Abstract
The role of adenosine A3 receptor activation during ischaemia-like conditions produced by oxygen and glucose deprivation (OGD) was evaluated with extracellular recordings from the CA1 region of rat hippocampal slices. In all, 7 min of OGD evoked tissue anoxic depolarisation (AD, peak at ∼7 min from OGD start, n=20) and were invariably followed by irreversible loss of electrically evoked field epsps (fepsps, n=42).
The selective adenosine A3 antagonists 3-propyl-6-ethyl-5[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate (MRS 1523, 1–100 nM, n=31), N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzeneacetamide (MRS 1220, 100 nM, n=7), N-(2-methoxyphenyl)-N′-[2-(3-pyrindinyl)-4-quinazolinyl]-urea, (VUF 5574, 100 nM, n=3) and 5-[[(4-pyridyl)amino]carbonyl]amino-8-methyl-2-(2-furyl)-pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine hydrochloride (1 nM, n=4), prevented the irreversible failure of neurotransmission induced by 7 min OGD (n=45) and the development of AD in 20 out of 22 monitored slices.
When tested on OGD episodes of longer duration (8–10 min, n=18), 100 nM MRS 1523 prevented or delayed the appearance of AD and exerted a protective effect on neurotransmission for episodes of up to 9 min duration. In the absence of AD, the fepsp recovery was almost total, regardless of OGD episode duration.
These findings support the notion that A3 receptor stimulation is deleterious during ischaemia and suggest that selective A3 receptor block may substantially increase the resistance of the CA1 hippocampal region to ischaemic damage.
Keywords: Adenosine, A3 receptors, cerebral ischaemia, hippocampal slices, synaptic potential, anoxic depolarisation
Introduction
Ischaemic episodes occurring in the mammalian central nervous system result in the impairment of neurotransmission and, with the duration of ischaemia, in increasingly severe tissue damage.
The impairment in neurotransmission is, however, not directly correlated with cell death and is reversible if the oxygen and glucose supply is restored within a narrow time window (Latini et al., 1999; Pugliese et al., 2003). While the disappearance of synaptic activity is the earliest detectable functional sign of tissue suffering, the absence of recovery after ischaemia interruption clearly indicates irreversible neurone damage.
Rapid anoxic depolarisation (AD) of a sizeable population of brain cells is observed with prolonged ischaemic episodes and its appearance is strictly correlated with neuronal and glial damage (see Somjen, 2001) during ischaemia, contributing also to the extension of cell damage to the so-called ‘ischaemic penumbral area' (Touzani et al., 2001). Therefore, it appears that pharmacological treatments directed to prevent or to delay AD would result in substantial neuroprotection (Obeidat & Andrew, 1998; Jarvis et al., 2001; Somjen, 2001).
One of the early events occurring during oxygen and glucose deprivation (OGD) caused by an ischaemic episode is the release of substantial amounts of adenosine (Latini & Pedata, 2001) which, through the activation of specific receptors (see Fredholm et al., 2001), is believed to exert important neuromodulatory effects relevant to the outcome of the ischaemic episode. Four subtypes of adenosine receptors, A1, A2A, A2B, and A3, all coupled to the effector system through heterotrimeric G proteins, have been identified (Fredholm et al., 2001) and are expressed in the brain (Dixon et al., 1996). Converging experimental data support the notion that activation of adenosine A1 receptors during ischaemic episodes results in neuroprotection of the brain tissue (see Abbracchio & Cattabeni, 1999; Phillis & Goshgarian, 2001) predominantly by reducing excitatory (glutamatergic) transmission. The role of the other adenosine receptors in cerebral ischaemia, and in particular of A3 receptors, is still controversial (see Von Lubitz, 1999). In fact, it has been demonstrated that mice lacking the A3 adenosine receptors show an increase in neurodegeneration in response to repeated episodes of hypoxia (Fedorova et al., 2003). Consistent with these reports, Hentschel et al. (2003) demonstrate, in rat cortical neurones, that the selective activation of A3 adenosine receptors during hypoxia is involved in the inhibition of excitatory neurotransmission indicating that the A3 receptors also contribute to neuroprotective action of adenosine brought about by A1 receptors. Similarly, at a cardiac level, most evidence indicates that A3 receptors are involved in protection of the ischaemic heart (Fredholm et al., 2005). On the other hand, acute A3 receptor stimulation has been shown to exacerbate the damage caused by a concomitant ischaemic episode in vivo (Von Lubitz et al., 1994), indicating a deleterious role of these adenosine receptors during cerebral ischaemia. The observation that A3 receptor activation during OGD limits the beneficial effects of ischaemic preconditioning on the resistance of synaptic transmission to ischaemia-like insults in hippocampal slices (Pugliese et al., 2003) is consistent with this hypothesis.
The role of A3 receptors in the brain under normoxic conditions appears to be equally controversial. In fact, in different brain regions in vitro, selective A3 receptor stimulation induces either an inhibitory (Brand et al., 2001) or a facilitatory effect (Dunwiddie et al., 1997; Fleming & Mogul, 1997; Macek et al., 1998; Costenla et al., 2001; Laudadio & Psarropoulou, 2004) on excitatory neurotransmission. These opposite effects may sustain a protective or a deleterious role of A3 receptors, respectively, during ischaemia. Therefore, the overall modulatory effects of A3 receptors on neurotransmission during cerebral ischaemia are not well defined.
In the present work, we used selective A3 receptor antagonists to investigate the role of A3 adenosine receptors on synaptic transmission during severe (7 min or longer duration) OGD episodes aimed at reproducing in vitro the consequences of interruption of blood flow following cardiac arrest or occlusion of intracranial vessels. A preliminary account of this work has been communicated (Pugliese et al., 2004).
Methods
All animal procedures were carried out according to the European Community Guidelines for Animal Care, DL 116/92, application of the European Communities Council Directive (86/609/EEC). Experiments were carried out on rat hippocampal slices, prepared as previously described (Pugliese et al., 2003).
