Abstract
The effects of the inhibitors of glycogen synthase kinase-3β (GSK-3β), TDZD-8 and SB 415286, which can substantially reduce the systemic inflammation associated with endotoxic shock in vivo, have now been investigated on the acute colitis provoked by trinitrobenzene sulphonic acid (TNBS) in the rat.
Administration of the GSK-3β inhibitor TDZD-8 (0.1, 0.33 or 1.0 mg kg−1, s.c., b.i.d., for 3 days) caused a dose-dependent reduction in the colonic inflammation induced by intracolonic TNBS assessed after 3 days, both as the area of macroscopic involvement and as a score using 0–10 scale.
Likewise, following administration of the GSK-3β inhibitor SB 415286 (0.1, 0.33 or 1.0 mg kg−1, s.c., b.i.d., for 3 days), the extent and degree of the TNBS-provoked colonic inflammation was reduced.
Administration of either TDZD-8 or SB 415286 reduced the fall in body weight following challenge with TNBS at each dose level studied.
The increase in myeloperoxidase activity, an index of neutrophil infiltration into the TNBS-induced inflamed colon, was significantly inhibited by both TDZD-8 and SB 415286 at each dose level.
The increase in the levels of the proinflammatory cytokine, TNF-α, in the inflamed colon was also significantly inhibited by either compound at the highest doses evaluated.
The elevated levels of the transcription factor NF-κB subunit p65, as determined by Western blot in the nuclear extracts from the TNBS-provoked inflamed colonic tissue, were dose-dependently reduced by TDZD-8 or SB 415286 treatment.
These findings demonstrate that two chemically distinct selective inhibitors of the activity of GSK-3β reduce the inflammation and tissue injury in a rat model of acute colitis. The mechanisms underlying this anti-inflammatory action may be related to downregulation of NF-κB activity, involved in the generation of proinflammatory mediators.
Keywords: Glycogen synthase kinase-3β, GSK-3β inhibitors, NF-κB, NF-κB p65, SB 415286, TDZD-8, TNBS colitis, TNF-α
Introduction
Glycogen synthase kinase-3 (GSK-3), a serine–threonine protein kinase involved in glycogen metabolism, is now considered to play an important role in the regulation of many cellular functions, including the control of cell division and apoptosis (Ali et al., 2001; Frame & Cohen, 2001). It has been proposed that one of the two isoforms, GSK-3β, can influence the activity of the nuclear factor (NF)-κB (Ali et al., 2001; Frame & Cohen, 2001), a known key transcription factor for the genes involved in the production of proinflammatory mediators, including the cytokine, tumour necrosis factor α (TNF-α) (Karin et al., 2004). This concept was first based on the findings that GSK-3β gene-deleted mice exhibit a phenotype comparable to that of mice in which the gene for NF-κB subunit p65, or the IκB kinase 2 involved in the activation of NF-κB, had been deleted (Hoeflich et al., 2000). Deletion of the GSK-3β gene had no effect on TNF-α-induced IκBα degradation, but did prevent the activation of NF-κB (Hoeflich et al., 2000). Other studies in vitro have now confirmed a regulatory influence of GSK-3β on the activity of NF-κB in a range of systems (Schwabe & Brenner, 2002; Demarchi et al., 2003; Buss et al., 2004; Takada et al., 2004).
Recent in vivo studies have demonstrated that potent, selective inhibitors of GSK-3β, including TDZD-8 (Martinez et al., 2002; Barry et al., 2003; Meijer et al., 2004) and SB 415286 (Coghlan et al., 2000; Cross et al., 2001; Gross et al., 2004), can substantially reduce the renal dysfunction and hepatocellular injury that results from challenge with endotoxin in combination with peptidoglycan (Dugo et al., 2005). Specifically, these GSK-3β inhibitors reduced the systemic inflammatory response, the associated tissue injury, the phosphorylation of Ser 536 on the NF-κB subunit p65 and the expression of NF-κB-dependent genes in the lung tissue of rats following coadministration of endotoxin and peptidoglycan (Dugo et al., 2005).
In the present study, to explore further the possible role of GSK-3β in the modulation of different inflammatory conditions in vivo, the effects of two selective GSK-3β inhibitors, TDZD-8 and SB 415286, have now been evaluated in a well-established rat model of colitis provoked by the hapten, trinitrobenzene sulphonic acid (TNBS). This model exhibits many of the macroscopic, histological and immunological features of inflammatory bowel disease (IBD) with neutrophilic involvement seen in patients (Boughton-Smith et al., 1988a, 1988b; Morris et al., 1989; Yamada et al., 1992; Kiss et al., 1997; Galvez et al., 2000; Neurath et al., 2000; Whittle et al., 2003).
Thus, acute colonic inflammation was provoked by a single intracolonic instillation of the TNBS and the response evaluated after a 3-day period. The inflammatory response has been assessed as both area of involvement of the colonic segment and its severity as a macroscopic score. Body weight was measured, while colonic weight has been determined as a reflection of colonic oedema. Colonic myeloperoxidase (MPO) activity has been measured as an index of white cell infiltration (Boughton-Smith et al., 1988b). Since TNF-α is involved in IBD and its levels are increased in TNBS-induced colitis in the rat (Ameho et al., 1997; Ribbons et al., 1997; Sykes et al., 1999; Villegas et al., 2003), the effects on the GSK-3β inhibitors on the colonic TNF-α levels as an inflammatory biomarker were evaluated. Moreover, the effects of TDZD-8 and SB 415286 on the NF-κB subunit p65 levels in the nuclear extracts of the colonic tissue have also been determined to provide a possible mechanistic explanation to their actions.
Methods
Induction of colitis
The investigation was performed in accordance with the United Kingdom Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986, published by HMSO, London.
