Abstract
The interaction of a new nonribose ligand (LUF5831) with the human adenosine A1 receptor was investigated in the present study.
Radioligand binding experiments were performed in the absence and presence of diverse allosteric modulators on both wild-type (wt) and mutant (T277A) adenosine A1 receptors. Thermodynamic data were obtained by performing these assays at different temperatures. In addition, cyclic adenosine monophosphate (cAMP) assays were performed.
The presence of allosteric modulators had diverse effects on the affinity of LUF5831, N6-cyclopentyladenosine (CPA), a full agonist, and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), an inverse agonist/antagonist, for the adenosine A1 receptor. PD81,723, for example, increased the affinity of CPA, while the affinity of LUF5831 was decreased. However, the affinity of DPCPX was decreased even more. In addition, LUF5831 was shown to have an affinity for the mutant (T277A) adenosine A1 receptor (Ki=122±22 nM), whereas CPA's affinity was negligible. The results of temperature-dependent binding assays showed that the binding of LUF5831 was entropy driven, in between the behaviour of CPA binding to the high- and low-affinity states of the receptor, respectively.
The inhibition of the forskolin-induced production of cAMP through activation of the wt adenosine A1 receptor showed that LUF5831 had a submaximal effect (37±1%) in comparison to CPA (66±5%). On the mutant receptor, however, neither CPA nor LUF5831 inhibited cAMP production.
This study indicates that the nonribose ligand, LUF5831, is a partial agonist for the adenosine A1 receptor.
Keywords: Adenosine A1 receptor; allosteric modulation; LUF5831; thermodynamics; PD81,723; T277A mutant
Introduction
Extracellular adenosine plays an important physiological role and mediates a large variety of effects, for example, on the cardiovascular, immune, and central nervous systems (Ralevic & Burnstock, 1998). These effects are mediated by adenosine receptors, which belong to the large family of membrane-bound G-protein-coupled receptors (GPCRs) (Fredholm et al., 2001). The adenosine receptors have been subclassified into four subtypes, A1, A2A, A2B and A3, according to their molecular, biochemical and pharmacological properties. The adenosine A1 and A3 receptors are coupled to the enzyme adenylate cyclase in an inhibitory fashion via a Gi protein, whereas the A2A and A2B receptors stimulate this enzyme via a Gs protein.
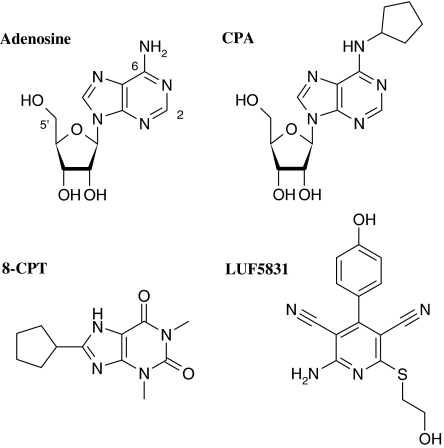
So far, agonists for the adenosine receptors have all been derivatives of the endogenous ligand adenosine. Chemical modification of the adenosine structure (Figure 1), particularly at the N6, C2, and C5′ positions, has proven feasible with N6-cyclopentyladenosine (CPA, Figure 1), a selective, high-affinity agonist for the adenosine A1 receptor, as a reference example (Müller, 2001). The ribose moiety was thought necessary for agonistic behaviour, as manipulation of this moiety yielded a lower intrinsic activity at the adenosine A1 receptor (Siddiqi et al., 1995; Van Calenbergh et al., 1997; Soudijn et al., 2003).
Figure 1.
Chemical structures of adenosine (endogenous ligand), CPA (full agonist), 8-CPT (inverse agonist) and LUF5831.
Recently, however, a series of novel ligands for the adenosine receptor, all pyridine-3,5-dicarbonitriles and thus structurally unlike adenosine, has been described in patent literature, some of them claimed to be agonists (Rosentreter et al., 2001; 2003). We decided to follow-up on these findings and started a synthetic program from which LUF5831 (Figure 1) emerged (Chang et al., 2005).
For the present study, we decided to analyse the pharmacological properties of LUF5831 in radioligand-binding studies and second messenger assays, always in comparison to the reference full agonist and adenosine-look-alike CPA. Radioligand-binding studies were performed on the wild-type (wt) human adenosine A1 receptor in the absence or presence of allosteric modulators and on a mutant (T277A) A1 receptor. This mutant receptor recognizes antagonists/inverse agonists, but has a remarkably low affinity for agonists such as CPA (Townsend-Nicholson & Schofield, 1994; Dalpiaz et al., 1998). The temperature dependence of the ligand–receptor interaction was also studied, yielding the thermodynamic terms ΔG0, ΔH0 and ΔS0 for the binding of both compounds. In addition, the effects of both CPA and LUF5831 on the inhibition of adenylate cyclase were determined as a measure of intrinsic activity.
Methods
Cell culture
Chinese hamster ovary (CHO) cells expressing the wt human adenosine A1 receptor were grown in a 1 : 1 mixture of Dulbecco's modified Eagle's medium (DMEM) and Ham's F12 medium containing 10% bovine calf serum, streptomycin (50 μg ml−1), penicillin (50 IU ml−1) and G418 (0.2 mg ml−1) at 37°C in 5% CO2. CHO cells expressing the mutant T277A human adenosine A1 receptor were grown as described above, but without G418. The cells were subcultured twice weekly at a ratio of 1 : 20 or 1 : 30 for the cells expressing the wt or the mutant receptors, respectively. For membrane preparation the cells were transferred to large 14-cm diameter plates (Dalpiaz et al., 1998).
Membrane preparation
Cells were detached from the plates by scraping them into 5 ml PBS, collected and centrifuged at 700 × g (3000 r.p.m.) for 5 min. Pellets derived from 20 plates were pooled and resuspended in 20 ml of ice-cold 50 mM Tris-HCl buffer, pH 7.4. An UltraThurrax was used to homogenize the cell suspension. Membranes and the cytosolic fraction were separated by centrifugation at 100,000 × g (31,000 r.p.m.) in a Beckman Optima LE-80 K ultracentrifuge at 4°C for 20 min. The pellet was resuspended in 10 ml of the Tris buffer and the homogenization and centrifugation step was repeated. Tris buffer (10 ml) was used to resuspend the pellet and the membranes were stored in 500 μl aliquots at −80°C. Membrane protein concentrations were measured using the BCA (bicinchoninic acid) method (Smith et al., 1985).
