Abstract
Analysis of the P2Y family of nucleotide-activated G-protein-coupled receptors has been compromised by the lack of selective high-affinity, high-specific-radioactivity radioligands. We have pursued quantification of the P2Y1 receptor through the development of a series of selective P2Y1 receptor antagonists.
Recently, we synthesized 2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine 3′,5′-bisphosphate (MRS2500), a selective, competitive antagonist that exhibits a Ki of 0.8 nM in competition-binding assays with [3H]MRS2279. A 3′-monophosphate precursor molecule, MRS2608, was radiolabeled at the 5′ position with 32P using polynucleotide kinase and [γ32P]ATP to yield [32P]MRS2500.
[32P]MRS2500 bound selectively to Sf9 insect cell membranes expressing the human P2Y1 receptor (Sf9-P2Y1), but did not detectably bind membranes expressing other P2Y receptors. P2Y1 receptor binding to [32P]MRS2500 was saturable with a KD of 1.2 nM. Agonists and antagonists of the P2Y1 receptor inhibited [32P]MRS2500 binding in Sf9-P2Y1 membranes with values in agreement with those observed in functional assays of the P2Y1 receptor.
A high-affinity binding site for [32P]MRS2500 (KD=0.33 nM) was identified in rat brain, which exhibited the pharmacological selectivity of the P2Y1 receptor. Distribution of this binding site varied among rat tissues, with the highest amount of binding appearing in lung, liver, and brain. Among brain regions, distribution of the [32P]MRS2500 binding site varied by six-fold, with the highest and lowest amounts of sites detected in cerebellum and cortex, respectively.
Taken together, these data illustrate the synthesis and characterization of a novel P2Y1 receptor radioligand and its utility for examining P2Y1 receptor expression in native mammalian tissues.
Keywords: P2Y1 receptor, competitive antagonist, radioligand, MRS2500, MRS2279
Introduction
Extracellular nucleotides signal through two classes of membrane-bound receptors to mediate a multiplicity of intracellular responses. The P2X receptors are ligand-gated ion channels and are primarily activated by ATP. The P2Y receptors are seven-transmembrane-spanning G-protein-coupled receptors, and are activated by adenine and uridine nucleotides. The P2Y receptor family consists of eight members that can be subclassified based on selectivity of G protein coupling and sequence homology. P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors couple to the Gαq class of guanine nucleotide-binding proteins, which signal downstream to trigger inositol lipid hydrolysis and subsequent mobilization of intracellular calcium. The P2Y11 receptor also couples to the Gαs family of G proteins to stimulate adenylyl cyclase. The P2Y12, P2Y13, and P2Y14 receptors exhibit high sequence homology and couple to the Gαi family of G proteins, resulting in inhibition of adenylyl cyclase activity (Burnstock, 1996; Burnstock & Knight, 2004).
The P2Y1 receptor is preferentially activated by ADP, while ATP is a weak partial agonist, and UTP and UDP are inactive (Schachter et al., 1996; Leon et al., 1997; Palmer et al., 1998). This receptor plays an essential role in ADP-promoted platelet aggregation by triggering shape change and an initial, reversible phase of aggregation (Jantzen et al., 1999). P2Y1 receptor mRNA has been detected in numerous tissues (Janssens et al., 1996; Leon et al., 1996); however, a direct study of this receptor and its related physiology historically has been difficult due to the lack of a reliable radioligand-binding assay.
We have developed a series of competitive antagonists that selectively inhibit P2Y1 receptor-promoted signaling. Adenosine derivatives with phosphate groups at the 5′ and 2′ or 3′ positions of the ribose ring were initially identified as selective, competitive antagonists (Boyer et al., 1996). Structure–activity studies for adenosine bisphosphate derivatives substituted at various positions of the adenine and ribose rings along with molecular modeling and site-directed mutagenesis have led to the development of non-nucleotide-antagonists that are highly selective for the P2Y1 receptor, exhibit low nanomolar potency for inhibiting downstream receptor signaling, and display limited susceptibility to metabolism by surface-localized nucleotide hydrolyzing enzymes (Jiang et al., 1997; Boyer et al., 1998; 2002; Camaioni et al., 1998; Moro et al., 1998; Nandanan et al., 1999; 2000; Kim et al., 2000; 2001; 2002). One of these molecules, 2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([3H]MRS2279), was developed into an antagonist radioligand for the P2Y1 receptor by a multi-step radiosynthetic scheme (Waldo et al., 2002). While the development of a radioligand-binding assay using this molecule provides a reliable tool for quantification of recombinant P2Y1 receptors and screening of new P2Y1 receptor ligands, its low specific activity (89 Ci mmol−1) and intermediate affinity for the P2Y1 receptor (KD: 8 nM) limit its general application for broadly quantifying P2Y1 receptors in native mammalian tissues.
Recently, 2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2500) was synthesized as a competitive P2Y1 receptor antagonist that inhibited [3H]MRS2279 binding with an affinity (Ki=0.79 nM) 10 times greater than MRS2279 (Kim et al., 2003). We have chosen this molecule as a template to develop a higher-affinity, high-specific-radioactivity antagonist radioligand for the P2Y1 receptor. MRS2500 was synthesized in radioactive form by the facile, single-step kinase-catalyzed phosphorylation of a precursor molecule to yield [32P]MRS2500 with a theoretical specific activity of 9120 Ci mmol−1. In this study, we describe the synthesis of this novel radioligand, the development of a high-specific-activity radioligand-binding assay for the P2Y1 receptor, and the quantification of P2Y1 receptors in various tissues of the adult rat.
Methods
Animals
Adult male Harlan Sprague–Dawley rats weighing 300–400 g were group housed and maintained on a 12 : 12 h light : dark cycle with access to food and water ad libitum. Animals were killed by decapitation by a trained laboratory animal technician. All procedures were carried out in accordance with the guidelines of the University of North Carolina Institutional Animal Care and Use Committee.
Precursor for synthesis of MRS2500
The general synthetic approach for 2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′-monophosphate, MRS2608, a precursor of MRS2500, was described (Kim et al., 2003). The detailed synthesis will be published separately.
