Abstract
Oleamide (cis-9-octadecenoamide) exhibits some cannabimimetic responses despite its low affinities at the currently known cannabinoid receptors. Here we have investigated whether or not it is a vasorelaxant in rat small mesenteric arteries.
Oleamide elicited vasorelaxation (EC50=1.2±0.2 μM, Rmax=99.1±3.9%, n=8) which was reduced by endothelial removal. Nitric oxide synthase inhibition reduced the response (EC50=5.3±1.6 μM, Rmax=59.2±7.7%, n=7; P<0.01) as did blockade of Ca2+-sensitive K+ channels (KCa) with apamin plus charybdotoxin (both 50 nM) (EC50=2.1±0.2 μM, Rmax=58.4±1.9%, n=5; P<0.05). Desensitisation of vanilloid receptors with capsaicin (10 μM for 30 min) shifted the oleamide concentration–response curve ∼30-fold to the right (n=7; P<0.01). Pertussis toxin (400 ng ml−1 for 2 h) caused a two-fold shift in the response curve (EC50=2.2±0.4 μM, Rmax=66.8±4.5%, n=6; P<0.01).
Rimonabant (CB1 cannabinoid receptor antagonist; SR141716A; 3 μM) significantly inhibited relaxation induced by oleamide (EC50=3.5±0.3 μM, Rmax=75.1±1.9%; n=8; P<0.05). In contrast, neither the more selective CB1 receptor antagonist, AM251 (1 μM), nor the CB2 antagonist, SR144528 (1 μM), had significant effects. O-1918 (10 μM), a putative antagonist at a novel endothelial cannabinoid receptor (abnormal-cannabidiol site), markedly reduced the relaxation to oleamide (n=7; P<0.01).
It is concluded that oleamide responses in the rat isolated small mesenteric artery are partly dependent on the presence of the endothelium, activation of Ca2+-sensitive K+ channels (KCa) and involve capsaicin-sensitive sensory nerves. Oleamide may share a receptor (sensitive to rimonabant and O-1918, and coupled to KCa and Gi/o) with anandamide in this vessel. This might be distinct from both of the known cannabinoid receptors and the novel abnormal-cannabidiol site.
Keywords: Oleamide, rat mesenteric artery, cannabinoid receptors, rimonabant, SR141716A, O-1918, endothelium, EDHF
Introduction
Cannabimimetic and related lipid mediators have aroused great interest in their biological effects in the last decade. Anandamide, which is an ethanol amide of arachidonic acid, was proposed to be an endocannabinoid by Devane et al. (1992). More recently, oleamide (cis-9-octadecenoamide) has been identified in the cerebrospinal fluid of sleep-deprived cats (Cravatt et al., 1995). It is structurally similar to the prototypical endocannabinoid, anandamide (cf. Figure 1) and is degraded by the same enzyme, fatty acid amide hydrolase (FAAH; Bisogno et al., 1998). Thus, oleamide is likely to have common, as well as perhaps distinct, pathways of action with anandamide.
Figure 1.
Structures of anandamide and oleamide.
Like anandamide and some other CB1 receptor agonists, oleamide has cannabimimetic effects in vivo including hypothermia (Ledent et al., 1999; Huitron-Resendiz et al., 2001; Lichtman et al., 2002) which has been proposed to be mediated through the activation of CB1 receptors (Ledent et al., 1999). However, several reports have indicated that oleamide exhibits negligible interaction with the currently known CB1 or CB2 receptors (Lichtman et al., 2002; Bradshaw & Walker, 2005). Furthermore, in CB1 receptor-knockout mice oleamide causes hypothermia and some other effects that might be considered cannabimimetic (Lichtman et al., 2002). More recently, Leggett et al. (2004) have produced evidence that oleamide acts as a full agonist at CB1 receptors in vitro but the concentrations required may not be of physiological importance (∼3–100 μM; Fowler, 2004). As a result, the classification of oleamide as an endocannabinoid has been very controversial. Indeed, its low affinity at the currently known cannabinoid receptors led Lambert & Di Marzo (1999) to put forward the interesting proposal that oleamide shows some of its cannabimimetic actions through an ‘entourage' effect. That is, since oleamide is a preferred substrate for FAAH, it might potentiate or prolong the effects of endocannabinoids such as anandamide by competitively inhibiting the enzyme FAAH. Nevertheless, the possibility that oleamide acts through activating specific receptors should not be excluded.
Modulation of vascular tone by cannabimimetic lipid mediators has attracted much attention in the last few years (for reviews, see Randall et al., 2002; Hiley & Ford, 2004). Since the first investigations of the effects of anandamide, many more cannabimimetic fatty acid derivatives have been added to the family, including 2-arachidonyl glycerol, N-arachidonoyl dopamine (NADA) and virodhamine (Mechoulam et al., 1995; Bisogno et al., 2000; Porter et al., 2002). Since anandamide and other endocannabinoids can relax rat isolated small mesenteric arteries (Kagota et al., 2001; Randall et al., 2002; O'Sullivan et al., 2004; Ho & Hiley, 2004), it is of interest to investigate oleamide as a potential vasorelaxant in isolated blood vessels.
In view of the fact that anandamide has been shown to cause vasorelaxation by many mechanisms (Randall et al., 2002; Hiley & Ford, 2004), this study has investigated the vascular effects of oleamide in the rat small mesenteric artery. Oleamide was found to be a vasorelaxant and so experiments were carried out to determine involvement in the responses of the endothelium, nitric oxide, and Ca2+-sensitive K+ channels (KCa) as well as VR1 vanilloid receptors and the putative endothelial cannabinoid receptor sensitive to O-1918 (Begg et al., 2003; Offertáler et al., 2003).