Radioligand-binding assays
Membrane aliquots containing 10 μg (CHOhA1-wt) protein were incubated in a total volume of 400 μl of 50 mM Tris-HCl, pH 7.4 at 25°C for 60 min in the presence or absence of GTP (guanosine-5′-triphosphate) (1 mM), PD81,723 (10 μM), SCH-202676 (10 μM) or NaCl (1 M). Membrane aliquots containing 20 μg (CHOhA1-mutT277A) protein were incubated in a total volume of 200 μl of 50 mM Tris-HCl, pH 7.4 at 25°C for 60 min. Saturation experiments were carried out using nine different concentrations of [3H]DPCPX ranging from 0.1 to 8.0 nM. Displacement experiments on CHOhA1-wt cell membranes were performed using either 12 or 24 concentrations of cold ligand in the presence of 1.6 nM [3H]DPCPX. Displacement experiments on CHOhA1-mutT277A cell membranes were carried out with 1.3 nM [3H]DPCPX. Nonspecific binding was determined in the presence of 10 μM CPA (CHOhA1-wt) or 0.1 mM 8-CPT (CHOhA1-mutT277A) and represented approximately 10% of the total binding. Incubations were terminated by dilution with ice-cold Tris-HCl buffer. Separation of bound from free radioligand was performed by rapid filtration through Whatman GF/B filters using a Brandel harvester. Filters were subsequently washed three times with ice-cold buffer. Filter-bound radioactivity was measured by scintillation spectrometry (LKB Wallac, 1219 Rackbeta) after addition of 3.5 ml of Packard Emulsifier Safe.
For the other adenosine receptor subtypes radioligand-binding studies (A2A and A3) and cAMP assays (A2B) were performed essentially as described by Beukers et al. (2004).
cAMP assays
CHOhA1-wt and CHOhA1-mutT277A cells were harvested, using trypsol (0.25% in PBS containing 4.4 mM EDTA) and, after centrifugation at 700 × g for 5 min, resuspended in medium. The cells were plated in 24-well plates (400 μl/well, 2 × 105 cells/well) and grown overnight as a monolayer at 37°C in 5% CO2. The medium was removed and the cells were washed two times with 500 μl DMEM, containing 50 mM HEPES, pH 7.4. Subsequently, the cells were incubated with 250 μl DMEM/HEPES, supplemented with adenosine deaminase (ADA) (final concentration 0.8 IU ml−1), rolipram (50 μM) and cilostamide (50 μM). After 30 min of incubation at 37°C, 50 μl of various ligands was added. CPA, 8-CPT and LUF5831 were tested at a concentration of 100 times their Ki values to determine their maximal effects at the CHOhA1-wt receptor. For the CHOhA1-T277A receptor, 8-CPT and LUF5831 were tested as described above. CPA, however, was tested at a concentration of 10−4 M due to its negligible affinity for this mutant receptor. After 10 min of incubation, 100 μl forskolin (final concentration 10 μM) was added. The cells were incubated for an additional 15 min at 37°C, and the incubation was terminated by quick aspiration of the medium. The cells were lysed by the addition of 200 μl ice-cold 0.1 M HCl. The plates were stored at −20°C until further use.
The amount of cAMP was determined by competition with [3H]cAMP for protein kinase A (PKA)-binding protein. A final volume of 200 μl containing 100 μl PKA in buffer supplemented with bovine serum albumine (BSA) (150 mM K2HPO4, 10 mM EDTA, 0.2% BSA, pH 7.5), 50 μl [3H]cAMP in buffer, 50 μl sample or cAMP standard (0–12 pmol) was incubated for 2.5 h on ice. The incubation was terminated by adding 2 ml of ice-cold Tris-HCl buffer (50 mM, pH 7.4), and bound radioactivity was separated by rapid filtration through Whatman GF/C filters with a Brandel harvester. Filters were washed twice with 1 ml of buffer. Filter-bound radioactivity was measured by scintillation spectrometry (LKB Wallac, 1219 Rackbeta) after addition of 3.5 ml of Packard Emulsifier Safe.
Thermodynamic data determination
The values of thermodynamic terms (ΔG0, ΔH0 and ΔS0) were obtained by measuring Ki values at 0°C and 25°C, followed by linear van ‘t Hoff plot regression (Dalpiaz et al., 1998). The standard free energy, ΔG0, was calculated according to ΔG0=−RT ln KA, where KA=1/Ki. The van ‘t Hoff equation ln KA=−ΔH0/RT+ΔS0/R gives a linear plot of ln KA versus 1/T. The standard enthalpy (ΔH0) can be calculated from the slope, −ΔH0/R, and the standard entropy from the intercept, ΔS0/R or as ΔS0=(ΔH0−ΔG0)/T, with T=298.15 K and R=8.314 JK−1 mol−1.
Data analysis
All binding data was analysed using the nonlinear regression curve-fitting program GraphPad Prism v. 4 (GraphPad Software Inc., San Diego, CA, U.S.A.). Inhibitory binding constants (Ki values) were derived from the IC50 values according to the Cheng and Prusoff equation Ki=IC50/(1+[C]/Kd) where [C] is the concentration of the radioligand and Kd its dissociation constant (Cheng & Prusoff, 1973). The Kd values of [3H]DPCPX at CHOhA1-wt and CHOhA1-mutT277A membranes at different conditions were obtained by computer analysis of saturation curves. All values obtained are means of at least three independent experiments performed in duplicate.
Materials
CPA, LUF5831, PD81,723 and SCH-202676 were synthesized in our laboratory, while 8-CPT was purchased from RBI Biochemicals Inc. (Natick, MA, U.S.A). ADA was purchased from Boehringer Mannheim (Germany), while forskolin and BSA were from Sigma (St Louis MO, U.S.A.). BCA and BCA protein assay reagent were purchased from Pierce Chemical Company (Rockford, IL, U.S.A.). PKA was isolated from bovine adrenal glands (Smit et al., 1994). [3H]DPCPX (specific activity 124 Ci mmol−1) was purchased from Amersham (‘s-Hertogenbosch, The Netherlands). [3H]cAMP (29.7 Ci mmol−1) was purchased from NEN (Du Pont Nemours, ‘s-Hertogenbosch, The Netherlands). All cell culture materials were taken from laboratory stocks.
