Abstract
Full remission should be the goal of antidepressant therapy; anything less leaves the patient with residual symptoms and an increased risk of relapse and recurrence. Most antidepressant agents offer similar rates of response, but there are some differences in the ability of different agents to promote a full remission. The greatest chance of achieving full remission occurs early in the course of treatment; thus, initial antidepressant strategies should be those that have the greatest therapeutic potential. Other strategies that may help improve the chances of achieving full remission include optimizing drug dosages and using combination and augmentation strategies. Failure to achieve full remission and early discontinuation of antidepressant therapy have been associated with a greater incidence of relapse and recurrence. Continued antidepressant therapy has clearly been shown to effectively reduce the probability of relapse and recurrence by about half compared with placebo. Therefore, once a patient achieves remission, it is important to continue the same antidepressant therapy for at least 6–12 months and, for many patients, considerably longer. Medication should continue at the dose that was initially effective because using low-dose maintenance therapy appears to decrease the protective benefits.
Medical subject headings: antidepressive agents; depressive disorder; dose–response relationship, drug; drug therapy; lithium carbonate; recurrence; remission induction; treatment outcome
Abstract
Toute thérapie aux antidépresseurs devrait viser à produire une rémission complète. Toute rémission incomplète laisse au patient des symptômes résiduels et un risque accru de rechute et de récidive. La plupart des antidépresseurs produisent des taux semblables de réponse, mais la capacité de différents agents de produire une rémission complète diffère un peu. C'est au début du traitement que la chance de rémission complète est la meilleure. Les traitements initiaux aux antidépresseurs devraient donc être ceux qui offrent le plus de potentiel thérapeutique. D'autres stratégies qui peuvent aider à améliorer les chances de rémission complète comprennent l'optimisation des posologies et le recours aux stratégies de combinaison et d'augmentation. On a établi un lien entre l'incapacité de produire une rémission complète et l'interruption précoce de la thérapie aux antidépresseurs, d'une part, et une incidence plus importante de rechutes et de récidives, de l'autre. Il a été démontré clairement que la thérapie continue aux antidépresseurs réduit d'environ la moitié la probabilité de rechute et de récidive comparativement au placebo. Par conséquent, lorsqu'un patient parvient au stade de la rémission, il importe de poursuivre la même thérapie aux antidépresseurs pendant au moins six à 12 mois, et beaucoup plus longtemps dans le cas de certains patients. Il faudrait poursuivre la médication à la posologie qui s'est révélée efficace au début parce qu'une thérapie d'entretien à faible dose semble réduire les avantages de la protection.
Introduction
An important paradigm shift among practising psychiatrists is the acceptance of unipolar depression, not as a single episode illness, but rather as a recurrent and often chronic debilitating condition.1 Between 15% and 20% of depressed patients experience a chronic course of illness, and up to 80% experience a recurrent course of illness.2,3,4 Antidepressant treatment for recurrent depression is intended to reduce the probability of, as well as the duration of, future episodes and to confer both the pharmacological and economic benefits of improved quality of life and reduced medical costs.5
The primary goal of patient and therapist in the treatment of depression is to achieve full remission. This is the optimal state for the patient's well being, and residual symptoms are known to predispose to further episodes of depression. A key question in the treatment of major depressive disorder (MDD) is how long to continue pharmacotherapy. Although antidepressant therapy may suppress symptoms, it may not immediately correct the underlying disorder.6,7 As a result, there may be a gap of several weeks or months between symptom control and episode resolution.8 Early discontinuation of therapy is likely to result in relapse for about 50% of patients.9,10
Response, remission, relapse and recurrence
Our definitions of response, remission, relapse and recurrence are consistent with those described by Frank and colleagues.11 The goal of therapy is full remission; anything less leaves patients with residual symptoms and an increased risk of recurrence. Full remission is the virtual elimination of symptoms and a return to premorbid levels of functioning.11 “Relapse” refers to the return of sufficient symptoms to cause an individual to again meet diagnostic criteria for the illness after a discrete period of wellness. In clinical trials, between 50% and 70% of patients respond to antidepressant therapy but only 25%–35% of patients experience full remission.12,13 This is very disturbing; less than half of treated patients achieve full remission, and even after a reasonable duration of treatment, most will experience some residual symptoms. A return to a premorbid level of functioning has probably been the most underemphasized portion of the treatment plan for depressed patients.
Since the distinction between continuation therapy (to prevent relapse) and maintenance therapy (to prevent recurrence) is somewhat arbitrary, these data will focus on the acute phase and long-term maintenance phase outcomes. Acute therapy is required to achieve full remission, and maintenance therapy is needed to sustain the fully remitted state.14
Time to response and remission
In general, evidence of improvement (variously defined as a decrease of 20% or 30% in Hamilton Rating Scale for Depression [HAM-D] baseline score) within 2 or 4 weeks of initiating treatment predicts a favourable outcome after 8 weeks of antidepressant treatment. The corollary is that significantly fewer patients who have not shown this modest level of early response go on to achieve response or remission at 8 weeks, compared with those in the “improvement and early response” group.15,16 In the first of 2 studies involving independent patient populations, Nierenberg et al15 reported that only 19% of those who did not show at least a 20% drop in HAM-D score at 4 weeks, and 6% of those who had not shown a similar drop at 6 weeks went on to achieve a response (50% reduction in HAM-D) after 8 weeks of treatment with fluoxetine (20 mg daily).15 In the second study involving a larger population of depressed subjects, the same research group reported that over 75% of eventual responders to 8 weeks of fluoxetine therapy had shown at least a 30% decrease in symptoms by week 4.16 Conversely, those who had not experienced improvement by weeks 4 or 6 had a 73%–88% chance of nonresponse by the end of the 8-week trial. Evidence of an early trajectory of response has also been reported for paroxetine and for venlafaxine.17,18
The same findings appear to be true for remission. Patients have the greatest chance of achieving full remission early in their treatment course. During the first 6 months of treatment, 50% of patients achieved full remission in a study conducted in the United Kingdom. Over time, the cumulative likelihood of full remission was reduced, and by months 13–15 only 5% of patients were in remission.19 In an Irish study, the probability of remission in 100 consecutive depressed inpatients was determined over 18 months.20 The cumulative probabilities of remission onset by 3 and 18 months were 67% and 82%, respectively, and there was a 25% probability of relapse, of which more than half occurred in the first 2 months after remission.