Methods
Myograph studies
Male Wistar rats (300–400 g; Charles River U.K. Ltd, Kent) were killed with an overdose of sodium pentobarbitone (120 mg kg−1, i.p.; Sagatal, Rhône Mérieux, Harlow, Essex, U.K.); all animal care and use was in accordance with the U.K. Animal (Scientific Procedures) Act 1986. The mesenteric arterial bed was removed rapidly and placed into cold Krebs–Henseleit buffer solution of the following composition (mM): NaCl, 118; KCl 4.7; MgSO4, 1.2; KH2PO4 1.2; NaHCO3, 25; CaCl2, 2.5; D-glucose, 5.5. The Krebs–Henseleit solution also contained 10 μM indomethacin (unless otherwise stated) and was bubbled with 95% O2 : 5% CO2 to give a pH of 7.4.
Small (third generation) mesenteric arteries (internal diameter, 312±4 μm; 136 vessels) were then dissected free and cleaned of adherent tissue. Segments (2 mm long) were mounted in a Mulvany–Halpern-type wire myograph (Danish Myo Technology, Aarhus, Denmark) maintained at 37°C in gassed (95% O2 : 5% CO2) Krebs–Henseleit solution and normalised as described previously (White & Hiley, 1997). Tension was measured and recorded on a PowerLab recording system (ADInstruments, Hastings, Sussex) connected to a Macintosh personal computer. The presence of a functional endothelium was tested by precontracting the vessels with methoxamine (10 μM), and then relaxing by carbachol (10 μM); endothelial integrity was designated by relaxations >90% of the methoxamine-induced precontraction. When the endothelium was not required, vessels were denuded by rubbing the intimal surface with a human hair, and successful endothelial removal was confirmed by a lack of vasorelaxant response (<10% of the precontraction) to carbachol.
Experimental protocols
After 30 min equilibration, vessels were precontracted with a submaximal concentration of methoxamine (10 μM). When a stable level of tone was achieved, a concentration–response curve to oleamide was constructed by cumulative addition of the drug; a vehicle control for oleamide was also obtained by adding an appropriate amount of the ethanol vehicle to precontracted vessels. Preliminary studies showed considerable variation in the relaxation responses to oleamide between vessels from different animals, with oleamide being less efficacious in some animals than in others. As a result, animals in which oleamide induced a reduced vasorelaxation (<20% at 30 μM) of the methoxamine-precontracted tone were discarded from the analysis of the effects of oleamide, while all other experiments were performed in a paired fashion, with control and test experiments being carried out on arteries from the same animal. No single preparation was exposed to more than one agonist or used for the construction of more than one concentration–response curve.
In experiments where the effect of a vasorelaxant was investigated in the presence of antagonists (rimonabant, AM251, SR144528 or O-1918) or apamin, charybdotoxin, arachidonyl trifluoromethyl ketone (ATFMK), NG-nitro-L-arginine methyl ester (L-NAME), or capsaicin, these agents were added to the organ bath 30 min before, and then were present during, the construction of the concentration–response curve. However, in the case of pertussis toxin, the preincubation was for 2 h. In experiments where vessels were incubated with these putative inhibitors, the tension of the precontraction was normalised to that obtained in the endothelial integrity test by lowering or increasing the concentration of methoxamine before construction of the concentration–relaxation curve to oleamide. The mean submaximal contraction with methoxamine in the rat mesenteric artery segments was 12.9±0.7 mN (34 vessels) in the test for endothelium as compared with 12.6±0.7 mN (34 vessels) when the putative inhibitors were present. In some experiments, the arteries were precontracted with high K+ (60 mM KCl) Krebs–Henseleit solution (prepared by equimolar substitution of NaCl for KCl in the standard Krebs–Henseleit buffer, as described previously; White & Hiley, 1997). The mean tension generated by 60 mM KCl (10.6±1.5 mN) was similar to the tone induced in the same vessels by methoxamine in the test for endothelium (12.1±2.0 mN; six vessels).
Data and statistical analysis
Relaxation responses are expressed as the percentage relaxation of the precontraction induced by 10 μM methoxamine or 60 mM KCl. Data are given as the mean±s.e.m. and n indicates the number of rats. When a defined maximum relaxant response was observed, the data were fitted to a logistic equation of the following form:
where R is the reduction in tone, A the concentration of the agonist, Rmax the maximal reduction of established tone, nH the slope function and EC50 the agonist concentration giving half the maximal relaxation. The curve fitting was carried out using KaleidaGraph (Synergy Software, Reading, PA, U.S.A.). Statistical comparisons of concentration–response curves were made by two-way analysis of variance of the whole data set, followed by the Bonferroni post hoc test for determining significant differences between treatment groups (StatView 4.5 for the Macintosh; Abacus Concepts, Inc., Berkeley, CA, U.S.A.). P-values less than 0.05 were considered to be statistically significant.
Drugs
Methoxamine hydrochloride, carbachol, charybdotoxin, L-NAME (NG-nitro-L-arginine methyl ester; Sigma Chemical Company, Poole, Dorset, U.K.) and apamin (Calbiochem, Nottingham, U.K.) were dissolved in deionised water. Indomethacin (Sigma) was dissolved in 5% w v−1 NaHCO3 solution. Arachidonoyl trifluoromethylketone (ATFMK; Alexis Corporation, Nottingham) was dissolved in 100% dimethyl sulphoxide (DMSO; Sigma). Oleamide (Tocris Cookson, Bristol), capsaicin (Sigma), rimonabant (a generous gift from Sanofi-Synthélabo, Montpellier, France), SR 144528 (N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide; also a generous gift from Sanofi-Synthélabo), AM 251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; Tocris Cookson) and O-1918 ((−)-1,3-dimethoxy-2-(3-3,4-trans-p-menthadien-(1,8)-yl)- orcinol; a generous gift from Dr George Kunos, National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health, Bethesda, MD, U.S.A.) were dissolved in 100% ethanol.