Slice preparation
Male Wistar rats (Harlan, Italy; Udine, Italy, 150–200 g body weight) were deeply anaesthetised with ether and decapitated with a guillotine. The hippocampi were rapidly removed and placed in ice-cold oxygenated (95% O2–5% CO2) artificial cerebral spinal fluid (aCSF) of the following composition (mM): NaCl 124, KCl 3.33, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 25 and D-glucose 10. Slices (400 μm nominal thickness) were cut with a McIlwain tissue chopper (The Mickle Lab. Engineering, Co. Ltd, Gomshall, U.K.) and kept in oxygenated aCSF for at least 1 h at room temperature. A single slice was then placed on a nylon mesh, completely submerged in a small chamber (0.8 ml) and superfused with oxygenated aCSF (30–32°C) at a constant flow rate of 2 ml min−1. The treated solutions reached the preparation in 90 s and this delay was taken into account in our calculations.
Extracellular recording
Test pulses (80 μs, 0.066 Hz) were delivered through a bipolar nichrome electrode positioned in the stratum radiatum. Evoked potentials were recorded with glass microelectrodes (2–10 MΩ, Clark Electromedical Instruments, Panghourne, U.K.) filled with 150 mM NaCl, and placed in the CA1 region of the stratum radiatum. The negative shifts were recorded in direct current (d.c.) mode. Responses were amplified (BM 622, Mangoni, Pisa, Italy), digitised (sample rate, 33.33 kHz), low-pass filtered (10 kHz), and stored for later analysis using LTP software facilities (version 2.30D, Anderson & Collingridge (2001), www.ltp-program.com). Stimulus–response curves were obtained by gradual increases in stimulus strength at the beginning of each experiment, when a stable baseline of evoked response was reached. The test stimulus pulse was then adjusted to produce field excitatory postsynaptic potentials (fepsps) whose amplitude was 40–50% of the maximum and was kept constant throughout the experiment. The amplitude of fepsp was routinely measured and expressed as the percentage of the average amplitude of the potentials measured during the 5 min preceding exposure of the hippocampal slices to in vitro ischaemia. In all the experiments, both the amplitude and initial slope of fepsp were measured, but since no appreciable differences between these two parameters were observed in the effect of drugs and of in vitro ischaemia, only the measure of the amplitude was expressed in the figures. The amplitude of AD was measured as the integral of tissue depolarisation in the first 2 min after AD peak.
Application of drugs and OGD
In vitro OGD was obtained by perfusing the slice for 7 min with glucose-free aCSF gassed with nitrogen (95% N2–5% CO2). At the end of the ischaemic period, slices were superfused with normal, glucose-containing, oxygenated aCSF.
All the A3 adenosine receptor antagonists used in our experiments were applied 10 or 20 min before, during OGD and 5 min after the end of ischaemic episode. On each experimental day, data were obtained either in the absence or in the presence of the adenosine antagonists, in slices taken from the same rat. The concentration used for each of the selective adenosine A3 receptor antagonists was chosen on the basis of Ki values on rat or human A3 receptors. 3-Propyl-6-ethyl-5[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate (MRS 1523), among all A3 antagonists used in our experiments, is the most potent and selective antagonist for the rat A3 receptors actually available in commerce (Ki value of 113 nM; Li et al., 1998; Muller, 2003).
Drugs
MRS 1523 was purchased from Sigma (Milano, Italy). MRS 1220 (N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide) and VUF 5574 (N-(2-methoxyphenyl)-N′-[2-(3-pyrindinyl)-4-quinazolinyl]-urea) were from Tocris (Bristol, U.K.). MRS 1523, MRS 1220 and VUF 5574 were dissolved in dimethylsulphoxide (DMSO) and stock solutions were made to obtain concentrations in DMSO of 0.05 and 0.01% in aCSF, respectively. Control experiments, carried out in parallel, showed that this concentration of DMSO did not affect either fepsp amplitude before OGD or the depression of synaptic potential induced by the following OGD. The hydrophilic A3 antagonist 5-[[(4-pyridyl)amino]carbonyl]amino-8-methyl-2-(2-furyl)-pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine hydrochloride (WSAB) was provided by Dr G. Spalluto.
Statistical analysis
Data were analysed using Prism 3.02 software (Graphpad Software, San Diego, CA, U.S.A.). All numerical data are expressed as the mean±s.e. Data were tested for statistical significance with two-tailed Mann–Whitney test or by analysis of variance (one-way ANOVA), as appropriate. When significant differences were observed, the Newman–Keuls multiple comparison test (one-way ANOVA) was inferred. A value of P<0.05 was considered significant.
Results
The role of A3 adenosine receptor stimulation by endogenous adenosine released during in vitro severe ischaemia-like episodes on synaptic transmission was investigated using selective A3 adenosine receptor antagonists. Electrically evoked fepsps were extracellularly recorded in the CA1 region of 124 hippocampal slices taken from 55 rats for monitoring the time course of the effects of OGD episodes of different duration on synaptic responses, both in control and treated slices. In a subset of experiments (n=53), the d.c. shift produced by AD was simultaneously recorded.
Selective block of A3 adenosine receptors prevents the irreversible impairment of neurotransmission induced by 7 min OGD
In a first series of experiments, we characterised the response of synaptic excitatory transmission to 7 min OGD, an ischaemia-like insult that in our experimental conditions has been shown to consistently produce an irreversible loss of synaptic transmission, but to be sensitive to the protective effects of ischaemic preconditioning (Pugliese et al., 2003).
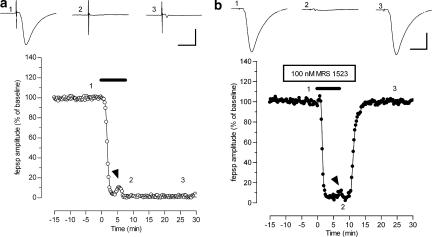
Figure 1a illustrates the effects of 7 min OGD on the amplitude of the synaptic responses evoked by stimulation of the CA1 stratum radiatum and recorded from the apical dendrite region of pyramidal cells. One 7 min OGD episode induced the disappearance of fepsps, which did not recover after prolonged superfusion with oxygenated, glucose-containing aCSF (up to 80 min, n=7; data not shown). The effect of 7 min OGD on fepsps was similar in 42 slices examined and the mean recovery of fepsp amplitude, after 7 min OGD episode, was 5±1%, n=42 (see also Figure 3).
Figure 1.