Results
Allosteric modulation of affinity constants
Radioligand saturation experiments
Saturation experiments were performed with [3H]DPCPX on CHO cells expressing the wt adenosine A1 receptor in the presence and absence of allosteric modulators. The binding of [3H]DPCPX was saturable and best characterized by a one-site competition model with a control Kd-value of 1.6 nM for the wt receptor (Table 1). From Table 1 it also follows that on CHOhA1-wt cell membranes the Kd-value of [3H]DPCPX was not affected in the presence of GTP (1 mM). This value in the presence of PD81,723 (10 μM) was increased approximately five-fold, whereas in the presence of SCH-202676 (10 μM) or NaCl (1 M), the Kd-value was decreased, approximately 2- and 3-fold, respectively. The data in Table 1 was used to derive Ki rather than IC50 values in the following paragraphs.
Table 1.
Kd-values of [3H]DPCPX in the presence of buffer; 1 mM GTP; 10 μM PD81,723; 10 μM SCH-202676, 1 M NaCl or at 0°C at CHO human wild-type adenosine A1 receptors
| Membranes | [3H]DPCPX Kd (nM)a | Shiftb |
|---|---|---|
| CHOhA1-wt | 1.6±0.1 | — |
| +GTP | 1.6±0.3 | 1.0 |
| +PD81,723 | 8.8±1.4 | 5.5 |
| +SCH-202676 | 0.79±0.08 | 0.49 |
| +NaCl | 0.55±0.08 | 0.34 |
| 0°C | 0.69±0.05c | 0.43 |
Saturation of specific [3H]DPCPX binding at wild-type human adenosine A1 receptors stably expressed on CHO cell membranes.
The shift is defined as the ratio of Kd-values in the presence and absence of an allosteric modulator or at 0°C, respectively.
Data from Dalpiaz et al. (1998).
Values are means (±s.e.m.) of three separate assays each performed in duplicate.
Radioligand displacement assays
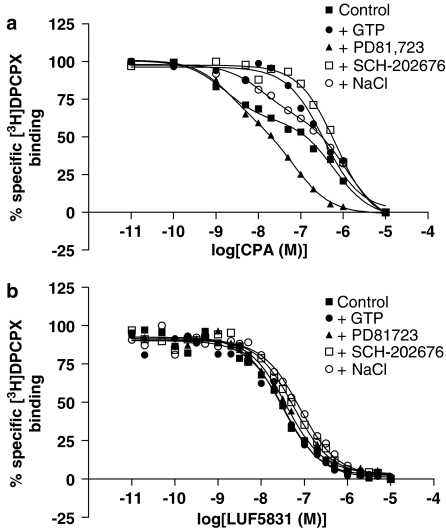
Displacement experiments of [3H]DPCPX on CHOhA1-wt cell membranes by CPA, as a reference full agonist, and LUF5831 were carried out in the presence or absence of 1 mM GTP, 10 μM PD81,723, 10 μM SCH-202676 or 1 M NaCl. For CPA a two-state receptor model with a higher (KH) and a lower affinity (KL) was sometimes statistically preferred over a one-state model, depending on the conditions applied. From Table 2 it follows that LUF5831 had a lower affinity (Ki value) for the A1 receptor than the reference full agonist CPA (KH value), 18 and 2.2 nM, respectively. Allosteric modulation of the adenosine A1 receptor resulted in diverse effects on binding affinity of these two ligands. In Figure 2 representative binding curves under the different conditions for CPA and LUF5831 are depicted. We used more data points to assess the effects of LUF5831 than with CPA, to assure an unequivocal interpretation of the effects of this unusual nonribose compound. In the presence of GTP the curve of CPA was less shallow than under control conditions, changing the two-state receptor interaction into one state. For LUF5831, however, this shift in binding state was not observed. In particular, PD81,723 had a marked and divergent effect on the binding affinity of CPA and LUF5831. PD81,723 increased the affinity of CPA for the low-affinity receptors, while the apparent affinity of LUF5831 was decreased in the presence of PD81,723. SCH-202676 decreased the affinity of both compounds, of CPA more than that of LUF5831, while shifting the interaction of CPA with the receptor to a one-state model. In the presence of NaCl the binding affinity of CPA appeared unaffected. For LUF5831, however, the affinity was decreased approximately two-fold. Apparently, the addition of allosteric modulators in some cases resulted in a shift from two affinity-binding states to one for CPA. For LUF5831, two-state binding was not observed under any of the conditions. Lastly, we determined the affinity of CPA and LUF5831 for the mutant (T277A) A1 receptor. We did not observe any displacement of [3H]DPCPX by CPA (1 μM). For LUF5831 we determined a Ki-value of 122±22 nM (one-state model) for this receptor, using a Kd value of 1.3 nM for [3H]DPCPX (Dalpiaz et al., 1998).
Table 2.
Affinities of CPA and LUF5831 at 25°C in the presence of buffer; 1 mM GTP; 10 μM PD81,723; 10 μM SCH-202676, 1 M NaCl or in buffer at 0°C at CHO human wild-type adenosine A1 receptors, expressed as Ki values
| Compound | KH or Ki (nM)a | KL (nM)a | % RH | Shiftb |
|---|---|---|---|---|
| CPA 25°C | 2.2±0.9 | 338±24 | 34±2 | — |
| +GTP | 191±24 | — | — | — |
| +NaCl | 2.4±0.3 | 309±23 | 26±4 | 1.1 |
| +PD81,723 | 2.3±0.4 | 93±22 | 40±3 | 1.0 |
| +SCH-202676 | 225±8 | — | — | — |
| CPA 0°C | 7.0±3.0 | 128±64 | 39±10 | 3.2 |
| LUF5831 25°C | 18±1 | — | — | — |
| +GTP | 23±4 | — | — | 1.3 |
| +NaCl | 33±5 | — | — | 1.8 |
| +PD81,723 | 32±4 | — | — | 1.8 |
| +SCH-202676 | 25±4 | — | — | 1.4 |
| LUF5831 0°C | 18±1 | — | — | 1.0 |
Displacement of specific [3H]DPCPX binding at human adenosine A1 receptors stably expressed on CHO cell membranes. Only data are shown, which according to computer analysis of the human adenosine A1 receptor-binding curves were statistically preferred under the different conditions.
The shift is defined as the ratio of KH-values (for CPA) or Ki-values (for LUF5831) in the presence and absence of an allosteric modulator, respectively.
Values are means (±s.e.m.) of three separate assays each performed in duplicate.
Figure 2.
Effects of the allosteric modulators on the displacement of [3H]DPCPX binding from human adenosine A1 receptors stably expressed on CHO cell membranes by CPA (a) or LUF5831 (b) in the absence or presence of GTP, PD81,723, SCH-202676 or NaCl. Representative graphs from one experiment performed in duplicate (see Table 2 for affinity values).