Male Wistar rats (200–240 g) were randomised before commencement of the study, housed in groups, and inspected and weighed every day. For the treatment groups, n=8 were used, with n=10 and 15 for the unchallenged control and the challenged DMSO groups. Food was withdrawn overnight for 12 h, prior to TNBS administration only, but the rats were allowed free access to drinking water. In these studies, a low intracolonic dose of 10 mg of TNBS was used to provoke a reproducible, yet not unduly severe, acute mucosal inflammation in the colon, which does not cause the mortality seen with higher doses of TNBS used by others (Woodruff et al., 2003). The colonic inflammation was determined after 3 days, a time when plateau levels of neutrophil infiltration and acute tissue inflammation following TNBS challenge are observed (Boughton-Smith et al., 1988a, 1988b). Thus, on the morning of the day of challenge, day 0, the rats were transiently anaesthetised with ether, and TNBS (10 mg in 0.25 ml of 50% ethanol) was instilled into the colon using a soft plastic catheter inserted 8 cm in the rat rectum. The rats were allowed to recover with free access to food and drinking water. At the end of the experiment, 3 days after TNBS administration (i.e. on the morning of day 3), the distal colon was dissected, photographed, processed and stored appropriately for subsequent analyses.
The primary parameters measured were the area of macroscopic inflammation and its severity score, MPO levels and TNF-α levels in segments of distal 8 cm of colon. In addition, the weight of the whole 8 cm colonic segment was measured as an indirect and nonspecific marker of oedema, while the body weight of the animals was determined as an indicator of general health.
The experimental compounds were administered subcutaneously twice daily (1 ml kg−1 in 10% dimethyl sulphoxide (DMSO), final concentration) commencing 2 h before TNBS administration and then 4 h later, and then twice a day on days 1 and 2 after challenge. The doses of TDZD-8 and of SB 415286 were 0.1, 0.33 and 1.0 mg kg−1, s.c., b.i.d., which were based on the previous in vivo studies with these agents (Dugo et al., 2005). A further group of rats that was challenged with TNBS received the DMSO vehicle alone, twice a day (1 ml kg−1 s.c.), while another group had no challenge or drug treatment and was used for baseline measurements.
Macroscopic analysis
The distal 8-cm portion of the colon (measured from the rectum) was removed, opened longitudinally and gently rinsed with ice-cold phosphate buffer (pH 7.4), blotted, weighed (Scaltec, Germany) and photographed (Samsung, Digimax 340, digital camera). The extent of macroscopically apparent inflammation, ulceration and tissue disruption was determined in a randomised manner from the colour images via computerised planimetry (Scion Image B4.02 version; Scion Corp.). The area of macroscopically visible mucosal involvement was calculated and expressed as the percentage of the total colonic segment area under study.
The tissue was then cut into longitudinal strips, each strip being thus 8 cm long, and included the whole of the zone of injury. This tissue was weighed, processed and the resulting supernatant stored at −20°C for the subsequent determination of MPO activity, protein levels, for the assay for TNF-α or for Western blot analysis.
Macroscopic score
In addition to the quantitative measurement of area of involvement, the degree of colonic inflammation was also assessed from the photographs in a randomised blinded fashion using a macroscopic score, utilising a 1–10 scale adapted from those used previously (Boughton-Smith et al., 1988a; Wallace et al., 1989):
- 0
No damage.
- 1
One region of localised inflammation or thickening; No ulcers.
- 2
Linear ulceration, but no significant inflammation.
- 3
Linear ulceration with inflammation at one site.
- 4
Two or more sites of ulceration and/or inflammation; ulcers present in at least one site.
- 5
Two or more sites of ulceration and inflammation with one major site of ulceration and inflammation extending 1 cm along the length of the colon.
- 6–10
Two or more sites of ulceration and inflammation with one major site of ulceration and inflammation extending 2, 3, 4, 5 or 6 cm along the length of the colon.
MPO activity
The MPO activity was determined in colonic tissue as previously described in this model (Kiss et al., 1997). The 8-cm longitudinal strips of the colon were weighed, homogenised (Ultra turrax, T25, 13,500 rev min−1; 2 × 30 s; 250 mg colon ml−1 buffer) in ice-cold phosphate buffer (50 mM, pH 6.0), freeze–thawed three times and then centrifuged twice (each time at 15,000 × g for 15 min at 4°C). Then, a 12-μl aliquot of the supernatant was mixed with 280 μl phosphate buffer (50 mM, pH 6) containing 0.167 mg ml−1 of O-adenosine dihydrochloride and the reaction initiated with 10 μl 0.03% hydrogen peroxide and assayed spectrophotometrically at 490 nm (Benchmark Microplate reader, Bio-Rad Labs) after 90 s of shaking. MPO was expressed as mU mg−1 protein.
TNF-α levels in the colon
The colonic tissue samples were thawed, weighed and homogenised (Ultra-turrax, T25, 2 × 30 s on ice; 250 mg colon ml−1 buffer) in a modified Greenburg buffer (300 mmol l−1 NaCl, 15 mmol l−1 Tris, 2 mmol l−1 MgCl2, 2 mmol l−1 Triton X-100, 20 ng ml−1 pepstatin A, 20 ng ml−1 leupeptin, 20 ng ml−1 aprotinin; pH 7.4). Tissue homogenates were lysed for 30 min on ice, and then centrifuged twice (10 min, 14,000 × g). The aliquots of the supernatant were stored at −20°C until use (Ten Hove et al., 2001).