The A3 adenosine receptor antagonist MRS 1523 protects hippocampal slices from irreversible fepsp depression induced by 7-min OGD. (a and b) Graphs: typical time courses of changes in fepsp amplitude evoked by 7-min OGD episodes (solid bar) in control (a) or in the presence of 100 nM MRS 1523 (b). Each point represents fepsp amplitude expressed as per cent of the mean baseline responses recorded before OGD application. Note the transient reappearance of synaptic potential during OGD (arrowheads) in (a and b). Upper traces: fepsp recordings taken at the times indicated by numbers in the corresponding graph. Note that after reperfusion in normal oxygenated aCSF, only the afferent volley recovered in controls, while a recovery of 77% was observed at the end of MRS 1523 application in normoxic conditions. A total recovery of fepsp after 15 min reperfusion in normal oxygenated aCSF was found in MRS 1523 (trace 3). Calibration bars: 0.5 mV, 10 ms.
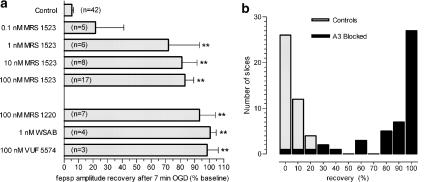
Figure 3.
Effects of selective A3 adenosine receptor antagonists on recovery of fepsp amplitude after 7-min OGD. (a) Column bars indicate the average recovery (mean±s.e.) of fepsps after 7-min OGD, recorded in hippocampal slices at 15 min reperfusion in normal, oxygenated aCSF. n indicates the number of slices tested and asterisks indicate P<0.05, one-way ANOVA, Newman–Keuls multiple comparison post hoc test, vs control and 0.1 nM MRS 1523-treated slices. (b) Distribution analysis of fepsp recovery in 87 slices receiving 7-min OGD episodes either in control or in the presence of A3 receptor antagonists (see a, 0.1 nM MRS excluded). Bars indicate the number of cells (ordinate) that showed a given recovery of fepsp (abscissa) from 7-min OGD episodes in control aCSF (n=42; white bars) or in the presence of A3 antagonists (n=45; black bars). Note that the large majority of treated slices (38 out of 45) show more than 50% recovery of fepsps.
The presence of selective adenosine A3 receptor antagonists prevented the irreversible disappearance of synaptic potentials induced by 7 min OGD. Thus, in the presence of MRS 1523 (100 nM, Figure 1b), a total recovery of synaptic response was observed within 10 min from OGD interruption. The mean recovery of fepsp amplitude after 7 min OGD in the presence of 100 nM MRS 1523 was 83±6%, (n=17, Figure 3a). Furthermore, if compared with those obtained in control conditions, in MRS 1523-treated slices the transient recovery of fepsp amplitude was delayed and the afferent fibre volley did not disappear at the end of 7-min OGD (Figure 1b, trace 2, see also Table 1).
Table 1.
Treatment with MRS 1523 produces a delay in the effects of OGD in the CA1 region of hippocampal slices
| (n) | Control | (n) | MRS 1523 | ||
|---|---|---|---|---|---|
| Initial fepsp disappearance time (s) | (50/50) | 177.9±7.2 | (41/41) | 254.3±10.9 | P<0.0001 |
| Transient fepsp recovery peak time (s) | (31/50) | 357±17 | (34/41) | 480±18 | P<0.0001 |
| Transient fepsp recovery duration (s) | (31/50) | 59±7 | (34/41) | 46±7 | P=0.0948 |
| Transient fepsp recovery amplitude (%) | (31/50) | 18.0±3.3 | (34/41) | 15.5±2.4 | P=0.4265 |
| Fibre volley disappearance time (s) | (34/50) | 372±18 | (19/41)a | 501±26 | P<0.0001 |
| AD peak time (s) | (20/20) | 436±17 | (22/33)b | 520±23 | P=0.0017 |
Data are from slices receiving 7 min or 30 min OGD in control (n=50) and 7, 8, 9, 10 or 30 min OGD in the presence of 100 nM MRS 1523 (n=41). Numbers in parentheses (n/n) indicate number of observations out of investigated slices. Time is calculated from OGD initiation. The amplitude of the transient fepsp recovery is expressed as per cent of baseline fepsp recorded before OGD application. Statistical significance was assessed by Mann–Whitney test.
Fibre volley did not disappear in any of the slices receiving 7-min OGD in the presence of MRS 1523.
AD was absent in 11 slices receiving 7–8-min OGD in the presence of MRS 1523.
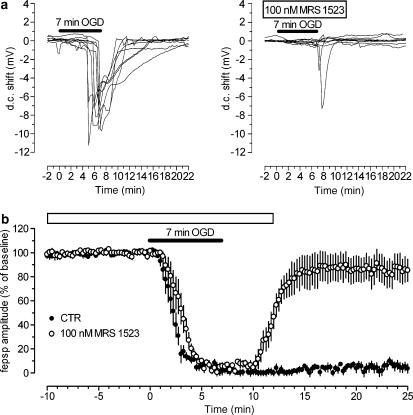
In a subset of experiments, we investigated the effects of 100 nM MRS 1523 on AD by comparing the time of peak and the magnitude of depolarising d.c. shifts caused by 7-min OGD in treated slices and in matched control slices from the same rats.
As illustrated in Figure 2a, in control conditions, 7-min OGD episodes always caused AD, recorded as negative d.c. shifts, with a mean peak latency of about 6.5 min (390±20 s) from the beginning of ischaemia and a peak amplitude of 8.9±0.6 mV (n=8). The duration of d.c. shifts was variable (range 5–15 min) and was always accompanied by complete and irreversible disappearance of fepsps. In the presence of MRS 1523 (100 nM, n=8), AD was virtually absent in seven out of eight preparations and, as shown in Figure 2b, the mean recovery of fepsp amplitude was significantly greater than in control slices taken from the same rats (85.8±13.3%, n=8 vs 3.3±2.5%; P<0.001). Interestingly, in one experiment where a sizeable AD (peak: −7.6 mV) was recorded, the recovery from OGD-evoked impairment in neurotransmission was only 22%.
Figure 2.