Enzymatic synthesis of [32P]MRS2500 from MRS2608
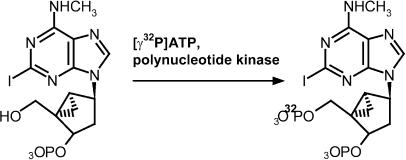
MRS2608 (50 nmol, 5 μl of a 10 mM solution in Tris, pH 7.5) was combined with 1.5 μl of 10 × reaction buffer (500 mM Tris-HCl, 100 mM MgCl2, 50 mM dithiothreitol, 1 mM spermidine, and 1 mM EDTA, pH 7.5), 1 mCi of [γ32P]ATP (7 μl, 0.16 nmol, 150 mCi ml−1), and 2 μl (20 U) of 3′-phosphatase-free polynucleotide kinase. The sample was mixed by pipetting and the kinase-catalyzed reaction was incubated at 37°C for 1 h. The entire reaction volume was then injected onto a Luna 5μ C18(2) column (4.6 × 250 mm) at a flow rate of 1 ml min−1 in a mobile phase of 5% acetonitrile/95% 0.1 M triethylammonium acetate (5% A/95% B). The column was washed for 30 min in 5% A/95% B to remove free [γ32P]ATP, and [32P]MRS2500 was eluted using a linear gradient of 5% A/95% B to 60% A/40% B over 50 min. [32P]MRS2500 eluted at 48 min, that is, 18 min after the start of the gradient (approximately 75% A/25% B). The precursor molecule, MRS2608, which was detected by UV (275 nM) eluted at 50 min. Fractions of 1 ml were collected during purification, and radioactivity in each fraction was quantified by liquid scintillation counting of a 5 μl aliquot of each fraction. [32P]MRS2500 has been purified by this procedure approximately 10 times, with a typical yield of approximately 20%. [32P]MRS2500 was stored at −20°C until use.
P2Y1 receptor expression in Sf9 insect cells
Sf9 insect cell membranes expressing recombinant P2Y receptors were prepared as described in detail previously (Waldo et al., 2002). Briefly, recombinant baculoviruses encoding epitope-tagged constructs of the human P2Y1, P2Y2, or P2Y12 receptors, or an avian P2Y receptor (Boyer et al., 1997) were constructed using established protocols. Suspension cultures of Sf9 cells were infected with recombinant baculoviruses, and plasma membranes were prepared from uninfected (wild type) or infected cells after cell lysis and high-speed centrifugation. The membranes were frozen in aliquots at −80°C.
Preparation of membranes from rat tissues
Adult male Harlan Sprague–Dawley rats were killed and organs were harvested and placed in 5 ml homogenization buffer (20 mM Hepes, pH 7.5, 145 mM NaCl, 5 mM MgCl2) per gram wet weight tissue. Whole organs or combined brain regions from groups of 2–6 rats were homogenized with a Polytron tissue disrupter for 45–60 s. Homogenized samples were centrifuged at 35,000 × g for 10 min. The resulting pellets were resuspended in 3 ml homogenization buffer per gram wet weight tissue and centrifugation was repeated two times. Final resuspensions were in homogenization buffer plus 5% glycerol and the samples were stored at −80°C. Protein concentrations were determined using the BCA protein assay.
Radioligand-binding assay
Membranes were typically incubated with 0.1–0.25 nM [32P]MRS2500 in assay buffer (20 mM Hepes, 145 mM NaCl, 5 mM MgCl2, pH 7.5) in a 25 μl reaction volume in 12 × 75 mm2 conical polypropylene tubes. Saturation-binding isotherms were generated at concentrations of [32P]MRS2500 ranging from 0.01 to 6 nM in a total volume of 20 μl. Incubations were from 15 to 45 min in an ice-water bath and were terminated by the addition of 3.5 ml of ice-cold assay buffer followed by vacuum filtration over Whatman GF/A glass microfiber filters. The filters were washed with 7 ml ice cold assay buffer and radioactivity on each filter was quantified by liquid scintillation counting. Specific binding was defined as total [32P]MRS2500 bound minus binding occurring in the presence of 10 or 100 μM MRS2179.
Materials
3′-phosphatase-free polynucleotide kinase was from Roche Diagnostics Corp., Indianapolis, IN, U.S.A. MRS2179 was from Tocris-Cookson, Inc., Ellisville, MO, U.S.A. [γ32P]ATP was from Perkin-Elmer, Inc., Boston, MA, U.S.A. All other drugs were from Sigma-Aldrich Corp., St Louis, MO, U.S.A. The Luna 5μ C18(2) HPLC column was from Phenomenex, Inc., Torrence, CA, U.S.A.
Data analysis
All experiments were carried out in duplicate or triplicate assays and were carried out at least three times or on samples from three individual animals. Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA, U.S.A.). Data are presented as the mean±s.e.m. from combined multiple experiments or in some cases as a data set from a typical experiment.
Results
Structure–activity relationships for a series of synthetic adenine nucleotide analogs have led to the development of a class of non-nucleotide adenosine bisphosphate derivatives that selectively inhibit the P2Y1 receptor (Boyer et al., 1998; Moro et al., 1998; Nandanan et al., 1999; Kim et al., 2002; 2003). The replacement of the ribose ring of adenosine 3′,5′-bisphosphate with a Northern-constrained cyclopentane structure and other modifications of the adenine base, including an N6-methyl addition, have yielded molecules that are highly selective for P2Y1 over other P2Y, P2X, and adenosine receptors. These non-nucleotide molecules are also presumed to circumvent the problem of nonspecific binding to the large number of other nucleotide-binding proteins present in mammalian cells. Recently, one of these molecules MRS2500, was found to interact with the P2Y1 receptor with subnanomolar affinity. This molecule was selected as the template for development of a high-specific-activity, 32P-labeled radioligand to quantify endogenous P2Y1 receptors in mammalian tissues.
