Abstract
Objective
To compare the cognitive functioning of a sample of patients experiencing their first episode of schizophrenia with that of patients with an established schizophrenia illness.
Design
Cross-sectional and longitudinal study.
Setting
The Calgary Early Psychosis Treatment and Prevention Program and an outpatient clinic in a department of psychiatry at a university-affiliated hospital.
Participants
One hundred and eleven patients who were experiencing their first episode of schizophrenia and 76 outpatients with an established schizophrenia illness.
Measures
The Positive and Negative Syndrome Scale for schizophrenia was administered to all subjects to determine levels of positive and negative symptoms. Executive functioning, information processing, visual memory, and immediate and delayed verbal memory were assessed.
Results
There were limited differences between the 2 groups in terms of cognitive functioning. Although the first-episode patients demonstrated generally superior scores, their performance was impaired.
Conclusions
These results support the findings of previous studies suggesting that first-episode patients demonstrate cognitive impairments similar to those of patients with an established schizophrenia illness.
Medical subject headings: disease progression, neurobehavioural manifestations, schizophrenia
Abstract
Objectif
Comparer le fonctionnement cognitif d'un échantillon de patients vivant leur premier épisode de schizophrénie à celui de patients dont la schizophrénie est établie.
Conception
Étude transversale et longitudinale.
Contexte
Le Programme de traitement rapide et de prévention de la psychose de Calgary et une clinique externe d'un service de psychiatrie d'un hôpital affilié à une université.
Participants
Cent onze patients vivant leur premier épisode de schizophrénie et 76 patients en service externe dont la schizophrénie est établie.
Mesures
On a soumis tous les sujets à l'échelle d'appréciation des symptômes positifs et négatifs pour la schizophrénie afin de déterminer le niveau des symptômes positifs et négatifs. On a évalué la fonction d'exécution, le traitement de l'information, la mémoire visuelle et la mémoire verbale immédiate et différée.
Résultats
On a constaté des différences limitées entre les deux groupes sur les plans du fonctionnement cognitif. Même si les patients vivant leur premier épisode ont obtenu des résultats en général supérieurs, ils avaient un déficit au niveau du rendement.
Conclusions
Ces résultats appuient les constatations d'études antérieures qui indiquent que les patients vivant leur premier épisode montrent des déficits de la cognition semblables à ceux des patients dont la schizophrénie est établie.
Introduction
It is well established that cognitive deficits are a core feature of schizophrenia1 and that people with schizophrenia have clear deficits in most aspects of cognitive functioning relative to both nonpsychiatric and other diagnostic control groups. Furthermore, it has been demonstrated that people experiencing their first episode of schizophrenia are already exhibiting cognitive deficits.2,3,4,5,6 Although these patients may not demonstrate as severe impairment as those who have experienced multiple episodes of schizophrenia, they are clearly more impaired than normal controls. In several studies first-episode (FE) patients showed cognitive impairment equivalent to the impairment of those who had been ill for several years, particularly in the area of memory.2,3,7,8,9 This impairment did not appear to be an effect of medication, as the deficits were also observed in FE subjects who were treatment naïve.10
The purpose of this study was to compare the cognitive functioning of a sample of FE subjects with that of patients who had experienced multiple episodes of schizophrenia. These FE patients were participants in an early-intervention program11 that offers optimal care as early as possible in the course of the illness.
Methods
Subjects
One hundred and eleven FE patients (74 men and 37 women) were recruited through the Calgary Early Psychosis Treatment and Prevention Program.11 These patients were experiencing their first episode of psychosis and had not previously received more than 3 months of adequate treatment.12 Although patients in the program have a range of schizophrenia spectrum diagnoses, the inclusion criteria for this study were a DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th edition)13 diagnosis of schizophrenia that was confirmed at a 1-year evaluation and completion of cognitive assessments at both initial entry to the program and the 1-year evaluation. Most patients (87%) in the FE sample were single, and their mean age was 24.7 (standard deviation [SD] 8.2) years. One hundred (90%) were taking second-generation antipsychotics, the mean dose of which (in chlorpromazine equivalents) was 372.9 mg/d. At the initial assessment, 50 (45%) of the FE patients had never experienced a psychiatric inpatient admission, and most were outpatients at the time of cognitive testing. By the time of the 1-year follow-up, we had lost approximately 20% of the subjects tested at the initial assessment: 11 (10%) had moved away or had chosen to go to a different treatment facility, and 11 (10%) had dropped out; their whereabouts were unknown. There was no difference in age, symptoms or cognition at initial assessment between those who remained in the program and those who did not complete the 1-year follow-up.