The TNF-α levels were determined with quantitative TNF-α solid-phase enzyme-linked immunosorbent assay (ELISA). The samples were measured spectrophotometrically at 450 nm, and were diluted 2 or 4 times with the buffer included in the kit. This commercially available kit used had a range of the standard curve of 0–2000 pg ml−1, with minimum detection level of 10 pg ml−1 of TNF-α. The TNF-α values were expressed as pg mg−1 protein.
Protein determination
Using a commercial protein assay kit, aliquots (20 μl) of the diluted samples (25 × or 50 × with distilled water) were mixed with 980 μl distilled water and 200 μl Bradford reagent added to each sample. After mixing and a 10-min incubation, the samples were assayed spectrophotometrically at 595 nm. Protein level was expressed as mg protein ml−1.
Western blotting for NF-κB p65
Protein extracts for analysis were prepared according to the method supplied with the nuclear extract kit (Active Motif Company, Carlsbad, U.S.A.) The colonic tissue samples were thawed, weighed and homogenised (Ultra-turrax, T8, 2 × 30 s on ice; 1 g colon per 3 ml buffer) in a hypotonic buffer supplied by the Active Motif company. Tissue homogenates were lysed for 15 min on ice, and then centrifuged (10 min, 850 × g). The pellets were suspended in hypotonic buffer, lysed for 15 min on ice, and then centrifuged (30 s, 14,000 × g). The pellets were suspended in the supplied complete lysis buffer containing 10 mM dithiothreitol, lysed for 30 min on ice and then centrifuged (10 min, 14,000 × g) to yield the nuclear fraction.
These protein extracts were stored at −80°C, the concentration of protein being measured using the Bradford method. Aliquots of the total nuclear proteins were denatured by mixing and boiling (3 min) v v−1 with 20 mM Tris, 3 mM EDTA, 2% sodium dodecyl sulphate (SDS), 10% O-mercaptoethanol and 20% glycerol. The samples (20 μg per 20 μl) were electrophoresed (100 V, 25 mA per gel) on 10% SDS–polyacrylamide gel, and transferred (100 V, 50 mA per gel, 2 h) to a nitrocellulose membrane (Hybond ECL Nitrocellulose membrane, Amersham, Pharmacia Biotech., Buckinghamshire, U.K.). At 1 h after blocking with phosphate buffer (pH 7.4), 0.25% Tween 20 (v v−1) and 5% nonfat dried milk, the membrane washed with phosphate buffer-Tween 20 (three times for 15 min) and probed with anti NF-κB p65 monoclonal antibody (1 : 1000; 2 h; Santa Cruz Biotechnology Inc.) at room temperature, washed with phosphate-buffered Tween 20 (three times for 15 min) and then incubated with horseradish peroxidase-conjugated bovine anti-mouse IgG secondary antibody (1 : 2000 dilution, 1 h; Santa Cruz Biotechnology Inc.) for 1 h at room temperature. A primary monoclonal antibody against β-actin (1 : 5000 dilution) was used as the loading control (Sigma Aldrich Company Ltd).
Membranes were developed using an enhanced Immobilon Western chemiluminescent HRP substrate (Millipore Corporation, Billerica, MA, U.S.A.) and scanned and analysed using the UVIPro Chemi imaging system (Uvitec Ltd, Cambridge, U.K.). Densitometric analysis was performed for each blot following normalisation with reference to the control β-actin and the data were expressed in terms of % of the challenged DMSO group.
Reagents and materials
TDZD-8 was obtained from Calbiochem (Merck Biosciences Ltd, Beeston, Nottingham, U.K.) and SB 415286 was obtained from Tocris Cookson Ltd (Avonmouth, Bristol, U.K.). These compounds were prepared freshly on each day of use in the vehicle, 10% DMSO (Sigma Aldrich Company Ltd; Poole, Dorset, U.K.).
The 2,4,6-TNBS was obtained from Fluka Chemie AG (Buchs, Switzerland). The Bradford protein assay was from Bio-Rad (Herts., U.K.). All other assay reagents were from Sigma Aldrich Company Ltd. The TNF-α ELISA assay kit was obtained from Hycult Biotechnology b.v. 5405 Uden, The Netherlands, while the nuclear extract kit was from Active Motif Company, Carlsbad, U.S.A. The primary and secondary antibodies for the Western blots were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, U.S.A.) and Sigma Aldrich Company Ltd (Poole, Dorset, U.K.).
Statistical evaluation
Results shown in the figures are expressed as mean±s.e.m. For statistical comparisons, the two-tailed Student's t-test and the analysis of variance with the Bonferoni test were used where appropriate. P<0.05 was taken as significant. In the graphs, statistical comparison is made against the values for the DMSO vehicle group.
Results
Effects on colonic macroscopic inflammation
Following intracolonic instillation of TNBS (10 mg), the area of colonic inflammation, determined 3 days after challenge in the control group of rats that had only received the DMSO vehicle (10%, 1 ml kg−1 b.i.d.), involved 59±6% (n=15) of the total colonic area of the segment studied (Figure 1). The macroscopic appearance was of areas of haemorrhagic necrosis, tissue ulceration and inflammation with hyperaemia, and was not different in extent from that induced by TNBS in a group of saline-treated rats (n=5; data not shown). There was no detectable macroscopic injury in the colons from the nonchallenged control group of rats (n=10; Figure 1).
Figure 1.
Effects of the vehicle DMSO (1 ml kg−1 s.c. twice a day for 3 days), TDZD-8 (0.1, 0.33 and 1.0 mg kg−1 s.c. twice a day for 3 days) or SB 415286 (0.1, 0.33 and 1.0 mg kg−1 s.c. twice a day for 3 days) on the area of colonic inflammation, expressed as % of the total colonic area of the segment (upper panel) and as a macroscopic score (1–10 scale; lower panel). Planimetric measurements and randomised score evaluation were taken from colonic tissue collected 3 days after intracolonic challenge with TNBS. The values from the nonchallenged control group are also shown. Results are expressed as mean±s.e.m.; n=8–15; **P<0.01, ***P<0.001 compared with the DMSO group.