Results
Preliminary experiments indicated that oleamide was less efficacious in some rats than in others, with considerable variation in the maximal relaxation between individual rats. Therefore, when the relaxation induced by 30 μM oleamide was <20% of the methoxamine-induced tone, the results for those animals in respect of oleamide were discarded; approximately 30% of the animals fell into this category. All other experiments were performed in a pairwise fashion, with control and test experiments being carried out on arteries from the same animal.
Effect of endothelial removal, nitric oxide synthase inhibition and K+ channel blockers
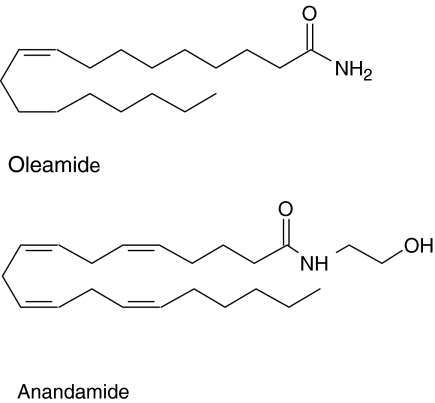
In those rats that were responsive to oleamide it caused a concentration-dependent relaxation of methoxamine-induced tone in the endothelium-intact small mesenteric artery (EC50=1.2±0.2 μM, Rmax=99.1±3.9%, n=8; Figure 2a). Removal of the endothelium markedly reduced the sensitivity of the vessels to oleamide, with about a 10-fold shift of the curve to the right (n=8; Figure 2a; P<0.01). Figure 2a also shows that the ethanol vehicle (up to 0.6% v v−1 final concentration in myograph bath) had a negligible relaxant effect.
Figure 2.
Concentration–response curves for relaxation of the methoxamine-induced tone by oleamide in the isolated small mesenteric artery of the rat. (a) Relaxation was determined in the presence and absence of a functional endothelium; n=8 for both. The effects of the appropriate amounts of ethanol (EtOH) vehicle are also shown, the final concentration of ethanol at the greatest volume used was 0.6% v v−1 (n=8). (b) Concentration–response curves for relaxation by oleamide were determined in the presence of L-NAME (300 μM, n=7) or the combination of charybdotoxin plus apamin (both 50 nM, n=5) with intact endothelium. Values are shown as means and vertical lines represent the s.e.m.
On the other hand, Figure 2b shows that the presence of the nitric oxide synthase inhibitor L-NAME (300 μM) alone significantly reduced the oleamide-induced relaxation (control: EC50=1.4±0.2 μM, Rmax=90.7±2.9%, n=7; L-NAME: EC50=5.3±0.7 μM, Rmax=59.2±7.7%, n=7; P<0.01). Similarly, the combination of apamin (a blocker of small conductance KCa; 50 nM) with charybdotoxin (a blocker of intermediate and large conductance KCa; 50 nM) caused significant inhibition of the relaxation (EC50=2.1±0.2 μM, Rmax=58.4±1.9%, n=5; Figure 2b; P<0.05). The relaxation induced by oleamide was also markedly reduced when the vessels were contracted by 60 mM KCl instead of 10 μM methoxamine (data not shown).
Effects of indomethacin and arachidonoyl trifluoromethyl ketone
Omission of indomethacin from the bathing solution had no significant effect on the response neither did inclusion of the fatty acid amide hydrolase inhibitor, ATFMK (10 μM), affect the response. Combination of the two agents also had no significant effect on the relaxation to oleamide in endothelium-intact vessels (control: EC50= 1.1±0.2 μM, Rmax=84.2±3.5%; in the absence of indomethacin: EC50=1.9±1.4 μM, Rmax=90.0±8.2%, n=4; in the presence of ATFMK alone: EC50=0.9±0.2 μM, Rmax=81.6±5.2%, n=4; in the absence of indomethacin and presence of ATFMK: EC50=1.1±0.2 μM, Rmax=93.6±4.9%; n=4).
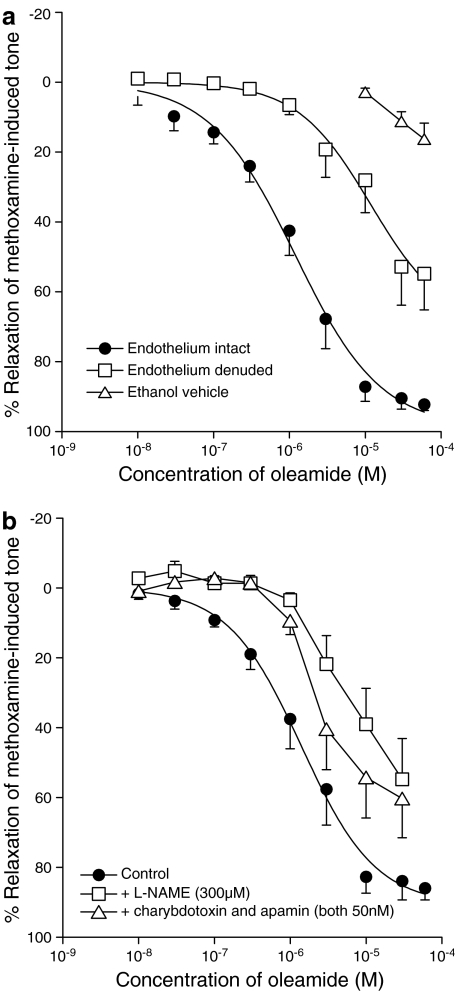
Effects of pretreatment with capsaicin or pertussis toxin
In experiments in vessels with an intact endothelium, functional desensitisation of the vanilloid receptor system by capsaicin (pretreatment with 10 μM for 30 min) markedly reduced the relaxation induced by oleamide. Figure 3a shows that there was an approximate 30-fold shift to the right of the concentration–response curve to oleamide after pretreatment with the vanilloid receptor agonist (n=7; P<0.01). Capsaicin pretreatment did not have any significant effect in the residual vasorelaxation induced by oleamide in endothelium-denuded experiments (control without endothelium: EC50= 7.9±0.5 μM, Rmax=49.7±9.5%; after capsaicin and without endothelium: EC50=8.0±2.0 μM, Rmax=50.3±5.4%, n=6).
