Abstract
We investigated the effects of rotigaptide (ZP123), a stable hexapeptide with antiarrhythmic properties, on gap junction mediated intercellular communication in contracting rat neonatal cardiac myocytes, HL-1 cells derived from cardiac atrium and in HeLa cells transfected with cDNA encoding Cx43-GFP, Cx32-GFP, Cx26-GFP, wild-type Cx43 or wild-type Cx26.
Intercellular communication was monitored before and after treatment with rotigaptide following microinjection of small fluorescent dyes (MW<1 kDa). The communication-modifying effect of rotigaptide was confined to cells expressing Cx43 since the peptide had no effect on dye transfer in HeLa cells expressing Cx32-GFP, Cx26-GFP or wild-type Cx26. In contrast, HeLa cells expressing Cx43-GFP exposed to 50 nM rotigaptide for 5 h showed a 40% increase in gap junction mediated communication.
Rotigaptide (50 nM) increased intercellular dye transfer in myocytes and atrial HL-1 cells, where Cx43 is the dominant connexin. However, it caused no change in cell beating rates of cardiac myocytes.
Western blot analysis showed that rotigaptide did not modify the overall level of Cx43 expression and changes in the phosphorylation status of the protein were not observed.
We conclude that the effects of rotigaptide were confined to cells expressing Cx43.
Keywords: Gap junction, connexin, arrhythmia, cardiac myocytes, antiarrhythmic peptide, intercellular communication
Introduction
Direct communication across gap junctions facilitates the functional coordination of cell networks (Loewenstein, 1981). Modifying the extent of cell to cell signalling across gap junction channels is likely to disrupt the synchronisation of individual activities of constituent cells in tissues and organs. Remodelling of gap junctions and changes in intercellular communication via these channels are associated with a number of pathophysiological conditions including ischaemia, cardiac arrhythmia, hypertension and atherosclerosis, as well as endothelial and epithelial wound healing events (Saez et al., 2003; Rummery & Hill, 2004; Severs et al., 2004). Connexins, the protein subunits assembled into gap junction intercellular channels, are therefore emerging as therapeutic targets (Dhein, 2004).
In the heart, gap junctions couple myocytes electrically and mechanically ensuring intercellular propagation of action potentials as well as Ca2+ waves, communication processes that underpin synchronous contraction at the cellular and organ level (Kleber & Rudy, 2004). To address cardiac arrhythmia, hexa-peptides such as AAP10 derived from bovine atria and a stable analogue ZP123, now termed rotigaptide, have been developed to modify gap junctional channel properties and intercellular communication (Muller et al., 1997; Dhein et al., 2003). Rotigaptide promoted electrical coupling between ventricular myocytes without changing membrane conductance (Xing et al., 2003). The peptide attenuated gap junction closure and preserved intercellular coupling during acidosis (Eloff et al., 2003; Kjolbye et al., 2003; Xing et al., 2003; Haugan et al., 2005). Most importantly, rotigaptide reduced the inducibility of re-entry ventricular tachycardia during acute ischaemia in dogs (Xing et al., 2003). Indeed, these properties suggest that rotigaptide could be a prototype of a new class of antiarrhythmic drug.
Connexins are a highly conserved 20 member family of membrane proteins that assemble into dodecameric gap junction channels (Willecke et al., 2002). All connexins share a common topology, traversing the lipid bilayer four times with two highly conserved extracellular loops. The carboxyl terminus is located intracellularly and varies in sequence and length between the different connexins (Evans & Martin, 2002). The carboxyl tail of Cx43 is subject to post-translational phosphorylation (Lampe & Lau, 2004), the extent of which is dynamically regulated and appears to influence channel permeability (Harris, 2001).
Cells in most tissue express different combinations of connexins. Connexin 43 is the dominant connexin expressed in mammalian heart. It is also highly expressed in many other tissues including skin where Cx26 and up to 10 other connexins are also differentially expressed in the dermal cell layers (Di et al., 2001; Willecke et al., 2002). Cx32 is found in several tissues including the nervous system and liver (Willecke et al., 2002). Each connexin subtype has distinctive permeability properties with different connexins exhibiting differing abilities to transfer small fluorescent marker dyes of varying molecular mass to neighbouring cells. For example, Cx43 gap junctions are permeable to most molecules <1 kDa in size. By contrast, Cx26 gap junctions are more selective and are permeable to small dyes such as Lucifer yellow (MW 457 Da) but are impermeable to larger marker dyes such as Alexa594 (MW 780 Da) (Nicholson et al., 2000). Differences in the permeabilities of Cx26, Cx32 and Cx43 channels to secondary messengers including cAMP, adenosine and IP3 have also been reported (Niessen et al., 2000; Harris, 2001; Goldberg et al., 2002). Such properties are believed to reflect specific physiological requirements of different tissues. The dynamic role that connexin membrane channels play in various physiological and pathophysiological conditions makes agents that target gap junctional communication potentially useful therapeutic tools.
In the present study, we investigated the effects of rotigaptide on the functionality and protein expression profiles of Cx43 channels in three model systems; rat neonatal cardiac myocytes, HL-1 cells (Claycomb et al., 1998) and HeLa cells transfected and selected to express different connexins. The lack of effect of rotigaptide on the permeability of Cx32-GFP and Cx26 gap junctions in HeLa cell model systems allowed the connexin specificity of rotigaptide action to be examined.
Methods
Preparation and culture of primary rat neonatal myocytes
Hearts from fifty 2 to 5-day-old Wistar rats were harvested, minced and single cells dissociated by serial trypsin treatment (Webster et al., 1999). After trypsin digestion, cells were washed and preplated for 1 h in Dulbecco's modified essential medium (DMEM), supplemented with 5% foetal calf serum (FCS) and penicillin/streptomycin (P/S) (100 μg ml−1). Nonattached cells were replated in 60 mm tissue culture dishes (Greiner bio-one, Germany) at 4 × 106 cells/dish in the same medium as outlined above, supplemented with 0.1 mM bromodeoxyuridine (BrDu). After 4 days, cells were transferred to serum-free DMEM supplemented with P/S (100 μg ml−1), BrDu (0.1 mM), vitamin B12 (1.5 μM) and insulin (20 μg ml−1). Cells (>95% myocytes) were used after 5–7 days. The preplating step and BrDu supplementation ensured the background of nonmyocardial cells (mainly fibroblasts) was minimal. The resultant cultures of rat neonatal cardiac myocytes contracted synchronously at >100 beats min−1 (bpm).
Cell culture
HL-1 atrial cardiomyocytes were maintained in Claycomb medium, supplemented with 10% FCS, P/S (100 μg ml−1), epinephrine (0.1 mM) and L-glutamine (2 mM) as previously described (Claycomb et al., 1998). HeLa cells selected to express Cx43-GFP, Cx32-GFP or Cx26-GFP were prepared as described (Paemeleire et al., 2000) and maintained in DMEM, supplemented with 10% FCS, P/S (100 μg ml−1), amphotericin (100 μg ml−1), L-glutamine (2 mM) and geneticin sulphate (G-418-sulphate 4 mg ml−1). HeLa cells expressing wild-type Cx26 or Cx43 were maintained in cDMEM supplemented with 0.5 μg ml−1 puromycin (Elfgang et al., 1995).