Treatment with TDZD-8 (0.1, 0.33 or 1.0 mg kg−1, s.c., twice a day for 3 days) caused a dose-dependent reduction in the area of inflammation observed on day 3, that was significant at each dose level (P<0.05) as shown in Figure 1. Administration of SB 415286 (0.1, 0.33 or 1.0 mg kg−1 s.c., twice a day for 3 days) also caused a reduction in the extent of colonic inflammation, with significant inhibition being observed at the two higher doses (Figure 1).
When the degree of the colonic inflammation was evaluated using a 1–10 macroscopic score, findings comparable to the quantitative area data were obtained. Thus, the increased score observed following challenge in the DMSO vehicle group was dose-dependently reduced by treatment TDZD-8 (0.1, 0.33 or 1.0 mg kg−1, s.c., twice a day), as shown in Figure 1. Likewise, treatment with SB 415286 (0.1, 0.33 or 1.0 mg kg−1, s.c., twice a day) reduced the macroscopic score, which was significant at the two higher doses (Figure 1).
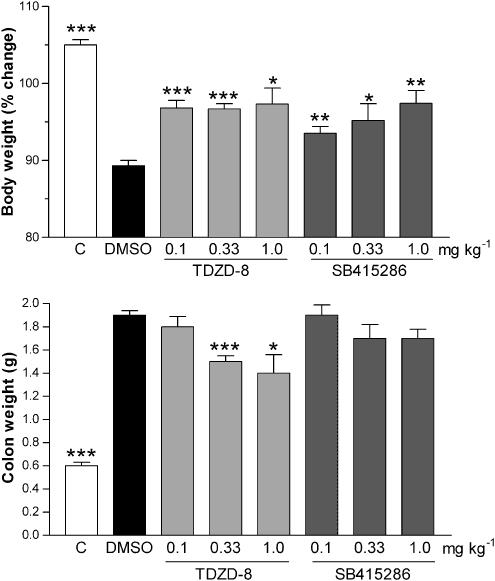
Effects on body and colon weight
Following challenge with TNBS, there was a progressive fall in body weight over the 3-day treatment period in the DMSO vehicle-treated group, being reduced to 98±0.5, 93±0.7 and 90±1% of the prechallenged weight on days 1, 2 and 3 postchallenge. The nonchallenged group gained weight over the 3-day experimental period (102±0.4, 103±0.5 and 105±1%, respectively; P<0.001 compared with the DMSO challenged group).
The fall in body weight determined 3 days following challenge with TNBS was attenuated by TDZD-8, the effects being significant at all three dose levels at this time point (Figure 2). There were no consistent significant differences in body weight between the various groups at the earlier time points. Treatment with SB 415286 significantly (P<0.01) attenuated the fall in body weight 3 days after TNBS challenge at all dose levels studied, as shown in Figure 2.
Figure 2.
Effects of the vehicle DMSO (1 ml kg−1 s.c. twice a day for 3 days), TDZD-8 (0.1, 0.3 and 1.0 mg kg−1 s.c. twice a day for 3 days) or SB 415286 (0.1, 0.33 and 1.0 mg kg−1 s.c. twice a day for 3 days) on the change in body weight, expressed a % change in body weight immediately prior to challenge (upper panel) and the weight of the total colon segment expressed as g (lower panel). Measurements of body weight and the standard colonic segment weight were made 3 days after intracolonic challenge with TNBS. The values from the nonchallenged control group are also shown. Results are expressed as mean±s.e.m.; n=8–15; **P<0.01, ***P<0.001 compared with the DMSO group.
As an indirect index of inflammatory oedema in the colonic tissue, the weight of the standard colonic segments was determined at the end of the treatment period. As shown in Figure 2, the colonic weight in the groups receiving DMSO and challenged with TNBS was significantly higher than that of nonchallenged colon for a comparable tissue segment. Treatment with TDZD-8 at the two higher doses significantly reduced the increase in colonic weight (Figure 2). However, treatment with SB 415286 failed to reduce significantly the increase in colon weight (Figure 2).
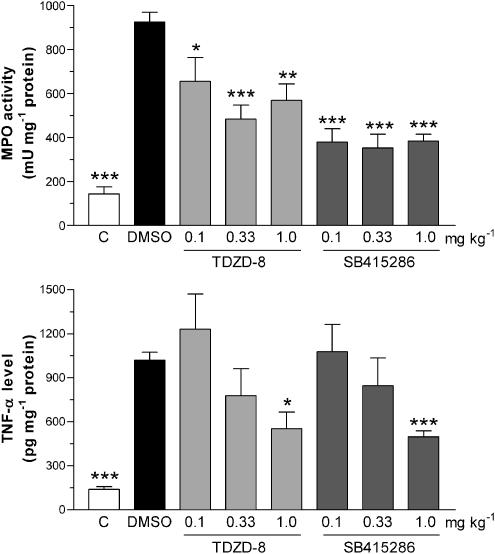
Effects on colonic MPO levels
The level of MPO activity in the colonic tissue was substantially increased in the DMSO vehicle group following TNBS challenge, compared with that from the nonchallenged control group, as shown in Figure 3.
Figure 3.