The A3 adenosine receptor antagonist MRS 1523 minimises AD and protects CA1 hippocampus from irreversible fepsp depression induced by 7-min OGD. (a) AD was recorded as the negative d.c. shift in response to 7-min OGD (solid bars) in control conditions (n=8) and in the presence of 100 nM MRS 1523 (open bar, n=8). MRS 1523 significantly prevented AD in seven out of eight slices. (b) Graph shows the time course of 7-min OGD effect on fepsp amplitude, expressed as per cent of baseline, in control aCSF (filled circles; mean±s.e., n=8) and in the presence of 100 nM MRS 1523 (unfilled circles, mean±s.e., n=8). Note that in the presence of the A3 antagonist, the time course of fepsps depression during OGD was significantly delayed (see Table 1) in comparison to corresponding times in the absence of the drug (control).
The protective effect of MRS 1523 on OGD-evoked irreversible depression of fepsps was detectable at concentrations as low as 0.1 nM and the recovery of fepsp amplitude became statistically significant with concentrations of 1–100 nM of the antagonist (Figure 3a). The apparent EC50 value for MRS 1523 was 0.25 nM (95% CL 0.05–1.2 nM). However, since within the time of the antagonist application (10–20 min) it might have not reached equilibrium at the receptor level in slices, the EC50 value is likely to be underestimated. Similar beneficial effects on the recovery of fepsps from 7-min OGD were exerted by the selective adenosine A3 receptor antagonists MRS 1220, VUF 5574 and WSAB, all chemically different from MRS 1523 (Kim et al., 1996; van Muijlwijk-Koezen et al., 2000; Maconi et al., 2002). As shown in Figure 3a, all A3 antagonists prevented synaptic impairment and allowed for complete synaptic recovery within 15 min from OGD interruption. In addition, all the A3 antagonists tested prevented or significantly delayed AD after 7-min OGD (data not shown).
The overall effect of A3 antagonism on fepsp recovery after 7-min OGD, compared with that observed in control preparations, is summarised in Figure 3b. As illustrated in the frequency histogram, 39 out of 45 slices treated with any of the A3 antagonists at effective concentrations (all except 0.1 nM MRS 1523), had a substantial (>50%) recovery of fepsp amplitude after 7-min OGD, while synaptic activity in controls (n=42) never recovered beyond 20% of responses recorded before OGD.
Selective block of A3 adenosine receptors delays the effects of prolonged OGD application on synaptic transmission
In the experiments performed with 7-min OGD, the inhibitory effect of A3 antagonists on the development of AD seemed to be in relation to the significant recovery of fepsp amplitude induced by these drugs.
Furthermore, as illustrated in Figures 1 and 2, OGD episodes produced a typical sequence of neurophysiological effects that followed the initial disappearance of fepsps and that comprised a transient recovery of fepsp response, the disappearance of the afferent fibre volley and the development of an AD. All these phenomena were significantly delayed and/or reduced in the presence of A3 receptor antagonists, as shown in Table 1, where the effects of 100 nM MRS 1523 on OGD of different duration are summarised.
The possible correlation of these effects of A3 receptor antagonists with the recovery of the fepsp after OGD interruption and the time window in which A3 receptor block may play a role in limiting the deleterious effects of severe ischaemia were investigated by applying OGD episodes longer than 7 min.
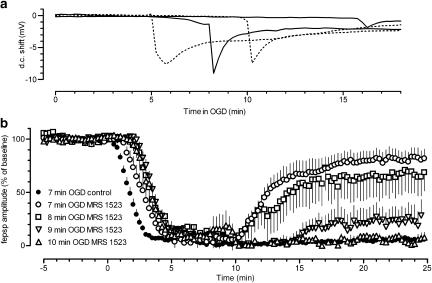
To delimit the time window in which A3 antagonists could delay the appearance of AD, we applied 30-min OGD in the presence of 100 nM MRS 1523 (Figure 4a). Compared to the effects observed in matched control slices, the A3 receptor antagonist significantly increased the latency of AD peak from 7.28 min (437±31 s) in control to 9.5 min (571±70 s) in MRS 1523 (P<0.01, Mann–Whitney test, two-tailed), without significantly affecting either the average magnitude (Figure 4) or the peak amplitude of AD (6.2±1.2 mV, n=6 vs 7.3±0.3 mV, n=8 in control, P=0.49, two-tailed Mann–Whitney test). No recovery of fepsps was recorded after interruption of 30-min OGD (not shown).
Figure 4.
Treatment with MRS 1523 postpones the appearance of AD and broadens the time window for CA1 fepsp recovery after OGD interruption. (a) Time window for OGD (30 min)-elicited ADs in the absence (dotted lines) or presence of 100 nM MRS 1523 (continuous lines). Traces are recordings of negative d.c. shifts in response to 30 min OGD (ADs) taken from control experiments (n=6) or in MRS 1523 (n=6). For the sake of clarity, only the earliest and the most delayed ADs observed in each group of slices are shown. Note that in MRS 1523 both the earliest and the latest ADs were delayed compared to the corresponding ADs in controls. AD magnitude, expressed as the integral of tissue depolarisation measured for 2 min after AD peak in MRS 1523-treated slices (3.2±0.6 vs, n=6), was not statistically different from that of controls (3.6±0.7 vs, n=8, P=0.49, Mann–Whitney, two-tailed). (b) Time course of fepsp amplitude, expressed as per cent of baseline, during OGD of different duration applied in the absence (n=31) or in the presence of 100 nM MRS 1523 (7 min OGD: n=17; 8 min OGD: n=6; 9 min OGD: n=7; 10 min OGD: n=5). Open bar indicates the time of MRS 1523 application. Values are mean±s.e. Note that in the presence of the A3 antagonist, the time course of fepsps depression during OGD was significantly delayed (see Table 1) in comparison to corresponding times in the absence of the drug (control). The recovery of fepsp amplitude following 7-, 8-, and 9-min OGD in slices treated with MRS 1523 was significantly greater than in controls subject to 7-min OGD (P<0.05, one-way ANOVA, Newman–Keuls multiple comparison post hoc test).
In a final set of experiments, we monitored the recovery of fepsps while recording AD latency and amplitude following OGDs of different duration (8–10 min) in the presence of 100 nM MRS 1523.