This implies a window of opportunity during which the patient has a greater likelihood of achieving remission. Remission occurs early in the treatment process, suggesting the need for aggressive first-line strategies that are capable of producing full remission. Given that functional improvement lags behind symptom improvement, the faster remission can be achieved, the sooner the patient will experience a restored quality of life.
Achieving full remission
Optimization, augmentation and combination therapy
A number of strategies should be considered for patients who fail to achieve full remission with an initial therapeutic trial. During initial therapy, the patient should be monitored and the pharmacotherapy optimized.21 This includes assessing adherence and comorbidity and adjusting the dose. In a US survey of 801 practising clinicians designed to assess strategies used in cases where selective serotonin reuptake inhibitors (SSRIs) are ineffective, 84% of respondents chose to increase the dose.22 This can be particularly useful with agents that have a linear dose–response curve. Yet, in a UK survey of a large prescription database for antidepressant use, less than 5% of family practitioners had actually increased the initial dose.23 Switching agents was the choice of only 7% of respondents; however, this may be preferred when the side-effect burden is too high with high-dose monotherapy. Although data are limited on the efficacy and safety of combination strategies, augmentation or combination was the choice of 10% of clinicians. When asked about their preferred agent, 30% chose bupropion and 22% lithium. Largely on the basis of anecdotal evidence, the use of augmentation or combination strategies is recommended for partial responders, whereas in the absence of any response, switching medications may be preferred.21
Strategies that involve combining pharmacotherapy and psychotherapy, such as cognitive behaviour therapy or interpersonal psychotherapy may also enhance remission rates. Some patients, particularly those who have been depressed for a long time, may need additional help to stop the negative thought patterns they have developed. In a small pilot study, patients who received cognitive behaviour therapy for residual symptoms after successful drug therapy showed significantly increased remission rates.24
Antidepressant choice
Aggressive initial treatment should be implemented with drugs that offer the greatest therapeutic potential and the best chance to induce full remission.25 In general, antidepressants from the various classes have not shown significantly different response rates. A meta-analysis of 102 trials involving 10 706 patients demonstrated no significant differences in overall efficacy, as measured by effect sizes, between SSRIs and tricyclic antidepressants (TCAs).26 In addition, no significant differences in efficacy were noted between any of the individual SSRIs and individual TCA comparators. However, TCAs may be more effective in inpatients, and amitriptyline was more effective than SSRI comparators. A systematic review of 186 randomized controlled trials also showed superior overall efficacy of amitriptyline compared with other TCAs and SSRIs even when dropouts were included as treatment failures.27
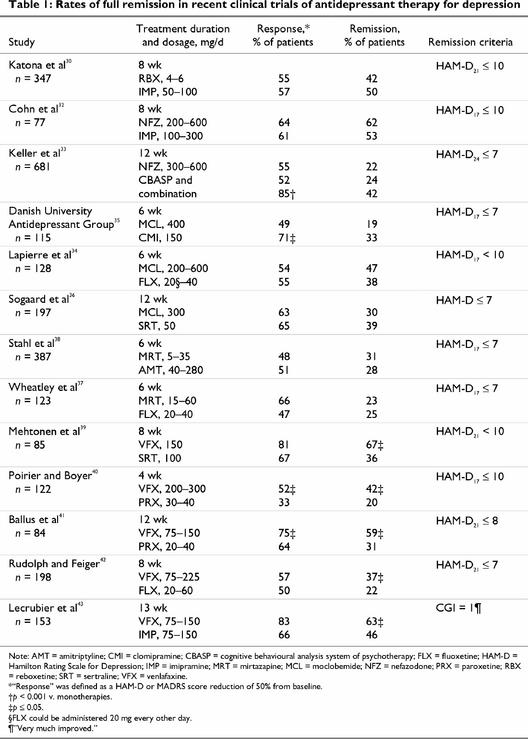
Table 1 reviews some of the recent clinical trials that have focused on achieving full remission, rather than just response. Separate trials were identified comparing reboxetine (not yet available in Canada), nefazodone, moclobemide, mirtazapine and venlafaxine to TCAs or SSRIs. Therapy with bupropion has demonstrated response rates comparable to various SSRIs (e.g., sertraline and paroxetine),28,29 but no studies reporting rates of full remission were identified. Comparisons among studies should be made with caution because study parameters including patient types, dosing and severity of illness varied significantly. No agent significantly and consistently was associated with higher remission rates than their TCA or SSRI comparator, with the exception of venlafaxine.
Table 1
Treatment with SSRIs generally results in remission rates of about 20%–39%, whereas remission rates with TCAs were 46%–53%. Remission rates with reboxetine, a selective norepinephrine reuptake inhibitor, were about 42%.30 Nefazodone acts primarily as a serotonergic 5-HT2 receptor antagonist as well as a weaker serotonin reuptake inhibitor.31 It exhibited rates of full remission of 62% in one study but only 22% in another study with a more stringent definition of remission.32,33
Moclobemide is a reversible inhibitor of monoamine oxidase A. Remission rates of 30%–47% have been reported with moclobemide treatment, which is comparable to those seen with SSRIs and the tricyclic clomipramine.34,35,36 However, in one of these studies, moclobemide was associated with a higher rate of nonresponse or worsening of depression than clomipramine.35 Mirtazapine acts to increase serotonin and norepinephrine by blocking presynaptic receptors. The studies involving mirtazapine did not report significantly higher remission rates for mirtazapine than for the SSRI fluoxetine37 or the tricyclic amitriptyline.38
Remission rates for venlafaxine, a dual-acting serotonin and norepinephrine reuptake inhibitor, range from 37% to 67%. These rates are significantly higher than those of comparator SSRIs and the tricyclic imipramine.39,40,41,42,43 The higher rates of full remission with venlafaxine were replicated in a recent meta-analysis of 8 randomized trials comparing venlafaxine and SSRIs in over 2000 patients.44 Patients had a 50% greater likelihood of achieving remission when treated with venlafaxine than they did when treated with an SSRI.