Dye transfer across gap junctions
Rat neonatal cardiac myocytes and HeLa cells were seeded at a density of 4 × 106 cells per 60 mm dish and 1 × 106 cells per 60 mm dish, respectively. Cx-GFP protein expression in HeLa cells was enhanced by addition of 5 mM sodium butyrate to the medium 18 h prior to the experiments (George et al., 1998). Direct cell–cell communication was assessed before and after treatment of the cells with rotigaptide by studying transfer of Lucifer yellow (charge −2, MW 457 Da), Alexa-488 (charge −1, MW 570 Da) or Alexa-594 (charge −1 MW 758 Da) following intracytoplasmic microinjection of dye using an Eppendorf Femtojet 5247 device. Experiments were repeated in triplicate with >30 cells injected per experiment. At 5 min after injection, cells were fixed in 4% w v−1 paraformaldehyde for 5 min and washed in phosphate-buffered saline pH 7.4 (PBS) prior to the percentage of injections resulting in dye transfer to different numbers of neighbouring cells being assessed on an Zeiss Axiovert 200 fluorescence microscope using appropriate filter sets (Zeiss, U.K.) (Martin et al., 2004).
Cell cytometric analysis
HeLa cells expressing Cx43-GFP were plated onto six-well dishes (1 × 105 cells) and were exposed to 50 or 250 nM rotigaptide for periods of 1 or 5 h. Cells were harvested by trypsinisation, washed in PBS and resuspended to a final concentration of 1 × 106 ml−1. Cells (1 × 105) were added to 500 μl of fluorescent activated cell sorting (FACS) sheath fluid (Becton Dickson, CA, U.S.A.) and subjected to FACS analysis after excitation at 488 nm. Data was exported to a Microsoft Excel spreadsheet and analysed. Experiments were repeated three times and data presented as mean±s.e.m.
Western blot analysis
Cells grown on 60 mm dishes (4 × 106 rat neonatal cardiac myocytes; 1 × 106 HeLa cells) were treated for periods of 1 to 5 h with 50, 100 or 250 nM rotigaptide, or 25 μM 18 α-glycyrrhetinic acid (18αGA) prior to harvesting in 200 μl of ice-cold lysis buffer (1% w v−1 SDS, 1 mM DTT, 1 mM sodium orthovanadate, 4 ng ml−1 leupeptin, 4 ng ml−1 apoprotinine and 1 mM phenylmethylsulphonyl fluoride in PBS), followed by sonication. Equal amounts of protein (100 μg rat neonatal cardiac myocyte lysates; 50 μg HeLa cell lysates) were analysed by SDS–PAGE (10% w v−1) and transferred onto nitrocellulose in transfer buffer (20 mMNa2CO3) for 3 h at 300 mA. The transfer efficiency was assessed by Ponceau S staining of the blots prior to further probing with a polyclonal antibody to Cx43 (1 : 4000 dilution) that recognises multiple phosphorylated/nonphosphorylated isoforms of Cx43 and a secondary goat anti-rabbit horseradish peroxidase antibody (1 : 2000 dilution). In some experiments, blots were also probed with a polyclonal antibody that recognises site-specific phosphorylation of serine 368 on the carboxyl tail of Cx43 (1 : 1000 dilution). To standardise protein expression, blots were also probed with a monoclonal antibody to α-tubulin (1 : 10 000 dilution) and a secondary goat anti-mouse horseradish peroxidase antibody (1 : 2000 dilution). Blots were developed by enhanced chemiluminescene (ECL) and quantified using a Biorad GS-700 densitometer (Martin et al., 2004).
Connexin 43 phosphorylation assays
Protein extracts (30 μg) were digested with 60 U of calf intestinal phosphatase for 1 h at 37°C and analysed by SDS–PAGE and Western blotting as described above. In some experiments, cells were treated with 100 ng ml−1 TPA (12-O-tetradecanoylphorbol 13-acetate), a protein kinase C activator, for 30 min prior to harvesting the cells to serve as a positive control when analysing serine 368 phosphorylation of Cx43.
Materials
DMEM, supplements for cell growth and other tissue culture reagents were supplied by Invitrogen (Glasgow, U.K.). G418-sulphate and Calf Intestinal Phosphatase were purchased from Promega, (Southhampton U.K.) and Lucifer yellow, Alexa-488, Alexa-594 were obtained from Molecular Probes (Leiden, The Netherlands). The Cx43 monoclonal and P368 antibodies were obtained from Chemicon (Chandlers Ford, U.K.). Other connexin antibodies for Western blotting were obtained from Zymed (Cambridge, U.K.). The goat anti-mouse and rabbit horseradish peroxidase were supplied by BioRad (Hemel Hempsted, U.K.) and the ECL system from Pierce (Tattenhall, U.K.). Rotigaptide was supplied by Zealand Pharma and dissolved in water to give a 50 μM stock and 18 αGA was dissolved in DMSO to give a 25 mM stock. All other reagents were obtained from Sigma (Poole, U.K.).
Statistical analysis
Data were evaluated by ANOVA followed by Dunnett's multiple comparison test, with P<0.05 being considered significant.
Results
Rotigaptide increases gap junctional communication in rat neonatal cardiac myocytes
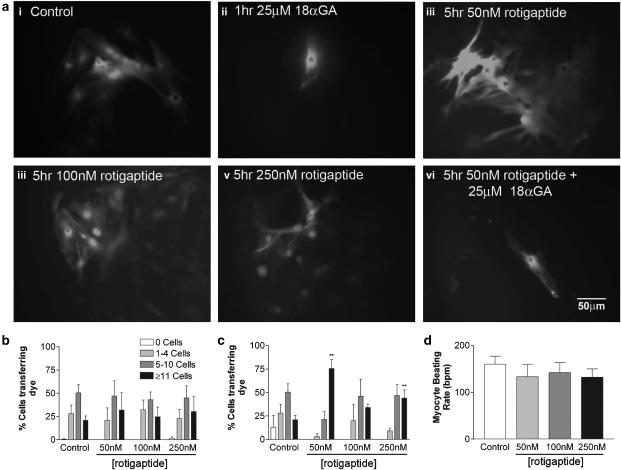
Rat neonatal cardiac myocytes were efficiently coupled with 20.9±4.82 of cells transferring dye to ⩾11 neighbouring cells. Rotigaptide treatment at 50, 100 or 250 nM for 1 h had no significant effect on Alexa 488 dye transfer (Figure 1a and b; Table 1). Exposure of the cells to 50 nM rotigaptide for 5 h increased dye spread approximately four-fold, with 75.6±9.7% of cells transferring dye to ⩾11 cells (Figure 1a and c; Table 1). Treatment of the cells for 5 h with 250 nM rotigaptide, also significantly increased intercellular coupling (Figure 1a and c; Table 1). Although a slight increase in coupling was also observed following treatment with 100 nM rotigaptide this was not significant (Table 1). There was no further modification of dye spread at these increased doses, suggesting that 50 nM is the optimal concentration at which rotigaptide operates in this system or that any further enhancement is not readily distinguished by monitoring spread of dye.
