Abstract
Objective
To report computed tomographic (CT) scan ratings of various aspects of brain morphology of a large representative sample of patients with a first episode of schizophrenic psychosis and to compare these ratings with those from a previously reported sample of patients with chronic schizophrenia.
Methods
A brain CT scan was performed on 114 patients with a diagnosis of first episode of schizophrenia or schizophreniform psychosis. Ratings on sulcal and ventricular enlargement and sylvian fissure were obtained using the Computed Tomographic Rating Scale for Schizophrenia. The influence of age, sex, age of onset, duration of illness and clinical psychopathology on CT ratings was assessed using bivariate correlations and multiple regression analyses. The CT ratings were also compared with those from a sample of patients with chronic schizophrenia.
Results
First-episode patients showed a modest enlargement of sulci and ventricles and a reversed asymmetry of the sylvian fissure. Age was the only independent predictor of these regional changes. Clinical symptoms, sex or duration of untreated psychosis showed no relation to CT ratings. A comparison of first-episode patients with chronically ill patients, with the effect of age covaried, revealed the sylvian fissure was significantly larger (right and left sides) in the chronically ill patients.
Conclusions
Patients with a first episode of schizophrenic psychosis showed evidence of morphological changes generally associated with chronic schizophrenia. Such changes are not likely related to sex, clinical symptoms or duration of untreated psychosis, but are influenced by age. Changes in the ventricles and sulcal size are unlikely to be progressive, suggesting a neurodevelopmental origin, whereas changes in the area of the sylvian fissure may be of a more degenerative nature.
Medical subject headings: antipsychotic agents; brain; cerebral cortex; cerebral ventricles; chronic disease; schizophrenia; tomography, x-ray computed
Abstract
Objectif
Faire rapport d'évaluations tomodensitométriques de divers aspects de la morphologie du cerveau d'un important échantillon représentatif de patients qui ont été victimes d'un premier épisode de psychose schizophrénique et comparer ces évaluations à celles d'un échantillon de patients atteints de schizophrénie chronique qui ont déjà fait l'objet de rapports.
Méthodes
On a soumis à une tomodensitométrie du cerveau 114 patients chez lesquels on avait diagnostiqué un premier épisode de schizophrénie ou de psychose schizophréniforme. On a évalué les élargissements des sillons et des ventricules, ainsi que du sillon latéral, au moyen de l'échelle d'évaluation tomodensitométrique de la schizophrénie. On a évalué l'influence de l'âge, du sexe, de l'âge à l'apparition de la maladie, de sa durée et de la psychopathologie clinique sur les évaluations tomodensitométriques au moyen de corrélations simples et d'analyses à régressions multiples. On a aussi comparé les évaluations tomodensitométriques à celles d'un échantillon de patients atteints de schizophrénie chronique.
Résultats
On a constaté, chez les patients victimes d'un premier épisode, un léger élargissement des sillons et des ventricules, et une asymétrie inverse du sillon latéral. L'âge était le seul prédicteur indépendant de ces changements régionaux. Les symptômes cliniques, le sexe ou la durée de la psychose non traitée n'ont révélé aucun lien avec les évaluations tomodensitométriques. Une comparaison entre les patients victimes d'un premier épisode et des patients atteints de la maladie chronique, où l'effet de l'âge a constitué une des deux variables, a révélé que le sillon latéral était beaucoup plus large (côtés droit et gauche) chez les patients atteints de la maladie chronique.
Conclusions
Les patients victimes d'un premier épisode de psychose schizophrénique montraient des signes de changements morphologiques généralement associés à la schizophrénie chronique. Ces changements ne sont probablement pas reliés au sexe, aux symptômes cliniques ou à la durée de la psychose non traitée, mais l'âge est un facteur. Il est peu probable que les changements de dimensions des ventricules et des sillons soient progressifs, ce qui indique une origine neurodéveloppementale, tandis que les changements dans la région du sillon latéral peuvent être de nature plus dégénérative.