Thermodynamic experiments
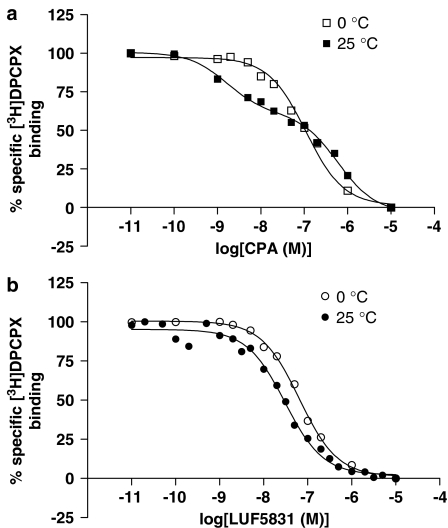
Displacement experiments of [3H]DPCPX by CPA and LUF5831 on CHOhA1-wt cell membranes were carried out at 0°C and 25°C. The Kd value of [3H]DPCPX at 0°C was determined to be 0.69±0.05 nM (Dalpiaz et al., 1998). The various displacement curves are shown in Figure 3. Apparently, a decrease in temperature had no effect on the curve and therefore on the affinity of LUF5831 (Table 2). The affinity of CPA decreased approximately three-fold for receptors in the high-affinity state (KH), whereas the affinity for the low-affinity state of the receptors increased with a similar value (Table 2).
Figure 3.
Effects of temperature on the displacement of [3H]DPCPX binding from human adenosine A1 receptors stably expressed on CHO cell membranes by CPA (a) at 0 and 25°C or LUF5831 (b) at 0 and 25°C. Representative graphs from one experiment performed in duplicate (see Table 2 for affinity values).
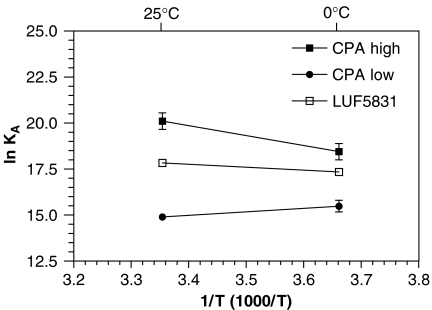
From the affinities shown in Table 2, the equilibrium binding association constants, KA, were calculated. The temperature dependence of CPA and LUF5831 at the different affinity states is illustrated by Van ‘t Hoff plots, which are shown in Figure 4. The KA value, the slope and the intercept of the Van ‘t Hoff plots were used to calculate the values of the thermodynamic terms (ΔG0, ΔH0 and ΔS0) as described in Methods above (Table 3). The values of the equilibrium standard enthalpy, ΔH0, and entropy, ΔS0 show that: (1) the interaction of CPA with the high-affinity state of the receptor was endothermic and totally entropy driven; (2) the binding of CPA to the low-affinity state of the receptor was exothermic and essentially enthalpy driven; and (3) the binding of LUF5831 to the receptor was endothermic and again entropy driven.
Figure 4.
Van ‘t Hoff plots showing the effect of temperature on the equilibrium binding association constants, KA, for CPA (high-affinity state), CPA (low-affinity state) and LUF5831. Graphs from three experiments performed in duplicate (see Table 3 for thermodynamic parameters).
Table 3.
Thermodynamic parameters for CPA and LUF5831 binding at CHO human wild-type adenosine A1 receptors, expressed as ΔG0, ΔH0 and ΔS0
| Compound | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (J mol−1 K−1) |
|---|---|---|---|
| CPA (high) | −50±1 | 45±17 | 318±60 |
| CPA (low) | −37±1 | −16±9 | 71±31 |
| LUF5831 | −44±1 | 13±11 | 193±7 |
ΔG0=Gibbs energy, ΔH0=standard enthalpy and ΔS0=standard entropy. Values are given at 298.15 K and means (±s.e.m.) are calculated from three separate assays performed in duplicate.
Radioligand displacement assays at other human adenosine receptors
Additionally, affinity (A2A and A3) or potency (A2B) of LUF5831 and CPA were determined at the other human adenosine receptor subtypes (Table 4). The affinity values were derived from a one-site competition analysis of the binding data. Both ligands, CPA and LUF5831, were shown to have the highest affinity for the adenosine A1 receptor. Further experiments also showed that LUF5831 had a higher selectivity towards the adenosine A1 receptor than CPA, except for the A2B receptor (Table 4).
Table 4.
Affinities of CPA and LUF5831 at the wild-type human adenosine A2A, A2B and A3 receptor subtypes, expressed as Ki values or % displacement at 1 μM
| Ki (nM) or % displacement at 1 μM | ||||||
|---|---|---|---|---|---|---|
| Compound | hA2Aa | A2A/A1b | hA2Bc | A2B/A1b | hA3d | A3/A1b |
| CPA | 131±14 | 60 | 203,000±97,000e | 92,272 | 281±56f | 128 |
| LUF5831 | 37% | >185 | 780±380 | 144 | 0% | >185 |
Displacement of specific [3H]ZM241385 binding at human adenosine A2A receptors expressed on HEK293 cell membranes.
The selectivity was calculated using the KH values for CPA and LUF5831 at the adenosine A1 receptor (see Table 2) and where % displacement is given, >1 μM was used as affinity value to calculate the selectivity.
Activities (EC50 value) on cAMP production in CHO cells expressing human A2B receptors.
Displacement of specific [125I]AB-MECA binding at human adenosine A3 receptors expressed on HEK293 cell membranes.
Data from de Zwart et al. (1998).
Data from van Tilburg et al. (1999).
Values are means (±s.e.m.) of three separate assays each performed in duplicate.
Inhibition of cAMP production via the wt and mutant receptor
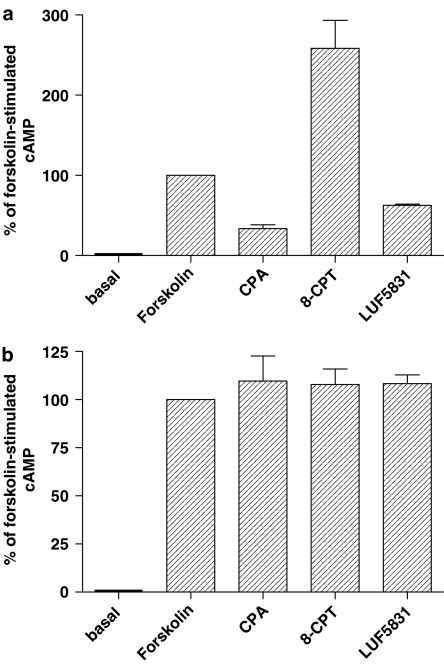
CPA and LUF5831 were further tested in a cAMP assay on both CHOhA1-wt and CHOhA1-mutT277A cells. 8-CPT was also examined in this assay. Here, cAMP was produced upon stimulation with 10 μM forskolin (=100% cAMP production) in order to examine agonist activation of the receptor. From Figure 5a it appears that CPA caused a stronger inhibition than LUF5831, with 66±5 and 37±1% reduction of cAMP levels, respectively. 8-CPT caused a substantial increase of cAMP levels, and is thus best classified as an inverse agonist in this system. We also determined the potency of both CPA and LUF5831. Both compounds inhibited the forskolin-induced cAMP production with EC50 values of 7.8±0.9 and 102±14 nM for CPA and LUF5831, respectively (data not shown). While CPA, LUF5831 and 8-CPT all modulated cAMP production on the wt receptor, neither inhibition nor stimulation of the mutant receptor occurred (Figure 5b).