Effects of the vehicle DMSO (1 ml kg−1 s.c. twice a day for 3 days), TDZD-8 (0.1, 0.33 and 1.0 mg kg−1 s.c. twice a day for 3 days) or SB 415286 (0.1, 0.33 and 1.0 mg kg−1 s.c. twice a day for 3 days) on the colonic MPO activity, expressed as mU mg−1 protein (upper panel) and colonic TNF-α levels determined by ELISA and, expressed as pg mg−1 protein (lower panel), in homogenates of colonic tissue collected 3 days after intracolonic challenge with TNBS. The values from the nonchallenged control group are also shown. Results are expressed as mean±s.e.m.; n=8–15; **P<0.01, ***P<0.001 compared with the DMSO group.
Administration of TDZD-8 caused a significant reduction in the TNBS-elevated MPO activity at all of the dose levels (Figure 3). Treatment with SB 415286 likewise caused a significant reduction in the elevated MPO activity at all doses, although no clear dose–response relationship was observed (Figure 3).
Effects on colonic TNF-α levels
The levels of TNF-α in the colonic tissue from DMSO vehicle group were significantly increased some four-fold compared to that from the nonchallenged control group following intrarectal TNBS, as shown in Figure 3. These elevated TNF-α levels were dose-dependently reduced following administration of TDZD-8, being significant at the highest dose (Figure 3). Treatment with SB 415286 also caused a dose-dependent inhibition of TNF-α levels in the inflamed colon, and, as with TDZD-8, was significant at the higher dose (Figure 3).
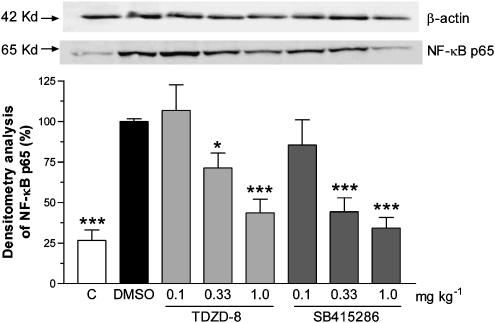
Western blot of NF-κB p65
The levels of the NF-κB p65 subunit protein in the nuclear fractions of the colonic tissue were substantially elevated following induction of inflammation by TNBS challenge after 3 days, compared to the control group, as determined by Western blotting techniques (Figure 4, upper panel). The levels of NF-κB p65 protein were dose-dependently reduced in the nuclear fractions of the colons from animals that had received either TDZD-8 or SB 415286, as shown in Figure 4 (upper panel). This reduction, as estimated by densitometric analysis, was significant with both compounds at their two higher doses (Figure 4, lower panel).
Figure 4.
Effects of the vehicle DMSO (1 ml kg−1 s.c. twice a day for 3 days), TDZD-8 (0.1, 0.33 and 1.0 mg kg−1 s.c. twice a day for 3 days) or SB 415286 (0.1, 0.33 and 1.0 mg kg−1 s.c. twice a day for 3 days) on NF-κB p65 subunit protein detected in nuclear fractions prepared from colonic tissue collected 3 days after intracolonic challenge with TNBS. Data from the nonchallenged control group are also shown. The upper panel shows an example of a Western blot following probing with the antibody to the NF-κB p65, for each treatment group. The lower histogram is the data derived from the Western blots following densitometry analysis, with the results shown as NF-κB p65 levels compared to the challenged DMSO group, mean±s.e.m.; n=8–15; *P<0.05, ***P<0.001.
Discussion
In order to evaluate further the anti-inflammatory potential of the GSK-3β inhibitors that have been recently identified in vivo in models of shock (Dugo et al., 2005), the effects of two such compounds, TDZD-8 and SB 415286, have now been evaluated in a rat model of IBD. These compounds are known to be active on GSK-3β in vitro in the nanomolar range, with IC50 values (concentrations causing 50% inhibition) of 2 and 0.08 μM for TDZD-8 and SB 415286, respectively (Coghlan et al., 2000; Martinez et al., 2002; Meijer et al., 2004). The compounds, TDZD-8 and SB 415286, which respectively interact noncompetitively or competitively at ATP-binding sites, also appear to be selective for this kinase. Thus, TDZD-8 did not inhibit a range of other kinases such as protein kinase A and C, casein kinase II and cyclin-dependent kinase 1 in sub-millimolar concentrations (Martinez et al., 2002). Likewise, SB 415286 did not show inhibitory activity on a panel of 24 other serine/threonine and tyrosine kinases (Coghlan et al., 2000). The full pharmacological profile and selectivity of these agents, however, awaits further evaluation. Previous studies in the rat have suggested that both compounds have similar potency for pharmacological activity in vivo (Dugo et al., 2005), as found in the current study using TNBS-induced colitis.
Oral administration of the thiadiazolidinone TDZD-8 (Martinez et al., 2002) produced a dose-dependent reduction in the extent of colonic macroscopic inflammation and ulceration, both in terms of the area of mucosal involvement as determined by planimetry and also its severity using a scoring system, which was significant at all the doses evaluated. To confirm that this activity was indeed the consequence of GSK-3β inhibitory activity, the actions of a structurally distinct hydroxyphenylamino-nitrophenyl-pyrrole-dione compound, SB 415286 (Coghlan et al., 2000), were also evaluated. This compound likewise showed a reduction in the extent of colonic macroscopic inflammation and score. The findings with these two chemically distinct inhibitors thus strongly suggest an involvement of the GSK-3β pathway in the pathogenesis of the colitis.
The increased weight of the colonic segments following TNBS challenge was significantly reduced by TDZD-8, presumably reflecting a diminution of the tissue disruption and subsequent oedema, although why this was not observed with SB 415286 on this secondary parameter of colitis is as yet unclear. More direct measurement of inflammatory oedema may be required to resolve this apparent discrepancy. The fall in body weight seen in the vehicle-treated groups over the 3 days following TNBS challenge was reduced by both compounds at each dose level, which may be the consequence of the reduction in colonic tissue disruption and hence the attenuation of any appetite loss.