Introduction
Despite significant support for abnormal brain morphology in individuals with schizophrenia, the precise nature of the abnormalities remains unclear. The most consistent finding to date from computed tomography (CT) and magnetic resonance imaging (MRI) studies has been slightly enlarged lateral ventricles, possibly indicative of volumetric reduction of grey matter in the frontal and temporal regions, although the amygdala and hippocampus have also been implicated.1 Other, less replicated findings include increased size of third ventricle,2 cortical sulcal enlargement,3,4,5 focal reduction in the size of frontal lobes6,7 and involvement of cerebellum8,9 and cortical–subcortical–cerebellar circuitry.10 Reverse asymmetry of the sylvian fissure has also been observed on CT scans of patients with familial schizophrenia.11
The equivocal results regarding the precise abnormalities of brain morphology in patients with schizophrenia are likely influenced by factors such as sex,12,13,14,15,16 patient's age,17,18,19 age of onset of psychosis,17 clinical symptoms,1,5,20 length21 and stage of illness2 and prolonged exposure to neuroleptics.22
Most studies have involved patients with a history of multiple hospital admissions and many years of neuroleptic treatment. The long duration of the disease process and neuroleptic use may have an impact on volumetric measures of specific brain structures.21,22 Several recent studies have examined brain structural change in patients who experience a first episode of schizophrenia using MRI23,24,25,26 or CT.18,27 MRI studies have generally reported an enlargement of ventricular size,24 an involvement of the corpus callosum and limbic structures25 and smaller left hippocampal volumes. CT studies have reported enlarged cortical sulci21 and enlarged ventricles.27
Although MRI provides relatively precise information on volumetric measurements of specific regions of interest, the patient samples in studies of first-episode schizophrenic psychosis are generally small (range 10–50) and selected mostly from hospital inpatients. However, CT studies can be conducted on larger samples of most new cases of first-episode psychosis within a defined population base.2,28 Results of such studies show an enlargement of the third ventricle in patients who receive a diagnosis of schizophrenia, but not in those with other psychotic disorders,2 and a significantly higher ventricular brain ratio (lateral ventricles) unrelated to any clinical symptoms.28 In the latter study by Iacono et al,28 patients with other psychotic disorders were not included. However, neither study2,28 examined the effect of duration of illness before the first treatment was initiated or the independent effect of age and age of onset.
If morphological changes in the brain are a result of a neurodevelopmental process, such changes should be present at the time of the first episode of the illness and not be progressive. On the other hand, a degenerative process concurrent with the onset of psychosis would involve a progression of atrophic changes after the onset of the illness.
We report results of the CT evaluations of a large community sample of patients who were assessed for their first episode of schizophrenia or schizophreniform psychosis and compare these results with a sample of patients who had been previously treated for schizophrenia. The objectives of this study were:
· to examine the extent of brain changes present in the early stages of the illness before significant exposure to antipsychotic treatment and compare this with brain changes seen in patients who had been treated for several years in the same treatment program and
· to assess the influence of age, sex, age of onset of symptoms and duration of illness on the level of cerebral atrophy ratings in first-episode patients.
It was hypothesized that:
· there would be no significant difference on any of the CT scan measures of cerebral atrophy between recently diagnosed first-episode patients who meet diagnostic criteria for schizophrenia or schizophreniform psychosis and patients treated for several years,
· first-episode patients with an early age of onset (i.e., children or adolescents under the age of 18 years) would show higher levels of cerebral atrophy than patients with a later age of onset (i.e., adults, 18 years and older) after controlling for the effect of age at the time of the scan and
· among first-episode patients, there would be no sex difference in the level of cerebral atrophy after controlling for age.
Methods
The data for this study were obtained from a larger evaluation study of an early intervention program at a teaching general hospital for patients with psychoses. Most of first-episode cases of schizophrenia and related disorders in this catchment area are treated in this program; it is the only first-episode psychosis program in this jurisdiction in a system where there is no parallel general hospital or a private health care facility. Patients received their initial assessment and treatment either as outpatients or inpatients (40% and 60%, respectively).
Over a 4-year period, a total of 163 patients presented for treatment of a first episode of psychosis; 119 met Diagnostic and Statistical Manual of Mental Disorders, fourth edition, (DSM-IV) criteria for schizophrenia or schizophreniform psychosis. Patients with affective psychosis, schizoaffective disorder and substance-induced psychosis were excluded, leaving 114 patients (79 males, 35 females) who agreed to participate in the assessment and treatment of their first episode of psychosis and signed an informed consent form. The informed consent was provided to indicate patients' agreement to complete a set of clinical assessments and investigations (including a CT scan) and to participate in treatment after the patient had been judged competent to consent to treatment. Patients who were initially deemed incompetent to consent to treatment did not sign their consent until they were deemed competent to do so by their clinicians. This delayed the completion of a CT scan for an average of 4.3 months for 31 (27%) patients. Five patients refused to sign consent or participate in further assessment and treatment.
Clinical assessment and treatment data, including investigations, were collected as part of an evaluation of early intervention in schizophrenia. These data are considered part of clinical care, and as such, approval had been obtained from the institutional research ethics board to collect them. A separate approval was obtained for use of these data for the purposes of other studies.
Brain morphology ratings
As part of the assessment protocol, CT scans were performed on a Picker PQ-2000 scanner as soon after a patient's contact with psychiatric services as was clinically feasible. The scanner bore a tilt with a variation of 0°–23° parallel to frontal bone plane. The degree of tilt was noted before rating each scan. Slice thickness was 8 mm. CT images were first reviewed by a neuroradiologist to exclude the presence of any structural findings such as a brain tumour.
Brain atrophy scores were obtained using the CT Rating Scale for Schizophrenia (CTRSS).29 The scale was designed to assess different regions of the brain that are likely to be of particular importance in schizophrenia, as well as any enlargement of sulci and ventricles. Ratings obtained for first-episode patients were compared with similarly obtained (i.e., CTRSS) ratings for a sample of patients with schizophrenia who had been treated for several years, had met diagnostic criteria for schizophrenia (according to the DSM-III-R) and had CT scans on the same scanner using identical methods.