Figure 5.
Bar graph representation of receptor activity of 1 μM CPA, 3.3 μM 8-CPT and 1 μM LUF5831 on CHOhA1-wt (a) or of 100 μM CPA, 3.3 μM 8-CPT and 13.5 μM LUF5831 on CHOhA1-mutT277A (b) cells expressed as a percentage of forskolin-induced cAMP production. Graph from three experiments performed in four-fold.
Discussion
The present study examines the affinity and activity of a new nonribose agonist, LUF5831, on the human adenosine A1 receptor under a variety of conditions. First, radioligand saturation experiments were performed to obtain Kd values of [3H]DPCPX, a selective inverse agonist for the A1 receptor, in the presence and absence of diverse allosteric modulators. In most cases the affinity of the radioligand was affected by the addition of allosteric modulators.
Next, radioligand displacement assays showed that on wt adenosine A1 receptors LUF5831 was less potent than CPA, with a Ki value of 18 nM for LUF5831 and a KH value of 2.2 nM for CPA. Further experiments in the absence and presence of GTP yielded the so-called GTP-shift of CPA and LUF5831 on CHOhA1-wt cells, a measure for the intrinsic activity of a compound (Kent et al., 1980; Ehlert, 1985). For both compounds a GTP-shift was observed, though in a different manner. For CPA the favoured mode of binding shifted towards a one-state competition model with a negligible fraction of high-affinity receptors. The shift of the binding model for CPA suggests the compound is an agonist, because the uncoupling of the G-protein from the receptors by GTP, and the concomitant disappearance of high-affinity receptors, induced a lower apparent affinity. Although the favoured binding model for LUF5831 was already one-state in the absence of GTP, the addition of GTP still caused a minor shift to a lower affinity. This experiment, therefore, showed that CPA, and LUF5831 to a lesser extent, are ligands with intrinsic activity on the wt A1 receptor, indicative of their agonistic profile. There was no GTP effect on [3H]DPCPX binding (Table 1), suggestive of the radioligand's characteristics as antagonist/inverse agonist. All in all, the different binding modes of the ligands can be explained by the ternary complex model (Lefkowitz et al., 1981).
Besides GTP, PD81,723 can also be used to discriminate between different classes of ligands (Kourounakis et al., 2001). The effects of PD81,723 have been extensively studied, showing this compound acts selectively as an allosteric modulator for the adenosine A1 receptor (Kollias-Baker et al., 1994; 1997; Dennis et al., 1996; Mizumura et al., 1996). We showed that there is a significant difference between the shift in Ki values of CPA and LUF5831 in the presence of PD81,723. The affinity of the full agonist CPA for the low-affinity state of the receptor (KL) increased three-fold, while we observed an almost two-fold decrease in the affinity of LUF5831 (Table 2). The affinity of the radioligand DPCPX decreased even more, approximately 5.5-fold, in the presence of PD81,723 (Table 1). This supports the findings that PD81,723 increases the affinity of agonists, but decreases the affinity of antagonists/inverse agonists (Kourounakis et al., 2001). The shift of LUF5831 was in between that of an agonist and an antagonist/inverse agonist, indicating that this compound might be a partial agonist. Another difference between the effect of PD81,723 on the binding of CPA and LUF5831 is that for CPA the two-state competition model was maintained with approximately the same amount of high-affinity receptors. For LUF5831, however, data analysis was best with a one-state competition model, without the presence of high-affinity receptors. In earlier experiments, we already found that PD81,723 slows the dissociation rate of the [3H]CCPA (2-chloro-N6-cyclopentyladenosine), a radiolabelled agonist, from the adenosine A1 receptor, which results in a higher affinity of this compound for the receptor (Van der Klein et al., 1999). Maybe PD81,723 somewhat accelerates dissociation of LUF5831 from the receptor, which would explain its lower affinity.
SCH-202676 has been shown to allosterically inhibit the binding of agonists and antagonists to various GPCRs (Fawzi et al., 2001; Gao et al., 2004; Lanzafame & Christopoulos, 2004; Van den Nieuwendijk et al., 2004). Very recently we have demonstrated the compound to be a protein modifier rather than modulator (Göblyös et al., 2005). In this study, the binding of CPA in the presence of SCH-202676 was best described by a one-state model with a lower apparent affinity. On the contrary, the affinity of [3H]DPCPX was increased two-fold in its presence. Therefore, it seems that SCH-202676 not only decreases the affinity of an agonist, but also increases the affinity of inverse agonists. The affinity of LUF5831 was slightly decreased, resembling neither full agonist nor antagonist/inverse agonist.
Lastly, we examined the so-called sodium shift at the adenosine A1 receptor. On other GPCRs, for example, the α2-adrenergic and D2 and D4 dopamine receptors, but also on adenosine receptors, it has been shown that sodium ions regulate ligand binding (Guyer et al., 1990; Neve et al., 1991; Schetz & Sibley, 2001). The presence of sodium ions reduces the affinity of agonists supposedly by a conformational change of the receptor. The extent of decrease in receptor affinity for agonists by sodium ions parallels agonist intrinsic activity in inhibiting adenylate cyclase (Tsai & Lefkowitz, 1978; Horstman et al., 1990). However, this shift seems to be different from the GTP shift, because sodium ions do not entirely uncouple the receptors from the G-protein, like GTP does (Linden et al., 1988). When the sodium shift of LUF5831 is compared with that of CPA and the radioligand DPCPX, it becomes clear that LUF5831 seems to behave more like an agonist than an antagonist/inverse agonist (Table 2). In addition, the amount of high-affinity receptors for CPA seems largely unaffected in the presence of NaCl. These findings indicate that the receptor indeed does not uncouple from the G-protein, which has already been reported (Linden et al., 1988).