The multiepisode (ME) sample consisted of the 76 subjects (53 men and 23 women) who had been recruited for a study of cognitive and social functioning in schizophrenia.14 These subjects met DSM-III-R15 criteria for schizophrenia, had experienced more than one acute episode of psychosis and had been admitted more than once for an acute relapse, and their first admission to hospital for a psychotic episode had occurred more than 3 years before their participation in the previous study.14 They had been ill for between 4 and 32 years, and 61 (80%) had been ill for more than 7 years. These patients were significantly older than the FE group (35.0 [SD 10.0] yr, t = –5.14, p < 0.001). All were taking antipsychotics, for which the mean dosage (in chlorpromazine equivalents) was 435.6 mg/d, not significantly different from the FE group. However, these patients did differ from the FE group in terms of the types of medications being taken. Two-thirds (52 or 68%) were taking traditional antipsychotics, whereas 24 were taking second-generation antipsychotics (16 [21%] were taking risperidone, 7 [9%] were taking clozapine, and 1 [1%] was taking olanzapine). The mean number of psychiatric admissions for these patients was 5.0. There was no difference between the groups in age at onset or in level of education (grade 12).
Measures
Diagnosis of the FE patients was made by the clinical research team of the Calgary Early Psychosis Program according to the Structured Clinical Interview for DSM-IV and taking account of all available sources of information. All raters were experienced research clinicians who had used these measures in other research projects and who had demonstrated adequate reliability.
Assessment of cognitive functioning encompassed executive function (the Wisconsin Card Sorting Test [WCST]), early information processing (span of apprehension),16,17 visual attention (degraded stimulus continuous performance test),18,19 visual memory (Rey complex figure), and immediate and delayed verbal memory (logical memory subtest of the revised Wechsler Memory Scale). The Positive and Negative Syndrome Scale20 was used to obtain ratings for positive and negative symptoms. Reliability was adequate because these measures are used routinely in our research program. Alternative forms were not used.
Procedures
Formal consent was obtained from all subjects.
For the FE subjects, the first cognitive assessment took place within 1 month of admission to the program, once patients had demonstrated their capability of attending to the testing. Subsequent assessments were completed 1 year after admission to the program. ME subjects were assessed only once. Procedures for the ME subjects are described elsewhere.14
Results
Correlational analyses demonstrated no significant associations between positive symptoms and cognitive functioning. There were several significant associations between negative symptoms and cognitive functioning. For the ME group, negative symptoms were associated with WCST and delayed verbal memory results. For the FE group, negative symptoms were associated with the WCST and visual and verbal memory results at the initial assessment and with the WCST and verbal memory results at the 1-year assessment.
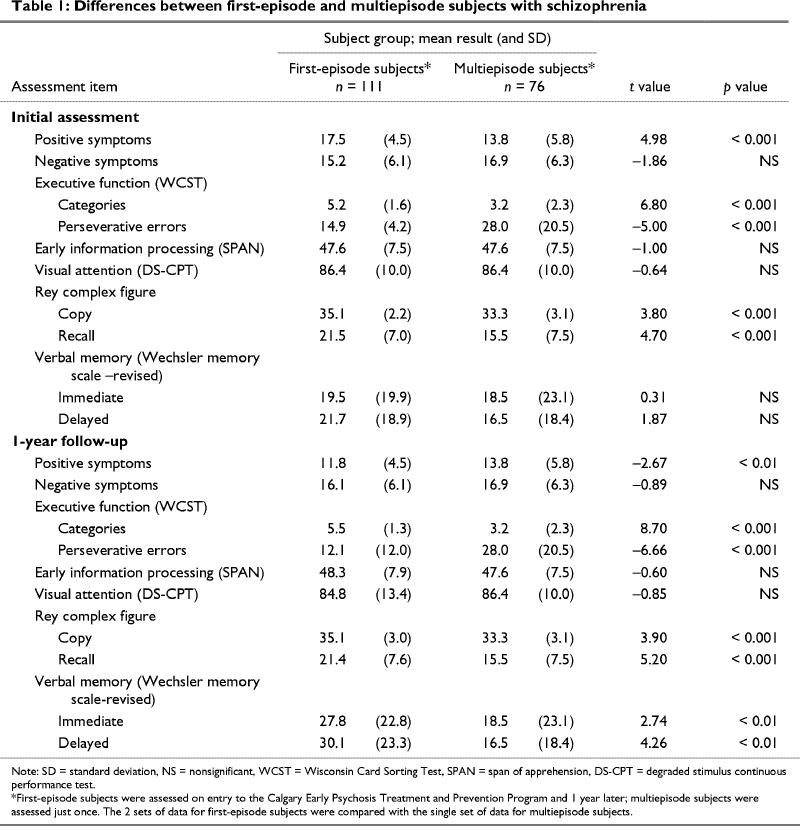
The FE subjects had more positive symptoms at the initial assessment and fewer positive symptoms at the 1-year assessment than the ME subjects had at their single assessment (Table 1). At both assessments, the FE subjects did better on the WCST and the Rey complex figure. At the 1-year assessment they demonstrated superior performance on verbal memory.