Figure 3.
Concentration–response curves for relaxation of methoxamine-induced tone in rat mesenteric vessels with an intact endothelium by oleamide after pretreatment (a) with capsaicin (10 μM for 30 min, n=7) or (b) with pertussis toxin (400 ng ml−1 for 2 h, n=6). Values are shown as the mean and vertical lines represent the s.e.m.
Figure 3b shows that preincubation with pertussis toxin (400 ng ml−1 for 2 h) significantly inhibited the relaxation to oleamide, causing an approximate two-fold shift in the concentration–response curve (control: EC50=1.0±0.8 μM, Rmax=83.1±2.5%; pertussis toxin: EC50=2.2±0.4 μM, Rmax=66.8±4.5%, n=6; Figure 3b; P<0.01).
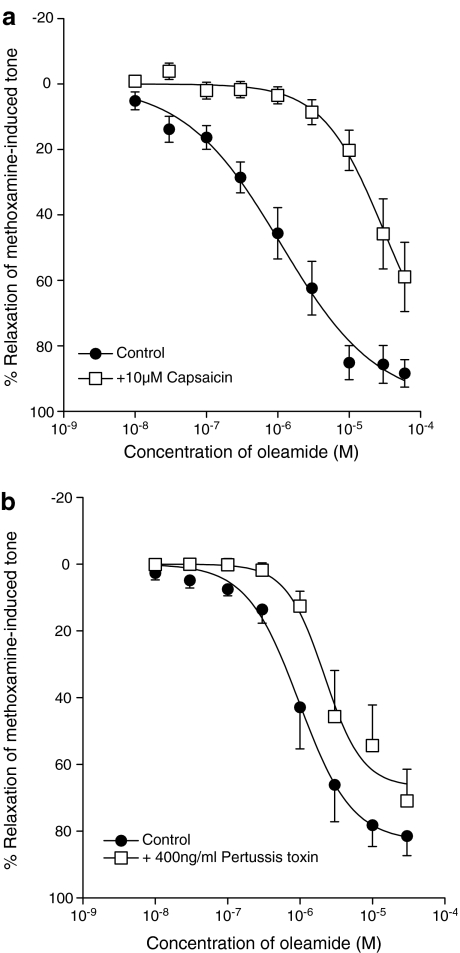
Effects of receptor antagonists on relaxations to oleamide
The cannabinoid receptor antagonist rimonabant (3 μM) significantly inhibited relaxation induced by oleamide as shown in Figure 4a, giving about a two-fold shift of the EC50 and a slight reduction in maximal response (control: EC50=1.6±0.3 μM, Rmax=96.3±5.1%; rimonabant: EC50=3.5±0.3 μM, Rmax=75.1±1.9%; n=8; Figure 4a; P<0.05). Lower concentrations of rimonabant (100 nM and 1 μM) did not produce any significant effects on the relaxation (100 nM control: EC50=5.4±1.3 μM, Rmax=76.0±7.6%; 100 nM rimonabant: EC50=7.0±1.6 μM, Rmax=77.4±3.6%; n=5; 1 μM control: EC50=5.7±0.5 μM, Rmax=81.8±2.4%; 1 μM rimonabant: EC50=6.6±0.5 μM, Rmax=74.8±6.9%; n=5).
Figure 4.
Concentration–response curves for relaxation of methoxamine-induced tone in rat mesenteric vessels with an intact endothelium by oleamide in the presence of (a) 3 μM rimonabant (n=8) or (b) 10 μM O-1918 (n=7). Values are shown as the mean and vertical lines represent the s.e.m.
In contrast, neither the more selective CB1 receptor antagonist, AM 251 (1 μM), nor the CB2 receptor antagonist, SR 144528 (1 μM) had any significant effect on the relaxation induced by oleamide (control for AM 251: EC50=1.6±0.3 μM, Rmax=95.1±6.3%; AM 251: EC50=1.9±0.6 μM, Rmax= 98.0±9.5%, n=4; P>0.1; control for SR 144528: EC50= 1.0±0.4 μM, Rmax=95.1±4.1%; SR 144528: EC50= 1.2±0.9 μM, Rmax=98.6±5.9%, n=4; P>0.5).
However, Figure 4b shows that O-1918 (10 μM), the putative antagonist for the novel endothelial cannabinoid receptor (abnormal-cannabidiol site), also produced a marked shift (approximately 25-fold) in the concentration–relaxation curve for oleamide (n=7; Figure 4b; P<0.01).