Areas rated
The following 13 areas of interest were assessed: lateral ventricle temporal horns, frontal horns and body; third ventricle; suprasellar cistern; temporal midbrain cistern; sylvian fissure; temporal lobe sulci; parietal sulci; lateral frontal sulci; medial frontal sulci; trigone; and cerebellum. All bilateral structures were rated separately on the right and left sides.
Photographic anchor points, provided by the authors of the scale, consist of 1–3 slices for each feature to be rated in the range of 1–7 points. The standards were developed from photographs of brain scans of a range of individuals, from young normal subjects to elderly patients with schizophrenia. The CTRSS method of rating CT scans is based on those developed for the study of dementia.30,31 The ratings have been shown to correlate highly with ventricle–brain ratios, sulcal and ventricular volumes and cross-sectional areas. A detailed explanation of this method of assessing CT scans is described by Smith et al.29
Mean composite ratings for ventricles and sulci were calculated as follows:
· total ventricular rating = sum of scores (body + frontal + temporal horns of lateral ventricles + third ventricle),
· total cortical sulci rating = sum of scores (medial frontal + lateral frontal + parietal + temporal sulci).
CT scans were rated by 2 authors (C.M. and M.L.) using the CTRSS, after they received training under the supervision of an experienced neuroradiologist (L.A.). Inter-rater reliability was established between the raters and between the raters and the neuroradiologist. The raters were blind to the patients' demographic and symptom status but, obviously, not to diagnosis.
Clinical ratings
The Scale for Assessment of Positive Symptoms (SAPS)32 and Scale for Assessment of Negative Symptoms (SANS)33 were used by psychiatrists to assess the severity of symptoms upon admission to the program. After the initial clinical assessment and as soon as the patient could tolerate the procedure, CT scan images were obtained. Seventy-three percent of patients received a CT scan before any antipsychotic medication was administered; the remainder had received enough medication to stabilize their symptoms before CT scans were performed (avg. length of treatment 4.3 mo).
To provide a measure of total symptom severity, total SAPS and SANS scores were also calculated by adding individual item scores and excluding global scores. Syndrome scores were calculated for 3 clinical syndromes (i.e., reality distortion, disorganization, psychomotor poverty) using scores on individual items on the SANS and SAPS (based on a previous factor analytic study).34
Demographic data were obtained for each patient as part of their initial assessment at the time of admission to the program. Age of onset of psychotic symptoms and duration of illness (time between onset of any psychotic symptoms and time of the scan) were derived from a detailed procedure using a modified version of the Interview for Retrospective Assessment of Onset of Schizophrenia.35
Data analysis
For all first-episode patients, CT scan ratings for the left and right sides of all bilateral structures (including sylvian fissure) were compared using a paired sample t-test. CT ratings were compared between male and female subjects using independent group t-tests after controlling for age. Comparisons between medicated and unmedicated patients were also carried out. The effects of age at the time of the scan, age of onset of psychosis and duration of illness on the CT scan ratings were also explored, first by using bivariate correlations and then by multiple regression analysis. The CT scan rating variables were also correlated with the clinical syndrome ratings. To reduce type 1 errors from multiple comparisons, we chose to consider differences and associations to be significant at the 1% level (i.e., p < 0.01).
All ratings for first-episode patients were contrasted with ratings for a sample of patients16 with a diagnosis of schizophrenia who had been treated and whose CT scans were also rated with the CTRSS using an analysis of covariance, controlling for the effect of age. The 2 groups of patients were also compared on the level of current symptoms, sex, marital status and level of education. Similar analyses were repeated for only those patients who met diagnostic criteria for schizophrenia (DSM-III-R).
Results
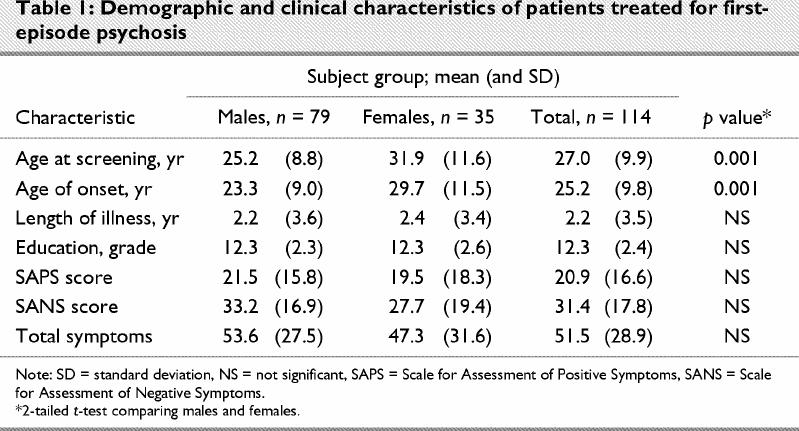
Demographic and clinical data for the sample are presented in Table 1. As would be expected from previous research, onset of psychosis occurred at an earlier age for male patients (p < 0.001), and males were younger at the time of the assessment than female patients (p < 0.001). There were no significant sex differences on the symptom scores, duration of untreated psychosis or any of the other demographic variables we assessed.