It has been hypothesized that the efficacy of antidepressants that act at more than one pharmacologic site (e.g., serotonin and norepinephrine reuptake inhibition) may be superior to that of SSRIs.45 However, in a meta-analysis of 105 trials, Freemantle et al46 did not find any relation between postulated mechanisms of action of antidepressants (i.e., serotonin or norepinephrine reuptake inhibition, 5-HT2 receptor antagonism or combined actions) and relative efficacy.46 This suggests that the question of how drug actions relate to efficacy may await a better understanding of downstream intracellular effects and also suggests that higher rates of remission with the dual-acting serotonin and norepinephrine reuptake inhibitor, venlafaxine, cannot be generalized to other agents simply on the basis of multiple mechanisms of action.
Given the risks associated with partial response, future studies comparing antidepressant strategies should turn their attention to full remission as an endpoint rather than simply response.
Factors affecting rates of relapse and recurrence
Residual symptoms
Epidemiological and clinical data support the goal of treating depressed patients to wellness or full remission.25 The persistence of residual symptoms after treatment is a sign of a poor prognosis and a primary risk factor for relapse.47,48,49 The impact of level of recovery on the probability of relapse was demonstrated in over 230 patients followed naturalistically for more than 10 years.47 Judd and colleagues reported that patients who had residual symptoms after recovery relapsed 3–5 times faster than those who had recovered fully. In addition, residual symptoms were reported in 19 (32%) of 60 patients who had remitted to below the threshold for major depression.48 This study also found residual symptoms to be strong predictors of early relapse, which occurred in 76% of patients with residual symptoms and 25% of those without.
Early discontinuation of antidepressant therapy
It has been suggested that antidepressants suppress depressive symptoms before acting on the pathophysiology underlying the disorder, during which time patients are vulnerable to a relapse if medication is withdrawn.8,50 Continuing antidepressant therapy for 4–9 months after the remission of acute symptoms has been demonstrated to reduce the likelihood of relapse or recurrence of depression.51,52
A study to assess adherence to guidelines and relapse, using a Medicaid database from 1989 to 1994, followed the progress of 4052 adult patients for between 6 months and 2 years.51 The guidelines were those published by the Agency for Health Care Policy and Research, which suggest acute treatment for 6–8 weeks, followed by 4–9 months of continuation treatment once depressive symptoms have resolved.52 These guidelines also call for switching or adding of antidepressants for patients who do not respond adequately during the acute phase. Less than 30% of the patients in this study received antidepressant treatment that was even minimally consistent with the guidelines, and 24% of patients experienced a relapse or recurrence during their follow-up period. Those patients who continued therapy with their initial antidepressant were the least likely to experience relapse or recurrence, while those who discontinued their antidepressant early were the most likely. Even after adjusting for severity of illness and comorbidities, the continuous use of a single antidepressant from the start of a depressive episode was related to the greatest reduction in probability of relapse or recurrence.51 The indicator of relapse or recurrence in this study was based on paid Medicaid claims, and this may mean that some patients who had a relapse or recurrence would not be counted as such.
Similar results were reported in an analysis of almost 7500 patients in a UK primary care database.23 The patients who continued their initial antidepressant had a significantly lower rate of relapse than those who discontinued antidepressant therapy within 120 days (20% v. 23%, p = 0.04). In this analysis, relapse or recurrence was defined as any reinitiation of antidepressant treatment or an event (i.e., suicide, referral to psychiatrist, admission to hospital or emergency room for mental disorder or electroconvulsive therapy [ECT]) during the 18-months after a 6-month treatment period. Relapse during the first 6 months, a time when the occurrence of relapse is generally high, was not included. It has also been demonstrated that many patients with mood disorders who received treatment for past episodes do not seek or receive care for subsequent ones.53 This may further contribute to significantly underestimated relapse rates in these studies.
In an attempt to prospectively determine the optimal length of therapy for patients with major depression, a long-term, placebo-controlled continuation study was carried out. Almost 400 patients who were treated to remission with fluoxetine were then randomly assigned to receive double-blind treatment in 1 of 4 groups: 50 weeks of placebo, 14 weeks of fluoxetine and then 36 weeks of placebo, 38 weeks of fluoxetine and then 12 weeks of placebo, or 50 weeks of fluoxetine.54 Relapse rates were significantly lower with fluoxetine compared with placebo after 24 total weeks of treatment (fluoxetine 26.4%, placebo 48.6%, p < 0.001) and after 38 total weeks of treatment (fluoxetine 9.0%, placebo 23.2%, p < 0.04). After 62 total weeks of treatment, relapse rates were not significantly different between the groups (fluoxetine 10.7%, placebo 16.2%). However, the study group in the last interval was small because of patient attrition over the year of treatment, and there was not enough statistical power to detect small treatment effects. In agreement with current guidelines, these results demonstrate that patients who are treated acutely with fluoxetine to achieve remission should continue treatment for at least an additional 26 weeks to minimize the risk of relapse.12
Maintenance dose
It is generally accepted that decreasing the effective dose during acute TCA therapy results in increased rates of relapse.11 This also appears to be the case with at least one SSRI. In a small, open-label study of the feasibility of reducing the dose of citalopram for long-term maintenance therapy,55 when responders to citalopram, 40 mg/day, continued with maintenance therapy at a dose of 20 mg/day, recurrence occurred in 50% of patients during the 2-year maintenance period. This study lacked a control group, but suggested that a dose reduction of citalopram during the maintenance phase was linked to a decrease in prophylaxis. Therefore, a full dose of antidepressant is strongly recommended in prophylactic therapy of patients with recurrent major depression. As the clinical cliché states: “The dose that gets you well, keeps you well.”