Effects in vessels from rats in which oleamide showed a reduced efficacy
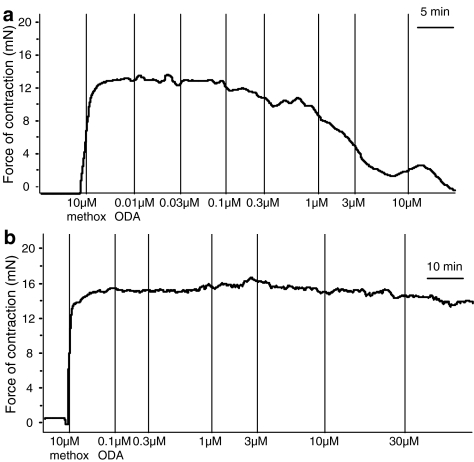
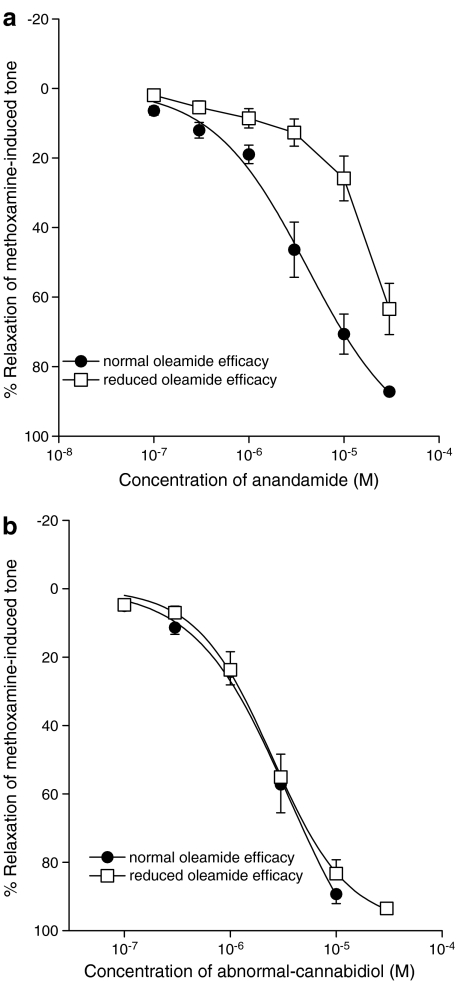
As noted above, oleamide was found to be less efficacious in vessels from some rats. Figure 5 contrasts the typical relaxant response induced by oleamide in rats where it exhibited a normal efficacy with that in rats which showed the lesser response. Low-response rats gave less than 20% relaxation of the induced tone at 30 μM oleamide, a concentration that usually induced near complete relaxation. When separate vessels from these same low-response rats were tested with anandamide, Figure 6a shows that reduced effects were also observed; anandamide showed a reduced potency and a significantly reduced relaxant response at a concentration of 30 μM, which usually causes complete relaxation, for example see White & Hiley (1997). In vessels with normal oleamide efficacy, the EC50 was 4.1±1.3 μM and the relaxation at 30 μM was 87.2±1.0% (n=6), while in rats showing reduced oleamide efficacy, the Rmax was 63.4±7.4% (n=5; P<0.01). However, vessels from these low-response rats relaxed completely to abnormal-cannabidiol (Figure 6b, normal oleamide efficacy: EC50=2.3±0.2 μM, Rmax=89.3±2.8%, n=7; reduced oleamide efficacy: EC50=2.5±0.2 μM, Rmax=98.3±2.5%, n=6; P>0.5).
Figure 5.
Typical traces of the relaxant responses induced by oleamide (ODA) in the small mesenteric artery from sensitive rats and lower sensitivity rats. (a) Responses recorded in a vessel from a rat in which oleamide showed normal efficacy. (b) Responses recorded in a vessel from a rat in which oleamide showed reduced efficacy.
Figure 6.
Concentration–response curves for relaxation of methoxamine-induced tone in rat mesenteric vessels with an intact endothelium by (a) anandamide in rats in which oleamide showed normal efficacy (n=6) and reduced efficacy (n=5) and by (b) abnormal-cannabidiol in rats in which oleamide showed normal efficacy (n=7) and reduced efficacy (n=6).
Discussion
The present study shows that oleamide induces vasorelaxation in the rat isolated small mesenteric artery. The response is partly endothelium-dependent and the amide acts through a number of different mechanisms involving not only the endothelium but also the vascular smooth muscle layer and the sensory nerves. Although oleamide shares its degradative enzyme, FAAH, with anandamide (Bisogno et al., 1998; Lambert & Di Marzo, 1999), and is potentially able to be metabolised rapidly, this study shows that the presence of neither the cyclooxygenase inhibitor indomethacin nor the FAAH inhibitor ATFMK, alone or in combination, had any significant effect on the relaxation induced by oleamide in responsive vessels. This suggests that vasoactive metabolites, such as prostanoid-like compounds, resulting from the metabolism of oleamide are not the major means of relaxation employed unlike as has been suggested for anandamide in bovine coronary artery (Pratt et al., 1998).
In endothelium-intact vessels, the presence of the nitric oxide synthase inhibitor L-NAME attenuated the relaxant response, suggesting that oleamide stimulates formation of nitric oxide by the endothelial cells. In many studies, it has been shown that nitric oxide is not involved in vasorelaxation to anandamide in rat small mesenteric artery (e.g. White & Hiley, 1997; O'Sullivan et al., 2005) although some studies have reported a role for nitric oxide, for example in the rat renal artery (Deutsch et al., 1997) or the rabbit isolated aorta (Mukhopadhyay et al., 2002). Nitric oxide also seems not to be involved in the relaxation responses produced by the putative endocannabinoids virodhamine (Ho & Hiley, 2004) and NADA (O'Sullivan et al., 2004). It is noteworthy that the oleamide homologue, oleoylethanolamide, is an agonist at the nuclear peroxisome proliferator-activated receptor-α (PPARα; Fu et al., 2003). PPARα has been identified in different vascular cell types including endothelial cells and smooth muscle cells, and Tabernero et al. (2002) have suggested that the activation of PPARα can lead to the generation of endothelial relaxant factors including nitric oxide in the mouse mesenteric bed. This might explain the sensitivity of the oleamide-induced relaxation to L-NAME although the time scale of responses to PPARα activation might be too slow relative to that of the responses reported here.