Table 1
CT ratings for first-episode patients
The intraclass correlation coefficient (ICC) for the 2 raters for the composite CT scan ratings (on 20 scans) was 0.83 (standard deviation [SD] 0.09) for the ventricles and 0.71 (SD 0.37) for the sulci. The level of agreement between the 2 raters on ventricular ratings varied between high for the temporal horn (ICC 0.85) and body of the lateral ventricle (ICC 0.80) to low for the third ventricle (ICC 0.45). Agreement on ratings of sulci was relatively higher for sylvian fissure (ICC 0.67) but lower for frontal sulci (ICC 0.53). Inter-rater reliability scores (i.e., ICCs) between the raters and the neuroradiologist were also high for the ventricles (0.86, SD 0.08) and sulci (0.70, SD 0.12).
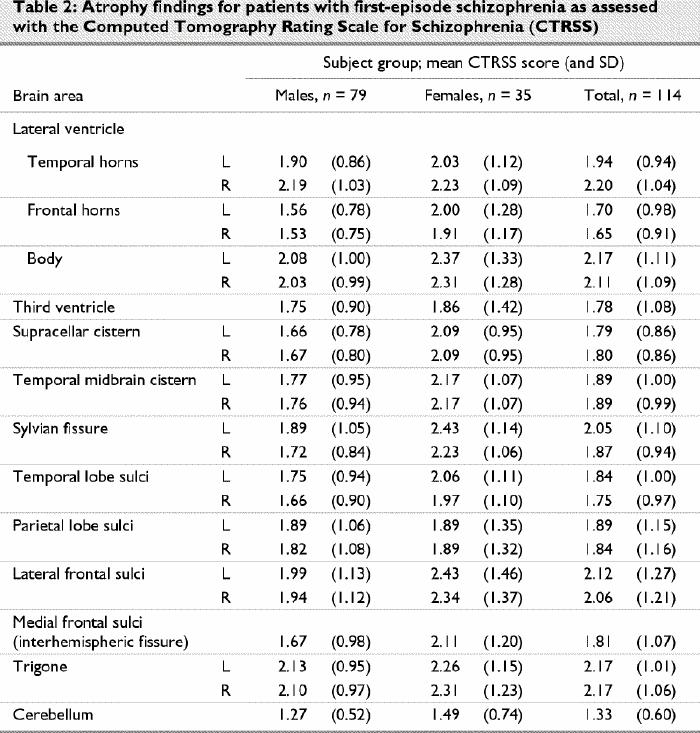
Table 2 shows the mean aggregate as well as left and right side ratings (for all bilateral areas rated) for all 13 brain regions. A comparison of ratings of right- and left-sided structures showed a significantly larger sylvian fissure on the left side than on the right (mean 2.05 v. 1.87, p < 0.001) and larger temporal horns on the right side than on the left (mean 2.20 v. 1.94, p < 0.001). Right–left differences were observed in both male and female patients but were not significant for any of the structures (p > 0.01). For further analysis of ratings of ventricles and sulci, mean composite ratings for right and left sides were used. Composite CT ratings for male and female patients were not significantly different after the effect of age was controlled for.
Table 2
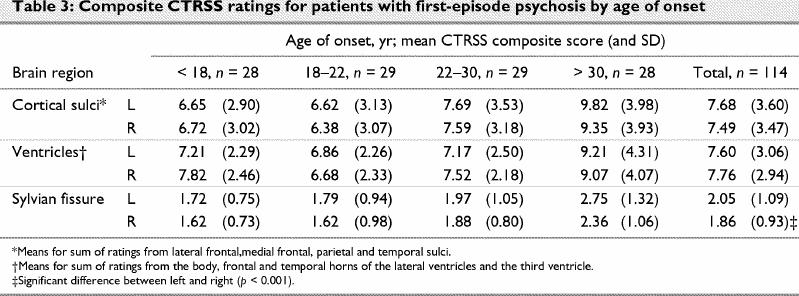
Composite ratings for ventricles and sulci showed mild enlargement, with total ratings of 7.49–7.76 on a possible range of 4–28 (Table 3). Sylvian fissure ratings were examined separate from the other sulci ratings. Comparisons of ratings of ventricular and sulcal enlargement and right and left sylvian fissure for first-episode patients who met the DSM-III-R criteria for schizophrenia (illness duration > 6 mo, n = 64) with those for patients who had been ill for less than 6 months (schizophreniform disorder, n = 50) failed to reveal any significant differences. DSM-III-R criteria were used so that further comparisons could be made with a sample of patients with chronic schizophrenia on whom results have been reported previously.16 A similar comparison of patients who had and those who had not received neuroleptic medication also failed to reveal any significant differences in CT ratings.