Synthesis of [32P]MRS2500
MRS2500 inhibited binding of the P2Y1 receptor radioligand [3H]MRS2279 with a Ki value of 0.79 nM, and inhibited 2MeSADP-promoted inositol phosphate accumulation with a calculated KB value of 1.74 nM (Kim et al., 2003). A precursor to MRS2500 was generated with the goal of synthesizing a high-specific-activity radioligand. The precursor molecule, MRS2608, contains a phosphate group at the 3′-position and a hydroxyl group at the 5′-position, which potentially allows phosphorylation by polynucleotide kinase using [γ32P]ATP as the 5′-phosphate donor.
Reaction conditions for polynucleotide kinase-catalyzed radiophosphorylation were optimized using unlabeled ATP and adenosine-3′-monophosphate (A3′MP) as the phosphate acceptor. The extent of phosphorylation was quantified using ion exchange chromatography. Since polynucleotide kinase is known to exhibit small amounts of 3′-phosphatase activity, reactions were carried out with a mutant form of the enzyme containing a C-terminal deletion that results in ablation of its 3′-phosphatase activity (Wang & Shuman, 2002). Lack of 3′-phosphatase activity was confirmed using A3′MP as substrate (data not shown). A3′MP was stable in the presence of 3′-phosphatase-free polynucleotide kinase in the absence of ATP at 37°C for up to 24 h; incubation of A3′MP with unmodified polynucleotide kinase under identical conditions resulted in the appearance of a small amount of adenosine (data not shown).
Reaction conditions that resulted in optimal phosphorylation of A3′MP were applied to 32P-phosphorylate MRS2608 to generate [32P]MRS2500 (Figure 1). Approximately 20% of the added [32P] radioactivity was routinely recovered in a single peak that eluted from the reversed-phase column with a retention time of 48 min. The retention time of the radioactive product corresponded to the retention time of purified, unlabeled MRS2500 (Kim et al., 2003) under the same mobile phase conditions. Contamination of purified [32P]MRS2500 with the precursor molecule MRS2608 was less than 1% in multiple purification procedures.
Figure 1.
Synthesis of [32P]MRS2500. MRS2608 (5 μl of a 10 mM solution) was combined with 1.5 μl of 10 × reaction buffer, 1 mCi of [γ32P]ATP (7 μl, 0.16 nmol, 150 mCi ml−1), and 2 μl (20 U) of 3′-phosphatase-free polynucleotide kinase. The sample was mixed by pipetting and incubated at 37°C for 1 h. The entire reaction volume was then injected onto a Luna 5μ C18(2) column for purification under mobile phase conditions as described in Methods.
Selectivity of [32P]MR2500 for the P2Y1 receptor
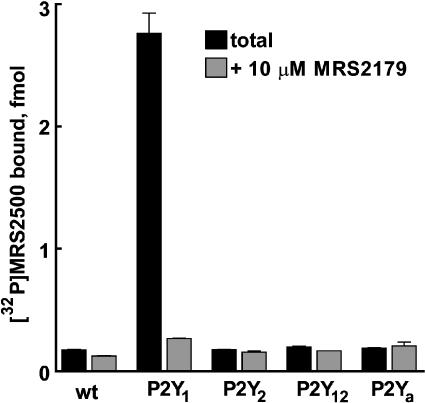
To determine selectivity of the novel radioligand for the P2Y1 receptor, [32P]MRS2500 binding was evaluated in membranes from wild-type Sf9 (Sf9-wt) insect cells or Sf9 insect cells expressing human P2Y1 (Sf9-P2Y1), P2Y2 (Sf9-P2Y2), or P2Y12 (Sf9-P2Y12) receptors or the avian P2Y receptor (Sf9-P2Ya) (Boyer et al., 1997). As shown in Figure 2, [32P]MRS2500 binding in Sf9-P2Y1 membranes was 15-fold higher than binding observed in Sf9-wt membranes, and was inhibited by 90% in the presence of the P2Y1-selective antagonist MRS2179 (10 μM). In contrast, [32P]MRS2500 binding in Sf9-P2Y2, Sf9-P2Y12, and Sf9-P2Ya membranes was essentially identical to that observed in Sf9-wt membranes and was not affected by MRS2179. These results indicate that [32P]MRS2500 binds specifically to P2Y1 receptors but not to other P2Y receptors in Sf9 membranes.
Figure 2.
[32P]MRS2500 binding in Sf9 membranes expressing P2Y receptors. Wild-type Sf9 membranes or membranes expressing the human P2Y1, P2Y2, or P2Y12 receptors or the avian P2Y receptor, P2Ya (10 μg each) were incubated with 220 pM [32P]MRS2500 in the presence or absence of 10 μM MRS2179 to determine nonspecific binding. Values are reported as total fmol [32P]MRS2500 bound±s.e.m. from a representative experiment (n=3).
High-affinity binding of [32P]MRS2500 to the P2Y1 receptor
Optimal conditions for radioligand binding were determined in preliminary experiments. Specific binding occurring at 4°C was at least as great as that observed at room temperature (data not shown), and therefore all subsequent binding analyses were carried out at 4°C. Time-course experiments revealed rapid association of [32P]MRS2500 such that steady-state binding occurred within 2 min at 4°C. Prebound radioligand dissociated rapidly upon addition of a saturating concentration of MRS2179 (10 μM), with half of the bound radioligand dissociating within approximately 90 s.
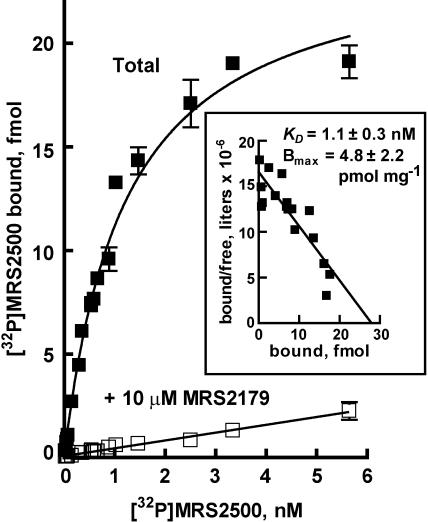
Saturation-binding analysis was performed to determine the affinity of [32P]MRS2500 for the recombinant human P2Y1 receptor expressed in Sf9 membranes (Figure 3). Saturation-binding isotherms exhibited one-site binding kinetics with a KD of 1.1±0.35 nM (n=3) and an average Bmax of 4.8±2.2 pmol receptor mg−1 protein in three experiments from a single membrane preparation.