The sensitivity of relaxation to the combination of the KCa inhibitors apamin and charybdotoxin indicates that these channels also play a role in the vasorelaxation responses to oleamide and suggest that oleamide can act through the mechanisms attributed to the endothelium-derived hyperpolarising factor (EDHF). This conclusion is supported by the further observation that the relaxant response to oleamide was greatly reduced if the vessels were precontracted with KCl rather than methoxamine. This characteristic sensitivity to the combination of apamin and charybdotoxin is shared by vasorelaxant agonists which act by causing endothelium-dependent relaxation and is a feature of the vasorelaxant actions of anandamide (White & Hiley, 1997). Indeed, Randall et al. (1996) proposed that anandamide was EDHF but this has been discounted (Plane et al., 1997; White & Hiley, 1997). Therefore, both anandamide and oleamide appear to be able to bring about vasorelaxation which is mediated in part by vascular smooth muscle hyperpolarisation that reduces Ca2+ influx through voltage-gated Ca2+ channels and hence vasorelaxation (Busse et al., 2002).
Another similarity with anandamide is that the relaxation response to oleamide was significantly reduced by functional desensitisation of sensory nerves. Zygmunt et al. (1999) and White et al. (2001) have shown that capsaicin, the pungent ingredient of chilli peppers, which causes activation of TRPV1 vanilloid receptors, reduces the vasorelaxation to anandamide in some (e.g. mesenteric artery), but not all (e.g. coronary artery), rat blood vessels. The stable analogue of anandamide, methanandamide, also acts through a similar mechanism (Ralevic et al., 2000) which suggests that it is not a breakdown product or metabolite that acts on the nerve endings. The present results suggest that activation of TRPV1 receptors, which are likely to be situated on perivascular sensory nerves, and the subsequent release of the vasoactive calcitonin gene-related peptide (CGRP), is also contributing to the relaxation responses induced by oleamide.
A residual relaxant response was observed after pretreatment with capsaicin, which shows that it must be acting through mechanisms other than TRPV1 receptor activation. Interestingly, the residual relaxation response produced by oleamide in endothelium-denuded vessels was not affected by the functional desensitisation of sensory nerves by capsaicin. The relationship between CGRP-induced vasodilatation and the endothelium is not entirely understood, nevertheless this evidence further supports the proposal that oleamide works through vasorelaxation mechanisms independent of both the endothelium and the sensory nerves. Oleamide has been shown to have negligible activity at both CB1 and CB2 receptors (Lichtman et al., 2002; Bradshaw & Walker, 2005) except, perhaps, at high concentrations in vitro (Leggett et al., 2004). Here it was found that, in the rat isolated small mesenteric artery, both AM 251 (a selective CB1 receptor antagonist; Lan et al., 1999) and SR 144528 (a CB2 receptor antagonist, used at a concentration 300-fold greater than the published Ki for CB2 receptors; Rinaldi-Carmona et al., 1998) had no significant effect on oleamide-induced relaxation. This further supports findings that oleamide does not mediate its major actions through currently known CB1 or CB2 cannabinoid receptors.
More interestingly, the relaxation to oleamide is sensitive to rimonabant (formerly SR 141716A) at a concentration (3 μM) below those at which it has been shown to cause nonspecific effects in the rat mesenteric artery (White & Hiley, 1998) but at which it antagonises the vascular relaxant effects of anandamide (White & Hiley, 1997). Rimonabant, apart from being a CB1 receptor antagonist (Showalter et al., 1996), is also an antagonist at the recently defined abnormal-cannabidiol receptor in the rat mesenteric artery (Járai et al., 1999; Ho & Hiley, 2004). However, responses to both anandamide (Randall et al., 1996; White & Hiley, 1997) and abnormal-cannabidiol (Ho & Hiley, 2003) were antagonised by 1 μM rimonabant, whereas this lower concentration had no significant effect on the responses to oleamide. The lack of effect of either 1 μM rimonabant, or of the lower concentration of 0.1 μM further shows that the receptor at which oleamide is acting is not the CB1 cannabinoid receptor as rimonabant shows a Kd of 12.3 nM for this receptor (Showalter et al., 1996), which means that these two concentrations should give shifts of approximately 80- and 7-fold, respectively.
Oleamide-induced relaxation was also significantly attenuated by O-1918, a structural analogue of cannabidiol that acts as an antagonist of the abnormal-cannabidiol receptor (Begg et al., 2003; Offertáler et al., 2003). The concentration of O-1918 used (10 μM) does not bind to cloned CB1 or CB2 receptors and has been shown to cause a 10-fold rightward shift of the concentration–relaxation curve for abnormal-cannabidiol in the rat isolated small mesenteric artery (Offertáler et al., 2003). This suggests that the abnormal-cannabidiol receptor might be involved in the oleamide vasorelaxant response. In this regard, it is noteworthy that anandamide has also been shown to cause mesenteric vasorelaxation by mechanisms apparently independent of CB1 and CB2 receptors (Ho & Hiley, 2003) and has been suggested to activate the abnormal-cannabidiol receptor (Járai et al., 1999; Offertáler et al., 2003).