Table 3
Age, age of onset and CT ratings
Bivariate correlations between age, age of onset, duration of illness and CT ratings revealed a significant relation between all CT variables and age (r = 0.32–0.45, p < 0.01–0.001) as well as age of onset (r = 0.30–0.43, p < 0.01–0.001). Age and age of onset were, however, highly correlated with one another (r = 0.90). No relations were seen between CT scan measures and length of illness.
Given the significant sex differences on age and age of onset, the data were further examined for an independent effect of each of age, sex and length of illness. Age and age of onset being highly correlated, only age, sex and length of illness were entered in a multiple regression analysis, with total ratings on ventricles and sulci and right and left sylvian fissure ratings as the dependent variables. Age was the only significant independent variable retained in the regression model. Older patients showed greater evidence of atrophy.
To further explore the relation between age, age of onset and CT ratings, the sample was split into 4 subgroups on the basis of age of onset (i.e., age < 18 yr, n = 28; age 18–22 yr, n = 29; age 23–30 yr, n = 29; age > 30 yr, n = 28). A univariate analysis of covariance with age as the covariate failed to show any significant between-group differences (Table 3). These analyses were repeated for the right and left sides separately with similar results. Age, therefore, appears to influence CT ratings in all regions independent of the effect of age of onset.
It was not possible to carry out separate analyses for subcategories of schizophrenia because nearly half of the patients only met criteria for schizophreniform disorder and most with a diagnosis of schizophrenia had mixed paranoid (psychotic), negative and disorganized symptoms. No patient met criteria for catatonic schizophrenia. We, therefore, examined correlation coefficients between clinical syndromes and CT scan variables but found no significant correlations.
First-episode versus previously treated patients
A comparison of sample characteristics showed, as expected, the patients who had been treated previously (n = 65) were older (mean age 33.0 yr [SD 9.6 yr] v. 27.2 yr [SD 10.2 yr], p < 0.001) and had a longer history of illness (mean 7.6 yr [SD 8.4 yr] v. 2.3 yr [SD 3.6 yr], p < 0.001) than the first-episode patients (n = 114). No differences were seen on any other demographic or clinical variables (details on patient characteristics for the patients previously treated for schizophrenia are provided in a previous publication16).
Age is known to influence the degree of atrophy, and this was further confirmed in our data on first-episode patients. Therefore, an analysis of covariance with age as a covariate was carried out to compare CT ratings of first-episode patients with those of previously treated (chronic) patients. Only the sylvian fissure was significantly larger in the previously treated patients than in first-episode patients (composite mean 2.68 v. 1.96, p < 0.003). This difference was significant for both the right (mean 2.54 v. 1.86, p < 0.002) and left sylvian fissure (mean 2.67 v. 2.05, p < 0.04).
The significant difference reported in the size of the sylvian fissure between first-episode psychosis and chronic schizophrenia samples could be accounted for by the inclusion of patients in the first-episode sample who met criteria for schizophreniform disorder. It is possible that some of these patients may not progress to meet the criteria for schizophrenia and, hence, may not be represented in a sample of patients who have had numerous hospital admissions and have received treatment for a number of years. We, therefore, compared the patients with chronic schizophrenia with those first-episode patients who had been ill for more than 6 months (n = 64) and, thus, met DSM-III-R criteria for schizophrenia. An analysis of covariance with age as the covariate revealed that differences between the first-episode and chronic samples on ratings of right and left sylvian fissure remained significant (right, p < 0.004; left, p < 0.03). We also repeated these analyses comparing the chronically ill sample with unmedicated first-episode patients. Results confirmed significant differences in the ratings of the right sylvian fissure (p < 0.01) but not for the left (p < 0.07). In contrast to the first-episode patients, a comparison of right and left sylvian fissure ratings (2.67 and 2.55, respectively) in the previously treated sample failed to reveal any significant differences. No significant differences were observed between first-episode and previously treated patients on sulci or ventricle ratings.
Discussion
In a large sample of patients presenting with a first episode of schizophrenic or schizophreniform psychosis, likely to be representative of an incidence sample, we found evidence of at least a mild degree of enlargement of cortical sulci and ventricles, the temporal horn being larger on the right than on the left side. The left sylvian fissure showed more enlargement than the right in first-episode patients, and a comparison with a more chronically ill patient sample suggested that enlargement of the sylvian fissure may be progressive. Patients with younger age of onset did not show a significantly greater level of cerebral atrophy. The variance in the ratings was explained mostly by age and not by variables such as sex, length of illness or type of clinical syndromes.