Antidepressant tolerance
Clinical folklore suggests that tolerance to antidepressants or “breakthrough depression” occurs with all antidepressants. This relapse or recurrence of depression while on antidepressant maintenance therapy has been experienced by patients treated with monoamine oxidase inhibitors, tricyclics and SSRIs; it is reported to occur in 9%–57% of patients.56 Proposed causes of this real or apparent tolerance include pharmacological tolerance, pharmacokinetic changes, unrecognized rapid cycling, prophylactic inefficacy and changes in disease due to drug therapy or independent of drug therapy. Noncompliance is often suggested as a probable cause of loss of efficacy, but reduced therapeutic contact and a loss of nonspecific “placebo effects” may also contribute to this “tachyphalaxis.”
Other factors that have been found to affect relapse and recurrence rates include comorbidities and severity of illness.51 Early onset of depression (< 20 years of age) and greater chronicity have also been associated with a poorer prognosis.57
Prevention of relapse and recurrence
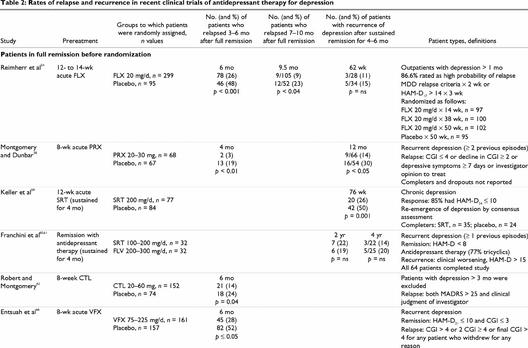
A number of studies have examined the rate of relapse or recurrence in patients who have responded to acute therapy (Table 2). In most studies, patients were required to achieve full remission during acute, open-label treatment to qualify for randomization to the double-blind relapse-prevention phase. In recurrence studies, patients generally must sustain a remission for 6 months before being randomly assigned to receive to the double-blind prevention phase.
Table 2
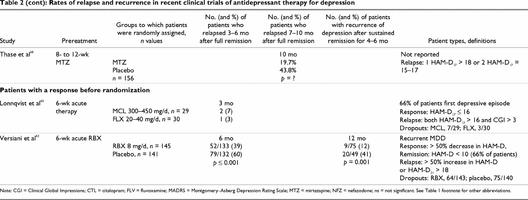
Table 2 continued
SSRIs
Fluoxetine, paroxetine, sertraline and citalopram have demonstrated efficacy in the prevention of relapse. Continuation therapy with fluoxetine significantly decreased relapse rates by about half at 24 and 38 total weeks of therapy compared with placebo.54 The efficacy of paroxetine in preventing relapse and recurrence was demonstrated in a study of 135 patients who had achieved full remission.58 Cumulatively, significantly more patients who continued paroxetine therapy remained in remission after 1 year (84%) than those who were switched to placebo (57%). There is also demonstrated efficacy for sertraline in the prevention of recurrence in 161 patients with chronic major or double depression.59 Over the 76 weeks of follow-up, recurrence was seen in 26% of the sertraline group compared with 50% of the placebo group. In a comparison of fluvoxamine and sertraline maintenance therapy, no significant differences were observed in cumulative recurrence rates at either the 2-year (about 20%) or 4-year follow-up (about 30%).60,61 Continuation therapy with citalopram was also found to significantly lower the rate of relapse at 6 months compared with placebo (14% v. 24%, respectively).62 Relapse rates may have been underestimated in this study, since criteria for relapse were severe and the relapse rate in the placebo group was lower than expected.
Reboxetine
Maintenance therapy with reboxetine (not available in Canada) for the prevention of relapse and recurrence of depression was evaluated in a 1-year study in patients with recurrent major depression.63 Full remission was defined as a HAM-D ≤ 10 and relapse as a ≥ 50% increase in HAM-D or a HAM-D ≥ 18. Relapse rates during the first 6 months of follow-up among patients who had responded to acute reboxetine therapy were significantly lower with reboxetine than with placebo (39% v. 60%, respectively). Patients who were relapse-free at 6 months remained in the trial for an additional 6 months. During this maintenance phase, 41% of patients in the placebo group compared with only 12% in the reboxetine group had experienced a recurrence at 1 year (p ≤ 0.001). Of the patients (n = 237) who were in full remission at the end of the 6 weeks of initial therapy, 48% of the placebo group compared with only 16% of the reboxetine group had relapsed at 1 year.
Nefazodone
Nefazodone demonstrated prevention of relapse of depression in a 1-year placebo substitution trial.64 Patients in full remission after 16 weeks of acute nefazodone therapy were randomly assigned to receive either nefazodone (n = 65) or placebo (n = 66) for a further 36 weeks. Remission was defined as a HAM-D17 < 10 and relapse as HAM-D17 ≥ 18 or discontinuation for lack of efficacy. At 9 months, relapse rates were significantly lower for patients randomly assigned to continue nefazodone treatment than they were for those switched to placebo (1.8% v. 18.3%, respectively, p = 0.009). Relapse rates were 19% with nefazodone and 51% with placebo (p = 0.028) when discontinuations due to lack of efficacy were included.
Moclobemide
In a comparison study, 59 patients who had responded (i.e., HAM-D ≤ 16) to double-blind treatment with 6 weeks of moclobemide or fluoxetine therapy agreed to continue and were randomly assigned to 12 weeks of continuation therapy with moclobemide or fluoxetine.65 No significant differences were seen between the groups. Relapse occurred in 2 patients (7%) in the moclobemide group and 1 (3%) in the fluoxetine group, but 23% of patients in the fluoxetine group and 10% in the moclobemide group dropped out of the study.
