old).
Daniels was the first to report that up to 50% of his narcoleptic patients gained a considerable amount of weight (5-45 kg) around the time of disease onset [5]. Importantly, however, he also noted that this phenotype was quite variable and more frequent in women. In our study of hypocretin-deficient human narcolepsy, we also observed similar tendency: Above 125% of body mass indexes (BMI; adjusted by age, gender and ethnicity) were observed in 9 out of 37 subjects (male/female is 18/19), and 8 of them were females (Fischer’s exact probability was p=0.0188, two-tailed) [21]. Although some other studies did not note the gender difference for weight gain in narcolepsy, a high incidence for obesity in females was also confirmed in our extended study with a large sample population (93 hypocretin deficient narcolepsy and 111 controls) (Arnulf manuscript in preparation). Therefore, this gender effect may generally be observed in both hypocretin-deficient mice and humans.
Another important result we obtained from the mouse experiments is the high levels of serum leptin in female TG mice. In human narcolepsy, Schuld et al. reported more than 50% decrease in fasting serum leptin, but no change in CSF leptin in 15 patients with narcolepsy-cataplexy (9/6) [26]. We observed normal to slightly increased CSF leptin (commensurate with increased BMI) in 38 narcoleptic subjects [21], and this may suggest an altered central penetration of leptin signaling in narcolepsy. Kok et al also reported that serum leptin levels were reduced in six male narcoleptic patients, compared to BMI-matched healthy male controls, when sampled over 24 hours [16]. Leptin decreases appetite and food intake, and leptin deficient animals are obese and hypoactive, while the majority of obese human subjects have increased serum leptin concentrations, suggesting some degree of leptin resistance (i.e. partial leptin resistance) [9, 19]. Schuld et al and Kok et al interpreted their data as that reduced circulating leptin in narcolepsy could lead to obesity [16, 26]. However, we did not observe any significant difference in serum-leptin, CSF-leptin and CSF: serum leptin ratio between hypocretin-deficient narcoleptic and control subjects in our extended study with larger sample population (Arnulf manuscript in preparation), including a subsample population under the design Schuld et al used. The discrepancy of these results is not known, but may be due to the type I error (due to the small sample size), and the heterogeneous nature of human sample population, such as differences in living environment, nutrition, and medication status. Variation in narcolepsy symptomatology, such as degree of sleep disturbance across samples, may affect leptin results [18]. All of the factors mentioned above have been reported to affect serum leptin levels.
In this regard, our animal data are more dependable than the human data since our results are obtained from littermate mice housed in the same environment, and animals have not received any medication. In contrast to human data, we observed higher serum leptin levels in female TG narcoleptic mice. This is not only secondary to the increase in body weight, since serum leptin levels were higher in TG mice even when compared with the levels of WT mice with similar body weight (see Fig. 4). Considering the fact that this tendency was not observed in male mice, it is likely that altered leptin signaling may functionally contribute to the weight gain in female narcoleptic TG mice. Although we have not evaluated the CSF leptin levels and CSF: serum leptin ratios in these mice, our results suggest a partial leptin resistance in female narcoleptic TG mice, in contrast to some earlier human findings.
The reason for the gender difference in obese tendency is not known. The leptin levels are influenced by various hormones and cytokines that are also under the influence of gender [3, 14], and leptin levels in females are higher than in males [3, 8, 14]. Similarly, higher levels of preprohypocretin mRNA in the hypothalamus of female rats compared to male rats are reported [15]. As mentioned earlier, leptin significantly affects hypocretin signaling [29]. Therefore, sexual dimorphism of hypocretin/leptin signaling likely exists, and alteration of this signaling may contribute to the gender difference in obesity. Detailed mechanisms underlying the gender difference should be further studied to better understand the physiology of obesity.