Other displacement experiments on a mutant (T277A) receptor showed that CPA lost all of its affinity for the receptor. This finding corresponds with earlier results (Townsend-Nicholson & Schofield, 1994; Dalpiaz et al., 1998). Interestingly, LUF5831 still bound; its affinity was only reduced five-fold in comparison to the wt receptor. Apparently, Thr277 contributes largely to the binding of CPA and is far less important for the binding of LUF5831. It has been suggested earlier that Thr277 has a specific interaction with the ribose group (Dalpiaz et al., 1998), which is lacking in LUF5831.
Discrimination between agonist and antagonist binding based on a thermodynamic evaluation has been reported for a number of GPCRs, including adenosine receptors (Borea et al., 1991; for review see Borea et al., 2004). Therefore, additional radioligand displacement studies were performed at 0°C to obtain thermodynamic parameters (ΔG0, ΔH0 and ΔS0) of the binding of CPA and LUF5831. First of all, a lowering of the temperature during the binding experiments seemed to have little (CPA) or no effect (LUF5831) on the affinity of both ligands (Table 2). Comparison of CPA's affinity (KH value) at 25°C and 0°C showed a decrease in affinity of approximately three-fold. The affinity of [3H]DPCPX was increased two-fold (Table 1). The thermodynamic data thus obtained characterizes the binding process and therefore discriminates between full (partial/inverse) agonists and antagonists. On the A1 receptor binding of an agonist has been shown to be completely entropy driven, whereas the binding of an antagonist is essentially enthalpy driven (IJzerman et al., 1994; Borea et al., 1995). The thermodynamic values of enthalpy and entropy of the binding of a partial agonist are intermediate between a full agonist and an antagonist (Borea et al., 1994; IJzerman et al., 1994; Dalpiaz et al., 1998). The slope of the line of LUF5831 in Figure 4 is between that of the slope of the lines corresponding to the high- and low-affinity binding state of CPA. This is yet another indication that LUF5831 acts as a partial agonist at the human adenosine A1 receptor, which supports the results found with the displacement experiments in the presence of PD81,723 or SCH-202676. Partial agonists are thought to be a valuable addition to the current repertoire of medicines. Van Schaick et al. (1998) demonstrated that partial agonists for the adenosine A1 receptor induce tissue selectivity of action. Partial agonists for the receptor caused a marked inhibition of lipolysis in freely moving rats with few cardiovascular effects. The full agonist CPA, however, produced pronounced effects in both tissues. Apparently, unwanted effects can be separated from desired effects with partial agonists.
We also examined the influence of the compound on cellular cAMP levels. Both compounds inhibited the forskolin-induced production of cAMP on the wt receptor. Here LUF5831 behaved as a partial agonist compared to CPA, the reference full agonist. The intrinsic activity of LUF5831 was approximately half that of CPA, while its EC50 value was approximately 12-fold lower. The ratio of the EC50 over the KH values showed that the decrease in potency was higher for LUF5831 than for CPA, 18-fold and four-fold, respectively. On the mutant (T277A) receptor, however, both CPA and LUF5831 were unable to inhibit forskolin-induced cAMP production. This is particularly surprising for LUF5831, since this compound had appreciable affinity for the mutant. One explanation could be that LUF5831's interaction with the receptor involves at least some amino acids that are different from those in the adenosine/CPA-binding site, and that the threonine residue at position 277 plays a vital role in receptor activation irrespective of the chemical nature of the agonist.
In conclusion, diverse radioligand-binding assays, thermodynamic analysis and cAMP determinations have been performed to elucidate the binding characteristics of LUF5831 at the human adenosine A1 receptor. The overall outcome of the experiments is that LUF5831 seems to be a partial agonist for this receptor. This is a surprising outcome, because the structure of this compound, unlike the endogenous ligand adenosine, does not include a ribose moiety. Until recently the ‘dogma' was that a compound without a ribose moiety could not act as an agonist at the adenosine receptor. In the present study this dogma has been firmly challenged.
Acknowledgments
We thank the Garvan Institute of Medical Research (Darlinghurst, Australia) for CHO cells stably transfected with the wild-type human adenosine A1 receptor, respectively.
Abbreviations
- ADA
adenosine deaminase
- cAMP
cyclic adenosine monophosphate
- CHO
Chinese hamster ovary
- CPA
N6-cyclopentyladenosine
- 8-CPT
8-cyclopentyltheophylline
- [3H]DPCPX
8-cyclopentyl-[3H]1,3-dipropylxanthine
- ΔG0
standard free energy
- GPCR
G-protein-coupled receptor
- GTP
guanosine-5′-triphosphate
- ΔH0
standard enthalpy
- KH
equilibrium binding dissociation constant for high-affinity state of the receptor
- KL
equilibrium binding dissociation constant for low-affinity state of the receptor
- PD81,723
2-amino-4,5-dimethyl-3-thienyl-[3(trifluoromethyl)-phenyl]methanone
- RH
fraction of receptors in high-affinity state
- ΔS0
standard entropy
- wt
wild type
References
- BEUKERS M.W., CHANG L.C., VON FRIJTAG DRABBE KUNZEL J.K., MULDER-KRIEGER T., SPANJERSBERG R.F., BRUSSEE J., IJZERMAN A.P. New, non-adenosine, high-potency agonists for the human adenosine A2B receptor with an improved selectivity profile compared to the reference agonist N-ethylcarboxamidoadenosine. J. Med. Chem. 2004;47:3707–3709. doi: 10.1021/jm049947s. [DOI] [PubMed] [Google Scholar]
- BOREA P.A., DALPIAZ A., VARANI K., GUERRA L., GILLI G. Binding thermodynamics of adenosine A2a receptor ligands. Biochem. Pharmacol. 1995;49:461–469. doi: 10.1016/0006-2952(94)00464-w. [DOI] [PubMed] [Google Scholar]
- BOREA P.A., VARANI K., DALPIAZ A., CAPUZZO A., FABBRI E., IJZERMAN A.P. Full and partial agonistic behaviour and thermodynamic binding parameters of adenosine A1 receptor ligands. Eur. J. Pharmacol. 1994;267:55–61. doi: 10.1016/0922-4106(94)90224-0. [DOI] [PubMed] [Google Scholar]
- BOREA P.A., VARANI K., GESSI S., MERIGHI S., DAL PIAZ A., GILLI P., GILLI G. Receptor binding thermodynamics at the neuronal nicotinic receptor. Curr. Top. Med. Chem. 2004;4:361–368. doi: 10.2174/1568026043451410. [DOI] [PubMed] [Google Scholar]