Figure 3.
Saturation-binding isotherm for [32P]MRS2500 binding to the human P2Y1 receptor. Sf9-P2Y1 membranes (10 μg per assay) were incubated for 45 min with the indicated concentrations of [32P]MRS2500 without or with the P2Y1R selective antagonist MRS2179 (10 μM). Values are reported as total fmol [32P]MRS2500 bound±s.e.m. from a representative experiment (n=3). Inset, Scatchard transformation of the data.
Pharmacological selectivity of [32P]MRS2500 binding
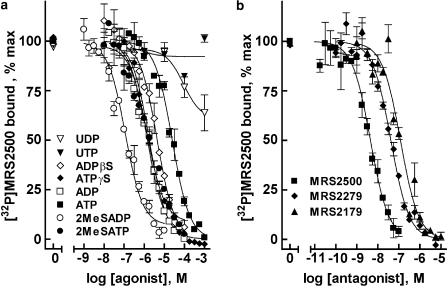
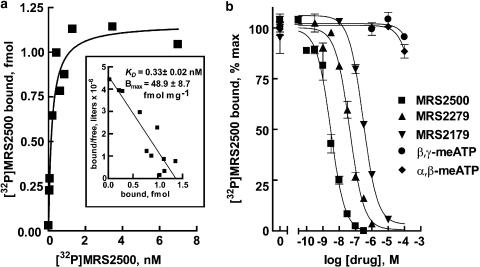
The capacity of several agonists and antagonists of the P2Y1 receptor and other P2Y receptors to compete with [32P]MRS2500 for binding was investigated in Sf9-P2Y1 membranes. Owing to the high specific activity of [32P]MRS2500, competition curves could be generated with minimal amounts of protein (250–500 ng), limiting the alteration of added nucleotides by membrane-bound nucleotide-metabolizing enzymes. Agonists known to bind to the P2Y1 receptor inhibited binding of [32P]MRS2500 in a concentration-dependent manner (Figure 4a). The rank order of potency observed was 2MeSADP>2MeSATP>ADP>ATPγS>ADPβS>ATP. This order was in agreement with the predicted potencies for the P2Y1 receptor based on previous observations of agonist-promoted P2Y1 receptor second-messenger signaling in cells continuously superfused with drug-containing medium (Palmer et al., 1998). Moreover, Ki values (Table 1) were in excellent agreement with values determined in competition assays with [3H]MRS2279, and the human P2Y1 receptor purified to homogeneity (Waldo & Harden, 2004). The P2Y1 receptor is known to bind adenine nucleotides specifically and is not activated by UTP or UDP; accordingly, uridine nucleotides did not compete with [32P]MRS2500 for binding to the P2Y1 receptor.
Figure 4.
Competition of P2Y1 receptor agonists and antagonists with [32P]MRS2500 for binding to P2Y1 receptor-expressing Sf9 membranes. (a) Sf9-P2Y1 membranes (250 ng per assay) were incubated with 100 pM [32P]MRS2500 and the indicated concentrations of P2Y1 receptor agonists. (b) Sf9-P2Y1 membranes (500 ng per assay) were incubated with 200 pM [32P]MRS2500 and the indicated concentrations of the P2Y1 receptor selective antagonists. Values are reported as % binding observed in the absence of competing ligand. Data shown are averages of triplicate samples±s.e.m. from a representative experiment.
Table 1.
Ki values for P2Y1 receptor agonists and antagonists in P2Y1 receptor-expressing Sf9 membranes
| Agonist | n | Ki (μM) | KB (nM) |
|---|---|---|---|
| 2MeSADP | 5 | 0.05±0.01 | |
| 2MeSATP | 5 | 0.49±0.10 | |
| ADP | 4 | 0.56±0.09 | |
| ATPγS | 3 | 1.07±0.11 | |
| ADPβS | 3 | 2.30±0.60 | |
| ATP | 3 | 14.0±6.0 | |
| UTP | 3 | >1000 | |
| UDP | 3 | >1000 | |
| MRS2500 | 3 | 2.35±0.48 | 1.74 (Kim et al., 2003) |
| MRS2279 | 5 | 46.5±7.9 | 8.91 (Boyer et al., 2002) |
| MRS2179 | 3 | 117±9 | 102 (Boyer et al., 1998) |
Values reported are the average of three or more experiments±s.e.m.
P2Y1 receptor antagonists were also investigated for their capacity to compete with [32P]MRS2500 for binding to the P2Y1 receptor. MRS2179, MRS2279 and MRS2500 inhibited [32P]MRS2500 binding with Ki values in good agreement with KB values determined for these same antagonists for inhibition of P2Y1 receptor-promoted second messenger signaling (Figure 4b, Table 1).
[32P]MRS2500 binding in rat brain
One of the potential advantages of a high-specific-activity radioligand is high sensitivity for detection of receptors in native tissues. To determine the utility of [32P]MRS2500 for detection of P2Y1 receptors in native tissues, membranes were prepared from brains of adult male Sprague–Dawley rats. As shown in Figure 5a, saturation-binding analysis revealed binding of [32P]MRS2500 to a homogenous population of binding sites in rat brain with high affinity (KD: 0.33±0.02 nM). An average Bmax value of 48.9±8.7 fmol receptor mg−1 protein was determined (n=3). To confirm the identity of this high affinity binding site as the rat P2Y1 receptor, pharmacological selectivity of P2Y1 receptor antagonists was examined. The P2Y1 selective antagonists, MRS2179, MRS2279, and MRS2500, competed for binding of [32P]MRS2500 in rat brain membranes with Ki values of 1.97±0.74, 27.4±8.4, and 267±72, respectively (n=3). These values were in agreement with values obtained at the recombinant human P2Y1 receptor (Table 2). Taken together, these data demonstrate that [32P]MRS2500 is useful for quantification of P2Y1 receptors in adult rat brains. Preliminary studies revealed a large amount of breakdown of nucleotides by brain homogenates; therefore, we have not pursued agonist competition binding further in these studies of native P2Y1 receptors.