Acknowledgements
We thank Edward Tuan, Takashi Tamura, Daniel Wu, Haruno Sengoku, and Timothy Cheng for assisting us weight measures of the animals. This research was supported by NIH Grant NS23724 and a grant for anorexia nervosa research from the Japanese Ministry of Health, Labour and Welfare.
Reference
- [1].Asakawa A, Inui A, Goto K, Yuzuriha H, Takimoto Y, Inui T, et al. Effects of agouti-related protein, orexin and melanin-concentrating hormone on oxygen consumption in mice. Int J Mol Med. 2002;10:523–5. [PubMed] [Google Scholar]
- [2].Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- [3].Considine RV. Regulation of leptin production. Rev Endocr Metab Disord. 2001;2:357–63. doi: 10.1023/a:1011896331159. [DOI] [PubMed] [Google Scholar]
- [4].Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur Arch Psychiatry Clin Neurosci. 2001;251:85–9. doi: 10.1007/s004060170057. [DOI] [PubMed] [Google Scholar]
- [5].Daniels LE. Narcolepsy. Medicine. 1934;13:1–122. [Google Scholar]
- [6].Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–53. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160:R7–12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- [8].Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–4. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- [9].Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- [10].Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- [12].Hara J, Yanagisawa M, Sakurai T. Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett. 2005;380:239–42. doi: 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- [13].Honda Y, Doi Y, Ninomiya R, Ninomiya C. Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep. 1986;9:254–9. doi: 10.1093/sleep/9.1.254. [DOI] [PubMed] [Google Scholar]
- [14].Janeckova R. The role of leptin in human physiology and pathophysiology. Physiol Res. 2001;50:443–59. [PubMed] [Google Scholar]
- [15].Johren O, Neidert SJ, Kummer M, Dominiak P. Sexually dimorphic expression of prepro-orexin mRNA in the rat hypothalamus. Peptides. 2002;23:1177–80. doi: 10.1016/s0196-9781(02)00052-9. [DOI] [PubMed] [Google Scholar]
- [16].Kok SW, Meinders AE, Overeem S, Lammers GJ, Roelfsema F, Frolich M, et al. Reduction of plasma leptin levels and loss of its circadian rhythmicity in hypocretin (orexin)-deficient narcoleptic humans. J Clin Endocrinol Metab. 2002;87:805–9. doi: 10.1210/jcem.87.2.8246. [DOI] [PubMed] [Google Scholar]
- [17].Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–7. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- [18].Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- [19].Munzberg H, Myers MG., Jr. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–70. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- [20].Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- [21].Nishino S, Ripley B, Overeem S, Nevsimalova S, Lammers GJ, Vankova J, et al. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol. 2001;50:381–8. doi: 10.1002/ana.1130. [DOI] [PubMed] [Google Scholar]
- [22].Niskanen LK, Haffner S, Karhunen LJ, Turpeinen AK, Miettinen H, Uusitupa MI. Serum leptin in obesity is related to gender and body fat topography but does not predict successful weight loss. Eur J Endocrinol. 1997;137:61–7. doi: 10.1530/eje.0.1370061. [DOI] [PubMed] [Google Scholar]
- [23].Overeem S, Kok SW, Pijl H, Lammers GJ, Meinders AE. Body weight and -composition in patients with narcolepsy versus idiopathic hypersomnia. SLEEP. 2002;25:A79. [Google Scholar]
- [24].Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- [26].Schuld A, Blum WF, Uhr M, Haack M, Kraus T, Holsboer F, et al. Reduced leptin levels in human narcolepsy. Neuroendocrinology. 2000;72:195–8. doi: 10.1159/000054587. [DOI] [PubMed] [Google Scholar]
- [27].Schuld A, Hebebrand J, Geller F, Pollmacher T. Increased body-mass index in patients with narcolepsy. Lancet. 2000;355:1274–5. doi: 10.1016/S0140-6736(05)74704-8. [DOI] [PubMed] [Google Scholar]
- [28].Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–58. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- [29].Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- [30].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]