Studies in both this experimental model of colitis (Boughton-Smith et al., 1988b; Morris et al., 1989; Sun et al., 2001) and in clinical IBD studies (Raab et al., 1993; Kristjansson et al., 2004) indicate the important involvement of neutrophils in these inflammatory processes in the colon. The macroscopic injury caused by TNBS challenge was accompanied by a substantial increase in the levels of MPO, an index of neutrophil infiltration. This increase in MPO activity was significantly inhibited by both TDZD-8 and SB 415286 at all dose levels investigated. These changes in this cellular biomarker of the acute inflammatory response in the colitis model thus confirm the anti-inflammatory activity of the two GSK-3β inhibitors.
Proinflammatory cytokines play a key pathological role in the inflammatory events underlying IBD and the clinical use of TNF-α modifying approaches, including use of the TNF-α antibody, infliximab, have proved very beneficial, particularly in Crohn's disease (Rutgeerts et al., 2004). As found in the current work, colonic TNF-α levels have been reported to be increased in TNBS-induced colitis in the rat (Ameho et al., 1997; Ribbons et al., 1997; Sykes et al., 1999; Villegas et al., 2003). Moreover, inhibitors of TNF-α synthesis (Bobin-Dubigeon et al., 2001; Ten Hove et al., 2001; Woodruff et al., 2003) can reduce the damage in this model. In the present work, the elevated levels of TNF-α in the colonic tissue were significantly reduced by treatment with either TDZD-8 or SB 415286, although this effect only reached significance at the higher doses. It is not yet clear whether this reflects a true dissociation between the more consistent effects of these compounds on tissue injury, which was achieved at lower doses than that on the levels of this cytokine. The reduction in the TNF-α levels may reflect an action of the GSK-3β inhibitors at these doses on the cellular transduction pathways promoting cytokine biosynthesis. However, it is also possible that the observed inhibition of TNF-α by the GSK-3β inhibitors is partly the consequence of the reduced area of tissue injury and inflammation, reducing the infiltration of cytokine-producing inflammatory cells at the high doses, although a fall in MPO levels was observed at all doses of these compounds.
The mechanisms by which TDZD-8 and SB 415286 can reduce the colitis remain to be identified more fully, but it is highly feasible that this reflects inhibition of the modulatory action of GSK-3β on transcriptional processes of proinflammatory genes facilitated by NF-κB (Ali et al., 2001; Frame & Cohen, 2001). Indeed, in the present work, both of these compounds substantially and dose-dependently reduced the NF-κB p65 levels in the nuclear fractions as determined by Western Blot analysis in the inflamed colonic tissue. These findings suggest that both TDZD-8 and SB 415286 attenuate the nuclear translocation of p65 and, hence, further suggest the reduced activation of the transcription factor NF-κB in vivo by these compounds through inhibition of GSK-3β. This is in agreement with recent findings using embryonic fibroblasts derived from GSK-3β gene-deleted mice showing suppressed activation of a number of cytoplasmic signalling intermediates and retention of p65 in the cytoplasm upon TNF-α stimulation (Takada et al., 2004). However, other in vitro (Hoeflich et al., 2000; Steinbrecher et al., 2005) and in vivo studies (Dugo et al., 2005) have reported that GSK-3β affects NF-κB activity through processes independent of p65 nuclear localisation. The heterogeneity and change in the number of cells present in the inflamed colonic tissue in the present study in vivo add to the complexity of interpreting the mechanism by which GSK-3β controls NF-κB activation.
The involvement of NF-κB regulated genes in experimental colitis has been clearly previously demonstrated by the use of antisense NF-κB, which reduced both the inflammation and the fibrosis associated with murine TNBS colitis (Neurath et al., 2000; Lawrance et al., 2003). In addition to GSK-3β, other kinases that can influence NF-κB activity include the G protein, Rho kinase (Segain et al., 2003) and the mitogen-activated protein kinase, p38 (Hollenbach et al., 2005). Inhibitors directed towards these latter kinases prevent activation of NF-κB and also reduce murine TNBS colitis (Segain et al., 2003; Hollenbach et al., 2005). Suppression of NF-κB activity has also been proposed to underlie the beneficial actions of a number of different agents in this colitis model, including the natural product, curcumin (Ukil et al., 2003; Jian et al., 2005), and the peroxisome proliferator-activated receptor γ (PPARγ) agonists, rosiglitazone and troglitazone (Desreumaux et al., 2001; Sanchez-Hidalgo et al., 2005). Interestingly, these latter thiadiazolidinedione PPARγ agonists have some chemical commonality with TDZD-8, although not with SB 415286. While it is not yet clear if TDZD-8 has any PPARγ ligand affinity, along with its potent activity as a GSK-3β inhibitor, that could influence its anti-inflammatory activity, it would seem unlikely that any such effects would extend in the present colitis model to the structurally dissimilar SB 415286.
The present pharmacological study has demonstrated that two distinct GSK-3β inhibitors can attenuate the acute colitis in the rat, with an inhibition of both macroscopic inflammation and the elevated biomarkers, MPO and TNF-α, and a reduction in the nuclear levels of NF-κB p65. Direct biochemical or molecular evidence for the involvement of GSK-3β in colitis is not yet available, although in a murine model of inflammation-related colon carcinogenesis mutations of β-catenin were noted at codons encoding serine and threonine that are targets for phosphorylation by GSK-3β (Kohno et al., 2005). If GSK-3β does act to upregulate the activity of NF-κB as a transcription factor in inflammation, then such approaches to the modulation of proinflammatory genes through GSK-3β inhibition may point to a novel therapeutic target for the treatment of colitis.