Figure 1.
The effect of rotigaptide and 18 αGA on Alexa-488 transfer and cell beating rate in rat neonatal cardiac myocytes. (a) Confluent monolayers of rat neonatal cardiac myocytes were viewed under a fluorescent microscope immediately after microinjection of Alexa-488: (i) control cells; (ii) control cells following 1 h pretreatment with 25 μM 18 αGA; (iii) following 5 h treatment with 50 nM rotigaptide; (iv) following 5 h treatment with 100 nM rotigaptide; (v) following 5 h treatment with 250, nM rotigaptide; (vi) 5 h treatment with 50 nM rotigaptide followed by 1 h 25 μM 18 αGA. *Injected cell. Bar=50 μM. Confluent monolayers of rat neonatal cardiac myocytes were microinjected with Alexa-488 under control conditions or following treatment with 50, 100 or 250 nM rotigaptide for 1 h (b) or for 5 h (c). Approximately 30 cells were injected per set of experiments, which were repeated in triplicate. **P<0.01 vs control. (d) Confluent monolayers of rat neonatal cardiac myocytes were viewed under a light microscope and cell beating rates were recorded under control conditions or following treatment with 50, 100 or 250 nM rotigaptide for 5 h (n=12).
Table 1.
Effect of rotigaptide on dye transfer properties of different cells and connexins
| Cell and Cx subtype expressed | Treatment | Dose | Time | Dye | % coupling±s.e.m. |
|---|---|---|---|---|---|
| aRat neonatal cardiac myocytes expressing Cx43 (transferring to 11 or more cells) | Control | — | 0 h | Alexa-88 | 20.90±4.82 |
| Rotigaptide | 50 nM | 1 h | Alexa-488 | 31.93±18.64 | |
| Rotigaptide | 100 nM | 1 h | Alexa-488 | 24.68±10.46 | |
| Rotigaptide | 250 nM | 1 h | Alexa-488 | 30.33±16.33 | |
| Rotigaptide | 50 nM | 5 h | Alexa-488 | 75.60±9.79** | |
| Rotigaptide | 100 nM | 5 h | Alexa-488 | 34.09±3.70 | |
| Rotigaptide | 250 nM | 5 h | Alexa-488 | 44.10±8.70** | |
| 18αGA | 50 μm | 1 h | Alexa-488 | 0** | |
| bHL-1 cardiomyocytes expressing Cx43 (transferring to 11 or more cells) | Control | — | 0 h | Alexa-488 | 1.67±1.67 |
| Rotigaptide | 50 nM | 5 h | Alexa-488 | 38.33±4.01** | |
| Rotigaptide | 100 nM | 5 h | Alexa-488 | 32.50±4.96* | |
| Rotigaptide | 250 nM | 5 h | Alexa-488 | 57.50±8.14** | |
| 18αGA | 50 μm | 30min | Alexa-488 | 0** | |
| HeLa cells expressing wild type Cx26 (transferring to 11 or more cells) | Control | — | 0 h | Alexa-488 | 15.5±3.9 |
| Rotigaptide | 50 nM | 5 h | Alexa-488 | 18.9±3.2 | |
| bHeLa cells expressing Cx43-GFP (transferring to 5 or more cells) | Control | — | 0 h | LY | 37.91±8.2 |
| Rotigaptide | 50 nM | 2–3 h | LY | 35.6±5.97 | |
| Rotigaptide | 50 nM | 5–6 h | LY | 69.1±7.12** | |
| Rotigaptide | 50 nM | o/n | LY | 56.7±6.84* | |
| Control | — | 0 h | Alexa-594 | 9.06±1.24 | |
| Rotigaptide | 50 nM | 5–6 h | Alexa-594 | 54.8±9.16* | |
| cHeLa cells expressing Cx32-GFP (transferring to 3 or more cells) | Control | — | 0 h | LY | 37.73±3.38 |
| Rotigaptide | 50 nM | 5–6 h | LY | 42.12±4.57 | |
| Rotigaptide | 100 nM | 5–6 h | LY | 27.13±2.62 | |
| Rotigaptide | 250 nM | 5–6 h | LY | 46.27±6.12 | |
| cHeLa cells expressing Cx26-GFP (transferring to 3 or more cells) | Control | — | 0 h | LY | 45.20±6.13 |
| Rotigaptide | 50 nM | 5–6 h | LY | 29.31±5.28 | |
| Rotigaptide | 100 nM | 5–6 h | LY | 27.75±0.25 | |
| Rotigaptide | 250 nM | 5–6 h | LY | 28.50±7.90 |
Confluent monolayers of rat neonatal cardiac myocytes, HL-1 cardiomyocytes and HeLa cells expressing Cx43-GFP, Cx32-GFP, Cx26-GFP or wild-type Cx26 were microinjected with Alexa-488, Lucifer yellow or Alexa-594 following treatment with rotigaptide at various concentrations and times as indicated in the table. The extent of dye transfer was recorded and the data here represented as % of cells transferring dye.
To ⩾11 neighbouring cells for rat neonatal cardiac myocytes and HL-1 cardiomyocytes.
To ⩾neighbouring cells for HeLa cells expressing Cx43-GFP.
To ⩾3 neighbouring cells for HeLa cells expressing Cx32-GFP and Cx26-GFP.
P<0.05,
P<0.01 compared with nontreated cells.
To demonstrate that intercellular transfer of dye occurred across gap junctions, cells were treated with 18 αGA for 1 h prior to microinjection and following exposure to 50 nM rotigaptide for 5 h (Figure 1a). Dye transfer was totally blocked under both conditions (Table 1). These results suggest that rotigaptide does not offset the effects of 18 αGA, a putative gap junction uncoupler (Davidson & Baumgarten, 1988). Despite the increase in intercellular coupling elicited by rotigaptide, the peptide had no significant effect on beating rates and their synchronicity in cardiac myocytes, which was maintained at approximately 150 bpm during each of the drug treatments (Figure 1d).
Rotigaptide increases gap junctional communication in HL-1 cells
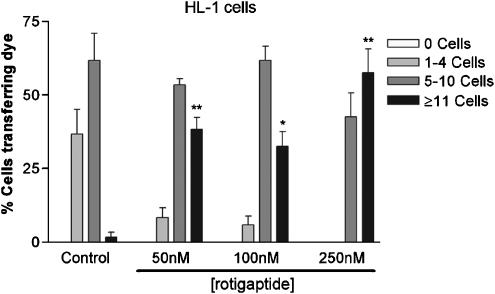
HL-1 cells displayed a lower degree of dye coupling than cardiac myocytes with 61.7±9.3% of cells transferring dye to 5–10 neighbouring cells and 1.7±1.7% of cells transferring dye to ⩾11 cells. Treatment of HL-1 cells with rotigaptide at 50, 100 or 250 nM for 5 h significantly increased transfer of dye to ⩾11 cells (Table 1, Figure 2).
Figure 2.