As summarised in Figure 4b, a significant recovery of fepsp amplitude was obtained after OGD insults of duration up to 9 min. No significant recovery was observed in five slices after 10 min OGD. Analysing the fepsp recovery in relation to AD appearance after OGD, it appeared that the degree of fepsp recovery after OGD in A3 antagonist-treated slices depended on the appearance of AD and not on the duration of OGD episodes. In fact, in the absence of AD a full recovery of fepsps was found in most preparations receiving 7 or 8 min OGD episodes and in one slice after 9 min OGD.
Interestingly, treatment with MRS 1523 allowed for a significantly better recovery of neurotransmission also in those slices in which AD was present, but peaked close to the end of OGD episodes (⩽45 s) or after the interruption of the OGD episode (25.6±5.5% mean±s.e., range 3–75%, n=14 vs control 7.2±2.8% mean±s.e., range 0–24.5%, n=17, P<0.005, Mann–Whitney test, two-tailed). Conversely, in control slices, no considerable recovery was found even in those preparations in which AD peaked after interruption of 7 min OGD episodes.
Therefore, it appears that in our experimental conditions the most relevant correlates for the degree of recovery of neurotransmission produced by A3 receptor block during OGD was the delay in AD development, which prolonged the time window allowed for the recovery of fepsps.
Discussion
The main finding of the present work is that the selective antagonism of A3 adenosine receptors reduces the deleterious effect induced by OGD on CA1 hippocampal neurotransmission and pyramidal cell survival.
In our experiments, the pretreatment of hippocampal slices with selective A3 adenosine receptor antagonists considerably delays the occurrence of AD and significantly protects from the irreversible disruption of excitatory neurotransmission caused by 7-min OGD episodes. A3 receptor antagonists exert a protective effect on OGD episodes of ⩽9 min duration, showing that other cellular mechanisms are implicated and predominate when AD takes place.
We used four selective A3 adenosine receptor antagonists with different chemical structure and lipid solubility (Kim et al., 1996; Jacobson et al., 1997; Li et al., 1998; van Muijlwijk-Koezen et al., 2000; Maconi et al., 2002; Muller, 2003) to assess the role of A3 receptor stimulation by endogenous adenosine released during OGD episodes. All compounds show similar protective effects on the 7 min OGD at nM concentration, ensuring specific involvement of A3 receptors in the observed effects.
MRS 1220, VUF 5574 and WSAB were reported to have higher affinity for the human A3 receptors (Kim et al., 1996; Jacobson et al., 1997; van Muijlwijk-Koezen et al., 2000; Maconi et al., 2002) and their effectiveness on native rat A3 receptors at very low concentration may be surprising. We do not have any straightforward explanation for this phenomenon. The tissue accumulation of the lipophilic MRS 1523, MRS 1220 and VUF 5574 is an unlikely explanation since WSAB is a hydrophilic compound (Maconi et al., 2002). On the other hand, receptor affinity of these compounds may also depend on specific conformations assumed by A3 receptors in the integer cell membrane and may differ from that assessed in receptor binding experiments on disrupted membranes. Alternatively, the paucity of A3 receptors in native tissue (Ji et al., 1994; von Lubitz, 1999) allows the speculation that occupancy of a substantial fraction of A3 receptors is required for evoking cell response(s). In this case, the block of a relatively small fraction of A3 receptors may be sufficient to greatly antagonise the effects of endogenous adenosine released during OGD (see also below).
Role of adenosine receptors in depression and disruption of CA1 hippocampal excitatory synaptic transmission during in vitro ischaemia
The depression of synaptic responses caused by OGD episodes of short duration (up to 5 min) in the CA1 region of the hippocampus is fully reversible (Latini et al., 1999; Pugliese et al., 2003). In contrast, the application of 7-min OGD elicits a complete and irreversible block of hippocampal neurotransmission, that persists upon slice reperfusion with normally oxygenated and glucose-containing aCSF (Pugliese et al., 2003).
A constant sequence of changes in neurotransmission occurs during the application of long OGD episodes and comprises (i) the early depression of evoked fepsps, (ii) a transient recovery of fepsps followed by (iii) the disappearance of synaptic responses and afferent fibre volley and (iv) AD. The whole sequence of events lasts about 6 min and therefore is typically recorded within the application of 7-min OGD.
Our results show that the block of A3 receptor-mediated effects by selective antagonists results in a significant delay of the sequence of electrophysiological changes, including disappearance of the afferent fibre volley and AD that are considered early electrophysiological signs of tissue suffering.
The earliest event observed during OGD was the disappearance of the electrically evoked fepsps that reflect the currents generated by the inflow of cations into CA1 pyramidal cell dendrites produced by activation of synaptic glutamate receptors. The predominant, although not exclusive, mechanism that accounts for the reduction in fepsps during the first 4–5 min of ischaemia is a decrease in glutamatergic neurotransmission caused by activation of adenosine A1 presynaptic receptors (Fowler, 1990; Gribkoff et al., 1990; Pedata et al., 1993; Latini et al., 1999).
A3 receptor stimulation per se does not produce any harmful effects in normoxic tissue. In fact, neither A3 receptor agonists nor adenosine disrupt CA1 neurotransmission in normally oxygenated slices (Dunwiddie et al., 1997). This implies that the main role of A3 receptor activation during OGD is to hasten the processes that lead to AD and that removal of these mechanisms prolongs the period of tissue survival to OGD, but cannot fully block the consequences of ischaemia.
In order to explain the mechanism by which A3 receptors may be contributing to failure of synaptic transmission during OGD, we can postulate that stimulation of A3 receptors by adenosine released during prolonged and severe ischaemia may enhance excitatory transmission on CA1 pyramidal neurones, accounting for increased neuronal excitability and consequent lack of protection of the ischaemic tissue. Stimulation of A3 receptors in the hippocampus may in fact: (i) counteract the inhibitory action of A1 adenosine receptors on excitatory neurotransmission (Dunwiddie et al., 1997); (ii) inhibit the presynaptic metabotropic glutamate receptor inhibitory function on excitatory transmission (Macek et al., 1998). Furthermore, A3 receptor-mediated stimulation of phospholipase C could contribute to neuronal damage through mobilisation of intracellular calcium (Abbracchio et al., 1995) and/or activation of PKC, resulting in an increase in excitability of CA1 neurones (Hu et al., 1987).