Acknowledgments
BJRW is supported by a grant from the William Harvey Research Foundation, and C.V. is supported by a Janos Bolyai Research Scholarship and by the Hungarian Ministry of Economy and Transport (GVOP-3.2.1-2004-04-0086/3.0).
Abbreviations
- GSK-3β
glycogen synthase kinase-3β
- IBD
inflammatory bowel disease
- NF-κB
nuclear factor kappa B
- MPO
myeloperoxidase
- PPARγ
peroxisome proliferator-activated receptor γ
- TNBS
trinitrobenzene sulphonic acid
- TNF-α
tumour necrosis factor α
References
- ALI A., HOEFLICH K.P., WOODGETT J.R. Glycogen synthase kinase-3: properties, functions, and regulation. Chem. Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- AMEHO C.K., ADJEI A.A., HARRISON E.K., TAKESHITA K., MORIOKA T., ARAKAKI Y., ITO E., SUZUKI I., KULKARNI A.D., KAWAJIRI A., YAMAMOTO S. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–493. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY F.A., GRAHAM G.J., FRY M.J., GIBBINS J.M. Regulation of glycogen synthase kinase 3 in human platelets: a possible role in platelet function. FEBS Lett. 2003;553:173–178. doi: 10.1016/s0014-5793(03)01015-9. [DOI] [PubMed] [Google Scholar]
- BOBIN-DUBIGEON C., COLLIN X., GRIMAUD N., ROBERT J.M., LE BAUT G., PETIT J.Y. Effects of tumour necrosis factor-alpha synthesis inhibitors on rat trinitrobenzene sulphonic acid-induced chronic colitis. Eur. J. Pharmacol. 2001;431:103–110. doi: 10.1016/s0014-2999(01)01410-8. [DOI] [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., WALLACE J.L., MORRIS G.P., WHITTLE B.J.R. The effect of anti-inflammatory drugs on eicosanoid formation in a chronic model of inflammatory bowel disease in the rat. Br. J. Pharmacol. 1988a;94:65–72. doi: 10.1111/j.1476-5381.1988.tb11500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUGHTON-SMITH N.K., WALLACE J.L., WHITTLE B.J.R. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988b;25:115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- BUSS H., DORRIE A., SCHMITZ M.L., FRANK R., LIVINGSTONE M., RESCH K., KRACHT M. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J. Biol. Chem. 2004;279:49571–49574. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- COGHLAN M.P., CULBERT A.A., CROSS D.A., CORCORAN S.L., YATES J.W., PEARCE N.J., RAUSCH O.L., MURPHY G.J., CARTER P.S., ROXBEE C.L., MILLS D., BROWN M.J., HAIGH D., WARD R.W., SMITH D.G., MURRAY K.J., REITH A.D., HOLDER J.C. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- CROSS D.A., CULBERT A.A., CHALMERS K.A., FACCI L., SKAPER S.D., REITH A.D. Selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. J Neurochem. 2001;77:94–102. doi: 10.1046/j.1471-4159.2001.t01-1-00251.x. [DOI] [PubMed] [Google Scholar]
- DEMARCHI F., BERTOLI C., SANDY P., SCHNEIDER C. Glycogen synthase kinase-3 beta regulates NF-kappa B1/p105 stability. J. Biol. Chem. 2003;278:39583–39590. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- DESREUMAUX P., DUBUQUOY L., NUTTEN S., PEUCHMAUR M., ENGLARO W., SCHOONJANS K., DERIJARD B., DESVERGNE B., WAHLI W., CHAMBON P., LEIBOWITZ M.D., COLOMBEL J.F., AUWERX J. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J. Exp. Med. 2001;193:827–838. doi: 10.1084/jem.193.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGO L., COLLIN M., ALLEN D.A., PATEL N.S., BAUER I., MERVAALA E.M., LOUHELAINEN M., FOSTER S.J., YAQOOB M.M., THIEMERMANN C. GSK-3beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit. Care Med. 2005;33:1903–1912. doi: 10.1097/01.ccm.0000178350.21839.44. [DOI] [PubMed] [Google Scholar]
- FRAME S., COHEN P. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALVEZ J., GARRIDO M., MERLOS M., TORRES M.I., ZARZUELO A. Intestinal anti-inflammatory activity of UR-12746, a novel 5-ASA conjugate, on acute and chronic experimental colitis in the rat. Br. J. Pharmacol. 2000;130:1949–1959. doi: 10.1038/sj.bjp.0703505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS E.R., HSU A.K., GROSS G.J. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ. Res. 2004;94:960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- HOEFLICH K.P., LUO J., RUBIE E.A., TSAO M.S., JIN O., WOODGETT J.R. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- HOLLENBACH E., VIETH M., ROESSNER A., NEUMANN M., MALFERTHEINER P., NAUMANN M. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J. Biol. Chem. 2005;280:14981–14988. doi: 10.1074/jbc.M500966200. [DOI] [PubMed] [Google Scholar]
- JIAN Y.T., MAI G.F., WANG J.D., ZHANG Y.L., LUO R.C., FANG Y.X. Preventive and therapeutic effects of NF-kappaB inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J. Gastroenterol. 2005;11:1747–1752. doi: 10.3748/wjg.v11.i12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARIN M., YAMAMOTO Y., WANG Q.M. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- KISS J., LAMARQUE D., DELCHIER J.C., WHITTLE B.J.R. Time-dependent actions of nitric oxide synthase inhibition on colonic inflammation induced by trinitrobenzene sulphonic acid in rats. Eur. J. Pharmacol. 1997;336:219–224. doi: 10.1016/s0014-2999(97)01246-6. [DOI] [PubMed] [Google Scholar]