- BOREA P.A., VARANI K., MALAGUTI V., GILLI G. Receptor binding at two different temperatures to discriminate agonist and antagonist behaviour of adenosine A1 receptor ligands in rat brain. J. Pharm. Pharmacol. 1991;43:866–868. doi: 10.1111/j.2042-7158.1991.tb03197.x. [DOI] [PubMed] [Google Scholar]
- CHANG L.C., VON FRIJTAG DRABBE KÜNZEL J.K., MULDER-KRIEGER T., SPANJERSBERG R.F., ROERINK S.F., VAN DEN HOUT G., BEUKERS M.W., BRUSSEE J., IJZERMAN A.P. A series of ligands displaying a remarkable agonistic–antagonistic profile at the adenosine A1 receptor. J. Med. Chem. 2005;48:2045–2053. doi: 10.1021/jm049597+. [DOI] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- DALPIAZ A., TOWNSEND-NICHOLSON A., BEUKERS M.W., SCHOFIELD P.R., IJZERMAN A.P. Thermodynamics of full agonist, partial agonist, and antagonist binding to wild-type and mutant adenosine A1 receptors. Biochem. Pharmacol. 1998;56:1437–1445. doi: 10.1016/s0006-2952(98)00202-0. [DOI] [PubMed] [Google Scholar]
- DE ZWART M., LINK R., VON FRIJTAG DRABBE KÜNZEL J.K., CRISTALLI G., JACOBSON K.A., TOWNSEND-NICHOLSON A., IJZERMAN A.P. A functional screening of adenosine analogues at the adenosine A2B receptor: a search for potent agonists. Nucleosides Nucleotides. 1998;17:969–985. doi: 10.1080/07328319808004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNIS D.M., RAATIKAINEN M.J., MARTENS J.R., BELARDINELLI L. Modulation of atrioventricular nodal function by metabolic and allosteric regulators of endogenous adenosine in guinea pig heart. Circulation. 1996;94:2551–2559. doi: 10.1161/01.cir.94.10.2551. [DOI] [PubMed] [Google Scholar]
- EHLERT F.J. The relationship between muscarinic receptor occupancy and adenylate cyclase inhibition in the rabbit myocardium. Mol. Pharmacol. 1985;28:410–421. [PubMed] [Google Scholar]
- FAWZI A.B., MACDONALD D., BENBOW L.L., SMITH-TORHAN A., ZHANG H., WEIG B.C., HO G., TULSHIAN D., LINDER M.E., GRAZIANO M.P. SCH-202676: An allosteric modulator of both agonist and antagonist binding to G protein-coupled receptors. Mol. Pharmacol. 2001;59:30–37. doi: 10.1124/mol.59.1.30. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., IJZERMAN A.P., JACOBSON K.A., KLOTZ K.N., LINDEN J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- GAO Z.G., GROSS A.S., JACOBSON K.A. Effects of the allosteric modulator SCH-202676 on adenosine and P2Y receptors. Life Sci. 2004;74:3173–3180. doi: 10.1016/j.lfs.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÖBLYÖS A., DE VRIES H., BRUSSEE J., IJZERMAN A.P. Synthesis and biological evaluation of a new series of 2,3,5-substituteD [1,2,4]-thiadiazoles as modulators of adenosine A1 receptors and their molecular mechanism of action. J. Med. Chem. 2005;48:1145–1151. doi: 10.1021/jm049337s. [DOI] [PubMed] [Google Scholar]
- GUYER C.A., HORSTMAN D.A., WILSON A.L., CLARK J.D., CRAGOE E.J., JR, LIMBIRD L.E. Cloning, sequencing, and expression of the gene encoding the porcine alpha 2-adrenergic receptor. Allosteric modulation by Na+, H+, and amiloride analogs. J. Biol. Chem. 1990;265:17307–17317. [PubMed] [Google Scholar]
- HORSTMAN D.A., BRANDON S., WILSON A.L., GUYER C.A., CRAGOE E.J., JR, LIMBIRD L.E. An aspartate conserved among G-protein receptors confers allosteric regulation of alpha 2-adrenergic receptors by sodium. J. Biol. Chem. 1990;265:21590–21595. [PubMed] [Google Scholar]
- IJZERMAN A.P., VAN DER WENDEN E.M., VON FRIJTAG DRABBE KÜNZEL J.K., MATHOT R.A., DANHOF M., BOREA P.A., VARANI K. Partial agonism of theophylline-7-riboside on adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1994;350:638–645. doi: 10.1007/BF00169369. [DOI] [PubMed] [Google Scholar]
- KENT R.S., DE LEAN A., LEFKOWITZ R.J. A quantitative analysis of beta-adrenergic receptor interactions: resolution of high and low affinity states of the receptor by computer modeling of ligand binding data. Mol. Pharmacol. 1980;17:14–23. [PubMed] [Google Scholar]
- KOLLIAS-BAKER C., RUBLE J., DENNIS D., BRUNS R.F., LINDEN J., BELARDINELLI L. Allosteric enhancer PD 81,723 acts by novel mechanism to potentiate cardiac actions of adenosine. Circ. Res. 1994;75:961–971. doi: 10.1161/01.res.75.6.961. [DOI] [PubMed] [Google Scholar]
- KOLLIAS-BAKER C.A., RUBLE J., JACOBSON M., HARRISON J.K., OZECK M., SHRYOCK J.C., BELARDINELLI L. Agonist-independent effect of an allosteric enhancer of the A1 adenosine receptor in CHO cells stably expressing the recombinant human A1 receptor. J. Pharmacol. Exp. Ther. 1997;281:761–768. [PubMed] [Google Scholar]
- KOUROUNAKIS A., VISSER C., DE GROOTE M., IJZERMAN A.P. Differential effects of the allosteric enhancer (2-amino-4,5-dimethyl-trienyl)[3-trifluoromethyl) phenyl]methanone (PD81,723) on agonist and antagonist binding and function at the human wild-type and a mutant (T277A) adenosine A1 receptor. Biochem. Pharmacol. 2001;61:137–144. doi: 10.1016/s0006-2952(00)00536-0. [DOI] [PubMed] [Google Scholar]
- LANZAFAME A., CHRISTOPOULOS A. Investigation of the interaction of a putative allosteric modulator, N-(2,3-diphenyl-1,2,4-thiadiazole-5-(2H)-ylidene) methanamine hydrobromide (SCH-202676), with M1 muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2004;308:830–837. doi: 10.1124/jpet.103.060590. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J., DE LEAN A., HOFFMAN B.B., STADEL J.M., KENT R., MICHEL T., LIMBIRD L. Molecular pharmacology of adenylate cyclase-coupled alpha- and beta-adrenergic receptors. Adv. Cyclic Nucleotide Res. 1981;14:145–161. [PubMed] [Google Scholar]
- LINDEN J., PATEL A., EARL C.Q., CRAIG R.H., DALUGE S.M. 125I-labeled 8-phenylxanthine derivatives: antagonist radioligands for adenosine A1 receptors. J. Med. Chem. 1988;31:745–751. doi: 10.1021/jm00399a010. [DOI] [PubMed] [Google Scholar]
- MIZUMURA T., AUCHAMPACH J.A., LINDEN J., BRUNS R.F., GROSS G.J. PD 81,723, an allosteric enhancer of the A1 adenosine receptor, lowers the threshold for ischemic preconditioning in dogs. Circ. Res. 1996;79:415–423. doi: 10.1161/01.res.79.3.415. [DOI] [PubMed] [Google Scholar]
- MÜLLER C.E. A1 adenosine receptors and their ligands: overview and recent developments. Farmaco. 2001;56:77–80. doi: 10.1016/s0014-827x(01)01005-9. [DOI] [PubMed] [Google Scholar]
- NEVE K.A., COX B.A., HENNINGSEN R.A., SPANOYANNIS A., NEVE R.L. Pivotal role for aspartate-80 in the regulation of dopamine D2 receptor affinity for drugs and inhibition of adenylyl cyclase. Mol. Pharmacol. 1991;39:733–739. [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- ROSENTRETER U., HENNING R., BAUSER M., KRAEMER T., VAUPEL A., HUEBSCH W., DEMBOWSKY K., SALCHER-SCHRAUFSTAETTER O., STASCH J.-P., KRAHN T., PERZBORN E. Substituted 2-thio-3,5-dicyano-4-aryl-6-aminopyridines and the use thereof as adenosine receptor ligands. WO01025210. 2001.