Venlafaxine
Venlafaxine demonstrated efficacy in the prevention of relapse in a 6-month, double-blind placebo-substitution trial.66 Patients with recurrent depression who achieved full remission after 8 weeks of acute venlafaxine therapy were randomly assigned to continue venlafaxine (n = 161) or switch to placebo (n = 157). Remission was defined as a HAM-D21 ≤ 10 and Clinical Global Impression Severity of Illness (CGI-S) ≤ 3 and relapse as CGI-S > 4 or 2 consecutive CGI-S ≥ 4 (moderately ill). Cumulative relapse rates at 3 and 6 months were 19% and 28% with venlafaxine compared with 44% and 52% with placebo (p < 0.001). When dropouts for unsatisfactory efficacy were included in survival estimates, cumulative probability of continued efficacy was also significantly higher with venlafaxine (74%) than with placebo (50%) (p = 0.0001).
Venlafaxine has also demonstrated efficacy as long-term maintenance therapy for the prevention of recurrence of depression.67 Patients who had a sustained response for at least 6 months during open-label venlafaxine therapy were randomly assigned to either venlafaxine (100–200 mg/d, n = 106) or placebo (n = 107) for 12 months. Recurrence, defined as CGI-S ≥ 4, HAM-D ≥ 20 or more than 2 HAM-D > 10, occurred in 51% of patients treated with placebo compared with only 20% of patients treated with venlafaxine (p = 0.0001). Significantly more patients in the venlafaxine group (50%) than in the placebo group (24%) completed the 1-year double-blind study.
Mirtazapine
Mirtazapine demonstrated efficacy for the prevention of relapse in a 40-week double-blind placebo-substitution trial.68 Patients who achieved remission after 8–12 weeks of open-label treatment with mirtazapine (n = 156) were randomly assigned to continue therapy or switch to placebo. Remission was defined as HAM-D ≤ 7 and relapse as HAM-D ≥ 18 at 1 visit or between 15 and 17 on 2 consecutive visits. Those patients who continued mirtazapine therapy had a significantly lower relapse rate than those in the placebo group (i.e., 20% v. 44%) after approximately 1 year of therapy.
Relapse and recurrence prevention with other strategies
Other antidepressant strategies have also been examined for their potential in preventing relapse or recurrence. Data suggest that lithium augmentation and continuation ECT may also be effective continuation strategies to prevent relapse in patients that respond to these strategies acutely.
Lithium augmentation
Lithium carbonate has been found to be effective in preventing manic recurrences in patients with bipolar disorder, but its effectiveness in combination with antidepressant drugs in preventing depressive disorders is less clear.69 Lithium augmentation has shown the potential for relapse prevention in a small study in patients with refractory depression. Patients who had responded to acute lithium augmentation during an open 6-week study (n = 30) were randomly assigned to placebo or to continue lithium augmentation for an additional 4 months.70 Antidepressant medication was continued throughout the study, with no significant differences between the 2 groups. Relapses (including 1 suicide) occurred in 7 (47%) of the 15 patients in the placebo group and none of the 14 patients who received lithium augmentation.
However, in a seminal study almost 2 decades earlier, lithium augmentation of antidepressant therapy was reported to be no more effective than antidepressant alone for preventing recurrences. One hundred and fifty patients, who were controlled on the combination of imipramine and lithium carbonate, were randomly assigned to receive either imipramine or lithium carbonate alone, the combination of lithium carbonate and imipramine, or placebo.71 Recurrence of depression occurred in 26% of patients who were taking the combination, 33% taking imipramine alone, 57% taking lithium alone, and 65% of those in the placebo group. However, the treatment effects were significantly related to the severity of the index episode. Also, only 36% of the patients with unipolar depression were judged to be treatment successes in this study.
Electroconvulsive therapy
ECT is an effective antidepressant in the acute, short-term treatment of depression, but its role as continuation or maintenance therapy has been underinvestigated. Continuation ECT (C-ECT) was found to exhibit a sustained prophylactic effect for a year after the index episode in a retrospective review of 33 courses of C-ECT in patients with affective illnesses conducted from an inpatient service between 1985 and 1991.72 The mean intertreatment interval was 10 days, and the average duration of treatment was 10 weeks. The rate of relapse with C-ECT was 33% over 1 year; this was 42% lower than the 95% relapse rate reported for patients maintained on continuation pharmacotherapy before the C-ECT program started. However, in a trial comparing pharmacotherapy or placebo continuation after ECT, at 6-month follow-up, relapse occurred in 65% of the placebo-treated patients, compared with 30% of the imipramine-treated patients and 10% of the paroxetine-treated patients.73
Similar long-term benefits were found in another retrospective, case-controlled study comparing C-ECT with antidepressant therapy to antidepressant therapy alone in patients with a positive response to acute ECT.74 The cumulative probability of continued effectiveness was significantly better with C-ECT compared with antidepressants alone at both 2 years (93% v. 52%, respectively) and 5 years (73% v. 18%, respectively).
Summary
There is evidence that full remission should be the goal of antidepressant therapy; patients with residual symptoms are at an increased risk of relapse and recurrence. Given that the greatest chance of achieving full remission occurs early in the course of treatment, initial antidepressant strategies should be those that have the greatest therapeutic potential.
In patients who achieve full remission, continued antidepressant therapy has clearly been shown to reduce the probability of relapse and recurrence by about half compared with placebo. Therefore, once a patient achieves remission, it is important to continue the same antidepressant for at least 6–12 months, and considerably longer for many patients, to prevent relapse or recurrence. Medication should continue at the dose that was initially effective because using low-dose maintenance therapy appears to decrease the protective benefits.