Figure 5.
[32P]MRS2500 binding in adult male rat brain. (a) Membranes prepared from adult male rat brain (30 μg per assay) were incubated for 45 min with the indicated concentrations of [32P]MRS2500 without or with the P2Y1R selective antagonist MRS2179 (100 μM). Inset, Scatchard transformation of the data. (b) Membranes from adult male rat brains (50 μg per assay) were incubated with 200 pM [32P]MRS2500 and the indicated concentrations of the indicated P2Y1 receptor antagonists. Values are reported as % binding observed in the absence of competing ligand. Data shown are averages of triplicate samples (a) or averages of triplicate samples±s.e.m. (b) from a representative experiment (n=3).
Table 2.
KD and Bmax values for [32P]MRS2500 binding in rat brain regions
| Region | KD (nM) | Bmax (fmol mg−1 protein) |
|---|---|---|
| Whole brain | 0.33±0.02 | 48.9±8.7 |
| Cerebellum | 0.55±0.07 | 112±17 |
| Cortex | 0.47±0.06 | 21.7±2.4 |
| Hippocampus | 0.44±0.09 | 32.2±7.8 |
| Hypothalamus | 0.43±0.09 | 55.8±11.0 |
| Midbrain | 0.38±0.01 | 74.8±9.4 |
Values reported are the average of three experiments±s.e.m.
Tissue distribution of the rat P2Y1 receptor
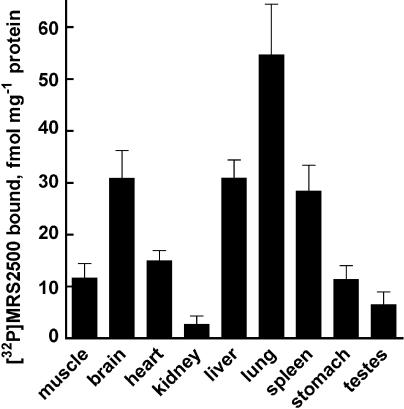
Having confirmed the utility of [32P]MRS2500 for labeling P2Y1 receptors in rat brain, we determined the relative density of P2Y1 receptors in a variety of rat tissues (Figure 6). Among tissues examined with a submaximal concentration of [32P]MRS2500 (4 nM), lung, liver, and brain exhibited the highest relative amounts of specific binding, with 55±10, 31±3, and 31±5 fmol [32P]MRS2500 bound mg−1protein, respectively. Heart, abdominal muscle, spleen, and stomach exhibited moderate receptor levels. Testes and kidney bound the least amounts of radioligand, 6.5±2.4 and 2.7±1.7 fmol [32P]MRS2500 bound mg−1 protein, respectively, and, in some cases, specific binding in these tissues was undetectable.
Figure 6.
[32]MRS2500 binding in adult rat tissues. Membranes prepared from various tissues of adult male Sprague–Dawley rats were incubated with 4 nM [32P]MRS2500 in the presence or absence of MRS2179. Specific binding was normalized to protein amounts. Values are reported as fmol [32P]MRS2500 bound per mg protein. Data shown are averages of triplicate samples±s.e.m. from a representative experiment (n=3).
P2Y1 receptor distribution in rat brain
P2Y1 receptor mRNA is abundantly expressed in brain, and this receptor has been implicated in a number of neuronal physiologies, including regulation of neurotransmission, anxiolysis, and protection of astrocytes from oxidative stress-induced damage (Kittner et al., 2003; Luthardt et al., 2003; Shinozaki et al., 2005). Saturation-binding analyses were performed in five major brain regions – cerebellum, cortex, midbrain, hypothalamus, and, hippocampus. Among the brain regions examined, cerebellum exhibited the highest number of binding sites with a Bmax value of 112±17 fmol [32P]MRS2500 bound per mg protein (Table 2). Midbrain, hypothalamus, and hippocampus displayed intermediate densities of binding sites, and cortex displayed the lowest number of binding sites with a Bmax value of 21.7±2.4 fmol [32P]MRS2500 bound per mg protein. Thus, P2Y1 receptor expression varies by approximately six-fold among the major brain regions examined.
Discussion
Study of the P2Y1 receptor has been significantly advanced by the development of selective pharmacological tools that directly target this signaling protein. In this report, we describe the synthesis and confirm the utility of [32P]MRS2500 as a novel high-affinity, high-specific-radioactivity antagonist radioligand for the P2Y1 receptor. [32P]MRS2500 binds selectively to the human P2Y1 receptor with a KD of 1.2 nM. We have used this high-affinity radioligand to quantify P2Y1 receptors in a variety of rat tissues, and among the tissues examined, relative receptor levels were highest in lung, liver, and brain. We also examined receptor levels in several major brain regions and found a six-fold range of expression, with the highest and lowest densities of receptors found in the cerebellum and cortex, respectively. To our knowledge, this is the first unambiguous demonstration of a broadly useful high-specific-activity radioligand for a P2Y receptor natively expressed in mammalian tissues. Given the availability of the precursor, MRS2608, the preparative method is sufficiently simple to allow its convenient synthesis.