Consistently, our data show that the action of A3 receptor antagonists is limited to a time window that extends survival to about 9 min of OGD. This time window seems to be related to the maximal delay allowed for the appearance of AD in the absence of A3 receptor stimulation.
The generation of AD is complex and multifactorial (see Somjen, 2001) and the mechanisms responsible for the delay in AD remain elusive. Interestingly, the time window of A3 receptor-mediated effects overlaps with the delay that can be obtained by treating the slices with glutamate receptor antagonists (Tanaka et al., 1997; Yamamoto et al., 1997; see also in Somjen, 2001). It is appealing to suggest that removal of the A3 receptor-mediated impairment of the feedback inhibition of glutamate release exerted by specific metabotropic glutamate receptor subtypes (Macek et al., 1998) may substantially decrease/delay the participation of the excitatory neurotransmitter in triggering the AD.
Our results are in contrast with those obtained in transgenic mice with a deletion of the A3 receptor. It has been demonstrated that after repeated brief exposure to carbon monoxide, mice lacking the A3 receptors are more vulnerable than control animals to hippocampal damage following hypoxia (Fedorova et al., 2003), suggesting a neuroprotective role of A3 receptors. The discrepancy about the functional role of A3 receptors in the brain during hypoxia or ischaemia could be due to the diversity of both the experimental conditions (hypoxia/ischaemia) and pharmacological profiles of these receptors across species.
The delay of the initial depression of fepsps caused by A3 antagonists indicates that A3 receptors are activated within the first 2 min of OGD despite the reported low affinity of adenosine for these receptors (about 5 μM: Zhou et al., 1992). However, as the estimated concentration of adenosine at the receptor level approaches 5 μM at the second minute of OGD and reaches 30–40 μM within the fifth minute of OGD (Latini et al., 1999), a substantial activation of A3 receptors may be achieved since the beginning of ischaemia. According to the Hill–Langmuir equation, implemented with the above reported values, the estimated occupancy of A3 receptors by endogenous adenosine would approach 50% within the second minute of OGD and be almost 90% at 5 min of OGD.
It is therefore conceivable that all the changes in neurophysiological parameters observed with A3 receptor antagonists result from block of cell mechanisms activated by A3 receptors and that their stimulation by adenosine released during OGD contributes to the development of ischaemia effects from the beginning of OGD, thus hastening the deleterious effects of ischaemia on neurotransmission.
A modest, transient, recovery of fepsps was observed in most preparations and fading of the recovery was accompanied by disappearance of the presynaptic fibre volley. This sequence of events has been ascribed to progressive increase in the extracellular K+ concentration that initially produces hyperexcitability of pyramidal cells followed by a depolarisation block of neurotransmission when extracellular K+ reaches 10 mM or higher concentration (Sick et al., 1987). After fibre volley disappearance and in the absence of any synaptic response, the large efflux of potassium into the extracellular space combined with activation of sodium and calcium channels, triggers sustained depolarisation of hippocampal cells that coincides with AD recorded in the CA1 region. Although similar to the spreading depression described by Leao (1951) and known to be harmless to the cerebral cortex under normoxic conditions, AD has been suggested to contribute to cell damage during ischaemia (see Somjen, 2001). Increased intracellular calcium and/or massive glutamate receptor activation are additional mechanisms that concur with potassium redistribution to produce AD (Tanaka et al., 1997; Yamamoto et al., 1997) and have been suggested to contribute to cell damage during ischaemia (see Somjen, 2001).
Therapeutic implications
In the brain, spreading depression is a phenomenon characterised by a slow transient cellular depolarisation moving at 3–4 mm min−1 over the surface of the cortex (Leao, 1951). A large efflux of potassium into the extracellular space coincides with the shift in the d.c. potential. The changes in brain homeostasis are transient and do not cause visible injury in normoxic conditions (Hansen & Nedergaard, 1988), but are correlated with tissue damage during ischaemia (see Somjen, 2001). Within 2 min of stroke onset, neurons and glia suddenly depolarise in the brain area where cerebral blood flow falls to 10% of control (Macdonald & Stoodley, 1998). In these conditions, the generation of AD may contribute to the extent and severity of neuronal damage. In particular, the propagation of the AD to the hypoxic/hypoglycemic region (penumbral area) surrounding the ischaemic core may extend the damage. Consistently, it has been demonstrated that one major factor contributing to neuronal death in the penumbra is the propagation of spreading depression waves (Koroleva & Bures, 1996). Because the penumbra constitutes potentially salvageable tissue, the molecular responses of the perifocal neurons to focal ischaemia and AD are of interest (Obeidat et al., 2000).
In our experiments, a substantial field depolarisation was recorded for several minutes (see e.g. Figures 2 and 4) after the AD peak and therefore even when OGD is interrupted immediately after the AD peak, hypoxia persists for few minutes after AD. Indeed, in our experimental conditions the recovery of pO2 to normal levels takes about 3–4 min (Pugliese et al., 2003).
This is important for the possible therapeutic outcome during ischaemia in vivo. It may be envisaged that the block of A3 receptors may increase the resistance of the brain tissue not only in the ischaemic core but also in the surrounding ‘penumbral' region. However, while the action of A3 block is of limited effectiveness in the ischaemic core (depending on the duration of the episode), more effective neuroprotection can occur in the surrounding regions, where the damage can be ascribed to the concomitant hypoxia/hypoglycemia and appearance of AD. The observation that a statistically significant recovery of fepsps occurred when AD peaked in concomitance or after interruption of OGD in the presence of A3 antagonists but not in control slices, supports the notion that A3 receptor block increases the resistance to the deleterious effect of AD in conditions of milder hypoglycaemia/hypoxia. The causal association of A3 receptor block, delayed AD appearance and better recovery from ischaemic episodes needs, however, further investigation.
Regardless of the exact mechanisms exerted by A3 receptors at a cellular level, it appears that the activation of these adenosine receptor subtypes during an ischaemic episode produces deleterious consequences for the survival of neuronal cells and that the block of A3 receptors may substantially increase the resistance of brain tissue to OGD occurring in ischaemia.