Footnotes
Competing interests: Dr. Kennedy has received research support from Pfizer, Astra-Zeneca, Organon and Boehringer Ingelheim; is on the speakers' bureaus of Lundbeck, Organon, Wyeth-Ayerst and GlaxoSmithKline; and serves on advisory boards for Pfizer, the Lundbeck Foundation, Eli Lilly, GlaxoSmithKline and Servier. Dr. McIntyre has received research support from Janssen-Ortho, Eli Lilly, GlaxoSmithKline, the Centre for Addiction and Mental Health Foundation and Wyeth-Ayerst Canada; is on the speakers' bureaus of GlaxoSmithKline, Lundbeck, Wyeth-Ayerst Canada, Organon, Janssen-Ortho, Eli Lilly, Pfizer, Astra-Zeneca Canada and Boehringer Ingelheim; and is a consultant for Bristol Myers Squibb, GlaxoSmithKline, Janssen-Ortho, Astra-Zeneca Canada and Wyeth-Ayerst Canada. Dr. Fallu has received research support from Bristol-Myers Squibb, Astra-Zeneca, Janssen-Ortho, GlaxoSmithKline, Lundbeck, Pfizer, Eli Lilly and Merck-Frosst; and has received speaker's fees from Wyeth-Ayerst, Organon, Servier, Bristol-Myers Squibb, Astra-Zeneca, Janssen-Ortho, GlaxoSmithKline and Lundbeck. Dr. Lam has received research support from Astra-Zeneca Canada, Eli Lilly Canada, Merck-Frosst Canada, Roche Canada and Servier Canada; and is on the speakers' bureaus of Cyberonics, Eli Lilly Canada, GlaxoSmithKline, Litebook, Lundbeck Canada, Organon Canada, Pfizer Canada and Wyeth-Ayerst Canada.
Correspondence to: Dr. Sidney Kennedy, University Health Network, 200 Elizabeth St., 8th Flr, Eaton Wing, Rm. 222, Toronto ON M5G 2C4; fax 416 340-4198; sidney.kennedy@uhn.on.ca
Submitted Jun. 11, 2001 Revised May 9, 2002 Accepted May 14, 2002
References
- 1.Judd LL. The clinical course of unipolar major depressive disorders. Arch Gen Psychiatry 1997;54:989-991. [DOI] [PubMed]
- 2.Angst J. How recurrent and predictable is depressive illness. In: Montgomery SA, Rouillon F, editors. Long-term treatment of depression. New York: John Wiley and Sons; 1992. p. 1-13.
- 3.Keller MD, Lavori P, Mueller T, Endicott J, Coryell W, Hirschfeld RM, et al. Time to recovery, chronicity and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry 1992;49:809-16. [DOI] [PubMed]
- 4.Surtess P, Barkely C. Future imperfect: the long-term outcome of depression. Br J Psychiatry 1994;164:327-41. [DOI] [PubMed]
- 5.Kamlet MS, Paul N, Greenhouse J, Kupfer D, Frank E, Wade M. Cost utility of maintenance treatment for recurrent depression. Control Clin Trials 1995;16:17-40. [DOI] [PubMed]
- 6.Angst J. The course of affective disorders. Psychopathology 1986;19(Suppl 2):47-52. [DOI] [PubMed]
- 7.Solomon DA, Keller M, Leon AC, Mueller TI, Shea MT, Warshaw M, et al. Recovery from major depression: a 10-year prospective follow-up across multiple episodes. Arch Gen Psychiatry 1997;54:1001-6. [DOI] [PubMed]
- 8.Prien RF, Kupfer DJ. Continuation drug therapy for major depressive episodes: how long should it be maintained? Am J Psychiatry 1986;143:18-23. [DOI] [PubMed]
- 9.Paykel ES, Priest RG. Recognition and management of depression in general practice: a consensus statement. BMJ 1992; 305:1198-202. [DOI] [PMC free article] [PubMed]
- 10.British Association for Psychopharmacology. Guidelines for treating depressive illness with antidepressants. J Psychopharmacol 1993;7:19-23. [DOI] [PubMed]
- 11.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry 1991;48(9):851-5. [DOI] [PubMed]
- 12.Depression Guideline Panel. Clinical Practice Guideline Number 5: Depression in primary care. Vol 2: Treatment of major depression. Rockville (MD): US Department of Health and Human Services, Agency for Health Care Policy and Research; 1993. AHCPR publication 93-0551.
- 13.Thase ME, Sullivan LR. Relapse and recurrence of depression: a practical approach for prevention. CNS Drugs 1995;4:261-77.
- 14.Kupfer DJ. Management of recurrent depression. J Clin Psychiatry 1993;54(2):29-33. [PubMed]
- 15.Nierenberg AA, McLean NE, Alpert JE, Worthington JJ, Rosenbaum JF, Fava M. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am J Psychiatry 1995;152:1500-3. [DOI] [PubMed]
- 16.Nierenberg AA, Farabaugh AH, Alpert JE, Gordon J, Worthington JJ, Rosenbaum JF, et al. Timing of onset of antidepressant response with fluoxetine treatment. Am J Psychiatry 2000;157:1423-8. [DOI] [PubMed]
- 17.Kennedy SH, Eisfeld BS, Meyer JH, Bagby RM. Antidepressants in clinical practice: limitations of assessment methods and drug response. Hum Psychopharmacol Clin Exp 2001;16:105-14. [DOI] [PubMed]
- 18.Entsuah R, Derivan A, Kikta D. Early onset of antidepressant action of venlafaxine: pattern analysis in intent-to-treat patients. Clin Ther 1998;20:517-26. [DOI] [PubMed]
- 19.Paykel ES. Remission and residual symptomatology in major depression. Psychopathology 1998;31:5-14. [DOI] [PubMed]
- 20.O'Leary D, Costello F, Gormley N, Webb M. Remission onset and relapse in depression. An 18-month prospective study of course for 100 first-admission patients. J Affect Disord 2000;57:159-71. [DOI] [PubMed]
- 21.Canadian Network for Mood and Anxiety Treatments. Guidelines for the diagnosis and pharmacological treatment of depression. 1st ed., rev. Toronto: CANMAT; 1999.