Development of selective P2Y1 receptor antagonists began with the identification of adenosine bisphosphate molecules as competitive antagonists. The presence of a 5′ phosphate group and an accompanying 2′ or 3′ phosphate group on the ribose moiety allowed recognition of these molecules by the P2Y1 receptor without receptor activation (Boyer et al., 1996). Removal of the 2′ hydroxyl group of the ribose entity eliminated interactions of adenosine-3′,5′-bisphosphate analog with adenosine receptors, and addition of a methyl group at the N6 position conferred an increase in P2Y1 receptor-binding affinity (Boyer et al., 1998). The discovery that interaction with the P2Y1 receptor was retained in bisphosphate analogs in which the ribose was replaced by acylic or heterocyclic moieties (Kim et al., 2000; 2001) was extended to the use of carbocyclic ribose-substituted heterocyclic bisphosphate analog constrained in either the Northern or Southern conformation by fusion of cyclopropane to a pseudosugar cyclopentane ring (Marquez et al., 1996; Ezzitouni & Marquez, 1997). These bisphosphate methanocarba analogs retained affinity for the P2Y1 receptor, and N-methanocarba derivatives of P2Y1 receptor agonists and antagonists were more than 100-fold more potent than their corresponding S-isomers (Nandanan et al., 2000; Kim et al., 2002). Molecular modeling studies of the P2Y1 receptor based on the structure of rhodopsin confirmed that the Northern conformation was energetically favored by ligands docked in the putative P2Y1 receptor ligand recognition site (Nandanan et al., 2000).
One goal of the development of non-nucleotide P2Y1 receptor antagonists was to reduce interaction of these molecules with other nucleotide-binding proteins, which hypothetically should be of value in our secondary goal of developing a useful radioligand for the P2Y1 receptor. Indeed, our studies of methanocarba analogs led to the synthesis of [3H]MRS2279, and the binding of [3H]MRS2279 to membranes prepared from Sf9 insect cells expressing recombinant human P2Y1 receptors fit the pharmacological properties of the P2Y1 receptor (Waldo et al., 2002). The [3H]MRS2279 radioligand-binding assay has allowed efficient screening of novel ligands for the P2Y1 receptor (Kim et al., 2002; Waldo et al., 2002) and has been applied to quantify the P2Y1 receptor during purification to homogeneity (Waldo & Harden, 2004). Although [3H]MRS2279 proved useful for quantification of P2Y1 receptors in human platelets (Waldo et al., 2002), its relatively low specific activity (89 Ci mmol−1) has limited its use in other tissues in which the receptor is endogenously expressed. Thus, development of [32P]MRS2500, which exhibits 10-fold higher affinity and 100-fold higher specific radioactivity than [3H]MRS2279, represents an important step in ligand development for the unambiguous study of P2Y1 receptor-binding sites in mammalian tissues.
Previous work has investigated the tissue distribution of the rodent P2Y1 receptor using in situ hybridization techniques (Tokuyama et al., 1995; Janssens et al., 1996; Leon et al., 1996; Moran-Jimenez & Matute, 2000). These studies suggest a broad expression pattern for the P2Y1 receptor among peripheral tissues and in rodent brain. Although in situ hybridization studies provide important insight into the relative distribution of this signaling protein, the relationship of mRNA to expressed functional receptors is unknown and is not likely to be constant. Antibodies that specifically recognize P2Y receptors would allow direct immunocytochemical quantification of receptor protein, but these tools also do not necessarily identify functional receptor-binding sites. Moreover, although antibodies against the P2Y1 receptor have been reported (Fong et al., 2002; Yoshioka et al., 2002; Franke et al., 2003; Scheibler et al., 2004), evidence for their selectivity is limited and their general reliability is uncertain.
The results described here illustrate that [32P]MRS2500 is a useful radioligand for quantification of functional P2Y1 receptor-binding sites across a wide range of mammalian tissues, and the remarkably high ratio of specific to nonspecific binding of this high-affinity, high-specific-activity radioligand allows reliable detection of binding sites to at least 1 fmol mg−1 protein. Application of [32P]MRS2500 revealed a broad expression pattern for the functional receptor protein among peripheral tissues and rodent brain. Interestingly, this pattern is similar to that previously reported for messenger RNA (Tokuyama et al., 1995; Janssens et al., 1996; Leon et al., 1996; Moran-Jimenez & Matute, 2000).
Tissue distribution data from our studies and other studies suggest potentially important physiological consequences of P2Y1 receptor signaling. The role of the P2Y1 receptor in ADP-promoted platelet aggregation is now well established (Gachet, 2001). However, its function remains largely undefined in the majority of tissues. Several studies have investigated the importance of P2Y1 receptor signaling in the central nervous system. ATP released from nerve terminals acts as an excitatory neurotransmitter through ionotropic P2X receptors (Cunha & Ribeiro, 2000). Roles for adenine nucleotides in other neural processes have been proposed, and potentially important consequences of signaling involving the P2Y1 receptor have been suggested. For example, activation of the P2Y1 receptor inhibits glutamate release, and P2Y1 receptor-mediated inhibition of NMDA receptor-promoted signaling occurs in prefrontal and parietal cortex (Luthardt et al., 2003; Rodrigues et al., 2005). Activation of the P2Y1 receptor also has been associated with anxiolysis, astrocyte protection, and oligodendrocyte proliferation and migration in rats (Kittner et al., 2003; Agresti et al., 2005; Shinozaki et al., 2005).
Our work illustrates that [32P]MRS2500 can be utilized to quantify P2Y1 receptors in very small tissue samples, and the relatively high affinity and high specific radioactivity of this radioligand also make it a good candidate for detection of these receptors using autoradiographic techniques. Previous studies have claimed autoradiographic detection of the rat P2Y1 receptor using [α33P]dATP or [35S]dATPαS as radioligands (Simon et al., 1997; Fong et al., 2002), but we have previously shown that the enormous amount of binding (10–50 pmol mg−1 protein) observed with these radioligands is nonspecific (Schachter & Harden, 1997). A 33P-labeled radioligand, [33P]MRS2179, was used previously to quantify P2Y1 receptors in human platelets (Baurand et al., 2001). We suspect that [33P]MRS2179 may not be a generally applicable radioligand since its affinity for the P2Y1 receptor is 100-fold lower affinity than the affinity of MRS2500. We have demonstrated here the high selectivity of [32P]MRS2500 for the P2Y1 receptor, and predict that this selectivity will allow for a more accurate analysis of brain P2Y1 receptor-binding sites.
The work described here demonstrates the development of a new molecular tool for quantification of the P2Y1 receptor with high sensitivity and illustrates that active P2Y1 receptor-binding sites are broadly distributed across rat tissues and brain. A reliable means for quantification of the P2Y1 receptor should lead to better understanding of the complex signaling and physiology associated with this important signaling protein.