- ROSENTRETER U., KRAEMER T., SHIMADA M., HUEBSCH W., DIEDRICHS N., KRAHN T., HENNINGER K., STASCH J.-P. Preparation of 2-heteroarylmethylthio-3,5-dicyano-4-phenyl-6-aminopyridines as adenosine receptor selective ligands. WO03008384. 2003.
- SCHETZ J.A., SIBLEY D.R. The binding-site crevice of the D4 dopamine receptor is coupled to three distinct sites of allosteric modulation. J. Pharmacol. Exp. Ther. 2001;296:359–363. [PubMed] [Google Scholar]
- SIDDIQI S.M., JACOBSON K.A., ESKER J.L., OLAH M.E., JI X.D., MELMAN N., TIWARI K.N., SECRIST J.A., 3RD, SCHNELLER S.W., CRISTALLI G. Search for new purine- and ribose-modified adenosine analogues as selective agonists and antagonists at adenosine receptors. J. Med. Chem. 1995;38:1174–1188. doi: 10.1021/jm00007a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMIT M.J., LEURS R., SHUKRULA S.R., BAST A., TIMMERMAN H. Rapid desensitization of the histamine H2 receptor on the human monocytic cell line U937. Eur. J. Pharmacol. 1994;288:17–25. doi: 10.1016/0922-4106(94)90005-1. [DOI] [PubMed] [Google Scholar]
- SMITH P.K., KROHN R.I., HERMANSON G.T., MALLIA A.K., GARTNER F.H., PROVENZANO M.D., FUJIMOTO E.K., GOEKE N.M., OLSON B.J., KLENK D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- SOUDIJN W., VAN WIJNGAARDEN I., IJZERMAN A.P. Medicinal chemistry of adenosine A1 receptor ligands. Curr. Top. Med. Chem. 2003;3:355–367. doi: 10.2174/1568026033392165. [DOI] [PubMed] [Google Scholar]
- TOWNSEND-NICHOLSON A., SCHOFIELD P.R. A threonine residue in the seventh transmembrane domain of the human A1 adenosine receptor mediates specific agonist binding. J. Biol. Chem. 1994;269:2373–2376. [PubMed] [Google Scholar]
- TSAI B.S., LEFKOWITZ R.J. Agonist-specific effects of monovalent and divalent cations on adenylate cyclase-coupled alpha adrenergic receptors in rabbit platelets. Mol. Pharmacol. 1978;14:540–548. [PubMed] [Google Scholar]
- VAN CALENBERGH S., VON FRIJTAG DRABBE KÜNZEL J.K., BLATON N.M., PEETERS O.M., ROZENSKI J., VAN AERSCHOT A., DE BRUYN A., DE KEUKELEIRE D., IJZERMAN A.P., HERDEWIJN P. N6-cyclopentyl-3'-substituted-xylofuranosyladenosines: a new class of non-xanthine adenosine A1 receptor antagonists. J. Med. Chem. 1997;40:3765–3772. doi: 10.1021/jm970176k. [DOI] [PubMed] [Google Scholar]
- VAN DEN NIEUWENDIJK A.M., PIETRA D., HEITMAN L., GÖBLYÖS A., IJZERMAN A.P. Synthesis and biological evaluation of 2,3,5-substituted [1,2,4]thiadiazoles as allosteric modulators of adenosine receptors. J. Med. Chem. 2004;47:663–672. doi: 10.1021/jm030863d. [DOI] [PubMed] [Google Scholar]
- VAN DER KLEIN P.A., KOUROUNAKIS A.P., IJZERMAN A.P. Allosteric modulation of the adenosine A(1) receptor. Synthesis and biological evaluation of novel 2-amino-3-benzoylthiophenes as allosteric enhancers of agonist binding. J. Med. Chem. 1999;42:3629–3635. doi: 10.1021/jm991051d. [DOI] [PubMed] [Google Scholar]
- VAN SCHAICK E.A., TUKKER H.E., ROELEN H.C., IJZERMAN A.P., DANHOF M. Selectivity of action of 8-alkylamino analogues of N6-cyclopentyladenosine in vivo: haemodynamic versus anti-lipolytic responses in rats. Br. J. Pharmacol. 1998;124:607–618. doi: 10.1038/sj.bjp.0701868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN TILBURG E.W., VON FRIJTAG DRABBE KÜNZEL J., DE GROOTE M., VOLLINGA R.C., LORENZEN A., IJZERMAN A.P. N6,5′-Disubstituted adenosine derivatives as partial agonists for the human adenosine A3 receptor. J. Med. Chem. 1999;42:1393–1400. doi: 10.1021/jm981090+. [DOI] [PubMed] [Google Scholar]