However, during the course of the study, it was noticed that oleamide was more efficacious in some rats than in others. In some rats, oleamide produced reduced relaxation responses (i.e. relaxation to 30 μM oleamide was <20% of the methoxamine-induced tone) and it is very noteworthy that, when the small mesenteric arteries from these rats were exposed to anandamide, a reduction in vasorelaxation response was also observed. This phenomenon could not be due to desensitisation as no vessel was exposed to more than one agonist. In contrast, responses produced by abnormal-cannabidiol were unchanged in the vessels from the same rats. It is unclear why oleamide and anandamide are less efficacious in some rats than in others. However, the lack of effect of the FAAH inhibitor ATFMK on responses to oleamide suggests that metabolism by FAAH, by which both anandamide and oleamide are degraded, does not limit the size of the response to the amide, at least in those vessels found to be responsive to it. However, it is possible that the nonresponsive rats might be characterised by a high rate of FAAH activity that could give rise to the observed differential efficacy of oleamide. The lack of effect of ATFMK on the responses to oleamide provides further evidence that the effects of oleamide are not likely to be just an ‘entourage effect' by which fatty acid endocannabinoids, such as anandamide, or their analogues act by competitively inhibiting the enzyme FAAH to potentiate or prolong the effects of endocannabinoids (Lambert & Di Marzo, 1999).
Several strands of evidence therefore suggest that oleamide might not act at the receptor for abnormal-cannabidiol in the rat mesenteric artery. Firstly, there is the differential effectiveness of oleamide, anandamide and abnormal-cannabidiol as vasorelaxants between individual rats. All rat vessels were relaxed by abnormal-cannabidiol but not all by oleamide. However, in those rats in which oleamide showed very low or no efficacy as a vasorelaxant, anandamide was a less potent vasorelaxant (Figure 6b). Secondly, there is the lesser antagonism by 1 μM rimonabant of responses to oleamide (it had no significant effect) and anandamide (it produces a two-fold shift; White & Hiley, 1997) relative to abnormal-cannabidiol (where it gave an eight-fold shift in the concentration–relaxation curve; Ho & Hiley, 2003). Also, O-1918 (at a concentration of 10 μM) gives a 10-fold shift to the right in the concentration–response curve of abnormal-cannabidiol while it gave a shift of about 26-fold in the case of anandamide (Offertáler et al., 2003) and about 25-fold for oleamide (this study). Together these points lead us to the suggestion that the abnormal-cannabidiol receptor might not be the endothelial receptor at which oleamide acts. The similarity of the shifts observed with 10 μM O-1918 in the concentration–relaxation curves for oleamide and anandamide suggest the possibility that oleamide might share a receptor with anandamide.
Oleamide-induced vasorelaxation was also found to be sensitive to pertusis toxin (an inhibitor of Gi/o protein activity), indicating that its receptor shares similar downstream pathways with other cannabinoid receptors (Felder et al., 1995). Recently, an orphan G-protein-coupled receptor, GPR55, has attracted interest as a potential novel cannabinoid receptor, although the limited characterisation of it so far suggests that it does not couple through Gi (see Bakeret al., 2006). Thus it seems unlikely at first sight that this orphan is the receptor for oleamide, but more rigorous investigation of the pharmacology of GPR55 will reveal if this is correct.
In conclusion, the present study shows that oleamide is a potent vasorelaxant in the isolated rat small mesenteric artery and, like anandamide, it shows multiplicity with regard to the mechanisms by which it produces its effects. The relaxation it induces is partly dependent on the presence of a functional endothelium and the activation of KCa channels, as well as involving perivascular capsaicin-sensitive sensory nerves. Further, results with rats which showed reduced responses induced by oleamide suggest that it might share a receptor with anandamide in the rat mesenteric artery which is distinct from both the currently known cannabinoid receptors as well as the putative endothelial abnormal-cannabidiol site in the rat mesenteric artery. This receptor is sensitive to both rimonabant and O-1918, and is possibly coupled to KCa and Gi/o.
Abbreviations
- ATFMK
arachidonoyl trifluoromethylketone
- DMSO
dimethyl sulphoxide
- EDHF
endothelium-derived hyperpolarising factor
- FAAH
fatty acid amide hydrolase
- KCa
Ca2+-sensitive K+ channels
- L-NAME
NG-nitro-L-arginine methyl ester
- NADA
N-arachidonoyl dopamine
- PPARα
peroxisome proliferator-activated receptor-alpha
References
- BAKER D., PRYCE G., DAVIES W.L., HILEY C.R.In silico patent searching reveals a new cannabinoid receptor Trends Pharmacol. Sci. 2006. doi:10.1016/j.tips.2005.11.003 [DOI] [PubMed]
- BEGG M., MO F.M., OFFERTÁLER L., BÁTKAI S., PACHER P., RAZDAN R.K., LOVINGER D.M., KUNOS G. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J. Biol. Chem. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., KATAYAMA K., MELCK D., UEDA N., DE PETROCELLIS L., YAMAMOTO S., DI MARZO V. Biosynthesis and degradation of bioactive fatty acid amides in human breast cancer and rat pheochromocytoma cells – implications for cell proliferation and differentiation. Eur. J. Biochem. 1998;254:634–642. doi: 10.1046/j.1432-1327.1998.2540634.x. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., MELCK D., BOBROV M., GRETSKAYA N.M., BEZUGLOV V.V., DE PETROCELLIS L., DI MARZO V. N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000;351:817–824. [PMC free article] [PubMed] [Google Scholar]