The effect of rotigaptide on Alexa-488 transfer in HL-1 cardiomyocytes. Confluent monolayers of HL-1 cardiomyocytes were microinjected with Alexa-488 under control conditions or following treatment with 50, 100 or 250 nM rotigaptide for 5 h. Approximately 30 cells were injected per set of experiments, which were repeated in triplicate. **P<0.01.
The connexin specificity of rotigaptide
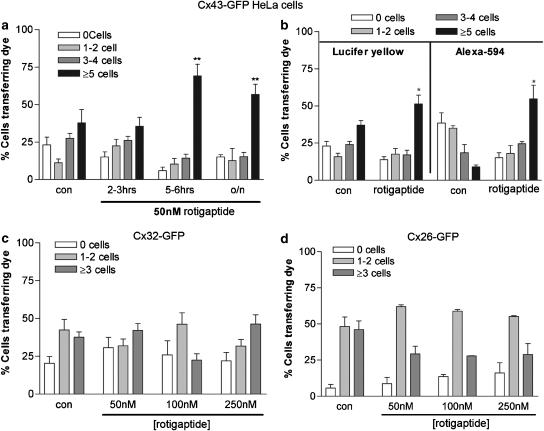
Untransfected HeLa cells are communication incompetent and do not transfer dyes to neighbouring cells (Elfgang et al., 1995; George et al., 1998). HeLa cells were made communication competent by transfection and selection of variants expressing Cx43-GFP, Cx32-GFP or Cx26-GFP, or wild-type Cx43 or Cx26. Following induction of Cx43-GFP expression with sodium butyrate, 37.9±8.2% of the transfected cells transferred dye to ⩾5 neighbours confirming previous results (Martin et al., 2001) (Figure 3, Table 1). Time course experiments, pursued to analyse the effects of rotigaptide (50 nM) on the efficiency of gap junctional coupling, showed that 2–3 h incubation with the peptide was without significant effect on the intercellular spread of Lucifer yellow (Figure 3a). However, after 5–6 h incubation with the peptide, increased dye transfer was observed, although overnight incubation did not result in further dye spread (Table 1). Similar findings were also observed with HeLa cells expressing wild-type Cx43, where preincubation with 50 nM rotigaptide for 5 h enhanced the spread of Alexa-594 to neighbouring cells (data not shown). Dye transfer was significantly reduced following preincubation of the cells with 18 αGA for 1 h prior to microinjection of dye under control conditions and following 5 h rotigaptide (50 nM) treatment; a similar result was obtained with cardiac myocytes (data not shown).
Figure 3.
The effect of rotigaptide on dye transfer in HeLa cells expressing Cx43-GFP, Cx32-GFP and Cx26-GFP. (a) Confluent monolayers of HeLa cells expressing Cx43-GFP were microinjected with Lucifer yellow under control conditions or following treatment with 50 nM rotigaptide for 2–3 h, 5–6 h or overnight. (b) Confluent monolayers of HeLa cells expressing Cx43-GFP were microinjected with Lucifer yellow or Alexa-594 under control conditions or following treatment with 50 nM rotigaptide for 5 h. (c) Confluent monolayers of HeLa cells expressing Cx32-GFP were microinjected with Lucifer yellow under control conditions or following treatment with 50, 100 or 250 nM rotigaptide for 5 h. (d) Confluent monolayers of HeLa cells expressing Cx26-GFP were microinjected with Lucifer yellow under control conditions or following treatment with 50, 100 or 250 nM rotigaptide for 5 h. *P<0.05, **P<0.01 in (a), (c) and (d). In (b), *P<0.05 between control cells and rotigaptide treated cells was determined by Student's t-test, for Lucifer yellow and Alexa-594 transfer, respectively.
To determine if rotigaptide had any selectivity on the molecular mass of molecules transferred the intercellular spread of Lucifer yellow (charge −2, MW 457 Da) and Alexa-594 (charge−1, MW 758 Da) was compared in HeLa cells expressing Cx43-GFP. Lucifer yellow was found to diffuse more efficiently through Cx43-GFP gap junctions than Alexa-594, with 37.9±8.2% of cells transferring Lucifer yellow whereas only 9±1.24% of cells transferred Alexa-594 to ⩾5 neighbouring cells, respectively. Transfer of both dyes increased significantly after 5 h treatment with rotigaptide (50 nM) (Figure 3b, Table 1). In general, Cx43-GFP channels transferred Lucifer yellow to a greater number of cells than Alexa-594, attributed to the increased MW of Alexa-594 and its lower transfer efficiency in control cells. Interestingly, the upregulation of dye transfer by rotigaptide was greater for Alexa594 than Lucifer yellow (Table 1).
HeLa cells expressing Cx32-GFP and Cx26-GFP exhibited lower dye coupling efficiency than Cx43-GFP transfected HeLa cells, with 37.3±3.4% of Cx32-GFP expressing cells transferring Lucifer yellow to ⩾3 neighbouring cells (Figure 3c, Table 1). This is a characteristic of these selected cell lines that may be related to the level of Cx expression in the cells. However, incubation with 50 nM rotigaptide for 5 h had no significant effect on the extent of Lucifer yellow transfer. Furthermore, increasing the dose of rotigaptide to 100 or 250 nM rotigaptide caused no significant change in dye transfer. Similar results were obtained with Cx26-GFP expressing cells (Figure 3d). Rotigaptide had no significant effect on the extent of transfer of Alexa 488 (charge −1, MW 570) in HeLa cells expressing wild-type Cx26 following 5 h incubation with 50 nM Rotigaptide, further confirming the lack of effect of rotigaptide on Cx26 (Table 1). Previous studies show that dye transfer properties of Alexa 488 and Lucifer yellow are very similar (Martin et al., 2005; Nicholson et al., 2000 unpublished observations). By contrast, Cx26 wild-type expressing cells were unable to efficiently transfer Alexa 594 (Martin et al., 2005; Nicholson et al., 2000; unpublished observations).
Effects of rotigaptide on connexin43-GFP expression levels
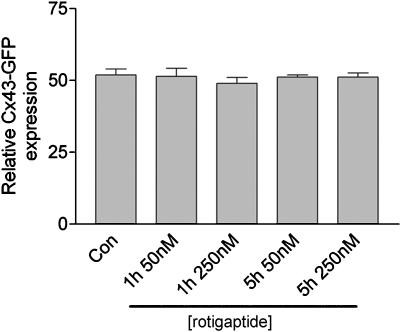
The inherent autofluorescent properties of Cx43-GFP permitted analysis of the effect of rotigaptide on Cx43-GFP expression in live cells by FACS analysis. The results showed that exposure of cells to rotigaptide did not change the overall level of Cx43-GFP fluorescence (Figure 4).
Figure 4.
The effect of rotigaptide on Cx43GFP expression. Cx43-GFP expressing HeLa cells were subject to FACS analysis and the % of cells expressing Cx43-GFP recorded following treatment for 1 and 5 h with 50 and 250 nM rotigaptide, respectively.