- KOHNO H., SUZUKI R., SUGIE S., TANAKA T. Beta-catenin mutations in a mouse model of inflammation-related colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran sodium sulfate. Cancer Sci. 2005;96:69–76. doi: 10.1111/j.1349-7006.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISTJANSSON G., VENGE P., WANDERS A., LOOF L., HALLGREN R. Clinical and subclinical intestinal inflammation assessed by the mucosal patch technique: studies of mucosal neutrophil and eosinophil activation in inflammatory bowel diseases and irritable bowel syndrome. Gut. 2004;53:1806–1812. doi: 10.1136/gut.2003.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWRANCE I.C., WU F., LEITE A.Z., WILLIS J., WEST G.A., FIOCCHI C., CHAKRAVARTI S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- MARTINEZ A., ALONSO M., CASTRO A., PEREZ C., MORENO F.J. First non-ATP competitive glycogen synthase kinase 3 beta (GSK-3beta) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer's disease. J. Med. Chem. 2002;45:1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- MEIJER L., FLAJOLET M., GREENGARD P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol. Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- MORRIS G.P., BECK P.L., HERRIDGE M.S., DEPEW W.T., SZEWCZUK M.R., WALLACE J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- NEURATH M., FUSS I., STROBER W. TNBS-colitis. Int. Rev. Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- RAAB Y., GERDIN B., AHLSTEDT S., HALLGREN R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut. 1993;34:1203–1206. doi: 10.1136/gut.34.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIBBONS K.A., THOMPSON J.H., LIU X., PENNLINE K., CLARK D.A., MILLER M.J. Anti-inflammatory properties of interleukin-10 administration in hapten-induced colitis. Eur. J. Pharmacol. 1997;323:245–254. doi: 10.1016/s0014-2999(97)00017-4. [DOI] [PubMed] [Google Scholar]
- RUTGEERTS P., VAN ASSCHE G., VERMEIRE S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology. 2004;126:1593–1610. doi: 10.1053/j.gastro.2004.02.070. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-HIDALGO M., MARTIN A.R., VILLEGAS I., ALARCON D.L.L. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces chronic colonic inflammation in rats. Biochem. Pharmacol. 2005;69:1733–1744. doi: 10.1016/j.bcp.2005.03.024. [DOI] [PubMed] [Google Scholar]
- SCHWABE R.F., BRENNER D.A. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G204–G211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- SEGAIN J.P., RAINGEARD D.L.B., SAUZEAU V., BOURREILLE A., HILARET G., CARIO-TOUMANIANTZ C., PACAUD P., GALMICHE J.P., LOIRAND G. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn's disease and experimental colitis. Gastroenterology. 2003;124:1180–1187. doi: 10.1016/s0016-5085(03)00283-x. [DOI] [PubMed] [Google Scholar]
- STEINBRECHER K.A., WILSON W., III, COGSWELL P.C., BALDWIN A.S. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol. Cell. Biol. 2005;25:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN F.F., LAI P.S., YUE G., YIN K., NAGELE R.G., TONG D.M., KRZESICKI R.F., CHIN J.E., WONG P.Y. Pattern of cytokine and adhesion molecule mRNA in hapten-induced relapsing colon inflammation in the rat. Inflammation. 2001;25:33–45. doi: 10.1023/a:1007023611478. [DOI] [PubMed] [Google Scholar]
- SYKES A.P., BHOGAL R., BRAMPTON C., CHANDER C., WHELAN C., PARSONS M.E., BIRD J. The effect of an inhibitor of matrix metalloproteinases on colonic inflammation in a trinitrobenzenesulphonic acid rat model of inflammatory bowel disease. Aliment. Pharmacol. Ther. 1999;13:1535–1542. doi: 10.1046/j.1365-2036.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- TAKADA Y., FANG X., JAMALUDDIN M.S., BOYD D.D., AGGARWAL B.B. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J. Biol. Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- TEN HOVE T., CORBAZ A., AMITAI H., ALONI S., BELZER I., GRABER P., DRILLENBURG P., VAN DEVENTER S.J., CHVATCHKO Y., TE VELDE A.A. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology. 2001;121:1372–1379. doi: 10.1053/gast.2001.29579. [DOI] [PubMed] [Google Scholar]
- UKIL A., MAITY S., KARMAKAR S., DATTA N., VEDASIROMONI J.R., DAS P.K. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br. J Pharmacol. 2003;139:209–218. doi: 10.1038/sj.bjp.0705241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLEGAS I., LA CASA C., ORJALES A., ALARCON D.L.L. Effects of dosmalfate, a new cytoprotective agent, on acute and chronic trinitrobenzene sulphonic acid-induced colitis in rats. Eur. J. Pharmacol. 2003;460:209–218. doi: 10.1016/s0014-2999(02)02949-7. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., MACNAUGHTON W.K., MORRIS G.P., BECK P.L. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- WHITTLE B.J.R., CAVICCHI M., LAMARQUE D.Assessment of anticolitic drugs in the trinitrobenzene sulfonic acid (TNBS) rat model of inflammatory bowel disease Inflammation Protocols: Methods in Molecular Biology 2003Totowa, NJ: Humana Press; 209–222.ed. Winyard, P.G. & Willoughby, D.A [DOI] [PubMed] [Google Scholar]
- WOODRUFF T.M., ARUMUGAM T.V., SHIELS I.A., REID R.C., FAIRLIE D.P., TAYLOR S.M. A potent human C5a receptor antagonist protects against disease pathology in a rat model of inflammatory bowel disease. J. Immunol. 2003;171:5514–5520. doi: 10.4049/jimmunol.171.10.5514. [DOI] [PubMed] [Google Scholar]
- YAMADA Y., MARSHALL S., SPECIAN R.D., GRISHAM M.B. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992;102:1524–1534. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]