Acknowledgments
We are indebted to Gary Waldo and Eduardo Lazarowski for advice and helpful discussion and to Catja van Heusden for technical assistance. We thank Todd O'Buckley, William Arendshorst, Andrea Olson, and David Bourdon for assistance with animal work. This work was supported by National Institutes of Health grants GM38213 and HL54889. D.H. is supported by a Howard Hughes Predoctoral Fellowship.
Abbreviations
- A3′MP
adenosine-3′-monophosphate
- MRS2279
2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2500
2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine 3′,5′-bisphosphate
References
- AGRESTI C., MEOMARTINI M.E., AMADIO S., AMBROSINI E., SERAFINI B., FRANCHINI L., VOLONTE C., ALOISI F., VISENTIN S. Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development. Glia. 2005;50:132–144. doi: 10.1002/glia.20160. [DOI] [PubMed] [Google Scholar]
- BAURAND A., RABOISSON P., FREUND M., LEON C., CAZENAVE J.P., BOURGUIGNON J.J., GACHET C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur. J. Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- BOYER J., WALDO G.L., HARDEN T.K. Molecular cloning and expression of an avian G protein-coupled P2Y receptor. Mol. Pharmacol. 1997;52:928–934. doi: 10.1124/mol.52.6.928. [DOI] [PubMed] [Google Scholar]
- BOYER J.L., ADAMS M., RAVI R.G., JACOBSON K.A., HARDEN T.K. 2-Chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br. J. Pharmacol. 2002;135:2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER J.L., MOHANRAM A., CAMAIONI E., JACOBSON K.A., HARDEN T.K. Competitive and selective antagonism of P2Y1 receptors by N6-methyl-2′deoxyadenosine-3′,5′-bisphosphate. Br. J. Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER J.L., ROMERO-AVILA T., SCHACHTER J.B., HARDEN T.K. Identification of competitive antagonists of the P2Y1 receptor. Mol. Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- BURNSTOCK G. P2 purinoreceptors: historical perspective and classification. Ciba Found. Symp. 1996;198:1–28. doi: 10.1002/9780470514900.ch1. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., KNIGHT G.E. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- CAMAIONI E., BOYER J.L., MOHANRAM A., HARDEN T.K., JACOBSON K.A. Deoxyadenosine bisphosphate derivatives as potent antagonists at P2Y1 receptors. J. Med. Chem. 1998;41:183–190. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA R.A., RIBEIRO J.A. ATP as a presynaptic modulator. Life Sci. 2000;68:119–137. doi: 10.1016/s0024-3205(00)00923-1. [DOI] [PubMed] [Google Scholar]
- EZZITOUNI A., MARQUEZ V.E. Conformationally locked carbocyclic nucleosides built on a bicycle[3.1.0]hexane template with a fixed Southern conformation. Synthesis and antiviral activity. J. Chem. Soc. Perkin Trans. 1997;1:1073–1078. [Google Scholar]
- FONG A.Y., KRSTWE E.V., BARDEN J., LAWRENCE A.J. Immunoreactive localization of P2Y1 receptors within the rat and human nodose ganglia and rat brainstem: comparison with [α33P]deoxyadenosine-5′-triphosphate autoradiography. Neuroscience. 2002;113:809–823. doi: 10.1016/s0306-4522(02)00237-3. [DOI] [PubMed] [Google Scholar]
- FRANKE H., KITTNER H., GROSCHE J., ILLES P. Enhanced P2Y1 receptor expression in the brain after sensitization with d-amphetamine. Psychopharmacology (Berlin) 2003;167:187–194. doi: 10.1007/s00213-002-1386-6. [DOI] [PubMed] [Google Scholar]
- GACHET C. ADP receptors of platelets and their inhibition. Thromb. Haemost. 2001;86:222–232. [PubMed] [Google Scholar]
- JANSSENS R., COMMUNI D., PIROTTO S., SAMSON M., PARMENTIER M., BOEYNAEMS J.M. Cloning and tissue distribution of the human P2Y1 receptor. Biochem. Biophys. Res. Commun. 1996;221:588–593. doi: 10.1006/bbrc.1996.0640. [DOI] [PubMed] [Google Scholar]
- JANTZEN H.M., GOUSSET L., BHASKAR V., VINCENT D., TAI A., REYNOLDS E.E., CONLEY P.B. Evidence for two distinct G-protein-coupled ADP receptors mediating platelet activation. Thromb. Haemost. 1999;81:111–117. [PubMed] [Google Scholar]
- JIANG Q., GUO D., LEE B.X., VAN RHEE A.M., KIM Y.C., NICHOLAS R.A., SCHACHTER J.B., HARDEN T.K., JACOBSON K.A. A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol. Pharmacol. 1997;52:499–507. doi: 10.1124/mol.52.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM H.S., BARAK D., HARDEN T.K., BOYER J.L., JACOBSON K.A. Acyclic and cyclopropyl analogues of adenosine bisphosphate antagonists of the P2Y1 receptor: structure–activity relationships and receptor docking. J. Med. Chem. 2001;44:3092–3108. doi: 10.1021/jm010082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM H.S., OHNO M., XU B., KIM H.O., CHOI Y., JI X.D., MADDILETI S., MARQUEZ V.E., HARDEN T.K., JACOBSON K.A. 2-Substitution of adenine nucldotide analogues containing a bicycle[3.1.0]hexane ring system locked in a Northern conformation: enhanced potency as P2Y1 receptor antagonists. J. Med. Chem. 2003;46:4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM H.S., RAVI R.G., MARQUEZ V.E., MADDILETI S., WIHLBORG A.K., ERLINGE D., MALMSJO M., BOYER J.L., HARDEN T.K., JACOBSON K.A. Methanocarba modification of uracil and adenine nucleotides: high potency of Northern ring conformation at P2Y1, P2Y2, P2Y4, and P2Y11 but not P2Y6 receptors. J. Med. Chem. 2002;45:208–218. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y.C., GALLO-RODRIGUEZ C., JANG S.Y., NANDANAN E., ADAMS M., HARDEN T.K., BOYER J.L., JACOBSON K.A. Acyclic analogues of deoxyadenosine 3′,5′-bisphosphates as P2Y1 receptor antagonists. J. Med. Chem. 2000;43:746–755. doi: 10.1021/jm9905211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITTNER H., FRANKE H., FISCHER W., SCHULTHEIS N., KRUGEL U., ILLES P. Stimulation of P2Y1 receptors causes anxiolytic-like effects in the rat elevated plus-maze: implications for involvement of P2Y1 receptor-mediated nitric oxide production. Neuropsychopharmacology. 2003;28:435–444. doi: 10.1038/sj.npp.1300043. [DOI] [PubMed] [Google Scholar]