Several previous studies have reported the presence of enlarged ventricles and cortical sulci in patients suffering from a first episode of schizophrenia. Madsen et al,21 in a CT scan study of a small sample of first-episode schizophrenia patients, found progressive atrophy in frontal and central brain regions at the 5-year reassessment. The reversed asymmetry of sylvian fissure, indicated by larger sylvian fissure on the left side, has also been reported previously in schizophrenia.8 Although our findings confirm cerebral atrophy in the frontal and temporal areas (enlargement of cortical sulci and ventricles) in a large sample of first-episode psychosis patients, a comparison with a chronically ill patient population suggests that, after controlling for the effect of age, only the enlargement of the sylvian fissure (right and left) may be progressive. This progression appears to be greater on the right than the left side, with the result that the difference between the size of right and left sylvian fissure is no longer significant in chronic patients. However, the loss of normal asymmetry is maintained in the chronic sample while losing the reversed asymmetry. This could imply that structures influenced by enlargement of the sylvian fissure (temporal lobe structures), especially on the right, may be subject to continued effects of the illness or be influenced by prolonged exposure to neuroleptic medication, whereas ventricular and sulcal enlargement (reflecting mostly loss of frontal grey matter) is more static in the years after initial treatment. More precise volumetric measurement studies of first-episode schizophrenia and schizophreniform psychosis using MRI scans have shown significant reductions in temporal lobe structures such as the left hippocampus26,36 and left superior temporal gyrus37 as well as bilateral reduction in the size of the hippocampus.38 Razi et al23 have suggested that volumetric reduction in limbic complex, especially the parahippocampal gyrus, may be related to an active degenerative process after the onset of the illness.
It has been suggested that patients with schizophrenia who meet the criteria for catatonic subtype show more diffuse frontal, parietal and temporal enlargement of cerebrospinal fluid space than normal controls, whereas patients with hebephrenic and paranoid subtypes are more likely to show enlargement of mostly upper and lower temporal sulci including the sylvian fissure.39 These results were reported on a sample of patients with chronic schizophrenia. We were unable to examine differences in sulcal or ventricular enlargement of subtypes of schizophrenia in our sample, partly because a large proportion of patients met criteria for only schizophreniform psychosis and most presented with mixed paranoid (psychotic), disorganized and negative symptoms. There were no patients with a pure catatonic schizophrenia. However, if the nature of symptoms is likely to reflect neuroanatomical structures, an examination of correlations between each symptom dimension and sulcal and ventricular enlargement should reveal such a relation. Our data failed to show any such relation with first-episode patients.
We confirmed the findings of Smith et al17 of a strong relation between age and ventricular size, but in our sample of first-episode patients, this effect was seen in relation to all CT measures and was independent of sex, age of onset and length of illness. When making comparisons between patients with different ages of onset, it is important to control for the effect of age. Our failure to replicate the relation between length of illness and the morphological measures reported by Smith et al17 is likely a reflection of differences in samples. Most (70%) of our sample had not received neuroleptic treatment, whereas the Smith et al17 sample comprised predominantly chronically ill patients. Length of illness in our sample reflected mostly a period of untreated psychosis. Our failure to replicate findings of Madsen et al21 of a relation between length of untreated psychosis and enlarged ventricles and sulci may also be related to sample differences. We have reported results on a large community sample of patients with a first episode of psychosis, which includes cases of schizophreniform psychosis.
Our findings are based on ratings of CT scan images using a visual scale, but one that has been shown to have acceptable levels of inter-rater reliability and validity.17,29 Our data indicate, however, that ratings of lateral ventricles may show greater agreement between raters than ratings of third ventricle or some sulcal areas such as frontal and parietal sulci. This could be related to the size of the area and anatomical landmarks available (e.g., in the case of lateral ventricle) or the degree of difficulty demarcating the area to be rated (e.g., sylvian fissure v. frontal or parietal sulci). Improved image resolution on MRI would likely provide more reliable data on specific morphological changes, but the more stringent criteria for subject selection and significantly higher costs restrict the use of MRI for assessing a large number of consecutive patients presenting with first episode of schizophrenic psychosis. The interpretation of our findings is also restricted by the absence of a matched normal control group. However, the ratings of CT scan measures are done in comparison with ratings that reflect a large variation of completely normal to severely affected brain morphology.
In conclusion, CT scan measures of cerebral structures assessed in a sample closely representative of incidence cases of schizophrenia or schizophreniform psychosis showed mild enlargement of ventricles and sulci and a reverse asymmetry of the sylvian fissure. These changes were mostly related to older age; there were no relations found between CT ratings and clinical syndromes, sex and length of illness. A comparison with a sample of patients with chronic schizophrenia suggests that only sylvian fissure enlargement may be progressive, either as a result of longer period of illness or prolonged exposure to neuroleptics.
Acknowledgments
The principal author is supported partially by an Ontario Mental Health Foundation Senior Research Fellowship. Dr. Mittal was supported as a student for this project through the Faculty of Medicine, University of Western Ontario, Student Research Award. The principal study of early intervention from which these data are derived is supported by an operating grant from the Canadian Institutes of Health Research.
Footnotes
Competing interests: None declared.