Acknowledgments
This work was supported by grants from the University of Florence, MIUR, Ente Cassa di Risparmio di Firenze, Italy (2002/1663 and 2004/0747) and EC (LSHM-CT-2004-503474).
Abbreviations
- aCSF
artificial cerebral spinal fluid
- AD
anoxic depolarisation
- DMSO
dimethylsulphoxide
- fepsp
field excitatory post synaptic potential
- MRS 1220
N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-yl]benzene acetamide
- MRS 1523
3-propyl-6-ethyl-5[(ethylthio)carbonyl]-2-phenyl-4-propyl-3-pyridinecarboxylate
- OGD
oxygen glucose deprivation
- VUF 5574
N-(2-methoxyphenyl)-N′-[2-(3-pyrindinyl)-4-quinazolinyl]-urea
- WSAB
5-[[(4-pyridyl)amino]carbonyl]amino-8-methyl-2-(2-furyl)-pyrazolo[4,3-e]1,2,4-triazolo[1,5-c]pyrimidine hydrochloride
References
- ABBRACCHIO M.P., BRAMBILLA R., CERUTI S., KIM H.O., VON LUBITZ D.K., JACOBSON K.A., CATTABENI F. G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol. Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- ABBRACCHIO M.P., CATTABENI F. Brain adenosine receptors as targets for therapeutic intervention in neurodegenerative diseases. Ann. NY Acad. Sci. 1999;890:79–92. doi: 10.1111/j.1749-6632.1999.tb07983.x. [DOI] [PubMed] [Google Scholar]
- ANDERSON W.W., COLLINGRIDGE G.L. A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Methods. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- BRAND A., VISSIENNON Z., ESCHKE D., NIEBER K. Adenosine A1 and A3 receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacology. 2001;40:85–95. doi: 10.1016/s0028-3908(00)00117-9. [DOI] [PubMed] [Google Scholar]
- COSTENLA A.R., LOPES L.V., DE MENDONCA A., RIBEIRO J.A. A functional role for adenosine A3 receptors: modulation of synaptic plasticity in the rat hippocampus. Neurosci. Lett. 2001;302:53–57. doi: 10.1016/s0304-3940(01)01633-0. [DOI] [PubMed] [Google Scholar]
- DIXON A.K., GUBITZ A.K., SIRINATHSINGHJI D.J., RICHARDSON P.J., FREEMAN T.C. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNWIDDIE T.V., DIAO L., KIM H.O., JIANG J.L., JACOBSON K.A. Activation of hippocampal adenosine A3 receptors produces a desensitization of A1 receptor-mediated responses in rat hippocampus. J. Neurosci. 1997;17:607–614. doi: 10.1523/JNEUROSCI.17-02-00607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEDOROVA I.M., JACOBSON M.A., BASILE A., JACOBSON K.A. Behavioral characterization of mice lacking the A3 adenosine receptor: sensitivity to hypoxic neurodegeneration. Cell Mol. Neurobiol. 2003;23:431–447. doi: 10.1023/A:1023601007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING K.M., MOGUL D.J. Adenosine A3 receptors potentiate hippocampal calcium current by a PKA-dependent/PKC-independent pathway. Neuropharmacology. 1997;36:353–362. doi: 10.1016/s0028-3908(97)83762-8. [DOI] [PubMed] [Google Scholar]
- FOWLER J.C. Adenosine antagonists alter the synaptic response to in vitro ischemia in the rat hippocampus. Brain Res. 1990;509:331–334. doi: 10.1016/0006-8993(90)90560-x. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., CHEN J.F., MASINO S.A., VAUGEOIS J.M. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., IJZERMAN A.P., JACOBSON K.A., KLOTZ K.N., LINDEN J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- GRIBKOFF V.K., BAUMAN L.A., VANDERMAELEN C.P. The adenosine antagonist 8-cyclopentyltheophylline reduces the depression of hippocampal neuronal responses during hypoxia. Brain Res. 1990;512:353–357. doi: 10.1016/0006-8993(90)90648-U. [DOI] [PubMed] [Google Scholar]
- HANSEN A.J., NEDERGAARD M. Brain ion homeostasis in cerebral ischemia. Neurochem. Pathol. 1988;9:195–209. doi: 10.1007/BF03160362. [DOI] [PubMed] [Google Scholar]
- HENTSCHEL S., LEWERENZ A., NIEBER K. Activation of A3 receptors by endogenous adenosine inhibits synaptic transmission during hypoxia in rat cortical neurons. Restor. Neurol. Neurosci. 2003;21:55–63. [PubMed] [Google Scholar]
- HU G.Y., HVALBY O., WALAAS S.I., ALBERT K.A., SKJEFLO P., ANDERSEN P., GREENGARD P. Protein kinase C injection into hippocampal pyramidal cells elicits features of long term potentiation. Nature. 1987;328:426–429. doi: 10.1038/328426a0. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A., PARK K.S., JIANG J.L., KIM Y.C., OLAH M.E., STILES G.L., JI X.D. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology. 1997;36:1157–1165. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARVIS C.R., ANDERSON T.R., ANDREW R.D. Anoxic depolarization mediates acute damage independent of glutamate in neocortical brain slices. Cereb. Cortex. 2001;11:249–259. doi: 10.1093/cercor/11.3.249. [DOI] [PubMed] [Google Scholar]
- JI X.D., GALLO-RODRIGUEZ C., JACOBSON K.A. A selective agonist affinity label for A3 adenosine receptors. Biochem. Biophys. Res. Commun. 1994;203:570–576. doi: 10.1006/bbrc.1994.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y.C., JI X.D., JACOBSON K.A. Derivatives of the triazoloquinazoline adenosine antagonist ( CGS15943) are selective for the human A3 receptor subtype. J. Med. Chem. 1996;39:4142–4148. doi: 10.1021/jm960482i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOROLEVA V.I., BURES J. The use of spreading depression waves for acute and long-term monitoring of the penumbra zone of focal ischemic damage in rats. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3710–3714. doi: 10.1073/pnas.93.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATINI S., BORDONI F., PEDATA F., CORRADETTI R. Extracellular adenosine concentrations during in vitro ischaemia in rat hippocampal slices. Br. J. Pharmacol. 1999;127:729–739. doi: 10.1038/sj.bjp.0702591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATINI S., PEDATA F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- LAUDADIO M.A., PSARROPOULOU C. The A3 adenosine receptor agonist 2-Cl-IB-MECA facilitates epileptiform discharges in the CA3 area of immature rat hippocampal slices. Epilepsy Res. 2004;59:83–94. doi: 10.1016/j.eplepsyres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- LEAO A.A. The slow voltage variation of cortical spreading depression of activity. Electroencephalogr. Clin. Neurophysiol. 1951;3:315–321. doi: 10.1016/0013-4694(51)90079-x. [DOI] [PubMed] [Google Scholar]