Table 1
Discussion
In this study, cognition was not associated with positive symptoms. As might be expected,21 there were several significant associations between negative symptoms and cognition in the FE group at both assessment times and in the ME group. At the initial assessment, the FE patients demonstrated more positive symptoms than the ME subjects because they were only in their first month of the treatment program. However, by 1 year, the FE subjects had significantly fewer positive symptoms than the ME subjects.
There were no differences in early information processing between the groups. Measures of early information processing have been described as vulnerability indictors,16,18 and it has been reported that deficits on such tasks are apparent in high-risk subjects as well as in patients at all stages of the schizophrenia illness.21,22 The FE subjects had superior results on verbal memory at the 1-year follow-up. However, these young patients had verbal memory scores that were, on average, at or below the 30th percentile, which clearly indicates impaired performance. The FE subjects performed consistently better on the WCST, a measure of executive functioning, and on the Rey complex figure, a measure of visual memory. Again, although performance on the Rey complex figure was superior for the FE group, the patients were performing, on average, below the 50th percentile; the ME subjects performed, on average, below the 25th percentile. However, the superior performance of the FE subjects at 1 year could be explained by the fact that alternative forms of the measures were not used. Thus, there may have been a learning effect over the 1-year period between assessments. In addition, a third of the ME subjects were receiving first-generation antipsychotics and may not have benefited from the suggested potential of second-generation antipsychotics to positively influence memory. This would only serve to emphasize the lack of difference in memory between these 2 groups.
Results of the WCST were somewhat different. A closer examination of the scores revealed that 74 (67%) of the FE subjects scored in the normal range (i.e., achieved 6 categories with a very low number of perseverative errors). The 37 FE patients (33%) who scored more poorly did not differ from the ME subjects and scored poorly on every other cognitive test. The same was not true of the other tests, for which deficits were noted across the FE group. These results are similar to those reported by Hutton et al,4 who found that FE schizophrenia patients have profound executive impairments at the beginning of the illness. However, these impairments were in planning and strategy use rather than attentional shift setting, which was generally unimpaired. The task used in the study by Hutton et al4 was analogous to the WCST, and 80% of the patients in their study performed adequately on the task. Thus, although executive functioning may be impaired in FE subjects, there appears to be a minority who not only have additional deficits in executive functioning but who may also have more profound deficits in other areas.
One limitation of the study was the use of multiple comparisons, which may not meet the correction for multiple testing. However, this possibility is unlikely to have an impact on the overall conclusion that the 2 groups are not that different.
In summary, the results of this study suggest that FE patients demonstrate cognitive impairments on some tasks that are as severe as those seen in people with an established schizophrenia illness, whereas on other tasks, they may demonstrate marginally superior performance to ME patients, but with scores that are still in the impaired range. The exception appears to be for performance on the WCST, a task widely used in the assessment of cognition in schizophrenia. This task appears to identify those with more significant impairments in the early stages of the illness. Further longitudinal work will be required to demonstrate whether this task continues to predict those patients who will have a poor course.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Jean Addington, Department of Psychiatry, Foothills Hospital, 1403 29th St. NW, Calgary AB T2N 2T9; fax 403 670-4008; jmadding@ucalgary.ca
Submitted Aug. 1, 2001 Revised Dec. 13, 2001 Accepted Jan. 7, 2002
References
- 1.Green MF. Schizophrenia from a neurocognitive perspective. Boston: Allyn & Bacon; 1998.