Correspondence to: Prof. Ashok K. Malla, Department of Psychiatry, University of Western Ontario, London Health Sciences Centre, Victoria Campus, 392 South St., London ON N6A 4G5; fax 519 667-6537; akmalla@uwo.ca
Submitted Feb. 15, 2001 Revised Jul. 23, 2001; Jan. 8, 2002; Mar. 7, 2002 Accepted Mar. 14, 2002
References
- 1.Chua SE, McKenna PJ. Schizophrenia — A brain disease? A critical review of structural and functional cerebral abnormality in the disorder. Br J Psychiatry 1995;166:563-82. [DOI] [PubMed]
- 2.Iacono WG, Smith GN, Moreau M, Beiser M, Fleming AE, Lin T, et al. Ventricular and sulcal size at the onset of psychosis. Am J Psychiatry 1988;145:820-4. [DOI] [PubMed]
- 3.Pfefferbaum A, Zipursky RB, Lim KO, Zatz LM, Stahl SM, Jernigan TL. Computed tomographic evidence for generalized sulcal and ventricular enlargement in schizophrenia. Arch Gen Psychiatry 1988;45:633-40. [DOI] [PubMed]
- 4.McCarley RW, Faux SF, Shenton M, LeMay M, Cane M, Ballinger R, et al. CT abnormalities in schizophrenia. A preliminary study of their correlations with P300/P200 electrophysiological features and positive/negative symptoms. Arch Gen Psychiatry 1989;46:698-708. [DOI] [PubMed]
- 5.Northoff G, Waters H, Mooren I, Schluter U, Diekmann S, Falkai P, et al. Cortical sulcal enlargement in catatonic schizophrenia: a planimetric CT study. Psychiatry Res 1999;91(1):45-54. [DOI] [PubMed]
- 6.Harvey I, Ron MA, DuBoulay G, Wicks D, Lewis SW, Murray RM. Reduction of cortical volume in schizophrenia on magnetic resonance imaging. Psychol Med 1993;23:591-604. [DOI] [PubMed]
- 7.Turetsky B, Cowell PE, Gur RC, Grossman RI, Shtasel DL, Gur RE. Frontal and temporal lobe brain volumes in schizophrenia. Arch Gen Psychiatry 1995;52:1061-70. [DOI] [PubMed]
- 8.Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry 2001;49(1):20-7. [DOI] [PubMed]
- 9.Levitt JJ, McCarley RW, Nestor PG, Petrescu C, Donnino R, Hirayasu Y, et al. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: clinical and cognitive correlates. Am J Psychiatry 1999;156(7):1105-7. [DOI] [PMC free article] [PubMed]
- 10.Andreasen NC, Paradiso S, O'Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24 (2):203-18. [DOI] [PubMed]
- 11.Honer WG, Bassett AS, Squires-Wheeler E, Falkai P, Smith GN, Lapointe JS, et al. The temporal lobes, reversed asymmetry and the genetics of schizophrenia. Neuroreport 1995;7 (1): 221-4. [DOI] [PMC free article] [PubMed]
- 12.Flaum M, Arndt S, Andresen NC. The role of gender in studies of ventricle enlargement in schizophrenia: a predominantly male effect. Am J Psychiatry 1990;147:1327-32. [DOI] [PubMed]
- 13.Andia AM, Zisook S, Heaton RK, Hesselink J, Jernigan T, Kuck J, et al. Gender differences in schizophrenia. Nerv Ment Dis 1995;183:522-8. [DOI] [PubMed]
- 14.Nasrallah HA, Schwarzkopf SB, Olson SC, Coffman JA. Gender differences in schizophrenia on MRI brain scans. Schizophr Bull 1990;16(2):205-10. [DOI] [PubMed]
- 15.Lauriello J, Hoff A, Wienke MH, Blankfeld H, Faustman WO, Rosenbloom M, et al. Similar extent of brain dysmorphology in severely ill women and men with schizophrenia. Am J Psychiatry 1997;154(6):819-25. [DOI] [PubMed]
- 16.Malla AK, Takhar J, Norman RMG, Assis L. Computerized tomography in schizophrenia: relationship with symptom dimensions and gender differences. J Psychiatry Neurosci 1999; 24 (2): 131-8. [PMC free article] [PubMed]
- 17.Smith GN, Kopala LC, Lapointe JS, MacEwan GW, Altman S, Flynn SW, et al. Obstetric complications, treatment response and brain morphology in adult-onset and early-onset males with schizophrenia. Psychol Med 1998;28(3):645-53. [DOI] [PubMed]
- 18.Schulz SC, Koller MM, Kishore PR, Hamer RM, Gehl JJ, Friedel RO. Ventricular enlargement in teenage patients with schizophrenia spectrum disorder. Am J Psychiatry 1983;140:1592-5. [DOI] [PubMed]
- 19.Tanaka Y, Hazama H, Kawahara R, Kobayashi K. Computerized tomography of the brain in schizophrenic patients: a controlled study. Acta Psychiatr Scand 1981;63:191-7. [DOI] [PubMed]