Effects of rotigaptide on connexin43 expression profiles in rat neonatal cardiac myocytes and connexin43 transfected HeLa cells
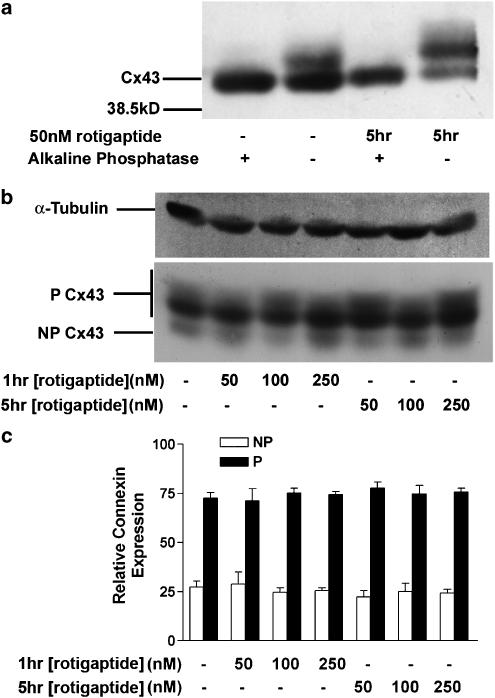
Immunoblot analysis of rat neonatal cardiac myocytes confirmed that Cx43 migrated electrophoretically as two phosphorylated forms (P1 and P2 bands; 43–45 kDa), with a minor band at 41 kDa (Figure 5a). Under control conditions Cx43-GFP migrated at ∼70 kDa and its intensity increased following 18–24 h treatment with sodium butyrate, that is, at times when dye transfer studies were performed (Figure 6a). A further band, at ∼81 kDa was confirmed as a phosphorylated isoform of Cx43-GFP in these cells (Figure 6a) since the chimera was dephosphorylated by alkaline phosphatase treatment. Similarly, Cx43 in rat neonatal cardiac myocytes also showed a 41 kDa band that was likely to be a nonphosphorylated form of Cx43 (Figure 5a), consistent with previous reports (Beardslee et al., 2000). Parallel data from HeLa cells expressing wild-type Cx43 gave similar results (data not shown).
Figure 5.
Analysis of the phosphorylation status of Cx43. (a) Cell lysates of rat neonatal cardiac myocytes under control conditions or following treatment with 50 nM rotigaptide for 5 h were digested with alkaline phosphatase followed by standard SDS–PAGE and Western blotting with an antibody targeted to Cx43. (b) Rat neonatal cardiac myocytes were treated with 50, 100 or 250 nM rotigaptide for 1 or 5 h and cell lysates analysed by standard SDS–PAGE and Western blotting with an antibody targeted to Cx43 and α-tubulin. Lane 1 – Control; lane 2 – 50 nM rotigaptide for 1 h; lane 3 – 100 nM rotigaptide for 1 h; lane 4 – 250 nM rotigaptide for 1 h; lane 5 – 50 nM rotigaptide for 5 h; lane 6 – 100 nM rotigaptide for 5 h; lane 7 – 250 nM rotigaptide for 5 h. (c) Rat neonatal cardiac myocytes were treated with 50, 100 or 250 nM rotigaptide for 1 or 5 h. The graph shows the combined densitometric analysis of four separate Western blots. P=Phosphorylated Cx43; NP=nonphosphorylated Cx43.
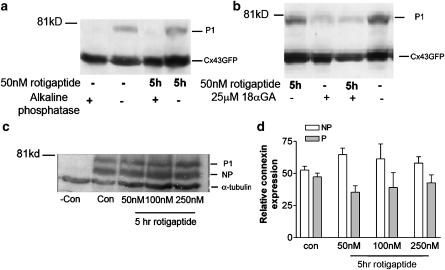
Figure 6.
The effect of rotigaptide and 18 αGA on Cx43-GFP expression. (a) Confluent monolayers of HeLa cells expressing Cx43-GFP were lysed under control conditions or following treatment with 50 nM rotigaptide for 5 h. Cell lysates were digested with alkaline phosphatase for 1 h at 37C followed by SDS–PAGE and Western blotting with antibodies to Cx43. (b) Cx43-GFP HeLa cells were treated with 50 nM rotigaptide and or 18 αGA. (c) Cx43-GFP HeLa cells were treated with 50, 100 or 250 nM rotigaptide for 5 h. (d) The graph shows the combined densitometric analysis of three separate Western blots following treatment of Cx43-GFP HeLa cells with 50, 100 or 250 nM rotigaptide for 5 h. P=Phosphorylated Cx43; NP=nonphosphorylated Cx43.
Rat neonatal cardiac myocytes treated with 50, 100, and 250 nM rotigaptide for 1 or 5 h showed little change in Cx43 phosphorylation status using an antibody that recognises multiple phosphorylated isoforms of Cx43 (Beardslee et al., 2000) (Figure 5b). Densitometric analysis of the gels confirmed that there was no significant difference between the phosphorylation status of Cx43 under control conditions or rotigaptide treatment detected by this antibody (Figure 5c).
The effect of rotigaptide on the phosphorylation status and expression of Cx43-GFP in HeLa cells was studied. After treatment with rotigaptide (50–250 nM) for 5 h, the phosphorylation status and level of Cx43-GFP expression remained unaltered (Figure 6c and d). However, preincubation of cells with the gap junction inhibitor 18 αGA reduced the phosphorylation of Cx43-GFP (Figure 6b); phosphorylation of Cx43 was similarly reduced following incubation with 18 αGA in the rotigaptide treated cells (Figure 6b).
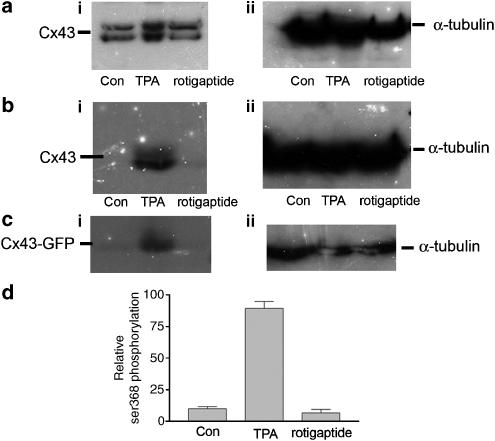
Rotigaptide is reported to stimulate protein kinase C (Dhein et al., 2003) and it is possible that the antibodies used in the current work failed to detect significant changes in phosphorylation at specific sites. We therefore investigated the effects of rotigaptide on Cx43 expression using an antibody specifically recognising the Ser368 phosphorylation site that is reported to be phosphorylated by protein kinase C (Lampe et al., 2000). Treatment of cells with TPA, a potent activator of protein kinase C (Lampe et al., 2000), confirmed that phosphorylation of Cx43 and Cx43-GFP at position ser368 occurred (Figure 7b and c). HeLa cells expressing wild-type Cx43 under control conditions, following TPA treatment and 5 h treatment with 50 nM rotigaptide were also probed with another antibody that detects multiple Cx43 isoforms (Rivedal et al., 1996). In both cases, there was no indication of alteration in the amount of Cx43 expressed following rotigaptide treatment, but it was noted that substantial phosphorylation occurs following TPA treatment (Figure 7a and b). Similar results were also obtained for Cx43 analysed from cardiac myocytes (data not shown).