- 22.Mischoulon D, Nierenberg AA, Kizilbash L, Rosenbaum JF, Fava M. Strategies for managing depression refractory to selective serotonin reuptake inhibitor treatment: a survey of clinicians. Can J Psychiatry 2000;45:476-81. [DOI] [PubMed]
- 23.Claxton AJ, Li Z, McKendrick J. Selective serotonin reuptake inhibitor treatment in the UK: risk of relapse or recurrence of depression. Br J Psychiatry 2000;177:163-8. [DOI] [PubMed]
- 24.Fava G, Grandi S, Zielezny M, Canestrari R, Morphy MA. Cognitive behavioral treatment of residual symptoms primary major depressive disorder. Am J Psychiatry 1994;151:1295-9. [DOI] [PubMed]
- 25.Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry 1999;60(Suppl 22):7-11. [PubMed]
- 26.Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord 2000;58:19-36. [DOI] [PubMed]
- 27.Barbui C, Hotopf M. Amitriptyline v. the rest: still the leading antidepressant after 40 years of randomised controlled trials. Br J Psychiatry 2001;178:129-44. [DOI] [PubMed]
- 28.Kavoussi RJ, Segraves RT, Hughes AR, Ascher JA, Johnston JA. Double-blind comparison of bupropion sustained release and sertraline in depressed outpatients. J Clin Psychiatry 1997; 58:532-7. [DOI] [PubMed]
- 29.Weihs KL, Settle EC Jr., Batey SR, Houser TL, Donahue RMJ, Ascher JA. Bupropion sustained release versus paroxetine for the treatment of depression in the elderly. J Clin Psychiatry 2000;61:196-202. [DOI] [PubMed]
- 30.Katona C, Bercoff E, Chiu E, Tack P, Versiani M, Woelk H. Reboxetine versus imipramine in the treatment of elderly patients with depressive disorders: a double-blind randomized trial. J Affect Disord 1999;55:203-213. [DOI] [PubMed]
- 31.Kent JM. SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet 2000;355:911-8. [DOI] [PubMed]
- 32.Cohn CK, Robinson DS, Roberts DL, Schwiderski UE, O'Brien K, Ieni JR. Responders to antidepressant drug treatment: a study comparing nefazodone, imipramine, and placebo in patients with major depression. J Clin Psychiatry 1996;57 (Suppl 2):15-8. [PubMed]
- 33.Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, et al. A comparison of nefazodone, the cognitive behavioural-analysis system of psychotherapy and their combination therapy for the treatment of chronic depression. N Engl J Med 2000;342:1462-70. [DOI] [PubMed]
- 34.Lapierre YD, Joffe R, McKenna, Bland R, Kennedy S, Ingram P, et al. Moclobemide versus fluoxetine in the treatment of major depressive disorder in adults. J Psychiatry Neurosci 1997;22:118-26. [PMC free article] [PubMed]
- 35.Danish University Antidepressant Group. Moclobemide: a reversible MAO-A inhibitor showing weaker antidepressant effect than clomipramine in a controlled multicentre study. J Affect Disord 1993;28:105-16. [DOI] [PubMed]
- 36.Sogaard J, Lane R, Latimer P, Behnke K, Christiansen PE, Nielsen B, et al. A 12-week study comparing moclobemide and sertraline in the treatment of outpatients with atypical depression. J Psychopharm 1999;13(4):486-414. [DOI] [PubMed]
- 37.Wheatley DP, van Moffaert M, Timmerman L, Kremer CM, and the Mirtazapine–Fluoxetine Study Group. Mirtazapine: efficacy and tolerability in comparison with fluoxetine in patients with moderate to severe depressive disorder. J Clin Psychiatry 1998;59:306-12. [PubMed]
- 38.Stahl S, Zivkov M, Reimitz PE, Panagides J, Hoff W. Meta-analysis of randomized, double-blind, placebo-controlled, efficacy and safety studies of mirtazapine versus amitriptyline in major depression. Acta Psychiatr Scand 1997;96(Suppl 391):22-30. [DOI] [PubMed]
- 39.Mehtonen OP, Sogaard J, Roponen R, Behnke K. Randomized, double-blind comparison of venlafaxine and sertraline in outpatients with major depressive disorder. J Clin Psychiatry 2000; 61:95-100. [DOI] [PubMed]
- 40.Poirier MF, Boyer P. Venlafaxine and paroxetine in treatment resistant depression. Br J Psychiatry 1999;175:12-6. [DOI] [PubMed]
- 41.Ballus C, Quiros G, de Flores T, de la Torre J, Palao D, Rojo L, et al. The efficacy and tolerability of venlafaxine and paroxetine in outpatients with depressive disorder or dysthymia. Int Clin Psychopharmacol 2000;15:43-8. [DOI] [PubMed]
- 42.Rudolph RL, Feiger AD. A double-blind, randomized, placebo-controlled trial of once-daily venlafaxine extended release (XR) and fluoxetine for the treatment of depression. J Affect Disord 1999;56:171-81. [DOI] [PubMed]
- 43.Lecrubier Y, Bourin M, Moon CAL, Schifano F, Blanchard C, Danjour Ph, et al. Efficacy of venlafaxine in depressive illness in general practice. Acta Psychiatr Scand 1997;95:485-93. [DOI] [PubMed]
- 44.Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry 2001;178:234-41. [DOI] [PubMed]
- 45.Stahl SM. Are two antidepressant mechanisms better than one? J Clin Psychiatry 1997;58:339-40. [DOI] [PubMed]
- 46.Freemantle N, Anderson IM, Young P. Predictive value of pharmacological activity for the relative efficacy of antidepressant drugs. Meta-regression analysis. Br J Psychiatry 2000;177:292-302. [DOI] [PubMed]
- 47.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord 1998;50:97-108. [DOI] [PubMed]
- 48.Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med 1995;25:1171-80. [DOI] [PubMed]
- 49.Lin EH, Katon WJ, VonKorff M, Russo JE, Simon GE, Bush TM, et al. Relapse of depression in primary care: rate and clinical predictors. Arch Fam Med 1998:7:443-9. [DOI] [PubMed]
- 50.Post F. Imipramine in depression [letter]. BMJ 1959;2:1252.
- 51.Melfi CA, Chawla AJ, Croghan TW, Hanna MP, Kennedy S, Sredl K. The effects of adherence to antidepressant treatment guidelines on relapse and recurrence of depression. Arch Gen Psychiatry 1998;55:1128-32. [DOI] [PubMed]
- 52.Depression Guidelines Panel. Treatment of major depression. In: Depression in primary care. Vol 2. Clinical practice guideline No. 5. Rockville (MD): US Dept of Health and Human Services, Public Health Service, and Agency for Health Care Policy and Research; 1993. AHCPR publication 93-0550.