- BRADSHAW H.B., WALKER J.M. The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 2005;144:459–465. doi: 10.1038/sj.bjp.0706093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concepts together. Tr. Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- CRAVATT B.F., PROSPERO-GARCIA O., SIUZDAK G., GILULA N.B., HENRIKSEN S.J., BOGER D.L., LERNER R.A. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., GOLIGORSKY M.S., SCHMID P.C., KREBSBACH R.J., SCHMID H.H., DAS S.K., DEY S.K., ARREAZA G., THORUP C., STEFANO G., MOORE L.C. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- FELDER C.C., JOYCE K.E., BRILEY E.M., MANSOURI J., MACKIE K., BLOND O., LAI Y., MA A.L., MITCHELL R.L. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995;48:443–450. [PubMed] [Google Scholar]
- FOWLER C.J. Oleamide: a member of the endocannabinoid family. Br. J. Pharmacol. 2004;141:195–196. doi: 10.1038/sj.bjp.0705608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FU J., GAETANI S., OVEISI F., LO VERME J., SERRANO A., RODRIGUEZ DE FONSECA F., ROSENGARTH A., LUECKE H., DI GIACOMO B., TARZIA G., PIOMELLI D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPARα. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- HILEY C.R., FORD W.R. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling. Biol. Rev. Camb. Philos. Soc. 2004;79:187–205. doi: 10.1017/s1464793103006201. [DOI] [PubMed] [Google Scholar]
- HO W.S., HILEY C.R. Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx. Br. J. Pharmacol. 2003;139:585–597. doi: 10.1038/sj.bjp.0705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO W.S., HILEY C.R. Vasorelaxant activities of the putative endocannabinoid virodhamine in rat isolated small mesenteric artery. J. Pharm. Pharmacol. 2004;56:869–875. doi: 10.1211/0022357023682. [DOI] [PubMed] [Google Scholar]
- HUITRON-RESENDIZ S., GOMBART L., CRAVATT B.F., HENRIKSEN S.J. Effect of oleamide on sleep and its relationship to blood pressure, body temperature, and locomotor activity in rats. Exp. Neurol. 2001;172:235–243. doi: 10.1006/exnr.2001.7792. [DOI] [PubMed] [Google Scholar]
- JÁRAI Z., WAGNER J.A., VARGA K., LAKE K.D., COMPTON D.R., MARTIN B.R., ZIMMER A.M., BONNER T.I., BUCKLEY N.E., MEZEY E., RAZDAN R.K., ZIMMER A., KUNOS G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGOTA S., YAMAGUCHI Y., NAKAMURA K., SUGIURA T., WAKU K., KUNITOMO M. 2-Arachidonoylglycerol, a candidate of endothelium-derived hyperpolarizing factor. Eur. J. Pharmacol. 2001;415:233–238. doi: 10.1016/s0014-2999(01)00833-0. [DOI] [PubMed] [Google Scholar]
- LAMBERT D.M., DI MARZO V. The palmitoylethanolamide and oleamide enigmas : are these two fatty acid amides cannabimimetic. Curr. Med. Chem. 1999;6:757–773. [PubMed] [Google Scholar]
- LAN R., LIU Q., FAN P., LIN S., FERNANDO S.R., MCCALLION D., PERTWEE R., MAKRIYANNIS A. Structure–activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J. Med. Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VALVERDE O., COSSU G., PETITET F., AUBERT J.F., BESLOT F., BOHME G.A., IMPERATO A., PEDRAZZINI T., ROQUES B.P., VASSART G., FRATTA W., PARMENTIER M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- LEGGETT J.D., ASPLEY S., BECKETT S.R., D'ANTONA A.M., KENDALL D.A. Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br. J. Pharmacol. 2004;141:253–262. doi: 10.1038/sj.bjp.0705607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHTMAN A.H., HAWKINS E.G., GRIFFIN G., CRAVATT B.F. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J. Pharmacol. Exp. Ther. 2002;302:73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., BEN-SHABAT S., HANUS L., LIGUMSKY M., KAMINSKI N.E., SCHATZ A.R., GOPHER A., ALMOG S., MARTIN B.R., COMPTON D.R., PERTWEE R.G., GRIFFIN G., BAYEWICH M., BARG J., VOGEL Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- MUKHOPADHYAY S., CHAPNICK B.M., HOWLETT A.C. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am. J. Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- OFFERTÁLER L., MO F.M., BÁTKAI S., LIU J., BEGG M., RAZDAN R.K., MARTIN B.R., BUKOSKI R.D., KUNOS G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- O'SULLIVAN S.E., KENDALL D.A., RANDALL M.D. Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA) Br. J. Pharmacol. 2004;141:803–812. doi: 10.1038/sj.bjp.0705643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'SULLIVAN S.E., KENDALL D.A., RANDALL M.D. The effects of Δ9-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br. J. Pharmacol. 2005;145:514–526. doi: 10.1038/sj.bjp.0706218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLANE F., HOLLAND M., WALDRON G.J., GARLAND C.J., BOYLE J.P. Evidence that anandamide and EDHF act via different mechanisms in rat isolated mesenteric arteries. Br. J. Pharmacol. 1997;121:1509–1511. doi: 10.1038/sj.bjp.0701361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER A.C., SAUER J.M., KNIERMAN M.D., BECKER G.W., BERNA M.J., BAO J., NOMIKOS G.G., CARTER P., BYMASTER F.P., LEESE A.B., FELDER C.C. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- PRATT P.F., HILLARD C.J., EDGEMOND W.S., CAMPBELL W.B. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am. J. Physiol. 1998;274:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., RANDALL M.D., ZYGMUNT P.M., MOVAHED P., HOGESTATT E.D. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br. J. Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., HARRIS D., KENDALL D.A., RALEVIC V. Cardiovascular effects of cannabinoids. Pharmacol. Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J.M., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUABOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIERE J.C., LE FUR G.L. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- SHOWALTER V.M., COMPTON D.R., MARTIN B.R., ABOOD M.E. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- TABERNERO A., SCHOONJANS K., JESEL L., CARPUSCA I., AUWERX J., ANDRIANTSITOHAINA R. Activation of the peroxisome proliferator-activated receptor α protects against myocardial ischaemic injury and improves endothelial vasodilatation. BMC Pharmacol. 2002;2:10. doi: 10.1186/1471-2210-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br. J. Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HO W.S., BOTTRILL F.E., FORD W.R., HILEY C.R. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br. J. Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]