Figure 7.
Analysis of Cx43 serine 368 phosphorylation. (a) (i) HeLa cells expressing wild-type Cx43 were probed with an antibody specifically targeted to the carboxyl tail of Cx43 under control conditions, treated with 100 ng ml−1 TPA for 30min or with 50 nM rotigaptide for 5 h prior to harvesting and Western blot analysis. (ii) α-Tubulin staining. (b) (i) Cx43 HeLa cells were probed with an antibody specifically targeted to serine 368 on the carboxyl tail of Cx43 under control conditions, treated with 100 ng ml−1 TPA for 30 min or with 50 nM rotigaptide for 5 h prior to harvesting and Western blot analysis. (ii) α-Tubulin staining. (c) (i) Cx43-GFP expressing HeLa cells were probed with an antibody specifically targeted to serine 368 on the carboxyl tail of Cx43 under control conditions, treated with 100 ng ml−1 TPA for 30 min or with 50 nM rotigaptide for 5 h prior to harvesting and Western blot analysis. (ii) α-Tubulin staining. (d) Densitometric analysis of ser 368 phosphorylation in Cx43-GFP expressing HeLa cells. All blots were performed on triplicate samples.
Discussion
We studied the specificity and effects of rotigaptide, an antiarrhythmic peptide, on intercellular communication across gap junctions in HeLa cell model systems and in cardio-specific cells. In heart, gap junctions provide low resistance intercellular channels that facilitate synchronous contraction of the myocytes (Kleber & Rudy, 2004). Since disturbed myocardial coupling is implicated in arrhythmogenesis (Xing et al., 2003; Severs et al., 2004), it is likely that drugs that modulate the extent of inter-myocyte communication via gap junctions may influence synchronisation and rhythmicity of contraction in the myocardium. The mechanism of action of such drugs should reveal new therapeutic approaches to understanding and correcting arrhythmia.
Rotigaptide is a stable analogue of the antiarrhythmic peptide AAP10 (Muller et al., 1997), its increased stability allowing protracted action by the use of D-amino acids that are less prone to degradation by proteases than L-amino acids. Rotigaptide has an in vitro half-life in human plasma of 14 days compared to 3–4 min for AAP10 (Kjolbye et al., 2003). Previous studies have shown that AAP10 enhanced conductance in adult cardiac myocytes and in HeLa cells expressing Cx43 (Dhein et al., 2001). Rotigaptide prevented re-entrant ventricular tachycardia during myocardial ischaemia in dogs (Xing et al., 2003). Although the effects of rotigaptide on cardiac electrophysiology and Cx43 mediated communication have been studied (Dhein et al., 2003; Eloff et al., 2003), its influence on other functional properties of gap junctions have not been investigated. Optimal doses of rotigaptide in these studies, were determined to be between 50 and 100 nM. In the present studies, we found that increasing the dose to 100 or 250 nM had no further enhancement of action.
The present results show that rotigaptide increased gap junctional intercellular communication in cells expressing Cx43 as monitored by the direct intercellular transfer of fluorescent dyes of different molecular mass. This increased communication was demonstrated in HeLa cells expressing wild-type Cx43 and Cx43-GFP, but was not observed in HeLa cells expressing wild-type Cx26, Cx26-GFP or Cx32-GFP. Although some connexins used in the present work were fused to GFP at its carboxyl terminus, HeLa cells expressing Cx43-GFP, Cx32-GFP and Cx26-GFP were shown previously to be electrically and dye coupled and to propagate intercellular Ca2+ waves to each other via gap junctions or connexin hemichannels (Bukauskas et al., 2000; Contreas et al., 2002). Fluorescent and nonfluorescent wild-type Cx43 expressing channels show similar functional characteristics in a variety of cell types, although subtle differences in channel gating have been reported (Bukauskas et al., 2000, 2002; Paemeleire et al., 2000). The present studies show that rotigaptide acts on both wild type and chimeric Cx43 but not on wild type or chimeric Cx26 channels or on chimeric Cx32 channels. Increased intercellular dye coupling was also observed in beating neonatal cardiac myocytes and a cell line derived from cardiac atria that possesses many cardio-specific properties (Claycomb et al., 1998). Cx43 is the dominant gap junction protein in both cell types (unpublished data). The enhancing effect on Cx43 dye coupling via gap junctions occurred after 5 h exposure to rotigaptide in a concentration independent manner. In contrast, the accelerated transmission of electrical signals following rotigaptide treatment was observed within 15 min using isolated guinea pig ventricular myocytes and rat atrial strip preparations (Xing et al., 2003; Haugan et al., 2005). These differences in the speed of action suggest that rotigaptide may firstly act rapidly on electrical coupling across gap junctions followed by an increase in the spread of larger molecules across the junctions and further support observations that dye movement and electrical communication via gap junctions are not always causally related. Despite the enhanced propagation of dye now reported and the increase in electrical coupling (Eloff et al., 2003; Haugan et al., 2005), rotigaptide had no effect on the overall synchronisation and contraction rate of the cardiac myocytes and the spatial localisation of Cx43 in cells was unaltered. Furthermore, even after exposure of cells to rotigaptide for 5 h the overall expression level of Cx43 in the HeLa cell model nor in neonatal cardiac myocytes was unchanged, suggesting that up to this time point rotigaptide is not affecting the dynamics of Cx43 life cycle (Martin & Evans, 2004).
Exposure of cells for 30 min to AAP10 and rotigaptide has been associated with enhanced protein kinase C activity in HeLa cells expressing Cx43, suggesting that rotigaptide may be phosphorylating Cx43 via a protein kinase C-dependent mechanism associated with enhanced electrical communication, and these studies implicated a 200 kDa plasma membrane protein (Weng et al., 2002; Dhein, 2004). However, these studies did not address Cx43 phosphorylation specifically. Phosphorylation of Cx43 has been associated with the control of gap junction mediated communication during connexin biogenesis and degradation (Laird, 2005) and in the response of cardiac myocytes to normoxia and hypoxia (Lampe & Lau, 2004; Turner et al., 2004). Activation of PKC results in phosphorylation of several serine sites including Ser368 and Ser 262 located on the carboxyl terminal tail of Cx43 (Lampe & Lau, 2004). However, no detectable changes in the phosphorylation status of Cx43 were observed following rotigaptide treatment by Western blot analysis, examined using a variety of antibodies that detect multiple Cx43 phosphorylated isoforms or a specific protein kinase C phosphorylation site (Serine, 368) (Lampe et al., 2000). Control experiments using the phorbol ester TPA, a potent inducer of protein kinase C (Lampe et al., 2000), showed that Cx43-GFP is subject to protein kinase C dependent phosphorylation at serine 368 further demonstrating that the attached GFP reporter protein does not modify normal Cx43 function. To ultimately determine whether rotigaptide specifically modulates the phosphorylation status of Cx43 on any of the multiple phosphorylation sites of the carboxyl terminus may require other techniques such as mass spectrometry, which would help to elucidate a concise mechanism of action of this peptide. It is also noteworthy that the Cx26-GFP and Cx32-GFP expressing HeLa cells were unaffected by rotigaptide and that these connexins are either not phosphorylated or are subject to minor phosphorylation (Lampe & Lau, 2004). Another potential, yet unexplored mechanism of action of rotigaptide action, is the effect it may have on Cx43 hemichannel function. The present experiments were performed under normal conditions of CO2, O2 and nutrient supply. It is possible that further mechanistic effects of rotigaptide are apparent under conditions of cell stress such as ischemia and those mimicking arrhythmia where further modifications of Cx43 by rotigaptide may be apparent and is subject to further investigation.