- LEON C., HECHLER B., VIAL C., LERAY C., CAZENAVE J.P., GACHET C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- LEON C., VIAL C., CAZENAVE J.P., GACHET C. Cloning and sequencing of a human cDNA encoding endothelial P2Y1 purinoreceptor. Gene. 1996;171:295–297. doi: 10.1016/0378-1119(96)00027-3. [DOI] [PubMed] [Google Scholar]
- LUTHARDT J., BORVENDEG S.J., SPERLAGH B., POELCHEN W., WIRKNER K., ILLES P. P2Y1 receptor activation inhibits NMDA receptor-channels in layer V pyramidal neurons of the rat prefrontal and parietal cortex. Neurochem. Int. 2003;42:161–172. doi: 10.1016/s0197-0186(02)00069-4. [DOI] [PubMed] [Google Scholar]
- MARQUEZ V.E., SIDDIQUI M.A., EZZITOUNI A., RUSS P., WANG J.Y., WAGNER R.W., MATTEUCI M.D. Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides. J. Med. Chem. 1996;39:3739–3747. doi: 10.1021/jm960306+. [DOI] [PubMed] [Google Scholar]
- MORAN-JIMENEZ M.J., MATUTE C. Immunohistochemical localization of the P2Y1 purinergic receptor in neurons and glial cells of the central nervous system. Brain Res. Mol. Brain. Res. 2000;78:50–58. doi: 10.1016/s0169-328x(00)00067-x. [DOI] [PubMed] [Google Scholar]
- MORO S., GUO D., CAMAIONI E., BOYER J.L., HARDEN T.K., JACOBSON K.A. Human P2Y1 receptor: molecular modeling and site-directed mutagenesis as tools to identify agonist and antagonist recognition sites. J. Med. Chem. 1998;41:1456–1466. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANDANAN E., CAMAIONI E., JANG S.Y., KIM Y.C., CRISTALLI G., HERDEWIJN P., SECRIST J.A., III, TIWARI K.N., MOHANRAM A., HARDEN T.K., BOYER J.L., JACOBSON K.A. Structure–activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists. J. Med. Chem. 1999;42:1625–1638. doi: 10.1021/jm980657j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANDANAN E., JANG S.Y., MORO S., KIM H.O., SIDDIQUI M.A., RUSS P., MARQUEZ V.E., BUSSON R., HERDEWIJN P., HARDEN T.K., BOYER J.L., JACOBSON K.A. Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. J. Med. Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER R.K., BOYER J.L., SCHACHTER J.B., NICHOLAS R.A., HARDEN T.K. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol. Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- RODRIGUES R.J., ALMEIDA T., RICHARDSON P.J., OLIVEIRA C.R., CUNHA R.A. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J. Neurosci. 2005;25:6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER J.B., HARDEN T.K. An examination of deoxyadenosine 5′(alpha-thio)triphosphate as a ligand. Br. J. Pharmacol. 1997;121:338–344. doi: 10.1038/sj.bjp.0701136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER J.B., LI Q., BOYER J.L., NICHOLAS R.A., HARDEN T.K. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoreceptor. Br. J. Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIBLER P., PESIC M., FRANKE H., REINHARDT R., WIRKNER K., ILLES P., NORENBERG W. P2X2 and P2Y1 immunofluorescence in rat neostriatal medium-spiny projection neurons and cholinergic interneurones is not linked to respective purinergic receptor function. Br. J. Pharmacol. 2004;143:119–131. doi: 10.1038/sj.bjp.0705916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINOZAKI Y., KOIZUMI S., ISHIDA S., SAWADA J., OHNO Y., INOUE K. Cytoprotection against oxidative stress-induced damage of astrocytes by extracellular ATP via P2Y1 receptors. Glia. 2005;49:288–300. doi: 10.1002/glia.20118. [DOI] [PubMed] [Google Scholar]
- SIMON J., WEBB T.E., BERNARD E.A. Distribution of [35S]dATPαS binding sites in the adult rat neuraxis. Neuropharmacology. 1997;36:1243–1251. doi: 10.1016/s0028-3908(97)00124-x. [DOI] [PubMed] [Google Scholar]
- TOKUYAMA Y., HARA M., JONES E.M., FAN Z., BELL G.I. Cloning of rat and mouse P2Y purinoreceptors. Biochem. Biophys. Res. Commun. 1995;211:211–218. doi: 10.1006/bbrc.1995.1798. [DOI] [PubMed] [Google Scholar]
- WALDO G.L., HARDEN T.K. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol. Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- WALDO G.L., CORBITT J., BOYER J.L., RAVI G., KIM H.S., JI X.D., LACY J., JACOBSON K.A., HARDEN T.K. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol. Pharmacol. 2002;62:1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG L.K., SHUMAN S. Mutational analysis defines the 5′-kinase and 3′-phosphatase active sites of T4 polynucleotide kinase. Nucleic Acids Res. 2002;30:1073–1080. doi: 10.1093/nar/30.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIOKA K., HOSODA R., KURODA Y., NAKATA H. Heterooligomerization of adenosine A1 receptors with P2Y1 receptors in rat brains. FEBS Lett. 2002;531:299–303. doi: 10.1016/s0014-5793(02)03540-8. [DOI] [PubMed] [Google Scholar]