- 20.Flaum M, O'Leary DS, Swayze VW, Miller DD, Arndt S, Andreasen NC. Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. J Psychiatr Res 1995; 29 (4): 261-76. [DOI] [PubMed]
- 21.Madsen AL, Karle A, Rubin P, Cortsen M, Andersen HS, Hemmingsen R. Progressive atrophy of the frontal lobes in first-episode schizophrenia: interaction with clinical course and neuroleptic treatment. Acta Psychiatr Scand 1999;100(5):367-74. [DOI] [PubMed]
- 22.Wyatt RJ. Neuroleptics and the natural course of schizophrenia. Schizophr Bull 1991;17(2):325-48. [DOI] [PubMed]
- 23.Razi K, Greene KP, Sakuma M, Kushner M, DeLisi LE. Reduction of the parahippocampal gyrus and the hippocampus in patients with chronic schizophrenia. Br J Psychiatry 1999; 174: 512-9. [DOI] [PubMed]
- 24.James AC, Crow TJ, Renowden S, Wardell AM, Smith DM, Anslow P. Is the course of brain development in schizophrenia delayed? Evidence from onsets in adolescence. Schizophr Res 1999; 40 (1): 1-10. [DOI] [PubMed]
- 25.DeQuardo JR, Keshaven MS, Bookstein FL, Bagwell WW, Green WD, Sweeney JA, et al. Landmark-based morphometric analysis of first-episode schizophrenia. Biol Psychiatry 1999; 45 (10): 1321-8. [DOI] [PubMed]
- 26.Velakoulis D, Pantelis C, McGorry PD, Dudgeon P, Brewer W, Cook M, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry 1999;56(2):133-41. [DOI] [PubMed]
- 27.Hoffler J, Braunig P, Kruger S, Ludvik M. Morphology according to cranial computed tomography of first-episode cycloid psychosis and its long-term course: differences compared to schizophrenia. Acta Psychiatr Scand 1997;96(3):184-7. [DOI] [PubMed]
- 28.Vazquez-Barquero JL, Cuest Nunez MJ, Quintana Pando F, De La Varga M, Herrera Castanedo S, Dunn G. Structural abnormalities of the brain in schizophrenia: sex differences in the Cantabria First Episode of Schizophrenia Study. Psychol Med 1995; 25: 1247-57. [DOI] [PubMed]
- 29.Smith GN, Flynn SW, Kopala LC, Bassett AS, Lapointe JS, Falkai P, et al. A comprehensive method of assessing routine CT scans in schizophrenia. Acta Psychiatr Scand 1997;96:395-401. [DOI] [PMC free article] [PubMed]
- 30.LeMay M, Stafford JL, Sandor T, Albert M, Haykal H, Zamani A. Statistical assessment of perceptual CT scan ratings in patients with Alzheimer-type dementia. J Comput Assist Tomogr 1986; 10: 802-9. [DOI] [PubMed]
- 31.Davis PC, Gray L, Albert M, Wilkinson W, Hughes J, Heyman A, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part III reliability of a standardized MRI evaluation of Alzheimer's disease. Neurology 1992;42:1676-80. [DOI] [PubMed]
- 32.Andreasen NC. The Scale for Assessment of Positive Symptoms (SAPS). Iowa City (IA): University of Iowa; 1984.
- 33.Andreasen NC. The Scale for Assessment of Negative Symptoms (SANS). Iowa City (IA): University of Iowa; 1983.
- 34.Malla AK, Norman RMG, Williamson P, Cortese L, Dias F. Three syndrome concept of schizophrenia: a factor analytic study. Schizophr Res 1993;10:143-50. [DOI] [PubMed]
- 35.Häfner H, Riecher-Rossler A, Fätkenheur B, Maurer K, Meissner S, Loffler W. Interview for the Retrospective Assessment of the Onset of Schizophrenia (IRAOS). Manneheim (Germany): Central Institute of Mental Health; 1992.
- 36.Barr WB, Ashtari M, Bilder RM, Degreef G, Liberman JA. Brain morphometric comparison of first-episode schizophrenia and temporal lobe epilepsy. Br J Psychiatry 1997;170:515-9. [DOI] [PubMed]
- 37.Keshaven MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, et al. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res 1998;32(3–4):161-7. [DOI] [PubMed]
- 38.Whitworth AB, Honeder M, Kremser C, Kemmler G, Felber S, Hausmann A, et al. Hippocampal volume reduction in male schizophrenic patients. Schizophr Res 1998;31(2–3):73-81. [DOI] [PubMed]
- 39.Northoff G, Waters H, Mooren I, Schlüter U, Diekmann S, Falkai P, et al. Cortical sulcal enlargement in catatonic schizophrenia: a planimetric CT study. Psychiatry Res 1999;91:45-54. [DOI] [PubMed]