Intercellular communication across gap junctions in cells expressing various connexins, including homotypic Cx43, 32 and 26, have been extensively studied electrophysiologically (Harris, 2001) and by dye coupling (Elfgang et al., 1995; Cao et al., 1998). These studies have shown that connexin channels show differential permeabilities to a range of dyes of differing charge and MW, including those currently studied. The present data indicate that rotigaptide selectively influences homotypic gap junctions constructed of Cx43, but is without effect on gap junctions constructed of Cx26 and 32. It should be noted that the extent of enhancement of dye spread by rotigaptide through Cx43 channels may be underestimated due to the limits of detection of the system as the dye spreads from the injected cell to neighbouring cells. Indeed, the detection limits also vary with individual dyes depending on their quantum yield and rates of photobleaching. Such differences in the nature of the fluorescent probes and molecule transfer rates may also partly explain the enhanced transfer of the larger Alexa 594 compared to Lucifer yellow following rotigaptide treatment. Further differences on the effect of rotigaptide on molecule transfer will only be resolved by analysing the direct rates of dye spread by timelapse microscopy and rate kinetic analysis. The present data show that rotigaptide enhances the spread of dyes of differing molecular mass, with there being preferential enhancement of transfer of larger sized molecules and suggest that the nature of molecule transfer may be an important aspect of the mechanism of rotigaptide action.
The highly specific effect of rotigaptide on gap junction intercellular communication is further supported by radioligand binding assays in which rotigaptide displayed only weak binding affinity to 80 different receptors and ion channels (Haugan et al., 2005). Further analysis of the selective action of rotigaptide on connexins, to supplement electrophysiological studies in cardiac tissue (Eloff et al., 2003), is expected to elucidate the biochemical mechanisms that result in the action of rotigaptide occurring at Cx43 gap junctions but not on junctions constructed of Cx26 and Cx32. The effects of rotigaptide on other connexins associated with the cardiovascular system (Cx40 and Cx45) remain to be explored.
The emergence of rotigaptide as an enhancer of intercellular communication across gap junctions constructed of Cx43 supports the view that it is a useful reagent to develop further as a therapeutic tool for modulating acute cardiac arrhythmias.
Acknowledgments
This work was supported by a grant from Zealand Pharma and the Royal Society to PEM and a BHF studentship to T.C. We thank Mr Scott Johnstone and Miss Jennifer Easton for research assistance and Dr Susan Jamieson for supply of the HeLa Cx26 and 43 transfected cells.
Abbreviations
- BrDu
bromodeoxyuridine
- Cx
Connexin
- DMEM
Dulbecco's modified essential medium
- ECL
enhanced chemiluminescene
- FACS
fluorescent activated cell sorting
- FCS
foetal calf serum
- GFP
green fluorescent protein
- PBS
phosphate-buffered saline
- P/S
penicillin/streptomycin
- TPA
12-O-tetradecanoylphorbol 13-acetate
- 18αGA
18α-glycyrrhetinic acid
References
- BEARDSLEE M.A., LERNER D.L., TADROS P.N., LAING J.G., BEYER E.C., YAMADA K.A., KLEBER A.G., SCHUESSLER R.B., SAFFITZ J.E. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ. Res. 2000;87:656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- BUKAUSKAS F.F., ANGELE A.B., VERSELIS V.K., BENNETT M.V. Coupling asymmetry of heterotypic connexin 45/connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7113–7118. doi: 10.1073/pnas.032062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUKAUSKAS F.F., JORDAN K., BUKAUSKIENE A., BENNETT M.V.L., LAMPE P.D., LAIRD D.W., VERSELIS V.K. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAO F.L., ECKERT R., ELFGANG C., NITSCHE J.M., SNYDER S.A., HULSER D.F., WILLECKE K., NICHOLSON B.J. A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J. Cell Sci. 1998;111:31–43. doi: 10.1242/jcs.111.1.31. [DOI] [PubMed] [Google Scholar]
- CLAYCOMB W.C., LANSON N.A, JR, STALLWORTH B.S., EGELAND D.B., DELCARPIO J.B., BAHINSKI A., IZZO N.J., JR HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTREAS J.E., SANCHEZ H.A., EUGENIN E.A., SPEIDEL D., THEIS M., WILLECKE K., BUKAUSKAS F.F., BENNETT M.V.L., SAEZ J.C. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIDSON J.S., BAUMGARTEN I.M. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap junction intecellular communication. Structure–activity relationships. J. Pharmacol. Exp. Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- DHEIN S. Pharmacology of gap junctions in the cardiovascular system. Cardiovac. Res. 2004;62:287–298. doi: 10.1016/j.cardiores.2004.01.019. [DOI] [PubMed] [Google Scholar]
- DHEIN S., LARSEN B.D., PETERSEN J.S., MOHR F.W. Effects of the new antiarrhythmic peptide ZP123 on epicardial activation and repolarization pattern. Cell Commun. Adhes. 2003;10:371–378. doi: 10.1080/cac.10.4-6.371.378. [DOI] [PubMed] [Google Scholar]
- DHEIN S., WENG S., GROVER R., TUDYKA T., GOTTWALD M., SCHAEFER T., POLONTCHOUK L. Protein kinase C alpha mediates the effect of antiarrhythmic peptide on gap junction conductance. Cell Adhes. Commun. 2001;8:257–264. doi: 10.3109/15419060109080734. [DOI] [PubMed] [Google Scholar]
- DI W.L., RUGG E.L., LEIGH I.M., KELSELL D.P. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Invest. Dermatol. 2001;117:958–964. doi: 10.1046/j.0022-202x.2001.01468.x. [DOI] [PubMed] [Google Scholar]
- ELFGANG C., ECKERT R., LICHTENBERGFRATE H., BUTTERWECK A., TRAUB O., KLEIN R.A., HULSER D.F., WILLECKE K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa-cells. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELOFF B.C., GILAT E., WAN X., ROSENBAUM D.S. Pharmacological modulation of cardiac gap junctions to enhance cardiac conduction. Evidence supporting a novel target for antiarrhythmic therapy. Circulation. 2003;108:3157–3163. doi: 10.1161/01.CIR.0000101926.43759.10. [DOI] [PubMed] [Google Scholar]