- LI A.H., MORO S., MELMAN N., JI X.D., JACOBSON K.A. Structure–activity relationships and molecular modeling of 3, 5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J. Med. Chem. 1998;41:3186–3201. doi: 10.1021/jm980093j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACEK T.A., SCHAFFHAUSER H., CONN P.J. Protein kinase C and A3 adenosine receptor activation inhibit presynaptic metabotropic glutamate receptor (mGluR) function and uncouple mGluRs from GTP-binding proteins. J. Neurosci. 1998;18:6138–6146. doi: 10.1523/JNEUROSCI.18-16-06138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD R.L., STOODLEY M. Pathophysiology of cerebral ischemia. Neurol. Med. Chir. 1998;38:1–11. doi: 10.2176/nmc.38.1. [DOI] [PubMed] [Google Scholar]
- MACONI A., PASTORIN G., DA ROS T., SPALLUTO G., GAO Z.G., JACOBSON K.A., BARALDI P.G., CACCIARI B., VARANI K., MORO S., BOREA P.A. Synthesis, biological properties, and molecular modeling investigation of the first potent, selective, and water-soluble human A(3) adenosine receptor antagonist. J. Med. Chem. 2002;45:3579–3582. doi: 10.1021/jm020974x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER C.E. Medicinal chemistry of adenosine A3 receptor ligands. Curr. Top Med. Chem. 2003;3:445–462. doi: 10.2174/1568026033392174. [DOI] [PubMed] [Google Scholar]
- OBEIDAT A.S., ANDREW R.D. Spreading depression determines acute cellular damage in the hippocampal slice during oxygen/glucose deprivation. Eur. J. Neurosci. 1998;10:3451–3461. doi: 10.1046/j.1460-9568.1998.00358.x. [DOI] [PubMed] [Google Scholar]
- OBEIDAT A.S., JARVIS C.R., ANDREW R.D. Glutamate does not mediate acute neuronal damage after spreading depression induced by O2/glucose deprivation in the hippocampal slice. J. Cereb. Blood Flow Metab. 2000;20:412–422. doi: 10.1097/00004647-200002000-00024. [DOI] [PubMed] [Google Scholar]
- PEDATA F., LATINI S., PUGLIESE A.M., PEPEU G. Investigations into the adenosine outflow from hippocampal slices evoked by ischemia-like conditions. J. Neurochem. 1993;61:284–289. doi: 10.1111/j.1471-4159.1993.tb03566.x. [DOI] [PubMed] [Google Scholar]
- PHILLIS J.W., GOSHGARIAN H.G. Adenosine and neurotrauma: therapeutic perspectives. Neurol. Res. 2001;23:183–189. doi: 10.1179/016164101101198316. [DOI] [PubMed] [Google Scholar]
- PUGLIESE A.M., COPPI E., CORRADETTI R., PEDATA F.The selective block of adenosine A3 receptors protects rat hippocampal slices from irreversibile synaptic depression elicited by oxygen and glucose deprivation 2004. Program No. 104.20. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2004
- PUGLIESE A.M., LATINI S., CORRADETTI R., PEDATA F. Brief, repeated, oxygen–glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors. Br. J. Pharmacol. 2003;140:305–314. doi: 10.1038/sj.bjp.0705442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SICK T.J., SOLOW E.L., ROBERTS E.L. Extracellular potassium ion activity and electrophysiology in the hippocampal slice: paradoxical recovery of synaptic transmission during anoxia. Brain Res. 1987;418:227–234. doi: 10.1016/0006-8993(87)90090-4. [DOI] [PubMed] [Google Scholar]
- SOMJEN G.G. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- TANAKA E., YAMAMOTO S., KUDO Y., MIHARA S., HIGASHI H. Mechanisms underlying the rapid depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. J. Neurophysiol. 1997;78:891–902. doi: 10.1152/jn.1997.78.2.891. [DOI] [PubMed] [Google Scholar]
- TOUZANI O., ROUSSEL S., MACKENZIE E.T. The ischaemic penumbra. Curr. Opin. Neurol. 2001;14:83–88. doi: 10.1097/00019052-200102000-00013. [DOI] [PubMed] [Google Scholar]
- VAN MUIJLWIJK-KOEZEN J.E., TIMMERMAN H., VAN DER GOOT H., MENGE W.M., FRIJTAG VON DRABBE KUNZEL J., DE GROOTE M., IJZERMAN A.P. Isoquinoline and quinazoline urea analogues as antagonists for the human adenosine A3 receptor. J. Med. Chem. 2000;43:2227–2238. doi: 10.1021/jm000002u. [DOI] [PubMed] [Google Scholar]
- VON LUBITZ D.K. Adenosine and cerebral ischemia: therapeutic future or death of a brave concept. Eur. J. Pharmacol. 1999;365:9–25. doi: 10.1016/s0014-2999(98)00788-2. [DOI] [PubMed] [Google Scholar]
- VON LUBITZ D.K., LIN R.C., POPIK P., CARTER M.F., JACOBSON K.A. Adenosine A3 receptor stimulation and cerebral ischemia. Eur. J. Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO S., TANAKA E., SHOJI Y., KUDO Y., INOKUCHI H., HIGASHI H. Factors that reverse the persistent depolarization produced by deprivation of oxygen and glucose in rat hippocampal CA1 neurons in vitro. J. Neurophysiol. 1997;78:903–911. doi: 10.1152/jn.1997.78.2.903. [DOI] [PubMed] [Google Scholar]
- ZHOU Q.Y., LI C., OLAH M.E., JOHNSON R.A., STILES G.L., CIVELLI O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]