- 2.Hoff AL, Riordan H, O'Donnell DW, Morris L, DeLisi LE. Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry 1992;149:898-903. [DOI] [PubMed]
- 3.Hoff AL, Riordan H, O'Donnell DW, Stritzke P, Neale C, Boccio A, et al. Anomalous lateral sulcus asymmetry and cognitive function in first-episode episode schizophrenia. Schizophr Bull 1992;18:257-72. [DOI] [PubMed]
- 4.Hutton SB, Puri BK, Duncun LJ, Robbins TW, Barnes TRE, Joyce EM. Executive function in first-episode schizophrenia. Psychol Med 1998;28:463-73. [DOI] [PubMed]
- 5.Mohamed S, Paulesen JS, O'Leary D, Arnndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry 1999;56:749-55. [DOI] [PubMed]
- 6.Øie M, Rund BR, Sundet K, Bryhn G. Auditory laterality and selective attention: normal performance in patients with early-onset schizophrenia. Schizophr Bull 1998:24(4):643-52. [DOI] [PubMed]
- 7.Albus M, Hubmann W, Ehrenberg CH, Forcht U, Mohr E, Sobizack N, et al. Neuropsychological impairment in first-episode and chronic schizophrenic patients. Eur Arch Psychiatry Clin Neurosi 1996;246:249-55. [DOI] [PubMed]
- 8.Albus M, Hubmann W, Sobizack N, Mohr F, Franz U, Hecht S, et al. Prospective 2-year follow-up study of cognition in first episode schizophrenic patients [abstract]. Schizophr Res 1998;29:51.
- 9.Censits D, Ragland JD, Gur RC, Gur RE. Neuropsychological evidence supporting a neurodevelopmental mode of schizophrenia. Schizophr Res 1997;24:289-98. [DOI] [PMC free article] [PubMed]
- 10.Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, et al. Neuropsychological deficits in neuroleptic naïve patients with first-episode schizophrenia. Arch Gen Psychiatry 1994;51:124-31. [DOI] [PubMed]
- 11.Addington J, Addington D. Early intervention for psychosis: the Calgary Early Psychosis Treatment and Prevention Program. Can Psychiatr Assoc Bull 2001;33:241-56.
- 12.Larsen TK, McGlashan TH, Moe LC. First-episode schizophrenia: I. Early course parameters. Schizophr Bull 1996;22:241-56. [DOI] [PubMed]
- 13.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: American Psychiatric Association; 1994.
- 14.Addington J, Addington D. Neurocognitive and social functioning in schizophrenia. Schizophr Bull 1999;25:173-83. [DOI] [PubMed]
- 15.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed, revised. Washington: American Psychiatric Association; 1987.
- 16.Asarnow RF, Granholm E, Sherman T. Span of apprehension in schizophrenia. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of schizophrenia: neuropsychology, psychophysiology and information processing. 5th ed. Amsterdam: Elsevier; 1991. p. 335-70.
- 17.Asarnow RF, Neuchterlein KH. UCLA forced-choice span of apprehension test, version 4 [software manual]. Los Angeles: University of California, Los Angeles; 1992.
- 18.Nuechterlein KH. Vigilance in schizophrenia and related disorders. In: Steinhauer SR, Gruzelier JH, Zubin J, editors. Handbook of schizophrenia: neuropsychology, psychophysiology and information processing. 5th ed. Amsterdam: Elsevier; 1991. p. 397-433.
- 19.Nuechterlein KH, Asarnow RF. UCLA continuous performance test, version 4 [software manual]. Los Angeles: University of California, Los Angeles; 1992.
- 20.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261-76. [DOI] [PubMed]
- 21.Addington J. Negative symptoms and the relationship to cognitive functioning. In: Sharma T, Harvey P, editors. Cognition in schizophrenia. Oxford (UK): Oxford University Press; 2000. p. 191-209.
- 22.Addington J, Addington D. Attentional vulnerability indicators in schizophrenia and bipolar disorder. Schizophr Res 1997; 23:197-204. [DOI] [PubMed]