- EVANS W.H., MARTIN P.E. Gap junctions: structure and function (Review) Mol. Membr. Biol. 2002;19:121–136. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- GEORGE C.H., MARTIN P.E., EVANS W.H. Rapid determination of gap junction formation using HeLa cells microinjected with cDNAs encoding wild-type and chimeric connexins. Biochem. Biophys. Res. Commun. 1998;247:785–789. doi: 10.1006/bbrc.1998.8835. [DOI] [PubMed] [Google Scholar]
- GOLDBERG G.S., MORENO A.P., LAMPE P.D. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J. Biol. Chem. 2002;277:36725–36730. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- HARRIS A.L. Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- HAUGAN K., OLSEN K.B., HARTVIG L., PETERSEN J.S., HOSTEIN-RATHLOU K.H., HENNAN J.K., NIELSEN M.S. The antiarrhythmic peptide analog ROTIGAPTIDE prevents atrial conduction slowing during metabolic stress. J. Cardiovasc. Electrophysiol. 2005;16:537–545. doi: 10.1111/j.1540-8167.2005.40687.x. [DOI] [PubMed] [Google Scholar]
- KJOLBYE A.L., KNUDSEN C.B., JEPSEN T., LARSEN B.D., PETERSEN J.S. Pharmacological characterization of the new stable antiarrhythmic peptide analog Ac-D-Tyr-D-Pro-D-Hyp-Gly-D-Ala-Gly-NH2 (ZP123): in vivo and in vitro studies. J. Pharmacol. Exp. Ther. 2003;306:1191–1199. doi: 10.1124/jpet.103.052258. [DOI] [PubMed] [Google Scholar]
- KLEBER A.G., RUDY Y. Basic mechanisms of cardiac impulse propagation and association with arrhythmias. Physicol. Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- LAIRD D.W. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim. Biophys. Acta. 2005;1711:172–182. doi: 10.1016/j.bbamem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- LAMPE P.D., LAU A.F. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPE P.D., TENBROEK EM BURT J.M., KURATA W.E., JOHNSON R.G., LAU A.F. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J. Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEWENSTEIN W.R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol. Rev. 1981;61:829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- MARTIN P.E.M., BLUNDELL G., AHMAD S., ERRINGTON R.J., EVANS W.H. Multiple pathways in the trafficking and assembly of Connexin 26, 32 and 43 into gap junctional intercellular communication channels. J. Cell Sci. 2001;114:3845–3855. doi: 10.1242/jcs.114.21.3845. [DOI] [PubMed] [Google Scholar]
- MARTIN P.E., HILL N.S., KRISTENSEN B., ERRINGTON R.J., GRIFFITH T.G. Ouabain exerts biphasic effects on connexin functionality and expression in vascular smooth muscle cells. Br. J. Pharmacol. 2004;141:374–384. doi: 10.1038/sj.bjp.0705671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN P.E., EVANS W.H. Incorporation of connexins into plasma membranes. Cardiovasc. Res. 2004;62:378–387. doi: 10.1016/j.cardiores.2004.01.016. [DOI] [PubMed] [Google Scholar]
- MARTIN P.E., WALL C., GRIFFITH T.M. Effects of connexin-mimetic peptides on gap junction functionality and connexin expression in cultured vascular cells. Br. J. Pharmacol. 2005;144:617–627. doi: 10.1038/sj.bjp.0706102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER A., GOTTWALD M., TUDYKA T., LINKE W., WILLECKE K., DHEIN S. Increase in gap junction conductance by an antiarrhythmic peptide. Eur. J. Pharmacol. 1997;327:65–72. doi: 10.1016/s0014-2999(97)89679-3. [DOI] [PubMed] [Google Scholar]
- NICHOLSON B., WEBER P.A., CAO F., CHANG H., LAMPE P., GOLDBERG G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz. J. Med. Biol. Res. 2000;33:369–378. doi: 10.1590/s0100-879x2000000400002. [DOI] [PubMed] [Google Scholar]
- NIESSEN H., HARZ H., BEDNER P., KRAMER K., WILLECKE K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J. Cell Sci. 2000;113:1365–1372. doi: 10.1242/jcs.113.8.1365. [DOI] [PubMed] [Google Scholar]
- PAEMELEIRE K., MARTIN P.E.M., COLEMAN S.L., FOGARTY K.E., CARRINGTON W.A., LEYBAERT L., TUFT R.A., EVANS W.H., SANDERSON M.J. Intercellular calcium waves in HeLa cells expressing GFP-labelled connexin 43, 32 or 26. Mol. Biol. Cell. 2000;11:1815–1827. doi: 10.1091/mbc.11.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVEDAL E., MOLLERUP S., HAUGEN A., VIKHAMAR G. Modulation of gap junctional intercellular communication by EGF in human kidney epithelial cells. Carcinogenesis. 1996;17:2321–2328. doi: 10.1093/carcin/17.11.2321. [DOI] [PubMed] [Google Scholar]
- RUMMERY N.M., HILL C.E. Vascular gap junctions and implications for hypertension. Clin. Exp. Pharmacol. Physiol. 2004;31:659–667. doi: 10.1111/j.1440-1681.2004.04071.x. [DOI] [PubMed] [Google Scholar]
- SAEZ J.C., BERTHOUD V.M., BRANES M.C., MARTINEZ A.D., BEYER E.C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- SEVERS N.J., COPPEN S.R., DUPONT E., YEH H.I., Ko Y.S., MATSUSHITA T. Gap junction alterations in human cardiac disease. Cardiovasc. Res. 2004;62:368–377. doi: 10.1016/j.cardiores.2003.12.007. [DOI] [PubMed] [Google Scholar]
- TURNER M.S., HAYWOOD G.A., ANDREKA P., YOU L., MARTIN P.E., EVANS W.H., WEBSTER K.A., BISHOPRIC N.H. Reversible connexin 43 dephosphorylation during hypoxia and reoxygenation is linked to cellular ATP levels. Circ. Res. 2004;95:726–733. doi: 10.1161/01.RES.0000144805.11519.1e. [DOI] [PubMed] [Google Scholar]
- WEBSTER K.A., WEBSTER K.A., DISCHER D.J., KAISER S., HERNANDEZ O., SATO B., BISHOPRIC N.H. Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J. Clin. Invest. 1999;104:239–252. doi: 10.1172/JCI5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENG S., LAUVEN M., SCHAEFER T., POLONTCHOUK L., GROVER R., DHEIN S. Pharmacological modification of gap junction coupling by an antiarrhythmic peptide via protein kinase C activation. FASEB J. 2002;16:114–116. doi: 10.1096/fj.01-0918fje. [DOI] [PubMed] [Google Scholar]
- WILLECKE K., EIBERGER J., DEGEN J., ECKARDT D., ROMUALDI A., GULDENAGEL M., DEUTSCH U., SOHL G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- XING D., KJOLBYE A.L., NIELSEN M.S., PETERSEN J.S., HARLOW K.W., HOLSTEIN-RATHLOU N.H., MARTINS J.B. ZP123 increases gap junctional conductance and prevents reentrant ventricular tachycardia during myocardial ischemia in open chest dogs. J. Cardiovasc. Electrophysiol. 2003;14:510–520. doi: 10.1046/j.1540-8167.2003.02329.x. [DOI] [PubMed] [Google Scholar]