- 53.Kessler R, McGonagle K, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III psychiatric disorders in the United States. Arch Gen Psychiatry 1994;51:8-19. [DOI] [PubMed]
- 54.Reimherr FW, Amsterdam JD, Quitkin FM, Rosenbaum JF, Fava M, Zajecka J, et al. Optimal length of continuation therapy in depression: a prospective assessment during long-term fluoxetine treatment. Am J Psychiatry 1998;155:1247-53. [DOI] [PubMed]
- 55.Franchini L, Zanardi R, Gasperini M, Smeraldi E. Two-year maintenance treatment with citalopram, 20 mg, in unipolar subjects with high recurrence rate. J Clin Psychiatry 1999;60:861-5. [DOI] [PubMed]
- 56.Bryne S, Rothschild A. Loss of antidepressant efficacy during maintenance therapy: possible mechanisms and treatments. J Clin Psychiatry 1998;59:279-287. [DOI] [PubMed]
- 57.McGrath PJ, Stewart JW, Petkova E, Quitkin FM, Amsterdam JD, Fawcett J, et al. Predictors of relapse during fluoxetine continuation or maintenance treatment of major depression. J Clin Psychiatry 2000;61:518-24. [DOI] [PubMed]
- 58.Montgomery SA, Dunbar G. Paroxetine is better than placebo in relapse prevention and the prophylaxis of recurrent depression. Int Clin Psychopharmacol 1993;8:189-95. [DOI] [PubMed]
- 59.Keller MB, Kocsis JH, Thase ME, Gelenberg AJ, Rush AJ, Koran L, et al. Maintenance phase efficacy of sertraline for chronic depression: a randomized controlled trial. JAMA 1998; 280:1665-72. [DOI] [PubMed]
- 60.Franchini L, Gasperini M, Zanardi R, Smeraldi E. Four-year follow-up study of sertraline and fluvoxamine in long-term treatment of unipolar subjects with high recurrence rate. J Affect Disord 2000;58:233-6. [DOI] [PubMed]
- 61.Franchini L, Gasperini M, Perez J, Smeraldi E, Zanardi R. A double-blind study of long-term treatment with sertraline or fluvoxamine for prevention of highly recurrent unipolar depression. J Clin Psychiatry 1997;58:104-7. [DOI] [PubMed]
- 62.Robert P, Montgomery SA. Citalopram in doses of 20–60 mg is effective in depression relapse prevention: a placebo-controlled 6-month study. Int Clin Psychopharmacol 1995; 10(Suppl 1):29-35. [PubMed]
- 63.Versiani M, Mehilane L, Gaszner P, Arnaud-Castiglioni R. Reboxetine, a unique selective NRI, prevents relapse and recurrence in long-term treatment of major depressive disorder. J Clin Psychiatry 1999;60:400-6. [DOI] [PubMed]
- 64.Feiger AD, Bielski RJ, Bremner J, Heiser JF, Trivedi M, Wilcox CS, et al. Double-blind, placebo-substitution study of nefazodone in the prevention of relapse during continuation treatment of outpatients with major depression. Int Clin Psychopharmacol 1999;14:19-28. [DOI] [PubMed]
- 65.Lonnqvist J, Sihvo S, Syvalahti E, Sintonen H, Kiviruusu O, Pitkanen H. Moclobemide and fluoxetine in the prevention of relapses following acute treatment of depression. Acta Psychiatr Scand 1995;91:189-94. [DOI] [PubMed]
- 66.Entsuah R, Agular L, Lei D, Rudolph R. Venlafaxine XR is superior to placebo in relapse prevention [poster no PO-15-012]. Presented at the European College of Neuropsychopharmacology (ECNP) meeting. September 2000. Munich, Germany.
- 67.Hackett D, Agular L, Rudolph R, et al. Venlafaxine prevents recurrence of depression. Presented at the European College of Neuropsychopharmacology (ECNP) meeting. September 2000. Munich, Germany.
- 68.Thase ME, Nierenberg AA, Keller MB, Panagides J; The Relapse Prevention Study Group. Efficacy of mirtazapine for prevention of depressive relapse: a placebo-controlled double-blind trial of recently remitted high-risk patients. J Clin Psychiatry 2001;62(10):782-8. [DOI] [PubMed]
- 69.Prien RF. Long-term prophylactic pharmacologic treatment of bipolar illness. In: Grinspoon L, editor. Psychiatry update: the American Psychiatric Association annual review. Vol. 2. Washington: American Psychiatric Press Inc.; 1983. p. 303-18.
- 70.Bauer M, Bschor T, Kunz D, Berghofer A, Strohle A, Muller-Oerlinghausen B. Double-blind, placebo-controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. Am J Psychiatry 2000;157:1429-35. [DOI] [PubMed]
- 71.Prien RF, Kupfer DJ, Mansky PA, Small JG, Tuason VB, Voss CB, et al. Drug therapy in the prevention of recurrences in unipolar and bipolar affective disorders. Report of the NIMH Collaborative Study Group comparing lithium carbonate, imipramine, and a lithium carbonate – imipramine combination. Arch Gen Psychiatry 1984;41:1096-104. [DOI] [PubMed]
- 72.Petrides G, Dhossche D, Fink M, Francis A. Continuation ECT: relapse prevention in affective disorders. Convuls Ther 1994; 10:189-94. [PubMed]
- 73.Lauritzen L, Odgaard K, Clemmesen L, Lunde M, Ohrstrom J, Black C, et al. Relapse prevention by means of paroxetine in ECT-treated patients with major depression: a comparison with imipramine and placebo in medium-term continuation therapy. Acta Psychiatr Scand 1996;94:241-51. [DOI] [PubMed]
- 74.Gagné GG Jr, Furman MJ, Carpenter LL, Price LH. Efficacy of continuation ECT and antidepressant drugs compared to long-term antidepressants alone in depressed patients. Am J Psychiatry 2000;157:1960-5. [DOI] [PubMed]